Abstract
In China, most of the high-sulfur iron ores have not been fully developed and utilized due to the lack of breakthrough progress in the research on the sulfur migration and the desulfurization mechanism during the roasting process. This study will focus on revealing the release and fixation mechanisms of sulfur during the roasting process to achieve the transformation of desulfurization from terminal treatment to process control. Experimental results show that as the roasting temperature increases, the release rate of SO2 also increases, reaching the maximum release rate at 900 °C. Simultaneously, it is found that at the same roasting temperature, the release rate and amount of SO2 under the O2/N2 atmosphere is significantly greater than that under the pure N2 and air atmospheres. Meanwhile, X-ray diffraction (XRD) is utilized to explore the phase composition of the roasted product and the sulfur release mechanism. In addition, the adsorption energy, stability and electron transfer of SO2 on the CaO surface are calculated through density functional theory (DFT), and the optimal adsorption active site perpendicular to the O atom (O-top) is also determined. Finally, the sulfur fixing agent CaO is used to study the SO2 fixation mechanism. When the concentration reaches 10%, the sulfur fixation efficiency reaches more than 80%. Therefore, this work will present basic knowledge and systematic guidance for the sulfur migration and release of high-sulfur iron ore under the oxidizing roasting process.
1. Introduction
There are various types of iron ore in China, among which high-sulfur iron ore resources are abundant and widely distributed. This type of ore is mainly composed of hematite (Fe2O3), magnetite (Fe3O4), pyrite (FeS2), quartz and other non-metallic minerals, in which the sulfur element mainly exists in the form of pyrite and sulfate. Pyrite is a naturally occurring material found in its concentrated form in nature and as an impurity in coal and many other minerals (shale, copper, limestone, uranium) [1,2]. The migration and transformation of sulfur in pyrite involves metallurgy, sulfuric acid preparation, coal burning, oil shale utilization and other industrial applications [3,4,5]. Most iron ore in China is rich in sulfur, and a series of physical and chemical transformations occur during the roasting process [6,7], which often leads to the high sulfur content of the iron concentrate produced and an excessive concentration of SO2 in flue gas dispersed into the environment [8,9]. Oxidizing roasting is an alternative method for the treatment of sulfur in pyrite, the principle and purpose of which is to oxidize the pyrite at high temperatures to release sulfur to reduce its content in the iron concentrate [10], thus reducing the cost of subsequent blast furnace ironmaking. Then, a sulfur fixing agent is used to fix and remove SO2 released from the discharged flue gas to meet the ultra-low emission standards (including SO2-35 mg/m3) stipulated in China’s environmental protection regulations [11,12]. Therefore, ascertaining the migration and release rules of the sulfur element during the roasting process of high-sulfur iron ore has important practical significance for the process control of sulfur emissions and efficient sulfur fixation. Many domestic and foreign scholars have carried out a lot of work on the migration direction of sulfur [13,14,15,16,17], but there are few studies on the migration and release of sulfur of high-sulfur iron ore during the roasting process. Wang et al. [18,19] investigates the effects of the roasting temperature, roasting time and dosage of reducing agent on sulfur migration in the process of direct reduction roasting of iron alum slag. The results show that Fe2(SO4)3 is decomposed at 400 °C and produces SO2 at 500 °C, and the sulfur element migrates from a solid phase to gas phase. Fe2O3, Fe3O4 and SO2 are confirmed to be the main final oxidation roasting products and dependent on experimental factors, such as the reaction temperature and reaction time [20]. In order to reduce the content of harmful sulfur in the separation concentrate and exhaust gas, related scholars tried to add a reinforcement sulfur agent to achieve this purpose and achieved some research results with scientific significance and application value, which has a good reference significance for research on sulfur fixation technology in the roasting process of high-sulfur iron ore. The common sulfur fixation agents in production are calcium sulfur fixation agents (CaO, MgO, CaCO3, etc.) [21,22,23], sodium sulfur fixation agents (NaOH, KOH, Na2CO3, etc.) [24] and other sulfur fixation agents [25]. However, due to the current research work mainly focused on the selection of sulfur fixation agents and the optimization parameters, the key scientific issues, such as the migration and release trend of sulfur and the sulfur fixation mechanism, have not been systematic and targeted research work. So, it is difficult to achieve breakthrough progress on the control of sulfur, resulting in the excessive investment of the sulfur fixation agent, unstable operation results and high cost. In recent years, theoretical calculations such as density functional theory (DFT) have been widely applied to deeply understand the reaction mechanism [26]. For instance, some researchers have used DFT calculation to accurately predict adsorption energy, electron transfer and stability, among other features [27,28]. Shen et al. evaluated the O-top position as the best adsorption site for CO2 on the surface of CaO (100) by using DFT calculation [29]. Therefore, theoretical calculation is a good auxiliary means to explore the mechanism of sulfur fixation.
This article is dedicated to revealing the removal rules of sulfur of high-sulfur iron ore through systematic exploration of the removal rules and oxidation mechanism at each stage under the roasting process. The restriction of the desulfurization reaction is determined to provide the necessary theoretical basis for improving the desulfurization efficiency, to optimize the oxidizing roasting process indicators and reduce consumption. Firstly, the distribution of sulfur elements in the solid phase and gas phase are studied to determine the migration trend of sulfur elements. Secondly, we obtain the release rate and release rule of sulfur in high-sulfur iron ore under different roasting parameters (sulfur content, roasting time, roasting temperature, oxygen concentration). Then, the migration mechanism of sulfur in the solid–gas phase and the fixed removal rule of SO2 are studied. Finally, we use DFT calculation to estimate the optimal active site of SO2 on the surface of CaO, adsorption energy, electron transfer and stability to further explore the mechanism of sulfur fixation. In summary, the mechanism of sulfur release and fixation in oxidizing roasting are studied in this paper, and the transition from end treatment to process control is realized.
2. Materials and Methods
The sulfur in the high-sulfur iron ore mainly exists in the form of pyrite and sulfate. The whole roasting process is carried out in an oxidizing atmosphere. Elemental sulfur and sulfur in sulfides are easily removed, but the decomposition of sulfate requires a higher temperature. Although the maximum roasting temperature in this study is set at 900 °C, this is less than the temperature at which the sulfate is decomposed. So, SO2 in the flue gas is mainly produced from FeS2 in the iron ore sample. Therefore, pyrite and hematite are used to prepare the sulfur-bearing iron ores in order to ensure the sample purity and reduce interference components, and the mechanism of sulfur release, migration and transformation during roasting is mainly discussed in this study.
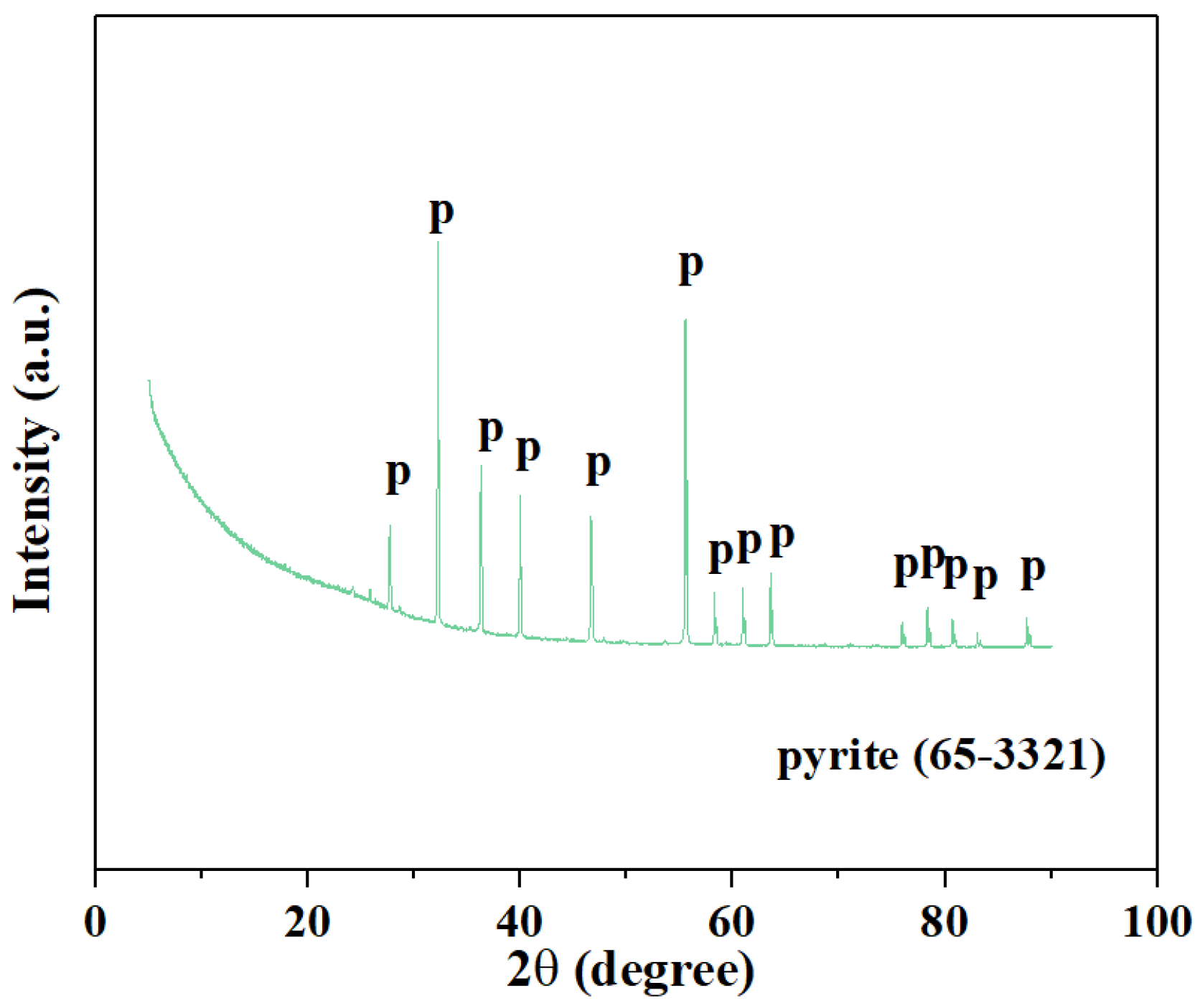
2.1. Material
The samples used in this study are powders composed of pyrite and hematite from southern China’s Jiangxi Province in a certain proportion. A vertical ball mill is used to prepare high-sulfur iron ore sample powder by comminution and sieving, and the sample is dried and preserved in a vacuum anaerobic before the experiment. The prepared micro–nano-grade sample has a large specific surface area and heat transfer efficiency, and has a high fluidity in the suspension fluidized state in the vertical furnace. X-ray diffraction analysis shows that the pyrite sample is a pure mineral, as in Figure 1 (JCPDs # 65-3321). In addition, the X-ray fluorescence characterization test shows that the pyrite contains a large amount of sulfur and iron, of which the content of sulfur is 53.6% and the content of iron is 44.42% (Table 1). Moreover, calcium oxide used for sulfur fixation and sodium hydroxide for the exhaust gas absorber were purchased from Beijing Bailingwei Technology Co., Ltd. (Beijing, China). All chemicals used are the analytical reagents received, and the solution is prepared using deionized water (18.25 MΩ cm) from an ultrapure water system. Furthermore, the simulated flue gas is composed of N2 (purity: 99.99%), O2 (purity: 99.99%) and air in this experiment, with the flow range for 0–600 mL/min.
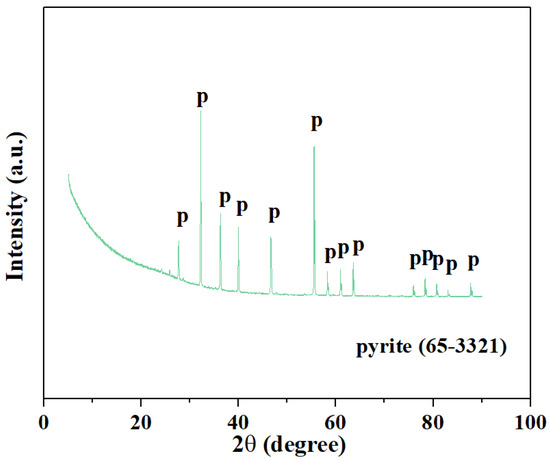
Figure 1.
X-ray diffraction analysis spectrum of pyrite.

Table 1.
Chemical composition analysis of the pyrite samples (wt%).
2.2. Experimental System
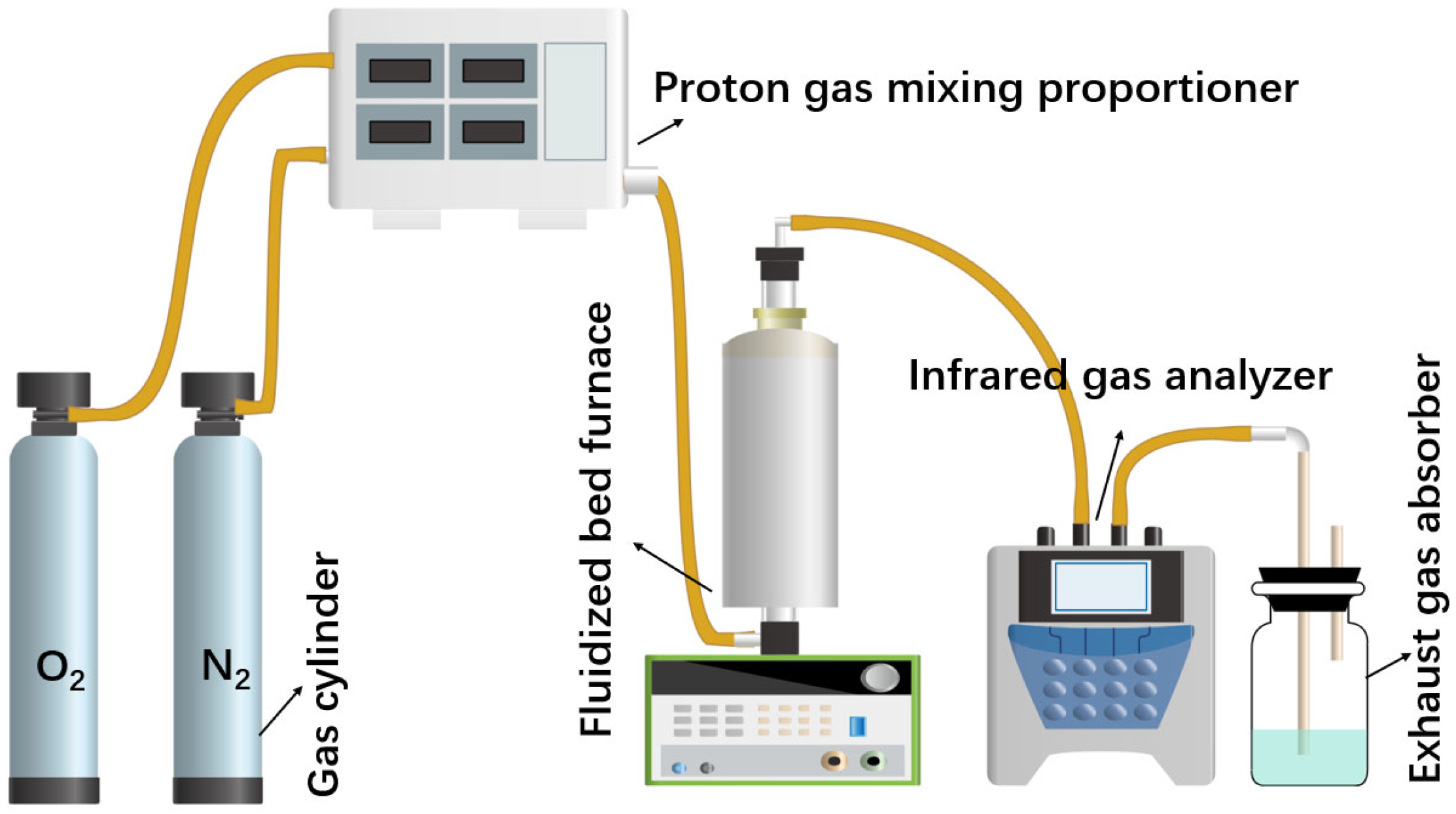
As can be seen in Figure 2, the experimental device is mainly composed of the flue gas preparation system, oxidizing roasting reaction system and measurement system. The simulated mixed flue gas used in this experiment is prepared by the O2 and N2 cylinders, and the purity of the gas is above 99.99%. The simulated gas is configured into different proportions of O2 and N2 by using a proton gas mixing proportioner according to the experimental requirements. The key part of the oxidizing roasting device is the vertical fluidized bed furnace, which is constructed by placing a transparent quartz tube with a diameter of 30 mm in the center. Firstly, the oxidizing roasting experiments of high-sulfur iron ore are carried out in a fluidized bed furnace to explore the optimal parameters of the sulfur release reaction. The configured high-sulfur iron ore is positioned on the fixed asbestos in a quartz tube for the roasting experiment in the temperature range of 400–900 °C, with the roasting time 30 min. Before the experiment, the furnace is first heated to the set temperature of the experiment, and the reaction gas is passed into the furnace for 5 min to ensure a stable and uniform atmosphere in the furnace. In the sulfur fixation experiment, high-sulfur iron ore is mixed with the sulfur fixation agent placed in the pipe. The SO2 (after drying) release concentration is on-line measured by the infrared gas analyzer, and the measuring result is recorded. Finally, the exhaust gas can be further processed through the exhaust gas absorber loaded with lye. For the collection of solid samples after roasting, because the sample is still in the quartz tube, it is necessary to continue to pass high-purity N2 to isolate the sample from the outside air, so that the sample can be prevented from continuing to react, and the quartz tube and solid sample can be quickly cooled. When the solid sample is cooled to room temperature in the N2 atmosphere, the solid product is removed and collected for subsequent testing. Subsequently, the different sulfur content, volume fraction ratio of O2/N2, roasting atmosphere, roasting temperature and roasting time are studied to reveal the release law, transfer path and release mechanism of sulfur in the sulfur-bearing iron ores.
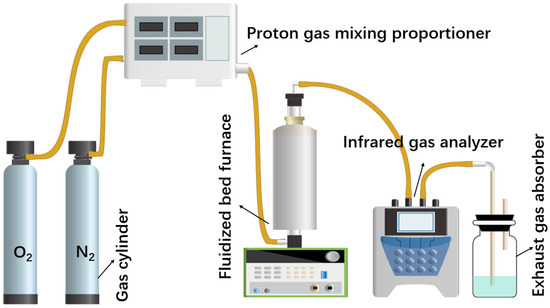
Figure 2.
Schematic diagram of roasting experimental setup.
The phases of the raw samples and oxidation roasted products at various stages are detected using a Rigaku D/max-2500HB XRD analyzer using Cu Kα radiation in the θ/2θ coupled mode with a 0.02° step size. The concentration of SO2 released from the flue gas is measured by the on-line infrared gas analyzer (Gasboard-3000plus).
2.3. DFT Computation
DFT calculations are performed using the DMol3 module of the Materials studio (MS) within the generalized gradient approximation (GGA) to simulate the SO2 adsorption on the pristine CaO (100) surface. The adsorption energy (Eads) is calculated following Equation (1) [30].
where , , and symbolize the energy of the adsorbate—substrate, adsorbate and substrate in the adsorbate—substrate system.
3. Results
3.1. Release Characteristics of Sulfur under Different Roasting Conditions
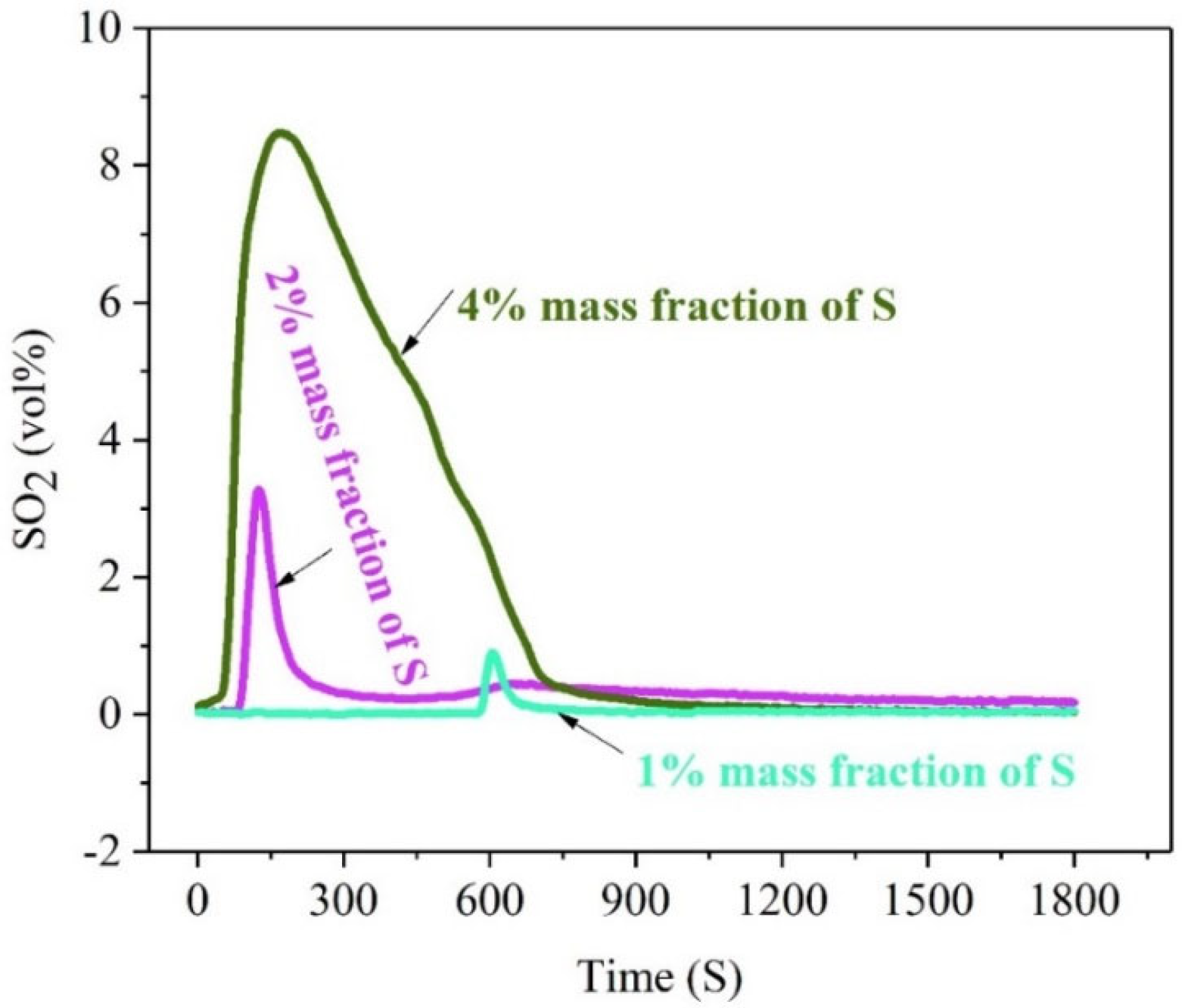
Figure 3 illustrates the release rule of sulfur of high-sulfur iron ore in the oxidizing roasting process with different S-content.
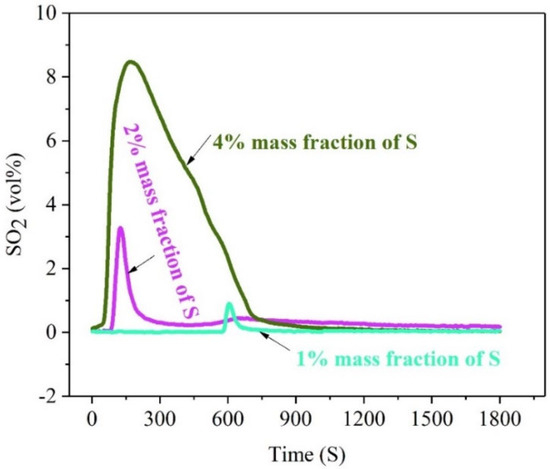
Figure 3.
The concentration-time curve of SO2 release from high-sulfur iron ore at different mass fractions of sulfur element (N2—470 mL/min, O2—130 mL/min, temperature—500 °C, roasting time—30 min).
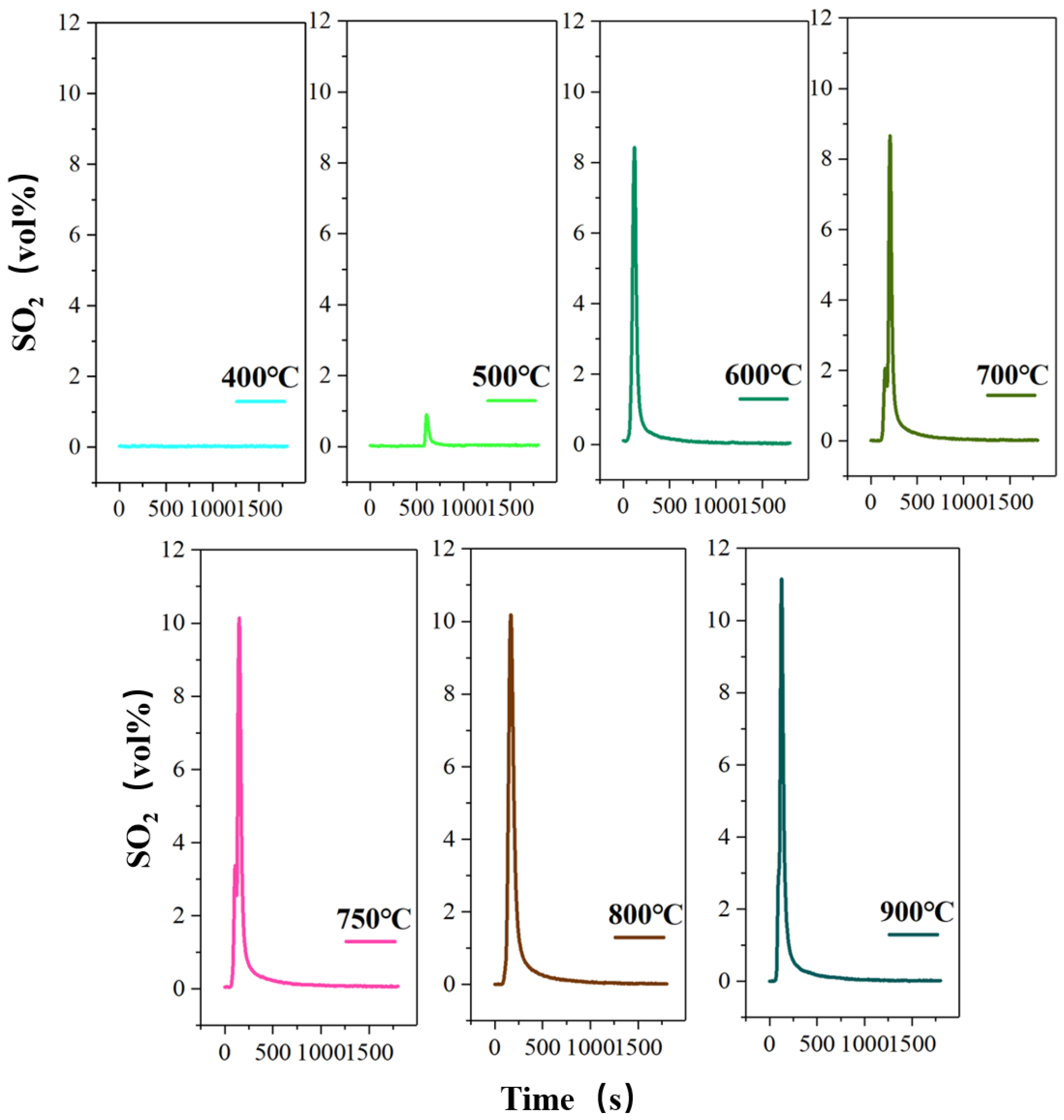
The processing of pyrite in the high-sulfur iron ore decomposition is an endothermic process, which can be considered as a heat control process. Therefore, the influence of the roasting temperature on the S-release rate is investigated under the same roasting time for 30 min, as shown in Figure 4. However, this study also investigates the migration and release of sulfur in a pure nitrogen without-oxygen atmosphere at different roasting temperatures, as shown Figure 5.
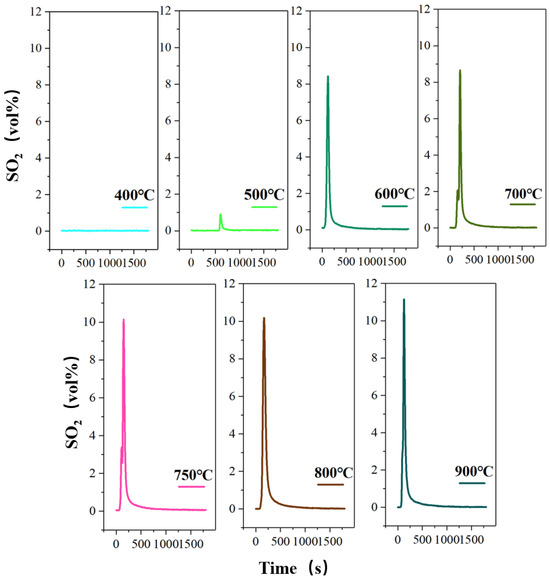
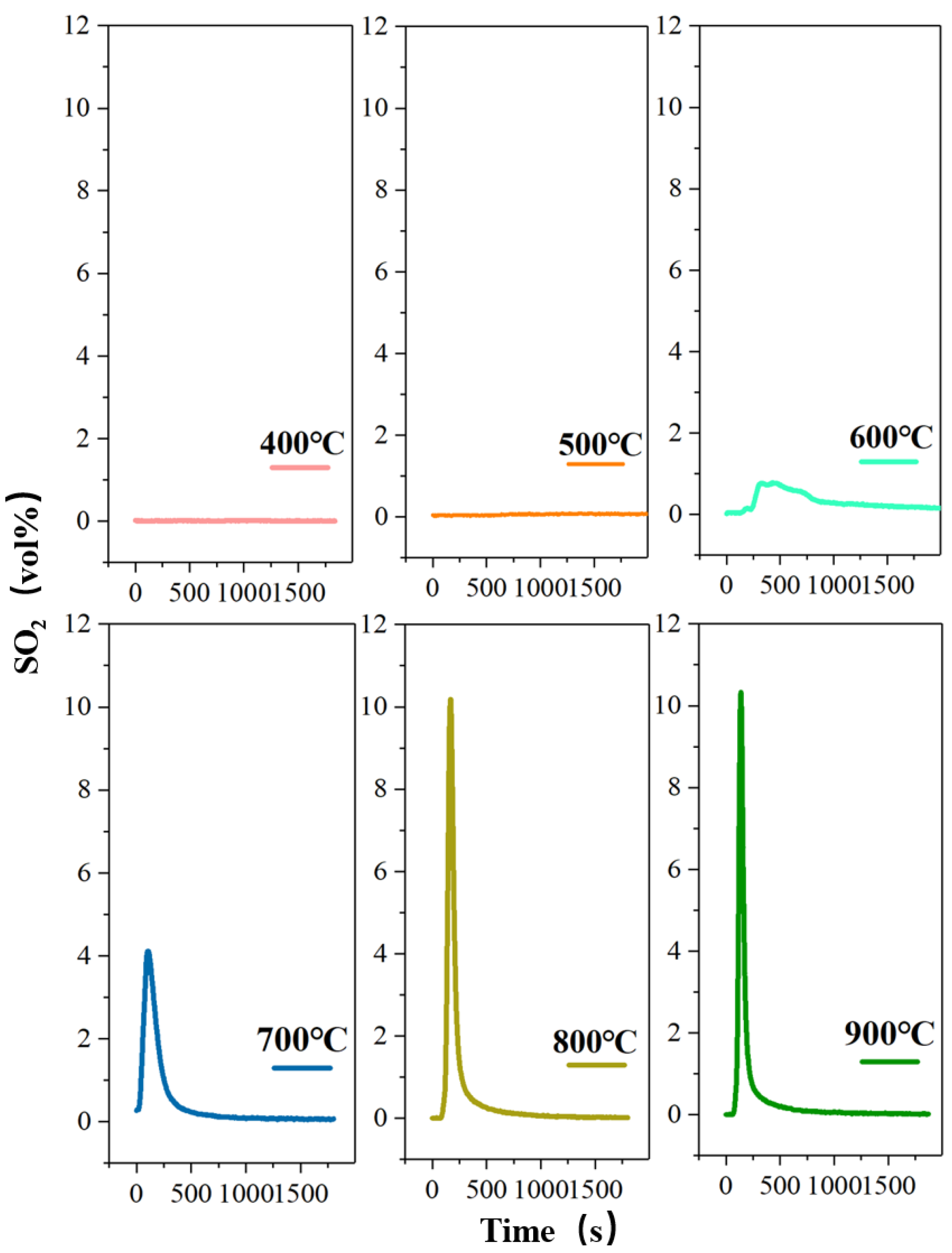
Figure 4.
The concentration-time curve of SO2 release from high-sulfur iron ore at different roasting temperatures in the presence of oxygen (N2—470 mL/min, O2—130 mL/min, S—1wt%, roasting time—30 min).
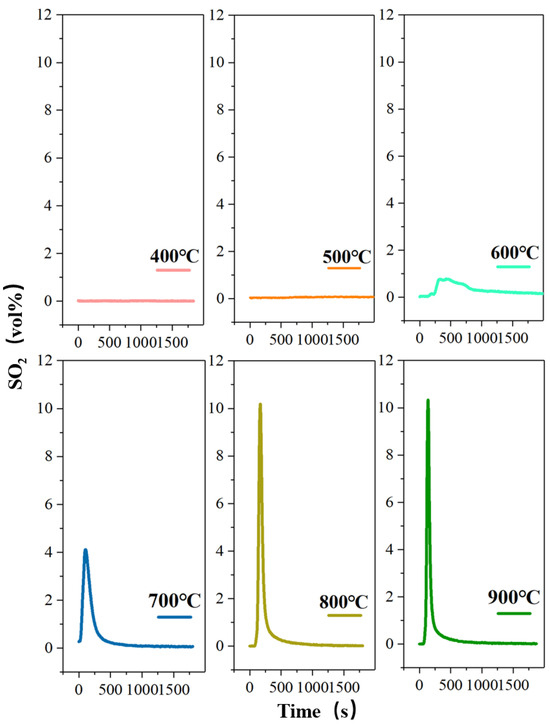
Figure 5.
The concentration-time curve of SO2 release from high-sulfur iron ore at different roasting temperatures in the absence of oxygen (N2—500 mL/min, O2—0 mL/min, S—1wt%, roasting time—30 min).
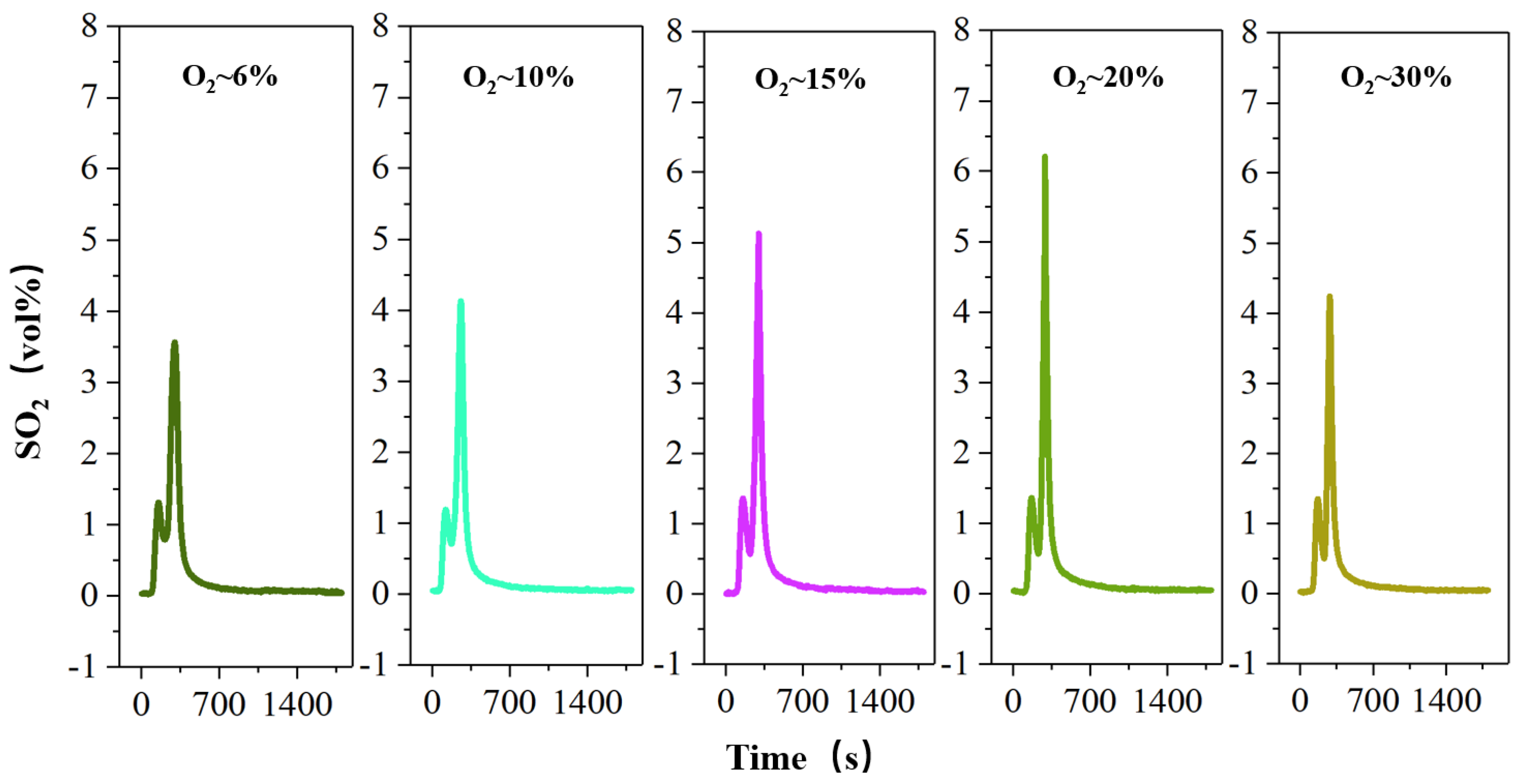
Oxygen plays an important role in the migration and release of the sulfur element under the roasting process, so Figure 6 investigates the effect of oxygen concentration on the release of sulfur during roasting.
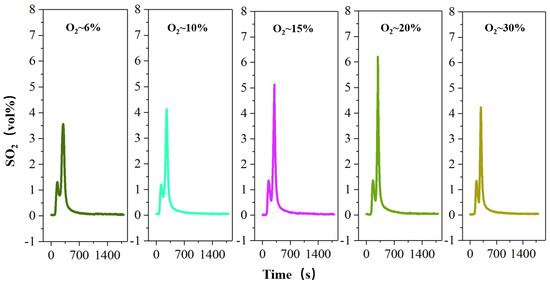
Figure 6.
The concentration-time curve of SO2 release from high-sulfur iron ore at different concentrations of oxygen (S—1 wt%, temperature—600 °C, roasting time—30 min).
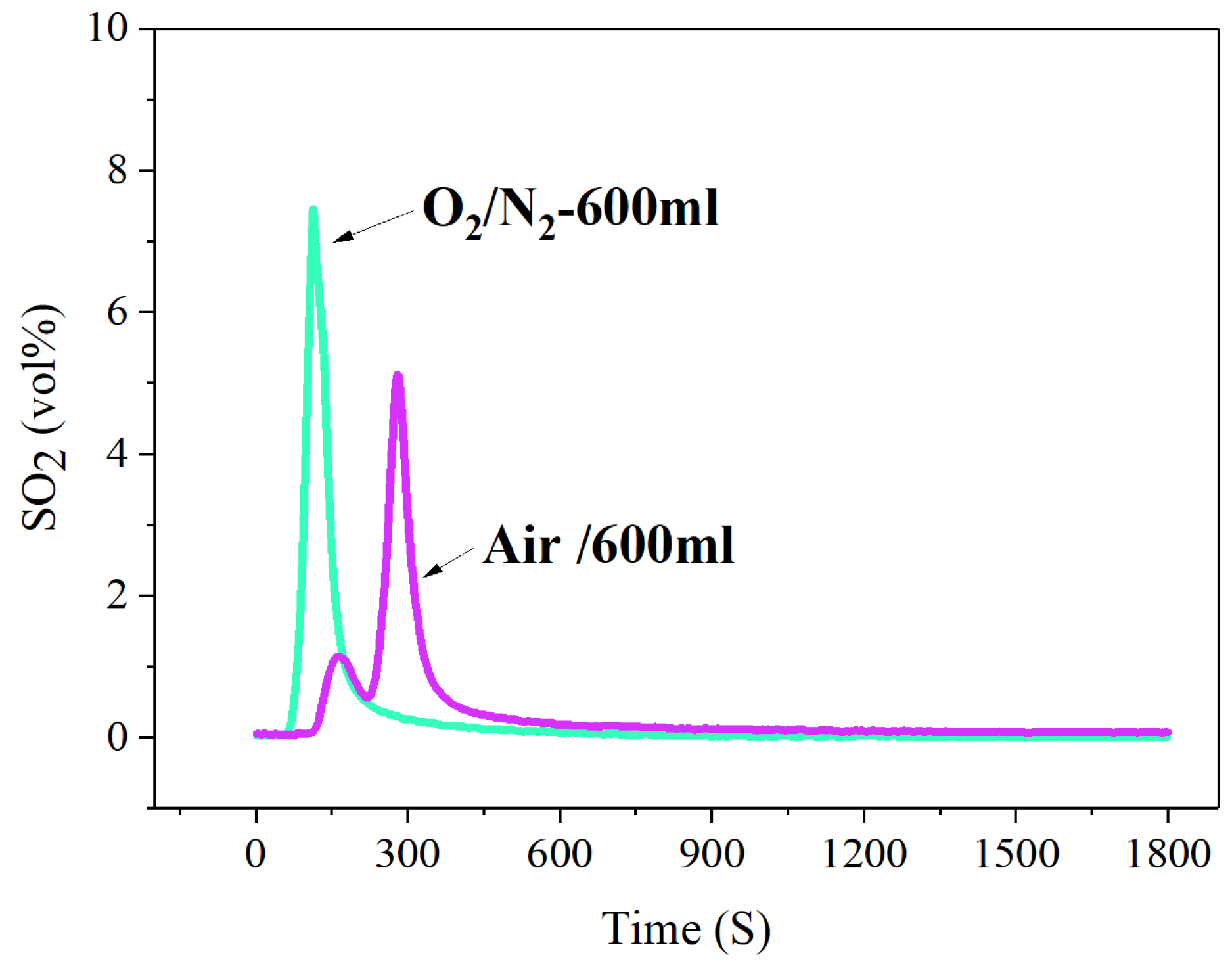
By comparing the release concentration and release rate of SO2 under a traditional air atmosphere and O2/N2 (O2-21%) atmosphere, it is obviously found that the release amount and release rate of SO2 under an O2/N2 atmosphere is significantly stronger than that under an air atmosphere, as shown in Figure 7.
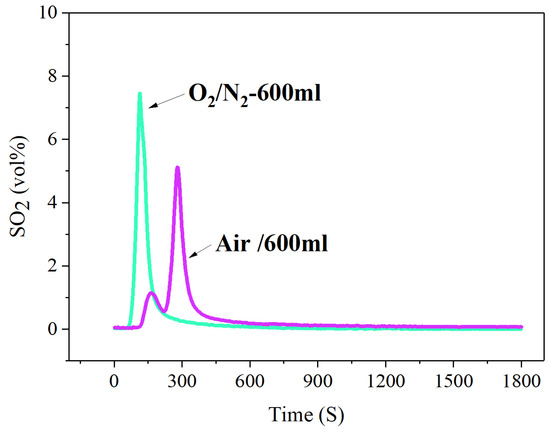
Figure 7.
The concentration-time curve of SO2 release from high-sulfur iron ore under different combustion atmosphere (S—1 wt%, temperature—600 °C, time—30 min, O2—21%, N2—79%).
3.2. Mechanism of Sulfur Element Release
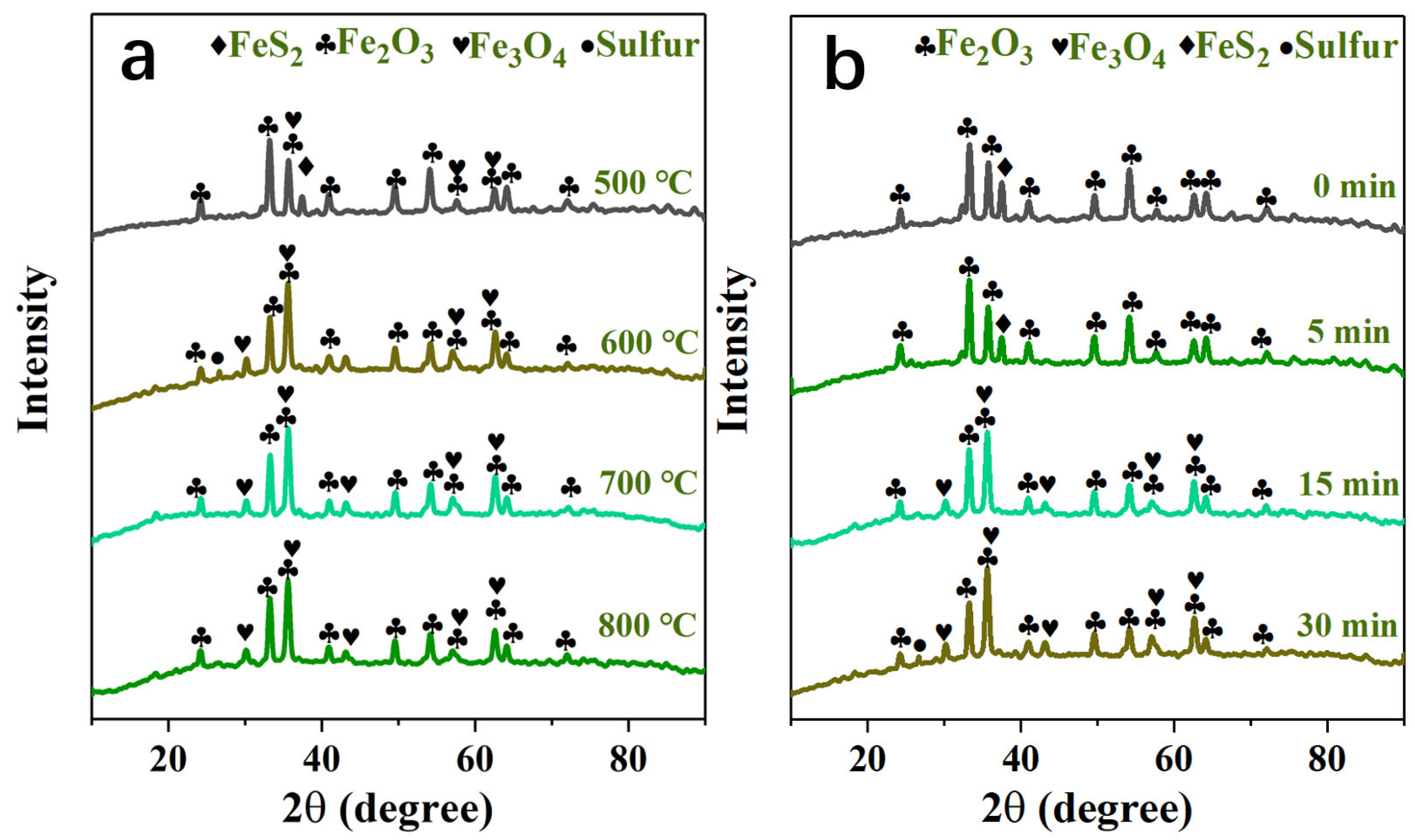
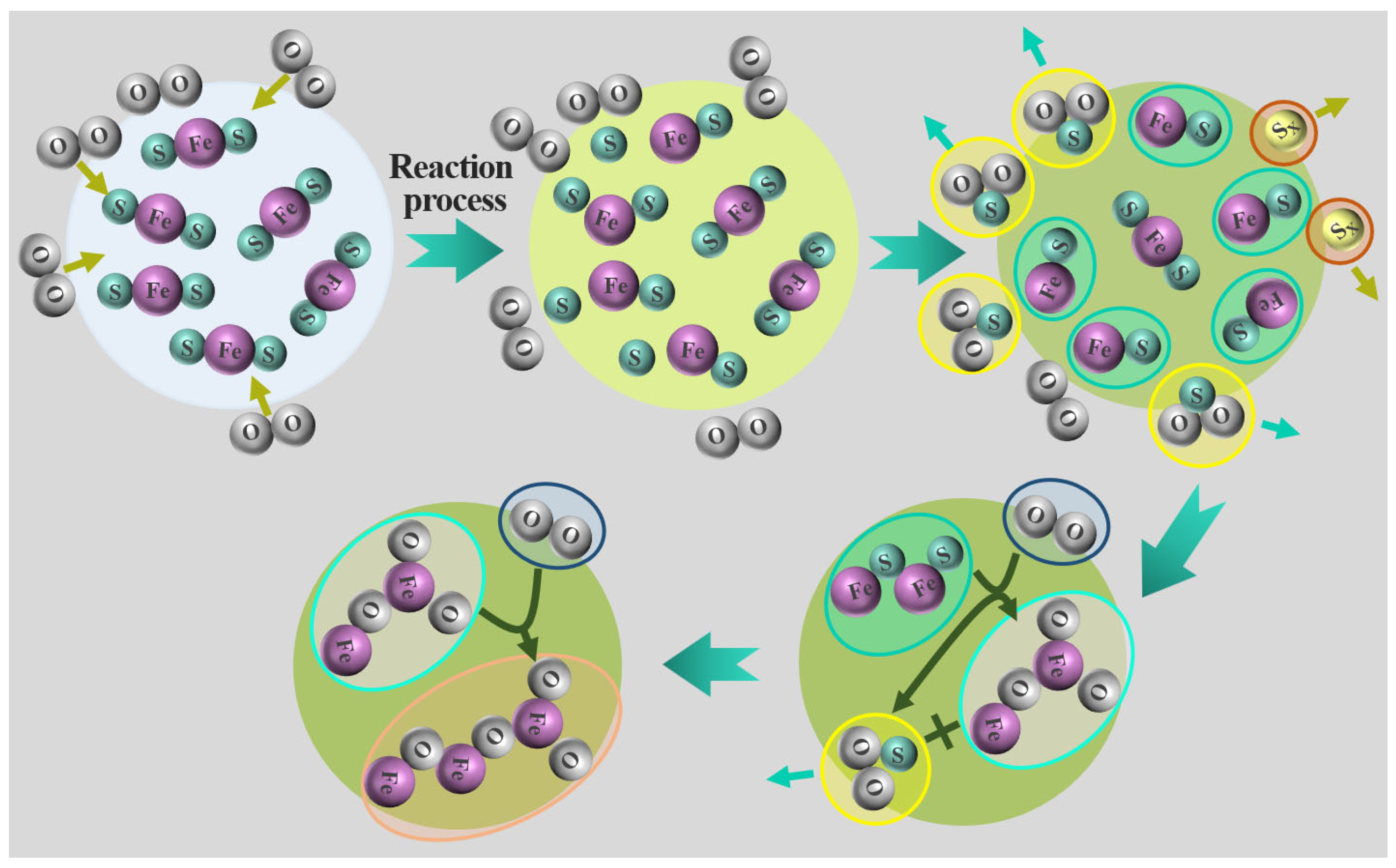
In order to improve the release rate of sulfur to prepare for the subsequent sulfur fixation process, the decomposition and oxidation mechanism analysis during the roasting process is very important and needs to be studied. The X-ray diffraction analysis spectrum of the oxidizing roasting product of high-sulfur iron ore is shown in Figure 8. The mechanism model of the desulfurization, decomposition and oxidation of pyrite particles in oxidizing roasting is shown in Figure 9.
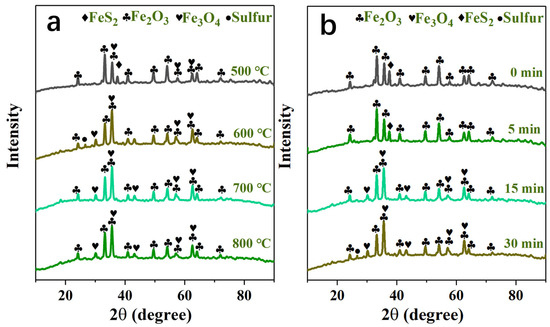
Figure 8.
(a) XRD patterns of products at different oxygen-roasting temperatures; (b) XRD patterns of products at different oxygen-roasting times under roasting temperature 600 °C.
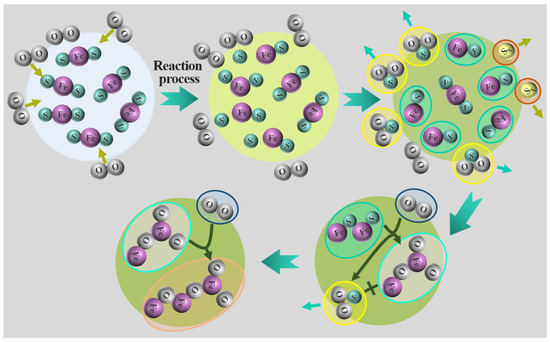
Figure 9.
Mechanism model of desulfurization, decomposition and oxidation of pyrite particles in oxidizing roasting.
3.3. Effect of Sulfur Fixative CaO on the Release of Sulfate and Mechanism Analysis
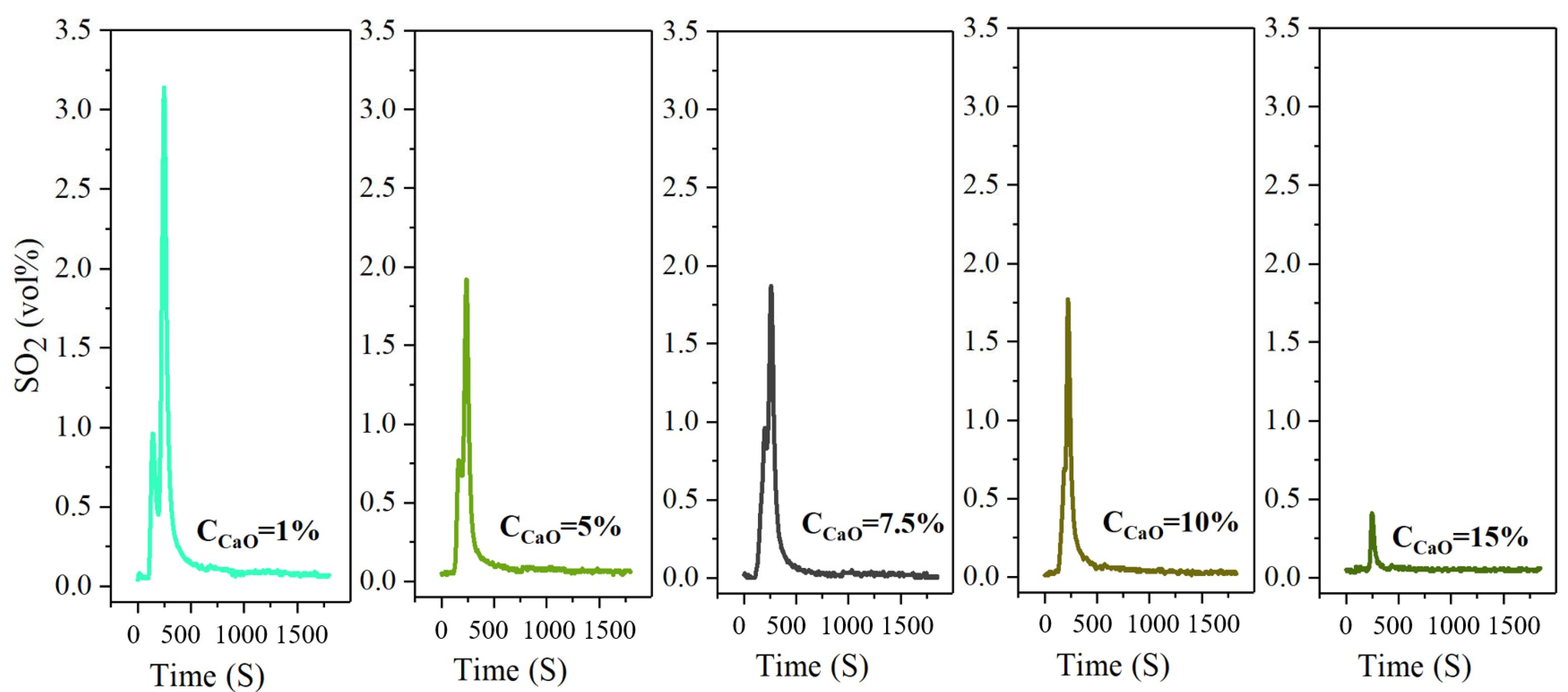
Calcium oxide (CaO) was selected as the fixative for SO2 because of its advantages of a wide source of raw materials, low cost and environmental friendliness. The law of sulfur release and the efficiency of sulfur fixation are investigated, as shown in Figure 10.
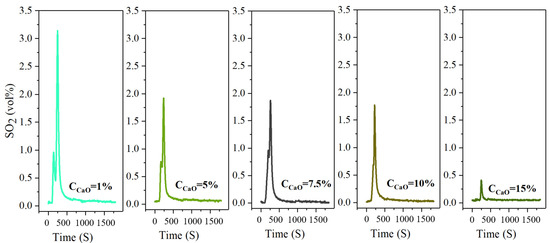
Figure 10.
The concentration-time curve of SO2 release from high-sulfur iron ore at different concentrations of sulfur fixer CaO (S—1 wt%, temperature—600 °C, time—30 min, O2—21%, N2—79%).
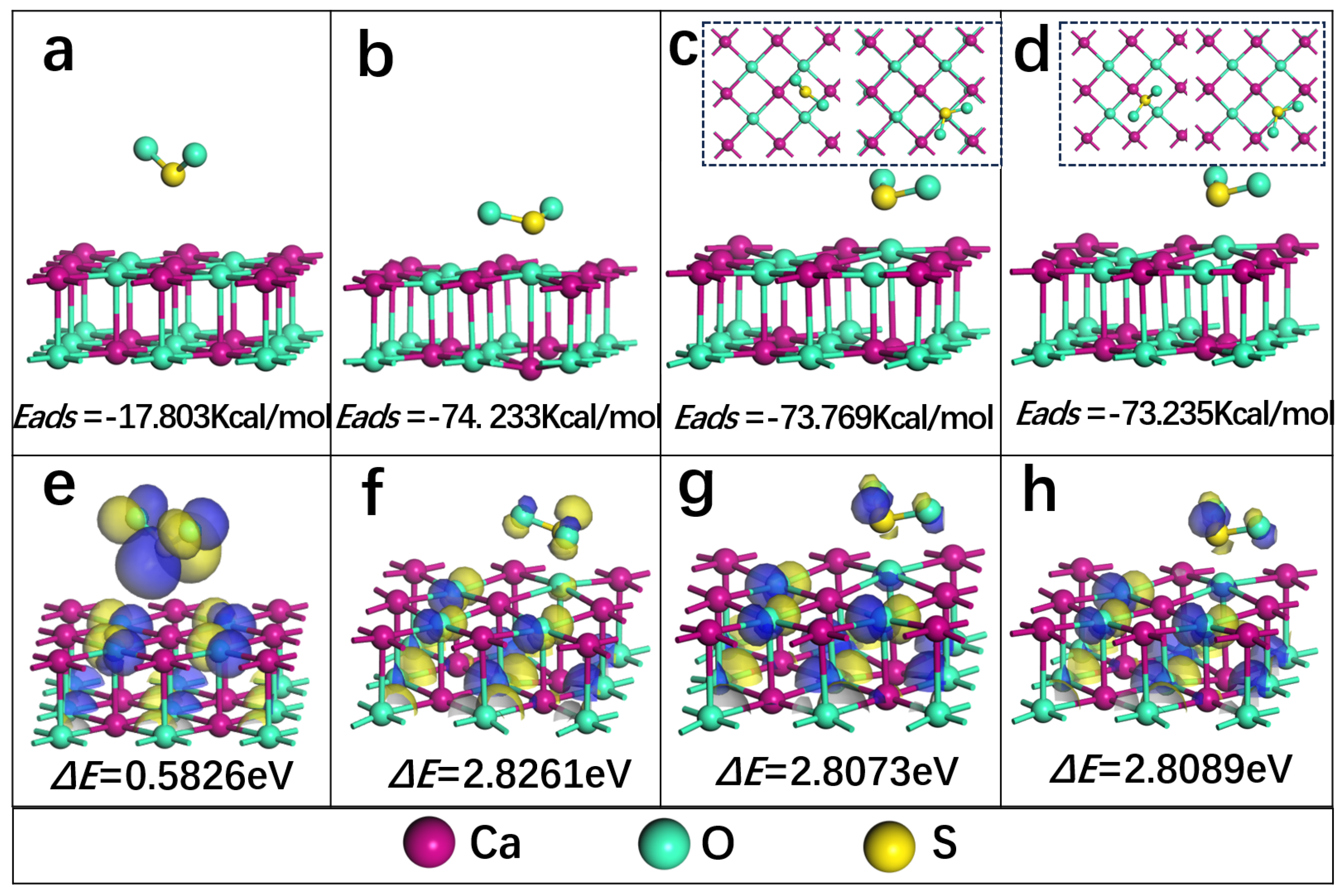
The above DFT calculation is applied to analyze the adsorption energy and stability of SO2 on the surface of CaO during sulfur fixation of calcium. Figure 11a–d show the adsorption stable configuration of Ca-top, O-top, hollow and bridge adsorption sites of SO2 on the surface of CaO (100), respectively. Figure 11e–h show the stability.
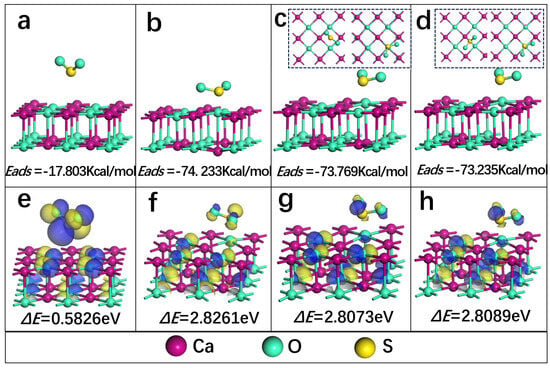
Figure 11.
(a–d) The adsorption stable configuration of Ca-top, O-top, hollow and bridge adsorption sites of SO2 on the surface of CaO (100), respectively (the illustrations in (c,d) represent top views of the position changes of before and after adsorption of SO2 on the CaO (100) surface); (e–h) The stability of the adsorption configuration at different adsorption sites depends on HOMO and LUMO. Note: For (e–h), the blue and yellow colors represent the positive and negative phases of the molecular orbitals of the compounds, respectively.
4. Discussion
4.1. Release Characteristics of Sulfur under Different Roasting Conditions
4.1.1. Effect of the SO2 Release on the Mass Concentration of Sulfur Element
Figure 3 illustrates that the release rule of sulfur of high-sulfur iron ore in the oxidizing roasting process with different S-content is consistent with a single peak, that is, the volume percentage of SO2 released increases with the advance of the roasting reaction and decreases rapidly after reaching the maximum value. However, there are obvious distinctions in the characteristics of peaks and the time when the maximum peak value appears, showing that the peak shape of 1% and 2% sulfur mass concentration is sharper, and the peak of 1% sulfur mass concentration appears latter. Under the same conditions of oxygen roasting (roasting temperature 500 °C and roasting time 30 min), the volume percentage of S-release increases with the increase insulfur content in high-sulfur iron ore. When the mass fraction of sulfur is 1% and 2%, the maximum volume percentage of SO2 released is 3.28% and 0.9%, respectively. Moreover, when the mass fraction of sulfur in the ore is as high as 4%, the maximum volume percentage of SO2 release is close to 8.47%, and the time of the SO2 high-value release lasted up to 12 min, which is much higher than the other two. This indicates that the higher the sulfur content is, the earlier the release peak appears and the larger the maximum peak is. The lower the sulfur content is, the sharper the release peak shape is.
4.1.2. Effect of the S-Release on the Different Roasting Temperatures
The processing of pyrite in the high-sulfur iron ore decomposition is an endothermic process, which can be considered as a heat control process. Wang et al. verified the phase transformation is strongly dependent on the reaction temperature during the oxidation of pyrite [31]. Therefore, the influence of the roasting temperature on the S-release rate is investigated under the same roasting time of 30 min, as shown in Figure 4. The peak mainly appears at about 200 s, and the peak shape is sharp. After about 800 s, the curve tends to be straight, and the release of SO2 is close to zero. However, when the roasting temperature is 500 °C, the peak is delayed due to the slow decomposition of sulfur caused by the low temperature. The results show that the oxygen roasting temperature has a significant effect on the volume percentage of SO2 released, that is, the maximum volume percentage gradually increases with the increase in roasting temperature. It can be found that at 400 °C, the concentration-time curve of SO2 release tends to be straight, and no obvious peak shape is produced. The measured volume percentage of SO2 approaches 0.03%, indicating that the pyrite begins to decompose and release the sulfur component at 400 °C, but only a small amount of SO2 is produced. This also shows that pyrite is stable and not decomposed below 400 °C. Then, as the temperature rises to 500 °C, there is a small spike in the release of SO2, with the maximum volume fraction of 0.9%. When the roasting temperature continues to rise to 600 °C, the release of SO2 shows an explosive increase, with the maximum volume fraction reaching 8.42%, which is due to the rapid fracture of the Fe-S bond in pyrite caused by the temperature rise realizing the quick release of sulfur [32,33]. The results also indicate that the extensive decomposition of pyrite occurs at 600 °C. Subsequently, the roasting temperature increases from 700 °C to 800 °C, and the maximum volume percentage increases from 8.66% to 10.18%. In this case, the volume percentage and the total release amount of SO2 do not change significantly, which indicates that the temperature rise has little effect on the decomposition of pyrite to SO2 when the temperature is higher than 700 °C. Finally, the highest SO2 release rate is obtained when the temperature is raised to 900 °C, accompanied by the maximum volume percentage of 11.15%. However, 600 °C should be the best oxygen roasting temperature from the perspective of energy saving.
In order to better understand the behavior of the sulfur element during the oxidizing roasting, this study also investigates the migration and release of sulfur in a pure-nitrogen-without-oxygen atmosphere at different roasting temperatures (Figure 5). At the roasting temperature 400 °C, there is no obvious change in the ore sample and almost no SO2 discharge. When the temperature rises to 500 °C, trace amounts of SO2 are detected with an infrared gas analyzer. Furthermore, it can be seen that the desulfurization reaction rate increases with the increase in roasting temperature. According to a previous study [34], the sulfur element in SO2 derives from the desulfurization reaction of FeS2, and pyrrhotite (FeSx) is the product of the desulfurization reaction. Also, the oxygen element in SO2 should come from Fe2O3, which causes Fe2O3 to generate Fe3O4. Compared to Figure 3 and Figure 4, the desulfurization effect under the O2/N2 roasting atmosphere is obviously stronger than that of under the nitrogen atmosphere at various roasting temperatures. Therefore, it can be concluded that the presence of oxygen in the roasting process promotes the formation and release of SO2, reducing the content of sulfur in the high-sulfur iron ore sample, which is conducive to the subsequent blast furnace ironmaking. Fe2O3 is deprived of oxygen, leading to formation of Fe3O4, which is conducive to the subsequent magnetic separation of iron ore. Therefore, for iron ore with low sulfur content, the nitrogen atmosphere can be tried. Compared with iron ore with higher sulfur content, better desulfurization efficiency is obtained by oxidizing roasting. In addition, yellow condensed sulfur is also found at the tail flue gas outlet, which indicates that the sulfur in the FeS2 is released in the form of elemental sulfur (Sx) in the N2 atmosphere.
4.1.3. Effect of the SO2 Release on the Concentration of Oxygen
Oxygen plays an important role in the migration and release of the sulfur element under the roasting process, so it is imperative to discuss the parameter of oxygen concentration (Figure 6). The results show that with the increase in oxygen concentration by 6% to 21%, the maximum volume fraction of SO2 produced gradually increases from 3.56% to 5.04%. With the increase in O2 concentration, the decomposition trend of FeS2 increases significantly. The main reason is that the reaction boundary radius of O2 and sulfur decrease with the increase in O2 concentration [35], so the heat absorbed by particles increases, which is conducive to the further decomposition of FeS2. Surprisingly, however, when the oxygen concentration increases to 30%, the release of SO2 does not continue to increase but shows a slight decrease to 4.24%. This is because the main solid roasting products of FeS2 roasting are FeS and Fe3O4 under the combustion condition of low O2 concentration, and the Fe3O4 increase in the product leads to the decrease in the slagging tendency of the solid product [36,37], which is conducive to sulfur migration and release. Nevertheless, under the combustion condition of high O2 concentration (O2-30%), the products of FeS2 roasting are Fe3O4 and Fe2O3, and the proportion of Fe2O3 in the product increases relative to the high oxygen concentration. Since the increase in Fe2O3 has no obvious improvement effect on the slagging tendency of the FeS2 roasting product [38], the product slagging prevents the diffusion of sulfur-internal particles to the surface and the diffusion of O2 to the interior. And because the S-release is controlled by the diffusion process, the release of SO2 is slightly reduced.
Furthermore, FeS2 undergoes the following chemical reaction under oxidizing roasting (Equation (2)) [39]. After the sulfur element in FeS2 is converted to SO2 gas, part of SO2 continues to react with O2 (Equation (3)) [39]. This shows that the higher the oxygen concentration, the more SO2 is converted to SO3. Therefore, as the oxygen concentration continues to increase to 30%, the release of SO2 decreases.
4.1.4. Effect of the SO2 Release under Air Atmosphere
By comparing the release concentration and release rate of SO2 under a traditional air atmosphere and O2/N2 (O2-21%) atmosphere, it is obviously found that the release amount and release rate of SO2 under an O2/N2 atmosphere is significantly stronger than that under an air atmosphere. It may be that the air contains water vapor, CO2 and other impurities. Water vapor can convert SO2 to sulfuric acid, and CO2 can make SO2 convert to carbon thiooxide (COS), both of which reduce the emission concentration of SO2, causing the results shown in Figure 7.
In addition, the presence of water vapor in the air causes FeS2 to react with H2O to form H2S and H2 (Equations (4)–(7)) [40,41,42]. Therefore, part of the sulfur released is converted to H2S in the atmosphere in the presence of H2O molecules, thereby reducing SO2 emissions.
It should be noted that the curve of SO2-release in the air atmosphere contains two peaks, with a small peak shape appearing at the initial stage of the reaction, while the SO2-release curve in the O2/N2 atmosphere has only one sharp peak in Figure 6. This is because the SO2 formed in the roasting process will go through the process of release, absorption and secondary release, showing the characteristics of SO2 emission in iron ore. Due to the air atmosphere containing water vapor, the released SO2 is absorbed to form sulfuric acid, which in turn forms sulfates and sulfites. The presence of water vapor provides good conditions for the absorption of SO2. However, with the process of the roasting reaction, the absorption capacity of SO2 is gradually weakened, and the SO2 is re-released into the flue gas. The above processes form the phenomena of SO2 release, absorption and secondary release during the sintering process, and also cause two peaks in the curve of SO2 release under the air atmosphere.
4.2. Mechanism of Sulfur Element Release
The mechanisms of the decomposition and oxidation of the high-sulfur iron ore sample roasting process under an O2/N2 atmosphere are studied. The X-ray diffraction analysis spectrum of the oxidizing roasting product of high-sulfur iron ore is shown in Figure 8. As can be seen in Figure 8a: when the oxidizing roasting temperature increases from 500 °C to 600 °C, the diffraction peak of FeS2 gradually weakens until it disappears, which is caused by the thermal decomposition of FeS2. Meanwhile, the diffraction peak of elemental sulfur is detected (Equation (8)), and an obvious diffraction peak of Fe3O4 appears. The appearance of the diffraction peak of Fe3O4 is due to the reaction of FeS2 and O2 producing FeO, and the generated intermediate-phase FeO is unstable at 200–550 °C, and then will be thermally decomposed into Fe3O4 [43]. When the oxidizing roasting temperature increases to 800 °C, solid-phase diffusion and crystallization need to occur at a higher temperature during the oxidation process due to the poor oxidation activity of Fe2O3, so the peak value of Fe2O3 detected has no significant changes. On the contrary, the diffraction peak of Fe3O4 gradually weakens, which may be caused by the reaction of Fe3O4 with O2 to produce Fe2O3. The above conclusion is consistent with those of Thauan Gomes et al., that is, the main products are Fe3O4 and Fe2O3 [20].
In addition, XRD is used to detect the phase changes of high-sulfur iron ore samples under different roasting times at the roasting temperature 600 °C, as shown in Figure 8b. The diffraction peaks of FeS2 and Fe2O3 are detected in the primary sample. The FeS2 content decreased at the reaction time 5 min, and with the advance of the reaction process, the diffraction peak of FeS2 vanishes, and no other sulfur-containing minerals are detected, indicating that most of the pyrite contained in the sample has undergone a desulfurization reaction. The sulfur element is mainly released in the form of SO2 gas, without a sulfur fixation reaction with other minerals. And the diffraction peak intensity of elemental sulfur increases with the progress of the reaction. In addition, the diffraction peak of Fe3O4 is detected at 15 min, which is due to the oxidative decomposition of FeS2 to produce Fe3O4. Notably, no FeSO4 is detected because the FeSO4 stability zone shrank as the temperature increased, hindering sulfate formation. Therefore, higher concentrations of oxygen and SO2 are required at high temperatures to produce sulfates and sulfites. Due to the low concentration of SO2 and oxygen involved in this study, the formation of FeSO4 is avoided.
Previous studies have pointed out that during the decomposition process of pyrite particles, part of the sulfur element is first lost on the surface, and the release of sulfur forms a porous structure on the surface of the particles, and the inside of the particles is still dense pyrite. Therefore, the FeS2 in high-sulfur iron ore particles first is decomposed into FeS and sulfur vapor Sx. And the sulfur vapor Sx reacts with oxygen entering the pore in the process of outward diffusion to produce SO2 in a secondary reaction (Equation (9)), which will expand the pore structure of the particles and accelerate the oxidation rate. FeS is then oxidized by O2 to Fe3O4 and Fe2O3 (Equations (10) and (11)). Part of the Fe3O4 then reacts with oxygen to form Fe2O3. No sulfate formation is detected during the reaction; in addition to the low oxidizing roasting temperature, it may also be that the SO2 produced in this reaction is low concentration and is easily carried out of the reactor by the incoming O2/N2 gaseous mixture. In addition, the FeS2 in high-sulfur iron ore particles is more likely to react directly with oxygen to form Fe3O4 and Fe2O3 rather than sulfate (Equations (12) and (13)) (Figure 9).
4.3. Effect of Sulfur Fixative CaO on the Release of Sulfate
Calcium oxide (CaO) has many advantages, such as a wide source of raw materials, low cost and environmental friendliness; it is especially easy for sulfation reaction. So, it is often used for high-temperature SO2 capture. In the following study, the law of sulfur release and the efficiency of sulfur fixation are investigated when the sulfur reinforcing agent CaO is added to the reaction system under the oxidizing roasting temperature of 600 °C. Figure 10 reveals that the release law of SO2 is consistent, and similar peak shapes are obtained at different concentrations of CaO added. During the whole roasting process, two sharp peaks of SO2-release appear, and the highest peak appears around 250 s. The first peak gradually decreases with the increase in CaO concentration and completely disappears when the CaO concentration is 15%. The sulfur in the high-sulfur iron ore is basically fixed, and the release curve tends to be a straight line. Through the detection, when the concentration of CaO is 1%, the highest SO2 emission volume percentage of 3.21% is significantly lower than 8.42% when no sulfur fixing agent is added, and the sulfur fixation efficiency is 35.93%. When the CaO concentration is increased to 5%, the maximum volume percentage of SO2 emission further decreases to 1.91%, and the sulfur fixation efficiency also increases to 56.19%. The concentration of CaO increases to 7.5% and 10%, and the maximum emission volume percentage of SO2 changes slightly to 1.87% and 1.77%, respectively, but the sulfur fixation efficiency enhances significantly to 64.44% and 70.02%, respectively. The concentration of CaO continues to increase to 15%, and the maximum emission volume percentage of SO2 reaches 0.42%. At this time, SO2 is almost completely fixed, and the maximum sulfur fixation efficiency is 82.48%. Figure 8 reveals that the SO2 release peak shape is bimodal, and the reaction process can be clearly divided into two stages. The first stage is the intrinsic reaction kinetics control stage, in which the surface of absorbent particles is exposed to a large amount of unreacted CaO. And this time, the sulfation reaction is mainly controlled by the intrinsic chemical reaction rate.
In the sulfation reaction, CaSO4 crystal nucleation islands are first formed on the surface of CaO, and the crystal nucleation gradually grows and fuses to form a product layer with the progress of the reaction. The product layer is not completely dense, but there are gaps through which SO2 gas can contact CaO inside the product layer [44]. However, with the extension of the reaction time, since the molar volume of CaSO4 (46 cm3/mol) is nearly three times that of CaO (16.9 cm3/mol), the CaSO4 product layer will gradually cover the CaO surface. On the one hand, the resulting product layer can clog pores that are too small. On the other hand, blocking the mass transfer channel for SO2 gas to diffuse into the small pores will increase the mass transfer resistance between SO2 gas and fresh CaO in the product layer, resulting in a significant reduction in the macroscopic reaction rate, and the sulfation reaction will be from a fast intrinsic reaction. The kinetic control stage transitions to the product layer diffusion control stage [45].
In this work, the most stable adsorption structure of SO2 on CaO (100) surface is studied with different adsorption sites. Figure 11a–d show the adsorption stable configuration perpendicular to the Ca atom (Ca-top), O-top, hollow and bridge adsorption sites of SO2 on the surface of CaO (100), respectively. It can be observed that the distance of SO2 to Ca-top on the CaO (100) adsorption surface increases after the optimization from 2.302 Å to 3.06 Å. Meanwhile, the adsorption energy obtained is −17.804 Kcal/mol (Equation (1)), which indicates weak chemisorption. In addition, the distance of SO2 to O-top on the CaO (100) adsorption surface decreases from 2.302 Å to 1.741 Å, and the SO2 on the hollow and bridge adsorption sites is shifted to the right above the O atom after adsorption. The adsorption energy obtained on O-top −74.233 Kcal/mol is much higher than the other two sites, −73.769 Kcal/mol and −73.235 Kcal/mol, indicating that the O-top position on the surface of CaO (100) requires less energy and is easier to reaches a stable structure easier when SO2 is adsorbed. Therefore, the above findings reveal that the O-top position is the best adsorption position for SO2 on the CaO (100) surface. They also show that SO2 is chemisorption at these three sites. The length of the S-O bond on the surface of CaO (100) with O-top as the adsorption site is shortened from 1.717 Å to 1.525 Å, which is consistent with the simulation result 1.50 Å of Wang et al. [46]. Moreover, the bong angle augments from 92.109° to 112.366°. And the structure of SO2 adsorbed on the surface of CaO (100) is similar to that of SO32−, which indicates that SO2 is adsorbed on the surface of CaO (100) in the form of sulfite by chemisorption.
Furthermore, the stability of the adsorption configuration at different adsorption sites is analyzed in terms of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) (ΔE = − ) [28]. By comparing the ΔE data in Figure 11e–h, it is found that the stability is ranked in order from largest to smallest: O-top > bridge > hollow > Ca-top. The result further supports the view that the O-top position on the surface of CaO (100) is the optimal adsorption site, and the adsorption configuration obtained is the most suitable and stable. The electron transfer between S and O-top atoms enhances the chemical bond formation and reactivity of the surface. After calculation analysis, four sites obtain a similar electron transfer, according to population analysis: O-top 0.277 e, bridge 0.286 e, hollow 0.265 e and Ca-top 0.257 Spaces exist and have been added, and the S atom obtains electrons. The above DFT calculation results presented the adsorption mechanism of SO2 on the surface of CaO during the sulfur fixation of calcium.
5. Conclusions
In order to reduce the sulfur content in high-sulfur iron ore, improving the efficiency of subsequent blast furnace ironmaking, the release behavior and fixation of sulfur under the oxygen roasting process is investigated. The roasting temperature, roasting time and sulfur dosage have obvious effects on the sulfur migration and release of FeS2. Experimental results reveal that as the roasting temperature increases, the release rate of SO2 also increases, reaching the maximum release rate at 900 °C. However, the total amount of release does not continue to increase with rising roasting temperatures, reaching a maximum at 800 °C. Meanwhile, it is found that at the same roasting temperature, the release rate of SO2 under the O2/N2 roasting atmosphere is significantly greater than that under the pure N2 and air atmospheres. The maximum volume fraction of SO2 increases with the increase in oxygen concentration, but an excessive oxygen concentration prevents the release of SO2. And the mechanism of sulfur migration and release for the high-sulfur iron ore roasting system is determined using XRD. Fe2O3 and Fe3O4 are the main final oxidation products, and no sulfate is detected throughout the process. Finally, the sulfur fixation reaction is studied: when the CaO concentration reaches 10%, 80% sulfur fixation efficiency can be obtained. And O-top is determined as the best adsorption activity site of SO2 on the CaO surface, according to the adsorption energy and stability calculated by the DFT theory. Moreover, the electron transfer is calculated as 0.277 e. This research will provide basic knowledge and guidance for the sulfur release of high-sulfur iron ore under the oxidizing roasting process, and enrich the theoretical system of sulfide roasting.
Author Contributions
X.C.: Validation, Formal Analysis, Data Curation, Writing—Original Draft, Review and Editing, Funding Acquisition; N.Z.: Methodology, Validation, Formal Analysis; Z.L.: Project Administration, Funding Acquisition; Z.W.: Formal Analysis, Investigation, Resources, Data Curation. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the National Natural Science Foundation of China (52104247) and the fund of the Educational Department of Liaoning Province of China (LJKZ0257).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the confidentiality required by the partner companies. OR Data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, X.J.; Wang, Y.Y.; Hu, X.M. Novel strategy of using a C/C electrodes electro-activated peroxymonosulfate to remove NO from simulated flue gas. Sep. Purif. Technol. 2021, 257, 117859. [Google Scholar] [CrossRef]
- Galina, N.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávila, I. Comparative study on combustion and oxy-fuel combustion environments using mixtures of coal with sugarcane bagasse and biomass sorghum bagasse by the thermogravimetric analysis. J. Energy Inst. 2019, 92, 741–754. [Google Scholar] [CrossRef]
- John, J.; Evans, C.; Johnson, B. Nitric acid treatment of a pyrite rougher concentrate to oxidise differentially the pyrite types and modify the Au:S ratio in a resulting cleaner concentrate. Miner. Eng. 2023, 202, 108270. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X. Rb-Sr, Sm-Nd, and Pb isotope systematics of pyrite: Implications for the age genesis of lode gold deposits. Geology 2001, 29, 711–714. [Google Scholar] [CrossRef]
- Dyni, J.R. Geology and Resources of Some World Oil-Shale Deposits; US Geological Survey: Reston, VA, USA, 2006; pp. 68–72.
- Lin, S.D.; Gao, L.; Yang, Y.; Chen, J. Dielectric properties and high temperature thermochemical properties of the pyrolusite-pyrite mixture during reduction roasting. J. Mater. Res. Technol. 2020, 9, 13128–13136. [Google Scholar] [CrossRef]
- Song, W.; Zhou, J.; Wang, B.; Li, S.; Han, J. New insight into investigation of reduction of desulfurization ash by pyrite for clean generation SO2. J. Clean. Prod. 2020, 253, 120026. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.M.; Ma, Y.C.; You, C.F. Sulfur fixation for raw coal with combined microwave irradiation and ultrafine Ca(OH)2 method. Fuel 2022, 330, 125570. [Google Scholar] [CrossRef]
- Makgato, S.S.; Chirwa, E.M.N. Recent developments in reduction of sulfur emissions from selected Waterberg coal samples used in South African power plants. J. Clean. Prod. 2020, 276, 123192. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Yang, H.; Lv, J. Oxidation mechanism of pyrite concentrates (PCs) under typical circulating fluidized bed (CFB)roasting condition sanded sign principles of PCs’CFB roaster. Chem. Eng. Process. Process Intensif. 2020, 153, 107944. [Google Scholar] [CrossRef]
- MEE (Ministry of Ecology and Environment of People’s Republic of China). Guideline on Available Technologies of Pollution Prevention and Control for Thermal Power Plant; Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2017. (In Chinese)
- NDRC (National Development and Reform Commission of People’s Republic of China). Upgrade and Retroft Plan for Coal-Fired Power Plants Aiming at Energy Savings and Emissions Reduction for 2014–2020; National Development and Reform Commission of People’s Republic of China: Beijing, China, 2014. (In Chinese)
- Paula, L.A.; Javier, G.G.; Fernando, G. Chemistry and phase evolution during roasting of toxic thallium-bearing pyrite. Chemosphere 2017, 181, 447–460. [Google Scholar]
- Yu, Y.; Li, L.; Wang, J.Y.; Li, K.Z.; Wang, H. Phase transformation of Sn in tin-bearing iron concentrates by roasting with FeS2 in CO-CO2 mixed gases and its effect son Sn separation. J. Alloys Compd. 2018, 750, 8–16. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, Y.; Guo, W.C.; Li, N.; Wang, Z.F. Phase transition and magnetic properties of low-grade limonite during reductive roasting. Vacuum 2019, 167, 163–174. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, G.; Lv, B. A novel high-sulfur fine coal clean desulfurization pretreatment: Microwave magnetic separation, high-gradient effect and magnetic strengthen. J. Clean. Prod. 2018, 202, 697–709. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Y.; Zhou, C. Fine coal desulfurization by magnetic separation and the behavior of sulfur component response in microwave energy pretreatment. Energy Fuels 2015, 29, 1243–1248. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Jing, B. Comprehensive recovery of lead, zinc, and iron from hazardous jarosite residues using direct reduction followed by magnetic separation. Int. J. Miner. Metall. Mater. 2018, 25, 123–130. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, W. Study on recovery of lead, zinc, and iron from jarosite residues and simultaneous sulfur fixation by direct reduction. Physicochem. Probl. Miner. Process. 2018, 54, 517–526. [Google Scholar]
- Thauan, G.; da Rafael, R.; Maykon, C. Pyrite roasting in modified fluidized bed: Experimental and modeling analysis. Chem. Eng. Sci. 2022, 261, 117977. [Google Scholar]
- Tosun, Y.I. Clean fuel-magnesia bonded coal briquetting. Fuel Process. Technol. 2007, 88, 977–981. [Google Scholar] [CrossRef]
- Li, J.; Fan, B.G.; Huo, R.P. Study on quenching hydration reaction kinetics and desulfurization characteristics of magnesium slag. J. Clean. Prod. 2018, 190, 12–23. [Google Scholar]
- Luo, C.; Zheng, Y.; Xu, Y. Wet mixing combustion synthesis of CaO-based sorbents for high temperature cyclic CO2 capture. Chem. Eng. J. 2015, 267, 111–116. [Google Scholar] [CrossRef]
- Zhang, D.K. Interactions between sodium, silica and sulfur in a low-rank coal during temperature-programmed pyrolysis. J. Fuel Chem. Technol. 2005, 33, 513–519. [Google Scholar]
- Wang, S.; Fan, S.; Fan, L. Effect of cerium oxide doping on the performance of CaO-based sorbents during calcium looping cycle. Environ. Sci. Technol. 2015, 49, 5021–5027. [Google Scholar] [CrossRef]
- Pan, F.; Ji, H.; Du, P.; Huang, T.; Wang, C.; Liu, W. Insights into catalytic activation of peroxymonosulfate for carbamazepine degradation by MnO2 nanoparticles in-situ anchored titanate nanotubes: Mechanism, ecotoxicity and DFT study. J. Hazard. Mater. 2021, 402, 123779. [Google Scholar] [CrossRef]
- Ma, M.; Chen, L.; Zhao, J.; Liu, W.; Ji, H. Efficient activation of peroxymonosulfate by hollow cobalt hydroxide for degradation of ibuprofen and theoretical study. Chin. Chem. Lett. 2019, 30, 2191–2195. [Google Scholar] [CrossRef]
- Li, X.R.; Yan, P.W.; Li, L.N.; Chen, Y.; Chen, Z.L. Activation of peroxymonosulfate by natural pyrite for effective degradation of 2,4,6-trichlorophenol in water: Efficiency, degradation mechanism and toxicity evaluation. Sep. Purif. Technol. 2023, 322, 124253. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.M.; Cao, Q.Z. Density functional theory studies on the adsorption of CO2 on different CaO surfaces. Chin. J. Struct. Chem. 2013, 32, 1715–1723. [Google Scholar]
- Feng, Z.Y.; Chen, N.; Liu, T.; Feng, C.P. KHCO3 activated biochar supporting MgO for Pb (II) and Cd(II) adsorption 3 from water: Experimental study and DFT calculation analysis. J. Hazard. Mater. 2022, 426, 128059. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Liu, Q.; Yang, H. Experimental studies on phase transformation during pyrite concentrate oxidation under circulating fluidized bed (CFB) roasting conditions. Ind. Eng. Chem. Res. 2011, 50, 14168–14174. [Google Scholar] [CrossRef]
- Panneerselvam, M.; Rao, K. Novel microwave method for the synthesis and sintering of mullite from kaolinite. Chem. Mater. 2003, 15, 2247–2252. [Google Scholar] [CrossRef]
- Ebadzadeh, T. Effect of mechanical activation and microwave heating on synthesis and sintering of nano-structured mullite. J. Alloys Compd. 2010, 489, 125–129. [Google Scholar] [CrossRef]
- Arnold, R.G. Mixtures of hexagonal and monoclinic pyrrhotite and the measurement of the metal content of pyrrhotite by X-ray diffraction. Am. Mineral. 1966, 51, 1221–1227. [Google Scholar]
- Wu, J.; Yu, D.; Zeng, X.; Yu, X. Impacts of CO2 on the pyrite–kaolinite interaction and the product sintering strength, Proceedings of the Combustion Institute. Proc. Combust. Inst. 2019, 37, 4479–4486. [Google Scholar] [CrossRef]
- Srinivasachar, S.; Boni, A.A. A kinetic model for pyrite transformations in a combustion environment. Fuel 1989, 68, 829–836. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, L.; Zhang, Z. Iron transformation and ash fusibility during coal combustion in air and O2/CO2 medium. Energy Fuels 2012, 26, 3150–3155. [Google Scholar] [CrossRef]
- Li, Y.; Gupta, R.; Wall, T. Fragmentation behavior of pyrite and calcite during high-temperature processing and mathematical simulation. Energy Fuels 2001, 15, 389–394. [Google Scholar]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Sydorovych, Y.Y.E.; Gaivanovych, V.; Martynets, E. Desulfurization of Donetsk Basin coals by air-steam mixture. Fuel 1996, 75, 78–80. [Google Scholar] [CrossRef]
- Pysh’yev, S.; Shevchuk, K.; Chmielarz, L. Effect of the water-vapor content on the oxidation desulfurization of sulfur-rich coal. Energy Fuels 2007, 21, 216–221. [Google Scholar] [CrossRef]
- Pysh’Yev, S.; Gunka, V. Study of the oxidation desulfurization process of coal with different metamorphism degrees. J. Fuel Chem. Technol. 2012, 40, 129–137. [Google Scholar] [CrossRef]
- Peng, Z.W.; Mourisj, H. Microwave Absorption characteristics of conventionally heated nonstoichiometric ferrous oxide. Metall. Land Mater. Trans. A 2001, 42, 2259–2263. [Google Scholar] [CrossRef]
- Duo, W.; Laursen, K.; Lim, J. Crystallization and fracture: Product layer diffusion in sulfation of calcined limestone. Ind. Eng. Chem. Res. 2004, 43, 5653–5662. [Google Scholar] [CrossRef]
- Laursen, K.; Duo, W.; Grace, J.R. Sulfation and reactivation characteristics of nine limestones. Fuel 2000, 79, 153–163. [Google Scholar] [CrossRef]
- Wang, W.J.; Fan, L.L.; Wang, G.P.; Li, Y.H. CO2 and SO2 sorption on the alkali metals doped CaO (100) surface: A DFT-D study. Appl. Surf. Sci. 2017, 425, 972–977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).