Al-Si Order and Chemical Composition Model across Scapolite Solid Solutions with Evidence from Rietveld Structure Refinements
Abstract
1. Introduction
2. Scapolite Crystal Structure
3. Scapolite Solid Solutions
4. Space Groups and Antiphase Domain Boundaries (APBs)
5. Al-Si Order and Compositional Model for Scapolite Solid Solutions
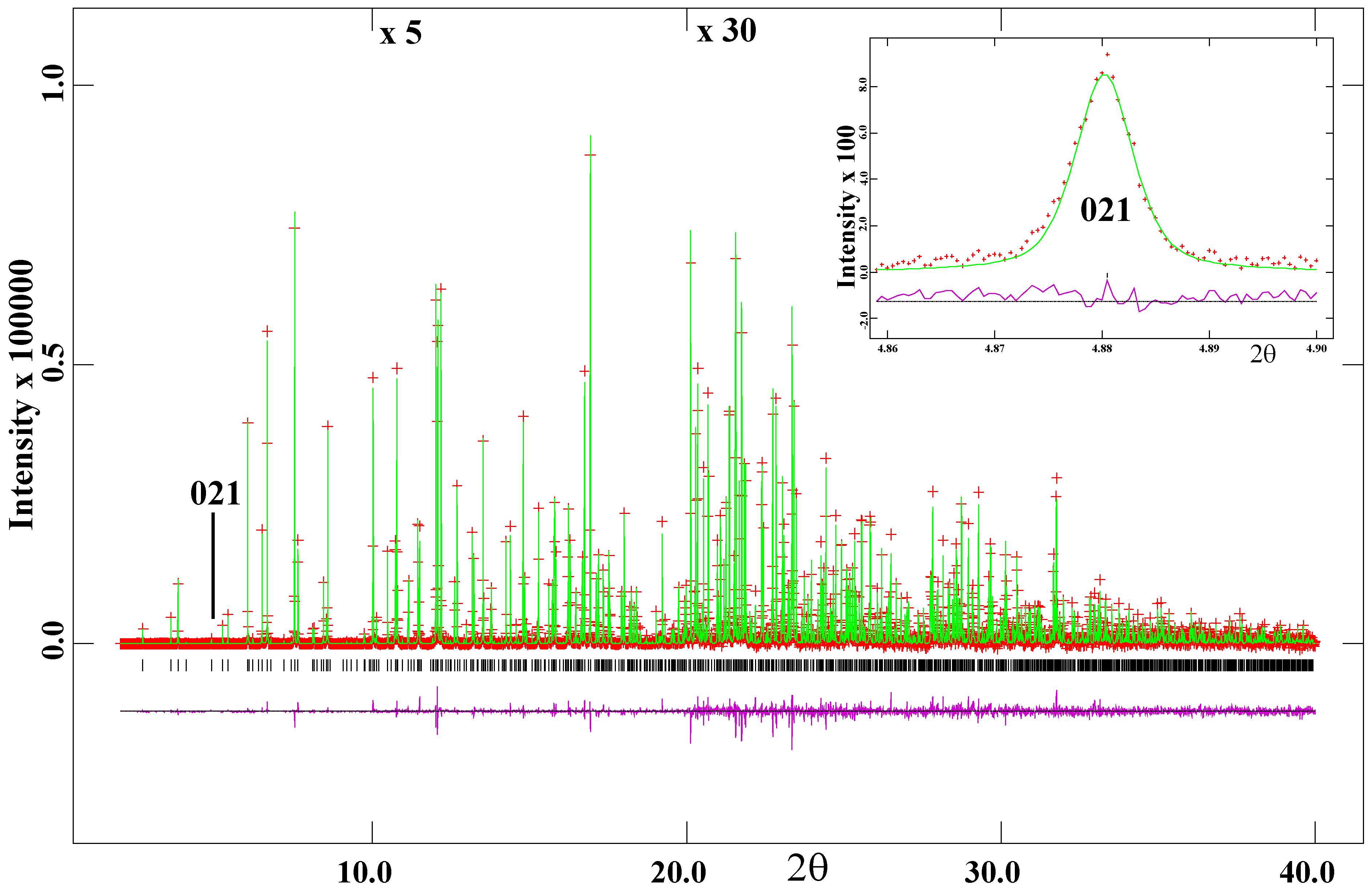
6. Scapolite Samples and Experimental Methods
7. Results and Discussion
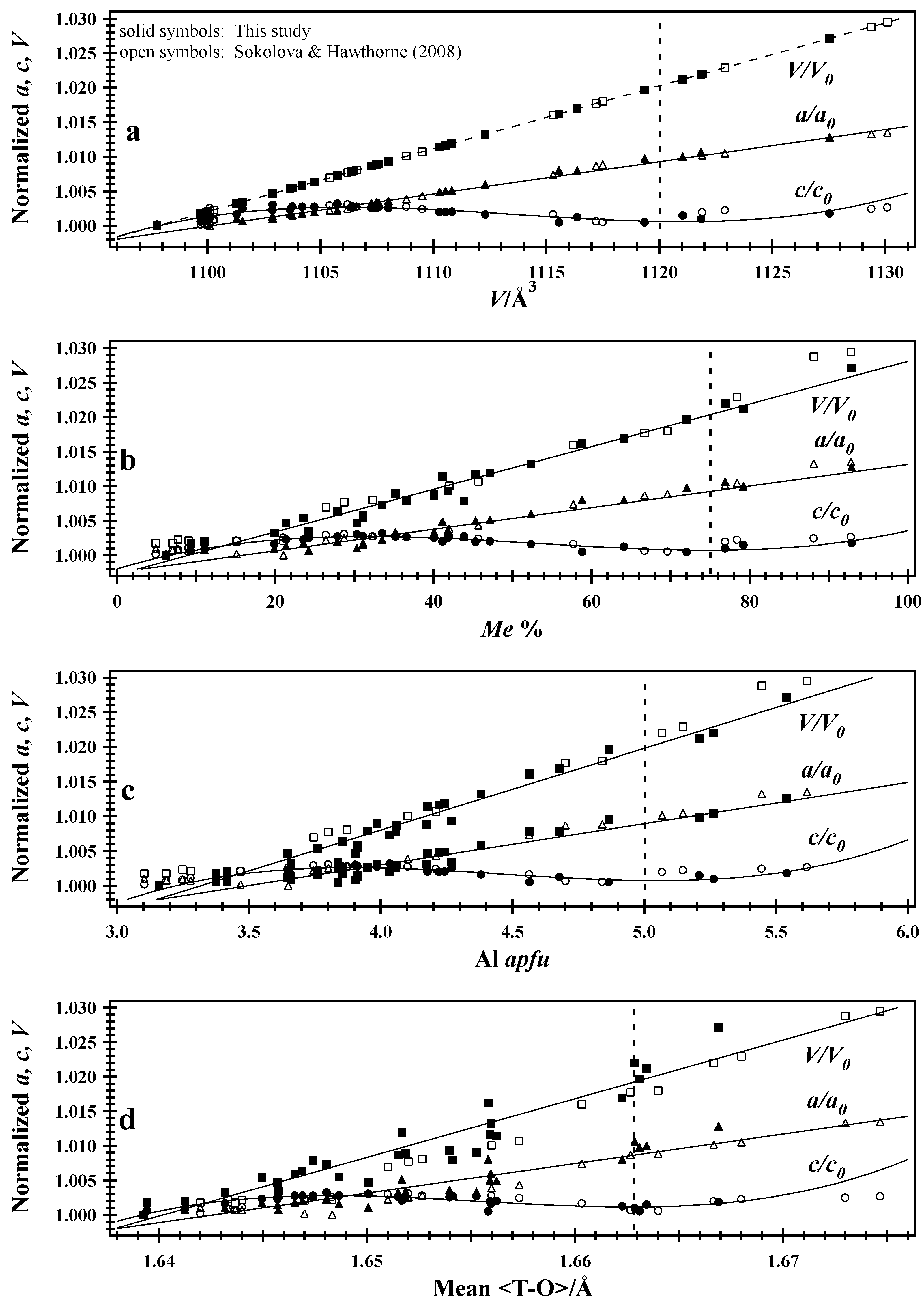
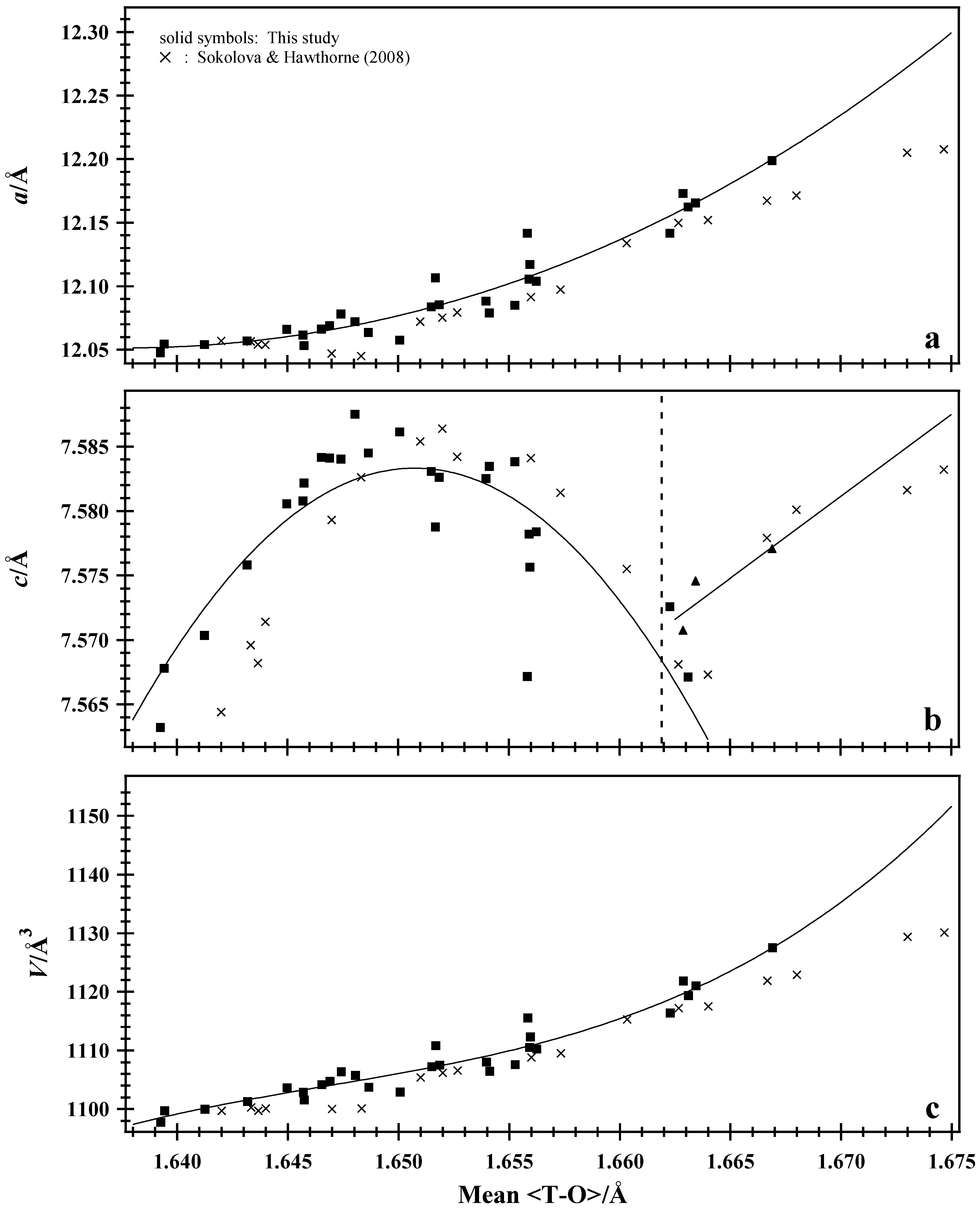
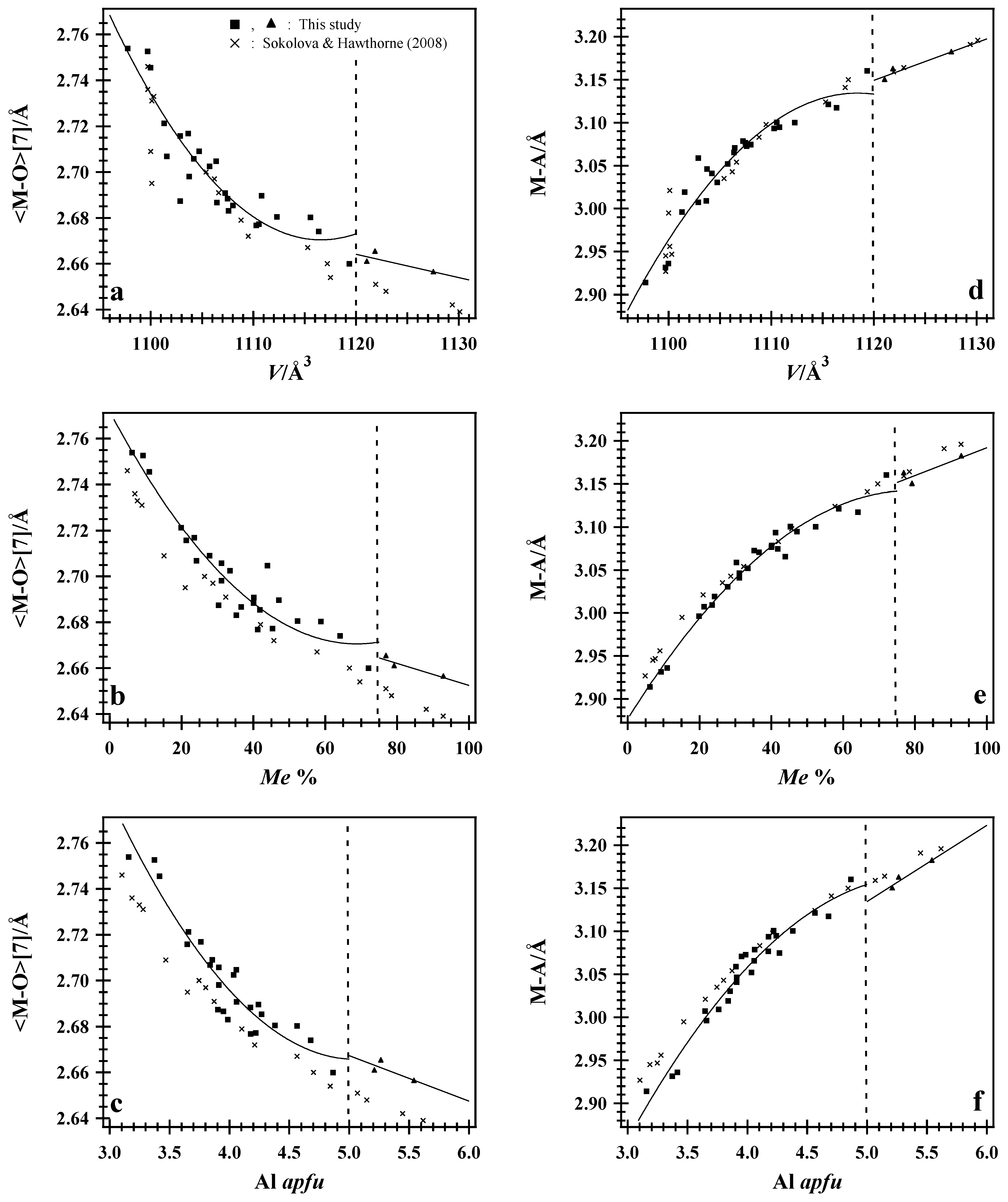
7.1. Normalized Unit-Cell Paramters
7.2. Anion Groups
7.3. Average <M–O> and <M–A> Distances
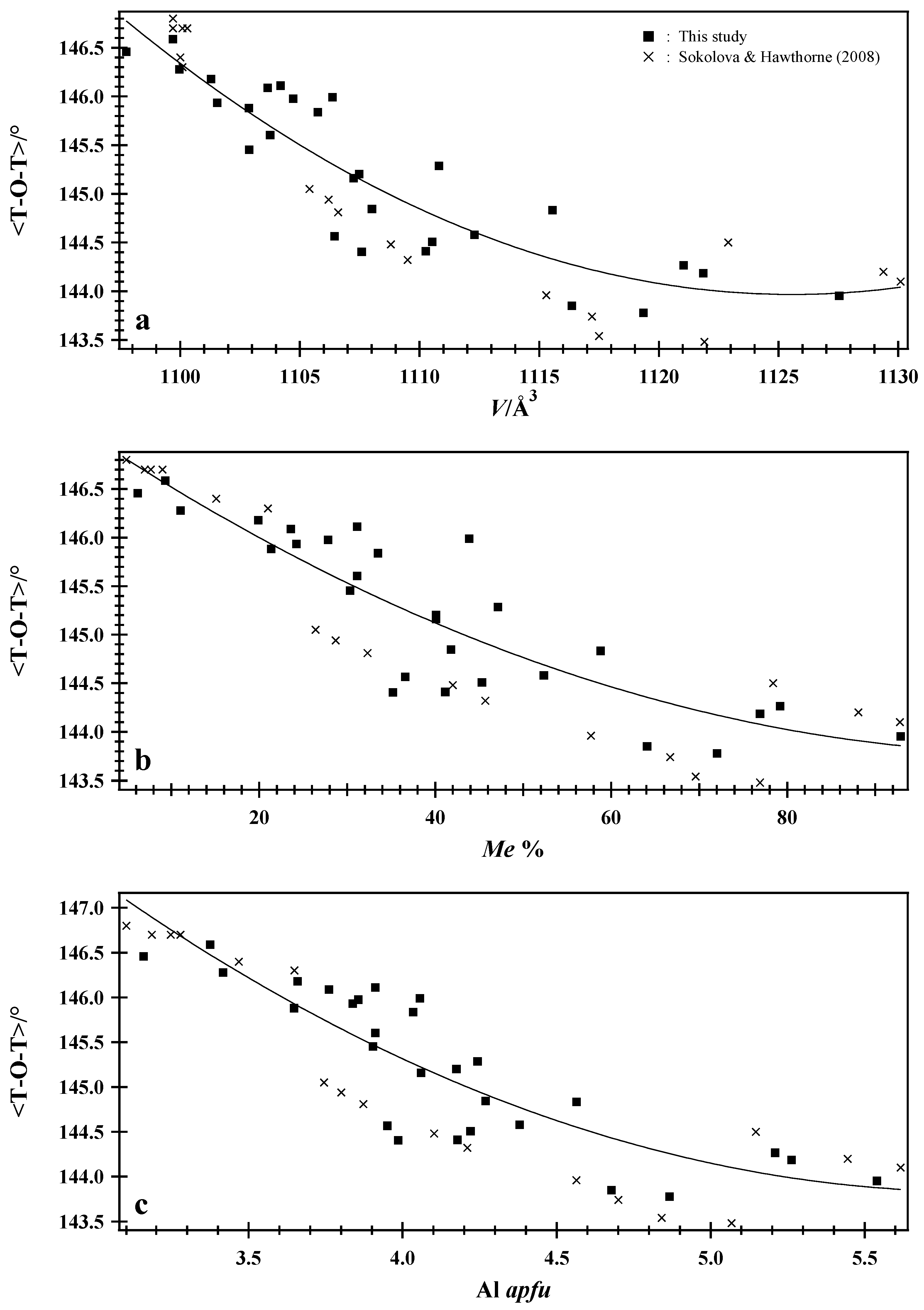
7.4. Average <T–O–T> Bridging Angle and <T–O> Distance
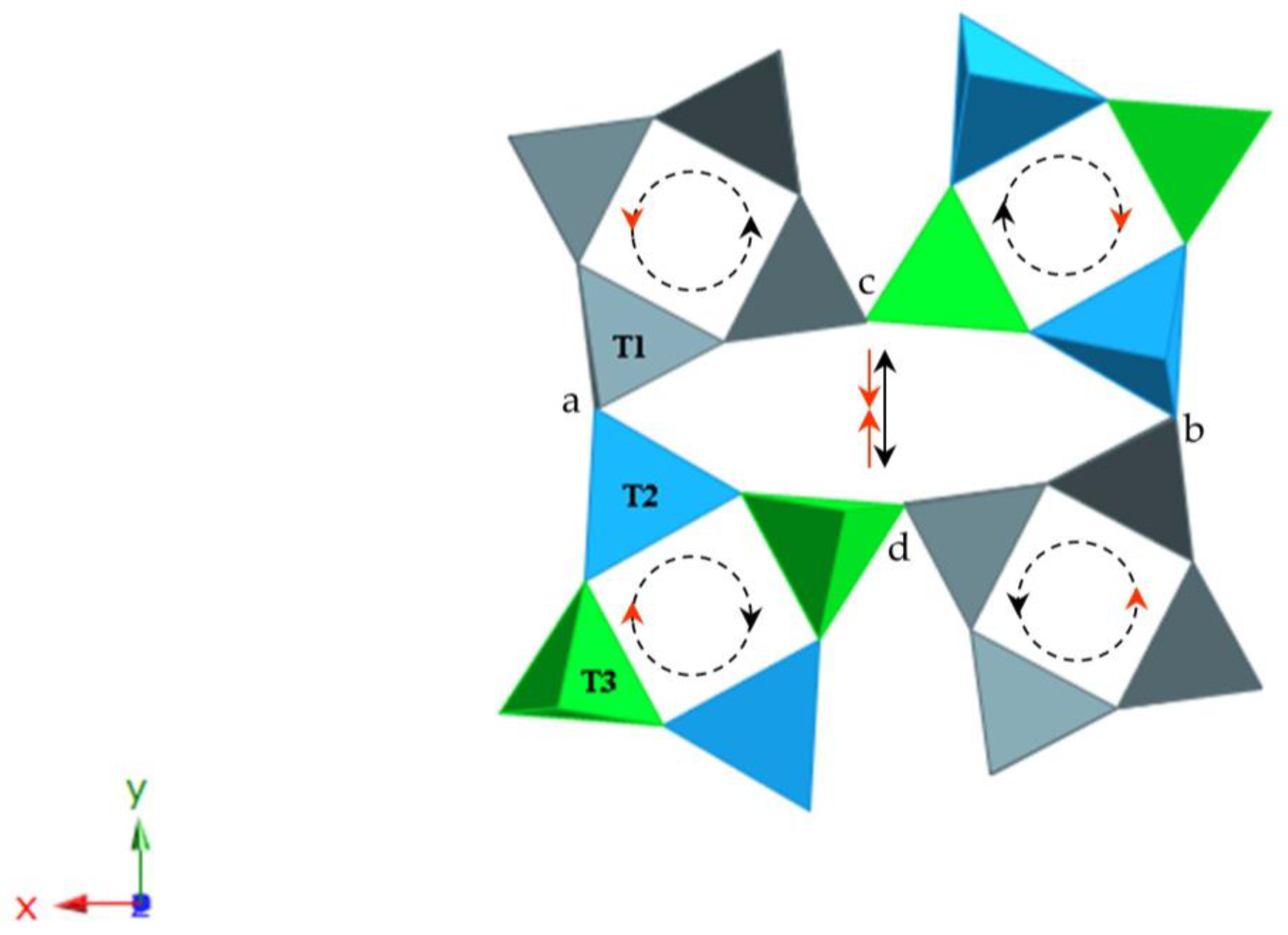
7.5. Oval-Shaped Channels and Tetrahedral Rotations
8. Concluding Remarks
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lovering, J.F.; White, A.J.R. Granulitic and eclogitic inclusions from basic pipes at Delegate, Australia. Contrib. Mineral. Petrol. 1969, 21, 9–52. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Palmer, M.R.; Xue, C.J.; Li, Y.H. Halogen-rich scapolite-biotite rocks from the Tongmugou Pb-Zn deposit, Qinling, north-western China: Implications for the ore-forming process. Mineral. Mag. 1994, 58, 543–552. [Google Scholar] [CrossRef]
- Kullerud, K.; Erambert, M. Cl-scapolite, Cl-amphibole, and plagioclase equilibria in ductile shear zones at Nusfjord, Lofoten, Norway: Implications for fluid compositional evolution during fluid-mineral interaction in the deep crust. Geochim. Cosmochim. Acta 1999, 63, 3829–3844. [Google Scholar] [CrossRef]
- Moecher, D.P.; Essene, E.J. Phase-equilibria for calcic scapolite, and implications of variable Al-Si disorder for P-T, T-XCO2, and a-X relations. J. Petrol. 1990, 31, 997–1024. [Google Scholar] [CrossRef]
- Moecher, D.P.; Essene, E.J. Calculation of CO2 activities using scapolite equilibria: Constraints on the presence and composition of a fluid phase during high-grade metamorphism. Contrib. Mineral. Petrol. 1991, 108, 219–240. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.; Kadiyski, M.; Nikolova, R. Further on the Choice of Space Group for Scapolite Group Members and Genetic Considerations about the Si-Al Ordering in Their Framework Construction. Minerals 2024, 14, 556. [Google Scholar] [CrossRef]
- Lotti, P.; Gatta, G.D.; Gigli, L.; Krüger, H.; Kahlenberg, V.; Meven, M.; Comboni, D.; Milani, S.; Merlini, M.; Liermann, H.-P. Thermal and combined high-temperature and high-pressure behavior of a natural intermediate scapolite. Am. Mineral. 2024, 109, 243–254. [Google Scholar] [CrossRef]
- Rao, Y.; Guo, Q.; Zhang, S.; Liao, L. Comparative Study on Gemmological Characteristics and Luminescence of Colorless and Yellow Scapolites. Crystals 2023, 13, 462. [Google Scholar] [CrossRef]
- Shendrik, R.; Kaneva, E.; Pankratova, V.; Pankrushina, E.; Radomskaya, T.; Gavrilenko, V.; Loginova, P.; Pankratov, V. Intrinsic luminescence and radiation defects in scapolite. Chem. Phys. Lett. 2024, 838, 141081. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; John Wiley: New York, NY, USA, 1992. [Google Scholar]
- Teertstra, D.K.; Schindler, M.; Sherriff, B.L.; Hawthorne, F.C. Silvialite, a new sulfate-dominant member of the scapolite group with an Al-Si composition near the I4/m-P42/n phase transition. Mineral. Mag. 1999, 63, 321–329. [Google Scholar] [CrossRef]
- Shaw, D.M. The geochemistry of scapolite. Part I. Previous work and general mineralogy. J. Petrol. 1960, 1, 218–260. [Google Scholar] [CrossRef]
- Evans, B.W.; Shaw, D.M.; Haughton, D.R. Scapolite stoichiometry. Contrib. Mineral. Petrol. 1969, 24, 293–305. [Google Scholar] [CrossRef]
- Hassan, I.; Buseck, P.R. HRTEM characterization of scapolite solid solutions. Am. Mineral. 1988, 73, 119–134. [Google Scholar]
- Antao, S.M.; Hassan, I. Thermal behavior of scapolite Me79.6 and Me33.3. Can. Mineral. 2002, 40, 1395–1401. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Increase in Al-Si and Ca-Na disorder with temperature in scapolite Me32.9. Can. Mineral. 2008, 46, 1577–1591. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Unusual Al-Si ordering in calcic scapolite, Me79.6, with increasing temperature. Am. Mineral. 2008, 93, 1470–1477. [Google Scholar] [CrossRef]
- Antao, S.M. Quartz: Structural and thermodynamic analyses across the α↔ β transition with origin of negative thermal expansion (NTE) in β quartz and calcite. Acta Crystallogr. 2016, 72, 249–262. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Mulder, W.H.; Lee, P.L.; Toby, B.H. In situ study of the R-3c→R-3m orientational disorder in calcite. Phys. Chem. Miner. 2009, 36, 159–169. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Parise, J.B. Cancrinite: Crystal structure, phase transitions, and dehydration behavior with temperature. Am. Mineral. 2006, 91, 1117–1124. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Mulder, W.H.; Lee, P.L. The R-3c→R-3m transition in nitratine, NaNO3, and implications for calcite, CaCO3. Phys. Chem. Miner. 2008, 35, 545–557. [Google Scholar] [CrossRef]
- Antao, S.M.; Mohib, S.; Zaman, M.; Marr, R.A. Ti-rich andradites: Chemistry, structure, multi-phases, optical anisotropy, and oscillatory zoning. Can. Mineral. 2015, 53, 133–158. [Google Scholar] [CrossRef]
- Antao, S.M.; Benmore, C.J.; Li, B.; Wang, L.; Bychkov, E.; Parise, J.B. Network rigidity in GeSe2 glass at high pressure. Phys. Rev. Lett. 2008, 100, 115501. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.D.; Antao, S.M.; Chupas, P.S.; Lee, P.L.; Shastri, S.D.; Parise, J.B. Quantitative high-pressure pair distribution function analysis of nanocrystalline gold. Appl. Phys. Lett. 2005, 86, 061910. [Google Scholar] [CrossRef]
- Lin, S.B. Crystal chemistry and stoichiometry of the scapolite group. Acta Geol. Taiwan 1975, 18, 36–48. [Google Scholar]
- Lin, S.B.; Burley, B.J. On the weak reflections violating body-centered symmetry in scapolites. Tschermaks Mineral.-Petrol. Mitteilungen 1973, 20, 28–44. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Hawthorne, F.C. The crystal chemistry of the scapolite-group minerals. I. Crystal structure and long-range order. Can. Mineral. 2008, 46, 1527–1554. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Sokolova, E.V. The crystal chemistry of the scapolite-group minerals. II. The origin of the I4/m ←→ P42/n phase transition and the nonlinear variations in chemical composition. Can. Mineral. 2008, 46, 1555–1575. [Google Scholar] [CrossRef]
- Sherriff, B.L.; Sokolova, E.V.; Kabalov, Y.K.; Jenkins, D.M.; Kunath-Fandrei, G.; Goetz, S.; Jäger, C.; Schneider, J. Meionite: Rietveld structure-refinement, 29Si MAS and 27Al SATRAS NMR spectroscopy, and comments on the marialite-meionite series. Can. Mineral. 2000, 38, 1201–1213. [Google Scholar] [CrossRef]
- Sherriff, B.L.; Sokolova, E.V.; Kabalov, Y.K.; Teertstra, D.K.; Kunath-Fandrei, G.; Goetz, S.; Jäger, C. Intermediate scapolite: 29Si MAS and 27Al SATRAS NMR spectroscopy and Rietveld structure-refinement. Can. Mineral. 1998, 36, 1267–1283. [Google Scholar]
- Sokolova, E.V.; Gobechiya, E.R.; Zolotarev, A.A.; Kabalov, Y.K. Refinement of the crystal structures of two marialites from the Kukurt deposit of the east Pamirs. Crystallogr. Rep. 2000, 45, 934–938. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Kabalov, Y.K.; Sherriff, B.L.; Teertstra, D.K.; Jenkins, D.M.; Kunath-Fandrei, G.; Goetz, S.; Jäger, C. Marialite: Rietveld structure-refinement and 29Si MAS and 27Al satellite transition NMR spectroscopy. Can. Mineral. 1996, 34, 1039–1050. [Google Scholar]
- Teertstra, D.K.; Sherriff, B.L. Scapolite cell-parameter trends along the solid-solution series. Am. Mineral. 1996, 81, 169–180. [Google Scholar] [CrossRef]
- Zolotarev, A.A. Once more on isomorphic schemes and isomorphic series in the scapolite group. Zap. Vser. Mineral. Obs. 1996, 125, 69–73. [Google Scholar]
- Zolotarev, A.A.; Petrov, T.G.; Moshkin, S.V. Peculiarities of chemical compositions of the scapolite group minerals. Zap. Vser. Mineral. Obs. 2003, 132, 63–84. [Google Scholar]
- Seto, Y.; Shimobayashi, N.; Miyake, A.; Kitamura, M. Composition and I4/m-P42/n phase transition in scapolite solid solutions. Am. Mineral. 2004, 89, 257–265. [Google Scholar] [CrossRef]
- Phakey, P.P.; Ghose, S. Scapolite: Observation of anti-phase domain structure. Nat. Phys. Sci. 1972, 238, 78–80. [Google Scholar] [CrossRef]
- Antao, S.M. Unit-cell parameters for scapolite solid solutions and a discontinuity at Me75, ideally NaCa3[Al5Si7O24](CO3). Powder Diffr. 2013, 28, 253–259. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Complete Al-Si order in scapolite Me37.5, ideally Ca3Na5[Al8Si16O48]Cl(CO3) and implications for antiphase domain boundaries (APBs). Can. Mineral. 2011, 49, 581–586. [Google Scholar] [CrossRef]
- Chamberlain, C.P.; Docka, J.A.; Post, J.E.; Burnham, C.W. Scapolite—Alkali atom configurations, antiphase domains, and compositional variations. Am. Mineral. 1985, 70, 134–140. [Google Scholar]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Antao, S.M.; Hassan, I.; Parise, J.B. Aluminate sodalite: Phase transitions and high-temperature structural evolution of the cubic phase. Can. Mineral. 2004, 42, 1047–1056. [Google Scholar] [CrossRef]
- Teertstra, D.K.; Sherriff, B.L. Substitutional mechanisms, compositional trends and the end-member formulae of scapolite. Chem. Geol. 1997, 136, 233–260. [Google Scholar] [CrossRef]
- Aitken, B.G.; Evans, H.T.; Konnert, J.A. The crystal-structure of a synthetic meionite. Neues Jahrb. Fur Mineral. -Abh. 1984, 149, 309–324. [Google Scholar]
- Levien, L.; Papike, J.J. Scapolite crystal chemistry: Aluminum-silicon distributions, carbonate group disorder, and thermal expansion. Am. Mineral. 1976, 61, 864–877. [Google Scholar]
- Lin, S.B.; Burley, B.J. The crystal structure of meionite. Acta Crystallogr. 1973, 29, 2024–2026. [Google Scholar] [CrossRef]
- Lin, S.B.; Burley, B.J. Crystal structure of a sodium and chlorine-rich scapolite. Acta Crystallogr. 1973, 29, 1272–1278. [Google Scholar] [CrossRef]
- Oterdoom, W.H.; Wenk, H.R. Ordering and composition of scapolite—Field observations and structural interpretations. Contrib. Mineral. Petrol. 1983, 83, 330–341. [Google Scholar] [CrossRef]
- Papike, J.J.; Stephenson, N.C. The crystal structure of mizzonite, a calcium- and carbonate-rich scapolite. Am. Mineral. 1966, 51, 1014–1027. [Google Scholar]
- Papike, J.J.; Zoltai, T. The crystal structure of a marialite scapolite. Am. Mineral. 1965, 50, 641–655. [Google Scholar]
- Peterson, R.C.; Donnay, G.; Lepage, Y. Sulfate disorder in scapolite. Can. Mineral. 1979, 17, 53–61. [Google Scholar]
- Ulbrich, H.H. Structural refinement of the Monte Somma scapolite, a 93% meionite. Schweiz. Mineral. Und Petrogr. Mitteilungen 1973, 53, 385–393. [Google Scholar]
- Buseck, P.R.; Iijima, S. High resolution electron microscopy of silicates. Am. Mineral. 1974, 59, 1–21. [Google Scholar]
- Lin, S.B.; Burley, B.J. The crystal-structure of an intermediate scapolite—Wernerite. Tschermaks Mineral. -Petrol. Mitteilungen 1974, 21, 196–215. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Wang, J.; Lee, P.L.; Toby, B.H. State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Parise, J.B. Sodalite: High temperature structures obtained from synchrotron radiation and Rietveld refinements. Am. Mineral. 2004, 89, 359–364. [Google Scholar] [CrossRef]
- Lee, P.L.; Shu, D.; Ramanathan, M.; Preissner, C.; Wang, J.; Beno, M.A.; Von Dreele, R.B.; Ribaud, L.; Kurtz, C.; Antao, S.M.; et al. A twelve-analyzer detector system for high-resolution powder diffraction. J. Synchrotron Radiat. 2008, 15, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Toby, B.H.; Lee, P.L.; Ribaud, L.; Antao, S.M.; Kurtz, C.; Ramanathan, M.; Von Dreele, R.B.; Beno, M.A. A dedicated powder diffraction beamline at the advanced photon source: Commissioning and early operational results. Rev. Sci. Instrum. 2008, 79, 085105. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. Generalized Structure Analysis System; Report No. LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Ulbrich, H.H. Crystallographic data and refractive indicies of scapolites. Am. Mineral. 1973, 58, 81–92. [Google Scholar]
- Eugster, H.P.; Protska, H.J.; Appleman, D.E. Unit cell dimensions of natural and synthetic scapolites. Science 1962, 137, 853–854. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Lucchesi, S. The thermal behavior of scapolites. Am. Mineral. 1982, 67, 1229–1241. [Google Scholar]
- Baker, J. Thermal expansion of scapolite. Am. Mineral. 1994, 79, 878–884. [Google Scholar]
- Hassan, I.; Grundy, H.D. The crystal structures of sodalite-group minerals. Acta Crystallogr. 1984, 40, 6–13. [Google Scholar] [CrossRef]

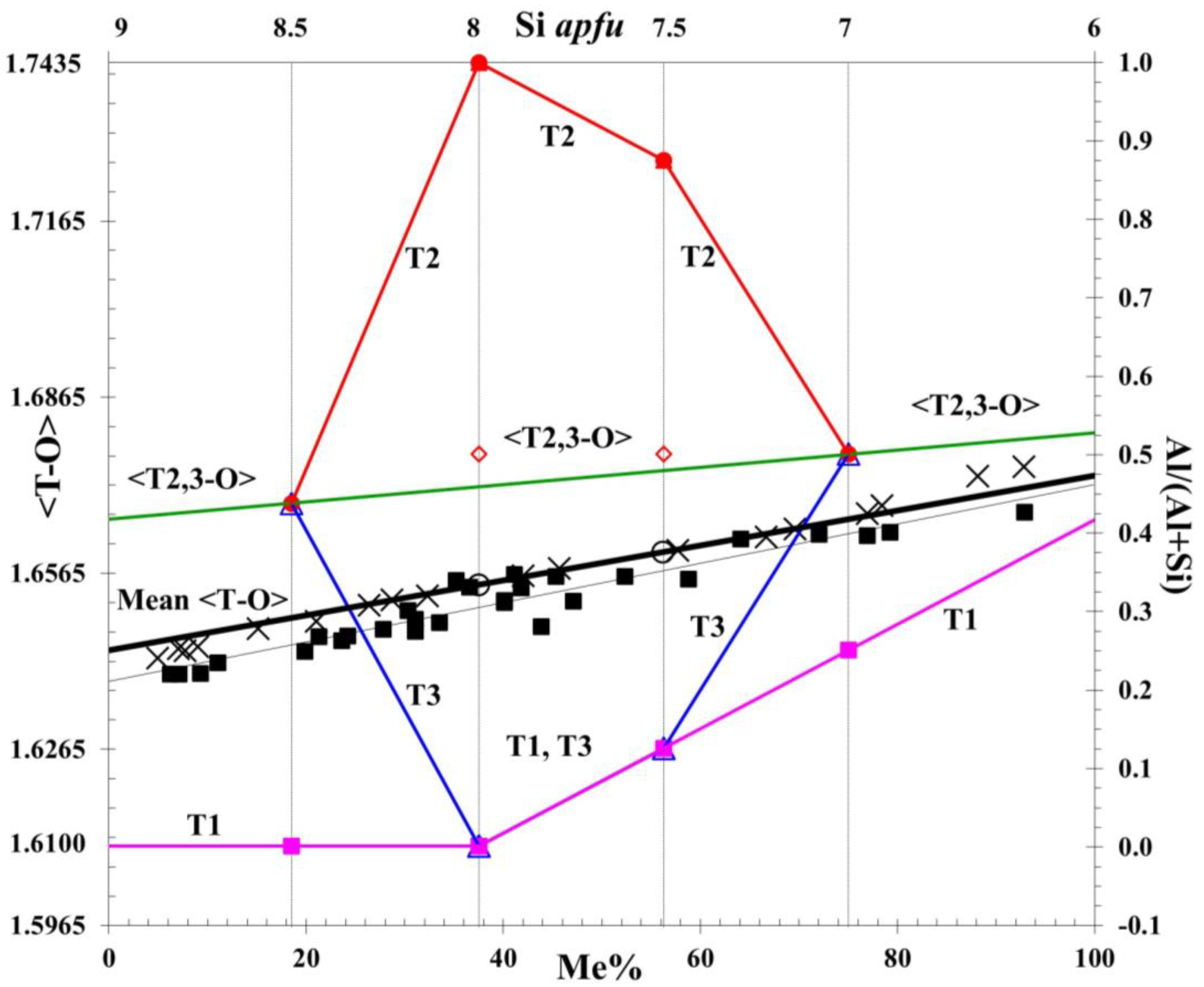

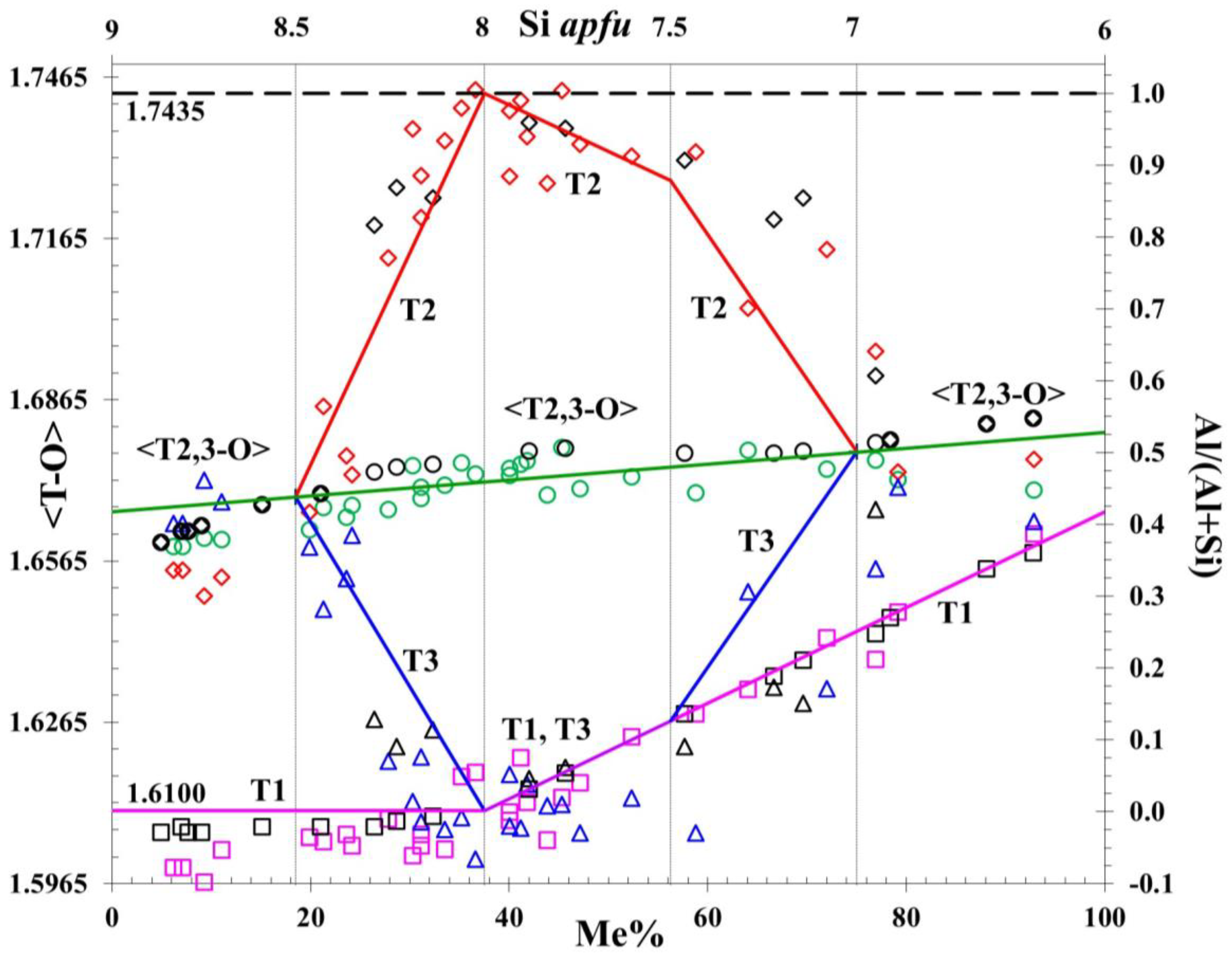
| Me% | Formulae and Clusters | T1 Site | T2 Site | T3 Site | Mean <T–O> |
|---|---|---|---|---|---|
| 0 | Na8[Al6Si18O48]Cl2 | 8Si | 3Al + 5Si | 3Al + 5Si | |
| [Na4·Cl]3+ = 1 | 1Si | 0.375Al + 0.625Si | =T2 | ||
| Tetrahedral distances | 1.6100 Å | 1.6601 Å | 1.6601 Å | 1.6434 | |
| 9.38 | Na7.25Ca0.75[Al6.5Si17.5O48]Cl1.75(CO3)0.25 | 8Si | 3.25Al + 4.75Si | 3.25Al + 4.75Si | |
| [Na4·Cl]3+:[NaCa3·CO3]5+ = 1.75:0.25 | 1Si | 0.406Al + 0.594Si | =T2 | ||
| Tetrahedral distances | 1.6100 Å | 1.6642 Å | 1.6642 Å | 1.6461 | |
| 18.75 | Na6.5Ca1.5[Al7Si17O48]Cl1.5(CO3)0.50 | 8Si | 3.5Al + 4.5Si | 3.5Al + 4.5Si | |
| [Na4·Cl]3+:[NaCa3·CO3]5+ = 1.5:0.5 | 1Si | 0.438Al + 0.563Si | =T2 | ||
| Tetrahedral distances | 1.6100 Å | 1.6701 Å | 1.6701 Å | 1.6501 | |
| 37.5 | Na5Ca3[Al8Si16O48]Cl(CO3) | 8Si | 8Al | 8Si | |
| [Na4·Cl]3+:[NaCa3·CO3]5+ = 1:1 | 1Si | 1Al | 1Si | ||
| Tetrahedral distances | 1.6100 Å | 1.7435 Å | 1.6100 Å | 1.6545 | |
| 56.25 | Na3.5Ca4.5[Al9Si15O48]Cl0.5(CO3)1.5 | 1Al + 7Si | 7Al + 1Si | 1Al + 7Si | |
| [Na4·Cl]3+:[NaCa3·CO3]5+ = 0.5:1.5 | 0.125Al + 0.875Si | 0.875Al + 0.125Si | 0.125Al + 0.875Si | ||
| Tetrahedral distances | 1.6267 Å | 1.7268 Å | 1.6267 Å | 1.6601 | |
| 75 | Na2Ca6[Al10Si14O48](CO3)2 | 2Al + 6Si | 4Al + 4Si | 4Al + 4Si | |
| [NaCa3·CO3]5+ = 1 | 0.25Al + 0.75Si | 0.5Al + 0.5Si | =T2 | ||
| Tetrahedral distances | 1.6434 Å | 1.6768 Å | 1.6768 Å | 1.6656 | |
| 87.5 | NaCa7[Al11Si13O48](CO3)2 | 2.7Al + 5.3Si | 4.15Al + 3.85Si | 4.15Al + 3.85Si | |
| [NaCa3·CO3]5+:[Ca4·CO3]6+ = 1:1 | 0.338Al + 0.663Si | 0.519Al + 0.481Si | =T2 | ||
| Tetrahedral distances | 1.6567 Å | 1.6793 Å | 1.6793 Å | 1.6718 | |
| 100 | Ca8[Al12Si12O48](CO3)2 | 3.4Al + 4.6Si | 4.3Al + 3.7Si | 4.3Al + 3.7Si | |
| [Ca4·CO3]6+ = 1 | 0.425Al + 0.575Si | 0.538Al + 0.463Si | =T2 | ||
| Tetrahedral distances | 1.6667 Å | 1.6834 Å | 1.6834 Å | 1.6779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antao, S.M. Al-Si Order and Chemical Composition Model across Scapolite Solid Solutions with Evidence from Rietveld Structure Refinements. Minerals 2024, 14, 812. https://doi.org/10.3390/min14080812
Antao SM. Al-Si Order and Chemical Composition Model across Scapolite Solid Solutions with Evidence from Rietveld Structure Refinements. Minerals. 2024; 14(8):812. https://doi.org/10.3390/min14080812
Chicago/Turabian StyleAntao, Sytle M. 2024. "Al-Si Order and Chemical Composition Model across Scapolite Solid Solutions with Evidence from Rietveld Structure Refinements" Minerals 14, no. 8: 812. https://doi.org/10.3390/min14080812
APA StyleAntao, S. M. (2024). Al-Si Order and Chemical Composition Model across Scapolite Solid Solutions with Evidence from Rietveld Structure Refinements. Minerals, 14(8), 812. https://doi.org/10.3390/min14080812







