Raman–Infrared Spectral Correlation of an Artificially Space-Weathered Carbonaceous Chondrite Meteorite
Abstract
1. Introduction
Space Weathering Effects
2. Methods
3. Results
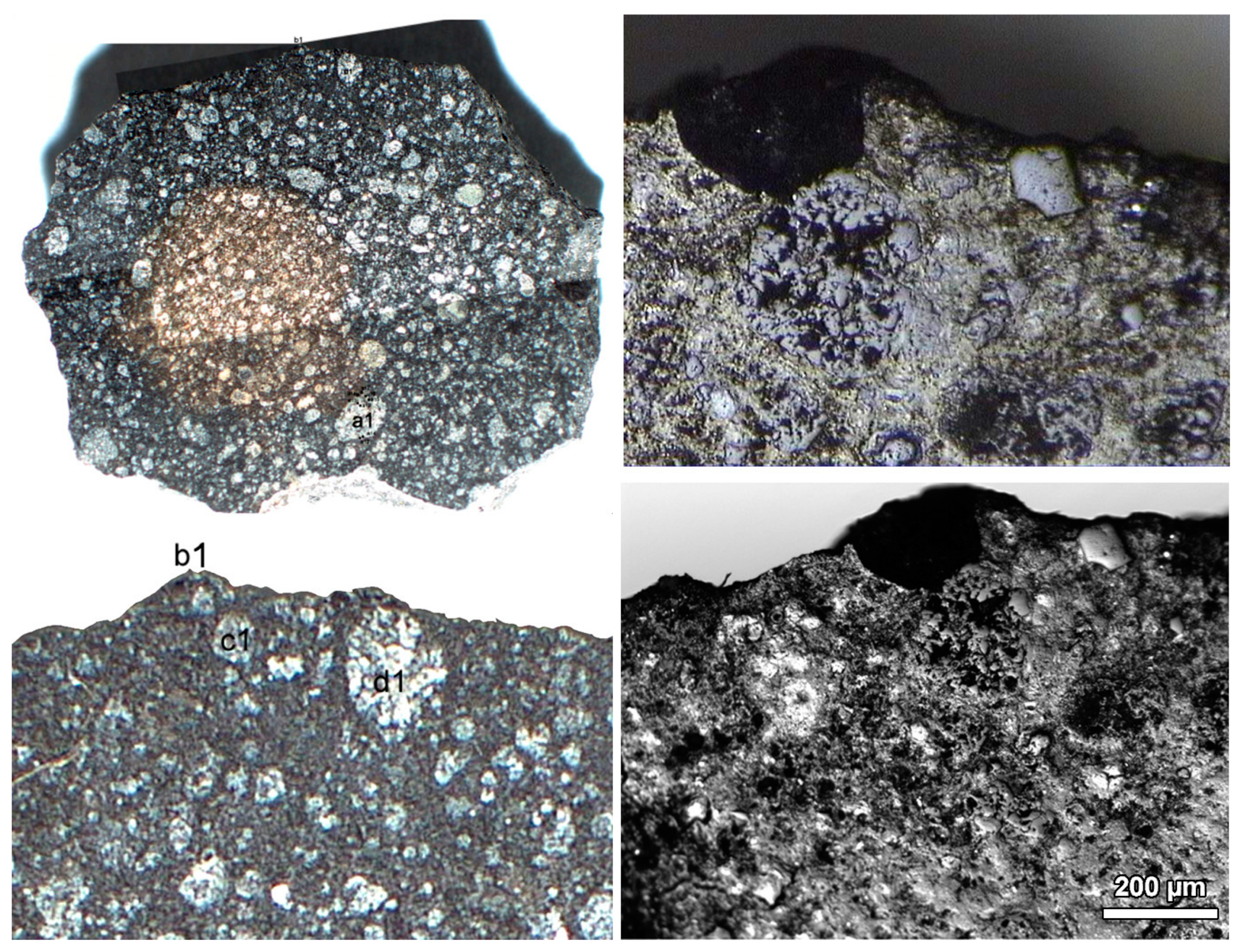
3.1. Mineral Identification
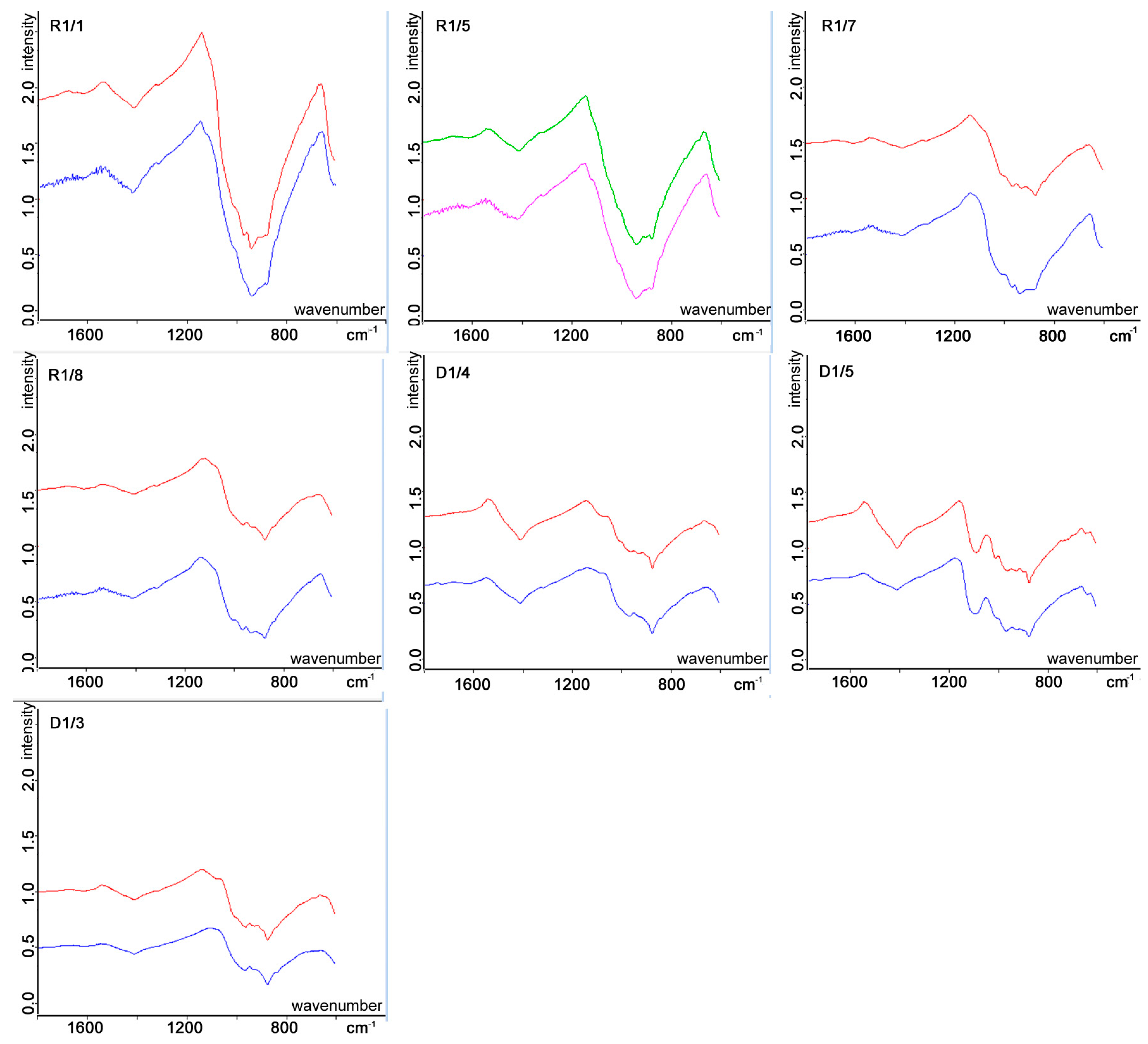
3.2. Raman Spectral Changes
- In the R1/1 area (Figure 2f), the spectrum after irradiation is characterized by decreasing intensity and increasing peak shift toward higher Raman shift in cm−1 values and increasing FWHM values. The change of band 821 is +3 (peak shift), +3 cm−1 (increase in FWHM), while of 846 cm−1 band these values are +1 cm−1 in position, and +3 cm−1 values in FWHM. The minor band at 954 cm−1 shows a peak shift of +6 cm−1 and an increase in FWHM with +5 cm−1.
- In the R1/5 area (Figure 2d), the band of calcite (1086 cm−1, which is probably Earth-based weathering product) can be observed before the irradiation, which band disappears after the irradiation. In the case of bands 826 cm−1 and 844 cm−1, these shifted to a higher wavenumber with +3 cm−1. The olivine is characterized by less intense bands than the R1/1 area and higher FWHM already before the irradiation. The band of organic material at 1355 cm−1 disappears after the irradiation.
- The R1/6 area (Figure 2g) is characterized by lower intensity bands than the spectrum before irradiation and higher FWHM values for olivine after the irradiation. The 817 cm−1 and 849 cm−1 major bands of olivine show an increase in peak shift by +2 cm−1 after the irradiation. The increase in FWHM is +5 cm−1 for band 817 cm−1, and +3 cm−1 for band 849 cm−1. The minor band at 955 cm−1 shows a decreasing intensity and shifted with +9 cm−1 to a higher wavenumber.
- The R1/7 (Figure 2h) is characterized by lower intensity bands and higher FWHM values for olivine after the irradiation. The band 816 cm−1 shifted with +4 cm−1, the band 847 cm−1 with +3 cm−1 to a higher wavenumber after the irradiation. The band 816 cm−1 has +5 cm−1 and the band 847 cm−1 has +6 cm−1 increase in FWHM. The organic material at band 1375 cm−1 disappears after the irradiation.
- The R1/8/7 (Figure 2e) spectrum has bands at 816 cm−1 and 844 cm−1 before irradiation with low intensity bands. The band at 844 cm−1 disappeared after the irradiation. The increase in FWHM values is +4 cm−1, and peak shift is +2 cm−1 of the band 816 cm−1 which can be identified after the irradiation.
- The Raman spectra of pyroxene are displayed at Figure 2a–c.
- The pyroxene in the b1/2 area (Figure 1 shows a decrease in intensity and increase in the FWHM value. The major band at 1009 cm−1 has +3 cm−1 peak shift and +7 cm−1 increase in the FWHM value. The minor band 658 cm−1 disappeared, and the band 680 cm−1 shifted with +6 cm−1 higher wavenumber.
- The d1 area (R4) contains pyroxene spectra. The R4/8 (Figure 2c) spectrum of pyroxene shows a decreasing intensity after the irradiation. The minor band at 667cm−1 disappeared after the irradiation, and other bands were characterised by an increased peak shift after the irradiation: +1 (band 346 cm−1); +3 (bands 689, 1014 cm−1). The band 689 cm−1 shows +9 cm−1, and the band 1014 cm−1 shows +8 cm−1 increase in FWHM.
- The pyroxene spectrum at R4/9 (Figure 1a) shows a decreasing intensity after the irradiation. The minor band at 661 cm−1 disappeared after the irradiation, the peak shift of other 339 cm−1 and 1009 cm−1 bands were characterized by +2 cm−1, and the 683 cm−1 band +4 cm−1. These bands show increasing FWHM about +7 cm−1 of band 683 cm−1, and +3 cm−1 both at bands 339 cm−1 and 1009 cm−1.
3.3. Infrared Spectral Changes
3.4. Comparison of Raman and Infrared Changes
4. Discussion
4.1. Interpretation of Spectral Changes
4.2. Amorphization
5. Conclusions
- The disappearance of minor bands of pyroxene (660 cm−1) and of olivine (978 cm−1) indicates amorphization. The increase in FWHM of minor bands of olivine and pyroxene also indicates structural disordering by the IR spectra.
- The decreasing peak position (negative peak shift) of the major IR bands of olivine (880 cm−1) and pyroxene (1050 cm−1) indicates Mg loss in infrared spectra (Table 3). There are a lot of other works also dealing with these data (Brunetto et al. [2,19,39,46], Lantz et al. [1,17], Lazzarin et al. [18], Vernazza et al. [16]). The Mg loss can be observed better in the IR spectra than in the Raman spectra.
- The increasing peak position (positive peak shift) and FWHM of pyroxene and of olivine in Raman spectra indicate structural disordering after the irradiations (Table 3). The disappearance of calcite peaks can be observed in one Raman spectrum after the irradiation.
- The positive peak shift with increasing FWHM and disappearance of minor bands in minerals by Raman spectroscopy after the irradiation experiments were first observed in our work.
- The positive peak shift, increasing FWHM, and decreasing minor bands which were observed in our Raman data after the irradiation were not observed in other works related to irradiation experiments. The positive peak shift and disappearance of minor bands were mentioned in the references only relating to shock metamorphic features. The new Raman data we provide here are complementary and coincide in trend with the also Raman-based results acquired not from irradiated but from shock-based alteration of meteorite minerals—pointing to the perspective regarding the connection between shock deformation and irradiation-modified aspects of changes in the mineral lattice.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lantz, C.; Brunetto, R.; Barucci, M.A.; Fornasier, S.; Baklouti, D.; Bourçois, J.; Godard, M. Ion irradiation of carbonaceous chondrites: A new view of space weathering on primitive asteroids. Icarus 2017, 285, 43–57. [Google Scholar] [CrossRef]
- Brunetto, R.; Loeffler, M.J.; Nesvorný, D.; Sasaki, S.; Strazzulla, G. Asteroid Surface Alteration by Space Weathering Processes. In Asteroids IV; Michel, P., De Meo, F.E., Bottke, W.F., Eds.; University of Arizona Press: Tucson, AZ, USA, 2015. [Google Scholar]
- Chapman, C.R. Space weathering of asteroid surfaces. Annu. Rev. Earth Planet. Sci. 2004, 32, 539–567. [Google Scholar] [CrossRef]
- DeMeo, F.E.; Burt, B.J.; Marsset, M.; Polishook, D.; Burbine, T.H.; Carry, B.; Binzel, R.P.; Vernazza, P.; Reddy, V.; Tang, M.; et al. Connecting asteroids and meteorites with visible and near-infrared spectroscopy. Icarus 2022, 380, 114971. [Google Scholar] [CrossRef]
- Krämer, R.L.; Beck, P.; Gattacceca, J.; Eschrig, J. Visible-infrared spectroscopy of ungrouped and rare meteorites brings further constraints on meteorite-asteroid connections. Icarus 2021, 362, 114393. [Google Scholar] [CrossRef]
- Keller, L.P.; McKay, D.S. Discovery of vapor deposits in the lunar regolith. Science 1993, 261, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.P.; McKay, D.S. The nature and origin of rims on lunar soil grains. Geochim. Et Cosmochim. Acta 1997, 61, 2331–2341. [Google Scholar] [CrossRef]
- Hapke, B. Space weathering from Mercury to the asteroid belt. J. Geophys. Res. Planets 2001, 106, 10039–10073. [Google Scholar] [CrossRef]
- Pieters, C.M.; Fischer, E.M.; Rode, O.; Basu, A. Optical effects of space weathering: The role of the finest fraction. J. Geophys. Res. Planets 1993, 98, 20817–20824. [Google Scholar] [CrossRef]
- Noguchi, T.; Nakamura, T.; Kimura, M.; Zolensky, M.E.; Tanaka, M.; Hashimoto, T.; Konno, M.; Nakato, A.; Ogami, T.; Fujimura, A.; et al. Incipient space weathering observed on the surface of Itokawa dust particles. Science 2011, 333, 1121. [Google Scholar] [CrossRef] [PubMed]
- OPUS Spectroscopy Software. Quick Reference Guide; Version 7; Bruker Optik GmbH: Ettlingen, Germany, 2017. [Google Scholar]
- Matsumoto, T.; Tsuchiyama, A.; Miyake, A.; Noguchi, T.; Nakamura, M.; Uesugi, K.; Takeuchi, A.; Suzuki, Y.; Nakano, T. Surface and internal structures of a space-weathered rim of an Itokawa regolith particle. Icarus 2015, 257, 230. [Google Scholar] [CrossRef]
- Thompson, M.S.; Zega, T.J.; Becerra, P.; Keane, J.T.; Byrne, S. The oxidation state of nanophase Fe particles in lunar soil: Implications for space weathering. Meteorit. Planet. Sci. 2016, 51, 1082–1095. [Google Scholar] [CrossRef]
- Nittler, L.R.; Starr, R.D.; Lim, L.; Mccoy, T.J.; Burbine, T.H.; Reedy, R.C.; Trombka, J.I.; Gorenstein, P.; Squyres, S.W.; Boynton, W.V.; et al. X-ray fluorescence measurements of the surface elemental composition of asteroid 433 Eros. Meteorit. Planet Sci. 2001, 36, 1673. [Google Scholar] [CrossRef]
- Loeffler, M.J.; Dukes, C.A.; Baragiola, R.A. Irradiation of olivine by 4 keV He+: Simulation of space weathering by the solar wind. J. Geophys. Res. 2009, 114, E03003. [Google Scholar] [CrossRef]
- Dukes, C.A.; Baragiola, R.A.; McFadden, L.A. Surface modification of olivine by H+ and He+ bombardment. J. Geophys. Res.: Planets 1999, 104, 1865–1872. [Google Scholar] [CrossRef]
- Vernazza, P.; Fulvio, D.; Brunetto, R.; Emery, J.P.; Dukes, C.A.; Cipriani, F.; Witasse, O.; Schaible, M.J.; Zanda, B.; Strazzulla, G.; et al. Paucity of Tagish Lake-like parent bodies in the Asteroid Belt and among Jupiter Trojans. Icarus 2013, 225, 517–525. [Google Scholar] [CrossRef]
- Lantz, C.; Brunetto, R.; Barucci, M.A.; Dartois, E.; Duprat, J.; Engrand, C.; Godard, M.; Ledu, D.; Quirico, E. Ion irradiation of the Murchison meteorite: Visible to mid-infrared spectroscopic results. Astron. Astrophys. 2015, 577, A41. [Google Scholar] [CrossRef]
- Lazzarin, M.; Marchi, S.; Moroz, L.V.; Brunetto, R.; Magrin, S.; Paolicchi, P.; Strazzulla, G. Space weathering in the main asteroid belt: The big picture. Astrophys. J. 2006, 647, L179. [Google Scholar] [CrossRef]
- Brunetto, R.; Lantz, C.; Ledu, D.; Baklouti, D.; Barucci, M.A.; Beck, P.; Delauche, L.; Dionnet, Z.; Dumas, P.; Duprat, J.; et al. Ion irradiation of Allende meteorite probed by visible, IR, and Raman spectroscopies. Icarus 2014, 237, 278–292. [Google Scholar] [CrossRef]
- Kaňuchová, Z.; Baratta, G.A.; Garozzo, M.; Strazzulla, G. Space weathering of asteroidal surfaces Influence on the UV-Vis spectra. Astron. Astrophys. 2010, 517, A60. [Google Scholar] [CrossRef]
- Kaňuchová, Z.; Boduch, P.; Domaracka, A.; Palumbo, M.E.; Rothard, H.; Strazzulla, G. Thermal and energetic processing of astrophysical ice analogues rich in SO2. Astron. Astrophys. 2017, 604, A68. [Google Scholar] [CrossRef]
- Kuebler, K.E.; Jolliff, B.L.; Wang, A.; Haskin, L.A. Extracting olivine (Fo–Fa) compositions from Raman spectral peak positions. Geochim. Et Cosmochim. Acta 2006, 70, 6201–6222. [Google Scholar] [CrossRef]
- Hapke, B.; Cassidy, W.; Wells, E. Effects of vapor-phase deposition processes on the optical, chemical, and magnetic properties OE the lunar regolith. Moon 1975, 13, 339–353. [Google Scholar] [CrossRef]
- Brucato, J.R.; Strazzulla, G.; Baratta, G.; Colangeli, L. Forsterite amorphisation by ion irradiation: Monitoring by infrared spectroscopy. Astron. Astrophys. 2004, 413, 395–401. [Google Scholar] [CrossRef]
- Demyk, K.; d’Hendecourt, L.; Leroux, H.; Jones, A.P.; Borg, J. IR spectroscopic study of olivine, enstatite and diopside irradiated with low energy H and He ions. Astron. Astrophys. 2004, 420, 233–243. [Google Scholar] [CrossRef]
- Rietmeijer, F.J. The irradiation-induced olivine to amorphous pyroxene transformation preserved in an interplanetary dust particle. Astrophys. J. 2009, 705, 791. [Google Scholar] [CrossRef]
- Durben, D.J.; McMillan, P.F.; Wolf, G.H. Raman study of the high-pressure behavior of forsterite (Mg2SiO4) crystal and glass. Am. Mineral. 1993, 78, 1143–1148. [Google Scholar]
- Kissel, J.; Krueger, F.R. Ion Formation by Impact of Fast Dust Particles and Comparison with Related Techniques. Applied Physics A Solids and Surfaces. Appl. Phys. A 1987, 42, 69–85. [Google Scholar] [CrossRef]
- Moroz, L.V.; Fisenko, A.V.; Semjonova, L.F.; Pieters, C.M.; Korotaeva, N.N. Optical effects of regolith processes on S-asteroids as simulated by laser shots on ordinary chondrite and other mafic materials. Icarus 1996, 122, 366–382. [Google Scholar] [CrossRef]
- Sasaki, S.; Nakamura, K.; Hamabe, Y.; Kurahashi, E.; Hiroi, T. Production of iron nanoparticles by laser irradiation in a simulation of lunar-like space weathering. Nature 2001, 410, 555–557. [Google Scholar] [CrossRef]
- Ferus, M.; Knížek, A.; Cassone, G.; Rimmer, P.B.; Changela, H.; Chatzitheodoridis, E.; Uwarova, I.; Žabka, J.; Kabáth, P.; Saija, F.; et al. Simulating asteroid impacts and meteor events by high-power lasers: From the laboratory to spaceborne missions. Front. Astron. Space Sci. 2023, 10, 1186172. [Google Scholar] [CrossRef]
- Morimitsu, S.; Sasaki, S.; Kaiden, H.; Hiro, T. Space weathering simulation with UV irradiation on olivine. In Proceedings of the Japan Geoscience Union Meeting, Chiba, Japan, 22–27 May 2022; pp. PPS07–PPS22. [Google Scholar]
- Ruesch, O.; Sefton-Nash, E.; Vago, J.; Kueppers, M. In situ fragmentation of lunar blocks and implications for impacts and solar-induced thermal stresses. Icarus 2020, 336, 113431. [Google Scholar] [CrossRef]
- Garzanti, E.; Resentini, A.; Andò, S.; Vezzoli, G.; Pereira, A.; Vermeesch, P. Physical controls on sand composition and relative durability of detrital minerals during ultra-long distance littoral and aeolian transport (N amibia and southern A ngola). Sedimentology 2015, 62, 971–996. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Biri, S.; Rácz, R.; Pálinkás, J. Status and special features of the Atomki ECR ion source. Rev. Sci. Instrum. 2012, 83, A341. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, J.; Moynier, F.; Qin, L.; O’De Alexander, C.M.; He, Y. Chromium Isotopic Evidence for an Early Formation of Chondrules from the Ornans CO Chondrite. Astrophys. J. 2019, 873, 8238. [Google Scholar] [CrossRef]
- Brunetto, R.; Lantz, C.; Nakamura, T.; Baklouti, D.; Le Pivert-Jolivet, T.; Kobayashi, S.; Borondics, F. Characterizing irradiated surfaces using IR spectroscopy. Icarus 2020, 345, 113722. [Google Scholar] [CrossRef]
- Van de Moortèle, B.; Reynard, B.; McMillan, P.F.; Wilson, M.; Beck, P.; Gillet, P.; Jahn, S. Shock-induced transformation of olivine to a new metastable (Mg, Fe) 2SiO4 polymorph in Martian meteorites. Earth Planet. Sci. Lett. 2007, 261, 469–475. [Google Scholar] [CrossRef]
- Huang, E.; Chen, C.H.; Huang, T.; Lin, E.H.; Xu, J.A. Raman spectroscopic characteristics of Mg-Fe-Ca pyroxenes. Am. Mineral. 2000, 85, 473–479. [Google Scholar] [CrossRef]
- Johnson, J.R.; Hörz, F.; Staid, M.I. Thermal infrared spectroscopy and modeling of experimentally shocked plagioclase feldspars. Am. Mineral. 2003, 88, 1575–1582. [Google Scholar] [CrossRef]
- Demyk, K.; Carrez, P.; Leroux, H.; Cordier, P.; Jones, A.P.; Borg, J.; Quirico, E.; Raynal, P.I.; d’Hendecourt, L. Structural and chemical alteration of crystalline olivine under low energy He+ irradiation. Astron. Astrophys. 2001, 368, L38–L41. [Google Scholar] [CrossRef]
- Sharp, T.G.; DeCarli, P.S. Shock effects in meteorites. Meteor. Early Sol. Syst. II 2006, 943, 653–677. [Google Scholar]
- Morlok, A.; Bischoff, A.; Patzek, M.; Sohn, M.; Hiesinger, H. Chelyabinsk–a rock with many different (stony) faces: An infrared study. Icarus 2017, 284, 431–442. [Google Scholar] [CrossRef]
- Brunetto, R.; Vernazza, P.; Marchi, S.; Birlan, M.; Fulchignoni, M.; Orofino, V.; Strazzulla, G. Modeling asteroid surfaces from observations and irradiation experiments: The case of 832 Karin. Icarus 2006, 184, 327–337. [Google Scholar] [CrossRef]
- Heymann, D.; Hörz, F. Raman-spectroscopy and X-ray diffractometer studies of experimentally produced diaplectic feldspar glass. Phys. Chem. Miner. 1990, 17, 38–44. [Google Scholar] [CrossRef]
- Pittarello, L.; Fritz, J.; Roszjar, J.; Lenz, C.; Chanmuang, N.C.; Koeberl, C. Partial amorphization of experimentally shocked plagioclase: A spectroscopic study. Meteorit. Planet. Sci. 2020, 55, 669–678. [Google Scholar] [CrossRef]

| IR Area | Raman Area | No. of Spec | Before Irradiation | After Irradiation | Produced Change | |||
|---|---|---|---|---|---|---|---|---|
| Raman Band Positions (cm−1) | FWHM (cm−1) | Raman Band Positions (cm−1) | FWHM (cm−1) | Peak Shift (cm−1) | FWHM (cm−1) | |||
| b1/2 | R1/1 | 1 | 821, 846, 954 | 8, 15, 8 | 824, 847, 960 | 11, 18, 13 | +3, +1, +6 | +3, +3, +5 |
| b1/2 | R1/5 | 2 | 826, 844 | 12, 22 | 829, 847 | 14, 24 | +3, +3 | +2, +2 |
| b1/2 | R1/6 | 3 | 826, 844, 948 | 6, 10, 17 | 829, 847, 955 | 12, 16, 23 | +3, +3, +7 | +6, +6, +6 |
| b1/2 | R1/7 | 5 | 816, 847 | 11, 15 | 820, 850 | 16, 22 | +4, +3 | +5, +6 |
| b1/2 | R1/8 | 6 | 816, 844 | 8, 9 | 818, d | 12, d | +2, d | +4, d |
| c1 | R2/1 | 2 | 658, 680, 1009 | 20, 13, 9 | d, 686, 1012 | d, 22, 14 | d, +6, +3 | d, +9. +5 |
| d1 | R4/8 | 4 | 346, 667, 689, 1014 | 14, 10, 21, 20 | 347, d, 692, 1017 | 25, d, 30, 28 | +1 d, +3, +3 | +11, +9, +8 |
| d1 | R4/9 | 5 | 339, 661, 683, 1009 | 22, 14, 21, 20 | 341, d, 687, 1011 | 25, d, 28, 23 | +2, d, +4, +2 | +3, d, +7, +3 |
| IR Area | Raman Area | Spec | Before Irradiation | After Irradiation | Difference | |||
|---|---|---|---|---|---|---|---|---|
| IR Peak Position (cm−1) | FWHM (cm−1) | IR Peak Position (cm−1) | FWHM (cm−1) | IR Peak Shift (cm−1) | FWHM (cm−1) | |||
| (b1/2) | R1/1 | 1 | 978, 894 | 7, 11 | 970, 845 | 11, 11 | −8, −39 | +4, 0 |
| (b1/2) | R1/5 | 2 | 978, 894 | 7, 11 | 887 | 6 | d, −7 | d, −5 |
| (b1/2) | R1/6 | 3 | 978, 894 | 7, 11 | 891 | 5 | d, −3 | d, −6 |
| (b1/2) | R1/7 | 5 | 894 | 11 | 887 | 5 | −7 | −6 |
| (b1/2) | R1/8 | 6 | 894 | 14 | 853, 970 | 11, 12 | −41, p | −3, p |
| c1 | R2/1 | 2 | 1046, 703, 667 | 26, 7, 16 | 1065 | 21 | +19, d, d | −5, d, d |
| d1 | R4/8 | 4 | 1046, 940, 913, 693, 660 | 31, 8, 7, 10, 7 | 1039, 914, 667, | 31, 50, 100 | −7, d, +1, d, +7 | 0, d, +43, +93 |
| d1 | R4/9 | 5 | 1064, 945, 664 | 40, 10, 80 | 1039, 667, | 26, 100 | −25, d, +3 | −14, d, +20 |
| Mineral | Raman Peak Shift | Raman FWHM Change | IR Peak Shift | IR FWHM Change |
|---|---|---|---|---|
| olivine major bands: 820, 840 cm−1—Raman, 894 cm−1—FTIR) | +3 cm−1 (820 cm−1), +3.2 cm−1 (840 cm−1) | +5 cm−1 (820 cm−1), +4.2 cm−1 (840 cm−1) | −24 cm−1 | −4.2 cm−1 |
| pyroxene major band: 1010 cm−1—Raman, 1046 cm−1 —FTIR | +3.6 cm−1 | +5.2 cm−1 | −6.5 cm−1 | −6 cm−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyollai, I.; Biri, S.; Juhász, Z.; Király, C.; Rácz, R.; Rezes, D.; Sulik, B.; Szabó, M.; Szalai, Z.; Szávai, P.; et al. Raman–Infrared Spectral Correlation of an Artificially Space-Weathered Carbonaceous Chondrite Meteorite. Minerals 2024, 14, 288. https://doi.org/10.3390/min14030288
Gyollai I, Biri S, Juhász Z, Király C, Rácz R, Rezes D, Sulik B, Szabó M, Szalai Z, Szávai P, et al. Raman–Infrared Spectral Correlation of an Artificially Space-Weathered Carbonaceous Chondrite Meteorite. Minerals. 2024; 14(3):288. https://doi.org/10.3390/min14030288
Chicago/Turabian StyleGyollai, Ildiko, Sándor Biri, Zoltán Juhász, Csilla Király, Richárd Rácz, Dániel Rezes, Béla Sulik, Máté Szabó, Zoltán Szalai, Péter Szávai, and et al. 2024. "Raman–Infrared Spectral Correlation of an Artificially Space-Weathered Carbonaceous Chondrite Meteorite" Minerals 14, no. 3: 288. https://doi.org/10.3390/min14030288
APA StyleGyollai, I., Biri, S., Juhász, Z., Király, C., Rácz, R., Rezes, D., Sulik, B., Szabó, M., Szalai, Z., Szávai, P., Szklenár, T., & Kereszturi, Á. (2024). Raman–Infrared Spectral Correlation of an Artificially Space-Weathered Carbonaceous Chondrite Meteorite. Minerals, 14(3), 288. https://doi.org/10.3390/min14030288






