Towards Safe Diatomite Sludge Management: Lead Immobilisation via Geopolymerisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Pb(II)-Rich Dt Sludge
2.3. Preparation of Geopolymers
2.4. Leaching Tests
2.5. Characterisation
3. Results and Discussion
3.1. Properties of Raw Materials
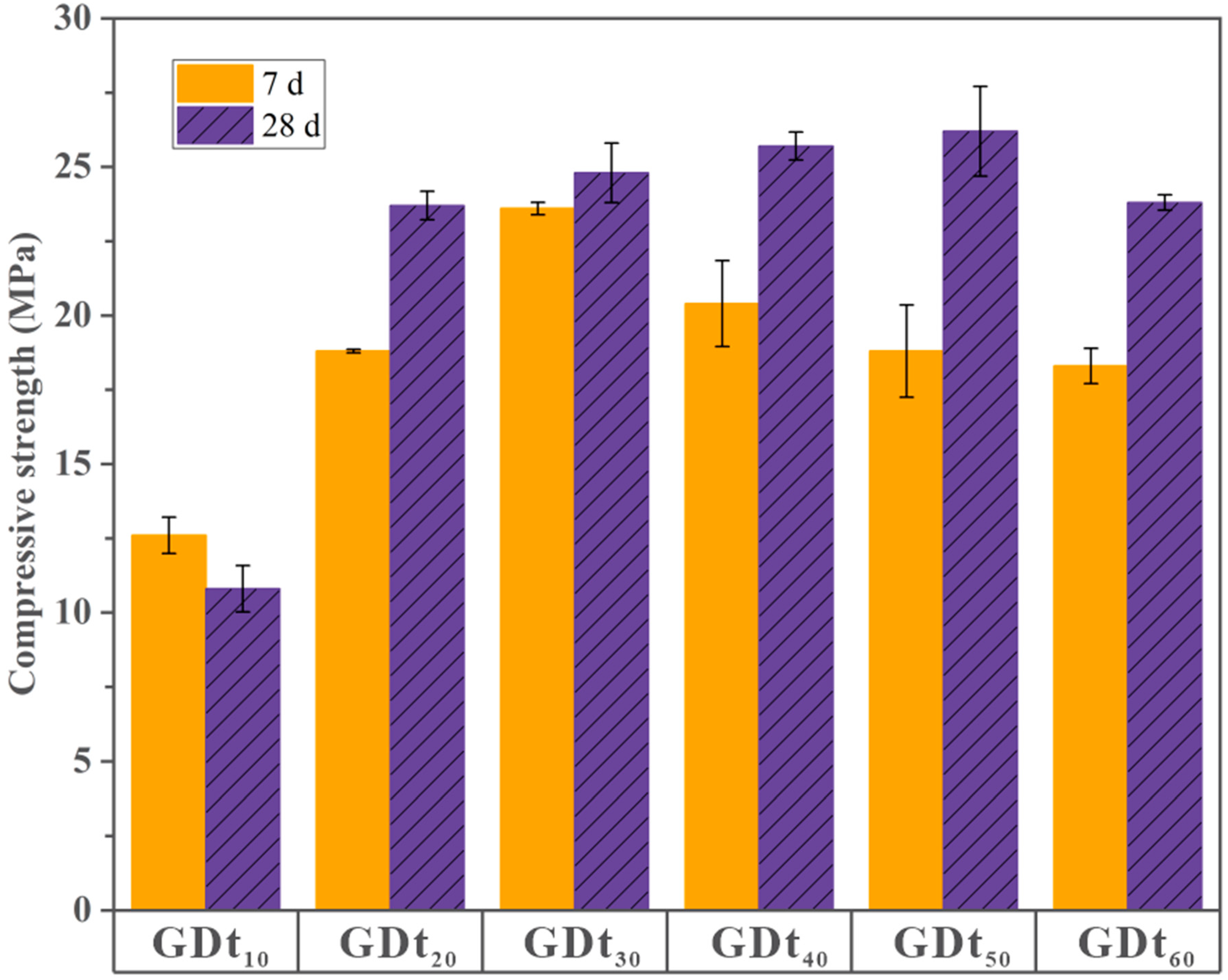
3.2. Compressive Strengths of Dt-Based Geopolymers
3.3. Geopolymer Solidified Body of Pb(II)-Rich Dt Sludge
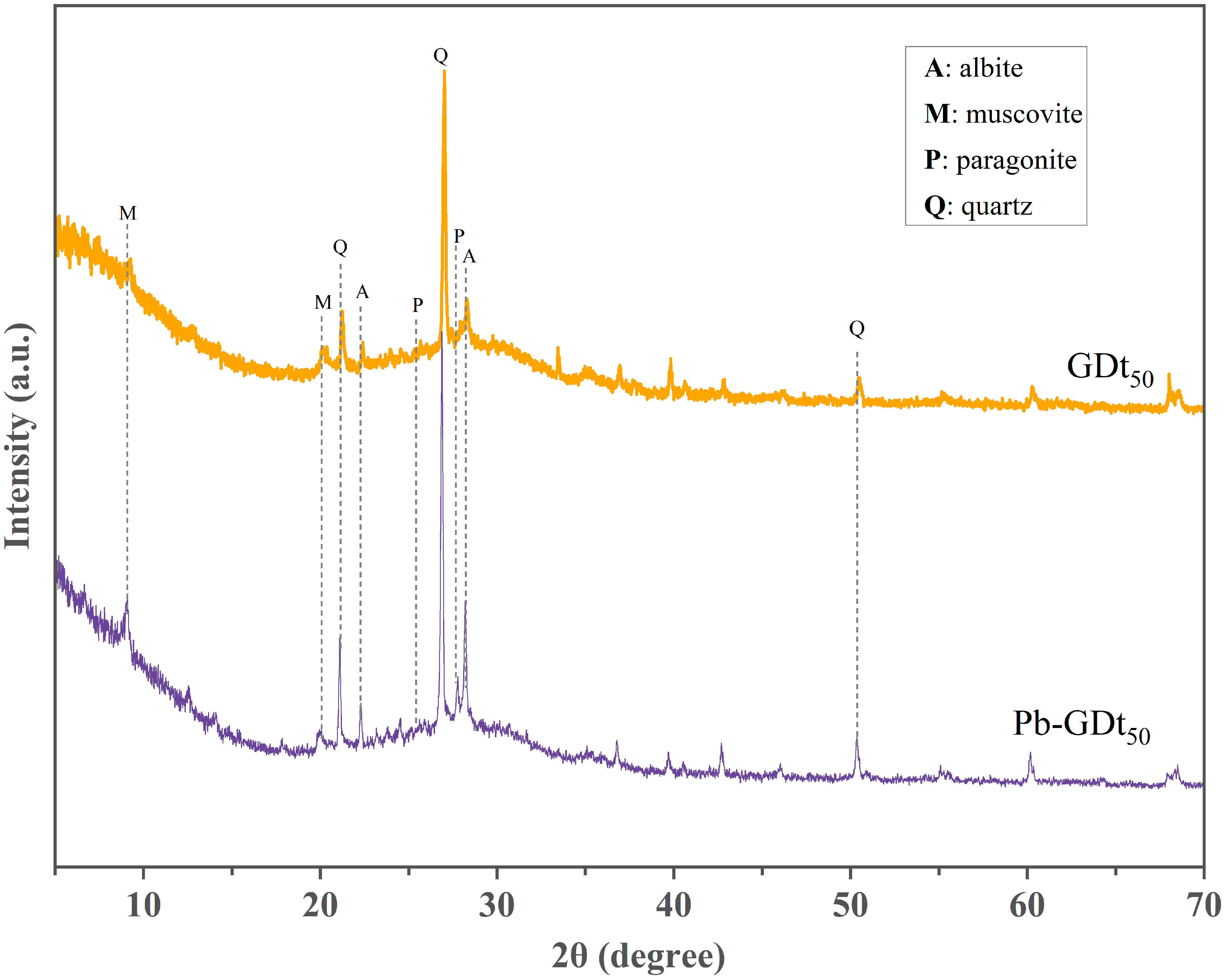
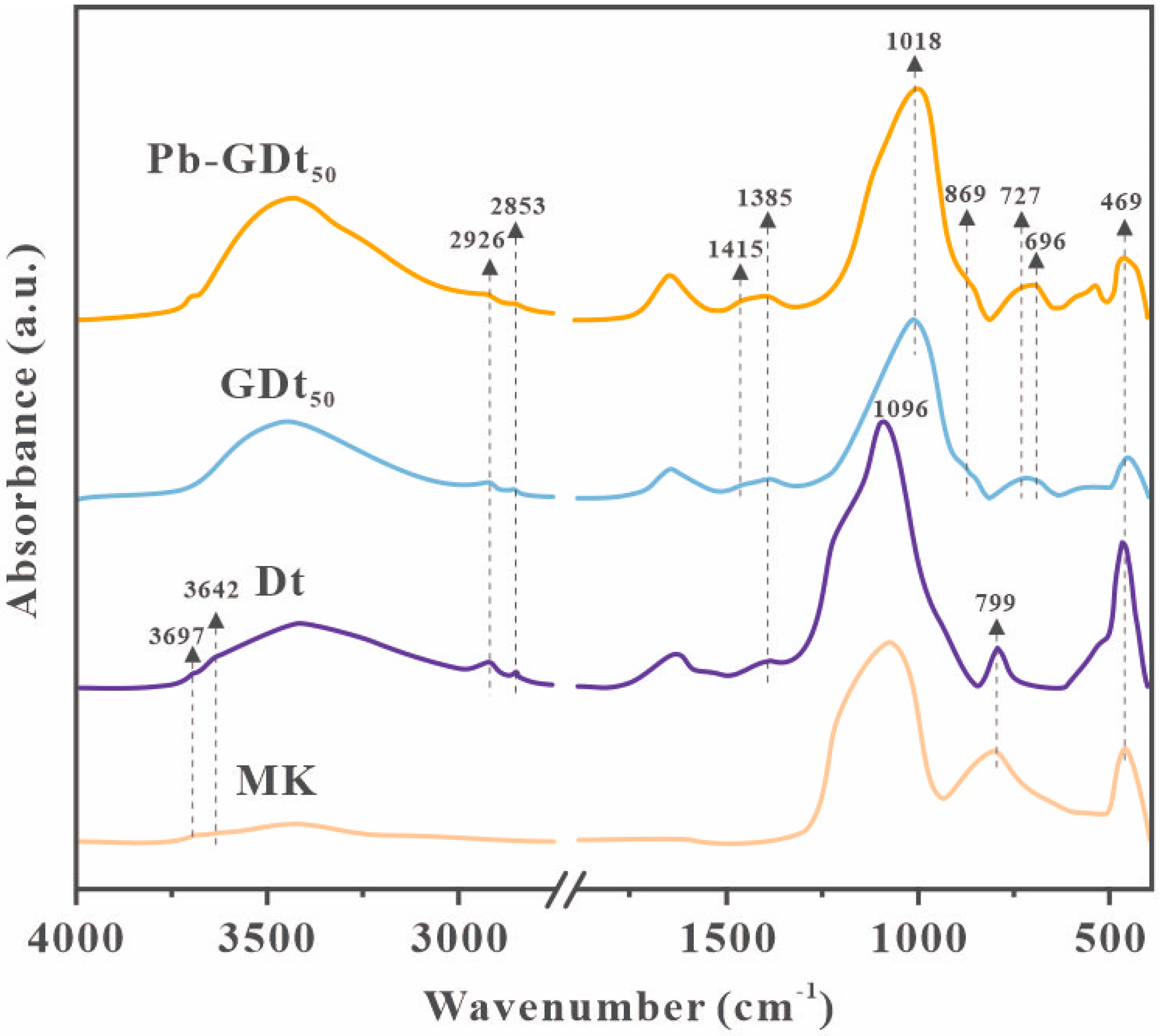
3.3.1. Mineral Composition
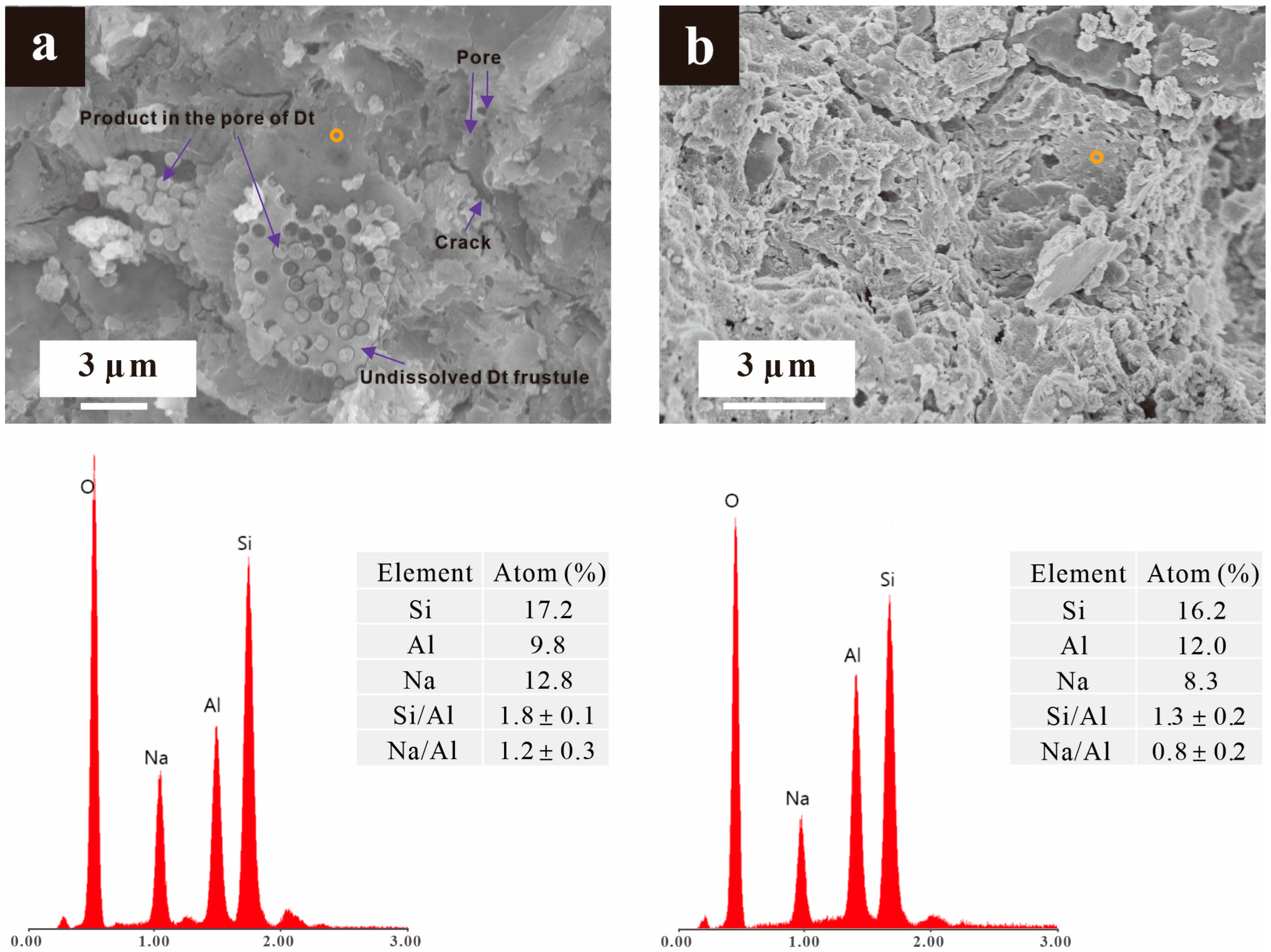
3.3.2. Microstructure
3.4. Stability
3.4.1. Compressive Strength
3.4.2. Leaching Behaviour
4. Conclusions
- The Pb(II) present in the pores of the undissolved diatom frustules underwent in situ binding with the activator, resulting in its encapsulation by the gels that were subsequently generated in the pores.
- The dissolution of frustules released Pb2+, which then participated in the condensation of silicon and aluminium monomers in the formation of soluble hydroxide under high alkalinity.
- The continuous consumption of the activator by metakaolin dissolution prompted the conversion of soluble hydroxides of Pb(II) to precipitates, which were encapsulated by the gels in the subsequent reaction.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Fan, M.D.; Yang, D.; Zhu, R.L.; Ge, F.; Zhu, J.X.; He, H.P. Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J. Hazard. Mater. 2010, 173, 614–621. [Google Scholar] [CrossRef]
- Khraisheh, M.A.M.; Al-Degs, Y.S.; Mcminn, W.A.M. Remediation of wastewater containing heavy metals using raw and modified diatomite. Chem. Eng. J. 2004, 99, 177–184. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; El Gamouz, A.; Frangie, S.; Martinez, V.; Valiente, L.; Webb, O.A. Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J. Hazard. Mater. 2012, 241, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Kigga, M.; Uthappa, U.T.; Rego, R.M.; Thendral, V.; Kumeria, T.; Jung, H.-Y.; Kurkuri, M.D. Naturally available diatomite and their surface modification for the removal of hazardous dye and metal ions: A review. Adv. Colloid Interface Sci. 2020, 282, 102198. [Google Scholar] [CrossRef] [PubMed]
- Malviya, R.; Chaudhary, R. Factors affecting hazardous waste solidification/stabilization: A review. J. Hazard. Mater. 2006, 137, 267–276. [Google Scholar] [CrossRef]
- Behera, S.K.; Mishra, D.P.; Singh, P.; Mishra, K.; Mandal, S.K.; Ghosh, C.N.; Kumar, R.; Mandal, P.K. Utilization of mill tailings, fly ash and slag as mine paste backfill material: Review and future perspective. Constr. Build. Mater. 2021, 309, 125120. [Google Scholar] [CrossRef]
- Gou, M.F.; Zhou, L.F.; Then, N.W.Y. Utilization of tailings in cement and concrete: A review. Sci. Eng. Compos. Mater. 2019, 26, 449–464. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- D’Angelo, A.; Vertuccio, L.; Leonelli, C.; Alzeer, M.I.M.; Catauro, M. Entrapment of Acridine Orange in Metakaolin-Based Geopolymer: A Feasibility Study. Polymers 2023, 15, 675. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Gowripalan, N. Mechanisms of Heavy Metal Immobilisation using Geopolymerisation Techniques—A review. J. Adv. Concr. Technol. 2018, 16, 124–135. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, G.; Hu, J.; Niu, R.; Wang, Z.; Liu, J.; Xing, F. Hazardous wastes used as hybrid precursors for geopolymers: Cosolidification/stabilization of MSWI fly ash and Bayer red mud. Chem. Eng. J. 2023, 474, 145966. [Google Scholar] [CrossRef]
- Tan, J.; De Vlieger, J.; Desomer, P.; Cai, J.; Li, J. Co-disposal of construction and demolition waste (CDW) and municipal solid waste incineration fly ash (MSWI FA) through geopolymer technology. J. Clean. Prod. 2022, 362, 132502. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Immobilization efficiency and mechanism of metal cations (Cd2+, Pb2+ and Zn2+) and anions (AsO43− and Cr2O72−) in wastes-based geopolymer. J. Hazard. Mater. 2020, 384, 121290. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.; Hohmann, M.; Posern, K.; Brendler, E. The suitability of thermally activated illite/smectite clay as raw material for geopolymer binders. Appl. Clay Sci. 2009, 46, 300–304. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Yuan, P.; Li, Y.; Wang, Q.; Deng, L.; Liu, D. Geopolymerization of halloysite via alkali-activation: Dependence of microstructures on precalcination. Appl. Clay Sci. 2020, 185, 105375. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, B.; Deng, L.; Yuan, P.; Li, M.; Wang, Q. Preparation of high-performance silico-aluminophosphate geopolymers using fly ash and metakaolin as raw materials. Appl. Clay Sci. 2021, 204, 106019. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Sinsiri, T.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. The effects of replacement fly ash with diatomite in geopolymer mortar. Comput. Concr. 2012, 9, 427–437. [Google Scholar] [CrossRef]
- Bagci, C.; Kutyla, G.P.; Kriven, W.M. Fully Reacted High Strength Geopolymer made with Diatomite as a Fumed Silica Alternative. Ceram. Int. 2017, 43, 14784–14790. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Chindaprasirt, P.; Sata, V.; Sinsiri, T. High calcium fly ash geopolymer containing diatomite as additive. Indian J. Eng. Mater. Sci. 2013, 20, 310–318. [Google Scholar]
- Yu, W.B.; Yuan, P.; Liu, D.; Deng, L.L.; Yuan, W.W.; Tao, B.; Cheng, H.F.; Chen, F.R. Facile preparation of hierarchically porous diatomite/MFI-type zeolite composites and their performance of benzene adsorption: The effects of NaOH etching pretreatment. J. Hazard. Mater. 2015, 285, 173–181. [Google Scholar] [CrossRef]
- ASTM D8155-17; Standard Practice for Shake Extraction of Solid Mining and Metallurgical Processing Waste with Water. ASTM: West Conshohocken, PA, USA, 2023.
- U.S. Environmental Protection Agency Office of Solid Waste. Hazard Waste Character Scoping Study; EPA: Washington, DC, USA, 1996; Volume 3, pp. 1–3.
- Huang, M.; Feng, H.; Shen, D.; Li, N.; Chen, Y.; Shentu, J. Leaching Behavior of Heavy Metals from Cement Pastes Using a Modified Toxicity Characteristic Leaching Procedure (TCLP). Bull. Environ. Contam. Toxicol. 2016, 96, 354–360. [Google Scholar] [CrossRef]
- Pereira, D.G.; Afonso, A.; Medeiros, F.M. Overview of Friedman’s Test and Post-hoc Analysis. Commun. Stat.-Simul. Comput. 2015, 44, 2636–2653. [Google Scholar] [CrossRef]
- Xue, B.; Jiang, Y.S.; Yang, D.F.; Shi, X.D. Carbonization Treatment of Diatomite with High Ignition Loss and Reinforcement of Natural Rubber. Chem. J. Chin. Univ. 2011, 32, 1617–1621. [Google Scholar]
- Lutynski, M.; Sakiewicz, P.; Lutynska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. A Review of the Classification of Opal with Reference to Recent New Localities. Minerals 2019, 9, 299. [Google Scholar] [CrossRef]
- Murray, H.H. Chapter 2 Structure and Composition of the Clay Minerals and their Physical and Chemical Properties. In Developments in Clay Science; Murray, H.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 7–31. [Google Scholar]
- Yang, Y.; Jaber, M.; Michot, L.J.; Rigaud, B.; Walter, P.; Laporte, L.; Zhang, K.; Liu, Q. Analysis of the microstructure and morphology of disordered kaolinite based on the particle size distribution. Appl. Clay Sci. 2023, 232, 106801. [Google Scholar] [CrossRef]
- Favier, A.; Habert, G.; Roussel, N.; d’Espinose de Lacaillerie, J.-B. A multinuclear static NMR study of geopolymerisation. Cem. Concr. Res. 2015, 75, 104–109. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Karuppaiyan, J.; Jeyalakshmi, R.; Kiruthika, S.; Wadaan, M.A.; Khan, M.F.; Kim, W. A study on the role of surface functional groups of metakaolin in the removal of methylene blue: Characterization, kinetics, modeling and RSM optimization. Environ. Res. 2023, 226, 115604. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. 29Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 2005, 21, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloid Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Esaifan, M.; Warr, L.N.; Grathoff, G.; Meyer, T.; Schafmeister, M.-T.; Kruth, A.; Testrich, H. Synthesis of Hydroxy-Sodalite/Cancrinite Zeolites from Calcite-Bearing Kaolin for the Removal of Heavy Metal Ions in Aqueous Media. Minerals 2019, 9, 484. [Google Scholar] [CrossRef]
- Rozek, P.; Krol, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Obiora, S.C.; Umeji, A.C. Petrographic evidence for regional burial metamorphism of the sedimentary rocks in the Lower Benue rift. J. Afr. Earth Sci. 2004, 38, 269–277. [Google Scholar] [CrossRef]
- Sitarz, M. The structure of liquation silico-phosphate glasses. J. Mol. Struct. 2008, 887, 229–236. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Yuan, P.; Deng, L.; Liu, D. Novel acid-based geopolymer synthesized from nanosized tubular halloysite: The role of precalcination temperature and phosphoric acid concentration. Cem. Concr. Compos. 2020, 110, 103601. [Google Scholar] [CrossRef]
- Zhang, B.; Yuan, P.; Guo, H.; Deng, L.; Li, Y.; Li, L.; Wang, Q.; Liu, D. Effect of curing conditions on the microstructure and mechanical performance of geopolymers derived from nanosized tubular halloysite. Constr. Build. Mater. 2020, 268, 121186. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Attenuated total reflectance Fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Schwartzman, A. The potential use of geopolymeric materials to immobilise toxic metals: Part II. Material and leaching characteristics. Miner. Eng. 1999, 12, 75–91. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhang, Z.; Wang, H.; Zhou, S.; Zhou, W. A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution. Fuel 2016, 185, 181–189. [Google Scholar] [CrossRef]
- Joussein, E.; Soubrand, M.; Pascaud, G.; Cogulet, A.; Rossignol, S. Immobilization of Pb from mine sediments in metakaolin-based geomaterials. Environ. Sci. Pollut. Res. 2019, 26, 14473–14482. [Google Scholar] [CrossRef] [PubMed]
- Alzeer, M.I.M.; MacKenzie, K.J.D. Synthesis and Catalytic Properties of New Sustainable Aluminosilicate Heterogeneous Catalysts Derived from Fly Ash. ACS Sustain. Chem. Eng. 2018, 6, 5273–5282. [Google Scholar] [CrossRef]
- Rodriguez-Vidal, F.J.; Ortega-Azabache, B.; Gonzalez-Martinez, A.; Bellido-Fernandez, A. Comprehensive characterization of industrial wastewaters using EEM fluorescence, FT-IR and 1H NMR techniques. Sci. Total Environ. 2022, 805, 150417. [Google Scholar] [CrossRef]
- Provis, J.L.; Yong, S.L.; Duxson, P. 5—Nanostructure/microstructure of metakaolin geopolymers. In Geopolymers; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 72–88. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic Polymeric New Materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y. Distribution of Pb(II) species in aqueous solutions. J. Colloid Interface Sci. 2003, 268, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhong, L.; Yang, X.; Bai, H.; Ren, B.; Zhao, Y.; Zhang, W.; Ju, X.; Wen, H.; Mao, S.; et al. Synthesis of rare earth tailing-based geopolymer for efficiently immobilizing heavy metals. Constr. Build. Mater. 2020, 254, 119273. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Xu, Y. Solidification/stabilization and immobilization mechanism of Pb(II) and Zn(II) in ettringite. Cem. Concr. Res. 2023, 174, 107350. [Google Scholar] [CrossRef]
- Yuan, P.; Wu, D.Q.; He, H.P.; Lin, Z.Y. The hydroxyl species and acid sites on diatomite surface: A combined IR and Raman study. Appl. Surf. Sci. 2004, 227, 30–39. [Google Scholar] [CrossRef]
- Chen, K.; Li, C.; Shi, L.; Gao, T.; Song, X.; Bachmatiuk, A.; Zou, Z.; Deng, B.; Ji, Q.; Ma, D. Growing three-dimensional biomorphic graphene powders using naturally abundant diatomite templates towards high solution processability. Nat. Commun. 2016, 7, 13440. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. Factors affecting the immobilization of metals in geopolymerized flyash. Metall. Mater. Trans. B Proc. Metall. Mater. Proc. Sci. 1998, 29, 283–291. [Google Scholar] [CrossRef]
- Moya, J.S.; Cabal, B.; Lopez-Esteban, S.; Bartolomé, J.F.; Sanz, J. Significance of the formation of pentahedral aluminum in the reactivity of calcined kaolin/metakaolin and its applications. Ceram. Int. 2024, 50, 1329–1340. [Google Scholar] [CrossRef]
- San Cristobal, A.G.; Castello, R.; Martin Luengo, M.A.; Vizcayno, C. Zeolites prepared from calcined and mechanically modified kaolins A comparative study. Appl. Clay Sci. 2010, 49, 239–246. [Google Scholar] [CrossRef]
- Lecomte, I.; Liégeois, M.; Rulmont, A.; Cloots, R.; Maseri, F. Synthesis and characterization of new inorganic polymeric composites based on kaolin or white clay and on ground-granulated blast furnace slag. J. Mater. Res. 2003, 18, 2571–2579. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, D.; van Deventer, J.S.J. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Tao, D.; Xu, Y.; Li, P.; Cui, H.; Zhai, J. Immobilization of simulated radionuclide 133Cs(+) by fly ash-based geopolymer. J. Hazard. Mater. 2013, 262, 325–331. [Google Scholar] [CrossRef]
- Saleh, H.M.; Tawfik, M.E.; Bayoumi, T.A. Chemical stability of seven years aged cement–PET composite waste form containing radioactive borate waste simulates. J. Nucl. Mater. 2011, 411, 185–192. [Google Scholar] [CrossRef]
- Valencia-Saavedra, W.G.; de Gutierrez, R.M.; Puertas, F. Performance of FA-based geopolymer concretes exposed to acetic and sulfuric acids. Constr. Build. Mater. 2020, 257, 119503. [Google Scholar] [CrossRef]

| Sample | Dt wt.% | MK wt.% | Molar Ratio | L/S | |
|---|---|---|---|---|---|
| Si/Al | Na/Al | ||||
| GDt10 | 10.0 | 90.0 | 1.2 | 0.6 | 0.6 |
| GDt20 | 20.0 | 80.0 | 1.3 | 0.6 | 0.6 |
| GDt30 | 30.0 | 70.0 | 1.5 | 0.7 | 0.6 |
| GDt40 | 40.0 | 60.0 | 1.8 | 0.8 | 0.6 |
| GDt50 | 50.0 | 50.0 | 2.1 | 0.9 | 0.6 |
| GDt60 | 60.0 | 40.0 | 2.6 | 1.1 | 0.6 |
| Sample | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | Na2O | MgO | TiO2 | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Dt | 67.90 | 4.92 | 0.41 | 2.67 | 0.88 | 0.56 | 0.60 | 0.21 | 1.11 | 20.74 |
| MK | 52.54 | 43.50 | 0.05 | 0.76 | 0.74 | 0.14 | 0.12 | 0.56 | 0.33 | 1.26 |
| Compressive Strength (MPa) | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|
| GDt10 | GDt20 | GDt30 | GDt40 | GDt50 | GDt60 | F | P | |
| 7 d | 12.6 c 1 | 18.8 b | 23.8 a | 20.4 b | 18.8 b | 18.3 b | 44.632 | 0.000 |
| 28 d | 10.8 b | 23.7 a | 24.6 a | 25.7 a | 26.2 a | 23.8 a | 85.327 | 0.000 |
| Leachate | Performance | |
|---|---|---|
| Deionised water | Leaching concentration (C) | 2.1 mg/L |
| Immobilisation rate (I) | 96.2% | |
| HOAc | Leaching concentration (C) | 2.8 mg/L |
| Immobilisation rate (I) | 94.9% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Huang, Z.; Zhang, B.; Yu, T.; Pantongsuk, T.; Yuan, P. Towards Safe Diatomite Sludge Management: Lead Immobilisation via Geopolymerisation. Minerals 2024, 14, 763. https://doi.org/10.3390/min14080763
Guo H, Huang Z, Zhang B, Yu T, Pantongsuk T, Yuan P. Towards Safe Diatomite Sludge Management: Lead Immobilisation via Geopolymerisation. Minerals. 2024; 14(8):763. https://doi.org/10.3390/min14080763
Chicago/Turabian StyleGuo, Haozhe, Zhihao Huang, Baifa Zhang, Ting Yu, Thammaros Pantongsuk, and Peng Yuan. 2024. "Towards Safe Diatomite Sludge Management: Lead Immobilisation via Geopolymerisation" Minerals 14, no. 8: 763. https://doi.org/10.3390/min14080763
APA StyleGuo, H., Huang, Z., Zhang, B., Yu, T., Pantongsuk, T., & Yuan, P. (2024). Towards Safe Diatomite Sludge Management: Lead Immobilisation via Geopolymerisation. Minerals, 14(8), 763. https://doi.org/10.3390/min14080763







