Revealing Juan de Oviedo y de la Bandera’s Artworks: The Case of the Polychrome of a Stone-Carved Sculpture from the Madre de Dios Convent Façade in Seville
Abstract
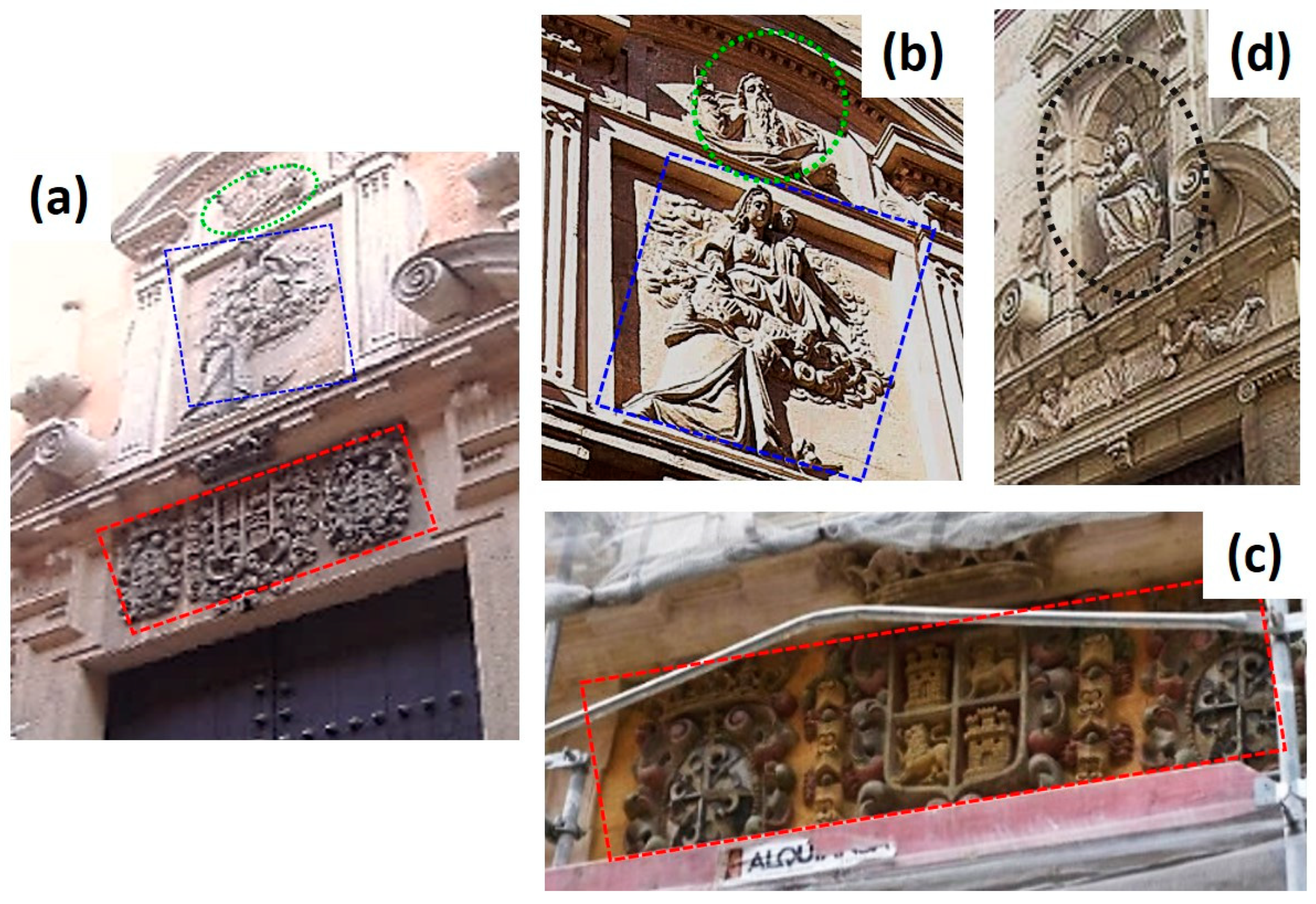
1. Introduction
2. Materials and Methods
3. Results and Discussion
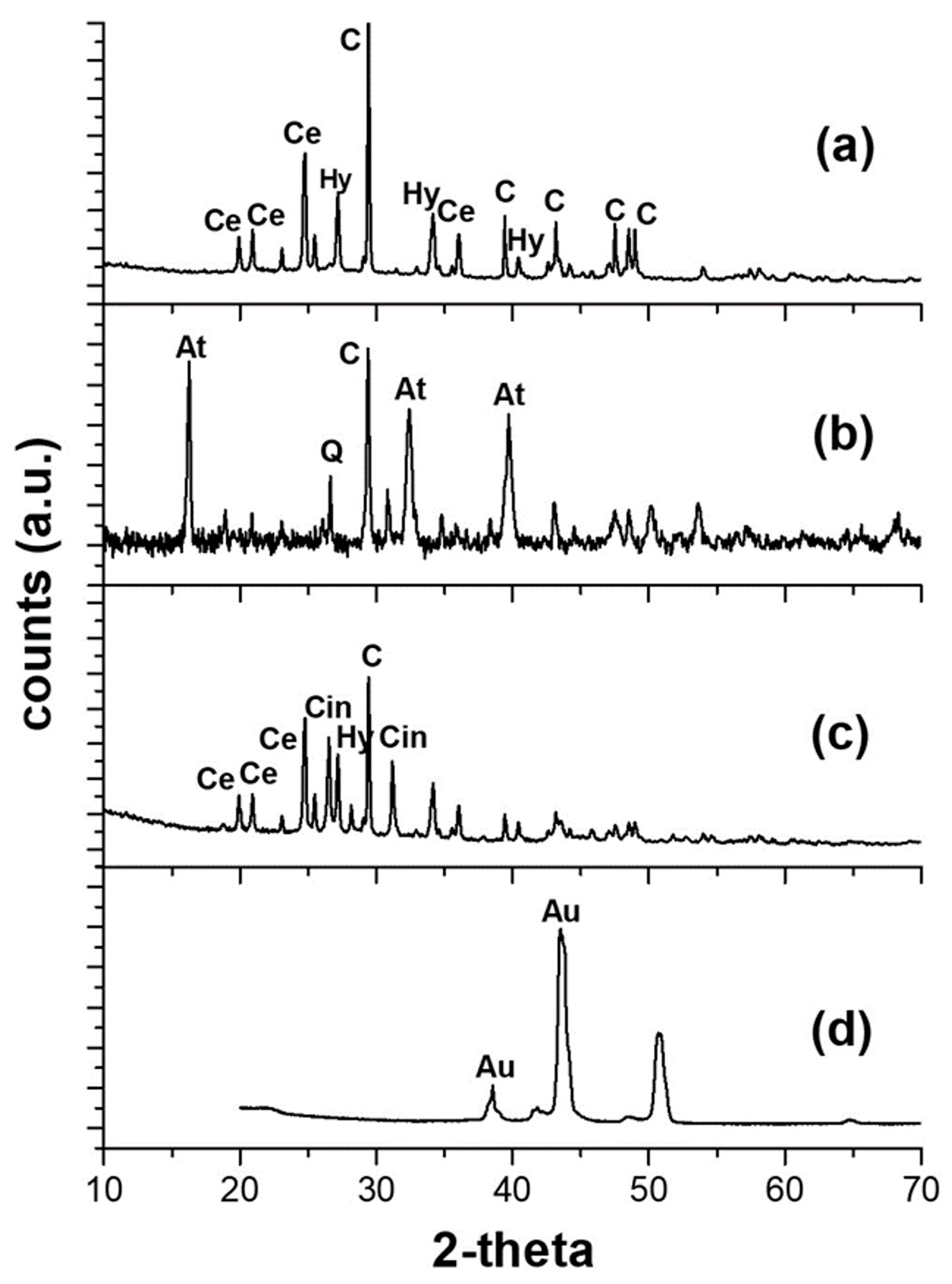
3.1. Consolidation Products
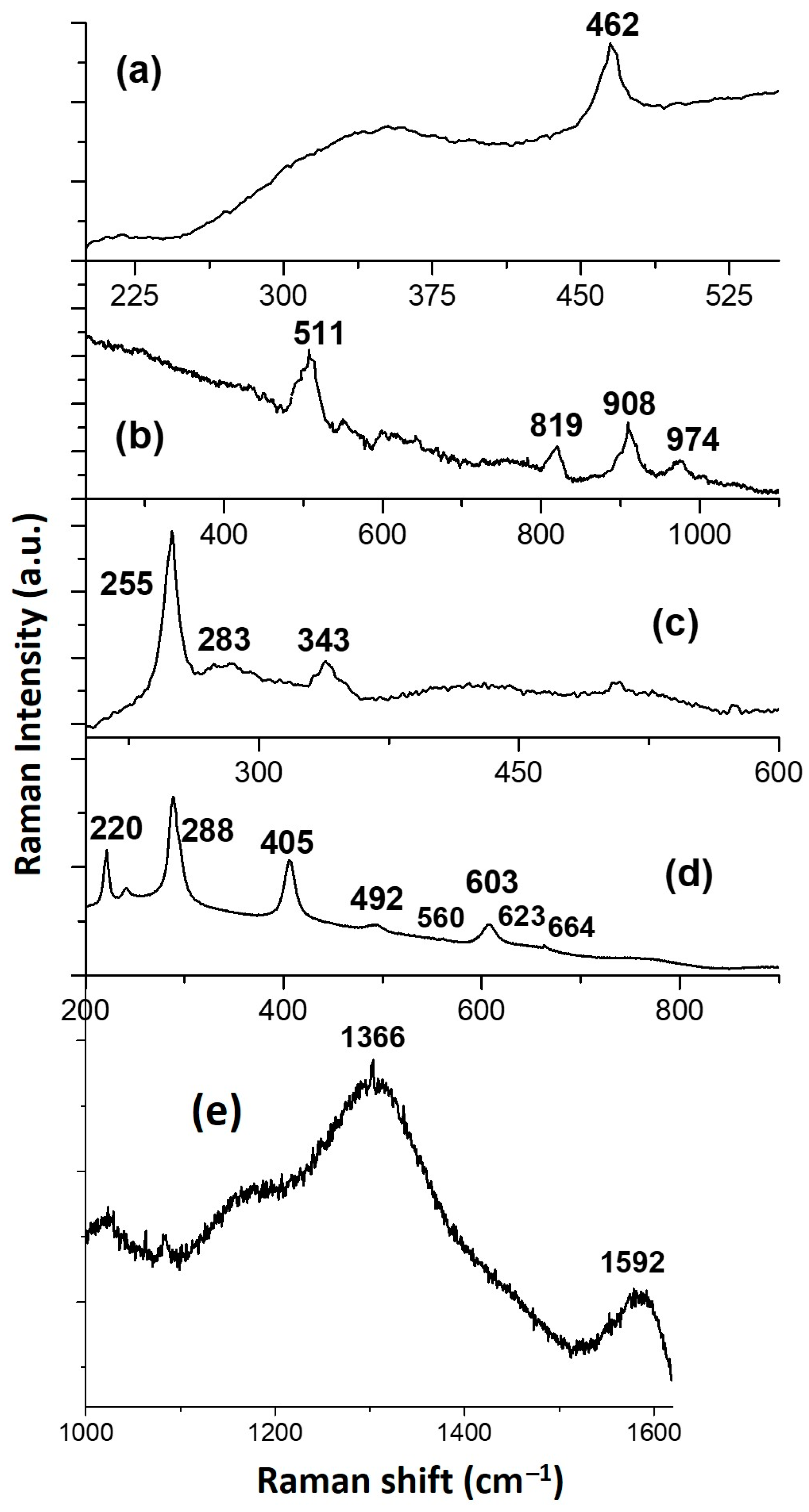
3.2. Study of the Polychrome and Preparatory Layers
3.2.1. Blue Colour
3.2.2. Green Colour
Sample 10
Sample 4
Sample 5
3.2.3. Turquoise Colour
3.2.4. Red Colour
Samples 3 and 2
Sample 11
3.2.5. Yellow Colour
3.2.6. Black Colour
3.2.7. Golden Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roldán, M.J. Iglesias de Sevilla; Almuzara: Córdoba, Spain, 2011; p. 336. [Google Scholar]
- Sevillaxm2. Convento Madre de Dios. Guía de Sevilla por Metro Cuadrado. Available online: https://sevillaxm2.com/convento-madre-de-dios/ (accessed on 13 February 2024).
- Vallejo, C. Juan de Oviedo y de la Bandera. Identidad e Imagen de Andalucía en la Edad Moderna. Área de Comunicaciones (Servicio de Informática). University of Almeria. 2016. Available online: https://www2.ual.es/ideimand/juan-de-oviedo-y-de-la-bandera-escultor-1565-1625/ (accessed on 22 January 2024).
- López, C. El Escultor y Arquitecto Juan de Oviedo y de la Bandera, 1565-Discurso de Ingreso en la Real Academia Sevillana de Bellas Artes de Santa Isabel de Hungría; Imprenta San Antonio: Sevilla, Spain, 1943. [Google Scholar]
- Pérez-Escolano, V. Juan de Oviedo y de la Bandera. In Artistas Andaluces y Artífices del Arte Andaluz; Publicaciones Comunitarias: Sevilla, Spain, 2011; Volume 37, pp. 214–257. [Google Scholar]
- Pérez-Escolano, V. Annus Mirabilis (1625): La muerte en Bahía de Juan de Oviedo y de la Bandera. Anu. De Estud. Am. 1981, 38, 467–477. [Google Scholar]
- Santos Márquez, A.J. Compañía artística entre Juan de Oviedo y Martínez Montañés. Una aportación inédita a sus respectivas biografías. Arch. Español De Arte 2011, 84, 163–170. [Google Scholar] [CrossRef]
- Robador, M.D.; Arroyo, F.; Perez-Rodriguez, J.L. Study and restoration of the Seville City Hall façade. Constr. Build. Mater 2014, 53, 370–380. [Google Scholar] [CrossRef]
- Duran, A.; Robador, M.D.; Jimenez de Haro, M.C.; Herrera, L.K.; Gimena, P.; Perez-Rodriguez, J.L. Seville City Hall Chapter Room ceiling decoration. Mater. De Construcción 2010, 60, 83–95. [Google Scholar] [CrossRef][Green Version]
- VV.AA. “Virgen del Buen Aire” Juan de Oviedo y la Bandera y Pedro Duque Cornejo. 1600-Palacio de San Telmo. Memoria Final de Intervención. Instituto Andaluz del Patrimonio Histórico. Centro de de Invertención en el Patrimonio; Junta de Andalucia: Sevilla, Spain, 2006. [Google Scholar]
- Parejo, J. El Convento de Madre de Dios de Sevilla ya Luce sin Andamios; Diario de Sevilla: Seville, Spain, 2021; Available online: https://www.diariodesevilla.es/sevilla/convento-Madre-Dios-Sevilla-luce-sin-andamios_0_1599141188.html (accessed on 22 January 2024).
- Melo, H.P.; Cruz, A.J.; Sanyova, J.; Valadas, S.; Cardoso, A.M. Paint, colour, and style: The contribution of minerals to the palette of the Descent from the Cross, attributed to the Portuguese painter Francisco Joao (act. 1558–1595). Minerals 2023, 13, 1182. [Google Scholar] [CrossRef]
- Blanco, A.C.; Alcántara, I.; Careaga, V.P.; Siracusano, G.; Maier, M.S. A multi-analytical approach for the characterization of painting materials and metal soap formation in two artworks by the Argentinian painter Antonio Berni. Minerals 2023, 13, 919. [Google Scholar] [CrossRef]
- Albero, D.; Cristobal, J.; Duran, A. Mineralogical Approaches to Archaeological Materials: Technological and Social Insights. MDPI: Basel, Switzerland, 2022; p. 226. [Google Scholar]
- Perez-Rodriguez, J.L.; Robador, M.D.; Duran, A. Composition and technological features of ceramics manufactured by Benito de Valladares in the seventeenth century from the Alcazar Palace in Seville, Spain. Eur. Phys. J. Plus 2022, 137, 469. [Google Scholar] [CrossRef]
- Duran, A.; Siguenza, M.B.; Franquelo, M.L.; Jimenez de Haro, M.C.; Justo, A.; Perez-Rodriguez, J.L. Murillo’s painting revealed by spectroscopic techniques and dedicated laboratory-made micro X-ray diffraction. Anal. Chim. Acta 2010, 671, 1–8. [Google Scholar] [CrossRef]
- Herrera, L.K.; Montalbani, S.; Chiavari, G.; Cotte, M.; Solé, V.A.; Bueno, J.; Duran, A.; Justo, A.; Perez-Rodriguez, J.L. Advanced combined application of µ-X-ray diffraction/µ-X-ray fluorescence with conventional techniques for the identification of pictorial materials from Baroque Andalusia paintings. Talanta 2009, 80, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Robador, M.D.; Mancera, I.; Perez-Maqueda, R.; Albardonedo, A. Study of the wall paintings of the Cenador del Leon in the Real Alcazar of Seville. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 082003. [Google Scholar] [CrossRef]
- Perez-Rodriguez, J.L.; Robador, M.D.; Centeno, M.A.; Siguenza, B.; Duran, A. Wall paintings studied using Raman spec troscopy: A comparative study between various assays of cross-sections and external layers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 602–609. [Google Scholar] [CrossRef]
- Duran, A.; Perez-Rodriguez, J.L. Revealing Andalusian wall paintings from the 15th century by mainly using infrared spectroscopy and calorimetry. Vib. Spectrosc. 2020, 111, 103153. [Google Scholar] [CrossRef]
- Calero-Castillo, A.I.; Garcia-Bueno, A.; Lopez-Cruz, O.; Medina-Florez, V.J. The plasterwork at courtyard of the maidens in the Alcazar of Seville. Initial contributions of the characterisation of materials and techniques. Al-Qanṭara 2016, 37, 129–141. [Google Scholar] [CrossRef]
- Lopez-Cruz, O.; Garcia-Bueno, A.; Medina-Florez, V.J. The evolution of the colour in the eaves of the façade of the palace of the king Pedro I, Royal Palace of Seville. Contribution of the study of materials to the identification of the conservation works undertaken throughout its history. Arqueol. Arquit. 2011, 8, 163–178. [Google Scholar] [CrossRef]
- Franquelo, M.L.; Duran, A.; Herrera, L.K.; Jimenez de Haro, M.C.; Perez-Rodriguez, J.L. Comparison between micro-Raman and micro-FTIR spectroscopy techniques for the characterization of pigments from Southern Spain cultural heritage. J. Mol. Struct. 2009, 924–926, 404–412. [Google Scholar] [CrossRef]
- Kriznar, A.; Moreno-Soto, J.; Gamero-Osuna, A.; Martín-de-Soto, A.; Ager, F.J.; Respaldiza, M.A. Material and technical análisis of La Inmaculada by Francisco Pacheco. Minerals 2023, 13, 541. [Google Scholar] [CrossRef]
- Reimer, L. Elemental Analysis and Imaging with X-Rays. In Scanning Electron Microscopy: Physics of Image Formation and Microanalysis; Springer Series in Optical Sciences (SSOS); Springer: Berlin/Heidelberg, Germany, 1998; Volume 45, pp. 365–403. [Google Scholar]
- Izzo, F.; Germinario, C.; Grifa, C.; Langella, A.; Mercurio, M. External reflectance FTIR dataset (4000–400 cm−1) for the identification of relevant mineralogical phases forming Cultural Heritage materials. Infrared Phys. Technol. 2020, 106, 103266. [Google Scholar] [CrossRef]
- Oancea, A.V.; Bodi, G.; Cernescu, A.; Spiridon, I.; Nicolescu, A.; Drobota, M.; Cotofana, C.; Simionescu, B.; Olaru, M. Protective coatings for ceramic artefacts exposed to UV ageing. npj Mater. Degrad. 2023, 7, 21. [Google Scholar] [CrossRef]
- Negri, A.; Nervo, M.; Di Marcello, S.; Castelli, D. Consolidation and adhesion of pictorial layers on a stone substrate: The study case of the Virgin with the Child from Palazzo Madama, in Turin. Coatings 2021, 11, 624. [Google Scholar] [CrossRef]
- Normand, L.; Duchêne, S.; Vergès-Belmin, V.; Dandrel, C.; Giovannacci, D.; Nowik, W. Comparative in Situ Study of Nanolime, Ethyl Silicate and Acrylic Resin for Consolidation of Wall Paintings with High Water and Salt Contents at the Chapter Hall of Chartres Cathedral. Int. J. Arch. Herit. 2020, 14, 1120–1133. [Google Scholar] [CrossRef]
- Giovanoli, R.; Mühlethaler, B. Investigation of discoloured smalt. Stud. Conserv. 1970, 15, 37–44. [Google Scholar] [CrossRef]
- Cavallo, G.; Riccardi, M.P. Glass-based pigments in paintings: Smalt blue and lead-tin yellow type II. Archaeol. Anthropol. Sci. 2021, 13, 199. [Google Scholar] [CrossRef]
- Nord, A.G.; Tronner, K. The frequent occurrence of atacamite in Medieval Swedish murals. Stud. Conserv. 2018, 63, 477–481. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Noya, D.; Montojo, C. Aesthetic effects on granite of adding nanoparticle TiO2 to Si-based consolidants (ethyl silicate or nano-sized silica). Coatings 2020, 10, 215. [Google Scholar] [CrossRef]
- Sepulveda, M.; Gutierrez, S.; Vallette, M.C.; Standen, V.G.; Arriaza, B.T.; Carcamo-Vega, J.J. Micro-Raman spectral identification of manganese oxides black pigments in archaeological context in Northern Chile. Herit. Sci. 2015, 3, 3447. [Google Scholar] [CrossRef]
- Gettens, R.J.; Stout, G.L. Painting Materials: A Short Encyclopaedia; Dover Publications: New York, NY, USA, 1966; p. 352. [Google Scholar]
- Duran, A.; Perez-Rodriguez, J.L.; Jimenez de Haro, M.C. Study of the gilding technique used in polychromed stone and ceramic by dedicated laboratory-made micro X-ray diffraction and complementary techniques. Anal. Bioanal. Chem. 2009, 394, 1671–1677. [Google Scholar] [CrossRef]
- Perez-Rodriguez, J.L.; Robador, M.D.; Albardonedo, A.; Duran, A. Gildings from Andalusia: Materials used in different types of artworks along centuries. J. Cult. Herit. 2018, 31, 112–121. [Google Scholar] [CrossRef]




| Colour | Sample | Micrographs | General/Punctual and Zones | Composition | Figures |
|---|---|---|---|---|---|
| Blue | 1 |  | Punctual zone 1 | Si, K, As, Co, Fe, Al, Ni | S2a |
| Punctual zone 2 | Si, Pb, K, Ca, As, Fe, Co, Al, Ni | S2b | |||
| General preparatory layers | Ca, Pb, Si, K | S2c | |||
| Green | 10 |  | General | Ca, Pb, Si, Cu, Cl, Fe, Al, Na | S3a |
| Punctual zone 1 | Cu, Cl, Pb, Ca | S3b | |||
| General preparatory layers | Ca, Pb, Al, Si | S3c | |||
| 4 |  | Punctual zone 1 | Si, K, Fe, Co, As | S3d | |
| Punctual zone 2 | Cu, Cl, Pb | S3e | |||
| Punctual zone 3 | Cu, Pb | S3f | |||
| 5 |  | Punctual zone 1 | C, Cu, Pb | S3g | |
| Punctual zone 2 | Cu, Cl, Pb, Ca | S3h | |||
| General preparatory layer | Pb | S3i | |||
| Stone support | Ca | S3j | |||
| Turquoise | 9 |  | General surface | Ti, C | S4a |
| General preparatory layers | Ca, S, Ti | S4b | |||
| Punctual zone 1 | Fe, Mn, Ca, Si | S4c | |||
| General | Ca, S, Pb, Fe, Ti, Mn, Si | S4d | |||
| Red | 3 |  | Punctual zone 1 | Hg, S | S5a |
| General | Hg, S, Si, Al, Na, K, Fe, Pb, Ca | S5b | |||
| 11 |  | Punctual zone 1 | Pb, Si, Fe, Mn, Ca, Al | S5c | |
| Punctual zone 2 | Fe, Mn, Pb, Si, Al | S5d | |||
| Yellow | 6 |  | General | Ca, Pb, Si, Fe | S6a |
| Black | 8 |  | General preparatory layers | Ca, Pb | S6b |
| Punctual zone 1 | P, Ca, Si, C | S6c | |||
| Golden | 7 |  | General surface | Au, Ca, Pb, Si, Al, Fe | S6d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rodríguez, J.L.; Robador, M.D.; Larrea, G.; Durán, A. Revealing Juan de Oviedo y de la Bandera’s Artworks: The Case of the Polychrome of a Stone-Carved Sculpture from the Madre de Dios Convent Façade in Seville. Minerals 2024, 14, 225. https://doi.org/10.3390/min14030225
Pérez-Rodríguez JL, Robador MD, Larrea G, Durán A. Revealing Juan de Oviedo y de la Bandera’s Artworks: The Case of the Polychrome of a Stone-Carved Sculpture from the Madre de Dios Convent Façade in Seville. Minerals. 2024; 14(3):225. https://doi.org/10.3390/min14030225
Chicago/Turabian StylePérez-Rodríguez, José Luis, María Dolores Robador, Garbiñe Larrea, and Adrián Durán. 2024. "Revealing Juan de Oviedo y de la Bandera’s Artworks: The Case of the Polychrome of a Stone-Carved Sculpture from the Madre de Dios Convent Façade in Seville" Minerals 14, no. 3: 225. https://doi.org/10.3390/min14030225
APA StylePérez-Rodríguez, J. L., Robador, M. D., Larrea, G., & Durán, A. (2024). Revealing Juan de Oviedo y de la Bandera’s Artworks: The Case of the Polychrome of a Stone-Carved Sculpture from the Madre de Dios Convent Façade in Seville. Minerals, 14(3), 225. https://doi.org/10.3390/min14030225







