Interactions of Acetylene-Derived Thioester Collectors with Gold Surfaces: A First-Principles Study
Abstract
1. Introduction
2. Methods
2.1. Model and Parameters
2.2. Adsorption Energy Calculation
2.3. Adsorption Experiments
3. Results and Discussion
3.1. Structure and Mulliken Population
3.2. Frontier Orbital Analysis
3.3. Adsorption Energy Comparison
3.4. Density of States Analysis
3.5. Electron Density Difference
3.6. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Ahn, J.; Lee, J. Gold deportment and leaching study from a pressure oxidation residue of chalcopyrite concentrate. Hydrometallurgy 2021, 201, 105583. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, S.H.; Song, X.L.; Pan, H.D. Gold occurrence of Jiaojia gold mine in Shandong province. Trans. Nonferrous Met. Soc. China 2011, 21, 2072–2077. [Google Scholar] [CrossRef]
- Arif, J.; Baker, T. Gold paragenesis and chemistry at Batu Hijau, Indoneisa: Implications for gold-rich porphyry copper deposits. Miner. Depos. 2004, 39, 523–535. [Google Scholar] [CrossRef]
- Asamoah, R.K. Specific refractory gold flotation and bio-oxidation products: Research overview. Minerals 2021, 11, 93. [Google Scholar] [CrossRef]
- Tagirov, B.R.; Filimonova, O.N.; Trigub, A.L.; Vikentyev, I.V.; Kovalchuk, E.V.; Nickolsky, M.S.; Chareev, D.A. The state of gold in phases of the Cu-Fe-S system: In situ X-ray absorption spectroscopy study. Geosci. Front. 2023, 14, 101533. [Google Scholar] [CrossRef]
- El-Sayed, S.; El-Shatoury, E.H.; Abdel-Khalek, N.A.; Abdel-Motelib, A.; Abdel-Khalek, M.A. Influence of Bacillus cereus-gold interaction on bio-flotation of gold in the presence of potassium butyl xanthate. Biointerface Res. Appl. Chem. 2021, 11, 13005–13018. [Google Scholar]
- Wang, S.; Zhang, L.; Lu, D.; Fu, Y. Identification of abnormal conditions for gold flotation process based on multivariate information fusion and double-channel convolutional neural network. Can. J. Chem. Eng. 2023, 101, 4523–4538. [Google Scholar] [CrossRef]
- Akop, C. Developing a Bulk Circuit Suitable for Chalcopyrite-Pyrite Ores with Elevated Pyrite Content in Copper-Gold Ore Treatment. Master’s Thesis, Sustainable Minerals Institute, The University of Queensland, Brisbane, QLD, Australia, 2014. [Google Scholar]
- Bas, A.D.; Larachi, F. The effect of flotation collectors on the electrochemical dissolution of gold during cyanidation. Miner. Eng. 2019, 130, 48–56. [Google Scholar] [CrossRef]
- Matveeva, T.N.; Gromova, N.K.; Lantsova, L.B. Experimental Proof of Applicability of Cyclic and Aliphatic Dithiocarbamate Collectors in Gold-Bearing Sulphide Recovery from Complex Ore. J. Min. Sci. 2021, 57, 123–130. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Oluwabunmi, K.E.; Adeleke, A.A.; Adetunji, A.R.; Jeje, S.O.; Abioye, A.A.; Adesina, O.A.; Ibitoye, F.P. 2 k Factorial Experiments on Factors that Influence the Recovery of Gold during the Upgrade of Ilesha-Itagunmodi Gold Ore through Froth Flotation. J. Miner. Mater. Charact. Eng. 2014, 2, 32–39. [Google Scholar]
- Tan, L.; Lin, Q.; Liu, P.; Fu, L. Studies on the flotation separation of a new thionocarbamate—ZL 4020. Min. Metall. Eng. 1996, 16, 26–29. [Google Scholar]
- Beattie, D.A.; Kempson, I.M.; Fan, L.J.; Skinner, W.M. Synchrotron XPS studies of collector adsorption and co-adsorption on gold and gold: Silver alloy surfaces. Int. J. Miner. Process. 2009, 92, 162–168. [Google Scholar] [CrossRef]
- Zhu, Y.M. Progress in Flotation Reagents Research in 2023. Nonferrous Metals (Mineral Processing Section); 2024, 1–43. Available online: http://118.89.52.29:8085/kcms/detail/11.1840.TF.20240131.1409.002.html (accessed on 23 February 2024).
- Ha, C.S.; Nagappan, S. Hydrophobic and Superhydrophobic Organic-Inorganic Nano-Hybrids; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Nosáľová, L.; Maliničová, L.; Kisková, J.; Timková, I.; Sedláková-Kaduková, J.; Pristaš, P. Cultivable microbiota associated with gold ore from the rozália gold mine, hodruša-hámre, Slovakia. Geomicrobiol. J. 2021, 38, 415–425. [Google Scholar] [CrossRef]
- Burdonov, A.; Vchislo, N.; Barakhtenko, V.; Sahabutdinova, T. Synthesized collectors flotation activity study based on fluorine containing and acetylene alcohols. Sustain. Dev. Mt. Territ. 2023, 15, 707–719. [Google Scholar] [CrossRef]
- Yushina, T.I. Justification of applying collectors from the class of unsaturated tertiary alcohols in flotation of gold-bearing sulphide ores. Non-ferrous Met. 2022, 2022, 3–10. [Google Scholar] [CrossRef]
- Merrill, M.D. First principles of instruction. Educ. Technol. Res. Dev. 2002, 50, 43–59. [Google Scholar] [CrossRef]
- Freysoldt, C.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Kresse, G.; Janotti, A.; Van de Walle, C.G. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86, 253. [Google Scholar] [CrossRef]
- Liu, W.; Miller, J.D.; Sun, W.; Hu, Y. Analysis of the selective flotation of elemental gold from pyrite using diisobutyl monothiophosphate. Minerals 2022, 12, 1310. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Zhong, H.; Zhang, B.; Liu, G.; Yang, Y. Density functional theory study on electronic structure of tetrahedrite and effect of natural impurities on its flotation property. Miner. Eng. 2021, 169, 106980. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Chauke, H.R.; Ngoepe, P.E. The effect of thiol collectors on nickel-rich (110) pentlandite surface using density functional theory. In Proceedings of the SAIP2017, the 62nd Annual Conference of the South African Institute of Physics, Stellenbosch, South Africa, 3–7 July 2017; pp. 95–100. [Google Scholar]
- Yuan, M.; Feng, X.; Yan, T.H.; Chen, J.; Ma, X.; Cunha, P.; Wang, Y. Superparamagnetic iron oxide-enclosed hollow gold nanostructure with tunable surface plasmon resonances to promote near-infrared photothermal conversion. Adv. Compos. Hybrid Mater. 2022, 5, 2387–2398. [Google Scholar] [CrossRef]
- Nenchev, G.; Diaconescu, B.; Hagelberg, F.; Pohl, K. Self-assembly of methanethiol on the reconstructed Au (111) surface. Phys. Rev. B 2009, 80, 081401. [Google Scholar] [CrossRef]
- He, J.; Sun, W.; Chen, D.; Gao, Z.; Zhang, C. Interface interaction of benzohydroxamic acid with lead ions on oxide mineral surfaces: A coordination mechanism study. Langmuir 2021, 37, 3490–3499. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Ren, Z.; Zheng, R.; Gao, H.; Chen, Z. The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates. Minerals 2023, 13, 1005. [Google Scholar] [CrossRef]
- Xu, B.; Wu, J.; Dong, Z.; Tao, J.; Qian, L.; Yang, Y. Flotation performance, structure–activity relationship and adsorption mechanism of a newly-synthesized collector for copper sulfide minerals in Gacun polymetallic ore. Appl. Surf. Sci. 2021, 551, 149420. [Google Scholar] [CrossRef]
- Cao, S.; Cao, Y.; Liao, Y.; Ma, Z. Depression mechanism of strontium ions in bastnaesite flotation with salicylhydroxamic acid as collector. Minerals 2018, 8, 66. [Google Scholar] [CrossRef]
- Corso, M.; Fernández, L.; Schiller, F.; Ortega, J.E. Au (111)-based nanotemplates by Gd alloying. ACS Nano 2010, 4, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, B.; Ao, Z.; An, T.; Wang, G. Atomic-scale identification of influencing factors of sodium dendrite growth on different current collectors. J. Mater. Chem. A 2020, 8, 10199–10205. [Google Scholar] [CrossRef]
- Loffreda, D. Theoretical insight of adsorption thermodynamics of multifunctional molecules on metal surfaces. Surf. Sci. 2006, 600, 2103–2112. [Google Scholar] [CrossRef]
- Sergiievskyi, V.P.; Jeanmairet, G.; Levesque, M.; Borgis, D. Fast computation of solvation free energies with molecular density functional theory: Thermodynamic-ensemble partial molar volume corrections. J. Phys. Chem. Lett. 2014, 5, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Amsler, J.; Plessow, P.N.; Studt, F.; Bucko, T. Anharmonic correction to adsorption free energy from DFT-based MD using thermodynamic integration. J. Chem. Theory Comput. 2021, 17, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Shen, Y.; Gao, H.; Chen, H.; Liu, C.; Chen, Z. Comparison of Sodium Oleate and Sodium Petroleum Sulfonate for Low-Temperature Flotation of Fluorite and the Collecting Mechanisms. Min. Met. Explor. 2021, 38, 2527–2536. [Google Scholar] [CrossRef]
- Baldridge, K.; Klamt, A. First principles implementation of solvent effects without outlying charge error. J. Chem. Phys. 1997, 106, 6622–6633. [Google Scholar] [CrossRef]
- Sure, R.; Brandenburg, J.G.; Grimme, S. Small atomic orbital basis set first-principles quantum chemical methods for large molecular and periodic systems: A critical analysis of error sources. ChemistryOpen 2016, 5, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S. Biotechnological avenues in mineral processing: Fundamentals, applications and advances in bioleaching and bio-beneficiation. Miner. Process. Extr. Metall. Rev. 2023, 44, 22–51. [Google Scholar] [CrossRef]
- Nie, Y.M.; Liu, S.X.; Dai, Q.H. Rough Flotation Research of Low Grade Gold Ore. Appl. Mech. Mater. 2014, 543, 3822–3825. [Google Scholar] [CrossRef]
- Janštová, S.; Janáková, I.; Čablík, V. Leaching of Gold from Fine-grained Flotation Tailings. GeoScience Eng. 2020, 66, 117–120. [Google Scholar] [CrossRef]
- Toktar, G.; Bakrayeva, A.; Abdyldayev, N.; Banks, G.E.; Kubaizhanov, A. Increase in the Free Finely-Dispersed Gold Recovery in the Flotation Cycle. J. Ecol. Eng. 2023, 24, 115–119. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Wang, F.L. Adsorption Selectivity of Fatty Acid Collector by Synthetic Magnetite. Appl. Mech. Mater. 2014, 552, 263–268. [Google Scholar] [CrossRef]
- Smart, R.S.; Amarantidis, J.; Skinner, W.M.; Prestidge, C.A.; La Vanier, L.; Grano, S.R. Surface Analytical Studies of Oxidation and Collector Adsorption in Sulfide Mineral Flotation; Springer: Berlin/Heidelberg, Germany, 2003; pp. 3–62. [Google Scholar]
- Ge, Y.Y.; Huang, L.; Xiong, X.H.; Yu, Y.F. Mechanism of a new collector alkyl polyamine ether adsorption on jasper and magnetite. J. Cent. South Univ. (Sci. Technol.) 2014, 45, 1377–1383. [Google Scholar]
- Terzi, M.; Kursun, I.; Cinar, M.; Ozdemir, O. Digital image processing (DIP) application on the evaluation of ironrich heavy mineral concentrates produced from river sand using a sequential mineral processing approach. Physicochem. Probl. Miner. Process. 2021, 57, 21–35. [Google Scholar] [CrossRef]
- Yang, K.; Lu, X.C.; Liu, X.D.; Hou, Q.F. Characterization technique ⅱ of mineral material based on probe gas adsorption isotherm: Nano-pore structure of porous material. Bull. Mineral. Petrol. Geochem. 2006, 25, 362–368. [Google Scholar]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. J. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [PubMed]
- Zierhut, A.; Leopold, K.; Harwardt, L.; Worsfold, P.; Schuster, M. Activated gold surfaces for the direct preconcentration of mercury species from natural waters. J. Anal. At. Spectrom. 2009, 24, 767–774. [Google Scholar] [CrossRef]

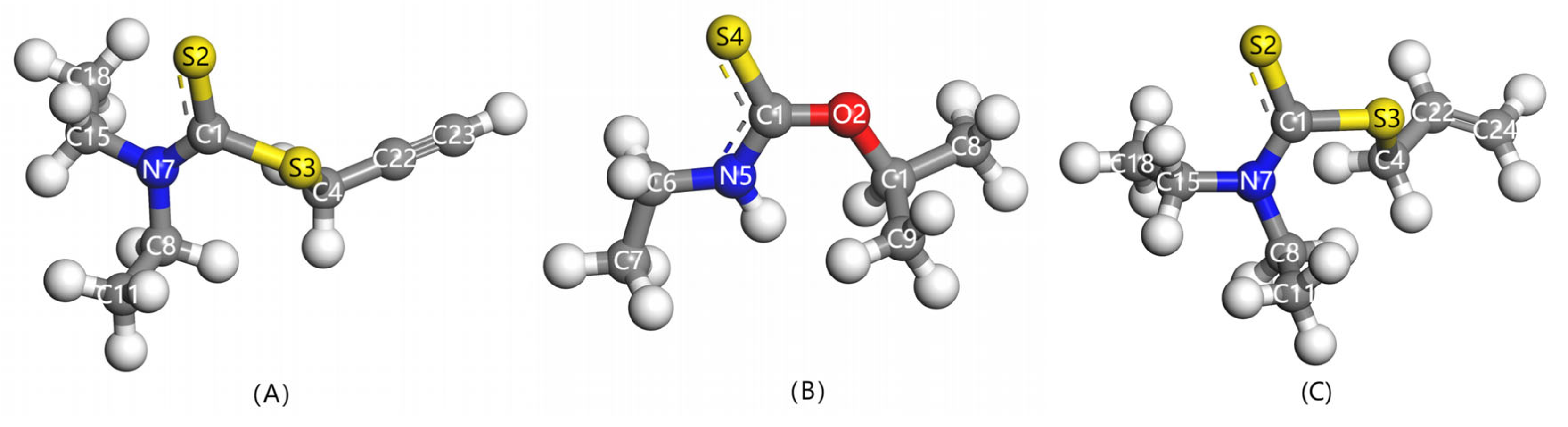


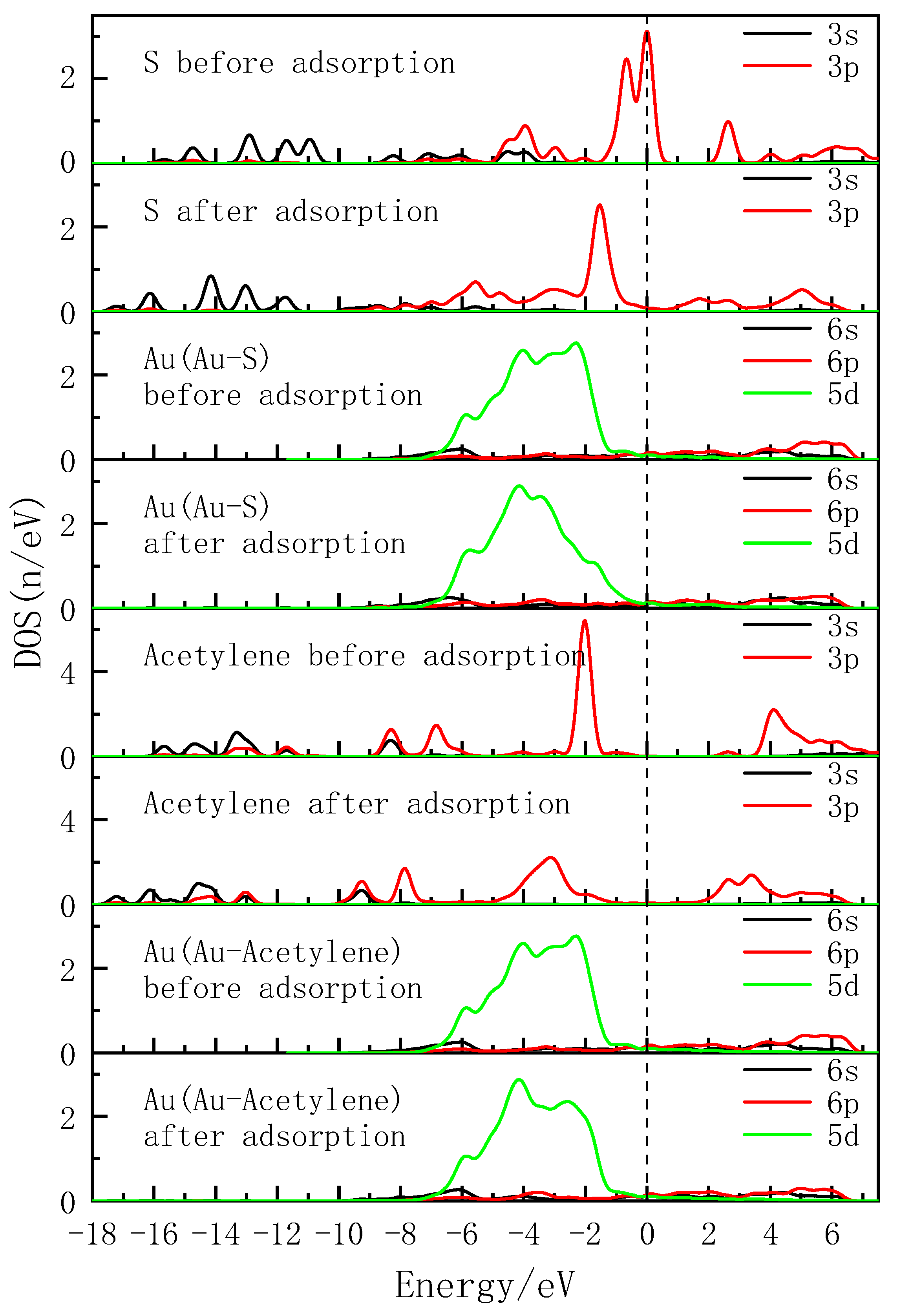
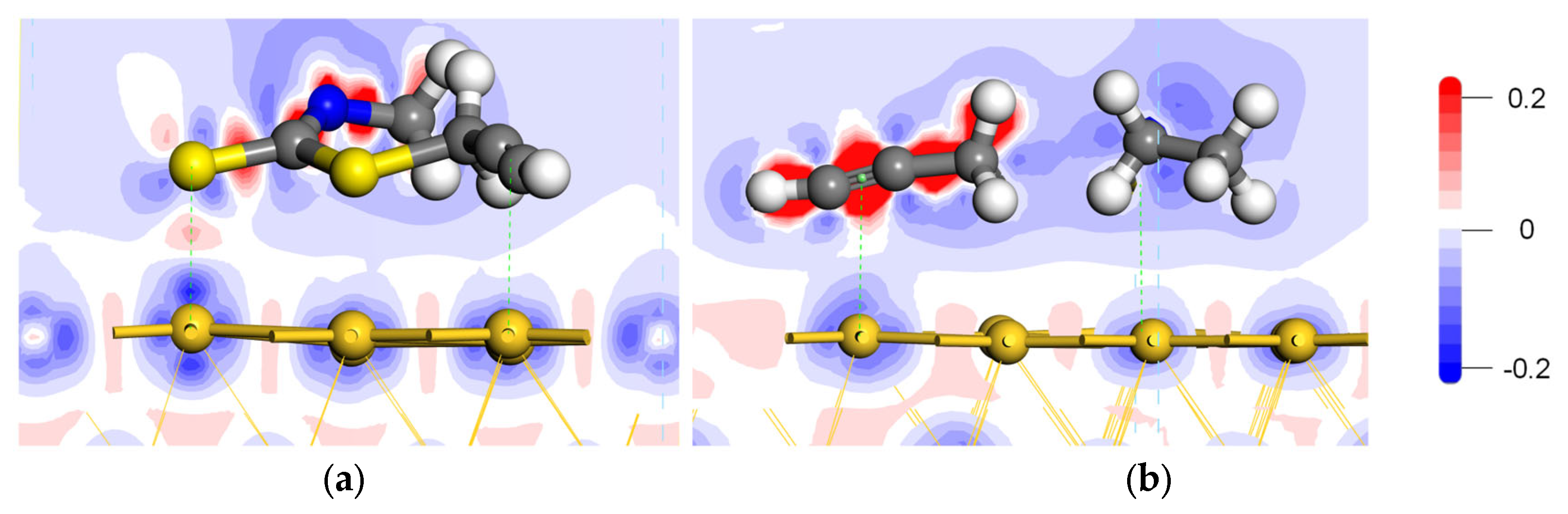

| Collectors | Bond Length (Å) | Mulliken Population of Bond (e) | Mulliken Population of Atom Charge (e) | ||||
|---|---|---|---|---|---|---|---|
| PDEC | C1-S2 | C1-S2 | S2 | C1 | N7 | S3 | C23 |
| 1.669 | 0.90 | −0.14 | −0.17 | −0.27 | 0.23 | −0.38 | |
| Z-200 | C3-S4 | C3-S4 | S4 | C3 | N5 | O2 | |
| 1.651 | 0.94 | −0.19 | 0.22 | −0.55 | −0.41 | ||
| Al-DECDT | C1-S2 | C1-S2 | S2 | C1 | N7 | S3 | C24 |
| 1.664 | 0.90 | −0.15 | −0.17 | −0.26 | 0.18 | −0.62 | |
| Collectors | Frontline Orbital Energy (eV) | ||
|---|---|---|---|
| HOMO | LUMO | LUMO + 1 | |
| PDEC | −4.708 | −2.077 | −0.608 |
| Z-200 | −4.515 | −0.812 | 0.510 |
| Al-DECDT | −4.456 | −1.942 | −0.895 |
| Collectors | Atoms | fw+ (e) | fw− (e) |
|---|---|---|---|
| PDEC | S2 | 0.280 | 0.421 |
| C1 | 0.079 | 0.017 | |
| N7 | 0.039 | 0.026 | |
| S3 | 0.158 | 0.154 | |
| C23 | 0.102 | 0.081 | |
| Z-200 | S4 | 0.365 | 0.572 |
| C3 | 0.154 | 0.078 | |
| N5 | 0.052 | 0.028 | |
| O2 | 0.064 | 0.013 | |
| Al-DECDT | S2 | 0.266 | 0.414 |
| C1 | 0.068 | 0.019 | |
| N7 | 0.040 | 0.026 | |
| S3 | 0.138 | 0.161 | |
| C24 | 0.065 | 0.034 |
| Collectors | Adsorption Energy (KJ/ mol) | S-Au Bond Length (Å) |
|---|---|---|
| PDEC | −71.46259119 | 2.580 |
| Z-200 | −58.05373004 | 2.521 |
| Al-DECDT | −59.43253585 | 2.557 |
| Atomic Label | Adsorption Status | s | p | d | Charge (e) |
|---|---|---|---|---|---|
| S | Before adsorption | 1.83 | 4.31 | 0.00 | −0.14 |
| After adsorption | 1.83 | 4.17 | 0.00 | 0.00 | |
| Au(Au-S) | Before adsorption | 0.91 | 0.53 | 9.65 | −0.09 |
| After adsorption | 0.85 | 0.72 | 9.62 | −0.18 | |
| C | Before adsorption | 1.15 | 3.23 | 0.00 | −0.38 |
| After adsorption | 1.17 | 3.19 | 0.00 | −0.36 | |
| Au(Au-C) | Before adsorption | 0.91 | 0.53 | 9.65 | −0.09 |
| After adsorption | 0.83 | 0.46 | 9.63 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Qi, Y.; Wei, D.; Zhang, F.; Wang, C. Interactions of Acetylene-Derived Thioester Collectors with Gold Surfaces: A First-Principles Study. Minerals 2024, 14, 238. https://doi.org/10.3390/min14030238
Qiu X, Qi Y, Wei D, Zhang F, Wang C. Interactions of Acetylene-Derived Thioester Collectors with Gold Surfaces: A First-Principles Study. Minerals. 2024; 14(3):238. https://doi.org/10.3390/min14030238
Chicago/Turabian StyleQiu, Xianyang, Yuechao Qi, Dezhou Wei, Faming Zhang, and Chenghang Wang. 2024. "Interactions of Acetylene-Derived Thioester Collectors with Gold Surfaces: A First-Principles Study" Minerals 14, no. 3: 238. https://doi.org/10.3390/min14030238
APA StyleQiu, X., Qi, Y., Wei, D., Zhang, F., & Wang, C. (2024). Interactions of Acetylene-Derived Thioester Collectors with Gold Surfaces: A First-Principles Study. Minerals, 14(3), 238. https://doi.org/10.3390/min14030238





