1. Introduction
Baryte (BaSO
4) is a mineral of critical industrial and geochemical significance, primarily used as a weighting agent in drilling fluids within the oil and gas industry [
1]. Globally, baryte mineralization occurs in a variety of geological settings, including Sedimentary-Exhalative (SEDEX) systems (e.g., Rammelsberg, Germany), Kuroko-type volcanogenic massive sulfide deposits (e.g., Hakurei, Japan), Mississippi Valley-Type (MVT) and Irish-type carbonate-hosted deposits (e.g., Walton, Canada, Alston Moor, UK), and hydrothermal veins (e.g., Wolkenhügel, Germany) [
1,
2,
3,
4,
5,
6]. In Cyclades, Greece, baryte is found in both epithermal and vein-type systems, such as the Mn-Ba-rich deposits of Milos [
7] and the high-grade baryte-sulfide veins within the Mykonos granitoid intrusion in the Cyclades, where estimated reserves exceed 6 Mt [
8].
While multiple formation mechanisms have been proposed for baryte deposits, including direct hydrothermal precipitation, replacement of carbonates in metasomatic fronts, and biogenic or bacterially mediated growth in marine settings, the processes that govern large-scale baryte mineralization remain under investigation. Hydrothermal baryte in carbonate rocks often results from the interaction of Ba
2+-rich fluids with SO
42−-bearing seawater under temperatures between 100 and 300 °C, producing zoned metasomatic columns characterized by baryte, pyrite, and associated silicates. Experimental studies have reproduced these assemblages by mixing Na
2SO
4 and BaCl
2 under controlled pH and temperature, revealing diffusion-controlled zoning and temperature-dependent phase stability. In parallel, laboratory experiments simulating baryte precipitation in seawater have shown that marine bacteria can mediate BaSO
4 nucleation details in [
1,
2,
3,
4,
5,
6,
7].
This study aims to experimentally investigate the physicochemical conditions that control baryte and sulfide ores in vein-type hydrothermal systems and compare them to numerical simulations. Mixing experiments that replicate Miocene seawater and Ba-rich magmatic/hydrothermal fluids were conducted to simulate fluid evolution in shallow-crustal, fault-controlled settings analogous to the Mykonos vein system. The interaction between magmatic fluids and host granitoids during cooling, combined with extensional tectonics, shaped ideal physicochemical conditions for baryte and sulfide co-precipitation. The roles of temperature, pressure, and redox conditions in baryte precipitation and its co-deposition with sulfates; sulfides such as pyrite, galena, sphalerite, and chalcopyrite; as well as native silver are exploited. These experiments are designed to validate a precipitating model for baryte–sulfide vein-type ores in order to improve our understanding of ore-forming mechanisms involving mixing, phase separation, and cooling in extensional tectonic settings characterized by magmatic to seawater fluid interaction.
2. Regional and Baryte Deposit Geology
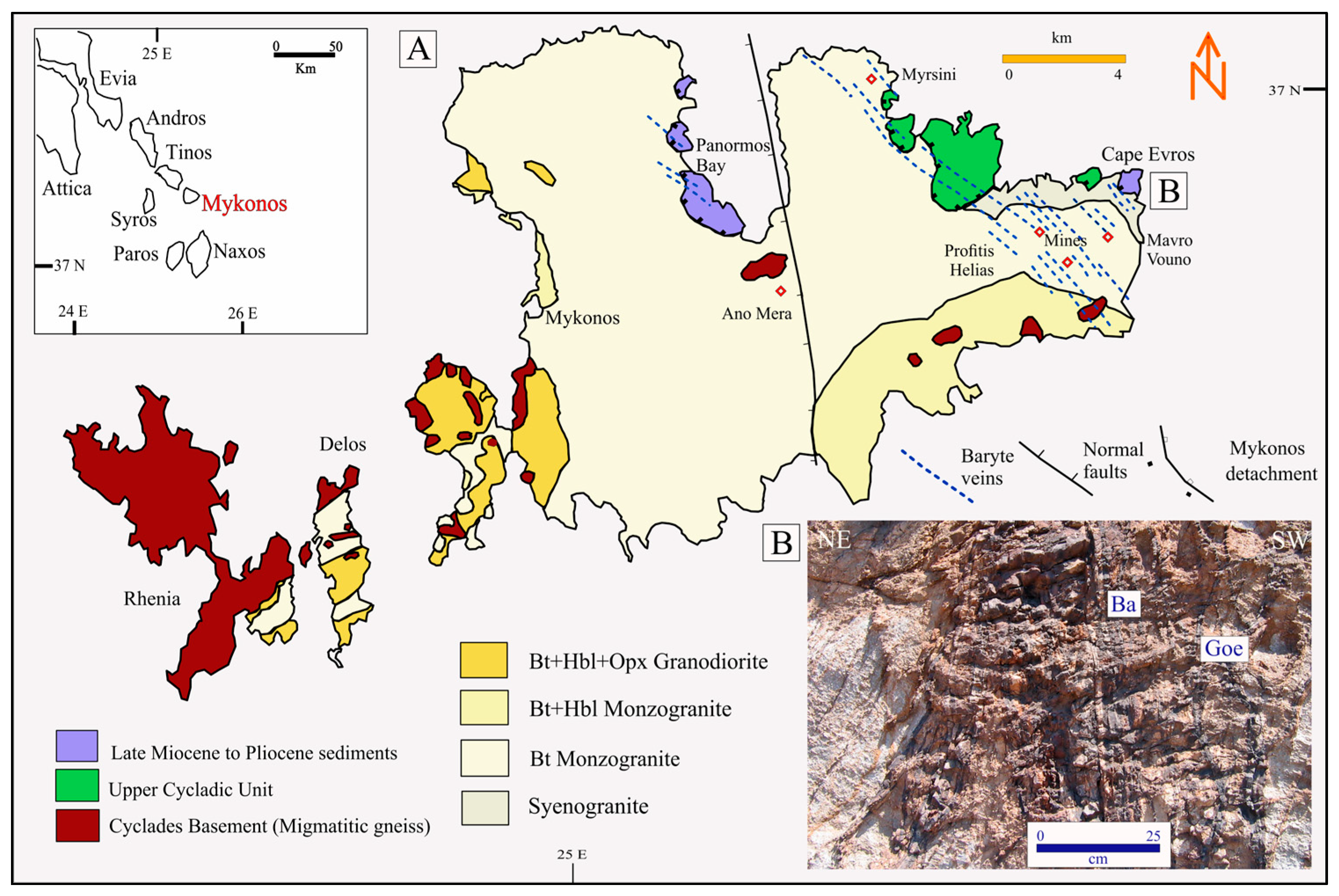
Mykonos Island, located within the Attico-Cycladic Massif, consists of three Alpine nappes: the Basal Unit, the Cycladic Blueschist Unit, and the Upper Cyclades Unit (
Figure 1A). The Basal Unit is a Late Triassic to Early Cretaceous succession of dolomite, phyllite, and quartzite [
9], overlain by the Cycladic Blueschist Unit, a Mesozoic continental margin assemblage consisting of a pre-Alpine basement and volcanic–sedimentary cover [
10,
11]. The Cycladic Blueschist Unit is, in turn, overlain by the Upper Cycladic Unit, which includes Late Cretaceous ophiolites [
12]. Miocene back-arc extension led to the exhumation of the Mykonos Metamorphic Core Complex through the activity of the North Cycladic Detachment System (NCDS), a major low-angle extensional fault with top-to-the-north or -northeast kinematics, active between ~14 and 9 Ma [
13,
14]. Intrusion of the ~13.5 Ma Mykonos pluton [
15] into the Metamorphic Core Complex occurred syn-tectonically during NCDS activity. The pluton is a multiphase, ENE-trending laccolith consisting of calc-alkaline, I-type granitoids, namely granodiorite, monzogranite, tonalite, quartz monzonite, and minor gabbro [
16,
17]. Its root zone is exposed on Delos Island, while the apical cupola, preserved in eastern Mykonos, is characterized by porphyritic monzogranite with orthoclase megacrysts. Dating, thermal, and structural data [
18] indicate that post-intrusion cooling occurred between ~12 and 11 Ma, modified by normal Late Miocene faulting and the formation of baryte tension gashes (~11 to 10 Ma [
19]). As first noted by [
20], a system of baryte-bearing dikes intruded syn-tectonically along NW-trending sinistral oblique-thrust faults [
21,
22]. Associated with these NW faults are less-developed ENE-trending dextral strike-slip faults. Fault kinematics and stress analysis point to two main deformation phases: an initial middle Miocene transpressional regime, followed by a late Miocene transtensional phase [
21]. The baryte gashes exploited structural discontinuities, such as foliation, cleavage, and detachment faults, facilitating hydrothermal fluid circulation in Mykonos granitoid.
The Cape Evros vein system (
Figure 1B) consists of over fifteen NW-NNW-striking, subvertical baryte veins, some exceeding 3 m in width and extending several kilometers in strike length [
8]. Hosted primarily in the Mykonos monzogranite, but also crosscutting the Cycladic Blueschist Unit and Upper Cyclades Unit greenschists and silica breccias, these veins contain multiple generations of mineralization and are often associated with brecciation and stockwork textures. Baryte is dominant across all paragenetic stages, accompanied by clear quartz, pyrite, sphalerite, chalcopyrite, and late-stage argentiferous galena. Mineralogical zoning from margin to core (Zones A–E) records systematic variations in texture and fluid composition—from fine-grained, sulfide-rich baryte at the margins to coarse, comb-textured baryte and Mn-oxides at the core. Zone A comprises fine-grained baryte ± muscovite with fine pyrite, sphalerite, chalcopyrite, and fragments of altered monzogranite. Zone B is characterized by medium-grained baryte and clear quartz ± chlorite, epidote, and muscovite, accompanied by fine chalcopyrite and colloform pyrite. Zone C contains medium-grained baryte with goethite, marcasite, and chalcopyrite. Zone D consists of medium- to coarse-grained baryte, goethite and coarse galena ± calcite, witherite, and muscovite. Zone E is dominated by coarse, comb-textured baryte with psilomelane, pyrolusite, hematite, ferrihydrite, and goethite. These compositional and textural differences warrant a thorough description to capture the progressive changes in fluid chemistry, redox state, and depositional conditions that controlled ore formation. Ore and SEM microscopy reveal over 40 ore and gangue minerals, including sulfosalts (geocronite, jordanite), native Ag-Au, and Pb-Sb-As-Te phases, formed through three hypogene stages and a subsequent supergene oxidation stage [
8,
19].
Hydrothermal alteration halos surrounding major veins at Cape Evros include the following: (i) an inner silica-rich breccia zone with baryte, quartz, hyalophane, and pyrite; (ii) a chlorite–muscovite–albite zone with epidote and minor feldspars; and (iii) an outer zone characterized by montmorillonite, goethite, pyrophyllite, and Fe-oxides. Geothermometric data indicate that mineralization occurred under shallow crustal, near-hydrostatic conditions at pressures of ~100 bars and temperatures between 275 °C and 325 °C. These fluids were sourced from oxidized, Ba-rich magmatic ore solutions derived from the alteration of the Mykonos granitoids, with additional contributions from Miocene seawater [
8,
19,
20].
3. Setting the Experiments
The Mykonos vein system that hosts the baryte–sulfide ores is defined by shallow emplacement depths, moderate hydrostatic pressures (~100 bars), and mesothermal temperatures ranging from ~275 °C to 330 °C. These conditions, constrained by mineral equilibria, fluid inclusion microthermometry, and structural context, reflect a low-pressure, high-permeability regime. The mineralization is spatially associated with zones of intense brecciation, alteration, and pervasive silicification within the apical zone of the Mykonos pluton. These features strongly suggest focused hydrothermal flow and repeated fracturing, creating transient supersaturated conditions conducive to baryte precipitation. Salinity and redox conditions were further defined through detailed fluid inclusion microthermometry and Raman spectroscopy, revealing a chemically complex fluid system dominated by H
2O-NaCl with minor CH
4, H
2S, SO
2, and trace CO
2 [
8]. Fluid salinities ranged from ~2 wt.% to ~15 wt.% NaCl eq. The co-occurrence of vapor-rich (V-L) and liquid-rich (L-V) inclusions, along with colloform and jigsaw textures in quartz, provided robust evidence for boiling, a critical mechanism for local baryte supersaturation [
8]. In addition, the chemical speciation, pH, and redox conditions of the ore fluid were critical for reproducing baryte precipitation experimentally [
8].
The literature on submarine hydrothermal systems and mid-crustal baryte deposits (e.g., [
23]) has emphasized that fluid boiling and degassing strongly affect sulfide, sulfate, and barium solubility, validating our inclusion of immiscibility and degassing mechanisms in the experimental approach. The geochemical and isotopic data from baryte and associated sulfides further constrained fluid composition and source. According to [
8,
23], the δ
34S values for baryte (i.e., +22 to +26 per mil) and sulfides (i.e., +4 to +25 per mil) indicate a dual sulfur source, meaning magmatic and crustal, while δ
18O and δD values from baryte (i.e., +0.6 to +5.1, and −34 per mil) and quartz (i.e., +6.4 to +9.5 and −80 to −75 per mil) suggest fluid mixing between magmatic–hydrothermal and seawater-derived fluids. Similarly, initial
87Sr/
86Sr ratios between 0.712 and 0.715 in baryte and quartz indicate interactions in the altered Mykonos monzogranite [
8]. By replicating the natural P-T-X conditions, i.e., pressures of ~100 bars, temperatures between 275 °C and 330 °C, salinities between ~2 wt.% and ~15 wt.% NaCl eq., and fluid mixing between oxidized and reduced components, we ensured that the experimental environment faithfully represented the hydrothermal system responsible for the Mykonos baryte veins. The documented role of feldspar alteration in liberating Ba, supported by EMPA data and comparable systems [
24,
25,
26,
27], reinforced our use of Ba-rich silicate phases as the barium source in the experiments. Prior studies (e.g., [
8,
26]) emphasized the importance of labile Ba release during organic and mineral breakdown in seawater for baryte precipitation, which complements our findings on Ba mobilization from alkali feldspars during phyllic alterations of the Mykonos monzogranite. Moreover, the observed boiling in the field guided the staged introduction of gas phases and temperature gradients in the lab, facilitating baryte nucleation under realistic saturation pathways. These constraints allowed us to not only reproduce baryte textures and mineral associations observed in Mykonos but also to assess the physicochemical thresholds critical for BaSO
4 precipitation in natural hydrothermal environments. The δ
34S
SO42− and δ
34S
H2S values further constrained the fluid sources and sulfur speciation, allowing us to mimic the dual magmatic and crustal sulfur inputs [
8]. Together, these field-based, petrographic, and geochemical observations directly informed the design of our baryte precipitation experiments, which were effectively reproduced in the laboratory setting.
4. Experimental Design and Modeling Rationale
The sample MY10 (zone A, Y/Ho ratio of 100 [
8],
Figure 1B) was crushed and separated into −16 and −200 mesh fractions. It contained baryte, Fe-oxides (mostly goethite), sphalerite, pyrite, and galena, with an average chemical composition of SiO
2 0.5 wt.%, Fe
2O
3 1.8 wt.%, BaO 63.7 wt.%, SO
3 32.7 wt.%, and Cu 395 ppm, Pb 337 ppm, Zn 44 ppm, and Ag 1.3 ppm. These minerals typically occur as intergrown aggregates, with sulfides commonly cemented within a baryte–Fe-oxide matrix and particle sizes ≥ 74 μm. The MY10 sample was roasted to enhance metal mobility (particularly for Pb, Zn, Cu, and Ag) and subsequently leached. Leaching was carried out using acidified NaCl and H
2SO
4 solutions (1 to 4 M NaCl, pH 3.5 to 6.2, adjusted with HCl) in 250 mL Erlenmeyer flasks for 12 h. The resulting leachate was then mixed with 1M Na
2SO
4 and 1 M H
2SO
4 solutions in a proportion of 1:1. Heating/cooling was maintained between 200 °C and 300 °C for 6 to 12 h, consistent with the post-boiling evolution of a hydrothermal fluid that had experienced a major boiling event at ~330 °C. The solid-to-liquid ratio and agitation time were optimized to maximize Ba
2+ extraction, with negligible improvement beyond 6 h of stirring. Iron was selectively removed from the leachate, by adding 10 mL of chlorine dioxide 0.5 M, to isolate and recover valuable base/precious metals (Zn, Cu, Pb, and Ag), and the Ba-rich solution was then filtered to remove residual solids.
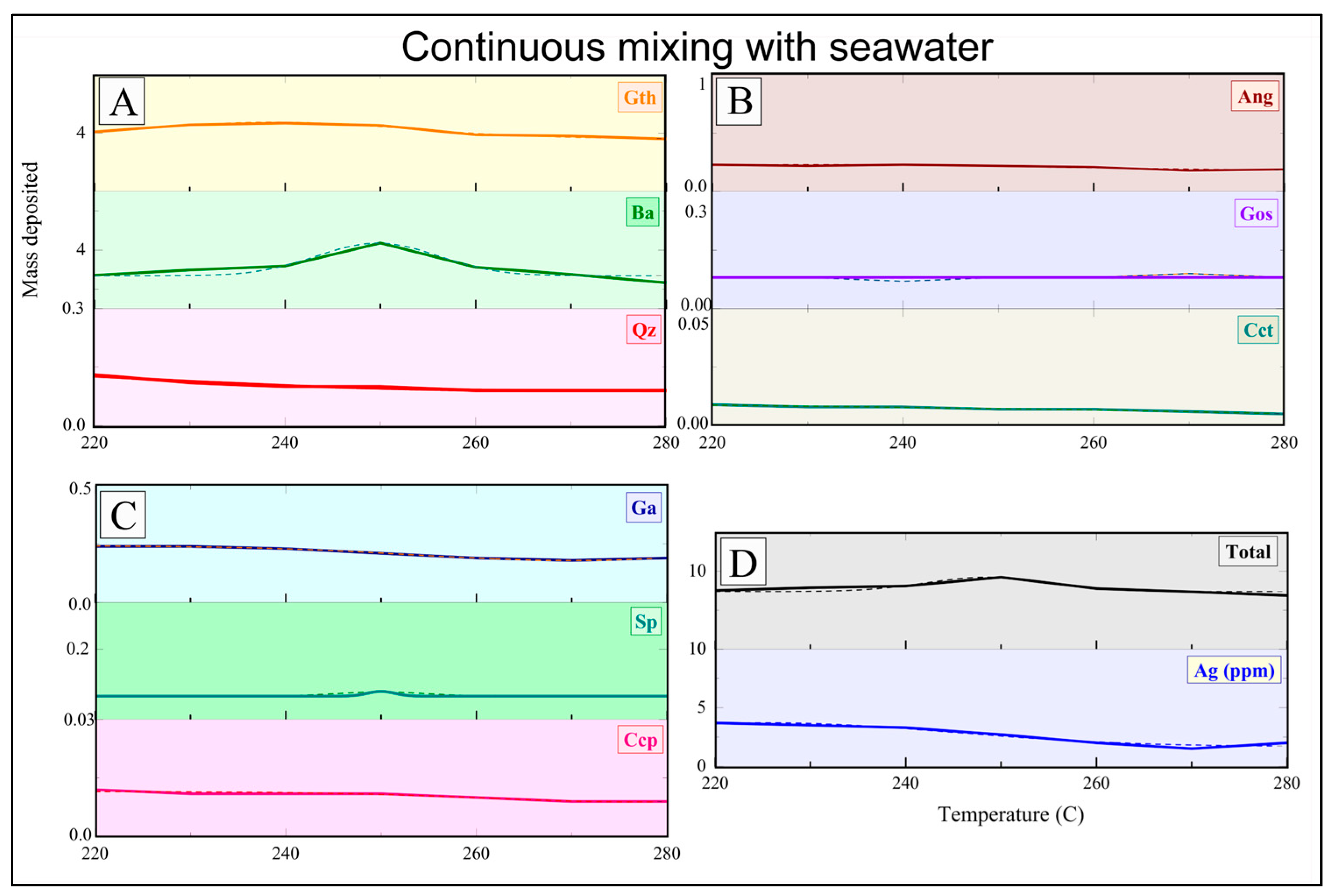
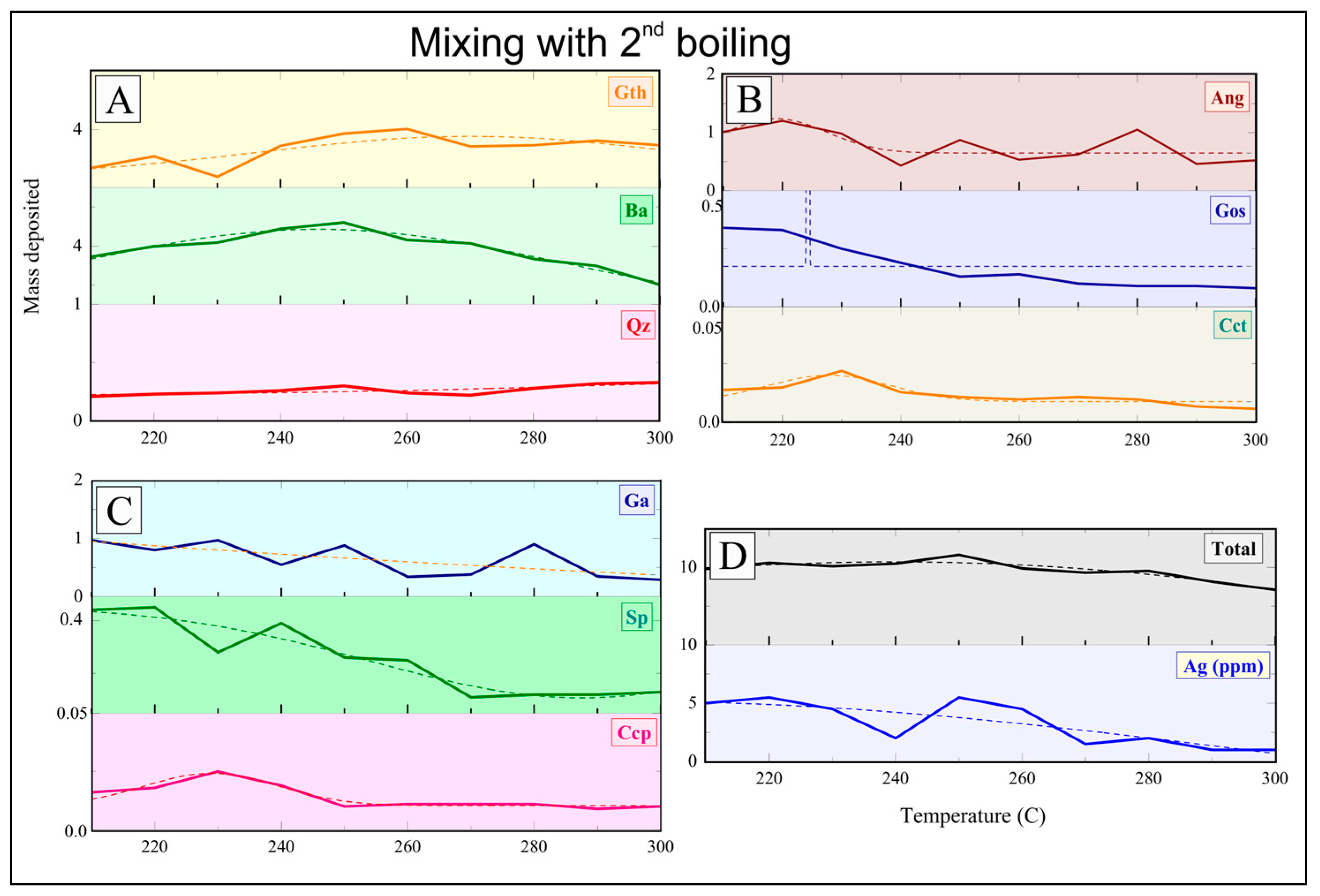
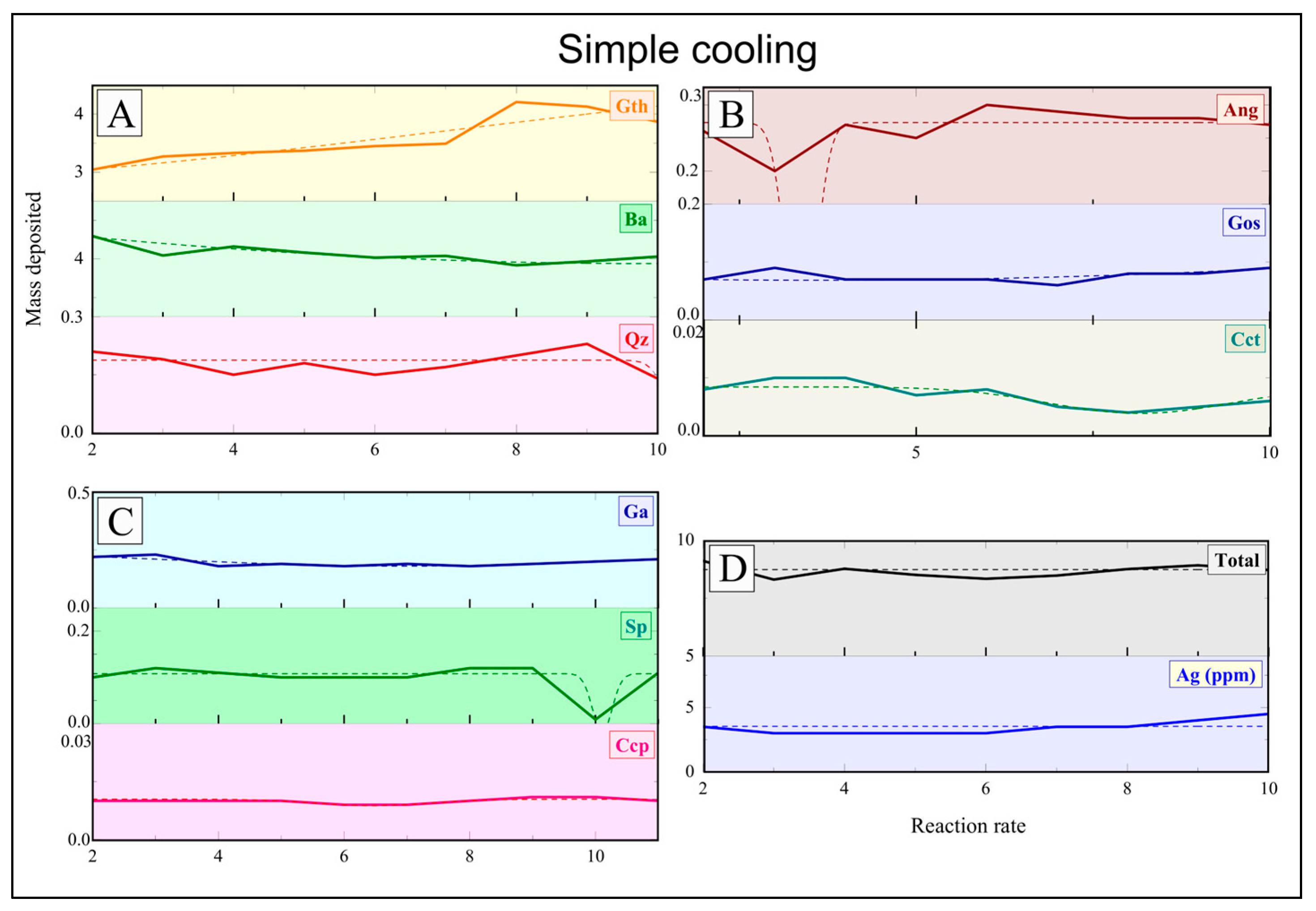
Baryte/sulfides/sulfates were precipitated by introducing pre-filtered Na
2SO
4 solution into the Ba-rich leachate under controlled conditions. Simulated scenarios included the following: (i) simple mixing of two fluid reservoirs, (ii) mixing accompanied by a second boiling phase at 250 °C caused by a sudden pressure release, and (iii) passive cooling post-mixing using a 10 °C step. Redox buffers such as sodium dithionite (reducing) and hydrogen peroxide (oxidizing) were used to reproduce the evolving fO
2 and fS
2 conditions observed in natural fluid inclusions. The precipitation setup employed a gravity-feed system (2–5 mL/h) to mimic slow natural mixing rates and to avoid the formation of fine-grained precipitates caused by turbulence. Stirring was avoided during precipitation to promote larger, well-formed baryte crystals, in line with prior experimental results ([
26,
27]). To ensure purity ≥95%, all glassware was cleaned using chromic–sulfuric acid and steam-rinsed or treated with 0.1 M EDTA solution. Solutions were aged and filtered before use to ensure chemical homogeneity. At the end of each experimental run, the hot suspension was filtered to avoid secondary crystallization during cooling. The solid products were dried and then were analyzed via SEM microscopy, whole-rock geochemistry, and XRD diffraction (
Supplementary Material, Figures S1–S4 and Tables S1 and S2).
X-ray diffractometry was carried out at the Department of Geology, University of Patras, using a Bruker D8 Advance diffractometer (Brucker AXS GmbH, Karlsruhe, Germany) with Ni-filtered Cu(Kα) radiation at 40 kV/40 mA, scanning the 2θ interval of 2–90° with a 0.015° step size. Mineral phases, including magnetite, pyrite, sphalerite, chalcopyrite, and galena, were identified with DIFFRACplus EVA 12® and quantified semi-quantitatively using the Rietveld method in TOPAS3. Coherent scattering domain (CSD) sizes of pyrite and sphalerite were further determined with the Bertaut–Warren–Averbach (BWA) technique in WINFIT, while grain sizes for analysis ranged between 5 and 50 μm. Mineral microanalyses were conducted using a JEOL JSM-6300 SEM (Jeol Ltd., Tokyo, Japan) equipped with energy-dispersive (EDS) and wavelength-dispersive (WDS) spectrometers, along with INCA software, at the Laboratory of Electron Microscopy and Microanalysis, University of Patras, Greece. The operating conditions included an accelerating voltage of 25 kV, a beam current of 3.3 nA, and a beam diameter of 4 μm. The total counting time was 60 s, with a dead time of 40%. Standards used for gangue and ore minerals included natural marialite (Cl), tourmaline (B, F), orthoclase (K), diopside (Ca, Si), ilmenite (Ti), rhodonite (Mn), fayalite (Fe), jadeite (Na), forsterite (Mg), corundum (Al), baryte (Ba), chlorite, epidote, plagioclase, and muscovite. Additional standards comprised natural chalcopyrite, tetrahedrite, tennantite, stibnite, pyrite, sphalerite, galena, hausmannite (Mn2+), manganite (Mn3+), pyrolusite (Mn4+), as well as synthetic CoNiAs, SnO2, and CdTe. Native metals, Ag, Au, Te, and Se, were also used. Detection limits were in the range of approximately 0.01%, and the accuracy was better than 5%. Geochemical analyses of the products were performed by ActLabs Ancaster, Ontario, Canada. Major and trace elements compositions were measured using ICP-OES and ICP-MS. The detection limits were 0.001 wt.% for MnO and TiO2 and 0.01 wt.% for the other major elements. For the trace elements, they were as follows: Au (2 ppb); Lu and Ge (4 ppb); Ir, Pr, Eu, and Tm (5 ppb); La, Ce, Nd, Sm, Gd, Tb, Dy, Ho, Er, Yb, Ta, Tl, Th, U, Se, and Te (0.1 ppm); Hf and In (0.2 ppm); Cd (0.3 ppm); Bi (0.4 ppm); Br, Ag, Cs, and Sb (0.5 ppm); Sc, Be, Co, Ga, Nb, Ni, Cr, Mo, W, Sn, and Hg (1 ppm); Sr, Y, and Rb (2 ppm); Ba (3 ppm); Zr (4 ppm); Pb, As, and V (5 ppm); and Cu (10 ppm) and Zn (30 ppm).
Geochemical modeling for the Mykonos baryte/sulfide/sulfate vein system was performed using XRD (Brucker AXS GmbH, Karlsruhe, Germany ) and bulk geochemical analyses in combination with chemical equilibrium calculations via the Solveq software [
28]. The initial composition of the baryte-enriched ore fluid was reconstructed from fluid inclusion microthermometry and Raman spectroscopy [
8]. These parameters were used as input for Solveq to model the fluid evolution under shallow and NaCl-saturated conditions. Ore fluid modification was tested, incorporating the physicochemical constraints observed in our laboratory experiments for continuous mixing, boiling at 250 °C, and simple cooling (350–200 °C, with a 5 °C step). The experimental results, especially the enhanced baryte and co-precipitated sulfide formation during boiling and mixing, were used to refine model parameters for Ba
2+, SO
42−, and base-metal activities. Chemical stability fields of sulfides, sulfates, and associated silicate phases were further evaluated using SUPCRT92 [
29], allowing for phase stability relationships to be compared directly with experimental precipitation products (
Supplementary Material, Figure S5). The close agreement between predicted and observed mineral assemblages helped to validate our experimental interpretation.