Occurrence of Differences between Alkali and Alkaline Earth Metals (AAEMs), Including Sodium, Potassium, Calcium, and Magnesium, in the Maceral Groups of the Xiheishan Coal, Zhundong Coalfield, Xinjiang, China
Abstract
1. Introduction
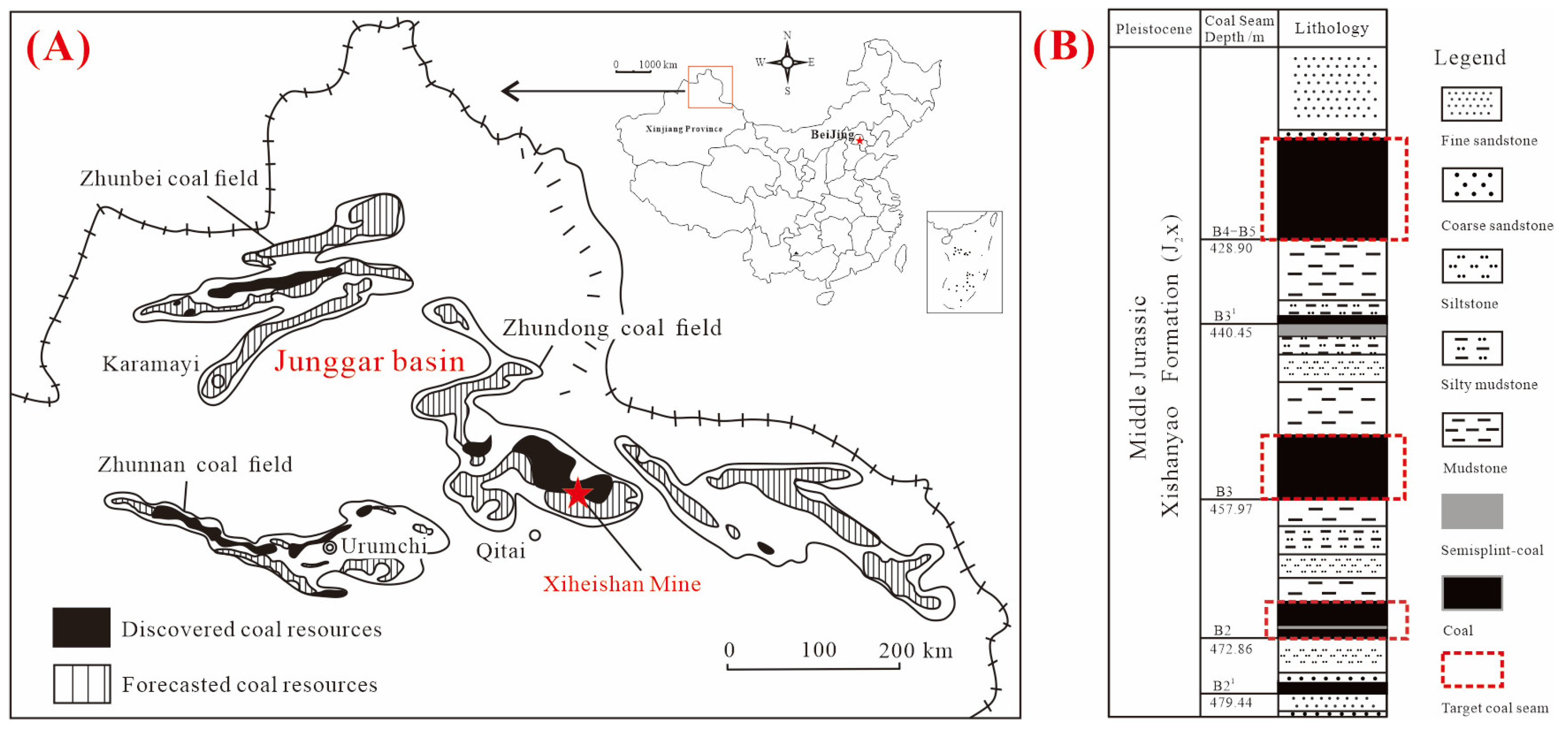
2. Geological Setting
3. Experimental Section
3.1. Sample Preparation
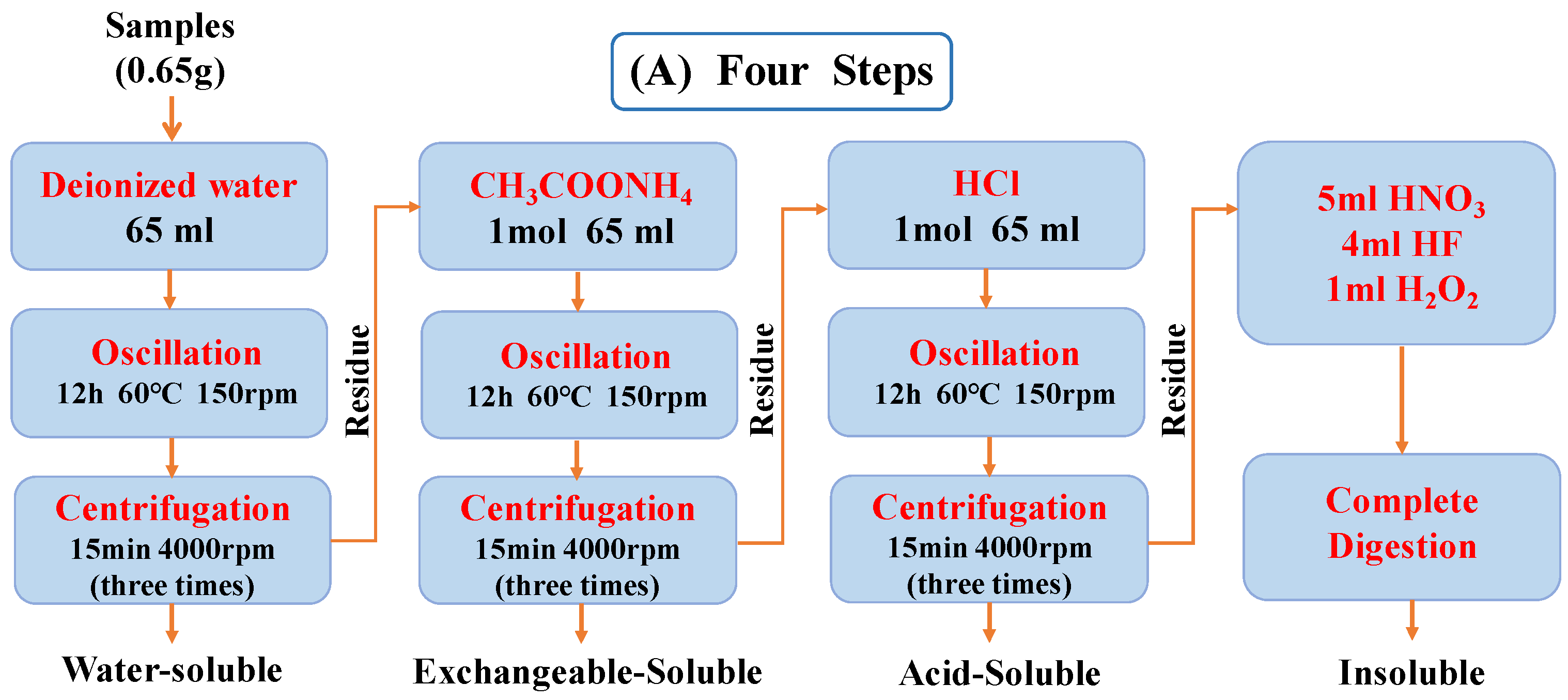
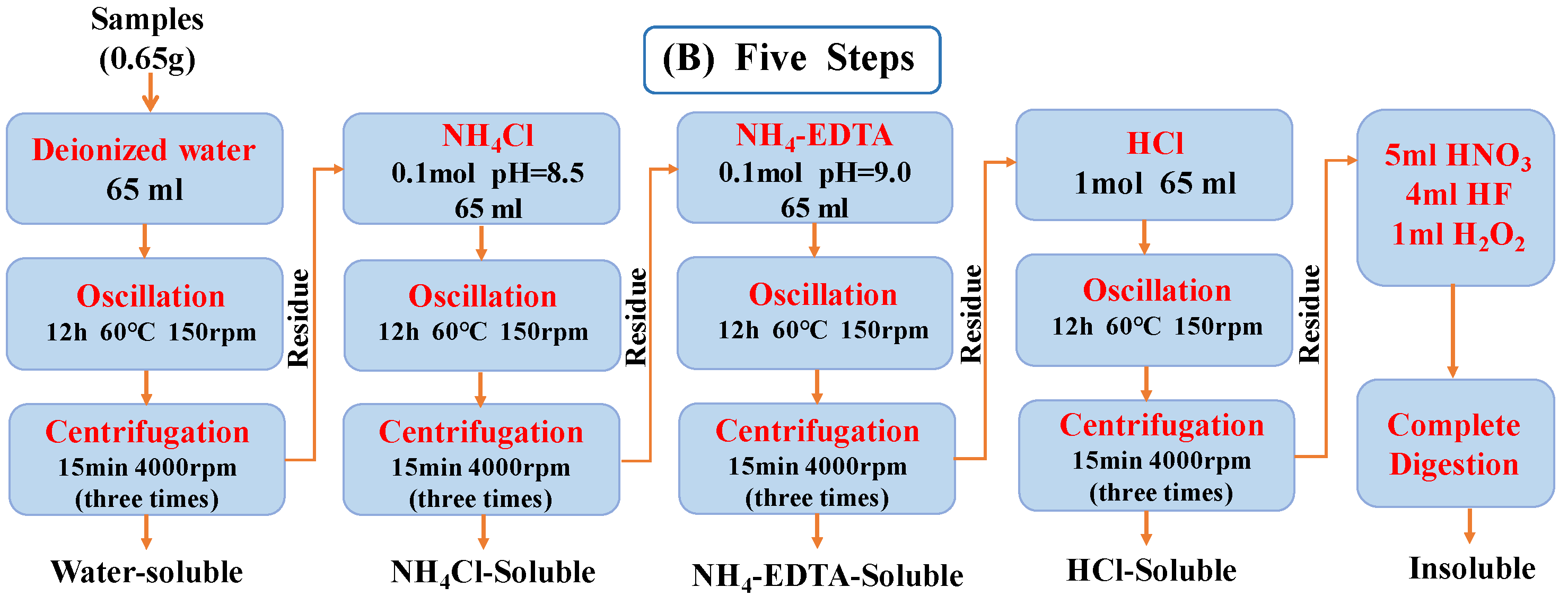
3.2. Sequential Extraction
3.3. Analytical Methods
4. Results and Discussion
4.1. Assessment of Coal Quality
4.2. Maceral Compositions of the Samples
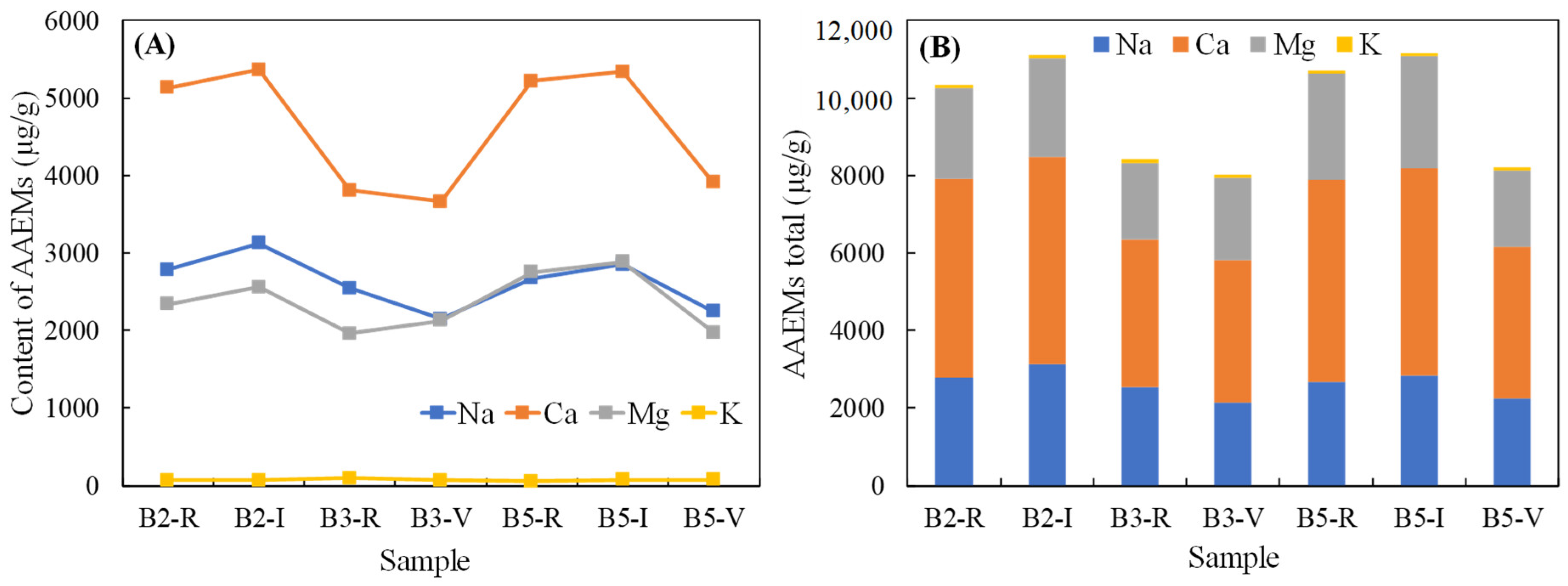
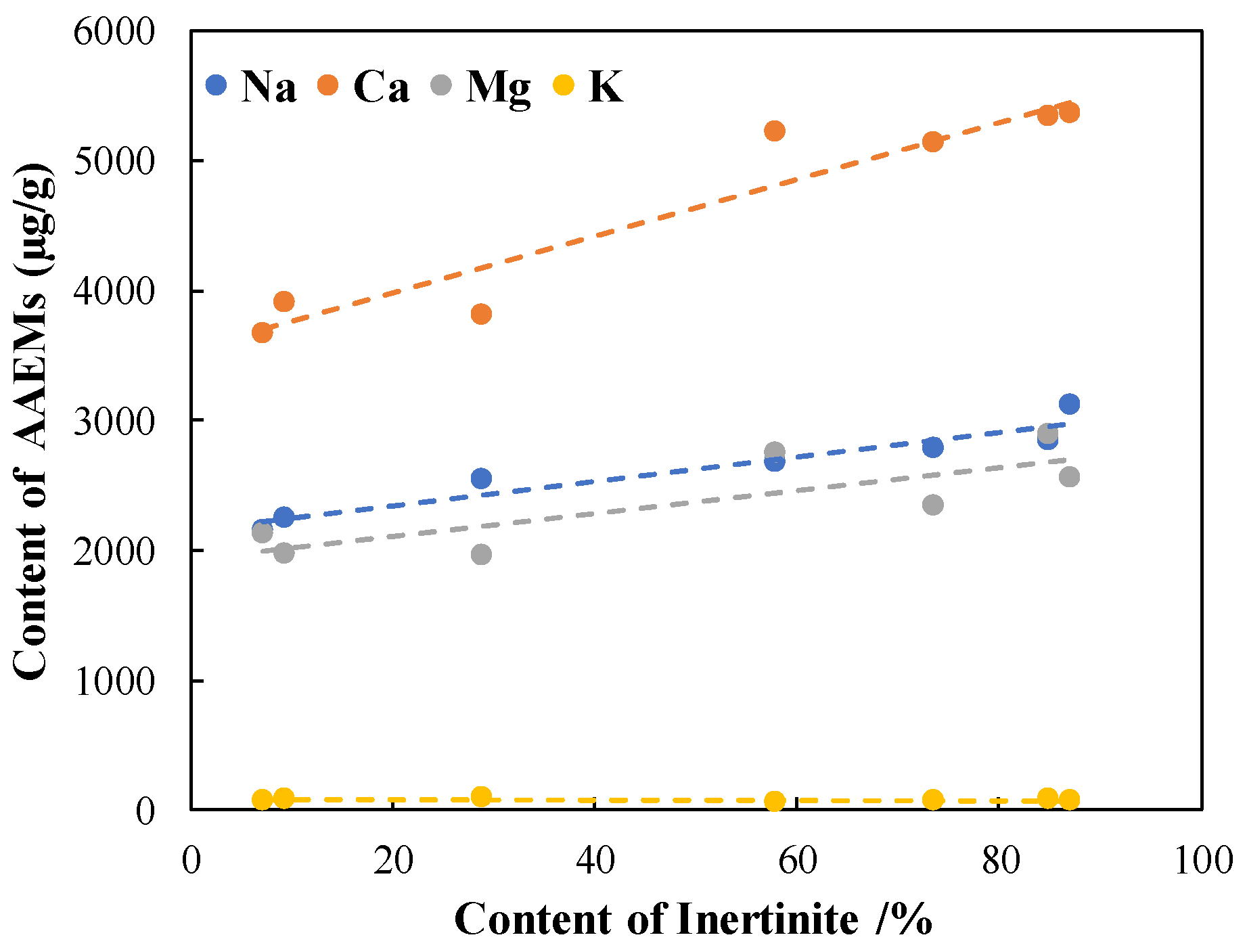
4.3. Contents of the AAEMs in the Samples
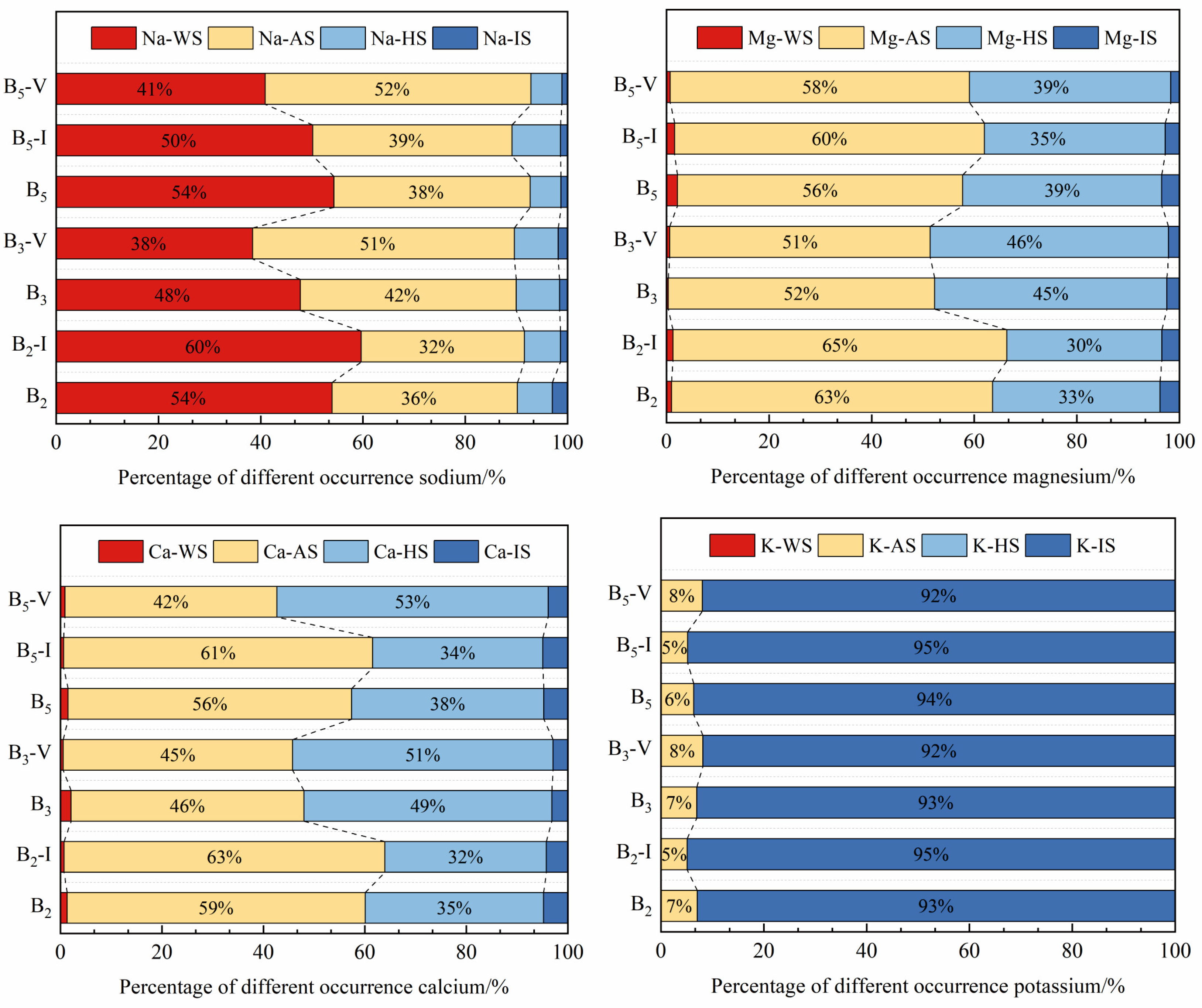
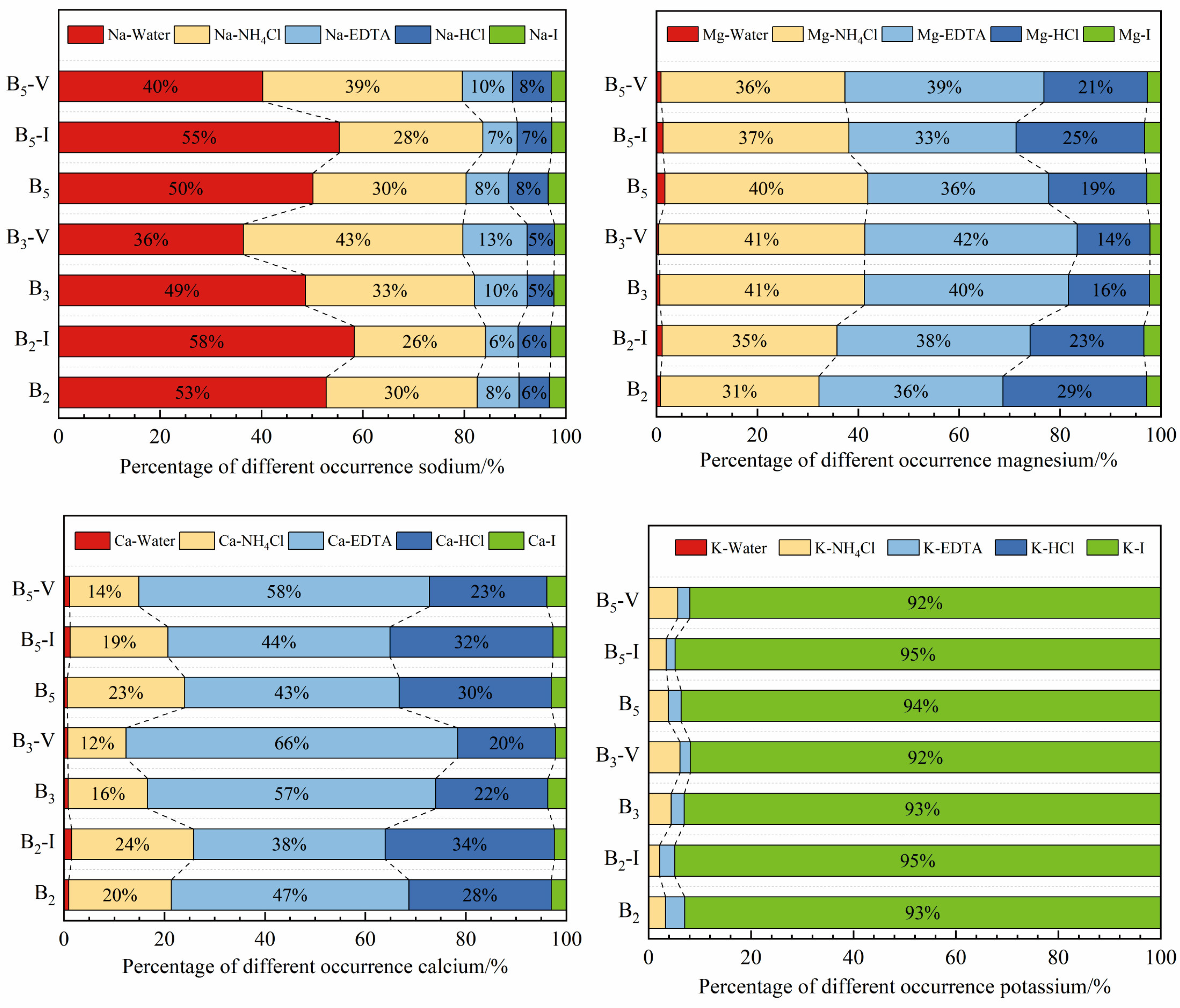
4.4. Sequential Extraction Results for the AAEMs
4.5. The Form in which Water-Soluble AAEMs Exist
4.6. Occurrence Characteristics of the AAEMs in Macerals
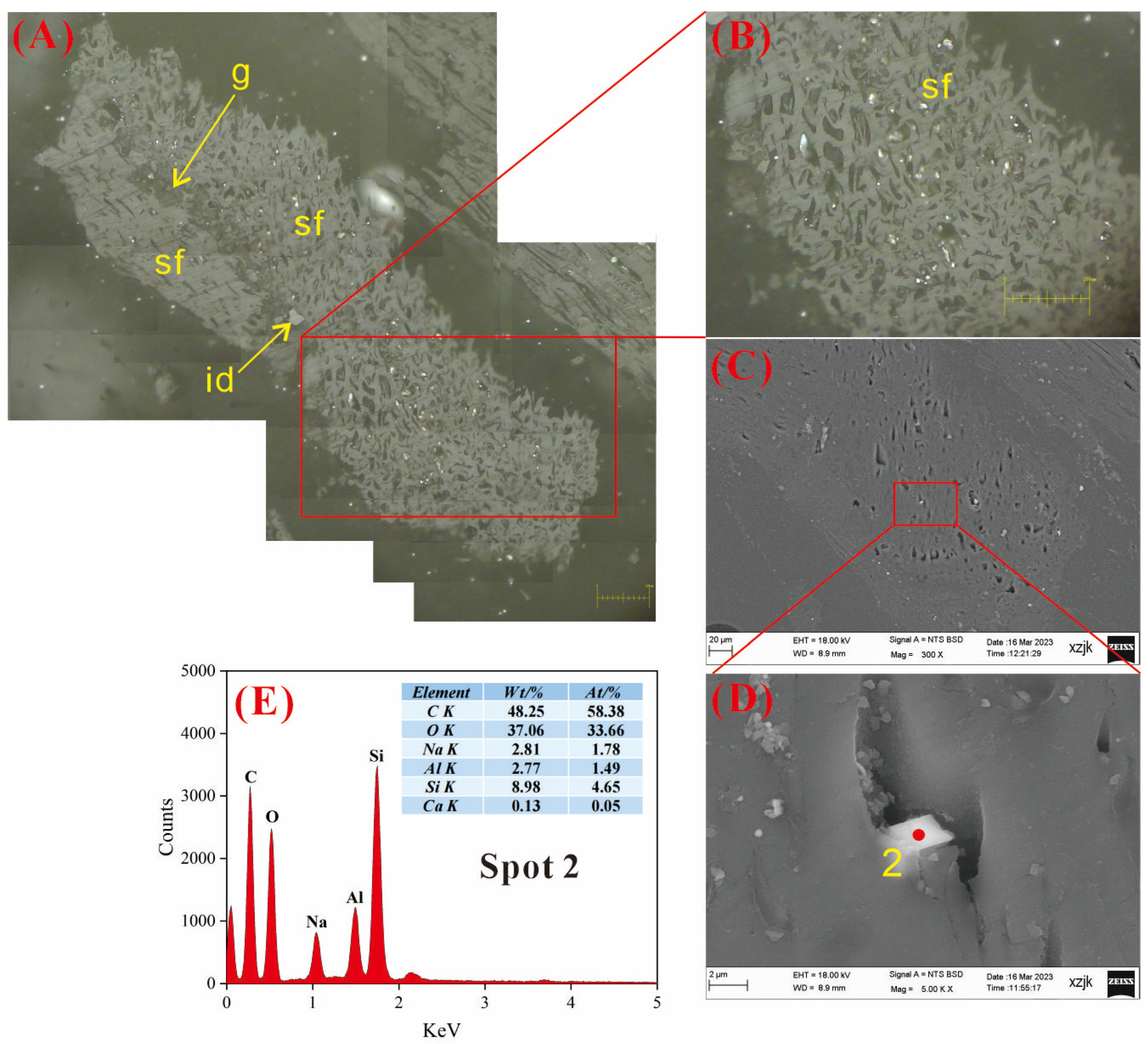
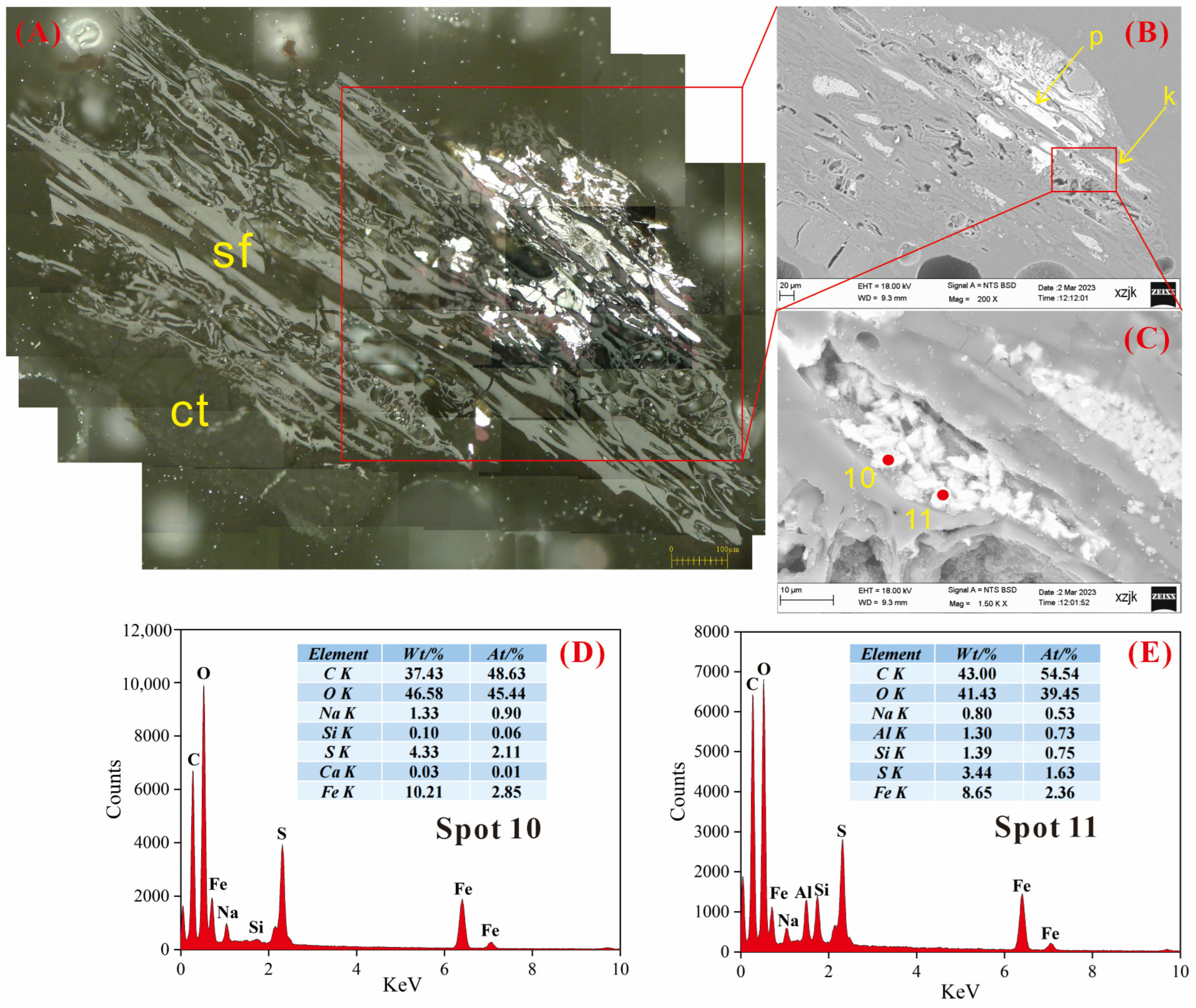
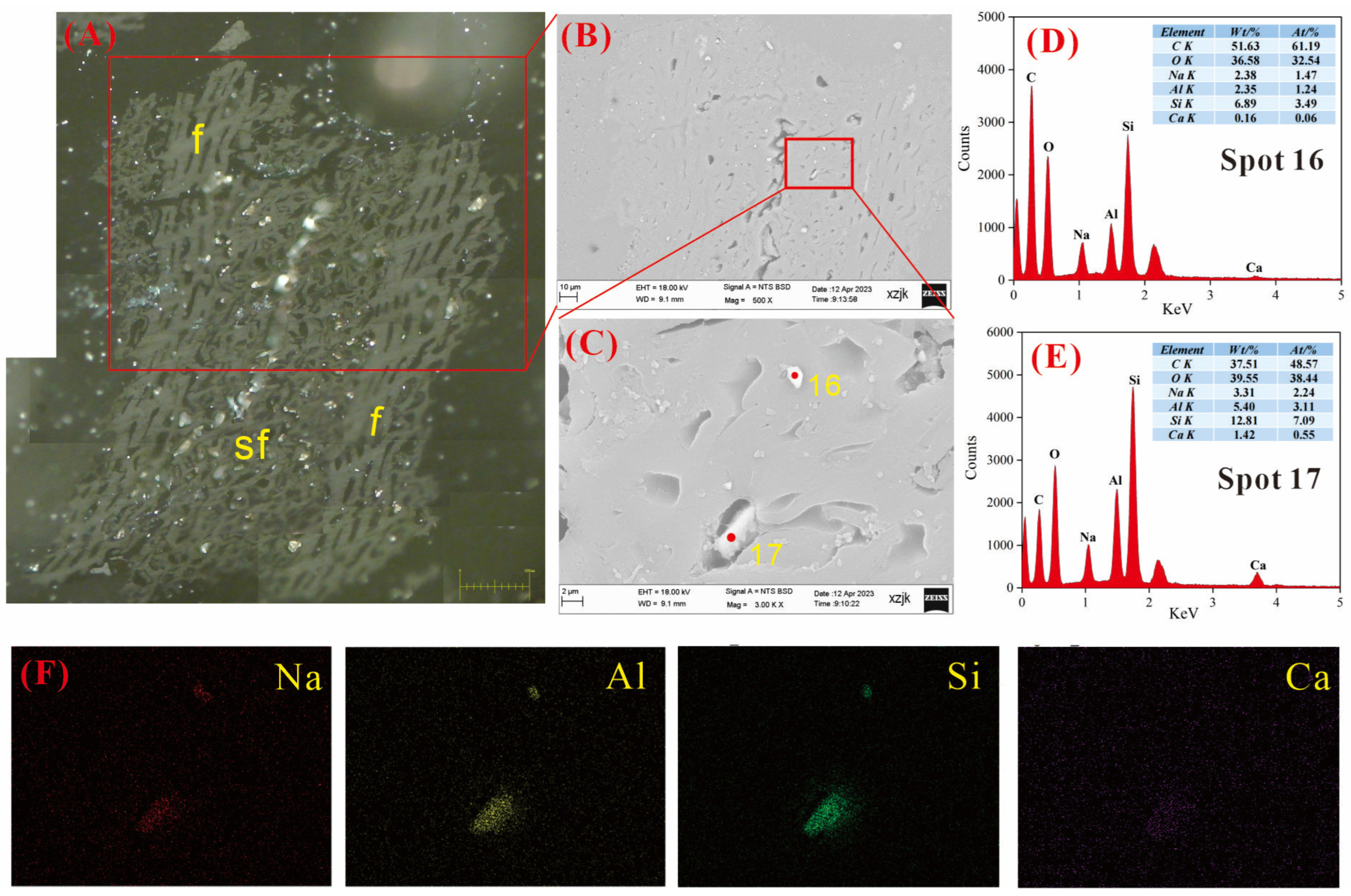
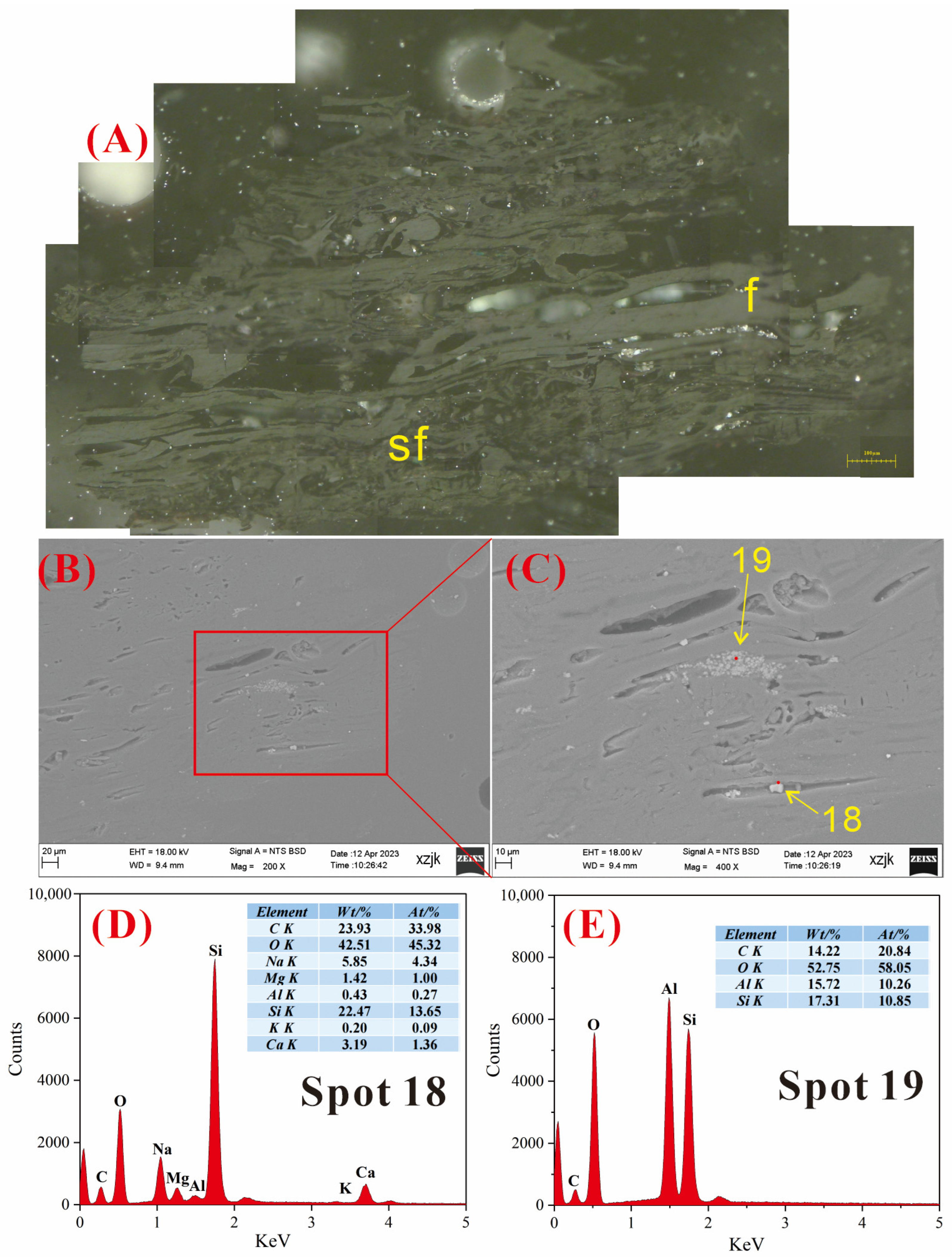
4.6.1. AAEMs Occurrence Characteristics of B2-R and B2-I
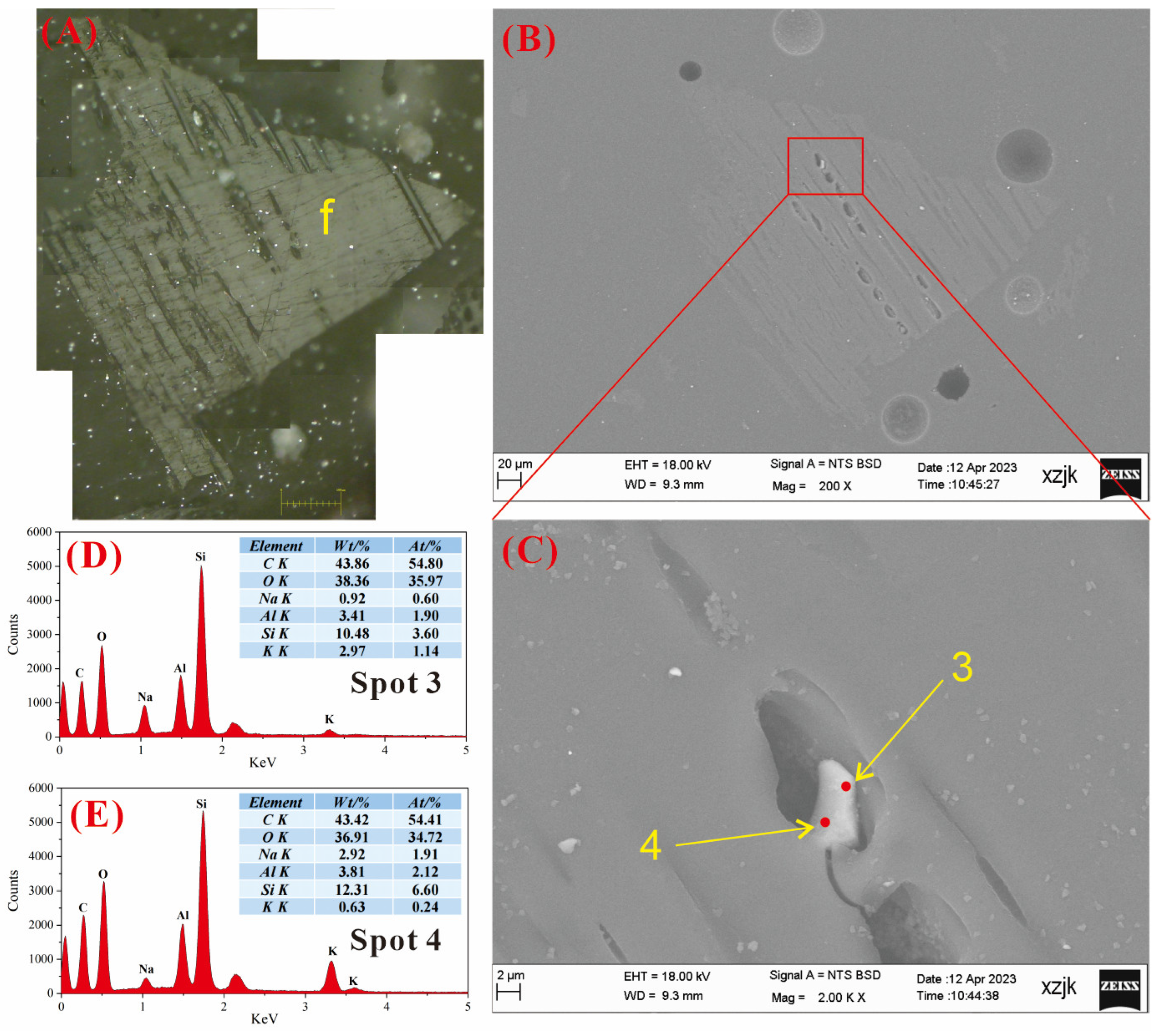
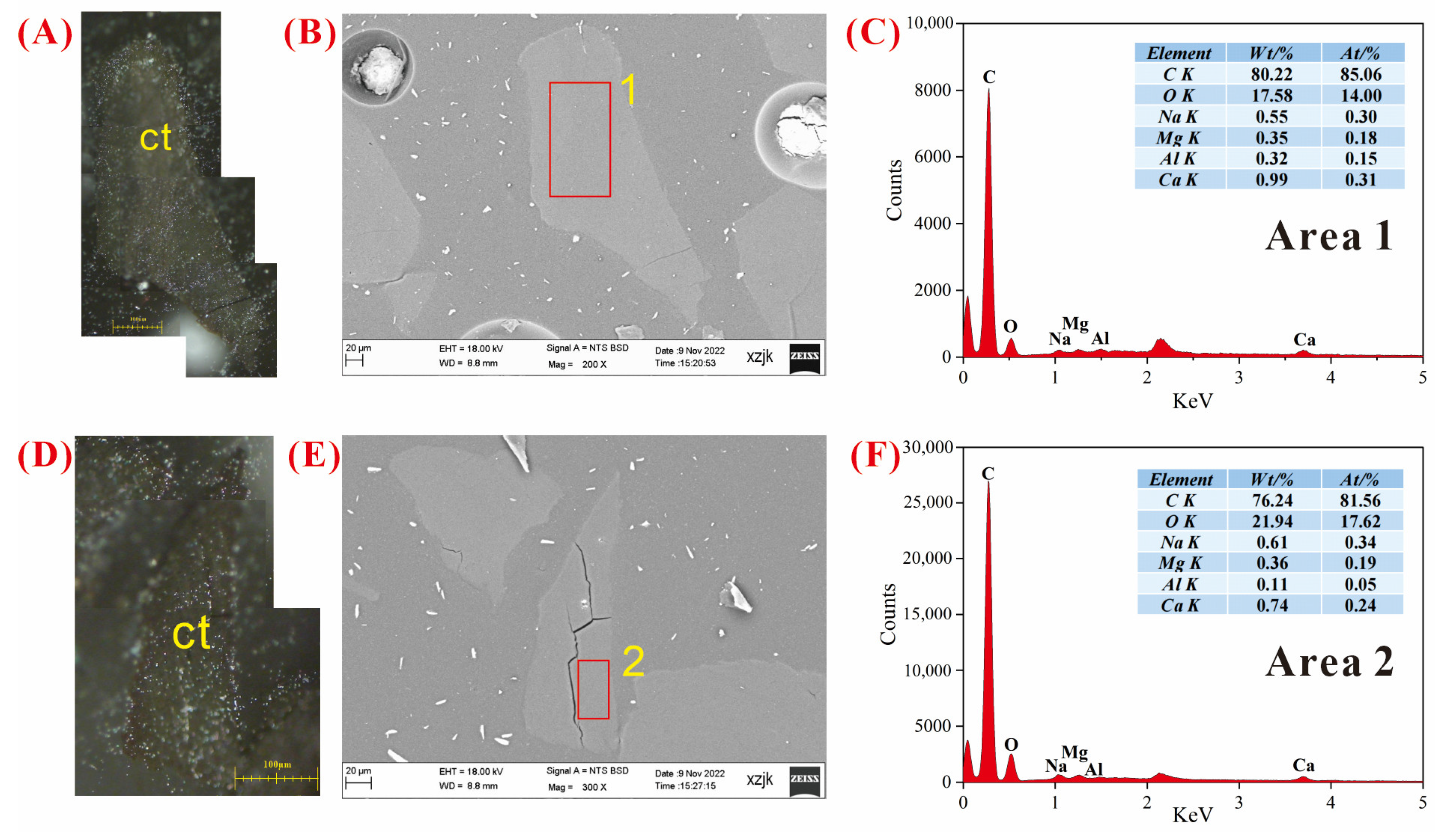
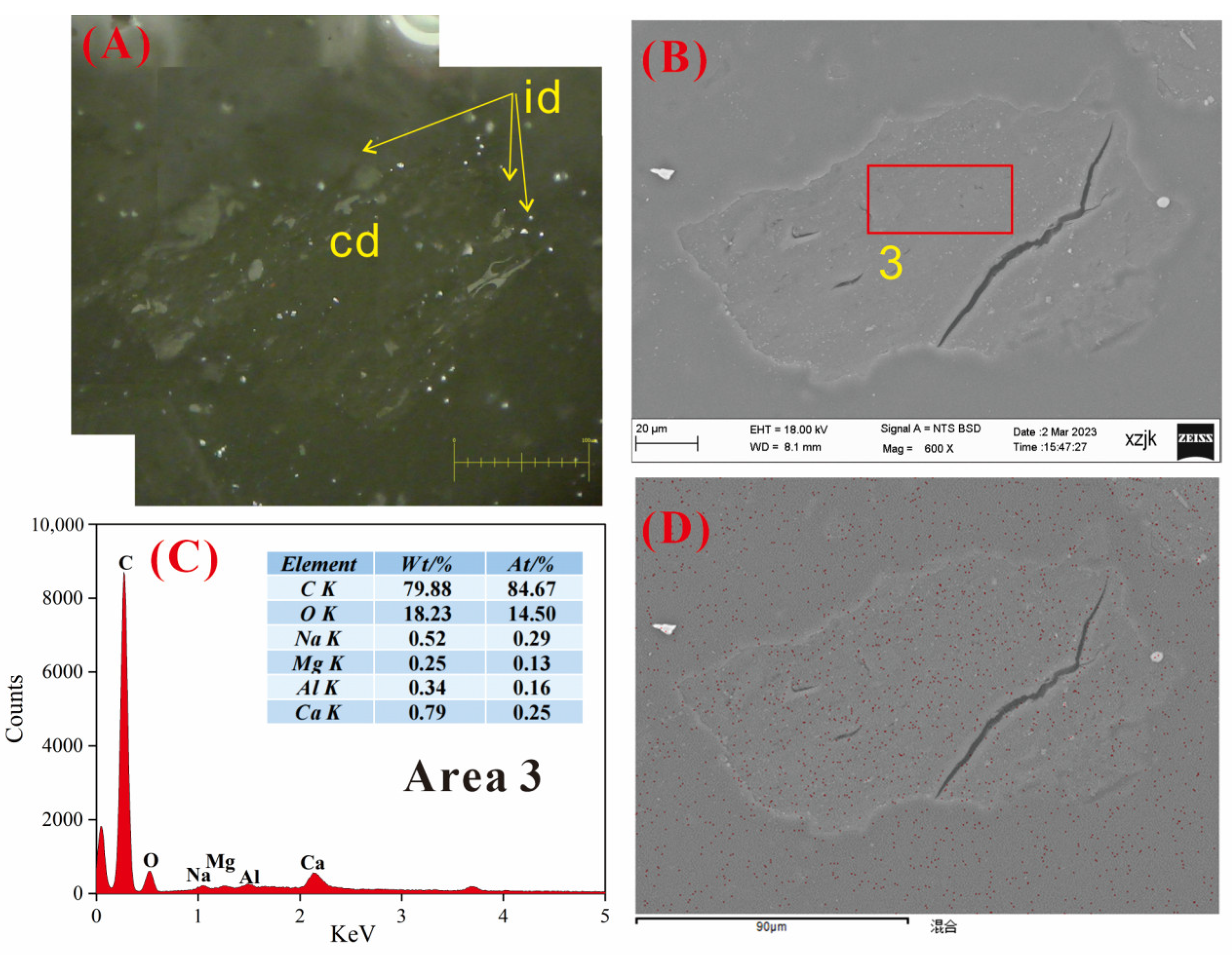
4.6.2. AAEMs Occurrence Characteristics of B3-R and B3-V
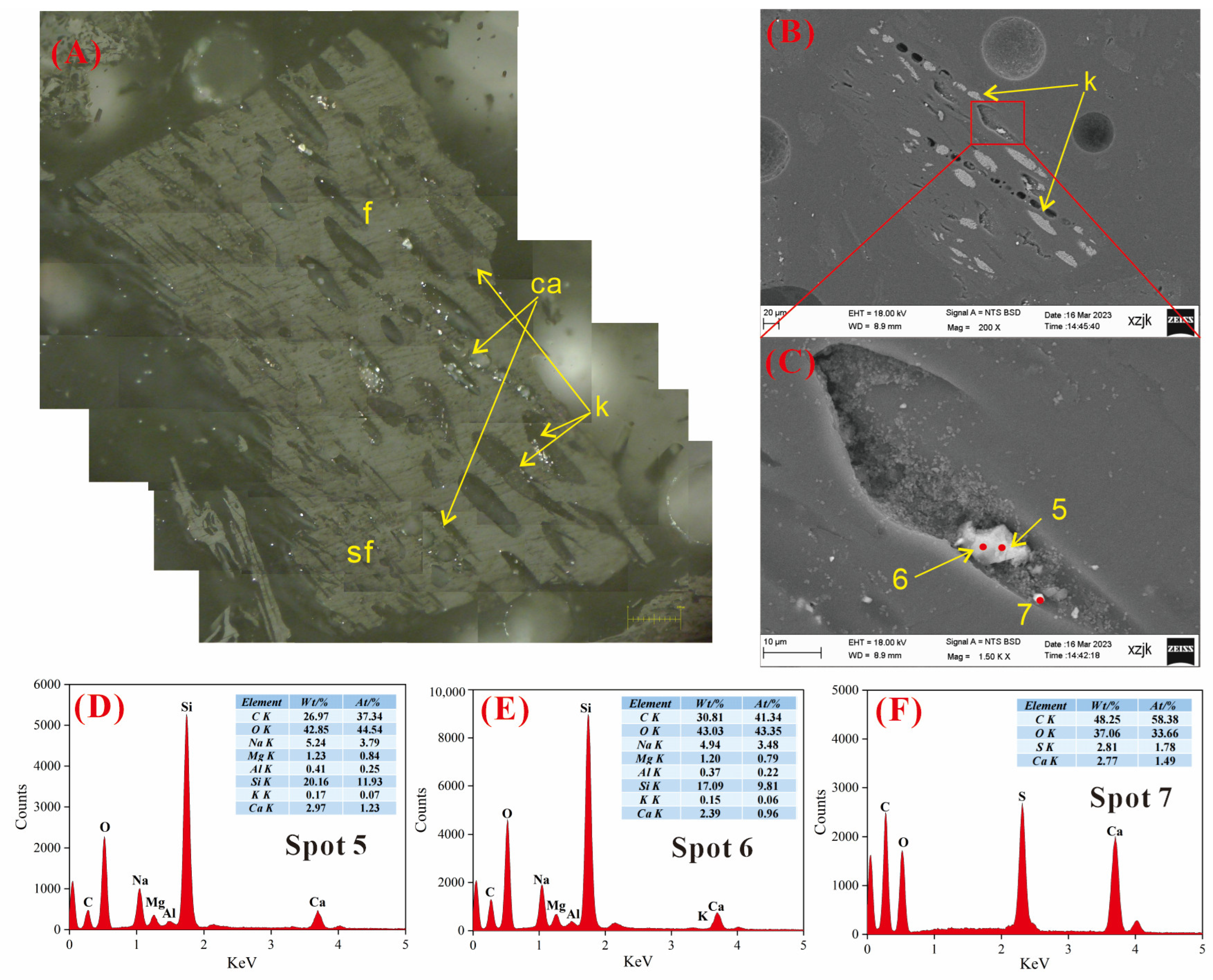
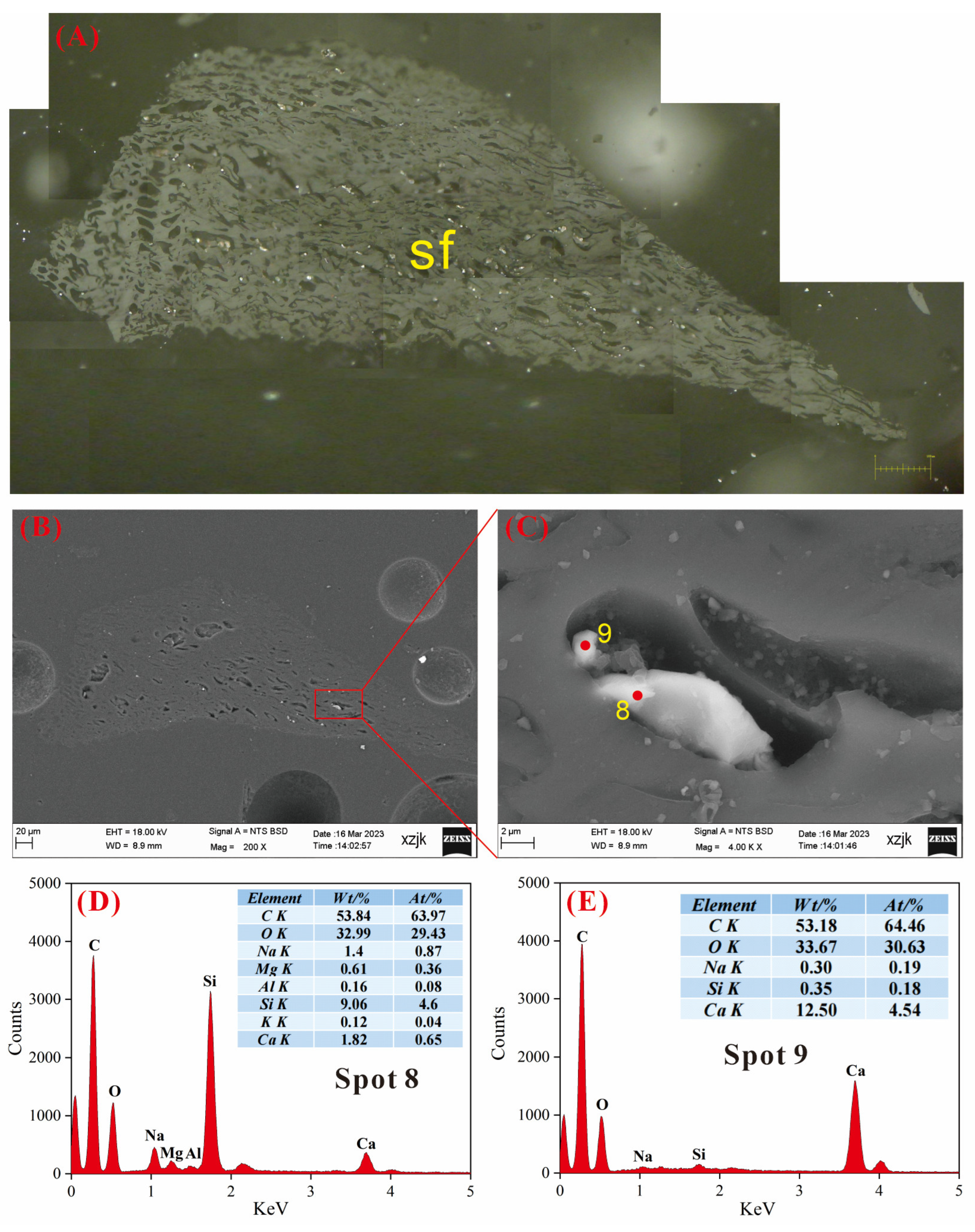
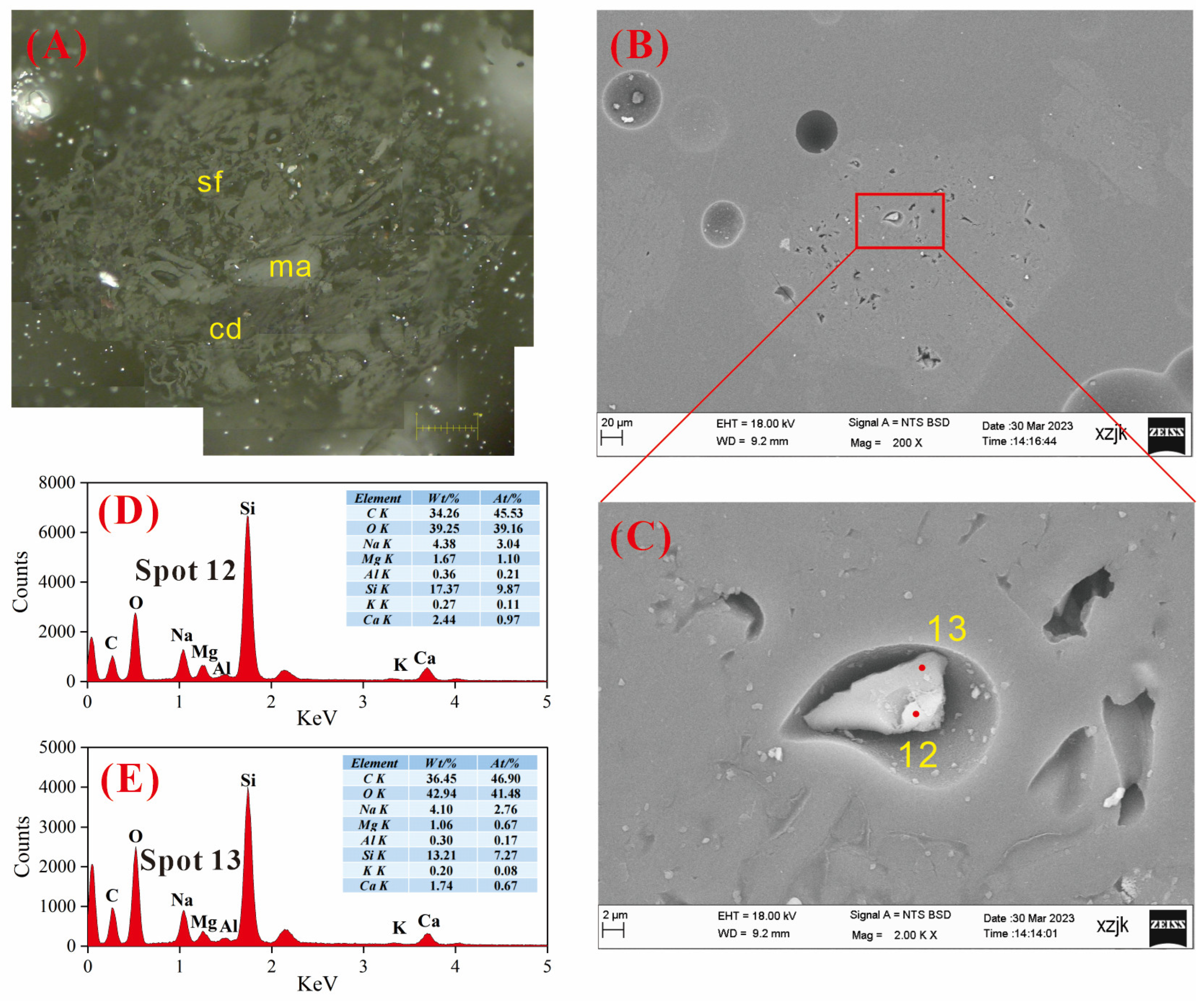
4.6.3. AAEMs Occurrence Characteristics of B5-R, B5-I, and B5-V
4.7. Differences between the Occurrence of AAEMs in Maceral Groups
5. Conclusions
- (1)
- The coal of the Xishanyao Formation in the Zhundong Xiheishan mining area has the characteristics of medium–high moisture, extremely low ash, medium–high volatility, and low sulfur. In the raw coal samples (B2-R, B3-R, B5-R), all the critical metal elements except for Sr are at deficit levels.
- (2)
- The AAEMs in the samples are dominated by Ca, Na, and Mg, and the contents of Na, Ca, and Mg showed a trend of increasing in line with the increase in the inertinite content. The total amount of AAEMs in the inertinite-enriched samples was significantly higher than the amounts in the rest of the samples, ranked in the following order: B5-I > B2-I > B2-R> B5-R > B3-R > B5-I > B3-I.
- (3)
- The sequential extraction results showed that occurrence mode patterns for Na in the raw coal samples and the inertinite-enriched samples were similar, with Na mainly being dominated by Na-Water, followed by organic sodium, including Na-NH4Cl and Na-EDTA, whereas Na in the vitrinite-enriched samples was dominated by organic sodium, of which Na-NH4Cl accounted for most of the samples, followed by Na-Water. The Na-Water in the sample mainly existed in the form of NaHCO3 and Na2SO4. Ca-EDTA is the main occurrence mode of Ca in all the samples, and the CA-EDTA contents in the vitrinite-enriched samples were higher than those in the other samples. Mg mainly existed in the form of Mg-NH4Cl and Mg-EDTA, which also occupied a relatively high proportion of the vitrinite-enriched samples. The main occurrence mode of K was K-I.
- (4)
- Under SEM-EDS observation, the localized enrichment of AAEMs was identified in raw coal samples and inertinite-enriched samples, with most of these examples found in the inertinite cell cavities. The highest localized enrichment of AAEMs was found in quartz crystals, where Na mainly existed in the form of NaHCO3 (Na-Water) (up to 5.85 wt%), and Ca and Mg accompanied it as dolomite and calcite minerals. Also, alkali feldspar minerals were found several times in the inertinite group. However, no localized enrichment of any AAEMs were found in the vitrinite-enriched samples. In summary, AAEMs were more inclined to be enriched in the inertinite group, likely due to the structural characteristics of having abundant pores.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Zhuang, X.; Alastuey, A.; Querol, X.; Li, J.H. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China. Int. J. Coal Geol. 2010, 82, 51–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhu, Z. Physical and chemical properties of fly ash from fluidized bed gasification of Zhundong coal. Fuel Chem. Technol. 2016, 44, 305–313. (In Chinese) [Google Scholar]
- Li, J.; Zhuang, X.; Querol, X.; Font, O.; Moreno, N.; Zhou, J.B.; Lei, G.M. High quality of Jurassic Coals in the Southern and Eastern Junggar Coalfields, Xinjiang, NW China: Geochemical and mineralogical characteristics. Int. J. Coal Geol. 2012, 99, 1–15. [Google Scholar] [CrossRef]
- Zhu, C.; Qu, S.; Zhang, J.; Wang, Y.; Zhang, Y.H. Distribution, occurrence and leaching dynamic behavior of sodium in Zhundong coal. Fuel 2017, 190, 189–197. [Google Scholar] [CrossRef]
- Li, G.D.; Li, S.Q.; Huang, Q.; Yao, Q. Fine particulate formation and ash deposition during pulverized coal combustion of high-sodium lignite in a down-fired furnace. Fuel 2015, 143, 430–437. [Google Scholar] [CrossRef]
- Wang, X.B.; Xu, Z.X.; Wei, B.; Zhang, L.; Tan, H.Z.; Yang, T.; Mikulcic, H.; Duic, N. The ash deposition mechanism in boilers burning Zhundong coal with high contents of sodium and calcium: A study from ash evaporating to condensing. Appl. Therm. Eng. 2015, 80, 150–159. [Google Scholar] [CrossRef]
- Yang, C.W.; Ding, H.; Bai, X.F.; Tang, Y.G.; He, J.; Yuan, D.Y. Research status of sodium geochemistry in high-sodium Coal in Xinjiang. Coal Sci. Technol. 2022, 50, 169–178. (In Chinese) [Google Scholar]
- Weng, Q.S.; Wang, C.A.; Che, D.F.; Fu, Z.W. Alkali metal occurrence mode and its influence on combustion characteristics in Zhundong coals. J. Combust. Sci. Technol. 2014, 20, 216–221. (In Chinese) [Google Scholar]
- Blaesing, M.; Mueller, M. Release of alkali metal, sulphur, and chlorine species from high temperature gasification of high-and low-rank coals. Fuel Process. Technol. 2013, 106, 289–294. [Google Scholar] [CrossRef]
- Song, G.L.; Yang, S.B.; Song, W.J.; Qi, X.B. Release and transformation behaviors of sodium during combustion of high alkali residual carbon. Appl. Eng. 2017, 122, 285–296. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Mao, Y.; Bi, J.C.; Zhu, M.M.; Zhang, Z.Z.; Zhang, L.; Zhang, D.K. Effect of CaCO3 addition on ash sintering behaviour during K2CO3 catalysed steam gasification of a Chinese lignite. Appl. Therm. Eng. 2017, 111, 503–509. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S.; Levi, T. Influence of AAEM species in coal and biomass on steam co-gasification of chars of blended coal and biomass. Renew. Energy 2017, 101, 356–363. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.Y.; Liu, D.H.; Guo, X.; Dong, A.X.; Xiong, Z.W.; Shi, D.Z.; Lv, J.F. Existence form of sodium in high sodium coals from Xinjiang and its effect on combustion process. J. Fuel Chem. Technol. 2013, 41, 832–838. [Google Scholar]
- Wu, L.; Qiao, Y.; Gui, B.; Wang, C.; Xu, J.Y.; Yao, H.; Xu, M.H. Effects of chemical forms of alkali and alkaline earth metallic species on the char ignition temperature of a loy yang coal under O2/N2 atmosphere. Energy Fuels 2012, 26, 112–117. [Google Scholar] [CrossRef]
- Li, R.; Chen, Q.; Zhang, H. Detailed investigation on sodium (Na) species release and transformation mechanism during pyrolysis and char gasification of high-Na Zhundong coal. Energy Fuels 2017, 31, 5902–5912. [Google Scholar] [CrossRef]
- Xie, K.C. Structure and Reactivity of Coal; Science Press: Beijing, China, 2002. [Google Scholar]
- Zhao, Y.; Hu, H.; Jin, L.; He, X.F.; Wu, B. Pyrolysis behavior of vitrinite and inertinite from Chinese Pingshuo coal by TG–MS and in a fixed bed reactor. Fuel Process. Technol. 2011, 92, 780–786. [Google Scholar] [CrossRef]
- brodzki, D.; Akar, A. Comparison by gc-ms of liquefaction extracts from coal maceral concentrates. Fuel 1995, 36, 407–415. [Google Scholar] [CrossRef]
- Guo, H.N.; Shi, H.; Wu, Y.X.; Lyu, J.F.; Zhang, Y. Mineral transformation during rapid heating and cooling of Zhundong coal ash. Fuel 2022, 310, 122269. [Google Scholar] [CrossRef]
- Zhang, D.L.; Zhou, J.B.; Zhuang, X.G.; Lei, D.M.; Song, Z.H. Xishanyao Formation Coal Facies Characteristics in Xiheishan Exploration Area, Eastern Junggar Coalfield, Xinjiang. Coal Geol. China 2014, 26, 1–7. (In Chinese) [Google Scholar]
- He, X.; Wang, W.F.; Yang, Y.T.; Zhou, C.C.; He, J.F.; Duan, P.P.; Lu, Q.F. Occurrence Relationship between Sodium and Maceral Groups in Subbituminous Coal: A Case Study on Zhundong Cola and Shenfu Coal. Minerals 2023, 13, 122. [Google Scholar] [CrossRef]
- He, X.; Sun, H.; Chen, X.W.; Zhao, B.; Zhang, X.X.; Komarneni, S. Charging mechanism analysis of macerals during triboelectrostatic enrichment process: Insights from relative dielectric constant, specific resistivity and X-ray diffraction. Fuel 2018, 225, 533–541. [Google Scholar] [CrossRef]
- He, X.; Sun, H.; Zhao, B.; Chen, X.W.; Zhang, X.X.; Komarneni, S. Tribocharging of macerals with various materials: Role of surface oxygen-containing groups and potential difference of macerals. Fuel 2018, 233, 750–768. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhuang, X.G.; Zhou, J.B.; Zeng, X.J.; A, M.N.; Ge, D.F.; Yang, S. Coal quality and its distribution of the eastern Junggar coalfield in Junggar basin, Xinjiang. Xinjiang Geol. 2013, 31, 89–93. [Google Scholar]
- Benson, S.A.; Holm, P.L. Comparison of inorganics in three low-rank coals. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 145–149. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Matsuoka, K.; Rosyadi, E.; Tomita, A. Mode of occurrence of calcium in various coals. Fuel 2002, 81, 1433–1438. [Google Scholar] [CrossRef]
- He, X.; Che, K.; Pan, J.; Sun, H.; Zhou, C.C.; Wang, W.F. Occurrence Mode of Sodium in Zhundong Coal, China: Relationship to Maceral Groups. Minerals 2023, 13, 1155. [Google Scholar] [CrossRef]
- Grigore, M.; Sakurovs, R. Inorganic matter in Victorian brown coals. Int. J. Coal Geol. 2016, 154, 257–264. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate analysis of coal. China Coal Industry Association: Beijing, China, 2008.
- GB/T 214-2007; Determination of total sulfur in coal. China Coal Industry Association: Beijing, China,, 2007.
- GB/T 19227-2008; Determination of nitrogen in coal. China Coal Industry Association: Beijing, China, 2008.
- GB/T 476-2008; Determination of carbon and hydrogen in coal. China Coal Industry Association: Beijing, China, 2008.
- ICCP. The new vitrinite classification (ICCP System 1994). Fuel 1998, 77, 349–358. [Google Scholar] [CrossRef]
- ICCP. The new inertinite classification (ICCP System 1994). Fuel 2001, 80, 459–471. [Google Scholar] [CrossRef]
- Pickel, W.; Kus, J.; Flores, D. Classification of liptinite-ICCP System 1994. Int. J. Coal Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef]
- Sykorova, I.; Pickel, W.; Christanis, K.; Wolf, M.; Taylor, G.H.; Flores, D. Classification of huminite-ICCP System 1994. Int. J. Coal Geol. 2005, 62, 85–106. [Google Scholar] [CrossRef]
- GB/T 6948-2008; Method of determining microscopically the reflectance of vitrinite in coal. China Coal Industry Association: Beijing, China, 2008.
- He, X.; Sun, H.; Ma, M.Y.; Zhang, X.X.; Wang, W.F. Enrichment Characteristics of Macerals during Triboelectrostatic Separation in the View of Surface Microstructure, Pore distribution, and Typical Electrical Parameters. ACS Omega 2021, 6, 18509–18517. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, X.X.; Jiao, Y.; Zhu, J.S.; Chen, X.W.; Li, C.Y.; Li, H.S. Complementary analyses of infrared transmission and diffuse reflection spectra of macerals in low-rank coal and application in triboelectrostatic enrichment of active maceral. Fuel 2017, 192, 93–101. [Google Scholar] [CrossRef]
- Weck, P.F.; Kim, E.; Wang, Y.; Kruichak, J.N.; Mills, M.M.; Matteo, E.N.; Pellenq, R.J.M. Model representations of kerogen structures: An insight from density functional theory calculations and spectroscopic measurements. Sci. Rep. 2017, 7, 7068. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Luo, Q.Z.; Li, N.; Li, H.B.; Wang, Q.; Gao, J. Occurrence characteristics of Carboniferous-Permian tar-rich coal and its influencing factors in Northern Shanxi. Coal Geol. Explor. 2021, 49, 50–61. (In Chinese) [Google Scholar]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y.P. Geochemistry of trace elements in chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Yuan, D.Y.; Ding, H.; He, J.; Bai, X.F.; Zhang, Y.H. Influence of surface saline-alkali soil on element characteristics of shallow coal seams in Xishanyao Formation. Coal Qual. Technol. 2023, 38, 48–55. (In Chinese) [Google Scholar]
- Bai, X.F.; Wang, Y.; Ding, H.; Zhu, C.; Zhang, Y.H. Occurrence state of sodium in coal of Zhundong. J. China Coal Sci. 2015, 40, 2909–2915. (In Chinese) [Google Scholar]
- He, X.; Wang, W.F.; Zhang, X.X.; Yang, Y.T.; Sun, H. Distribution characteristics and differences of oxygen-containing functional groups in macerals of low rank coal. J. China Coal Soc. 2021, 46, 2804–2812. [Google Scholar]
- Haus, R.; Prinz, S.; Priess, C. Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–67. [Google Scholar]
- Dai, S.F.; Zhang, W.G.; Ward, C.R.; Seredin, V.V.; Hower, J.C.; Li, X.; Song, W.J.; Wang, X.B.; Kang, H.; Zheng, L.C.; et al. Mineralogical and geochemical anomalies of Late Permian coals from the Fusui Coalfield, Guangxi province, southern China: Influences of terrigenous materials and hydrothermal fluids. Int. J. Coal Geol. 2013, 105, 60–84. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: Anupdated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Lu, Q.F.; Qin, S.J.; Wang, W.F.; Wu, S.H.; Shao, F.J. Mineralogy and Geochemistry of High-Sulfur Coals from the M8 Coal Seam, Shihao Mine, Songzao Coalfield, Chongqing, Southwestern China. Minerals 2024, 14, 95. [Google Scholar] [CrossRef]
- Dai, S.; Li, T.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhou, Y.P.; Zhang, M.Q.; Song, X.L.; Song, W.J.; Zhao, C.L. Origin of minerals and elements in the Late Permian coals, tonsteins, and host rocks of the Xinde Mine, Xuanwei, eastern Yunnan, China. Int. J. Coal Geol. 2014, 121, 53–78. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Liu, D.M.; Yang, Q.; Zhou, C.; Tang, D.Z.; Kang, X.D. Occurrence character istics and ge-ological genesis of pyrite in late Paleozoic coal in North China. Geochimica 1999, 28, 340–350. (In Chinese) [Google Scholar]
- Yu, L.D.; Sun, H.W.; Zhang, J.; He, X.H.; Xue, D.; Dan, W.; Sun, Y.Q. Hydrothermal alteration and element migration in the Yufeng gold deposit, Eastern Tianshan Orogen. Acta Petrol. Sin. 2020, 36, 14. (In Chinese) [Google Scholar]
- Zhao, L.; Ward, C.R.; French, D.; Graham, I.T. Mineralogy of the volcanic-influenced Great Northern coal seam in the Sydney Basin, Australia. Int. J. Coal Geol. 2012, 94, 94–110. [Google Scholar] [CrossRef]
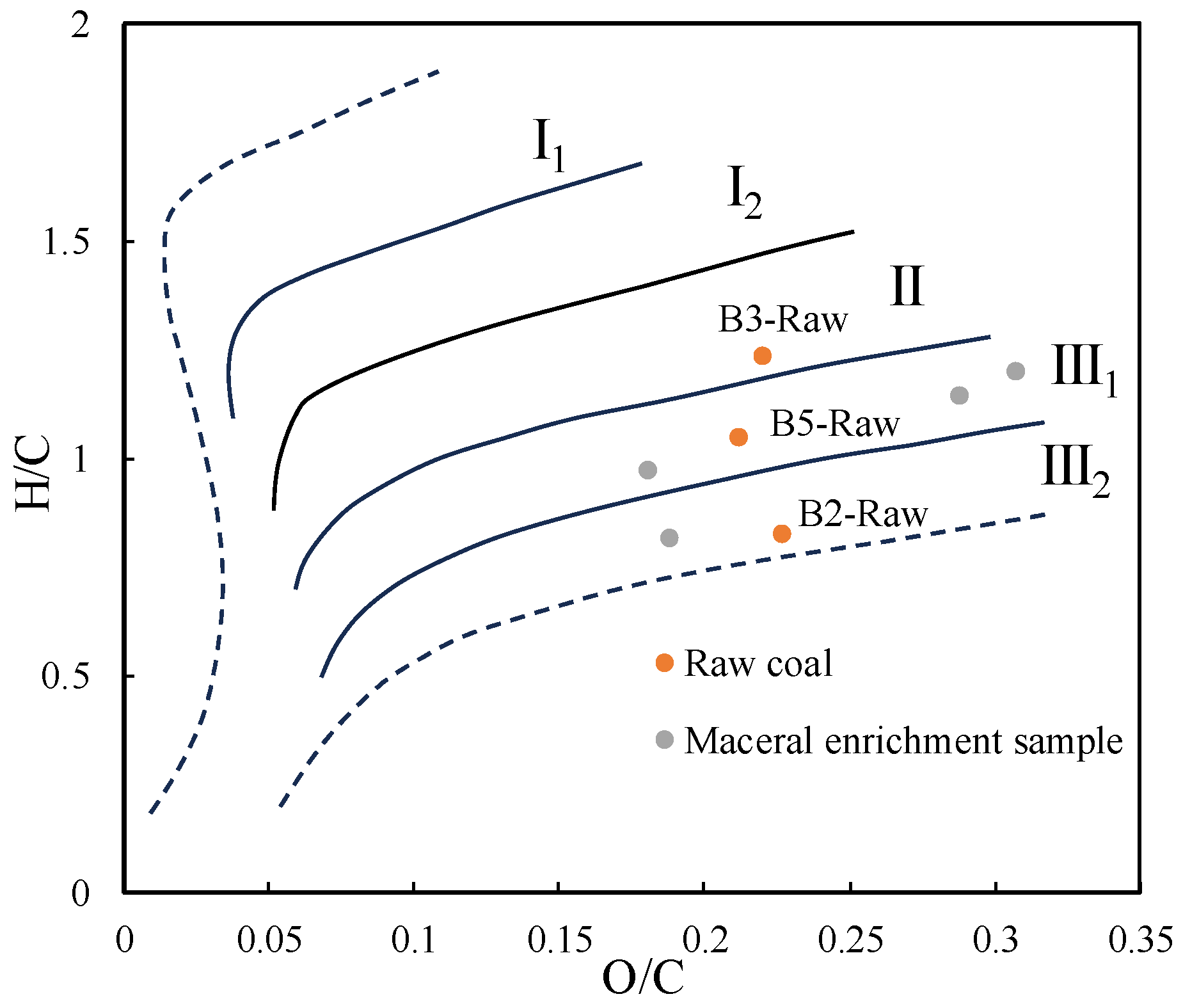


| Samples | Proximate Analyses (w%) | Ultimate Analyses (wad%) | VR | H/C | O/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCd | Cdaf | Hdaf | Odaf | Ndaf | St,d | ||||
| B2-R | 18.98 | 3.58 | 29.92 | 63.22 | 72.14 | 4.94 | 21.83 | 0.57 | 0.53 | 0.49 | 0.82 | 0.23 |
| B2-I | 17.17 | 2.54 | 28.56 | 64.62 | 75.1 | 5.08 | 18.89 | 0.53 | 0.40 | 0.51 | 0.81 | 0.19 |
| B3-R | 13.83 | 3.76 | 41.04 | 55.37 | 70.42 | 7.24 | 20.71 | 1.09 | 0.54 | 0.41 | 1.23 | 0.22 |
| B3-V | 10.76 | 3.25 | 46.60 | 50.63 | 66.73 | 6.34 | 25.63 | 0.83 | 0.48 | 0.38 | 1.14 | 0.29 |
| B5-R | 15.61 | 3.29 | 32.02 | 65.74 | 72.27 | 6.29 | 20.44 | 0.68 | 0.33 | 0.44 | 1.04 | 0.21 |
| B5-I | 17.12 | 2.63 | 28.91 | 64.22 | 74.95 | 6.05 | 18.07 | 0.55 | 0.38 | 0.48 | 0.97 | 0.18 |
| B5-V | 11.09 | 2.52 | 45.29 | 52.28 | 65.97 | 6.58 | 27.09 | 0.54 | 0.41 | 0.40 | 1.20 | 0.31 |
| Raw Coal | Li | Be | Sc | V | Cr | Co | Ga | Rb | Sr |
| B2-R | 11.00 | 0.04 | 0.16 | 0.98 | 2.08 | 0.54 | 0.14 | 0.37 | 215.00 |
| B3-R | 9.06 | 0.07 | 0.93 | 8.59 | 8.55 | 8.89 | 0.63 | 0.26 | 153.00 |
| B5-R | 5.53 | 0.03 | 0.18 | 0.61 | 0.94 | 0.36 | 0.17 | 0.20 | 181.00 |
| China [39] | 31.80 | 2.11 | 4.38 | 35.10 | 15.40 | 7.08 | 6.55 | 9.25 | 140.00 |
| World [39] | 12.00 | 2.00 | 3.90 | 25.00 | 16.00 | 5.10 | 5.80 | 14.00 | 110.00 |
| Raw coal | Nb | Mo | Cd | In | Cs | Ta | W | U | ∑REY |
| B2-R | 0.60 | 0.07 | 0.02 | 0.002 | 0.07 | 0.01 | 0.004 | 0.05 | 3.90 |
| B3-R | 1.64 | 0.87 | 0.01 | 0.006 | 0.03 | 0.06 | 0.140 | 0.18 | 11.70 |
| B5-R | 0.60 | 0.08 | 0.01 | 0.001 | 0.03 | 0.02 | 0.032 | 0.06 | 4.20 |
| China [39] | 22.30 | 3.08 | 0.25 | 0.05 | 1.13 | 0.62 | 1.08 | 2.43 | 137.90 |
| World [39] | 12.00 | 2.20 | 0.22 | 0.03 | 1.00 | 0.28 | 1.10 | 2.40 | 68.50 |
| Sample | Mineral Matter Free Basis (vol)/% | ||
|---|---|---|---|
| Vitrinite | Inertinite | Liptinite | |
| B2-R | 26.2 | 73.6 | 0.2 |
| B2-I | 12.5 | 87.1 | 0.4 |
| B3-R | 70.8 | 28.8 | 0.4 |
| B3-V | 92.4 | 7.2 | 0.4 |
| B5-R | 41.9 | 57.9 | 0.2 |
| B5-I | 14.7 | 84.9 | 0.4 |
| B5-V | 90.2 | 9.4 | 0.4 |
| Sample | Anion Concentration/(mg/L) | ||||
|---|---|---|---|---|---|
| Cl− | SO42− | NO3− | CO32− | HCO3 | |
| B2-R | 0.21 | 1.48 | - | - | 18.12 |
| B2-I | 0.15 | 1.12 | - | - | 20.45 |
| B3-R | 0.12 | 1.86 | - | - | 12.21 |
| B3-V | - | 0.85 | - | - | 7.66 |
| B5-R | 0.16 | 2.74 | - | - | 16.57 |
| B5-I | 0.18 | 1.06 | - | - | 19.15 |
| B5-V | 0.03 | 1.23 | - | - | 8.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, K.; Li, J.; Lu, Q.; Shao, F.; Wang, W.; Wang, W.; He, X. Occurrence of Differences between Alkali and Alkaline Earth Metals (AAEMs), Including Sodium, Potassium, Calcium, and Magnesium, in the Maceral Groups of the Xiheishan Coal, Zhundong Coalfield, Xinjiang, China. Minerals 2024, 14, 525. https://doi.org/10.3390/min14050525
Che K, Li J, Lu Q, Shao F, Wang W, Wang W, He X. Occurrence of Differences between Alkali and Alkaline Earth Metals (AAEMs), Including Sodium, Potassium, Calcium, and Magnesium, in the Maceral Groups of the Xiheishan Coal, Zhundong Coalfield, Xinjiang, China. Minerals. 2024; 14(5):525. https://doi.org/10.3390/min14050525
Chicago/Turabian StyleChe, Kexin, Jiaxin Li, Qingfeng Lu, Fengjun Shao, Wenlong Wang, Wenfeng Wang, and Xin He. 2024. "Occurrence of Differences between Alkali and Alkaline Earth Metals (AAEMs), Including Sodium, Potassium, Calcium, and Magnesium, in the Maceral Groups of the Xiheishan Coal, Zhundong Coalfield, Xinjiang, China" Minerals 14, no. 5: 525. https://doi.org/10.3390/min14050525
APA StyleChe, K., Li, J., Lu, Q., Shao, F., Wang, W., Wang, W., & He, X. (2024). Occurrence of Differences between Alkali and Alkaline Earth Metals (AAEMs), Including Sodium, Potassium, Calcium, and Magnesium, in the Maceral Groups of the Xiheishan Coal, Zhundong Coalfield, Xinjiang, China. Minerals, 14(5), 525. https://doi.org/10.3390/min14050525










