Recovery of Copper from Pregnant Leach Solutions of Copper Concentrate Using Aluminum Shavings
Abstract
1. Introduction
2. Materials and Methods
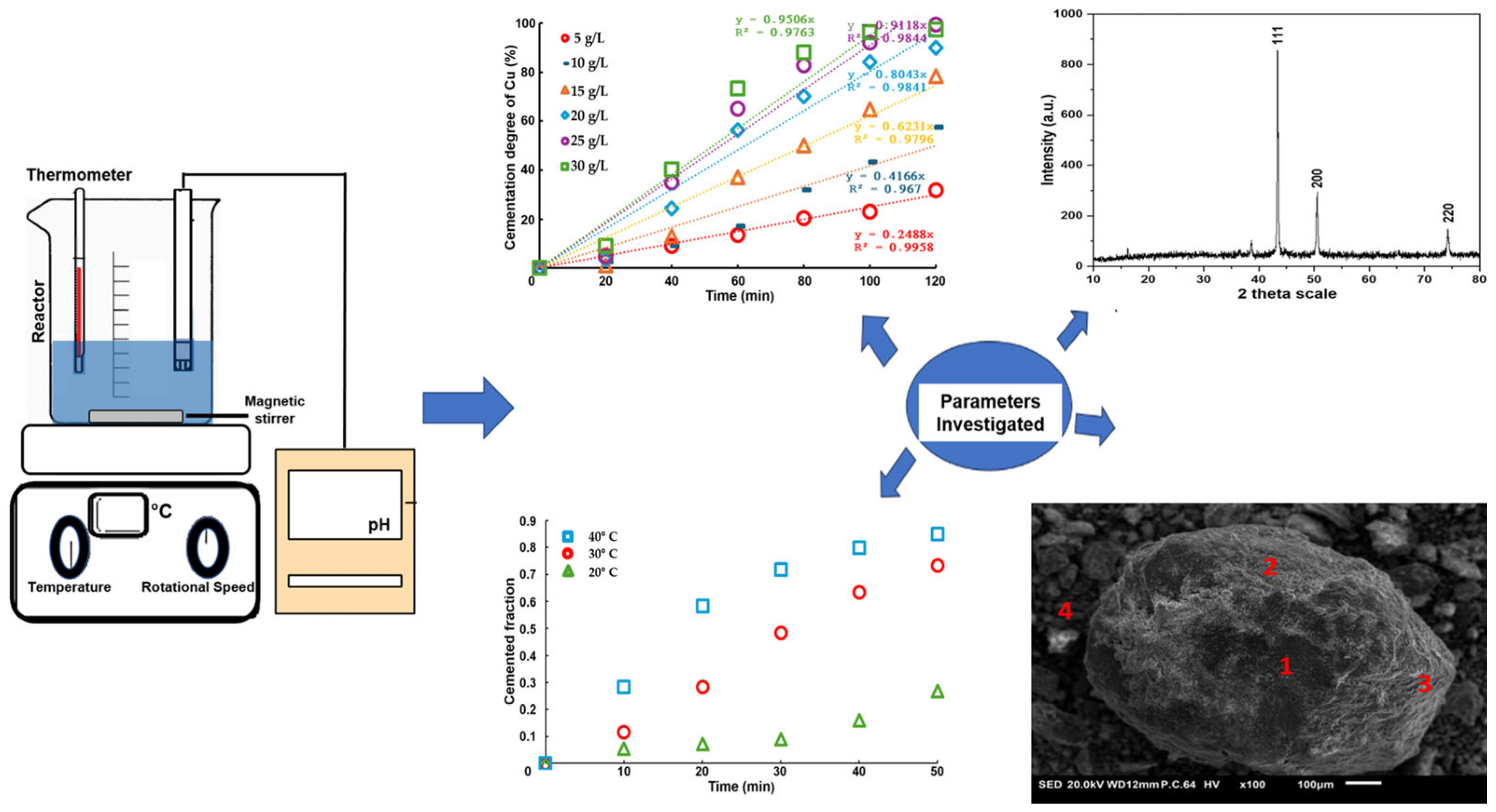
2.1. Experimental Development
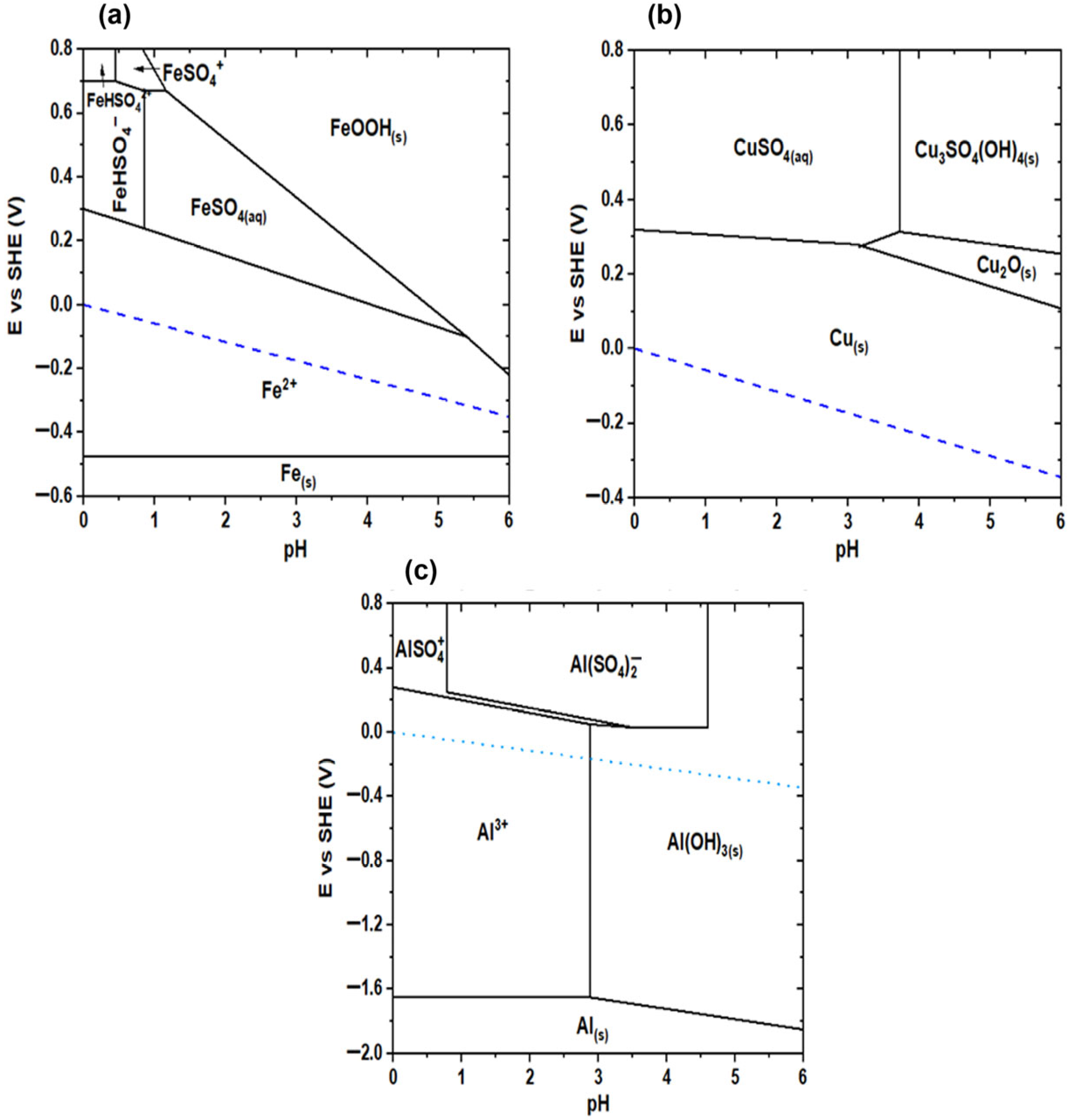
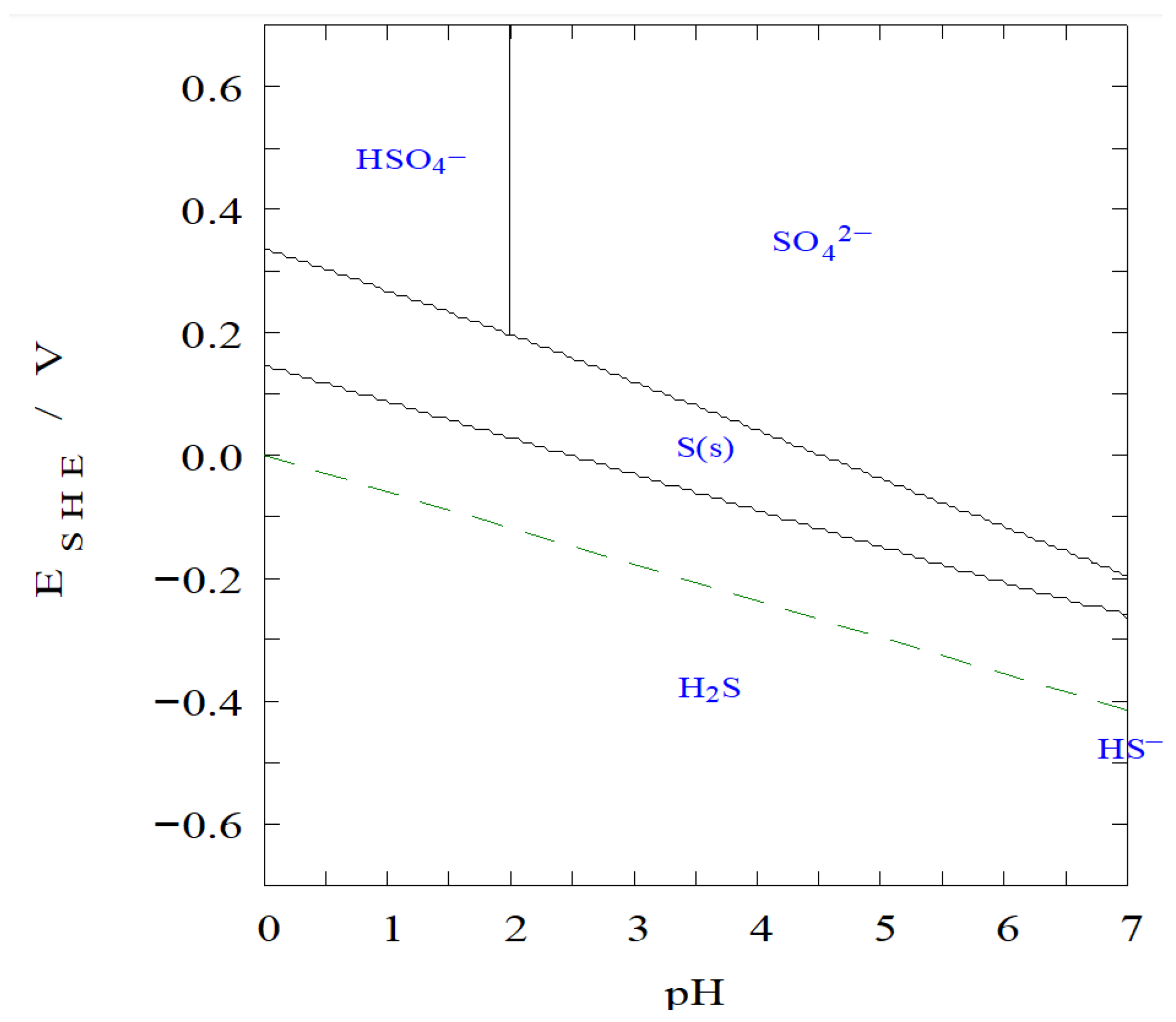
2.2. Thermodynamic Analysis
3. Results
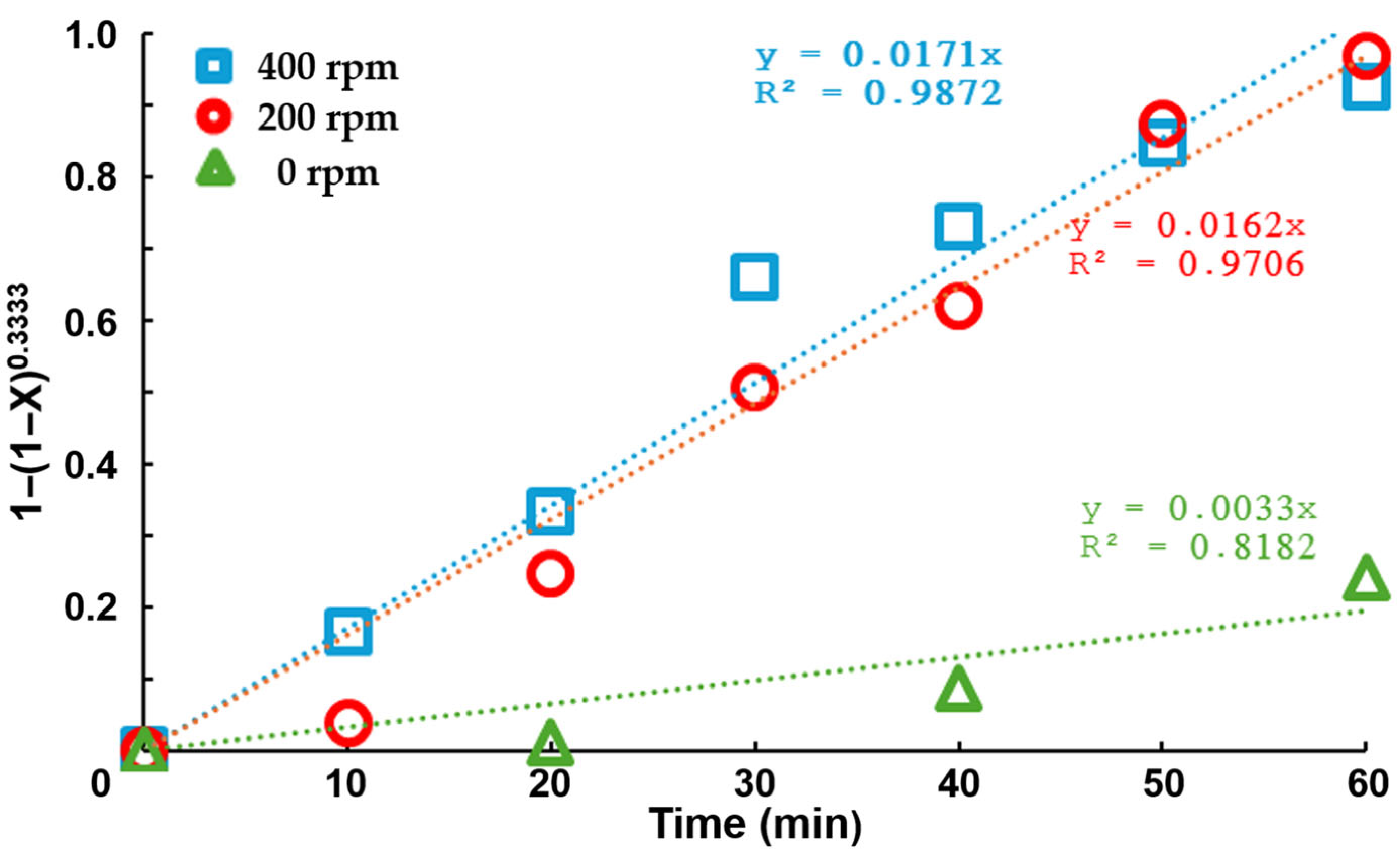
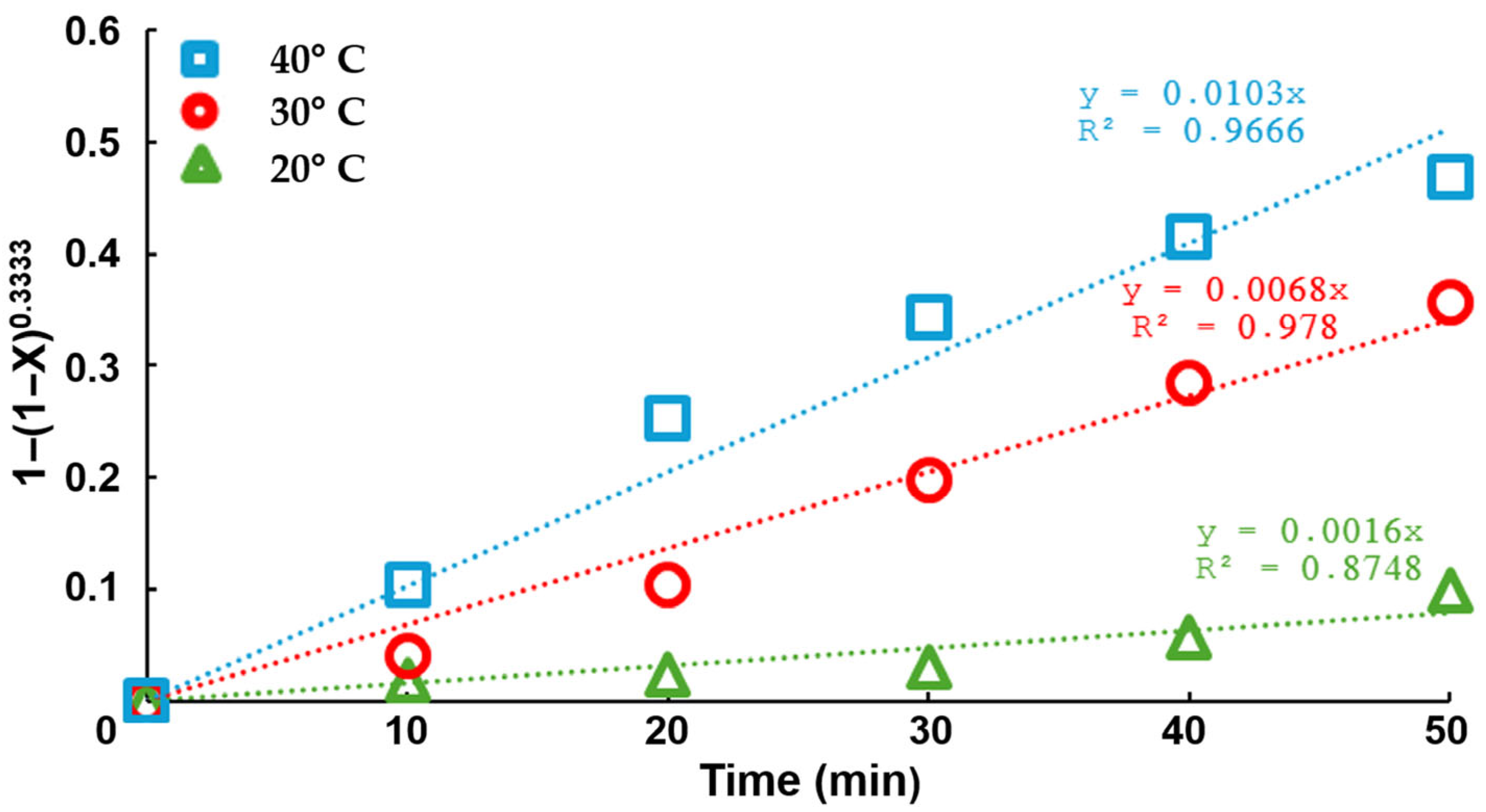
Cementation Test
4. Conclusions
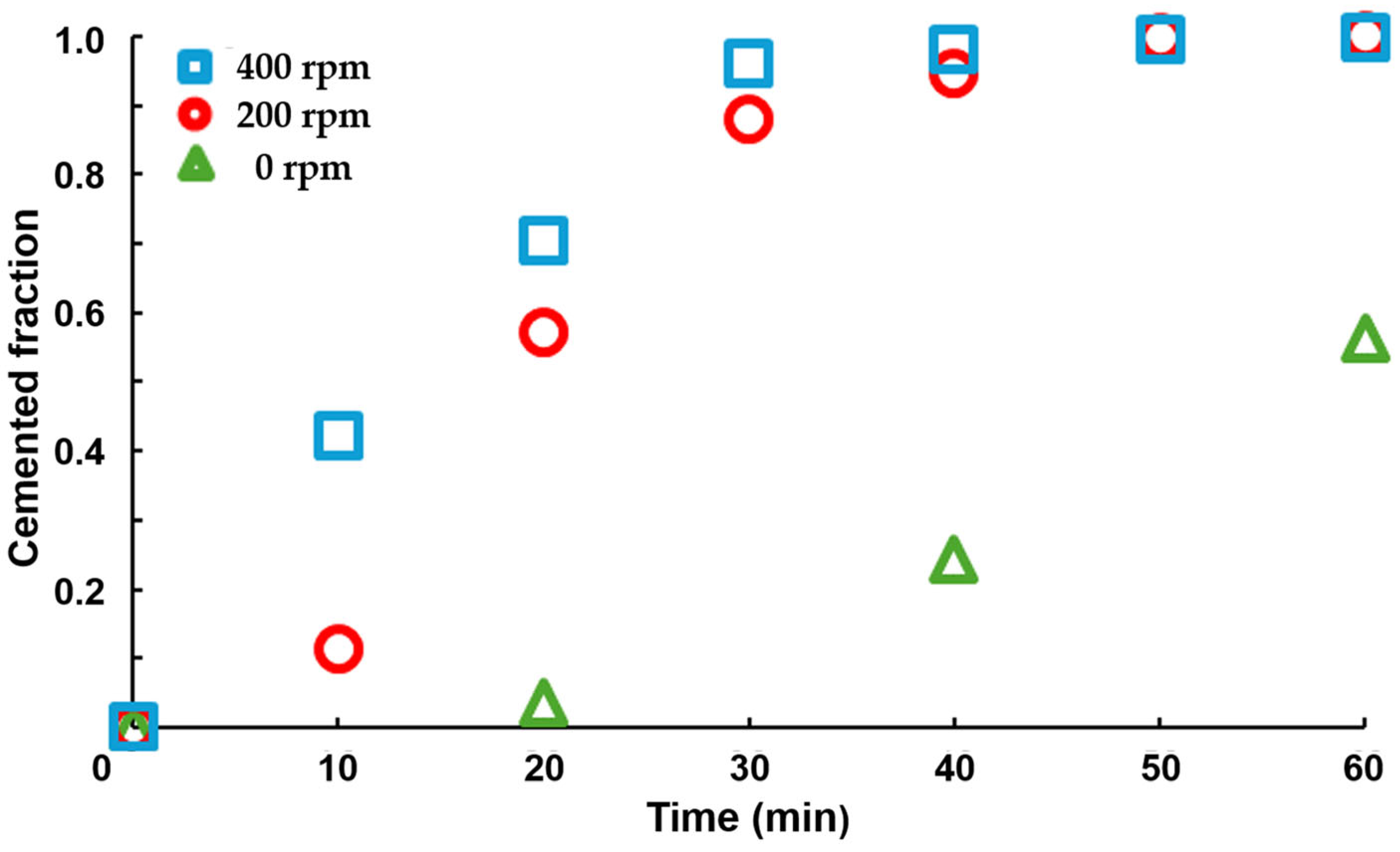
- The shrinking core model is adequately fitted to the experimental results. The lower stage is dependent on the operation conditions. In the absence of stirring, the limit step is the diffusion of the reactants to particles and the observed kinetics follow a zero-order model, indicating that the process control is not dominated by reactant diffusion but rather by the availability of the cementing agent.
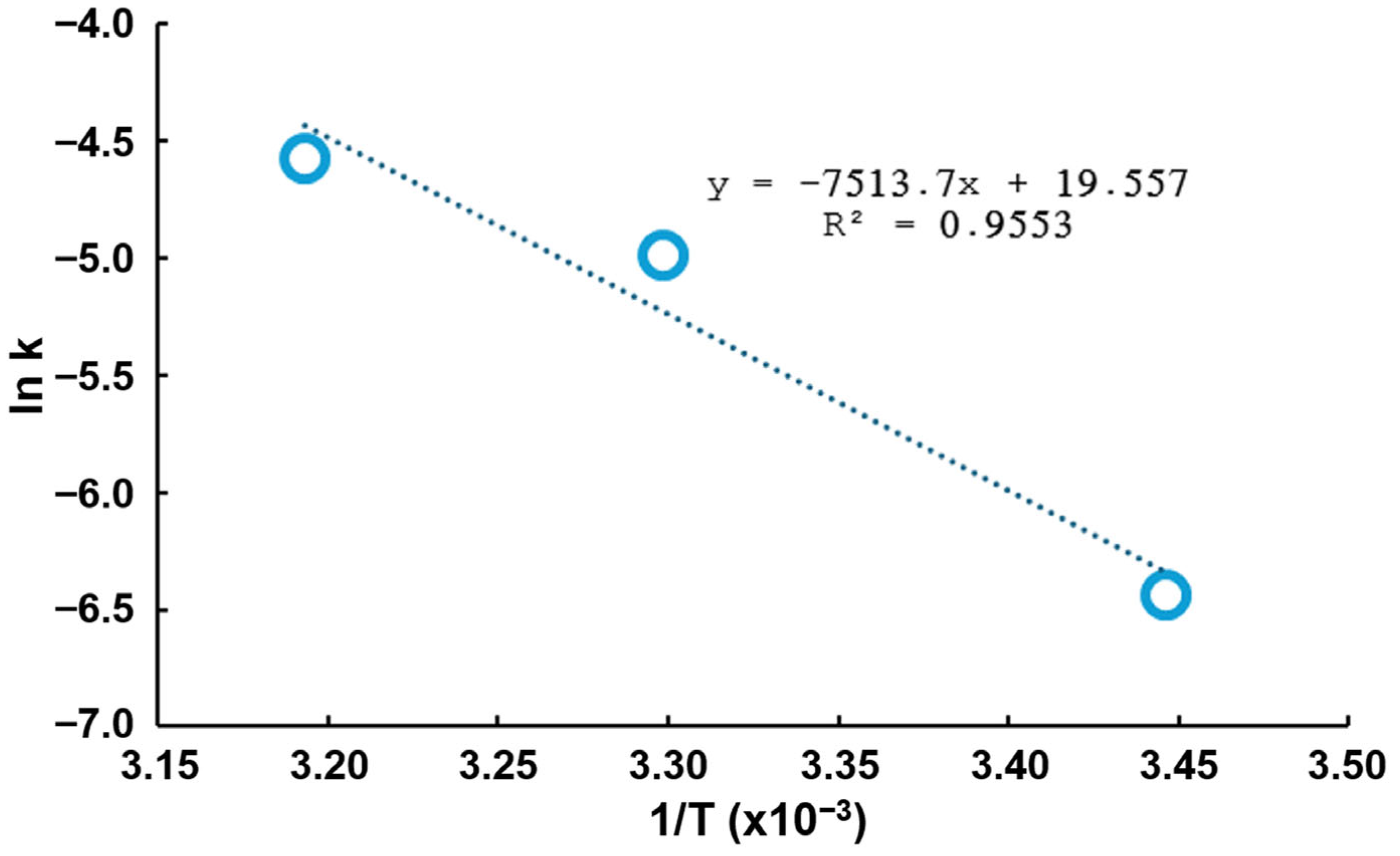
- Additionally, an activation energy of 62.6 kJ/mol was determined within the temperature range of 20 to 40 °C, suggesting that the chemical stage of the process predominantly controls the reaction rate. Increasing the temperature accelerated the cementation kinetics and enhanced the process efficiency by improving copper deposition on the aluminum surface, reducing the impurity content in the final precipitate.
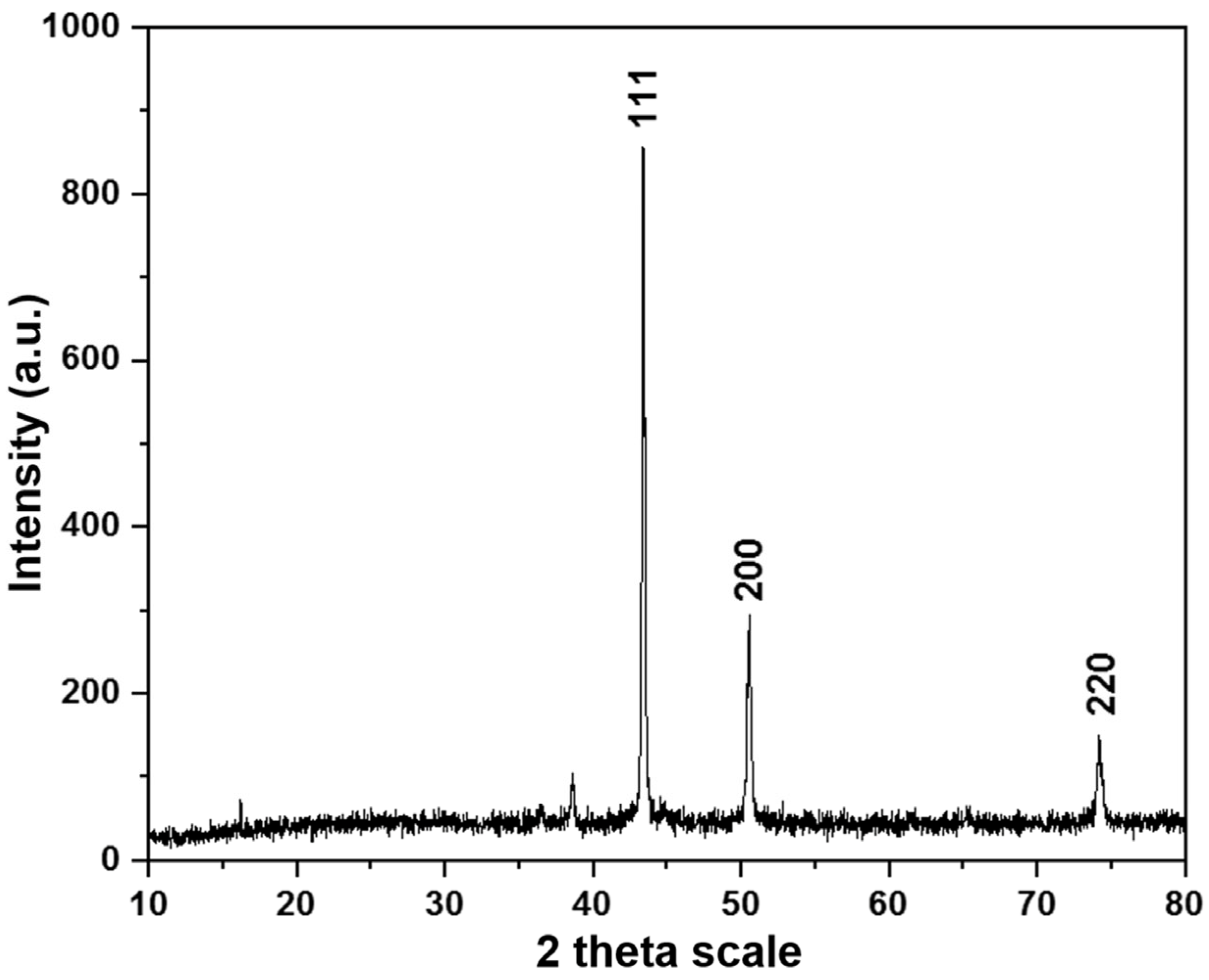
- X-Ray Diffraction analysis confirmed the formation of metallic copper with a face-centered cubic crystalline structure, verifying the purity of the precipitated copper. Overall, the results highlight the feasibility of using aluminum shavings as a cementing agent for copper recovery from Pregnant Leach Solutions, achieving a high efficiency and selectivity. However, further studies are recommended to assess process stability on a larger scale and under variable operating conditions to optimize its industrial application.
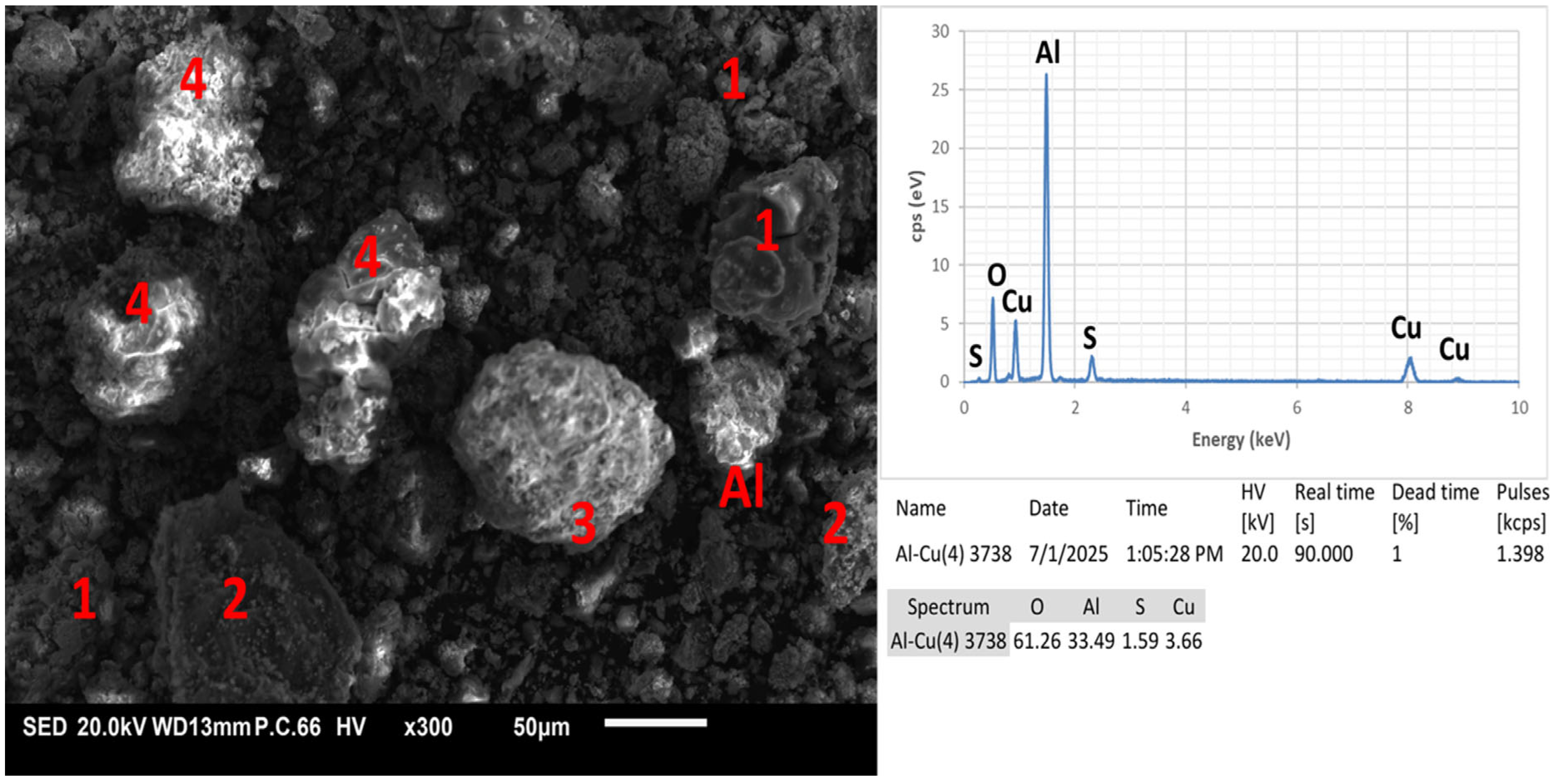
- Through morphological and compositional analysis using SEM-EDX, the presence of copper precipitates with varying degrees of surface oxidation was confirmed. Particles with a high oxygen and aluminum content were identified, suggesting the formation of aluminum hydroxides or oxides, likely as secondary products from the leaching reactions.
- The detection of elemental sulfur on the surface of copper particles indicates the possible partial oxidation of sulfur species or residues from the sulfidation process. The brightest particles in the micrographs exhibit a spectral profile characteristic of metallic aluminum, indicating that some aluminum remained unreacted during the experimental process. From a thermodynamic standpoint, this observation aligns with the stability domains predicted in the Pourbaix diagram for sulfur, where elemental sulfur is favored under the experimental conditions (pH 1.7 and –337 mV vs. SHE), being close to the stability window of S0. This proximity may have allowed the partial precipitation of sulfur, rather than its full conversion into soluble sulfide species such as H2S or HS−. The information obtained through SEM-EDX complements the XRD results, as it allows for a spatial visualization of elemental distribution and confirms the coexistence of multiple solid phases at the microscale.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirkıran, N.; Ekmekyapar, A.; Künkül, A.; Baysar, A. A kinetic study of copper cementation with zinc in aqueous solutions. Int. J. Miner. Process. 2007, 82, 80–85. [Google Scholar] [CrossRef]
- Vakylabad, A.B.; Scaffie, M.; Naseri, A.; Ranjabar, M.; Manafi, Z. A procedure for processing of pregnant leach solution (PLS) produced from a chalcopyrite-ore-bio-heap: CuO Nano-powder fabrication. Hydrometallurgy 2016, 163, 24–32. [Google Scholar] [CrossRef]
- Demirkiran, N.; Künkül, A. Recovering of copper with metallic aluminum. Trans. Nonferrous Met. Soc. China 2011, 21, 2778–2782. [Google Scholar] [CrossRef]
- Kazakova, N.; Lucheva, B.; Llev, P. A study on the cementation process of non-ferrous metals from brine leaching solution. J. Chem. Technol. Met. 2020, 55, 223–227. [Google Scholar]
- Costa, C.D.; Triana, M.J.H.; Avila, M.; Diz, V.E.; González, G.A. Evaluating the effectiveness of iron oxide (Fe3O4) nanoparticles vs. traditional chloride methods for copper cementation and recovery from industrial waste solutions by aluminium. Hydrometallurgy 2025, 236, 106528. [Google Scholar] [CrossRef]
- Aatach, M.; Simão, M.A.; Gaydardzhiev, S. Effects of ultrasound on the electrochemical cementation of copper onto iron. Miner. Eng. 2024, 213, 108750. [Google Scholar] [CrossRef]
- Djokic, S.S. Cementation of copper on aluminum in alkaline solutions. J. Electrochem. Soc. 1996, 4, 1300–1304. [Google Scholar] [CrossRef]
- Annamali, V.; Hiskey, J.B.; Murr, L.E. The effects of kinetic variables on the structure of copper deposits cemented on pure aluminum discs: A Scanning Electron Microscopic study. Hydrometallurgy 1978, 3, 163–180. [Google Scholar] [CrossRef]
- Karavasteva, M. Kinetics and deposit morphology of copper cementation onto zinc, iron and aluminum. Hydrometallurgy 2005, 76, 149–152. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, B.; Jordon, J. Standard Potentials in Aqueous Solutions; Dekker: New York, NY, USA, 1985. [Google Scholar]
- Milazzo, G.; Caroli, S.; Sharma, V.K. Tables of Standard Electrode Potentials; Wiley: London, UK, 1978. [Google Scholar]
- Dönmez, B.; Sevim, F.; Sarac, H. A kinetic study of the cementation of copper from sulphate solutions onto a rotating aluminum disc. Hydrometallurgy 1999, 53, 145–154. [Google Scholar] [CrossRef]
- Ahmed, I.M.; El-Nadi, Y.A.; Daoud, J.A. Cementation of copper from spent copper-pickle sulfate solution by zinc ash. Hydrometallurgy 2011, 110, 62–66. [Google Scholar] [CrossRef]
- Amin, N.K.; El-Ashtoukhy, E.-S.Z. Kinetic study of copper cementation onto zinc using a rotating packed bed cylindrical reactor. Can. J. Chem. Eng. 2011, 89, 609–616. [Google Scholar] [CrossRef]
- Costa, C.D.; Lustig, D.; D’Angelo, M.V.; González, G.A. Copper recovery by cementing from waste solutions derived from the manufacturing/printing industry. J. Environ. Chem. Eng. 2020, 8, 103989. [Google Scholar] [CrossRef]
- Fadali, O.A. Effect of Drag-Reducing Polymer on the Rate of Cementation of Copper Ion on Zinc Pellets. Chem. Eng. Technol. 2003, 26, 491–495. [Google Scholar] [CrossRef]
- Granata, G.; Tsendorj, U.; Liu, W.; Tokoro, C. Direct recovery of copper nanoparticles from leach pad drainage by surfactant-assisted cementation with iron powder. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123719. [Google Scholar] [CrossRef]
- Konaté, F.O.; Vitry, V.; Yonli, A.H. Leaching of base metals in PCBs and copper cementation by iron powder. J. Hazard. Mater. Adv. 2024, 15, 100449. [Google Scholar] [CrossRef]
- Solís-Marcial, O.J.; Lapidus, G.T. Study of the Dissolution of Chalcopyrite in Sulfuric Acid Solutions Containing Alcohols and Organic Acids. Electrochim. Acta 2014, 140, 434–437. [Google Scholar] [CrossRef]
- Solís-Marcial, O.J.; Lapidus, G.T. Chalcopyrite leaching in alcoholic acid media. Hydrometallurgy 2014, 147, 54–58. [Google Scholar] [CrossRef]
- NIST Standard Reference Database 46; Version 8. Critically Selected Stability Constants of Metal Complexes. National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2004.
- Eriksson, G. An algorithm for the computation of aqueous multicomponent, multiphase equilibria. Anal. Chim. Acta 1979, 112, 375–383. [Google Scholar] [CrossRef]
- Puigdomenech, I. Make Equilibrium Diagrams Using Sophisticated Algorithms (MEDUSA); Inorganic Chemistry, Royal Institute of Technology: Stockholm, Sweden, 2004. [Google Scholar]



| Stage | Concentration of Cu [g/L] | Concentration of Fe [g/L] | pH | Redox Potential vs. (SHE) (mV) |
|---|---|---|---|---|
| Initial | 53.5 | 51.7 | 1.9 | 637 |
| Final | 0.5 | 51 | 1.7 | −337 |
| Compound | |
|---|---|
| 0.0 | |
| −744.5 | |
| 0.0 | |
| −292.72 | |
| −1306.0 | |
| 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solís Marcial, O.J.; Nájera-Bastida, A.; Soriano-Vargas, O.; Ruelas Leyva, J.P.; Talavera-López, A.; Inchaurregui, H.; Zárate Gutiérrez, R. Recovery of Copper from Pregnant Leach Solutions of Copper Concentrate Using Aluminum Shavings. Minerals 2025, 15, 1048. https://doi.org/10.3390/min15101048
Solís Marcial OJ, Nájera-Bastida A, Soriano-Vargas O, Ruelas Leyva JP, Talavera-López A, Inchaurregui H, Zárate Gutiérrez R. Recovery of Copper from Pregnant Leach Solutions of Copper Concentrate Using Aluminum Shavings. Minerals. 2025; 15(10):1048. https://doi.org/10.3390/min15101048
Chicago/Turabian StyleSolís Marcial, Oscar Joaquín, Alfonso Nájera-Bastida, Orlando Soriano-Vargas, José Pablo Ruelas Leyva, Alfonso Talavera-López, Horacio Inchaurregui, and Roberto Zárate Gutiérrez. 2025. "Recovery of Copper from Pregnant Leach Solutions of Copper Concentrate Using Aluminum Shavings" Minerals 15, no. 10: 1048. https://doi.org/10.3390/min15101048
APA StyleSolís Marcial, O. J., Nájera-Bastida, A., Soriano-Vargas, O., Ruelas Leyva, J. P., Talavera-López, A., Inchaurregui, H., & Zárate Gutiérrez, R. (2025). Recovery of Copper from Pregnant Leach Solutions of Copper Concentrate Using Aluminum Shavings. Minerals, 15(10), 1048. https://doi.org/10.3390/min15101048






