Prevention of Silica Gel Formation for Eudialyte Study Using New Digestion Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Characterisation
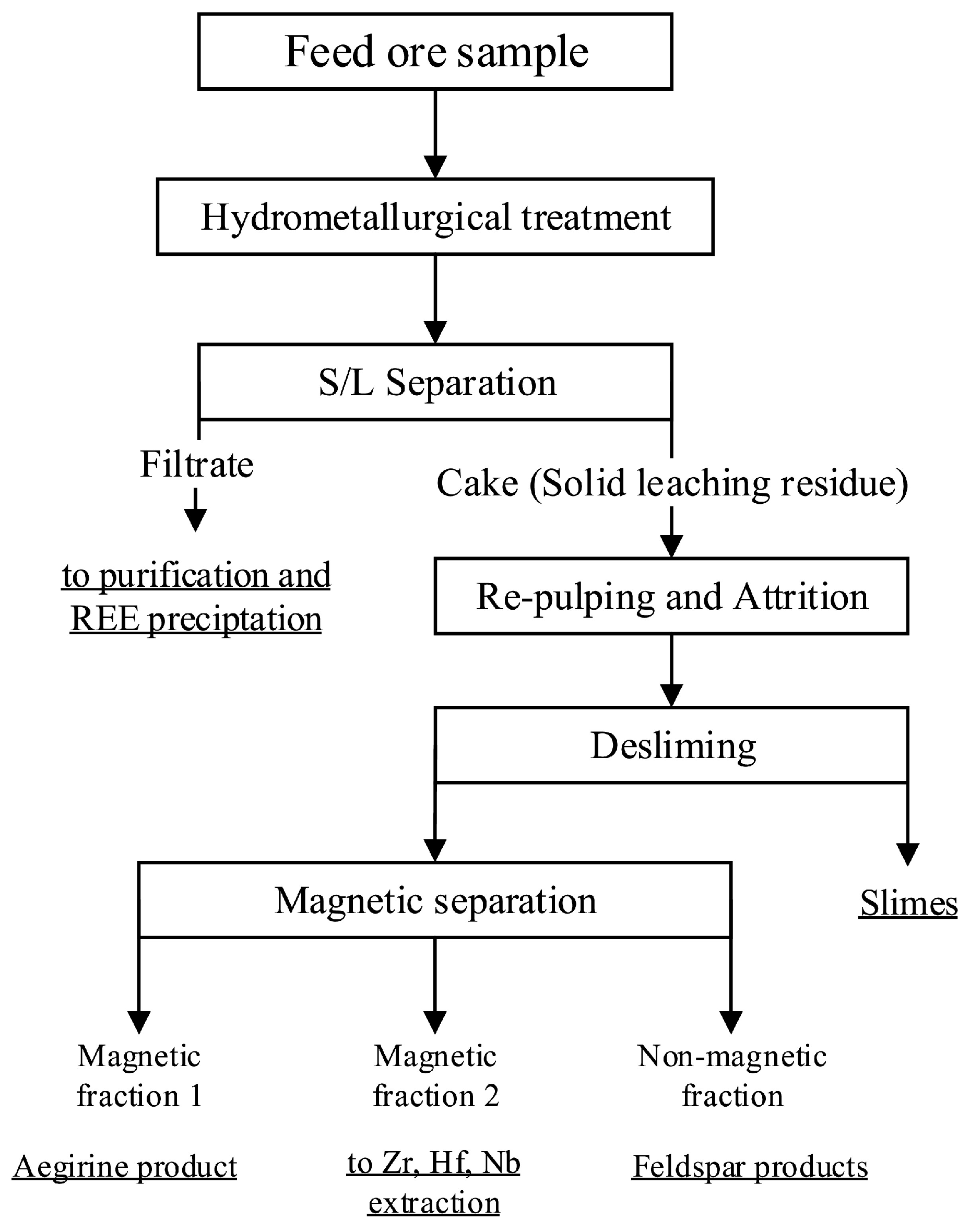
2.2.2. Hydrometallurgical Treatment
2.2.3. Magnetic Separation
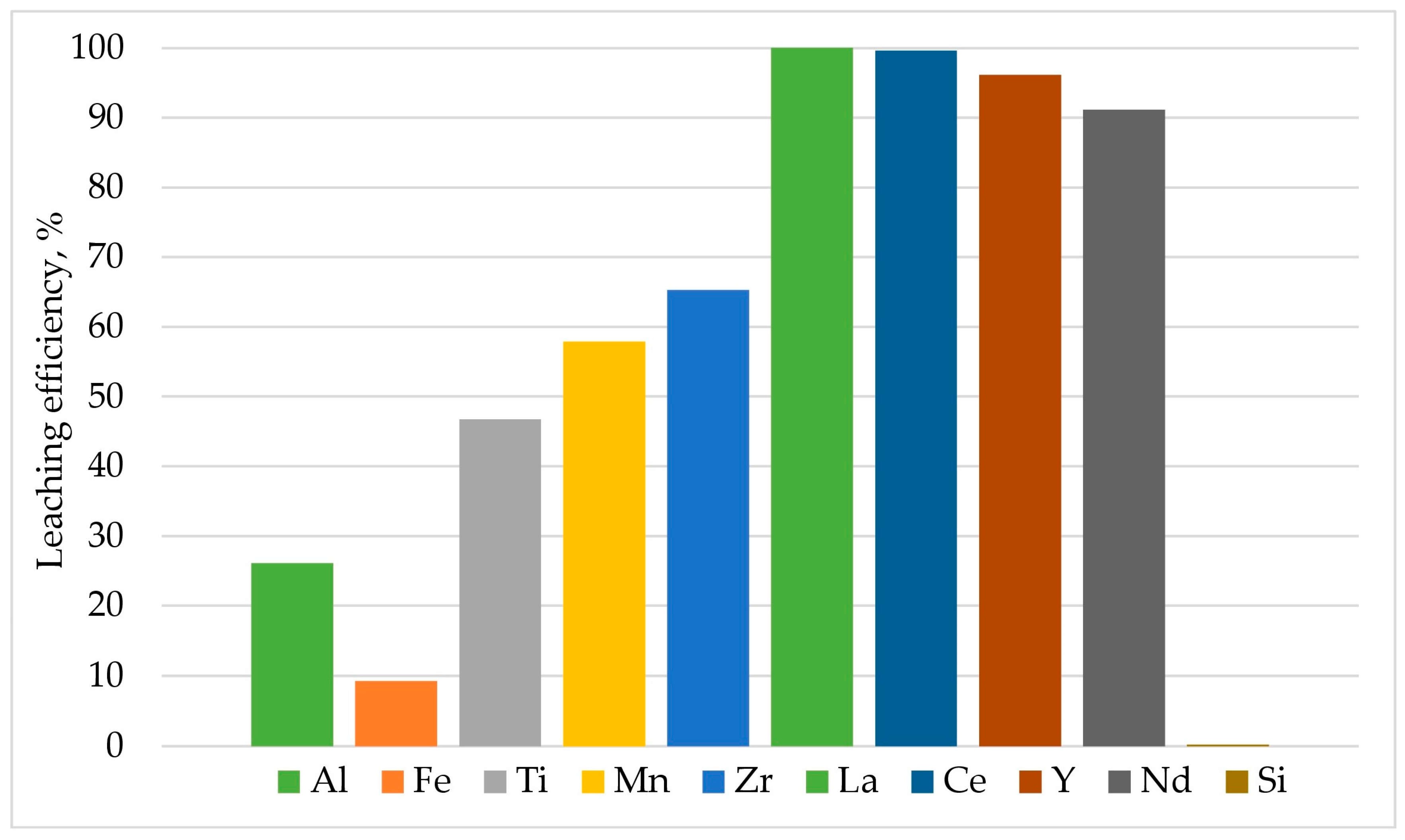
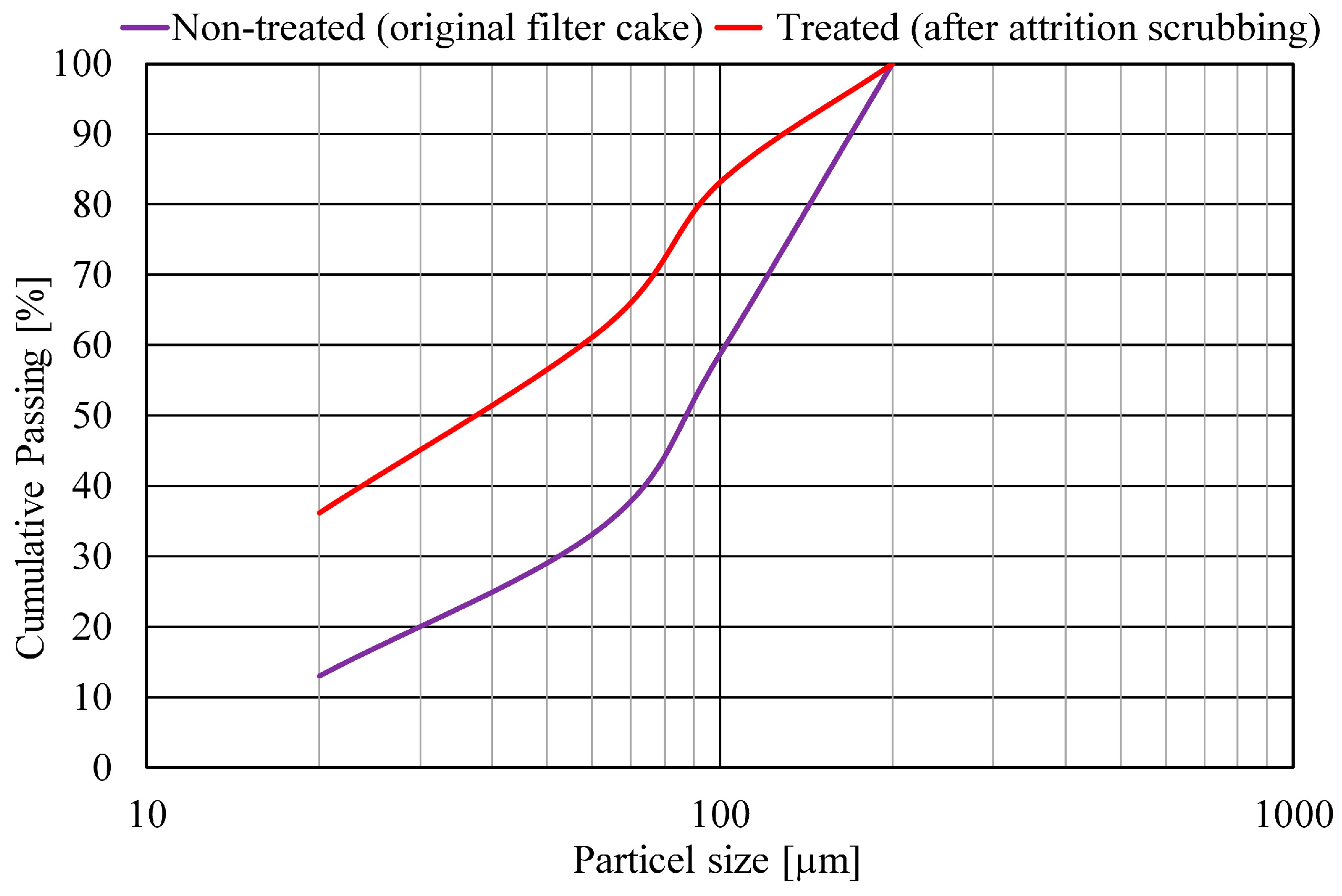
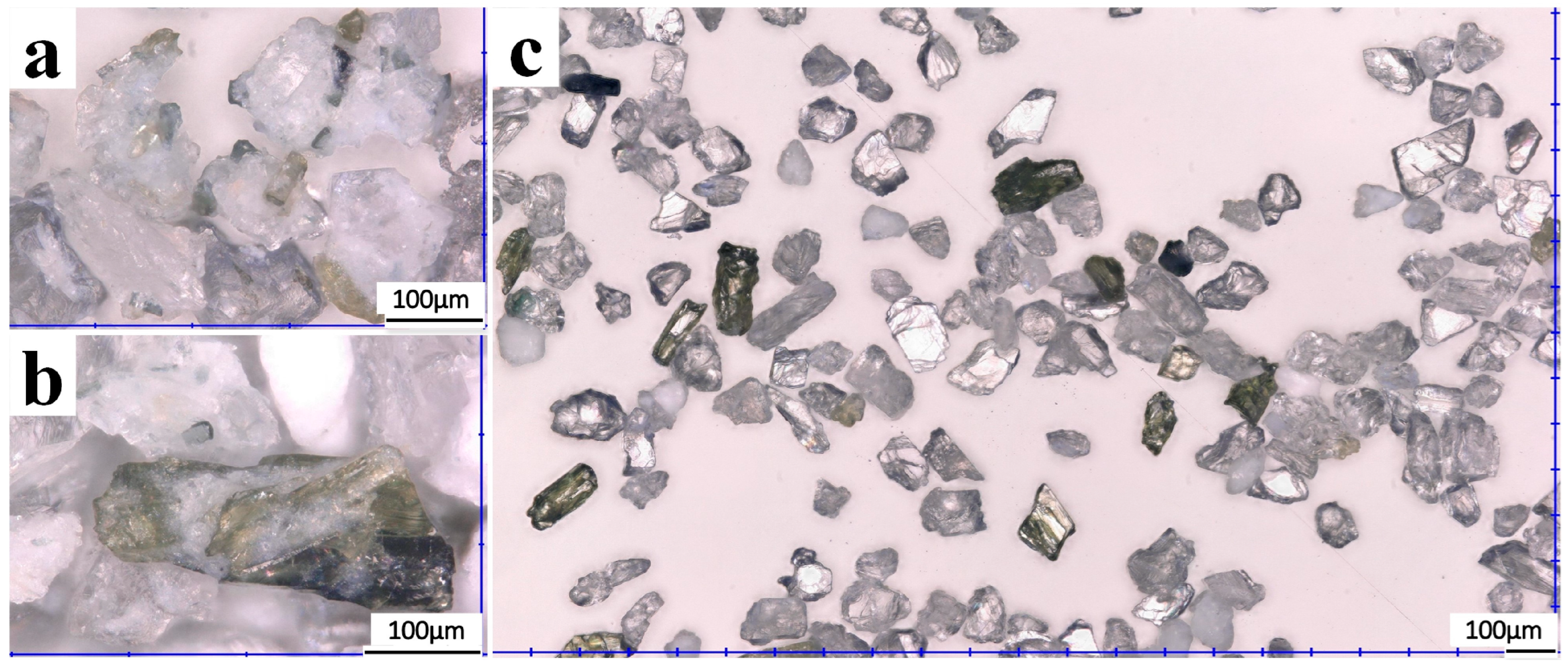
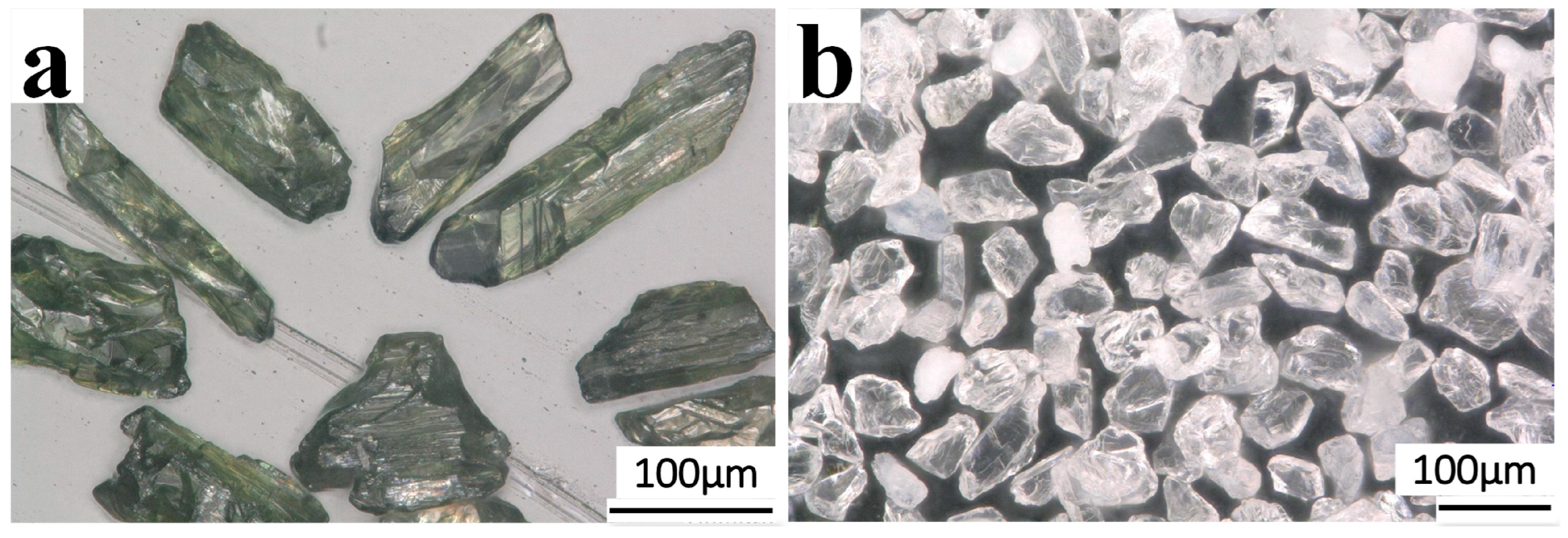
3. Results and Discussion
3.1. Hydrometallurgical Treatment
3.2. Magnetic Separation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dostal, J. Rare Earth Element Deposits of Alkaline Igneous Rocks. Resources 2017, 6, 34. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Mikhailova, J.A.; Pakhomovsky, Y.A.; Bazai, A.V.; Aksenov, S.M.; Kalashnikov, A.O.; Krivovichev, S.V. Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia. Minerals 2021, 11, 982. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. A global review on agpaitic rocks. Earth-Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Vaccarezza, V.; Anderson, C. An Overview of Beneficiation and Hydrometallurgical Techniques on Eudialyte Group Minerals. Min. Metall. Explor. 2020, 37, 39–50. [Google Scholar] [CrossRef]
- Zakharov, V.I.; Maiorov, D.V.; Alishkin, A.R.; Matveev, V.A. Causes of insufficient recovery of zirconium during acidic processing of lovozero eudialyte concentrate. Russ. J. Non-Ferr. Metals 2011, 52, 423–428. [Google Scholar] [CrossRef]
- Lebedev, V.N. Sulfuric Acid Technology for Processing of Eudialyte Concentrate. Russ. J. Appl. Chem. 2003, 76, 1559–1563. [Google Scholar] [CrossRef]
- Lebedev, V.N.; Shchur, T.E.; Maiorov, D.V.; Popova, L.A.; Serkova, R.P. Specific Features of Acid Decomposition of Eudialyte and Certain Rare-Metal Concentrates from Kola Peninsula. Russ. J. Appl. Chem. 2003, 76, 1191–1196. [Google Scholar] [CrossRef]
- Dibrov, I.A.; Chirkst, D.E.; Litvinova, T.E. Russ. J. Appl. Chem. 2002, 75, 195. [Google Scholar]
- Ma, Y.; Stopic, S.; Friedrich, B. Hydrometallurgical Treatment of an Eudialyte Concentrate for Preparation of Rare Earth Carbonate. Johns. Matthey Technol. Rev. 2019, 63, 2–13. [Google Scholar] [CrossRef]
- Stark, T.; Silin, I.; Wotruba, H. Mineral Processing of Eudialyte Ore from Norra Kärr. J. Sustain. Metall. 2017, 3, 32–38. [Google Scholar] [CrossRef]
- Silin, I.; Gürsel, D.; Büchter, C.; Weitkämper, L.; Wotruba, H. Recovery of Catapleiite and Eudialyte from Non-Magnetic Fraction of Eudialyte ore Processing of Norra Kärr Deposit. Minerals 2022, 12, 19. [Google Scholar] [CrossRef]
- Spooren, J.; Binnemans, K.; Björkmalm, J.; Breemersch, K.; Dams, Y.; Folens, K.; González-Moya, M.; Horckmans, L.; Komnitsas, K.; Kurylak, W.; et al. Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: Technology development trends. Resour. Conserv. Recycl. 2020, 160, 104919. [Google Scholar] [CrossRef]
- Agrawal, S.; Rayapusi, V.; Dhawan, N. Comparison of microwave and conventional carbothermal reduction of red mud for recovery of iron values. Miner. Eng. 2019, 132, 202–210. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical Process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Lorenz, W.; Gwosdz, W. Bewertungskriterien für Industrieminerale. Steine und Erden. Teil 7. Feldspate und andere Flussmittel. Geologisches Jahrbuch, Sonderhefte Reihe H 2004. Heft 10, BGR, Hannover, Germany. Available online: https://www.schweizerbart.de/publications/detail/isbn/9783510959143/Geologisches_Jahrbuch_Reihe_H_Heft_H10 (accessed on 21 January 2024).
- Militzer, A. Feldspar Potential in Namibia—Evaluation of Economic Suitability; BGR: Hannover, Germany, 2020. [Google Scholar]
- Popov, I.O.; Mitrofanov, Y.A.; Ustinov, S.M. Feasibility of using aegirine concentrate as a complex flux in copper metallurgy. Metallurgist 2012, 56, 64–70. [Google Scholar] [CrossRef]
- Pleshakov, Y.V.; Fedchenko, V.F.; Shishkin, S.P.; Suvorova, O.V. Aegirite concentrate—Valuable raw material in industry. Obogashcheniye Rud-Miner. Process. J. 2005, 5, 39–41. [Google Scholar]
- Brylyakov, Y.Y. Prospects for all-round utilization of apatite-nepheline ores from the Khibini deposits. Obogashcheniye Rud-Miner. Process. J. 2005, 3, 28–31. [Google Scholar]
- Stopic, S.; Dertmann, C.; Gulgans, U.; Friedrich, B. Neuer modularer Reaktor zum trockenen Aufschließen silikatreicher Erze und Konzentrate und für die Extraktion kritischer Metalle. Erzmetall-World Metall. 2019, 72, 3. [Google Scholar]
- Ma, Y.; Stopic, S.; Gronen, L.; Friedrich, B. Recovery of Zr, Hf, Nb from eudialyte residue by sulfuric acid dry digestion. Hydrometallurgy 2018, 181, 206–214. [Google Scholar] [CrossRef]
- Hedrich, S.; Breuker, A.; Martin, M.; Schippers, A. Rare earth elements (bio)leaching from zircon and eudialyte concentrates. Hydrometallurgy 2023, 219, 106068. [Google Scholar] [CrossRef]
- Judge, W. Azimi Recent progress in impurity removal during rare earth processing: A review. Hydrometallurgy 2020, 196, 104435. [Google Scholar] [CrossRef]




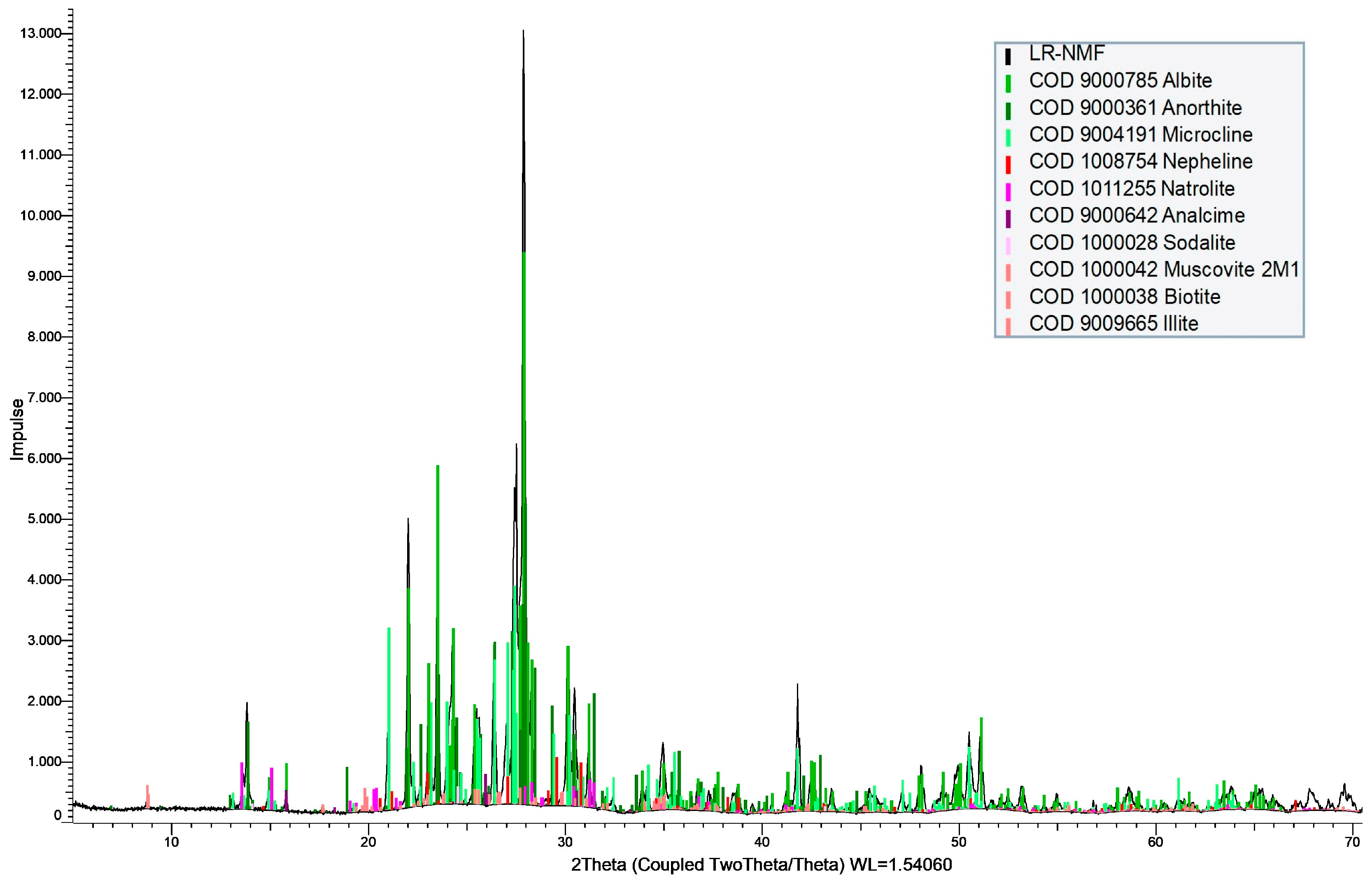
| Si | Na | K | Al | Zr | Ti | Ca | Fe | Mn | P | LREE (La–Sm) | HREE (Y, Eu–Lu) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25.15 | 6.85 | 3.16 | 8.65 | 0.79 | 0.12 | 1.31 | 3.27 | 0.14 | 0.045 | 0.2823 | 0.1514 |
| Parameter | Unit | Value |
|---|---|---|
| Initial Material | kg | 20 |
| Used acid (H2SO4) | % | 96 |
| Concentration | mol/L | 12 |
| Duration of dry digestion | min | 120 |
| Solid/liquid in dry digestion (H2SO4) | - | 1:2 |
| Solid/liquid in leaching (H2O) | - | 1:5 |
| Duration of leaching | min | 60 |
| SiO2 | Al2O3 | Na2O | K2O | CaO | MgO | Fe2O3 | MnO | ZrO2 | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 60.6 | 15.15 | 7.2 | 4.58 | 0.77 | 0.45 | 4.82 | 0.16 | 0.27 | 0.092 | 0.31 |
| Products | Yield, % | Fe2O3 | ZrO2 | TiO2 | |||
|---|---|---|---|---|---|---|---|
| Content, % | Recovery, % | Content, % | Recovery, % | Content, % | Recovery, % | ||
| Magnetic Fraction 1 (MF1) | 15.64 | 28.20 | 94.11 | 0.39 | 17.09 | 0.34 | 43.22 |
| Magnetic Fraction 2 (MF2) | 4.66 | 3.51 | 3.50 | 3.15 | 41.60 | 0.80 | 30.70 |
| Non-Magnetic Fraction (NMF) | 79.70 | 0.14 | 2.40 | 0.18 | 41.31 | 0.04 | 26.08 |
| Feed | 100.00 | 4.69 | 100.00 | 0.35 | 100.00 | 0.12 | 100.00 |
| Products | SiO2 | Al2O3 | Na2O | K2O | CaO | MgO | Fe2O3 | MnO | ZrO2 | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aegirine (MF1) | 48.28 | 1.53 | 8.35 | 0.86 | 1.78 | 2.78 | 28.15 | 0.62 | 0.39 | 0.36 | 0.40 |
| Feldspars (NMF) | 64.10 | 17.21 | 7.10 | 5.11 | 0.55 | 0.16 | 0.14 | 0.01 | 0.18 | 0.04 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silin, I.; Dertmann, C.; Cvetković, V.S.; Stopic, S.; Friedrich, B. Prevention of Silica Gel Formation for Eudialyte Study Using New Digestion Reactor. Minerals 2024, 14, 124. https://doi.org/10.3390/min14020124
Silin I, Dertmann C, Cvetković VS, Stopic S, Friedrich B. Prevention of Silica Gel Formation for Eudialyte Study Using New Digestion Reactor. Minerals. 2024; 14(2):124. https://doi.org/10.3390/min14020124
Chicago/Turabian StyleSilin, Ivan, Christian Dertmann, Vesna S. Cvetković, Srecko Stopic, and Bernd Friedrich. 2024. "Prevention of Silica Gel Formation for Eudialyte Study Using New Digestion Reactor" Minerals 14, no. 2: 124. https://doi.org/10.3390/min14020124
APA StyleSilin, I., Dertmann, C., Cvetković, V. S., Stopic, S., & Friedrich, B. (2024). Prevention of Silica Gel Formation for Eudialyte Study Using New Digestion Reactor. Minerals, 14(2), 124. https://doi.org/10.3390/min14020124










