Combined Scanned Macro X-Ray Fluorescence and Reflectance Spectroscopy Mapping on Corroded Ancient Bronzes
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Methods
2.3. Data Analysis
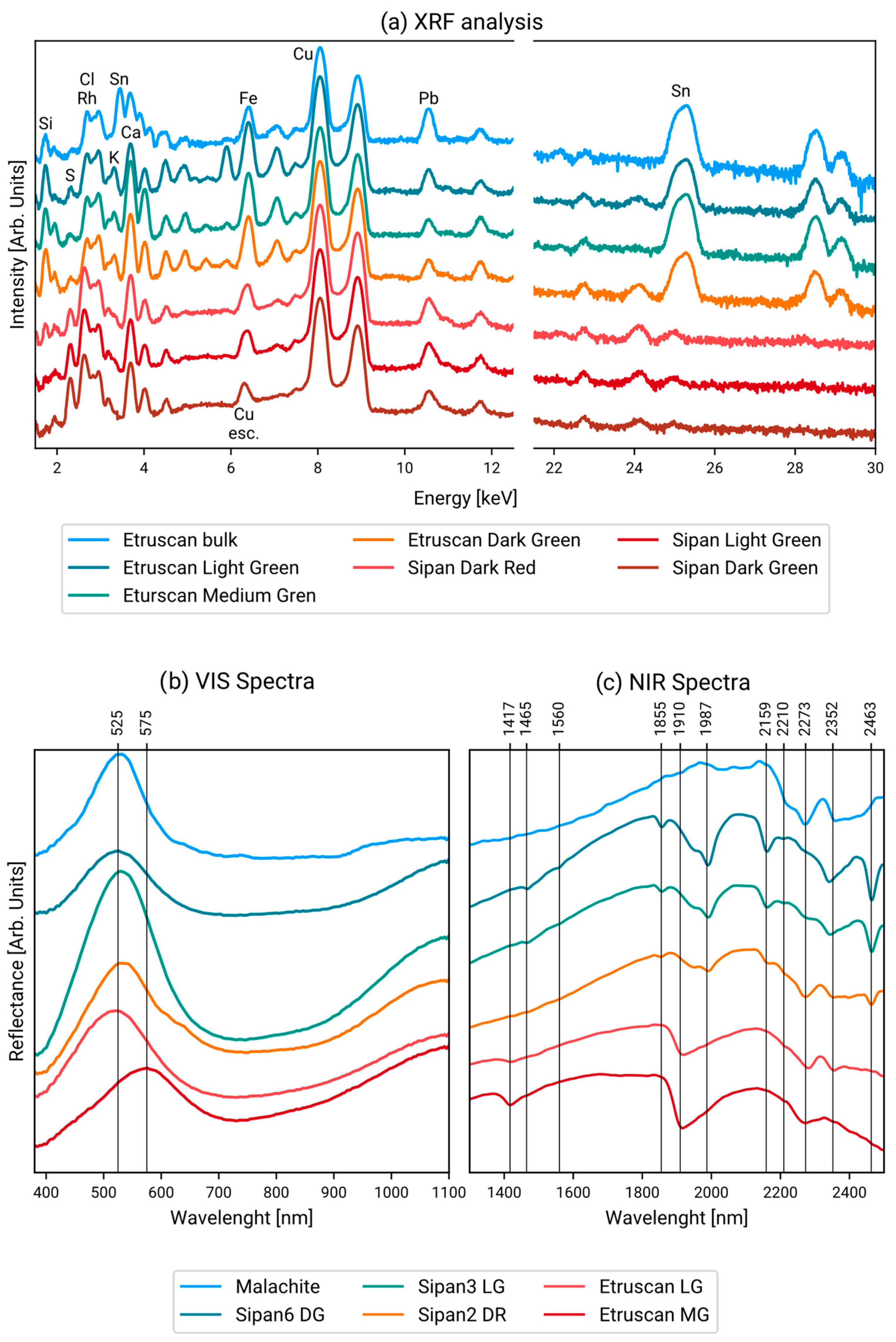
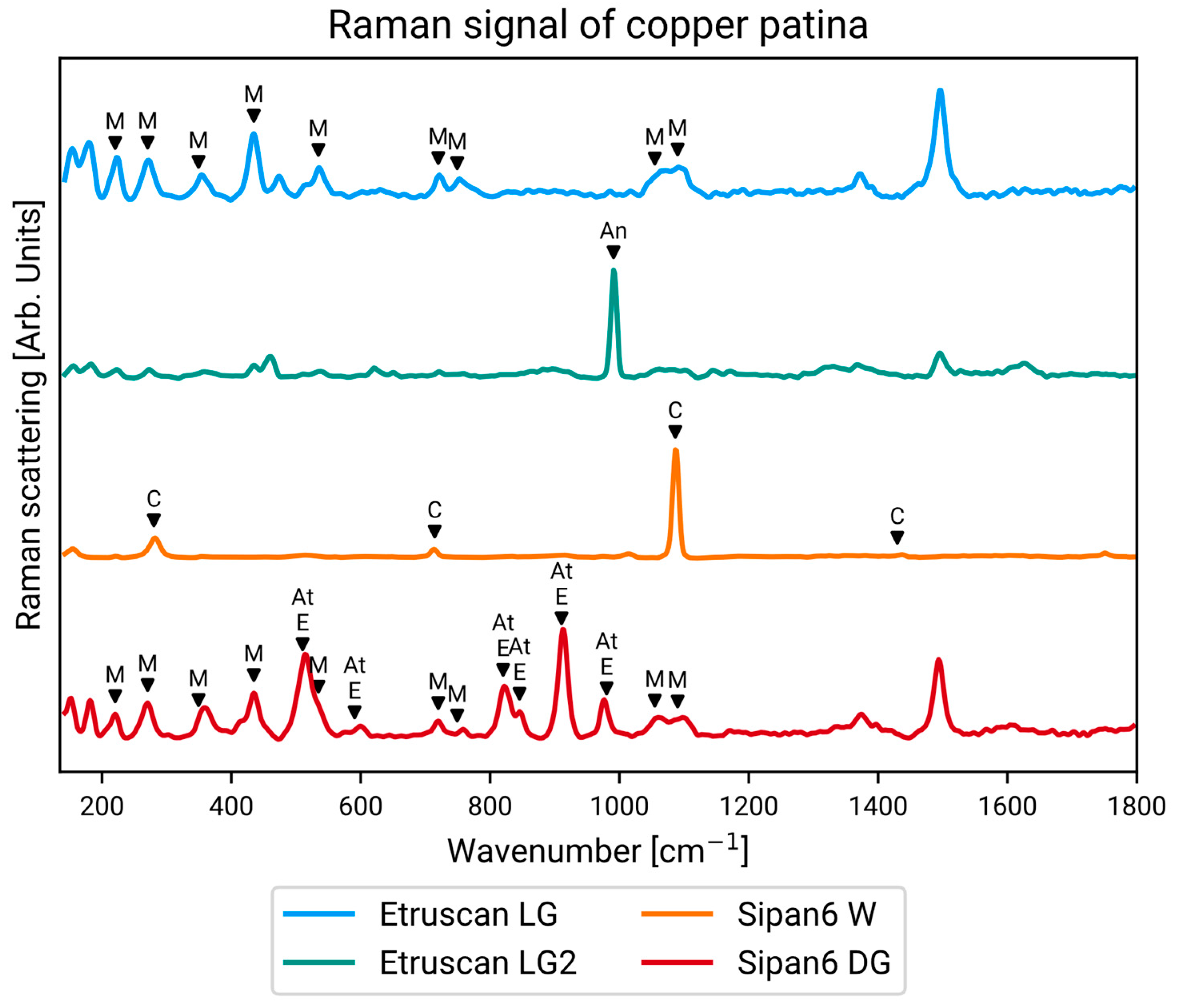
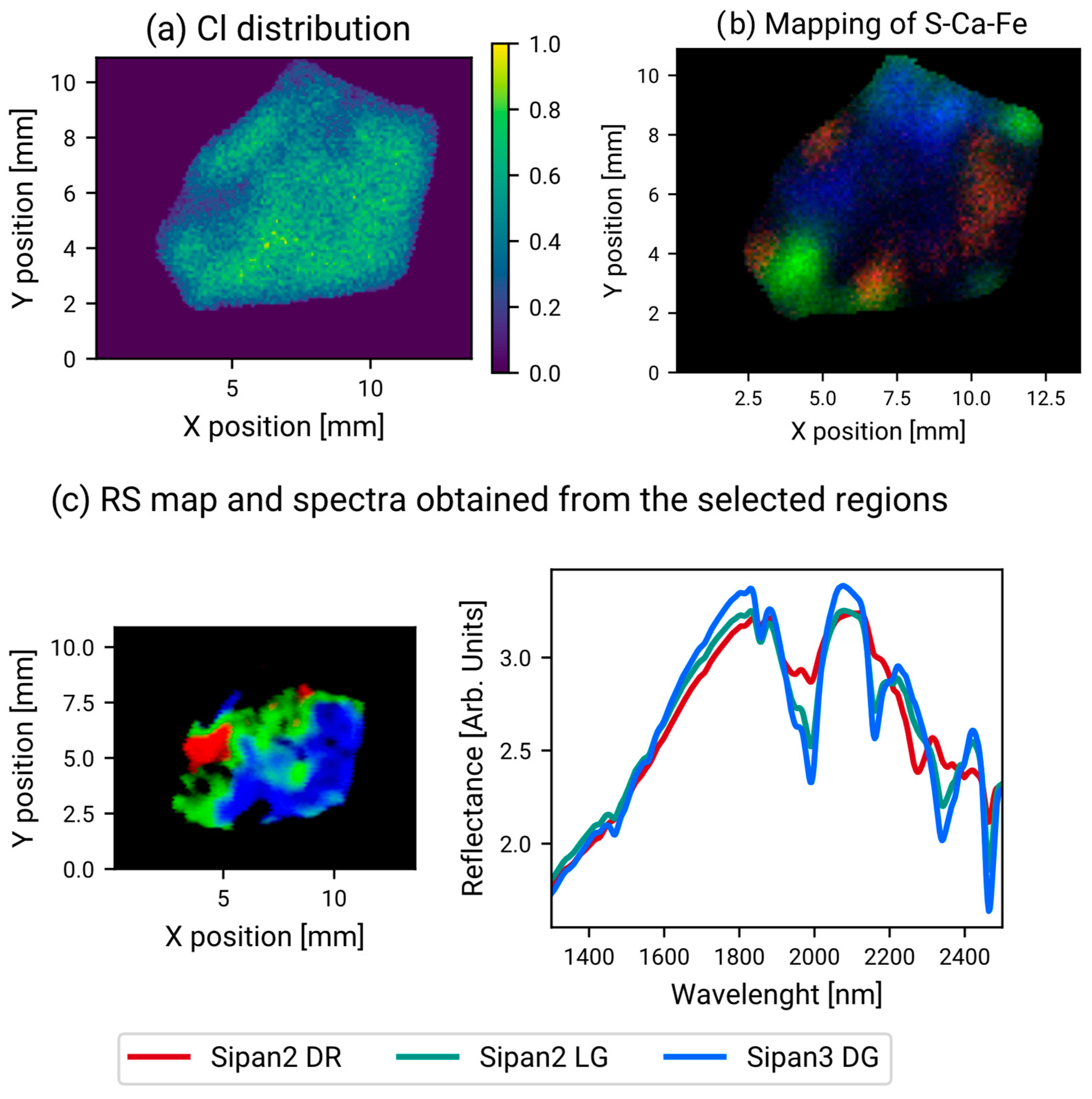
3. Results
3.1. Point Data Results
3.2. Mapping Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillmann, P.; Beranger, G.; Piccardo, P.; Matthiessen, H. Corrosion of Metallic Heritage Artefacts: Investigation, Conservation and Prediction of Long Term Behaviour; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-1-84569-301-5. [Google Scholar]
- Dillmann, P.; Neff, D.; Féron, D. Archaeological Analogues and Corrosion Prediction: From Past to Future. A Review. Corros. Eng. Sci. Technol. 2014, 49, 567–576. [Google Scholar] [CrossRef]
- Sandu, I.G.; Mircea, O.; Vasilache, V.; Sandu, I. Influence of Archaeological Environment Factors in Alteration Processes of Copper Alloy Artifacts. Microsc. Res. Tech. 2012, 75, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; Getty Publications: Los Angeles, CA, USA, 2002; ISBN 978-0-89236-638-5. [Google Scholar]
- Robbiola, L.; Fiaud, C.; Harch, A. Characterisation of Passive Layers of Bronze Patinas (Cu-Sn Alloys) in Relation with the Tin Content of the Alloy. In Proceedings of the European Symposium on Modifications of Passive Films, Paris, France, 15–17 February 1993; pp. 150–154. [Google Scholar]
- Robbiola, L.; Blengino, J.-M.; Fiaud, C. Morphology and Mechanisms of Formation of Natural Patinas on Archaeological Cu–Sn Alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Catelli, E.; Sciutto, G.; Prati, S.; Jia, Y.; Mazzeo, R. Characterization of Outdoor Bronze Monument Patinas: The Potentialities of near-Infrared Spectroscopic Analysis. Env. Sci. Pollut. Res. 2018, 25, 24379–24393. [Google Scholar] [CrossRef] [PubMed]
- Bettuzzi, M.; Casali, F.; Morigi, M.P.; Brancaccio, R.; Carson, D.; Chiari, G.; Maish, J. Computed Tomography of a Medium Size Roman Bronze Statue of Cupid. Appl. Phys. A 2015, 118, 1161–1169. [Google Scholar] [CrossRef]
- Privitera, A.; Corbascio, A.; Calcani, G.; Della Ventura, G.; Ricci, M.A.; Sodo, A. Raman Approach to the Forensic Study of Bronze Patinas. J. Archaeol. Sci. Rep. 2021, 39, 103115. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Galli, A.; Poldi, G. In Situ EDXRF Analyses on Renaissance Plaquettes and Indoor Bronzes Patina Problems and Provenance Clues. X-Ray Spectrom. Int. J. 2008, 37, 388–394. [Google Scholar] [CrossRef]
- Galli, A.; Bonizzoni, L. True versus Forged in the Cultural Heritage Materials: The Role of PXRF Analysis. X-Ray Spectrom. 2014, 43, 22–28. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Canevari, C.; Galli, A.; Gargano, M.; Ludwig, N.; Malagodi, M.; Rovetta, T. A Multidisciplinary Materials Characterization of a Joannes Marcus Viol (16th Century). Herit. Sci. 2014, 2, 15. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Caglio, S.; Galli, A.; Germinario, C.; Izzo, F.; Magrini, D. Identifying Original and Restoration Materials through Spectroscopic Analyses on Saturnino Gatti Mural Paintings: How Far a Noninvasive Approach Can Go. Appl. Sci. 2023, 13, 6638. [Google Scholar] [CrossRef]
- Galli, A.; Caccia, M.; Alberti, R.; Bonizzoni, L.; Aresi, N.; Frizzi, T.; Bombelli, L.; Gironda, M.; Martini, M. Discovering the Material Palette of the Artist: A p-XRF Stratigraphic Study of the Giotto Panel ‘God the Father with Angels’: Discovering the Pigment Palette Using a p-XRF Stratigraphic Analysis. X-Ray Spectrom. 2017, 46, 435–441. [Google Scholar] [CrossRef]
- Bottaini, C.; Brunetti, A.; Bordalo, R.; Valera, A.; Schiavon, N. Non-Destructive Characterization of Archeological Cu-Based Artifacts from the Early Metallurgy of Southern Portugal. Archaeol. Anthr. Sci. 2018, 10, 1903–1912. [Google Scholar] [CrossRef]
- Mezzi, A.; de Caro, T.; Riccucci, C.; Parisi, E.I.; Faraldi, F.; Vassiliou, P.; Grassini, S. Analytical Methodologies for the Investigation of Soil-Induced Degradation of Cu-Based Archaeological Artefacts. Surf. Interface Anal. 2012, 44, 953–957. [Google Scholar] [CrossRef]
- Gargano, M.; Galli, A.; Bonizzoni, L.; Alberti, R.; Aresi, N.; Caccia, M.; Castiglioni, I.; Interlenghi, M.; Salvatore, C.; Ludwig, N.; et al. The Giotto’s Workshop in the XXI Century: Looking inside the “God the Father with Angels” Gable. J. Cult. Herit. 2019, 36, 255–263. [Google Scholar] [CrossRef]
- Galli, A.; Caccia, M.; Caglio, S.; Bonizzoni, L.; Castiglioni, I.; Gironda, M.; Alberti, R.; Martini, M. An Innovative Protocol for the Study of Painting Materials Involving the Combined Use of MA-XRF Maps and Hyperspectral Images. Eur. Phys. J. Plus 2022, 137, 22. [Google Scholar] [CrossRef]
- Orsilli, J.; Galli, A.; Bonizzoni, L.; Caccia, M. More than XRF Mapping: STEAM (Statistically Tailored Elemental Angle Mapper) a Pioneering Analysis Protocol for Pigment Studies. Appl. Sci. 2021, 11, 1446. [Google Scholar] [CrossRef]
- Alfeld, M.; Mulliez, M.; Devogelaere, J.; de Viguerie, L.; Jockey, P.; Walter, P. MA-XRF and Hyperspectral Reflectance Imaging for Visualizing Traces of Antique Polychromy on the Frieze of the Siphnian Treasury. Microchem. J. 2018, 141, 395–403. [Google Scholar] [CrossRef]
- da Silva, A.T.; Legrand, S.; Van der Snickt, G.; Featherstone, R.; Janssens, K.; Bottinelli, G. MA-XRF Imaging on René Magritte’s La Condition Humaine: Insights into the Artist’s Palette and Technique and the Discovery of a Third Quarter of La Pose Enchantée. Herit. Sci. 2017, 5, 37. [Google Scholar] [CrossRef]
- Tapia, J.; Eveno, M.; Calligaro, T.; Pichon, L.; Laval, E.; Ravaud, E.; Reiche, I. Efficiency of Combined MA-XRF and CXRF to Give Nondestructive Insights about Changes of a Historical Painting. Eur. Phys. J. Plus 2023, 138, 46. [Google Scholar] [CrossRef]
- Solomon, S.D.; Rutkowsky, S.A.; Mahon, M.L.; Halpern, E.M. Synthesis of Copper Pigments, Malachite and Verdigris: Making Tempera Paint. J. Chem. Educ. 2011, 88, 1694–1697. [Google Scholar] [CrossRef]
- Švarcová, S.; Hradil, D.; Hradilová, J.; Čermáková, Z. Pigments—Copper-Based Greens and Blues. Archaeol. Anthr. Sci. 2021, 13, 190. [Google Scholar] [CrossRef]
- Scott, D.A. A Review of Copper Chlorides and Related Salts in Bronze Corrosion and as Painting Pigments. Stud. Conserv. 2000, 45, 39–53. [Google Scholar] [CrossRef]
- Galli, A.; Bonizzoni, L.; Sibilia, E.; Martini, M. EDXRF Analysis of Metal Artefacts from the Grave Goods of the Royal Tomb 14 of Sipán, Peru. X-Ray Spectrom. 2011, 40, 74–78. [Google Scholar] [CrossRef]
- HoÈrz, G.; Kallfass, M. The Treasure of Gold and Silver Artifacts from the Royal Tombs of SipaÂn, Peru Ð a Study on the Moche Metalworking Techniques. Mater. Charact. 2000, 45, 391–419. [Google Scholar] [CrossRef]
- Ingo, G.M.; Bustamante, A.D.; Alva, W.; Angelini, E.; Cesareo, R.; Gigante, G.E.; Zambrano, S.D.P.A.; Riccucci, C.; Di Carlo, G.; Parisi, E.I.; et al. Gold Coated Copper Artifacts from the Royal Tombs of Sipán (Huaca Rajada, Perù): Manufacturing Techniques and Corrosion Phenomena. Appl. Phys. A 2013, 113, 877–887. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Maloni, A.; Milazzo, M. Evaluation of Effects of Irregular Shape on Quantitative XRF Analysis of Metal Objects. X-Ray Spectrom. 2006, 35, 390–399. [Google Scholar] [CrossRef]
- Ager, F.J.; Ferretti, M.; Grilli, M.L.; Juanes, D.; Ortega-Feliu, I.; Respaldiza, M.A.; Roldán, C.; Scrivano, S. Reconsidering the Accuracy of X-Ray Fluorescence and Ion Beam Based Methods When Used to Measure the Thickness of Ancient Gildings. Spectrochim. Acta Part B At. Spectrosc. 2017, 135, 42–47. [Google Scholar] [CrossRef]
- Duée, C.; Orberger, B.; Maubec, N.; Laperche, V.; Capar, L.; Bourguignon, A.; Bourrat, X.; El Mendili, Y.; Chateigner, D.; Gascoin, S.; et al. Impact of Heterogeneities and Surface Roughness on pXRF, pIR, XRD and Raman Analyses: Challenges for on-Line, Real-Time Combined Mineralogical and Chemical Analyses on Drill Cores and Implication for “High Speed” Ni-Laterite Exploration. J. Geochem. Explor. 2019, 198, 1–17. [Google Scholar] [CrossRef]
- Kanngießer, B.; Malzer, W.; Mantouvalou, I.; Sokaras, D.; Karydas, A.G. A Deep View in Cultural Heritage—Confocal Micro X-Ray Spectroscopy for Depth Resolved Elemental Analysis. Appl. Phys. A 2012, 106, 325–338. [Google Scholar] [CrossRef]
- Nakano, K.; Tsuji, K. Nondestructive Elemental Depth Profiling of Japanese Lacquerware ‘Tamamushi-Nuri’ by Confocal 3D-XRF Analysis in Comparison with Micro GE-XRF. X-Ray Spectrom. 2009, 38, 446–450. [Google Scholar] [CrossRef]
- Šmit, Ž.; Janssens, K.; Proost, K.; Langus, I. Confocal μ-XRF Depth Analysis of Paint Layers. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 219–220, 35–40. [Google Scholar] [CrossRef]
- Trojek, T.; Prokeš, R.; Šefců, R.; Bilavčíková, H.; Čechák, T. Confocal X-Ray Fluorescence Spectrometer for in-Situ Analyses of Paintings. Radiat. Phys. Chem. 2017, 137, 238–242. [Google Scholar] [CrossRef]
- Cesareo, R.; de Assis, J.T.; Roldán, C.; Bustamante, A.D.; Brunetti, A.; Schiavon, N. Multilayered Samples Reconstructed by Measuring Kα/Kβ or Lα/Lβ X-Ray Intensity Ratios by EDXRF. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 312, 15–22. [Google Scholar] [CrossRef]
- Trojek, T.; Čechák, T.; Musílek, L. Recognition of Pigment Layers in Illuminated Manuscripts by Means of Kα/Kβ and Lα/Lβ Ratios of Characteristic X-Rays. Appl. Radiat. Isot. 2010, 68, 871–874. [Google Scholar] [CrossRef]
- Orsilli, J. AR-XRF Techniques for the Analysis of Cultural Heritage Layered Samples; University of Milano Bicocca: Milan, Italy, 2023. [Google Scholar]
- Orsilli, J.; Migliori, A.; Padilla-Alvarez, R.; Martini, M.; Galli, A. AR-XRF Measurements and Data Treatment for the Evaluation of Gilding Samples of Cultural Heritage. J. Anal. At. Spectrom. 2023, 38, 174–185. [Google Scholar] [CrossRef]
- Margreiter, R.; Baumann, J.; Mantouvalou, I.; Radtke, M.; Reinholz, U.; Strub, E. Investigations on Fire-Gilding. Archaeometry 2022, 64, 1465–1478. [Google Scholar] [CrossRef]
- Hauff, P. An Overview of VIS-NIR-SWIR Field Spectroscopy as Applied to Precious Metals Exploration. Spectr. Int. Inc. 2008, 80001, 303–403. [Google Scholar]
- Caccia, M.; Caglio, S.; Galli, A. Objective Interpretation of Ultraviolet-Induced Luminescence for Characterizing Pictorial Materials. Sci. Rep. 2023, 13, 20240. [Google Scholar] [CrossRef]
- Verri, G. The Application of Visible-Induced Luminescence Imaging to the Examination of Museum Objects. In Proceedings of the O3A: Optics for Arts, Architecture, and Archaeology II, Munich, Germany, 25 June 2009; SPIE: Bellingham, WA, USA, 2009; Volume 7391, pp. 37–48. [Google Scholar]
- Liu, W.; Li, M.; Wu, N.; Liu, S.; Chen, J. A New Application of Fiber Optics Reflection Spectroscopy (FORS): Identification of “Bronze Disease” Induced Corrosion Products on Ancient Bronzes. J. Cult. Herit. 2021, 49, 19–27. [Google Scholar] [CrossRef]
- Frost, R.L. Raman Spectroscopy of Selected Copper Minerals of Significance in Corrosion. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2003, 59, 1195–1204. [Google Scholar] [CrossRef]
- Database of Raman Spectroscopy, X-Ray Diffraction and Chemistry of Minerals. Available online: https://rruff.info/ (accessed on 28 December 2023).
- Python Software Foundation Python. Available online: https://www.python.org/ (accessed on 3 January 2022).
- Meneses, P.R. Spectral Correlation Mapper (SCM): An Improvement on the Spectral Angle Mapper (SAM); JPL Publication: Pasadena, CA, USA, 2000. [Google Scholar]
- Ricciardi, P.; Pallipurath, A.; Rose, K. ‘It’s Not Easy Being Green’: A Spectroscopic Study of Green Pigments Used in Illuminated Manuscripts. Anal. Methods 2013, 5, 3819–3824. [Google Scholar] [CrossRef]
- Bruni, S.; Caglio, S.; Guglielmi, V.; Poldi, G. The Joined Use of n.i. Spectroscopic Analyses—FTIR, Raman, Visible Reflectance Spectrometry and EDXRF—to Study Drawings and Illuminated Manuscripts. Appl. Phys. A 2008, 92, 103–108. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, W.; Liu, S.; Chen, J. Detecting Copper Trihydroxychlorides with Reflectance Spectroscopy and Machine Learning Methods. J. Cult. Herit. 2023, 59, 49–56. [Google Scholar] [CrossRef]
- Clementi, C.; Miliani, C.; Verri, G.; Sotiropoulou, S.; Romani, A.; Brunetti, B.G.; Sgamellotti, A. Application of the Kubelka—Munk Correction for Self-Absorption of Fluorescence Emission in Carmine Lake Paint Layers. Appl. Spectrosc. 2009, 63, 1323–1330. [Google Scholar] [CrossRef]
- Dupuis, G.; Menu, M. Quantitative Characterisation of Pigment Mixtures Used in Art by Fibre-Optics Diffuse-Reflectance Spectroscopy. Appl. Phys. A 2006, 83, 469–474. [Google Scholar] [CrossRef]
- Dal Fovo, A.; Fontana, R. Stratigraphic Mapping of Paintings by Multispectral Reflectography. Eur. Phys. J. Plus 2023, 138, 1112. [Google Scholar] [CrossRef]
- Bonizzoni, L.; Caglio, S.; Galli, A.; Poldi, G. Comparison of Three Portable EDXRF Spectrometers for Pigment Characterization. X-Ray Spectrom. 2010, 39, 233–242. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsilli, J.; Caglio, S. Combined Scanned Macro X-Ray Fluorescence and Reflectance Spectroscopy Mapping on Corroded Ancient Bronzes. Minerals 2024, 14, 192. https://doi.org/10.3390/min14020192
Orsilli J, Caglio S. Combined Scanned Macro X-Ray Fluorescence and Reflectance Spectroscopy Mapping on Corroded Ancient Bronzes. Minerals. 2024; 14(2):192. https://doi.org/10.3390/min14020192
Chicago/Turabian StyleOrsilli, Jacopo, and Simone Caglio. 2024. "Combined Scanned Macro X-Ray Fluorescence and Reflectance Spectroscopy Mapping on Corroded Ancient Bronzes" Minerals 14, no. 2: 192. https://doi.org/10.3390/min14020192
APA StyleOrsilli, J., & Caglio, S. (2024). Combined Scanned Macro X-Ray Fluorescence and Reflectance Spectroscopy Mapping on Corroded Ancient Bronzes. Minerals, 14(2), 192. https://doi.org/10.3390/min14020192







