Abstract
The usage of bismuth (Bi), a critical and strategic raw material, has increased in the last 10 years. At present, the knowledge of Bi geochemistry is too limited to develop accurate mine waste and water management strategies to prevent environmental impact. Therefore, its geochemistry was studied in historical tailings in Yxsjöberg, Sweden. Intact tailings cores and shore samples were geochemically and mineralogically analyzed. Groundwater was sampled between 2016 and 2021 and analyzed for 71 elements and (SO4, F, Cl). The results were correlated with metals and dissolved organic matter (DOC), which have been previously published. The total concentrations, sequential extraction and scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM–EDS) mapping indicated that Bi had been mobilized from the primary mineral bismuthinite (Bi2S3). In the oxidized tailings from both the cores and shore, Bi was hypothesized to have adsorbed to iron (Fe) (hydr)oxides, which prohibited high concentrations of Bi leaching into the groundwater and surface water. Dissolved Bi in groundwater was significantly correlated with DOC. In surface water, dissolved Bi was transported more than 5 km from the tailings. This study indicates that Bi can become mobile from legacy mine waste due to the oxidation of bismuthinite and either be scavenged by adsorption of Fe (hydr)oxides or kept mobile in groundwater and surface water due to complexation with DOC.
1. Introduction
According to the European Green Deal, net carbon dioxide emissions within the European Union (EU) should be reduced to zero by 2050. To achieve this, several metals and materials are needed as components of green technology. The EU is striving for higher domestic production of these materials to increase resilience for the green transition and stabilize political security [1]. The supply chain vulnerability and economic importance of metals and minerals have therefore led to the definition of 34 critical raw materials and 17 strategic raw materials [2].
Bismuth (Bi) is classified as both a critical and a strategic raw material, and its use has increased steadily over the last decade, mainly in pharmaceutical and cosmetic products (62%), low-melting alloys (28%) and metallurgical additives (10%), and to a lesser extent in coatings, pigments and semiconductors [3]. As a result of the increased usage, in Stockholm, Sweden, for example, the concentration of Bi in wastewater treatment plants has increased fivefold during the last 10 years [4].
Bismuth occurs naturally in low concentrations in the bedrock, mainly as bismuthinite (Bi2S3) [2,5]. Bismuthinite is found in a wide range of mineral deposits but is usually regarded as a non-economic byproduct [6]. The Critical Raw Materials Act, published in 2023, highlights the need for a rapid increase in the mining of critical and strategic raw materials in the EU [1]. Mining and mineral extraction generate large quantities of tailings, and if they are not managed adequately, adverse impacts on the water quality and ecosystems in the vicinity of the mine site can occur [7]. This is due to mine drainage containing high concentrations of metals and metalloids, usually associated with a low pH due to sulfide oxidation, also called acid mine drainage (AMD) [8]. Research projects world-wide have mainly focused on AMD and tailings from sulfide deposits, but recently [9] showed that metals such as Be, F and W can mobilize in neutral mine drainage from skarn tailings. The most common sources of Bi are skarn and vein deposits [6]. This raises the question of whether increased mining and usage could change the geochemical cycle of Bi in the terrestrial environment, but as of yet, the geochemical knowledge of Bi in near-surface environments is poor, and studies of natural systems are very limited. Concentrations of Bi in the terrestrial environment are assumed to be low [10], but this has not been fully elucidated. In Sweden, Bi is not usually incorporated into standard analyses of groundwater and surface water [11], nor is it regularly monitored in groundwater programs [12]. No guideline values exist for surface water [4,13,14] or drinking water [15,16,17,18].
Concerns have also been raised that the current ecotoxicological knowledge of Bi is inadequate [19,20]. For example, there is contradictory evidence regarding Bi toxicity, with some reports classifying the element as harmless to humans and ecosystems [21,22], while others have shown that Bi can negatively affect reproduction and/or be acutely toxic to earthworms [23,24]. Bismuthinite is assumed to be stable under reducing conditions, similar to galena (PbS), but the weathering rate in an oxic environment has not, as far as we are aware, been determined. To achieve sustainable mine waste and water management with low environmental impact in future mining projects, the geochemistry of Bi needs to be better understood.
The tungsten (W) skarn tailings at the Yxsjöberg legacy mine site in Sweden contain a complex matrix including calcite (CaCO3), fluorite (CaF2) and sulfides (e.g., pyrrhotite Fe(1−x)S), together with enriched concentrations of beryllium (Be) (284 mg/kg), Bi (496 mg/kg), copper (Cu) (946 mg/kg) and W (960 mg/kg) [25]. Elevated concentrations of dissolved Bi have been found in the surface water downstream from Yxsjöberg legacy mine waste [26]. For this reason, the historical mine tailings at Yxsjöberg provided an interesting setting for studying the mobility and fate of Bi. The aim of this investigation was to elucidate the stability of Bi minerals in the tailings, the transport of Bi in groundwater, any uptake of Bi in secondary minerals and Bi association with organic matter. These results were also compared to the concentrations of Bi released into the surface water downstream from the mining site.
2. Study Site
The Yxsjöberg legacy mine waste has been described previously in detail by [25]. Here, described briefly: Tailings were generated from mining W, Cu and fluorite (CaF2) from a skarn ore deposit, and the tailings are stored in the municipality of Ljusnarsberg, approximately 250 km northwest of Stockholm, Sweden (SWEREF99 TM (N, E): 6655949, 487511) (Figure 1). The region is characterized by winters with four months of snow and an annual precipitation of 730 mm per year. The monthly average temperatures range from −5 °C in January to 15 °C in July (data collected between 1901 and 2016 at Ställdalen, Sweden, 16 km from Yxsjöberg) [27].
2.1. The Ore Minerals and Mining Operations
As summarized by [25], “The skarn-type deposit was formed 1789 ± 2 Ma ago in relation to post-kinematic granitoid intrusions [28]. The main skarn minerals of the ore lenses were pyroxenes, amphiboles, garnets and fluorite. The ore minerals included scheelite, chalcopyrite, pyrrhotite, pyrite and smaller amounts of magnetite [29]. Other accessory minerals were calcite, helvite, molybdenite, wolframite, sphalerite, apatite, titanite, chlorite, epidote, allanite, zircon and hematite [28].” Underground mining took place over three periods (1918–1920, 1935–1963 and 1969–1989) [30,31]. The mining of scheelite began in 1918 and was extracted using roasting and gravity separation. Fluorite and chalcopyrite were recovered in 1956. Mineral processing, including crushing and grinding, was carried out on site, and the tailings were pumped through several pipes into two repositories, Smaltjärnen (1897–1963) and Morkulltjärnen (1969–1989) [30].
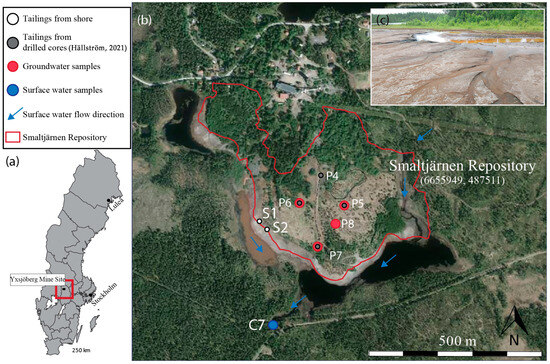
Figure 1.
(a) The location of the Yxsjöberg mine, northwest of Stockholm, Sweden. (b) The extent of the tailings (indicated by the red line) deposited in the Smaltjärnen repository between 1887 and 1963 [9]. (c) Secondary Iron (Fe) (hydr)oxides and gypsum (CaSO4·2H2O) minerals formed on the tailings shore [32]. The coordinates for Smaltjärnen Repository are given in SWEREF TM (N, E).
2.2. The Smaltjärnen Tailings Repository
This study focuses on the older repository, Smaltjärnen (Figure 1). The area into which the tailings were discharged consisted mainly of bogs and swamps [31]. At present, the Smaltjärnen repository covers 26 hectares and contains approximately 2.8 million tons of tailings discharged from 1887 to 1963. The repository descends from the industrial area to the east, south and southwest. The height difference between the industrial area and the downstream lake is 15–20 m, with a relatively uniform slope, except at the shore where it flattens out. The old repository was not remediated between active periods or directly after the closure of the mine. In 1994, a thin layer of sewage sludge was used to establish vegetation cover to suppress dust. At present, the vegetation varies over the repository, with high trees in the north and northwest areas and grass in the middle. Parts of the tailings are barren as a result of erosion.
3. Materials and Methods
3.1. Field Sampling and Environmental Mineralogy
Intact tailings cores (P4, P5, P7) were collected and described in detail by [25]. In brief, tailings core samples were taken from the surface down to the underlying strata using percussion drilling in 1.2-m Plexiglas tubes. A total of 99 tailings subsamples from all three cores, each 10–20 cm, were collected, and the chemical composition of each was analyzed by ALS Minerals, Piteå, Sweden, for 66 elements using the characterization packages CCP-PKG01 and F-ELE82, after pulverization with an agate mill (85% passing 75 µm). ALS Minerals classifies the elements into 8 groups, namely: “major elements, carbon, fluorine, LOI, sulfur, base metals, gold related trace elements, and resistive elements”. A full description of the methodology and analysis of each group can be found in [33].
Polished, uncovered thin sections of five subsamples from P4 were examined using point analysis using scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDS) (Oxford Instruments, High Wycombe, UK), using a high-resolution Zeiss MerlinTM FE-SEM (10 KeV and 1 µA) system equipped with the Oxford Aztec software.
Additionally, three samples (S1, S2:01, S2:02) were collected from the shore of the Smaltjärnen Repository in 2021 [32] and 2022. Subsamples of these were sent to ALS Scandinavia for analysis of the total concentrations of 71 elements using inductively coupled plasma (ICP) sector field mass spectrometry (SFMS) and fluoride (F−) ion chromatography (CSN ISO 10304-1) [34]. The samples were digested with an aqua regia and HF mixture with microwave-assisted digestion at 600 W for 60 min, and the concentrations were measured using ICP-SFMS (ELEMENT XR, ThermoScientific, Bremen, Germany).
The samples were analyzed mineralogically in three dimensions (3D) in low vacuum with the same instruments and settings as used for the thin sections, i.e., using a high-resolution Zeiss MerlinTM FE-SEM (10 KeV and 1 µA) system with the AZtec software.
3.2. Sequential Extraction of Tailings Samples
Five subsamples from core P4 were sent to SGS Canada Inc. (Lakefield, ON, Canada) for a seven-step sequential extraction, following the methodology of [35]; see Figure 2 for details. This procedure aim to extract: (1) water-soluble phases, (2) exchangeable phases, (3) easily reducible phases/minerals (e.g., Fe and Mn oxyhydroxides) and oxalate-extractable phases (e.g., Al oxyhydroxides), (4) reducible minerals (e.g., magnetite), (5) easily oxidizable phases/minerals (e.g., secondary sulfides), (6) resistant oxidizable minerals (e.g., primary sulfides) and (7) residues and silicates. The same sample was used during all steps; hence, the residual solid phase from one step was used in the next step.
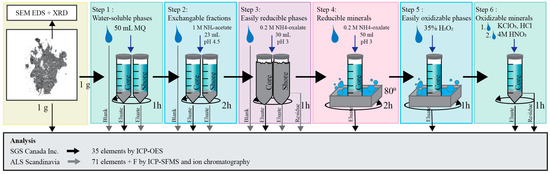
Figure 2.
The sequential extraction methodology [35] carried out on five samples from core P4 and two shore samples of Smaltjärnen tailings. Black arrows of eluate and residue symbolize analysis carried out at SGS Canada Inc. with ICP-OES, and gray arrows symbolize analysis carried out at ALS Scandinavia with ICP-SFMS.
All samples were pulverized until 85% passed 75 µm before the sequential extraction. For each eluate, the concentrations of 35 elements were analyzed with ICP-optical emission spectrometry (OES). The residue was analyzed after digestion with HNO3, HF and HClO4. The detection limit for elements extracted in each step was 10 mg/kg.
Additionally, samples S2:1 and S2:2 from the shore of Smaltjärnen were extracted at Luleå University of Technology using the first three steps of the seven-step sequential extraction methodology (Figure 2). The samples were sieved before the sequential extraction, and the eluates and residues from S2:1 and S2:2 were then sent to ALS Scandinavia for screening for 71 elements using ICP-SFMS (ELEMENT XR, ThermoScientific, Bremen, Germany) and F− ion chromatography (CSN ISO 10304-1) [34].
3.3. Groundwater and Surface Water Sampling
Groundwater from the tailings was collected from wells P5, P6, P7 and P8 (Figure 1) between 2016 and 2022. Well P4 was dry during all sampling occasions and could therefore not be sampled. In 2016, the groundwater in P8 was sampled once. In 2018, the groundwater in P7 was sampled monthly between May and October, while the groundwater in P5 was sampled two times and the groundwater in P6 was sampled three times. In 2021, wells P5, P7 and P8 were sampled in October. The groundwater was pumped using a portable Masterflex® peristaltic pump (Cole-Parmer® International, Chicago, IL, USA) connected to a silicon tube (9 mm). The water was pumped for 10–15 min to remove stagnant groundwater before the determination of pH, electrical conductivity (EC) and temperature. To avoid oxygenation of the groundwater, the tube was connected directly to a vacuum Sterifil® Aseptic System and Holder from Merck Millipore, with a diameter of 42 mm. The filters used were 0.22 µm cellulose acetate membrane filters that had been washed with 5% acetic acid for 72 h and rinsed with MilliQ water for 24 h [36]. Screening for 71 elements in the filtered groundwater and surface water (dissolved phases) was carried out by ALS Scandinavia using ICP-SFMS, while SO4 and F− were determined using ion chromatography (CSN ISO 10304-1) [34]. All analyses were carried out in duplicate, with appropriate blanks and standards for quality control. Particulate phases trapped on the filters used in surface water filtration were analyzed by ALS Scandinavia following the same procedure as for the dissolved phases after lithium metaborate and HNO3/HF/HCl digestion [37]. The filter holders were cleaned with 5% HNO3 between each sampling occasion, and blanks obtained after the cleaning process were used as controls. Each filter holder was used solely for samples collected from a specific sampling location, and new silicon tubes were used each time.
4. Results
The average Bi concentration of the total 99 samples from the tailings cores P4, P5 and P7 was 496 mg/kg, and the concentration of Bi in samples S1, S2:1 and S2:2 was 330, 345 and 385 mg/kg, respectively. These concentrations were between 1650 and 2500 times higher than the average Bi content of the Earth’s continental crust [38]. Bismuthinite was the major primary mineral hosting Bi, and in the tailings profile, it appeared mainly as small inclusions in silicate minerals (Figure 3a–f). Some of the bismuthinite grains had a porous and fractured structure (Figure 3e). Compared to the bismuthinite grains found in the tailings, the minerals at the tailings shore were single grains, not incorporated as inclusions in silicate minerals. These minerals at the shore appeared altered, with smooth edges and a porous structure (Figure 3g,h).
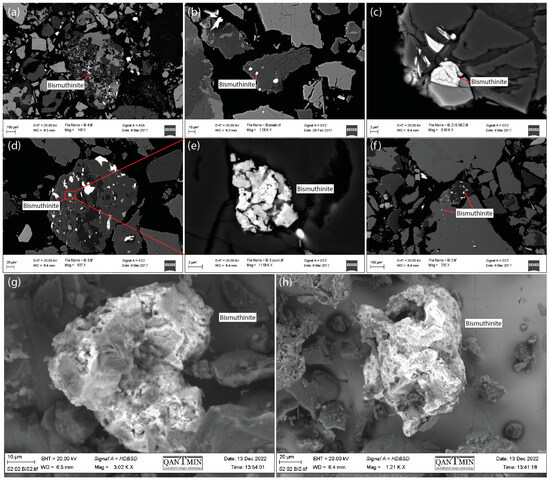
Figure 3.
Bismuthinite (Bi2S3) in the P4 tailings profile was observed mainly as small inclusions in silicate minerals (a–f). In the shore tailings, weathered bismuthinite was observed in three dimensions (g,h).
4.1. Sequential Depth Profile of the Tailings
The concentrations of water-soluble Bi (Step 1 in Table 1) were low, being below or close to the detection limit in all samples analyzed. In Step 2, relatively high concentrations of ‘exchangeable’, NH4 acetate-extractable Bi were observed in all five of the P4 samples. A maximum value of 197 mg/kg exchangeable Bi was found at a depth of 5 m. Lower concentrations of Bi were exchanged from the shore tailings (samples S2:1 and S2:2; 35 and 71 mg/kg, respectively). In Step 3, Bi was released in elevated concentrations in the uppermost tailings from P4 (30 cm depth) and in the shore tailings (S2:1 and S2:2): 57, 48 and 45 mg/kg, respectively. The highest Bi concentrations were found in Step 6, represented by primary sulfides, in both P4 and the shore residues. The results from all the P4, S2:1 and S2:2 samples mirrored the average content of the tailings.

Table 1.
Bismuth (Bi) concentrations in mg/kg in sequential extraction of five samples from the P4 core tailings and the S2:1 and S2:2 samples from the shore tailings.
4.2. Metals in the Groundwater
The pH of the groundwater at Smaltjärnen varied, with the highest pH found in P5 (6.8–7.0) and the lowest pH found in P7 (6.2–6.5) (Figure 4). The EC was anti-correlated with the pH, with the highest values found in P7 (maximum 2.8 mS/cm) and the lowest in P5 (minimum 2.0 mS/cm). Concentrations of the major elements aluminum (Al), calcium (Ca), iron (Fe), fluorine (F), potassium (K), magnesium (Mg), sodium (Na), sulfur (S) and silica (Si) in the groundwater are published by [39].
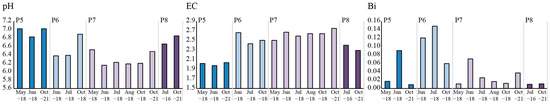
Figure 4.
pH, electrical conductivity (EC) (mS/cm) and Bi (µg/L) levels in the groundwater from wells P5, P6, P7 and P8, sampled between 2016 and 2021.
The concentrations of Bi were low but still higher than the detection limit (0.005 µg/L) in all groundwater wells, and the concentrations varied between the different groundwater wells and over time (Figure 4). The average Bi concentration in the groundwater at P5, P6, P7 and P8 was 0.04 ± 0.04, 0.11 ± 0.04, 0.03 ± 0.02 and 0.01 ± 0.001 µg/L, respectively. The highest concentration of Bi was found in P6 during July 2018 (maximum 0.15 µg/L).
5. Discussion
Bismuth was found in elevated concentrations in the Smaltjärnen tailings cores and at the shore of the repository and was more than 1500 times higher than the average levels found in the continental crust [38]. The dominant mineral hosting Bi was bismuthinite (Bi3S2), and the SEM-EDS point analysis of the tailings indicated that it occurred mainly as inclusions in unweathered silicate minerals. Even so, some inclusions of bismuthinite had a porous and fractured structure. The fractures could come from mechanical weathering or sample preparation, while the porous structure is likely to come from chemical weathering.
The Smaltjärnen tailings have been open to the atmosphere for more than 30 years, resulting in sulfide oxidation decreasing the pH from 8 to 4 in the upper parts of the tailings, with a subsequent release of Fe and sulfate (SO4) [25]. Calcite in the tailings has buffered the pH, generating neutral mine drainage and formation of Fe (hydr)oxides in the tailings cores, the tailings shore and in the downstream surface water [26,32,39]. Dissolved Ca, F and Fe and sulfate (with an average concentration of 588 ± 64, 37 ± 36, 39 ± 16 and 510 ± 95 mg/L, respectively) were the dominant ions in the groundwater, controlling the EC, with an average level of 2.4 mS/cm [39]. The tailings groundwater drained to the shore of the tailings. It has been hypothesized that the shore at Smaltjärnen Repository acts as a chemical barrier to metals (e.g., Be and W) released from tailings as a result of adsorption by secondary gypsum and Fe (hydr)oxides [32].
Only a very few studies are available regarding Bi geochemistry in mining waste and contaminated soils in the vicinity of mining areas [40,41], and studies regarding the adsorption of Bi to secondary gypsum or Fe-(hydr)oxides are lacking. Bismuth belongs to group 15 in the periodic table and exhibits similar geochemical behavior to arsenic (As) and antimony (Sb) [40]. The electron configuration of Bi3+ is similar to Pb2+, and it is well known that the reactivity of, for example, galena (PbS) is much lower than that of pyrite and pyrrhotite because of a more stable crystal structure and the lack of ferrous Fe [42]. Environmental mineralogy combined with sequential extraction indicated that Bi has been mobilized from bismuthinite in the Smaltjärnen tailings; see discussion below.
5.1. Bismuth Mobility in the Tailings and Transport to Groundwater
A comparison of the relative mobility of silver (Ag), Bismuth (Bi), indium (In), antimony (Sb) and tin (Sn) in column leaching tests using uncontaminated soil showed that Bi and In had the highest mobility [43]. Until now, Bi has been assumed to be a non-mobile element, replacing lead (Pb) in ammunition [4]. In the Smaltjärnen repository, previous studies of Bi concentrations by depth in tailings have indicated that Bi mainly acts as an immobile element [25], but peaks of Bi were found in P4 at a depth of 1.5 m, in P5 at 2 and 4 m and in P7 at 0.5 m (Figure 5).
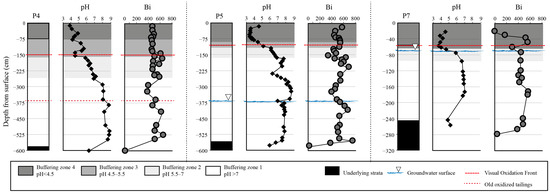
Figure 5.
pH and solid Bi concentrations (mg/kg) in the tailings profiles from cores P4, P5 and P7, modified after [25].
In the upper 40 cm of P7, Bi concentrations decreased stepwise and were below the detection limit at the very top of the tailings. In the top centimeters of the tailings of P7, all elements were low, except for carbon (C) [25]. Carbon is originally found in calcite in the tailings (5.7 wt.%) but has been depleted in the upper parts of the tailings when buffering acidity from sulfide oxidation. The enrichment of C in the upper parts of P7 could have an organic origin; however, the tailings there are not covered by vegetation, and no measurements of dissolved organic matter (DOC) have been performed there. One possibility is that Bi has been mobilized due to complexation with organic carbon and later physically removed from the tailings in P7. Looking at the morphology of the tailings, the area above P7 was eroded. Large volumes of water from the spring flood and autumn rain run horizontally in the northern area of the tailings (P4, P5, P6), and, when the slope flattens out at P7, the water starts to infiltrate the tailings, possibly leaching Bi–organic complexes from P7.
According to the sequential extraction from P4, higher concentrations of Bi were released from exchangeable phases throughout the tailings, indicating that either (1) 30%–40% of all Bi had been released from bismuthinite in the tailings and adsorbed as exchangeable fractions, or (2) bismuthinite is easily weathered in low-pH environments with high organic matter, which was the geochemical environment at the leaching step. A newly published paper by [44] showed that Bi forms exceptionally strong complexes with natural organic matter and with a wide range of organic acids. This supports alternative two, meaning that the acetic acid with a pH of 4 added in Step 2 likely affected the results of the sequential extraction. Hence, strong complexation between the acetic acid and Bi3+ could explain the high concentrations of Bi released during Step 2 of the sequential extraction. The results might not reflect the ‘true’ geochemical behavior of the Bi-containing mineral phases in mining waste unless large amounts of organic matter are present. However, the organic carbon concentrations in the tailings of P4 were very low (at or below the detection limit) [45], which makes it probable that the high acetate-extractable Bi is an artifact of the sequential extraction method. Knowledge regarding the importance of Bi–organic complexes is still at an early stage, and further studies are needed.
We hypothesize that the adsorption of Bi to secondary Fe (hydr)oxides played an important role in scavenging Bi and prohibiting it from reaching the groundwater (Figure 6). Thus, Bi was released by oxalate extraction (Step 3) in the upper parts of the tailings, which suggests the role of the adsorption of Bi to oxyhydroxides. In the upper parts, secondary gypsum, Fe (hydr)oxides and clay minerals were all present [25,39,45]. The Fe (hydr)oxides were too small to define specific minerals with the characterization techniques available in that study, e.g., Raman spectroscopy [39]. Water-soluble concentrations of Bi were only released in the tailings when the pH was high and also where Fe (hydr)oxides were absent, as can be seen in Figure 6. This suggests that Bi has been adsorbed to Fe (hydr)oxides in the upper parts of the tailings profile.
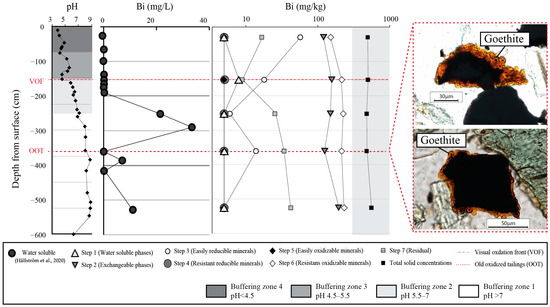
Figure 6.
pH and water-soluble Bi concentrations (µg/L) in tailings core P4, modified after [39], Bi released in core P4 during the seven-step sequential extraction (this study), and goethite presence determined by Raman spectroscopy in old oxidized tailings [39].
In the lower parts of the tailings, larger crystals of Fe (hydr)oxides were present in the old oxidized tailings (OOT) [39]. There, concentrations of water-soluble Bi were below the detection limit, and the easily reducible phase concentrations were higher (10 mg/kg) (Figure 6), again indicating an adsorption to oxyhydroxides. Raman spectroscopy measurements of the spherical crystal growth structure of Fe (hydr)oxides formed on sulfides and scheelite in the OOT were identified as goethite (α-FeO(OH)), with Raman bands at 88, 299, 383, 559, 684, 1000 and 1315 cm−1 [39]. However, more research is needed, e.g., with the extended X-ray absorption fine structure (EXAFS) spectroscopy, to confirm whether Bi has adsorbed to Fe (hydr)oxides.
5.2. Correlation between Dissolved Bi and DOC in Groundwater
Only low concentrations of dissolved Bi (maximum 0.11 µg/L) were detected in the groundwater of all four wells (Figure 4 and Figure 7). What is notable is that the dissolved Bi concentrations are very low compared to other trace metals in the tailings (e.g., Be, W and Zn) [9]. The average concentration of Bi in 1470 samples from 600 groundwater wells in Sweden between 2015 and 2021 was 0.04 µg/L [12], making the concentrations in Smaltjärnen only slightly elevated. Hence, Bi mobilized from bismuthinite in the tailings profile is assumed to have been adsorbed to Fe (hydr)oxides/goethite, prohibiting Bi from leaching in high concentrations into the Smaltjärnen groundwater. Looking at the groundwater trends, the dissolved concentrations of Bi did not behave in a way similar to the major elements. The lowest concentrations of Bi were detected when the EC was high. DOC concentrations in the groundwater were published by [45] when determining the isotopic signature of carbon (C) and oxygen (O) in the groundwater and surface water of Yxsjöberg and were sampled simultaneously as Bi concentrations.
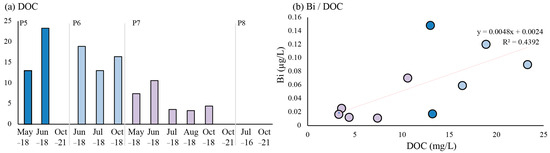
Figure 7.
(a) Dissolved organic compounds (DOC) data published by [45], and (b) the correlation between dissolved organic carbon (DOC) and Bi in the groundwater of wells P5, P6 and P7.
A correlation between dissolved Bi and DOC could be observed in the groundwater, with an R2 value of 0.44 (Figure 7). Regression analysis with a confidence level of 95% showed that the correlation between DOC and dissolved Bi was significant with a p-value of 0.04. However, the concentrations are low, and the data points are few. More research is needed to prove the relationship between Bi and DOC. With that in mind, these data indicate that low concentrations of Bi were kept in the dissolved phase (<0.22 µm) due to complexation with DOC in the groundwater. The significant relationship observed between Bi and DOC in the groundwater agrees with the findings of [44], indicating that Bi forms very strong complexes with natural organic matter.
5.3. Bismuth Mobility on the Tailings Shore and Transport to Surface Water
At the tailings shore, the average concentration of Bi was lower (353 mg/kg) than in the core samples (496 mg/kg). The bismuthinite found in the shore tailings was probably deposited directly from the processing plant rather than having been transported to the shore by physical movement through the repository. Orthophotos from 1963 show that there was a spigot point close to the sampling locations of S1 and S2 [25]. The lower concentrations of Bi at the shore may indicate that Bi was removed from the shore to the surface water downstream. However, no information is available regarding the contents of Bi that had been released at different spigot points during the active years of the mining operations, and the difference in concentrations could also arise from variations in gangue minerals in the ore. The bismuthinite grains found in the shore tailings showed strong signs of weathering, with high degrees of freedom, having an amorphous porous structure with soft edges and a weathered appearance (Figure 3).
Similar to the results from the core tailings profile, Bi was released in elevated concentrations from Step 2: exchangeable fractions (35 and 71 mg/kg), and in Step 3: the oxalate-extractable phases (48 and 45 mg/kg, respectively) of the sequential extraction. SEM–EDS measurements between each step of the sequential extraction showed that Fe had been removed after Step 3 [32], with subsequent higher concentrations of Fe in the eluate. The presence of Fe (hydr)oxides in the tailings, combined with a decrease of Fe in the SEM–EDS mapping after Step 3 and the increased concentrations of Bi and Fe in the eluates, indicate that Bi had been adsorbed/co-precipitated with Fe (hydr)oxides on the tailings shore. Low-vacuum 3D SEM-EDS pictures of S2 showed Fe (hydr)oxides with the same structure as seen within the OOT, which were proven to be goethite.
This means that the majority of Bi mobilized from bismuthinite on the tailings shore was scavenged by adsorption to goethite and transported by erosion to the surface water. There, a significant correlation (p-value 0.02) between Bi and Fe (hydr)oxides was observed, with an R2-value of 0.58 between suspended Bi and Fe after removing one outlier from C7. Including the C7 outlier, the R2 value between Bi and Fe was 0.36 (Figure 8). The surface water was sampled in 2018 for dissolved and suspended matter (see [26] for a detailed description of the methodology). The suspended matter with Bi adsorbed to Fe (hyrd)oxides settled to the sediments a few 100 m from the Yxsjöberg mine site. Thus, the concentrations of suspended Bi and Fe were elevated in the surface water close to the Yxsjöberg Mine site (average: 0.6 µg/L and 2.8 mg/L, respectively) compared to a reference (average: 0.1 µg/L and 0.9 mg/L) that was sampled by [26] in a nearby stream that belonged to another catchment area. After approximately 1 km, at C14, the Bi and Fe concentrations had decreased to concentrations below the reference value (0.1 µg/L and 0.5 mg/L).
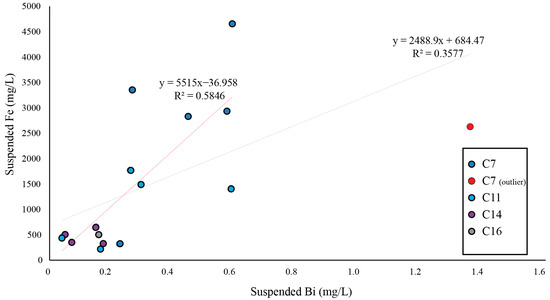
Figure 8.
Relationship between suspended Fe and Bi in the surface water downstream of the Smaltjärnen repository. The different R2 values represent the inclusion/exclusion of the C7 outlier marked in red. See Figure 9 for sample locations.
Studies of the adsorption of Bi to Fe (hydr)oxides in mine waste are, to the best of our knowledge, missing. The uptake of Bi in goethite and the presence of other Fe (hydr)oxides need to be investigated further with more detailed measurements than were possible in this study, for example, by using synchrotron instruments.
5.4. Relationship between Dissolved Bi and Organic Matter in Downstream Surface Water
The small part of Bi that did not adsorb to Fe (hydr)oxides was transported in the dissolved phase more than 5 km from the Yxsjöberg mine site. When correlating surface water data regarding DOC [45] and dissolved concentrations of Bi [26], a likely, but not significant, relationship between the two was observed (p-value 0.08), as can be seen in Figure 9. The lowest concentrations of Bi were detected when the EC levels were at their maximum, and the highest concentrations were detected during the spring flood (Figure 9) when all other elements were diluted by the high water flow [39]. The spring flood contained elevated concentrations of dissolved organic matter (DOC), and it is likely that organic matter has formed strong complexes with Bi, keeping it mobile in the downstream surface water with a neutral pH. Thus, dissolved Bi was transported more than 5 km from the mine site [26].
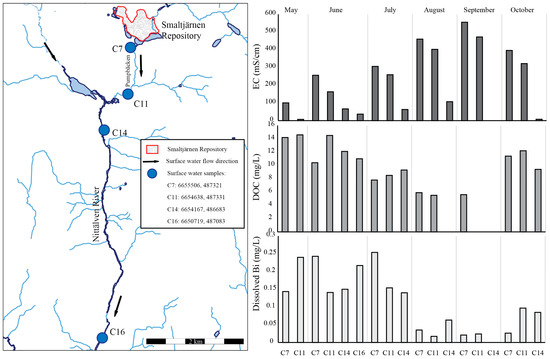
Figure 9.
Electrical conductivity (EC) data published by [26], DOC data published by [45] and dissolved Bi concentrations published by [26] in samples C7, C11, C14 and C16 from May to October. The coordinates for the sampling points are given in SWEREF TM (N, E).
6. Conclusions
The main bismuth (Bi) mineral present in the tungsten (W)-skarn tailings in the Smaltjärnen Repository from the Yxsjöberg mine was bismuthinite (Bi3S2). Environmental mineralogy combined with sequential extraction indicated that Bi had been mobilized from bismuthinite in the upper parts of the tailings, where the geochemical conditions were low-pH (minimum pH 4) and oxygenated. There, Bi was released from Step 3 of the sequential extraction, and iron (Fe) (hydr)oxides were present. Bi was also released from Step 3 from deeper tailings, where a second layer of old oxidized tailings (OOT) containing goethite was detected in previous studies. Thus, Bi is likely to have adsorbed to goethite and other iron (Fe) (hydr)oxides, which acted as a chemical barrier and prevented Bi from reaching the groundwater in high concentrations. Adsorption of Bi to Fe (hydr)oxides needs to be studied further. The groundwater contained low concentrations of dissolved Bi (maximum 0.15 μg/L). A significant correlation (p-value 0.036) between dissolved Bi and DOC was found in the groundwater, suggesting that Bi was complexed with DOC, which enhanced the mobility of dissolved Bi that had reached the groundwater.
The same geochemical process of bismuthinite weathering and adsorption to goethite occurred at the tailings shore, as indicated by strong weathered grains of Bi, formations of goethite and the release of Bi and Fe during Step 3 of the sequential extraction. A likely, but not significant, correlation between dissolved Bi and DOC was also observed in the surface water downstream of the tailings. An increase in organic matter during the spring flood might have increased the mobility of Bi as a result of complexation, but this needs to be investigated further.
The ability of Bi to form organic complexes might have influenced Step 2 of the sequential extraction, as ammonium acetate was used as a reagent. The results, which indicated that 30%–40% of the Bi in the tailings was in exchangeable phases, indicate a problem with the sequential extraction method used, which needs to be studied further.
Author Contributions
Conceptualization, L.P.B.H., methodology, L.P.B.H. and J.P.G.; software, L.P.B.H.; validation, L.P.B.H. and J.P.G.; resources, L.P.B.H.; writing—original draft preparation, L.P.B.H. and J.P.G.; writing—review and editing, L.P.B.H. and J.P.G.; visualization, L.P.B.H.; project administration, L.P.B.H.; funding acquisition, L.P.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Geological Survey of Sweden (DNR 36-2802/2021), Vinnova (Grant 215 06 631), and co-funded by the Center of Advanced Mining and Metallurgy (CAMM2) at LTU.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank our colleagues from Applied Geochemistry/Swedish School of Mines at Luleå University of Technology (LTU) and Soil Chemistry at the Swedish University of Agricultural Sciences in Sweden. A special thank you is dedicated to Lena Alakangas and Olof Martinsson at LTU for guidance, and to Peder Englund at NIRAS Sweden AB who carried out the drilling of intact tailings cores. Also, we thank our former colleagues within the collaboration of the ERA-MIN project and the REMinE project from Luleå University of Technology (LTU) in Sweden, Porto University in Portugal and the National Institute for Metals and Radioactive Resources in Romania.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Commission. Critical Raw Materials: Ensuring Secure and Sustainable Supply Chains for EU’s Green and Digital Future. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_23_1661 (accessed on 27 March 2023).
- European Commission. Study of the Critical Raw Materials for the EU 2023—Final Report. Available online: https://single-market-economy.ec.europa.eu/publications/study-critical-raw-materials-eu-2023-final-report_en (accessed on 27 March 2023).
- Eilu, P.; Bjerkgård, T.; Franzson, H.; Gautneb, H.; Häkkinen, T.; Jonsson, E. The Nordic Supply Potential of Critical Metals and Minerals for a Green Energy Transition; Nordic Innovation Report; 2021; ISBN 978-82-8277-115-3 (digital publication), ISBN 978-82-8277-114-6 (printed). Available online: https://www.nordicinnovation.org/2021/nordic-supply-potential-critical-metals-and-mineralsgreen-energy-transition (accessed on 27 March 2023).
- Amneklev, J.; Sörme, L.; Augustsson, A.; Bergbäck, B. The increase in bismuth consumption as reflected in sewage sludge. Water Air Soil Pollut. 2015, 226, 92. [Google Scholar] [CrossRef]
- Salminen, R.; Bidovec, M.; Demetriades, A.; De Vivo, B. Geochemical Atlas of Europe. Part 1: Background Information, Methodology and Maps, Espoo, Finland. ISBN 951-690-913-2. Available online: http://weppi.gtk.fi/publ/foregsatlas/maps_table.php (accessed on 6 October 2020).
- Deady, E.; Moon, C.; Moore, K.; Goodenough, K.M.; Shail, R.K. Bismuth: Economic geology and value chains. Ore Geol. Rev. 2022, 143, 104722. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. J. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. J. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Hällström, L.P.B. Source, Mobility and Fate of Critical Be, Bi, F and W from Historical Sulfidic-Oxidic Skarn Tailings: Re-Mining as Remediation Method? Ph.D. Dissertation, Luleå University of Technology, Luleå, Sweden, 2021. [Google Scholar]
- Murata, T. Bismuth solubility through binding by various organic compounds and naturally occurring soil organic matter. J. Environ. Sci. Health Part A 2010, 45, 746–753. [Google Scholar] [CrossRef]
- ALS Scandinavia. Analytical Services. Available online: www.alsglobal.se/en/environment (accessed on 25 August 2023).
- The Geological Survey of Sweden’s Environmental Monitoring of Groundwater. Available online: www.sgu.se/grundvatten/miljoovervakning-av-grundvatten/ (accessed on 25 August 2023).
- Swedish Agency Marine and Water Management. Report: The Swedish Agency Marine and Water Management regulations on classification and environmental quality standards regarding surface water. HVMFS 2019, 25, pp. 1–88. Available online: https://www.havochvatten.se/vagledning-foreskrifter-och-lagar/foreskrifter/register-vattenforvaltning/klassificering-och-miljokvalitetsnormer-avseende-ytvatten-hvmfs-201925.html (accessed on 23 January 2024).
- Swedish Agency Marine and Water Management. Report: Environmental toxins in Water—Classification of Surface Water Status. Guid. Appl. HVMFS 2013, 19, pp. 1–104. Available online: https://viss.lansstyrelsen.se/referencelibrary/54097/vagledn-miljogiftklassning-hvmfs201319.pdf (accessed on 23 January 2024).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption, Official Journal of the European Union, L435/1, 2020, pp. 1–62. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 23 January 2024).
- The Swedish National Food Agency’s Regulations on Drinking Water. Svenska Livsmedelsverkets Föreskrifter om Dricksvatten. LIVSFS 2022, 12, pp. 1-32. Available online: https://www.livsmedelsverket.se/globalassets/om-oss/lagstiftning/dricksvatten---naturl-mineralv-kallv/livsfs-2022-12_web_t.pdf (accessed on 23 January 2024).
- Australian Government; National Health and Medical Research Council; Natural Resources Management Ministerial Council. National Water Quality Management Strategy. Australia Drinking Water Guidelines 6; Version 3.4. Commonwealth of Australia 2011. 2017, pp. 1–1167. ISBN Online: 1864965118. Available online: https://www.nhmrc.gov.au/about-us/publications/australian-drinking-water-guidelines (accessed on 23 January 2024).
- Guidelines for Drinking Water Quality, 4th ed.; Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. Available online: https://fctc.who.int/publications/i/item/9789241549950 (accessed on 23 January 2024).
- Fahey, N.S.; Tsuji, L.J. Is there a need to re-examine the approval of bismuth shotshell as a non-toxic alternative to lead based on the precautionary principle? J. Environ. Monit. 2006, 8, 1190–1194. [Google Scholar] [CrossRef]
- Fahey, N.S.; Karagatzides, J.D.; Jayasinghe, R.; Tsuji, L.J. Wetland soil and vegetation bismuth content following experimental deposition of bismuth pellets. J. Environ. Monit. 2008, 10, 951–954. [Google Scholar] [CrossRef]
- Thomas, V.G. Chemical compositional standards for non-lead hunting ammunition and fishing weights. Ambio 2019, 48, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Mineral Tolerance of Animals; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Ghaffari, M.A.; Motlagh, B. In vitro effect of lead, silver, tin, mercury, indium and bismuth on human sperm creatine kinase activity: A presumable mechanism for men infertility. Iran. Biomed. J. 2011, 15, 38–43. [Google Scholar] [PubMed]
- Omouri, Z.; Hawari, J.; Fournier, M.; Robidoux, P.Y. Bioavailability and chronic toxicity of bismuth citrate to earthworm Eisenia andrei exposed to natural sandy soil. Ecotoxicol. Environ. Saf. 2018, 147, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hällström, L.P.B.; Alakangas, L.; Martinsson, O. Geochemical characterization of W, Cu and F skarn tailings at Yxsjöberg, Sweden. J. Geochem. Explor. 2018, 194, 266–279. [Google Scholar] [CrossRef]
- Hällström, L.P.B. Mobility of Be, Bi, F, Ga, Ge and W in Surface Water and the Water Quality Impact on Epilithic Diatoms Downstream of the Historical Yxsjöberg Mine Site, Sweden. Mine Water Environ. 2022, 41, 731–747. [Google Scholar] [CrossRef]
- SMHI. Available online: https://opendata-download-metobs.smhi.se/explore/?parameter=3 (accessed on 18 January 2018).
- Romer, R.L.; Öhlander, B. U-Pb age of the Yxsjöberg Tungsten Skarn deposit, Sweden. GFF 1994, 116, 161–166. [Google Scholar] [CrossRef]
- Ohlsson, L.G. Tungsten occurences in central Sweden. Econ. Geol. 1979, 74, 1012–1034. [Google Scholar] [CrossRef]
- Höglund, L.O.; Jones, C.; Lindgren, M. Consultant report: Prestudy of remediation options for tailings in Yxsjöberg. In Swedish: Förstudie för efterbehandling av sandmagasin i Yxsjöberg. Kemakta AR 2003, 23. Available online: https://scholar.google.com/scholar_lookup?title=F%C3%B6rstudie%20f%C3%B6r%20efterbehandling%20av%20sandmagasin%20i%20Yxsj%C3%B6berg%20-%20Kemakta%20AR%202003-23&publication_year=2004&author=L.O.%20H%C3%B6glund&author=C.%20Jones&author=M.%20Lindgren (accessed on 23 January 2024).
- Rothelius, E. Swedish Mineral Dressing Mills, Short Descriptions and Flowsheets; International Mineral Dressing Congress: Stockholm, Sweden, 1957; pp. 1–9. [Google Scholar]
- Hällström, L.P.B.; Flodin, E. Secondary Gypsum and HFO Affected the Mobility of Critical Metals (Be&W) in Historical Skarn Tailings, Yxsjöberg, Sweden; IMWA Conference Paper, Newport, Wales; 2023. Available online: https://www.imwa.info/docs/imwa_2023/IMWA2023_Hallstrom_185.pdf (accessed on 22 January 2024).
- ALS Global. Complete Characterization Packages. Available online: https://alsglobal.com/en/geochemistry/rock-characterisation/complete-characterisation-packages-packages (accessed on 10 January 2024).
- CSN, I. 10304-1 (757391) Water Quality–Determination of Dissolved Fluoride, Chloride, Nitrite, Orthophosphate, Bromide, Nitrate and Sulfate Ions Using Liquid Chromatography of Ions-Part 1: Method for Water with Low Contamination. 1995. Available online: https://app.nbn.be/data/r/platform/frontend/detail (accessed on 23 January 2024).
- Dold, B. Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J. Geochem. Explor. 2023, 80, 55–68. [Google Scholar] [CrossRef]
- Ödman, F.; Ruth, T.; Pontér, C. Validation of a field filtration technique for characterization of suspended particulate matter from freshwater. Part I. Major elements. Appl Geochem. 1999, 14, 301–317. [Google Scholar] [CrossRef]
- Ödman, F.; Ruth, T.; Rodushkin, I.; Pontér, C. Validation of a field filtration technique for characterization of suspended particulate matter from freshwater. Part II. Minor, trace and ultra trace elements. Appl. Geochem. 2006, 21, 2112–2134. [Google Scholar] [CrossRef]
- Krauskopf, K.B.; Bird, D.K. Introduction to Geochemistry; MacGraw-Hill: New York, NY, USA, 1995; p. 647. [Google Scholar]
- Hällström, L.P.B.; Alakangas, L.; Martinsson, O. Scheelite weathering and tungsten (W) mobility in historical oxidic-sulfidic skarn tailings at Yxsjöberg, Sweden. Environ. Sci. Pollut. Res. 2020, 27, 6180–6192. [Google Scholar] [CrossRef]
- Jung, M.C.; Thornton, I.; Chon, H. Arsenic, Sb and Bi contamination of soils, plants, waters and sediments in the vicinity of the Dalsung Cu–W mine in Korea. Sci. Total. Environ. 2002, 295, 81–89. [Google Scholar] [CrossRef]
- Wei, C.; Deng, Q.; Wu, F.; Fu, Z.; Xu, L. Arsenic, antimony, and bismuth uptake and accumulation by plants in an old antimony mine, China. Biol. Trace. Elem. Res. 2011, 144, 1150–1158. [Google Scholar] [CrossRef]
- Lottermoser, B. Mine Water. In Mine Wastes; Springer: Berlin/Heidelberg, Germany, 2003; pp. 83–141. [Google Scholar]
- Hou, H.; Takamatsu, T.; Koshikawa, M.K.; Hosomi, M.; Koshikawa, M.K. Migration of silver, indium, tin, antimony, and bismuth and variations in their chemical fractions on addition to uncontaminated soils. Soil Sci. 2005, 170, 624–639. [Google Scholar] [CrossRef]
- Kleja, D.B.; Gustafsson, J.P.; Kessler, V.; Persson, I. Bismuth (III) Forms Exceptionally Strong Complexes with Natural Organic Matter. Environ. Sci. Technol. 2022, 56, 3076–3084. [Google Scholar] [CrossRef]
- Salifu, M.; Aiglsperger, T.; Alakangas, L. Biogeochemical controls on 13CDIC signatures from Circum-Neutral pH Groundwater in Cu–W–F Skarn tailings to acidic downstream surface waters. Minerals 2020, 10, 758. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).