Effect of Microgravity on Rare Earth Elements Recovery by Burkholderia cepacia and Aspergillus niger
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microbe Incubation
2.2.1. In Normal Gravity
2.2.2. In Microgravity
2.3. Batch of Recovery Experiments
2.3.1. Recovery of REEs by B. cepacia
2.3.2. Recovery of REEs by A. niger
2.4. REEs Concentration Determined by ICP-OES
3. Results and Discussion
3.1. Recovery of REEs by B. cepacia
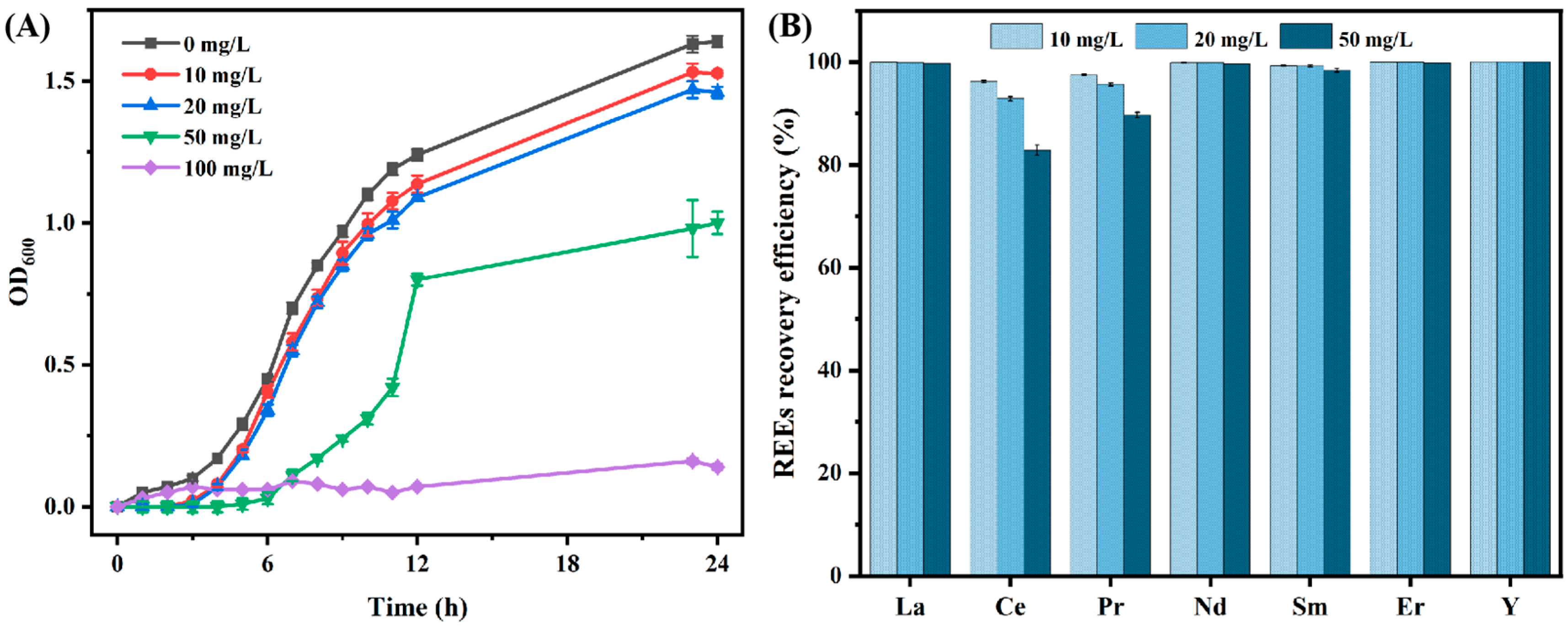
3.1.1. Effect of Different Initial REEs Concentrations on the Recovery Efficiency of REEs by B. cepacia
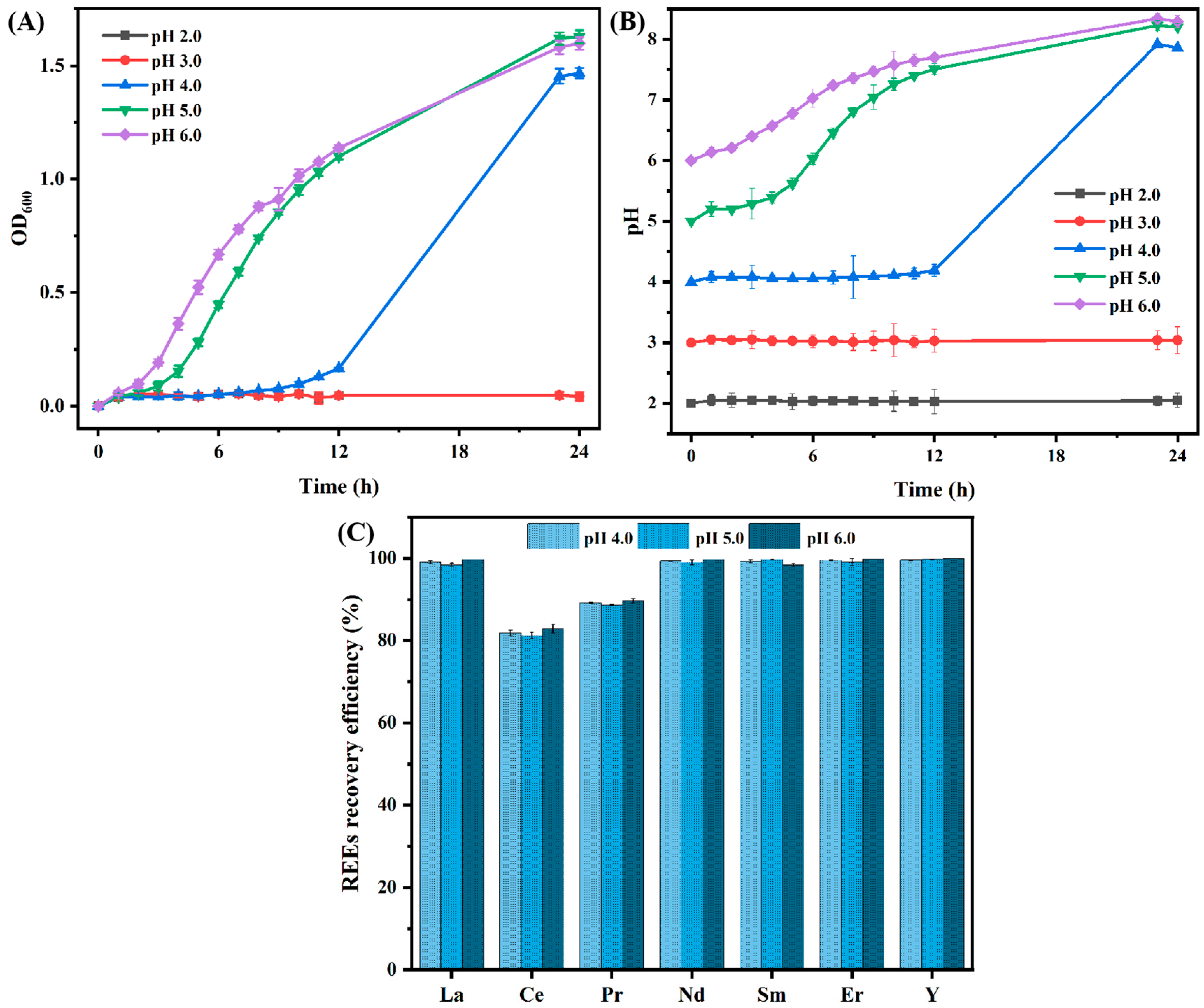
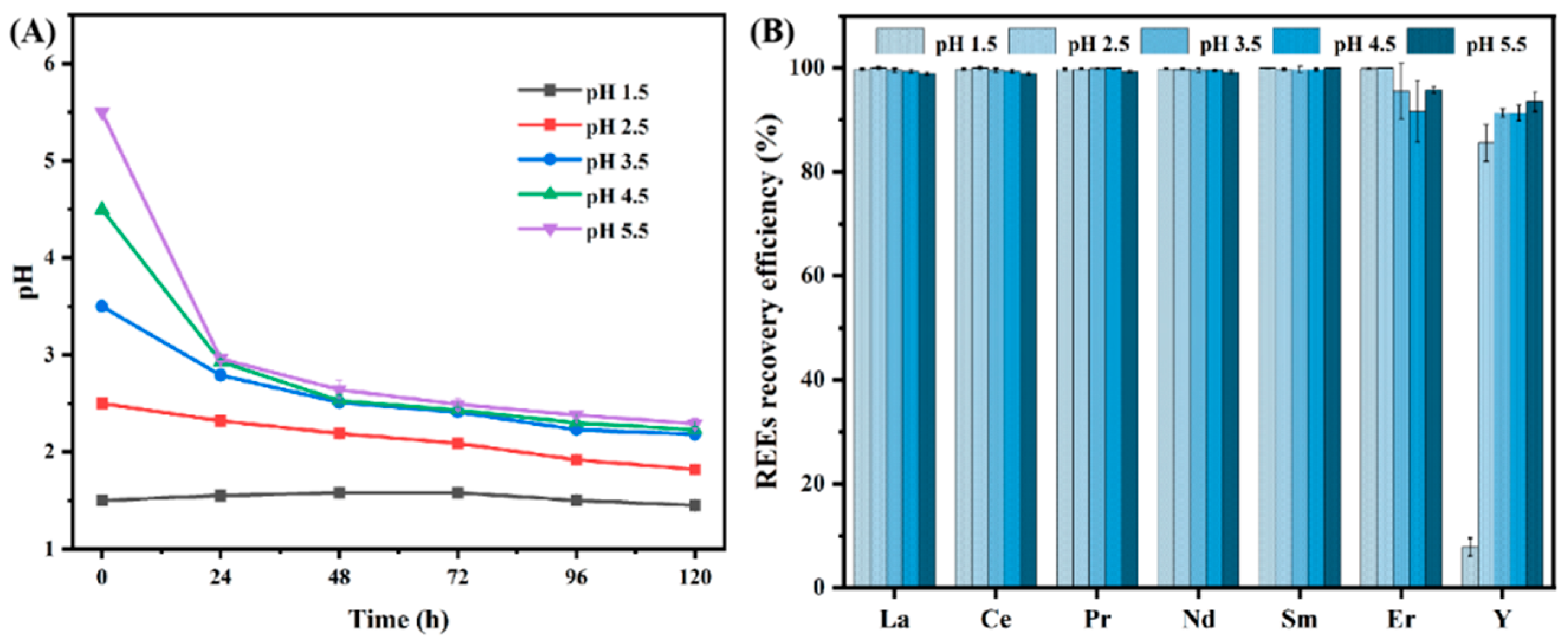
3.1.2. Effect of Different Initial pH Levels on the Recovery Efficiency of REEs by B. cepacia
3.2. Recovery of REEs by A. niger
3.2.1. Effect of Different Initial REEs Concentrations on the Recovery Efficiency of REEs by A. niger
3.2.2. Effect of Different Initial pH Levels on the Recovery Efficiency of REEs by A. niger
3.3. Microgravity Incubation
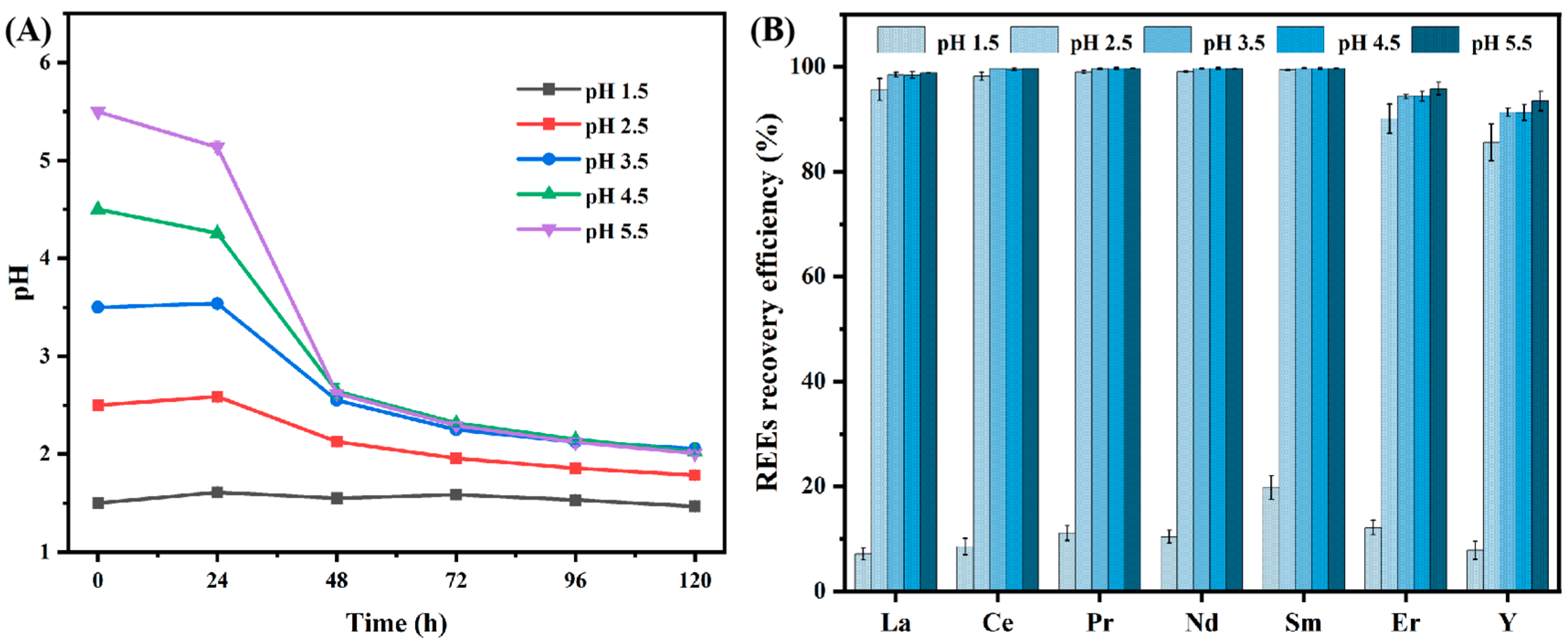
3.3.1. Effect of Microgravity Incubation on B. cepacia Biosorption Ability
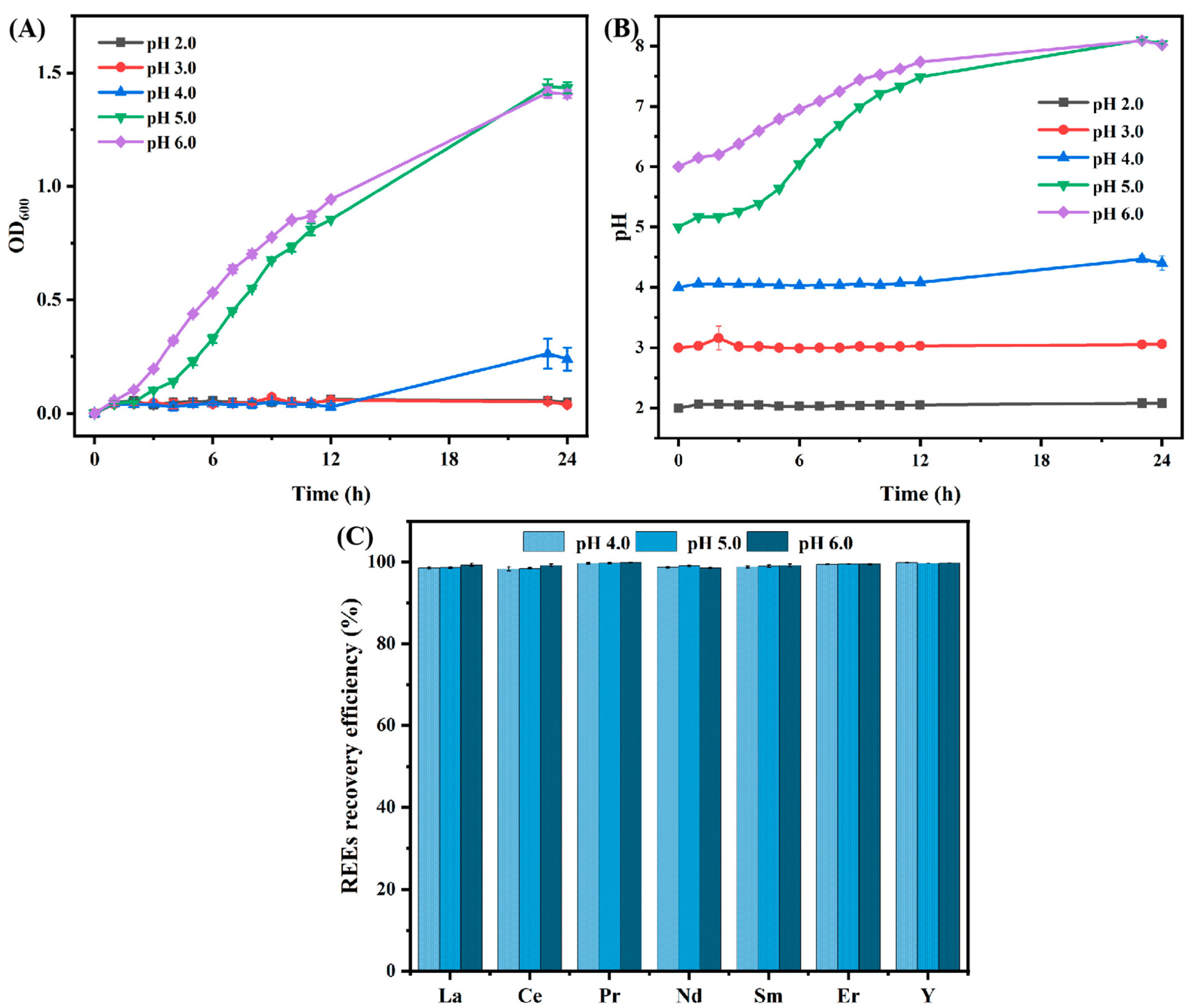
3.3.2. Effect of Microgravity Incubation on A. niger Biosorption Ability
3.4. Possible Recovery Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, X.Y.; Zhao, H.B.; Zhang, Y.S.; Shen, L.; Gu, G.H.; Qiu, G.Z.; Zhang, X.G.; Yu, H.; He, X.; Liu, C. Simulated bioleaching of ion-adsorption rare earth ore using metabolites of biosynthetic citrate: An alternative to cation exchange leaching. Miner. Eng. 2022, 189, 107900. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.B.; Shen, L.; Qiu, G.Z.; Wang, Y.Y.; Zhao, Y.; Zhao, H.; Shen, L.; Qiu, G.; Wang, Y. Study on the role of microbial metabolites in in-situ noncontact bioleaching of ion-adsorption rare earth ore. J. Environ. Manag. 2024, 368, 122184. [Google Scholar] [CrossRef]
- Brewer, A.; Chang, E.; Park, D.M.; Kou, T.Y.; Li, Y.; Lammers, L.N.; Jiao, Y.Q.; Brewer, A.; Chang, E.; Park, D.M.; et al. Recovery of Rare Earth Elements from Geothermal Fluids through Bacterial Cell Surface Adsorption. Environ. Sci. Technol. 2019, 53, 7714. [Google Scholar] [CrossRef]
- Das, N.; Das, D.; Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earths 2013, 31, 933. [Google Scholar] [CrossRef]
- Park, D.M.; Brewer, A.; Reed, D.W.; Lammers, L.N.; Jiao, Y.Q. Recovery of Rare Earth Elements from Low-Grade Feedstock Leachates Using Engineered Bacteria. Environ. Sci. Technol. 2017, 51, 13471. [Google Scholar] [CrossRef] [PubMed]
- Taggart, R.K.; Hower, J.C.; Hsu-Kim, H. Effects of roasting additives and leaching parameters on the extraction of rare earth elements from coal fly ash. Int. J. Coal Geol. 2018, 196, 106. [Google Scholar] [CrossRef]
- Tofan, L.; Bojoaga, C.N.; Paduraru, C.; Tofan, L.; Bojoaga, C.-N.; Paduraru, C. Biosorption the recovery and analysis of rare earth elements and platinum group metals from real samples. A review. Environ. Chem. Lett. 2022, 20, 1225. [Google Scholar] [CrossRef]
- Owusu-Fordjour, E.Y.; Yang, X.B. Bioleaching of rare earth elements challenges and opportunities: A critical review. J. Environ. Chem. Eng. 2023, 11, 110413. [Google Scholar] [CrossRef]
- Wu, M.X.; Liang, J.J.; Tang, J.; Li, G.; Shan, S.P.; Guo, Z.H.; Deng, L.; Wu, M.; Liang, J.; Tang, J.; et al. Decontamination of multiple heavy metals-containing effluents through microbial biotechnology. J. Hazard. Mater. 2017, 337, 189. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes-a review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhao, H.B.; Zhao, Y.; Shen, L.; Gu, G.H.; Qiu, G.Z.; Meng, X.; Zhao, H.; Zhao, Y.; Shen, L.; et al. Heap leaching of ion adsorption rare earth ores and REEs recovery from leachate with lixiviant regeneration. Sci. Total Environ. 2023, 898, 165417. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M.; Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, W.Z.; Liu, D.S.; Liu, T.; Wang, Z.D.; Jiang, L.; Zhou, W.; Liu, D.; Liu, T.; Wang, Z. Biosorption isotherm study of Cd2+, Pb2+ and Zn2+ biosorption onto marine bacterium Pseudoalteromonas sp SCSE709-6 in multiple systems. J. Mol. Liq. 2017, 247, 230. [Google Scholar] [CrossRef]
- Önal, S.; Baysal, S.H.; Ozdemir, G.; Onal, S.; Baysal, S.H.; Ozdemir, G. Studies on the applicability of alginate-entrapped Chryseomonas luteola TEM 05 for heavy metal biosorption. J. Hazard. Mater. 2007, 146, 417. [Google Scholar] [CrossRef]
- Nourbakhsh, M.N.; Kiliçarslan, S.; Ilhan, S.; Ozdag, H.; Nourbakhsh, M.N.; Kiliçarslan, S.; Ilhan, S.; Ozdag, H. Biosorption of Cr6+, Pb2+ and Cu2+ ions in industrial waste water on Bacillus sp. Chem. Eng. J. 2002, 85, 351. [Google Scholar] [CrossRef]
- Lu, N.Q.; Hu, T.J.; Zhai, Y.B.; Qin, H.Q.; Aliyeva, J.; Zhang, H.; Lu, N.; Hu, T.; Zhai, Y.; Qin, H.; et al. Fungal cell with artificial metal container for heavy metals biosorption: Equilibrium, kinetics study and mechanisms analysis. Environ. Res. 2020, 182, 109061. [Google Scholar] [CrossRef]
- Texier, A.C.; Andrès, Y.; Le Cloirec, P.; Texier, A.C.; Andrès, Y.; Le Cloirec, P. Selective biosorption of lanthanide (La, Eu, Yb) ions by Pseudomonas Aeruginosa. Environ. Sci. Technol. 1999, 33, 489. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Ohnuki, T.; Tanaka, K.; Kozai, N.; Kamiishi, E.; Utsunomiya, S.; Jiang, M.; Ohnuki, T.; Tanaka, K.; Kozai, N.; et al. Post-adsorption process of Yb phosphate nano-particle formation by Saccharomyces cerevisiae. Geochim. Cosmochim. Acta 2012, 93, 30. [Google Scholar] [CrossRef]
- Fein, J.B.; Martin, A.M.; Wightman, P.G.; Fein, J.B.; Martin, A.M.; Wightman, P.G. Metal adsorption onto bacterial surfaces: Development of a predictive approach. Geochim. Cosmochim. Acta 2001, 65, 4267. [Google Scholar] [CrossRef]
- Park, D.M.; Reed, D.W.; Yung, M.C.; Eslamimanesh, A.; Lencka, M.M.; Anderko, A.; Fujita, Y.; Riman, R.E.; Navrotsky, A.; Jiao, Y.Q. Bioadsorption of Rare Earth Elements through Cell Surface Display of Lanthanide Binding Tags. Environ. Sci. Technol. 2016, 50, 2735. [Google Scholar] [CrossRef]
- Johnson, D.B.; Johnson, D.B. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; Pierson, D.L.; Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; Pierson, D.L. Microbial responses to microgravity and other low-shear environments. Microbiol. Mol. Biol. Rev. 2004, 68, 345. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, D.G.; Huang, Y.; Liu, C.T.; Huang, B.; Li, D.-G.; Huang, Y.; Liu, C.-T. Effects of spaceflight and simulated microgravity on microbial growth and secondary metabolism. Mil. Med. Res. 2018, 5, 18. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L.; Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121. [Google Scholar] [CrossRef]
- Wilson, J.W.; Ott, C.M.; Bentrup, K.H.Z.; Ramamurthy, R.; Quick, L.; Porwollik, S.; Cheng, P.; McClelland, M.; Tsaprailis, G.; Radabaugh, T.; et al. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc. Natl. Acad. Sci. USA 2007, 104, 16299. [Google Scholar] [CrossRef]
- Wilson, J.W.; Ramamurthy, R.; Porwollik, S.; McClelland, M.; Hammond, T.; Allen, P.; Ott, C.M.; Pierson, D.L.; Nickerson, C.A. Microarray analysis identifies Salmonella genes belonging to the low-shear modeled microgravity regulon. Proc. Natl. Acad. Sci. USA 2002, 99, 13807. [Google Scholar] [CrossRef] [PubMed]
- Vukanti, R.; Model, M.A.; Leff, L.G.; Vukanti, R.; Model, M.A.; Leff, L.G. Effect of modeled reduced gravity conditions on bacterial morphology and physiology. Bmc Microbiol. 2012, 12, 4. [Google Scholar] [CrossRef]
- Kacena, M.A.; Merrell, G.A.; Manfredi, B.; Smith, E.E.; Klaus, D.M.; Todd, P.; Kacena, M.A.; Merrell, G.A.; Manfredi, B.; Smith, E.E.; et al. Bacterial growth in space flight: Logistic growth curve parameters for Escherichia coli and Bacillus subtilis. Appl. Microbiol. Biotechnol. 1999, 51, 229. [Google Scholar] [CrossRef]
- Benoit, M.R.; Li, W.; Stodieck, L.S.; Lam, K.S.; Winther, C.L.; Roane, T.M.; Klaus, D.M.; Benoit, M.R.; Li, W.; Stodieck, L.S.; et al. Microbial antibiotic production aboard the International Space Station. Appl. Microbiol. Biotechnol. 2006, 70, 403. [Google Scholar] [CrossRef]
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; Van Houdt, R.; Leys, N.; et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat. Commun. 2020, 11, 5523. [Google Scholar] [CrossRef]
- Gumulya, Y.; Zea, L.; Kaksonen, A.H.; Gumulya, Y.; Zea, L.; Kaksonen, A.H. In situ resource utilisation: The potential for space biomining. Miner. Eng. 2022, 176, 107288. [Google Scholar] [CrossRef]
- Akberdin, I.R.; Collins, D.A.; Hamilton, R.; Oshchepkov, D.Y.; Shukla, A.K.; Nicora, C.D.; Nakayasu, E.S.; Adkins, J.N.; Kaiyuzhnaya, M.G.; Akberdin, I.R.; et al. Rare Earth Elements Alter Redox Balance in Methylomicrobium alcaliphilum 20ZR. Front. Microbiol. 2018, 9, 2735. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Li, L.L.; Chen, A.W.; Zeng, Q.R.; Xia, H.; Gu, J.D.; Luo, S.; Li, L.; Chen, A.; Zeng, Q.; et al. Biosorption of diethyl phthalate ester by living and nonliving Burkholderia cepacia and the role of its cell surface components. Chemosphere 2017, 178, 187. [Google Scholar] [CrossRef] [PubMed]
- Vo, P.H.N.; Danaee, S.; Hai, H.T.N.; Huy, L.N.; Nguyen, T.A.H.; Nguyen, H.T.M.; Kuzhiumparambil, U.; Kim, M.; Nghiem, L.D.; Ralph, P.J. Biomining for sustainable recovery of rare earth elements from mining waste: A comprehensive review. Sci. Total Environ. 2024, 908, 168210. [Google Scholar] [CrossRef]
- He, N.; Ran, M.D.; Hu, L.; Jiang, C.Y.Z.; Liu, Y.Y.; He, N.; Ran, M.; Hu, L.; Jiang, C.; Liu, Y. Periplasmic space is the key location for Pb (II) biomineralization by Burkholderia cepacia. J. Hazard. Mater. 2023, 445, 130465. [Google Scholar] [CrossRef]
- Amini, M.; Younesi, H.; Bahramifar, N.; Lorestani, A.A.Z.; Ghorbani, F.; Daneshi, A.; Sharifzadeh, M.; Amini, M.; Younesi, H.; Bahramifar, N.; et al. Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus Niger. J. Hazard. Mater. 2008, 154, 694. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, H.; Shi, Q.Y.; Meng, X.Y.; Zhao, Y.; Qiu, G.Z.; Zhang, X.G.; Yu, H.; He, X.; He, H.J.; et al. Comparative chemical and non-contact bioleaching of ion-adsorption type rare earth ore using ammonium sulfate and metabolites of Aspergillus niger and Yarrowia lipolytica to rationalise the role of organic acids for sustainable processing. Hydrometallurgy 2023, 216, 106019. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhao, H.B.; Zhao, Y.; Shen, L.; Gu, G.H.; Qiu, G.Z. Effective recovery of rare earth from (bio)leaching solution through precipitation of rare earth-citrate complex. Water Res. 2023, 233, 119752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Shao, S.; Yu, X.; Kang, J.; Qiu, G.; Chen, Z.; Zhao, H.; Shen, L. Comparison of biosorption behavior and mechanism of La3+, Sm3+, Y3+, Nd3+, Er3+ by Aspergillus niger and Bacillus sp. J. Water Process Eng. 2024, 59, 104965. [Google Scholar] [CrossRef]
- Atasoy, M.; Ordóñez, A.A.; Cenian, A.; Djukic-Vukovic, A.; Lund, P.A.; Ozogul, F.; Trcek, J.; Ziv, C.; De Biase, D.; Atasoy, M.; et al. Exploitation of microbial activities at low pH to enhance planetary health. Fems Microbiol. Rev. 2024, 48, fuad062. [Google Scholar] [CrossRef]
- Roy, P.K.; Ha, A.J.W.; Mizan, M.F.R.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Nahar, S.; Kim, Y.K.; Ha, S.D.; Roy, P.K.; et al. Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poultry Science 2021, 100, 101209. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Inoue, N.; Takamatsu, Y.; Kamada, K.; Wakao, N.; Isobe, K.; Inoue, N.; Takamatsu, Y.; Kamada, K.; Wakao, N. Production of catalase by fungi growing at low pH and high temperature. J. Biosci. Bioeng. 2006, 101, 73. [Google Scholar] [CrossRef]
- Papagianni, M.; Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189. [Google Scholar] [CrossRef]
- Ma, J.J.; Li, S.Y.; Wang, J.X.; Jiang, S.T.; Panchal, B.; Sun, Y.Z. Bioleaching rare earth elements from coal fly ash by Aspergillus Niger. Fuel 2023, 354, 129387. [Google Scholar] [CrossRef]
- He, N.; Hu, L.; He, Z.G.; Li, M.K.; Huang, Y.J.; He, N.; Hu, L.; He, Z.; Li, M.; Huang, Y. Mineralization of lead by Phanerochaete chrysosporium microcapsules loaded with hydroxyapatite. J. Hazard. Mater. 2022, 422, 126902. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhou, H.; Qiu, G.Z.; Zhao, H.B.; Shen, L.; Zhou, H.; Qiu, G.; Zhao, H. A review of bioleaching combined with low-cost fermentation and metabolic engineering technology to leach rare earth elements. J. Environ. Chem. Eng. 2024, 12, 112117. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R.; Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship between host acidification and virulence of Penicillium spp. on apple and citrus fruit. Phytopathology 2004, 94, 44. [Google Scholar] [CrossRef]
- Castro-Wallace, S.; Stahl, S.; Voorhies, A.; Lorenzi, H.; Douglas, G.L.; Castro-Wallace, S.; Stahl, S.; Voorhies, A.; Lorenzi, H.; Douglas, G.L. Response of Lactobacillus acidophilus ATCC 4356 to low-shear modeled microgravity. Acta Astronaut. 2017, 139, 463. [Google Scholar] [CrossRef]
- Kim, H.W.; Rhee, M.S.; Kim, H.W.; Rhee, M.S. Low-shear modeled microgravity impacts the acid stress response and post-thermal stress behavior of acid-resistant, adaptable, and sensitive Escherichia coli O157:H7 strains. Food Control 2021, 121, 107603. [Google Scholar] [CrossRef]
- Fang, A.; Pierson, D.L.; Mishra, S.K.; Demain, A.L.; Fang, A.; Pierson, D.L.; Mishra, S.K.; Demain, A.L. Growth of Streptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl. Microbiol. Biotechnol. 2000, 54, 33. [Google Scholar] [CrossRef]
- Fang, A.; Pierson, D.L.; Mishra, S.K.; Koenig, D.W.; Demain, A.L. Secondary metabolism in simulated microgravity: Beta-lactam production by Streptomyces clavuligerus. J. Ind. Microbiol. Biotechnol. 1997, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Zhang, Q.; Dang, L.; Chen, N.A.; Yin, Z.; Ma, L.L.; Feng, Y.L.; Li, W.L.; Wei, Y.L.; Zhang, W.D.; et al. The interaction between Aspergillus brasiliensis and exposed copper circuits in the space microgravity environment. Corros. Sci. 2024, 234, 112132. [Google Scholar] [CrossRef]
- Kalpana, D.; Im, C.; Lee, Y.S. Comparative growth, cross stress resistance, transcriptomics of Streptococcus pyogenes cultured under low shear modeled microgravity and normal gravity. Saudi J. Biol. Sci. 2016, 23, 24. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, N.; Zhang, Z.; Meng, X.; Davaasambuu, S.; Zhao, H. Effect of Microgravity on Rare Earth Elements Recovery by Burkholderia cepacia and Aspergillus niger. Minerals 2024, 14, 1055. https://doi.org/10.3390/min14101055
He N, Zhang Z, Meng X, Davaasambuu S, Zhao H. Effect of Microgravity on Rare Earth Elements Recovery by Burkholderia cepacia and Aspergillus niger. Minerals. 2024; 14(10):1055. https://doi.org/10.3390/min14101055
Chicago/Turabian StyleHe, Ni, Zhongxian Zhang, Xiaoyu Meng, Sarangerel Davaasambuu, and Hongbo Zhao. 2024. "Effect of Microgravity on Rare Earth Elements Recovery by Burkholderia cepacia and Aspergillus niger" Minerals 14, no. 10: 1055. https://doi.org/10.3390/min14101055
APA StyleHe, N., Zhang, Z., Meng, X., Davaasambuu, S., & Zhao, H. (2024). Effect of Microgravity on Rare Earth Elements Recovery by Burkholderia cepacia and Aspergillus niger. Minerals, 14(10), 1055. https://doi.org/10.3390/min14101055






