Study on the Drying Characteristics of Moist Fine Lignite in a Dense Gas–Solid Separation Fluidized Bed
Abstract
1. Introduction
2. Materials and Methods
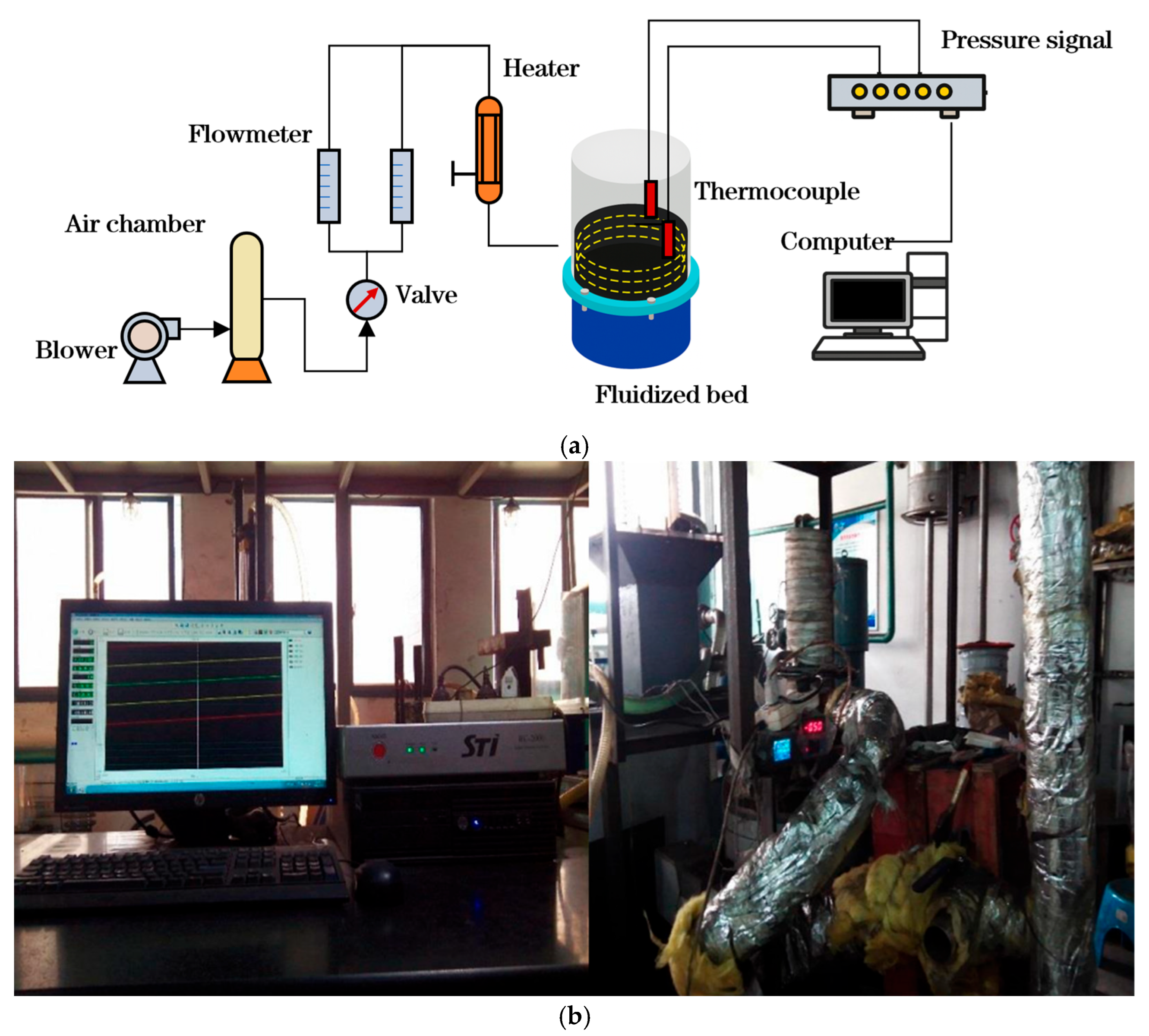
2.1. Experimental Setup
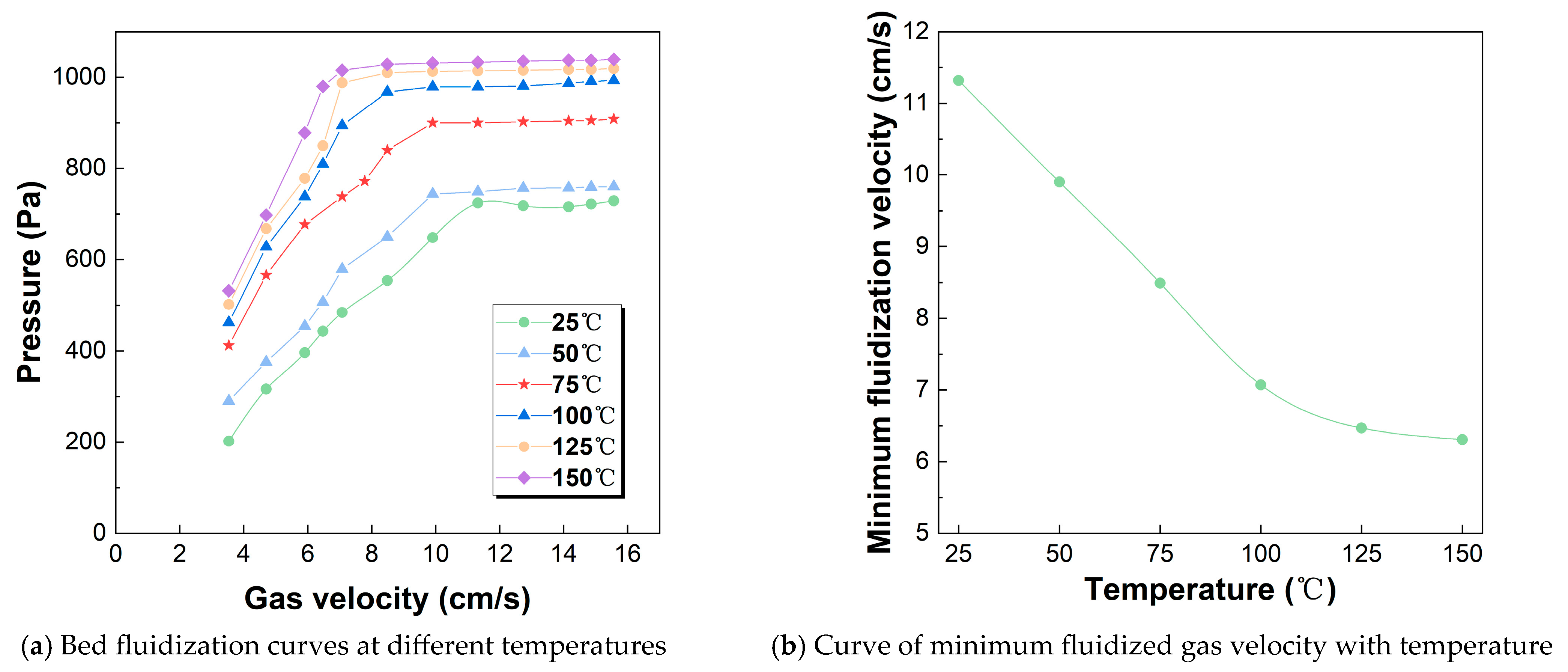
2.2. Determination of Minimum Fluidization Velocity
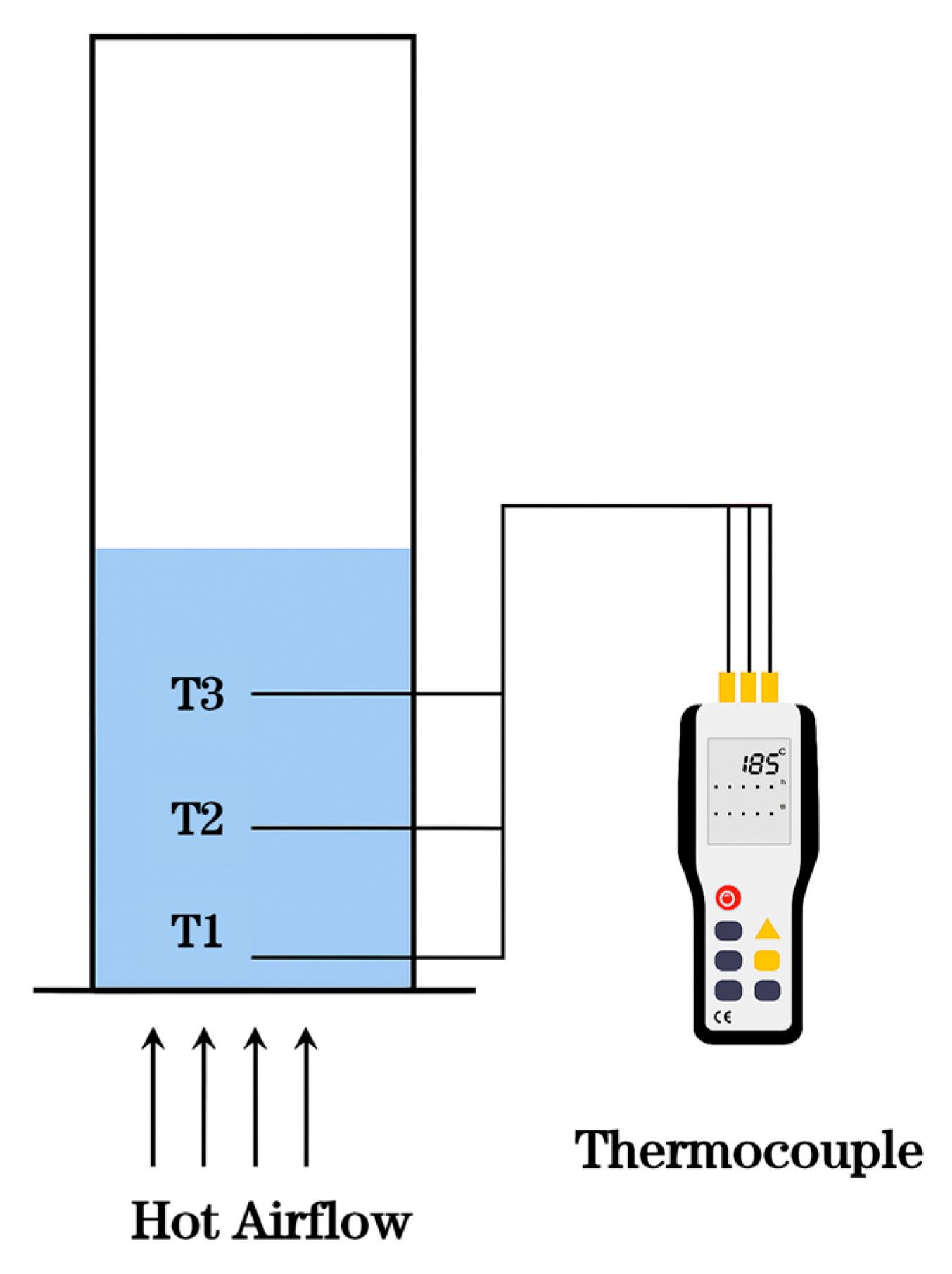
2.3. Temperature Measurement
2.4. Coal Drying Tests
2.5. Sample Characteristics
3. Results and Discussion
3.1. Fluidization Characteristics in a Hot Gas–Solid Fluidized Bed
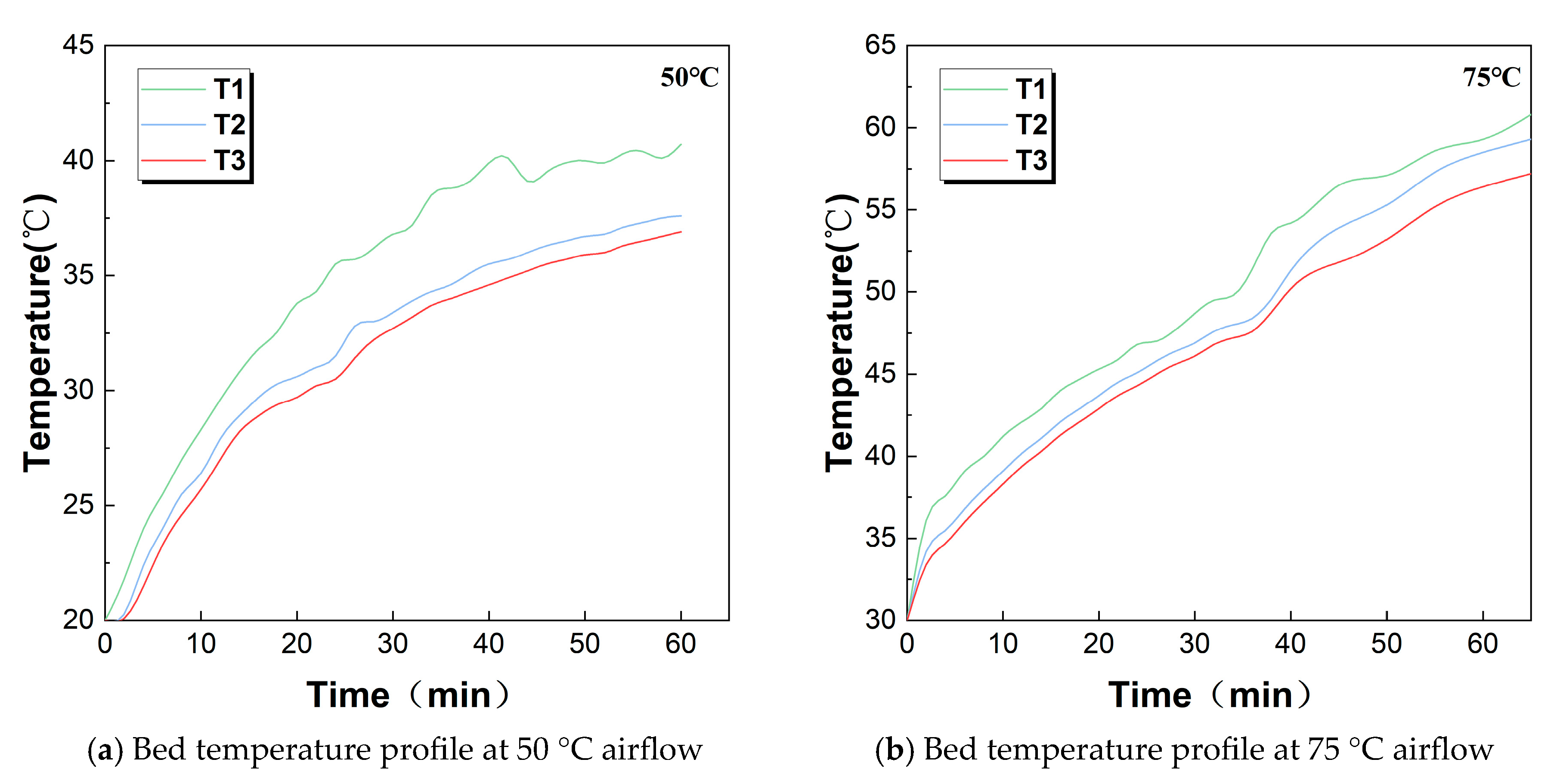
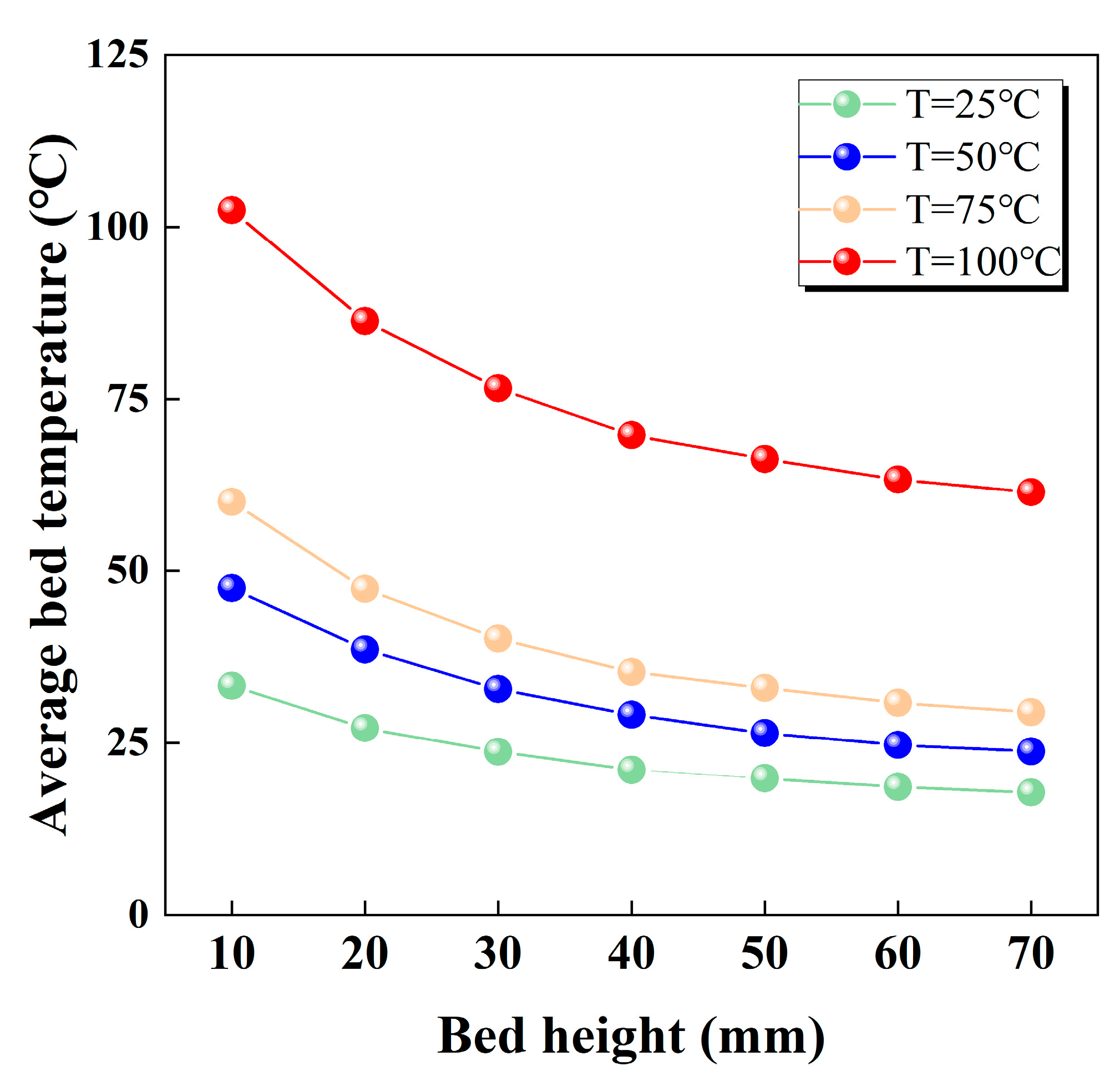
3.2. Heat Transfer Behavior in the Bed
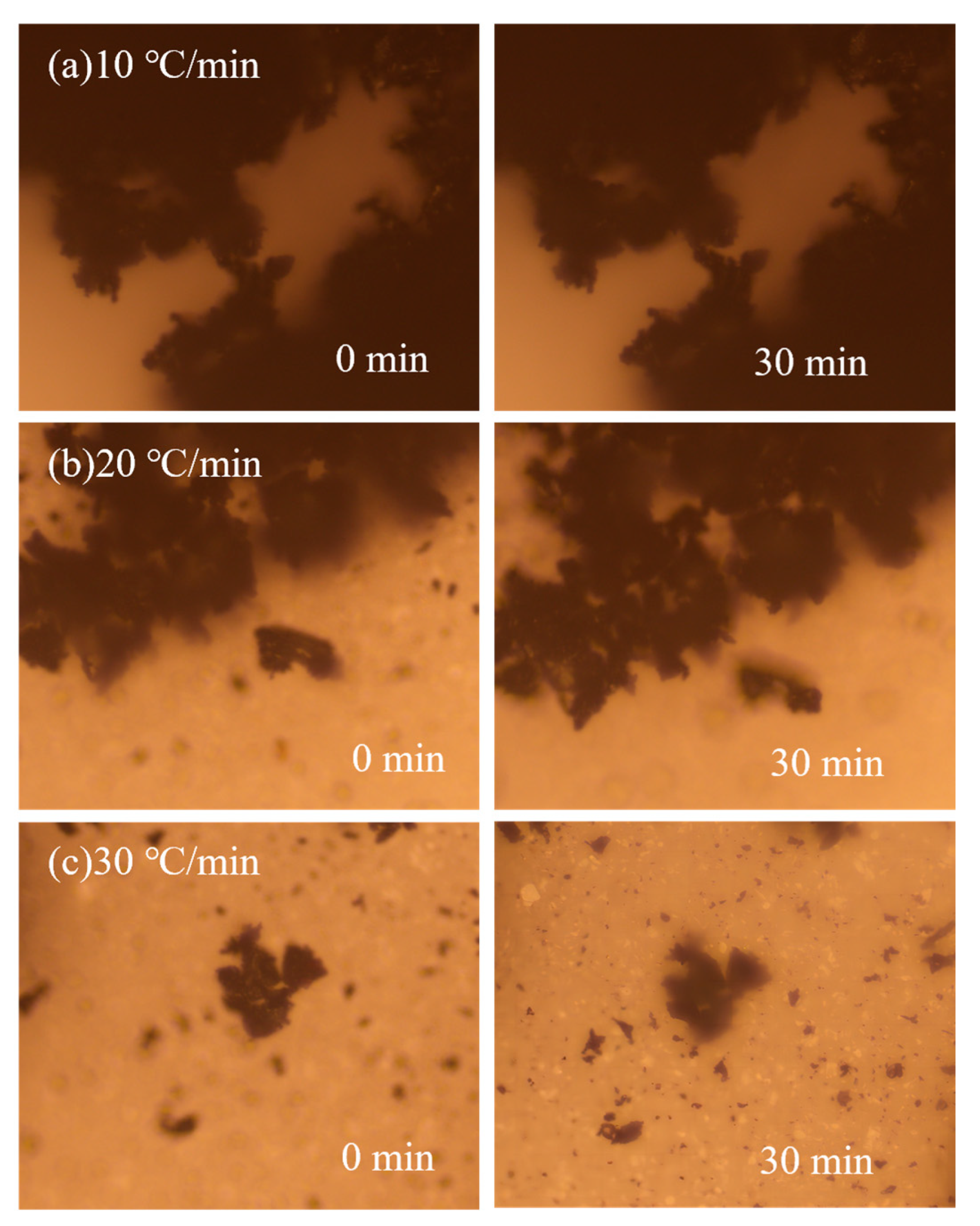
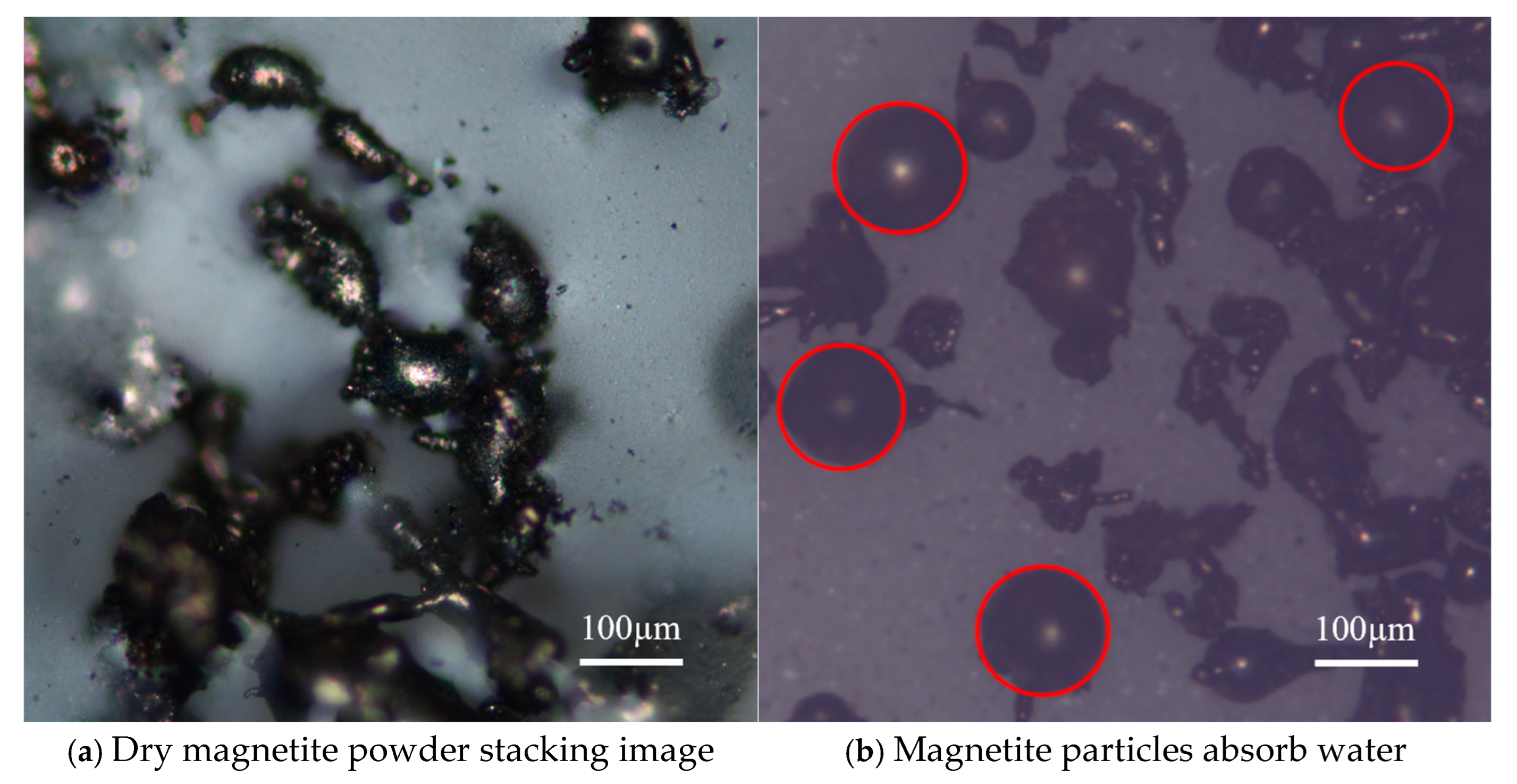
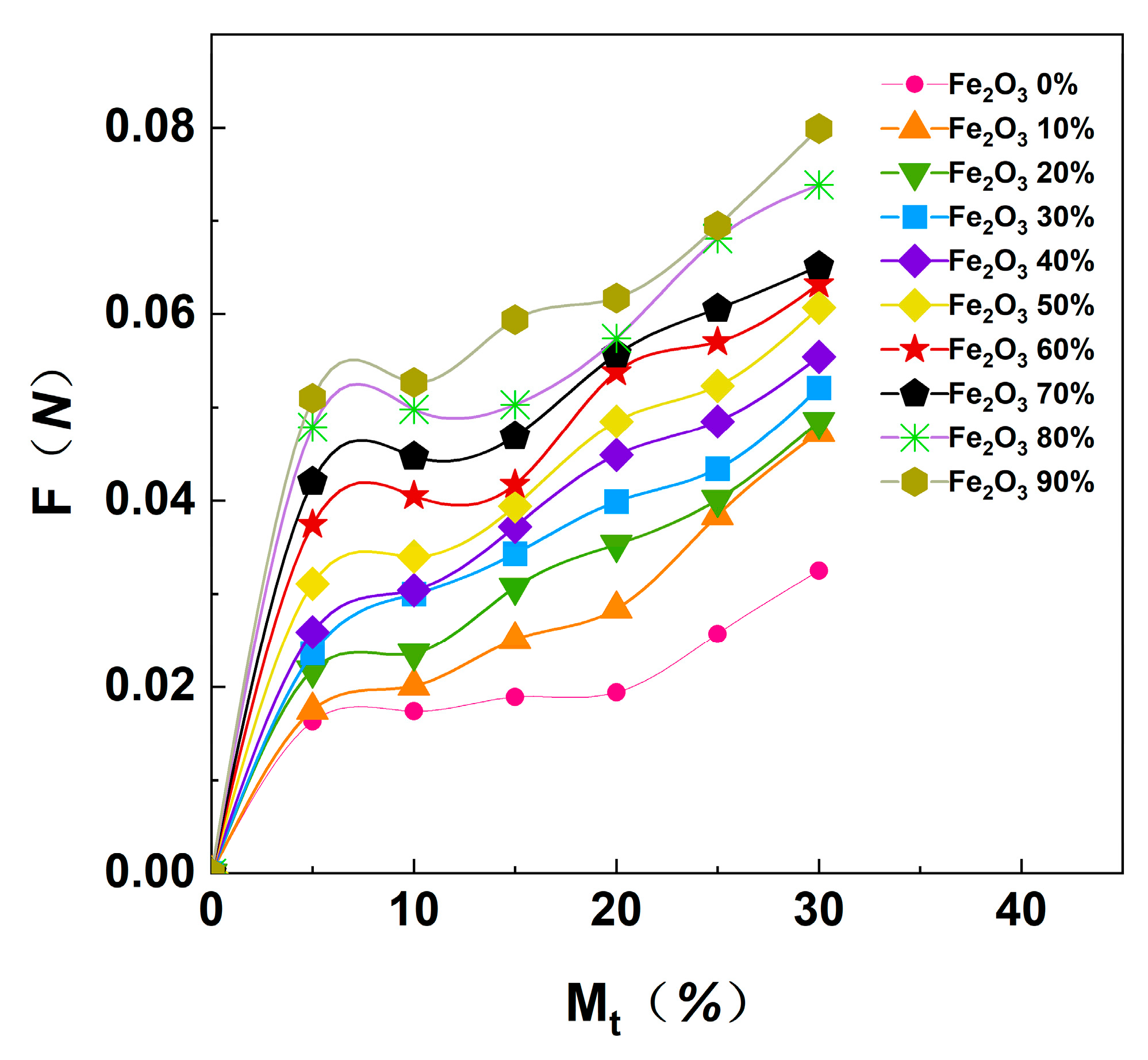
3.3. Analysis of the Drying Properties of Magnetite Powder–Coal Powder Agglomeration
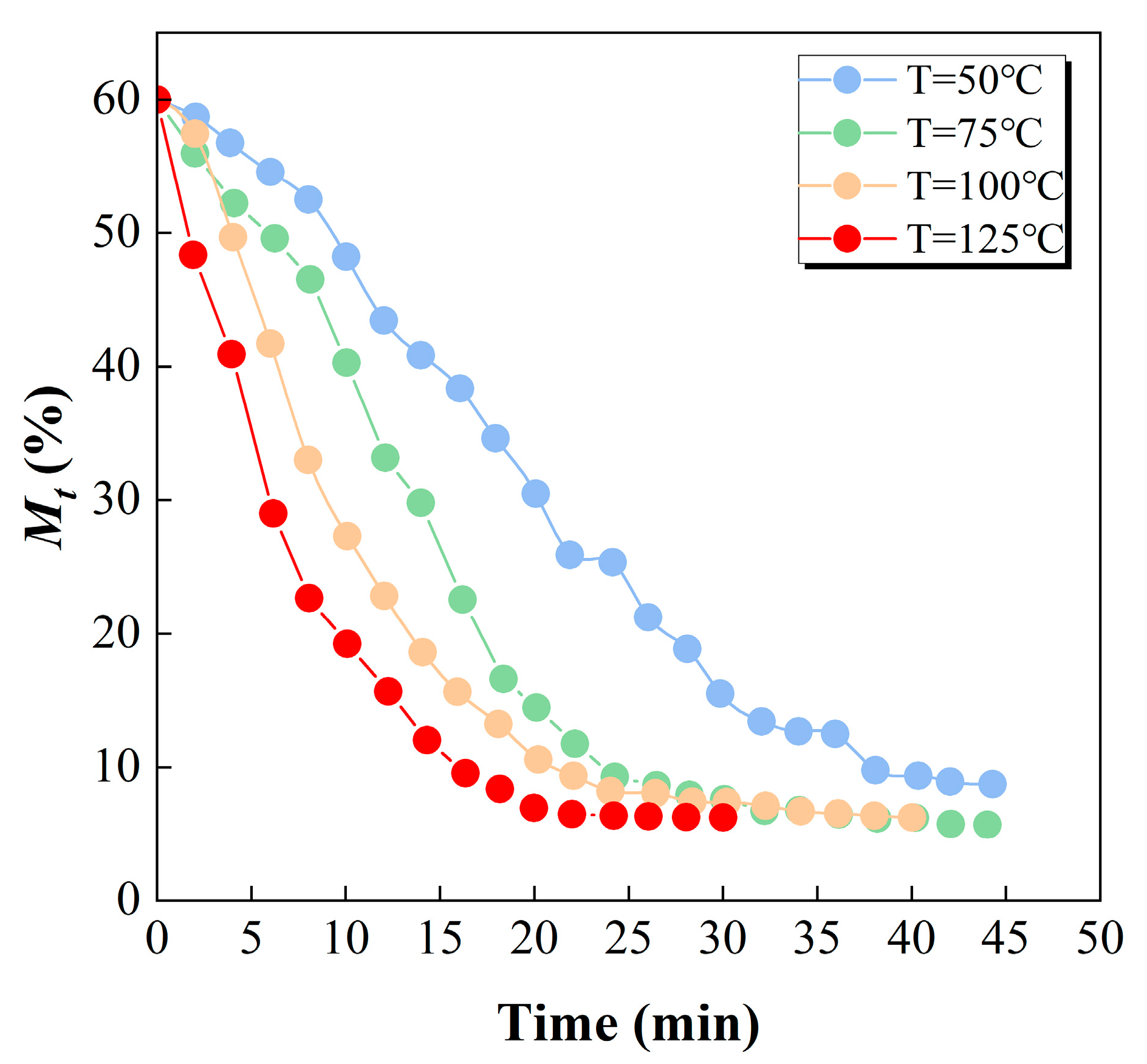
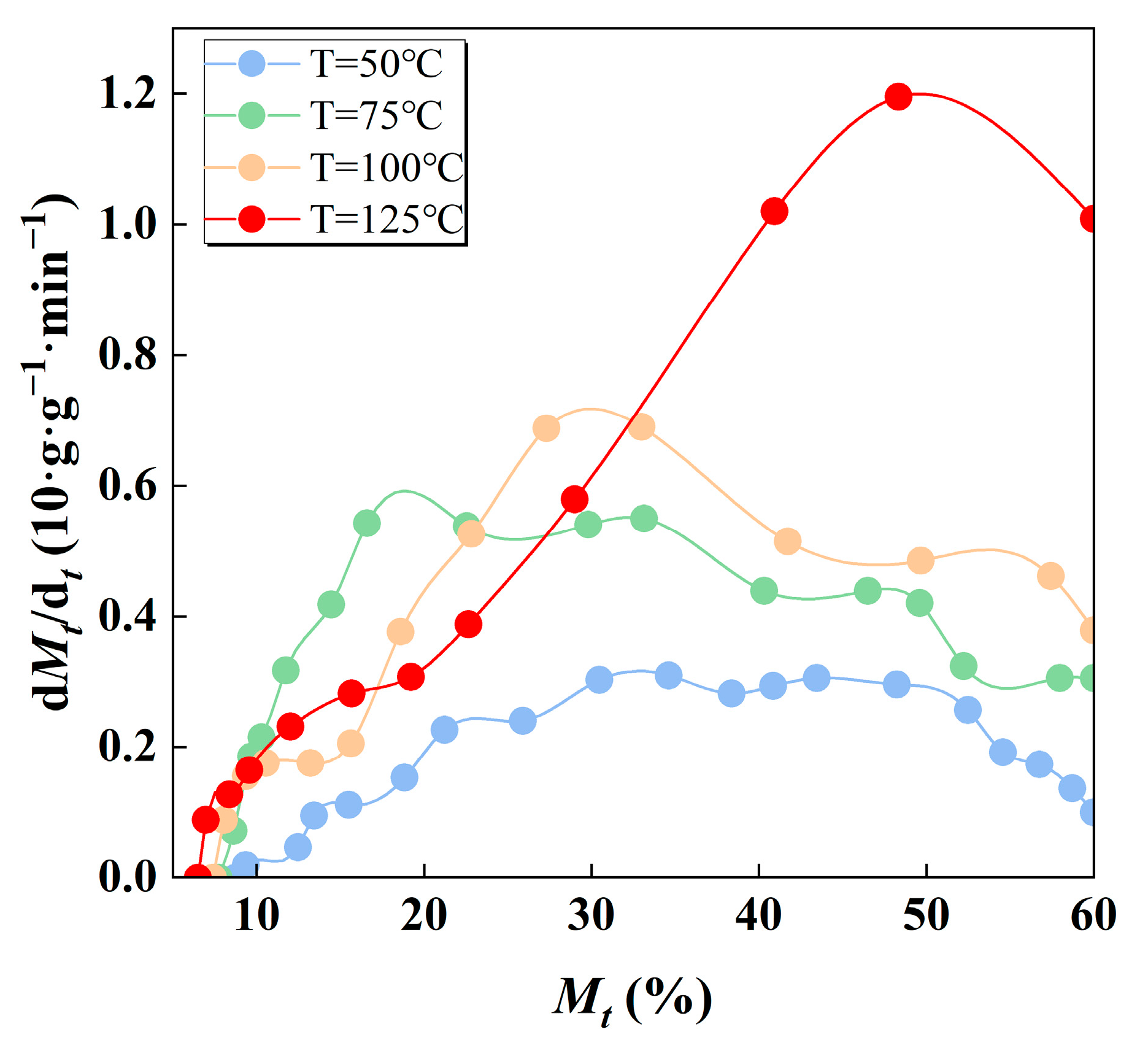
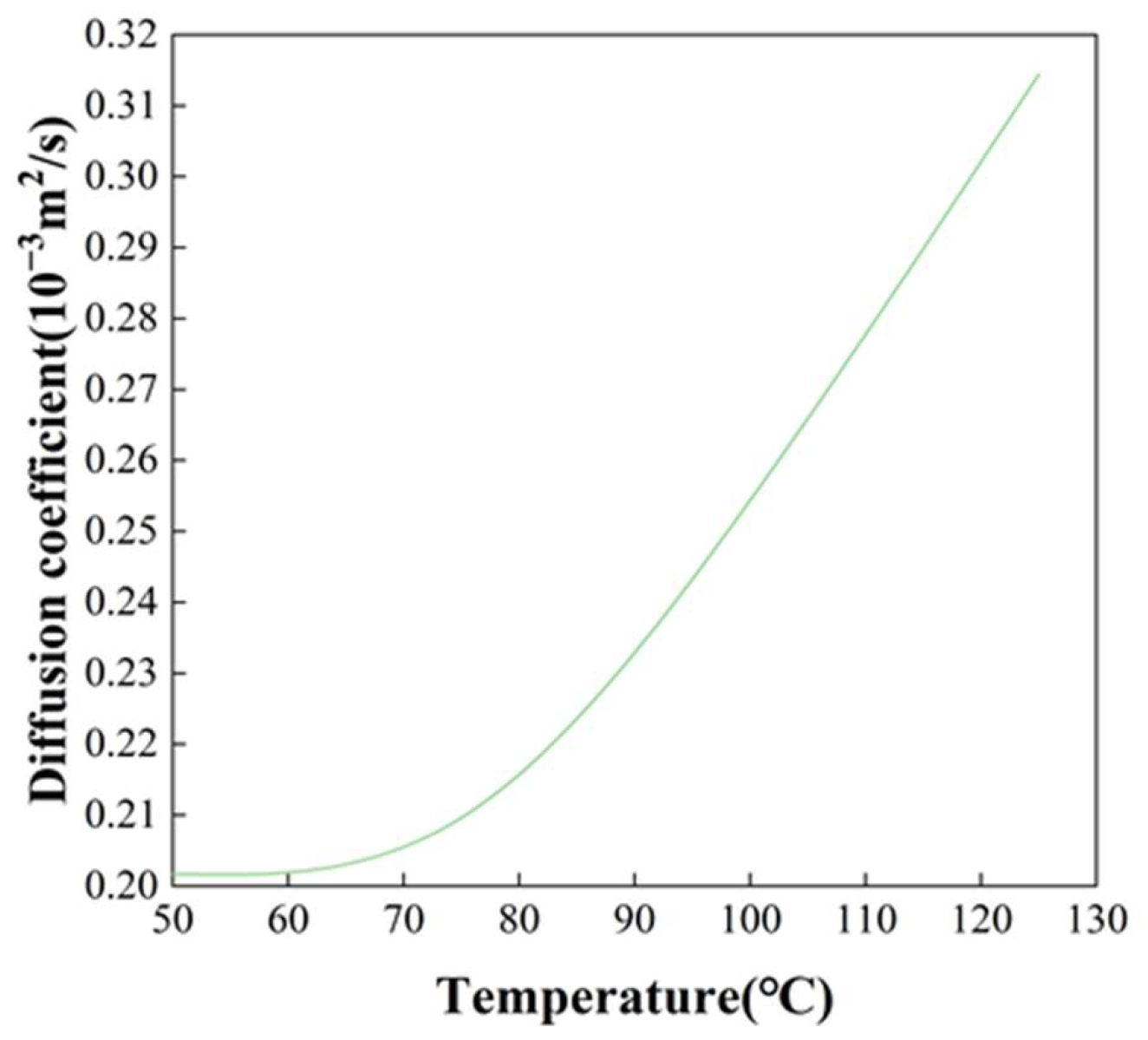
3.4. Drying Kinetics of Fine Coal Particles
3.5. Analysis of Calorific Value, Ash Content and Total Moisture of Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Mad | Moisture on the air-dry basis |
| Ad | Ash content on a dry basis |
| Vdaf | Volatile matter on a dry ash-free basis |
| FCad | Fixed carbon content on an air-dried basis |
| Cdaf | Carbon content on a dry ash-free basis |
| Hdaf | Hydrogen content on a dry ash-free basis |
| Odaf | Oxygen content on a dry ash-free basis |
| Ndaf | Nitrogen content on a dry ash-free basis |
| Sdaf | Sulfur content on a dry ash-free basis |
References
- You, C.F.; Wang, H.M.; Zhang, K. Moisture Adsorption Properties of Dried Lignite. Energy Fuels 2013, 27, 177–182. [Google Scholar] [CrossRef]
- Rao, Z.H.; Zhao, Y.M.; Huang, C.L.; Duan, C.L.; He, J.F. Recent developments in drying and dewatering for low rank coals. Prog. Energy Combust. Sci. 2015, 46, 1–11. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, G.Q.; Xie, R.L.; Zhao, Z.G.; Cui, P. A review on moisture re-adsorption of lignite treated using different drying techniques. Dry. Technol. 2022, 40, 1263–1277. [Google Scholar] [CrossRef]
- Nikolopoulos, N.; Violidakis, I.; Karampinis, E.; Agraniotis, M.; Bergins, C.; Grammelis, P.; Kakaras, E. Report on comparison among current industrial scale lignite drying technologies (A critical review of current technologies). Fuel 2015, 155, 86–114. [Google Scholar] [CrossRef]
- Pakowski, Z.; Adamski, R.; Kokocinska, M.; Kwapisz, S. Generalized desorption equilibrium equation of lignite in a wide temperature and moisture content range. Fuel 2011, 90, 3330–3335. [Google Scholar] [CrossRef]
- Wei, F.J.; Liao, J.J.; Chang, L.P.; Han, Y.N.; Bao, W.R. Transformation of functional groups during lignite heat-treatment and its effects on moisture re-adsorption properties. Fuel Process. Technol. 2019, 192, 210–219. [Google Scholar] [CrossRef]
- Yang, Y.K.; Liao, J.J.; Mo, Q.; Chang, L.P.; Bao, W.R. Evolution of Physical and Chemical Structures in Lignite during Dewatering Process and Their Effects on Combustion Reactivity. Energy Fuels 2019, 33, 3891–3898. [Google Scholar] [CrossRef]
- Lichota, J.; Plutecki, Z. Coal drying in power plants. Rynek Energii 2007, 6, 36–41. [Google Scholar]
- Wang, Y.; Zhang, Y.F.; Qiao, X.X.; Liu, J.; Li, T.; Xu, Y. Effect of moisture on dehydration and heat transfer characteristics of lignite in low temperature carbonization furnace. Fuel Process. Technol. 2016, 147, 18–25. [Google Scholar] [CrossRef]
- Wu, L.; Guan, Y.N.; Reddy, B.R.; Li, C.C.; Liew, R.K.; Zhou, J. Revelation of non-thermal effects on microwave drying of lignite: Reactor design and drying characteristics. Fuel 2025, 383, 133893. [Google Scholar] [CrossRef]
- Wu, L.; Shi, Y.Q.; Yang, F.; Reddy, B.R.; Zhang, W.Q.; Guan, Y.N.; Zhang, X.W.; Zhang, Q.L.; Zhou, J. Revelation of non-thermal effects on lignite microwave drying II: Impacts on the structure and the properties of dried lignite. Fuel 2025, 398, 135529. [Google Scholar] [CrossRef]
- Arbuzov, S.I.; Finkelman, R.B.; Il’enok, S.S.; Maslov, S.G.; Mezhibor, A.M.; Blokhin, M.G. Modes of Occurrence of Rare-Earth Elements (La, Ce, Sm, Eu, Tb, Yb, Lu) in Coals of Northern Asia (Review). Solid Fuel Chem. 2019, 53, 1–21. [Google Scholar] [CrossRef]
- Zhao, P.F.; Zhong, L.P.; Zhu, R.; Zhao, Y.M.; Luo, Z.F.; Yang, X.L. Drying characteristics and kinetics of Shengli lignite using different drying methods. Energy Convers. Manag. 2016, 120, 330–337. [Google Scholar] [CrossRef]
- Bergins, C. Kinetics and mechanism during mechanical/thermal dewatering of lignite. Fuel 2003, 82, 355–364. [Google Scholar] [CrossRef]
- Liu, H.P.; Chen, T.P.; Fang, L.X. Evolution of char structure during non-isothermal low temperature pyrolysis of ZhunDong coal by microwave heating: A comparative study with conventional heating. J. Energy Inst. 2020, 93, 1195–1206. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Gao, M.Q.; Miao, Z.Y.; Cheng, C.; Wan, K.J.; He, Q.Q. Physicochemical properties and combustion kinetics of dried lignite. Energy 2024, 289, 129928. [Google Scholar] [CrossRef]
- Hulston, J.; Favas, G.; Chaffee, A.L. Physico-chemical properties of Loy Yang lignite dewatered by mechanical thermal expression. Fuel 2005, 84, 1940–1948. [Google Scholar] [CrossRef]
- Seehra, M.S.; Kalra, A.; Manivannan, A. Dewatering of fine coal slurries by selective heating with microwaves. Fuel 2007, 86, 829–834. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Tang, L.G.; Luo, Z.F.; Liang, C.C.; Xing, H.B.; Duan, C.L.; Song, S.L. Fluidization Characteristics of a Fine Magnetite Powder Fluidized Bed for Density-Based Dry Separation of Coal. Sep. Sci. Technol. 2012, 47, 2256–2261. [Google Scholar] [CrossRef]
- Yu, X.D.; Luo, Z.F. Effect of 3–0 mm fine coal migration characteristics on high sulfur lignite separation in compound dry cleaning apparatus. Fuel 2023, 340, 127495. [Google Scholar] [CrossRef]
- Hansen-Carlson, J.; Das, A. Settling characteristics and separation features in a CrossFlow separator for fine coal beneficiation. Int. J. Coal Prep. Util. 2021, 41, 893–910. [Google Scholar] [CrossRef]
- Chalavadi, G.; Singh, R.K.; Das, A. Processing of coal fines using air fluidization in an air table. Int. J. Miner. Process. 2016, 149, 9–17. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Dong, Y.N.; Lv, G.N.; Dong, L.; Zhao, Y.M.; Duan, C.L.; Zhou, E.H.; Hua, W. Agglomeration behavior of the dense medium in a gas-solid separation fluidized bed. Fuel 2023, 339, 127383. [Google Scholar] [CrossRef]
- Lu, M.S.; Fang, M.X.; He, M.C.; Liu, S.X.; Luo, Z.Y. Insights into agglomeration and separation of fly-ash particles in a sound wave field. RSC Adv. 2019, 9, 5224–5233. [Google Scholar] [CrossRef] [PubMed]
- Haig, C.W.; Hursthouse, A.; McIlwain, S.; Sykes, D. The effect of particle agglomeration and attrition on the separation efficiency of a Stairmand cyclone. Powder Technol. 2014, 258, 110–124. [Google Scholar] [CrossRef]
| Sample | Size Range (mm) | Mean Particle Size (mm) | Content (%) | Particle Density (g/cm3) | Bulk Density (g/cm3) |
|---|---|---|---|---|---|
| Magnetite powder | −0.300 | 0.207 | 100 | 4.6 | 2.43 |
| 0.300~0.150 | 0.247 | 80 | 4.6 | 2.44 | |
| 0.150~0.074 | 0.147 | 15 | 4.6 | 2.35 | |
| −0.074 | 0.068 | 5 | 4.6 | 2.05 |
| Sample | Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad (%) | Ad (%) | Vdaf (%) | FCad (%) | Cdaf (%) | Hdaf (%) | Odaf (%) | Ndaf (%) | Sdaf (%) | |
| Coal | 6.13 | 16.93 | 36.34 | 49.64 | 62.51 | 3.36 | 0.79 | 10.64 | 0.71 |
| Gas Temperature (°C) | Fitting Equation | R2 | Deff (m2/s) |
|---|---|---|---|
| 50 | lnMR = 0.21496 − 0.04677t | 0.9787 | 0.00020171 |
| 75 | lnMR = 0.02926 − 0.06291t | 0.9434 | 0.00025197 |
| 100 | lnMR = −0.17198 − 0.06203t | 0.9271 | 0.00025440 |
| 125 | lnMR = −0.28033 − 0.07758t | 0.9182 | 0.00031442 |
| Product | Raw Coal | Cleaned Coal | Middling | Gangue |
|---|---|---|---|---|
| Ash/% | 16.93 | 7.85 | 18.4 | 40.26 |
| Moisture/% | 30.54 | 6.36 | 8.15 | 4.35 |
| Calorific value/cal·g−1 | 2180 | 4599 | 3124 | - |
| Yield/% | 100.00 | 63.38 | 12.74 | 23.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Wan, T.; Chen, T.; Ma, B.; Yao, Z.; Xu, B.; Wang, Q.; Xu, X. Study on the Drying Characteristics of Moist Fine Lignite in a Dense Gas–Solid Separation Fluidized Bed. Minerals 2025, 15, 1039. https://doi.org/10.3390/min15101039
Lei H, Wan T, Chen T, Ma B, Yao Z, Xu B, Wang Q, Xu X. Study on the Drying Characteristics of Moist Fine Lignite in a Dense Gas–Solid Separation Fluidized Bed. Minerals. 2025; 15(10):1039. https://doi.org/10.3390/min15101039
Chicago/Turabian StyleLei, Huicheng, Tengfeng Wan, Tingguan Chen, Bingbing Ma, Zongxu Yao, Bao Xu, Qingfei Wang, and Xuan Xu. 2025. "Study on the Drying Characteristics of Moist Fine Lignite in a Dense Gas–Solid Separation Fluidized Bed" Minerals 15, no. 10: 1039. https://doi.org/10.3390/min15101039
APA StyleLei, H., Wan, T., Chen, T., Ma, B., Yao, Z., Xu, B., Wang, Q., & Xu, X. (2025). Study on the Drying Characteristics of Moist Fine Lignite in a Dense Gas–Solid Separation Fluidized Bed. Minerals, 15(10), 1039. https://doi.org/10.3390/min15101039







