Whole-Rock Geochemistry and Mica Compositions in Lijiagou Pegmatite Spodumene Deposit, Western Sichuan, China
Abstract
1. Introduction
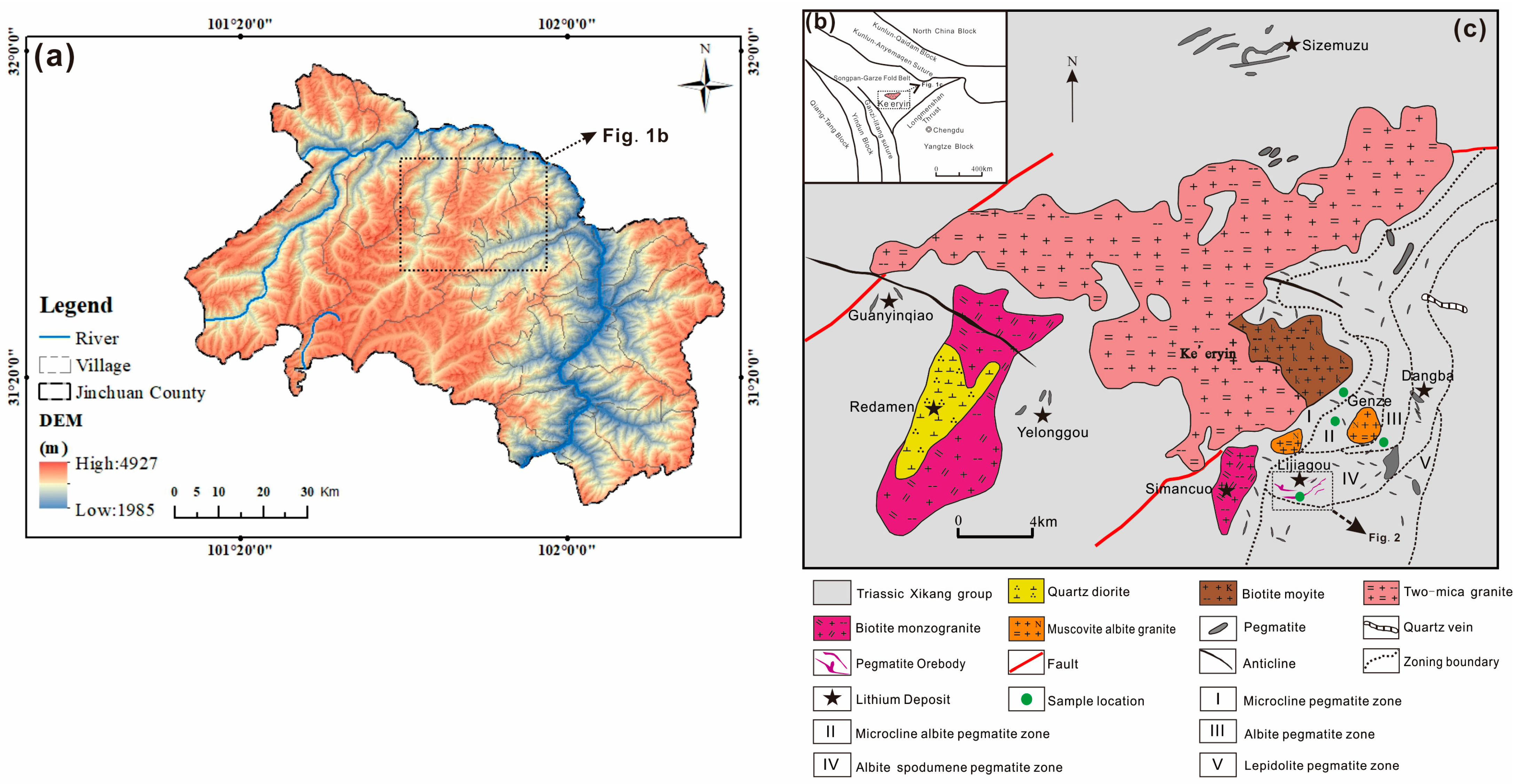
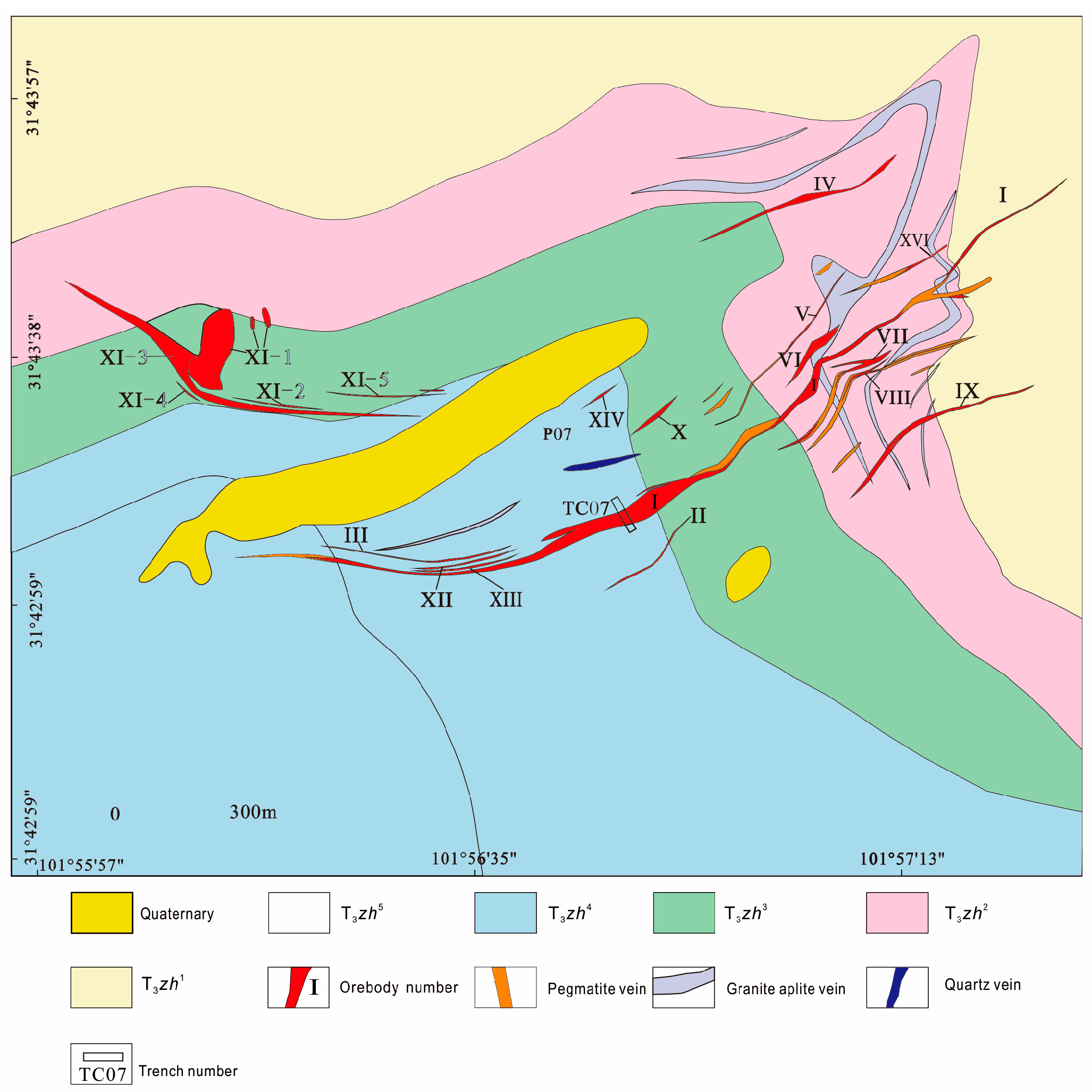
2. Geological Background
3. Sample and Petrographic Characteristics
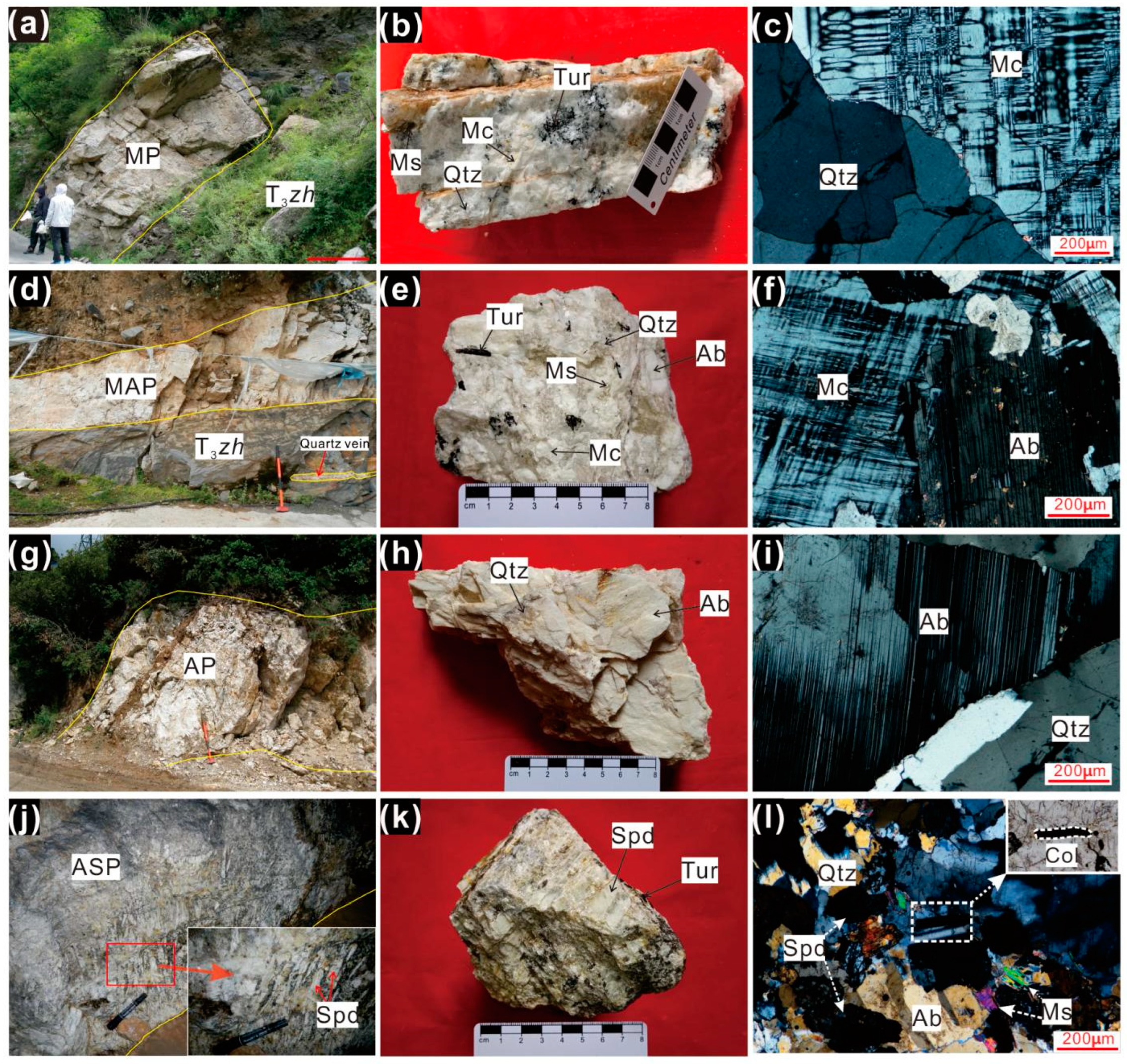
3.1. Petrographic Characteristics of the Pegmatite
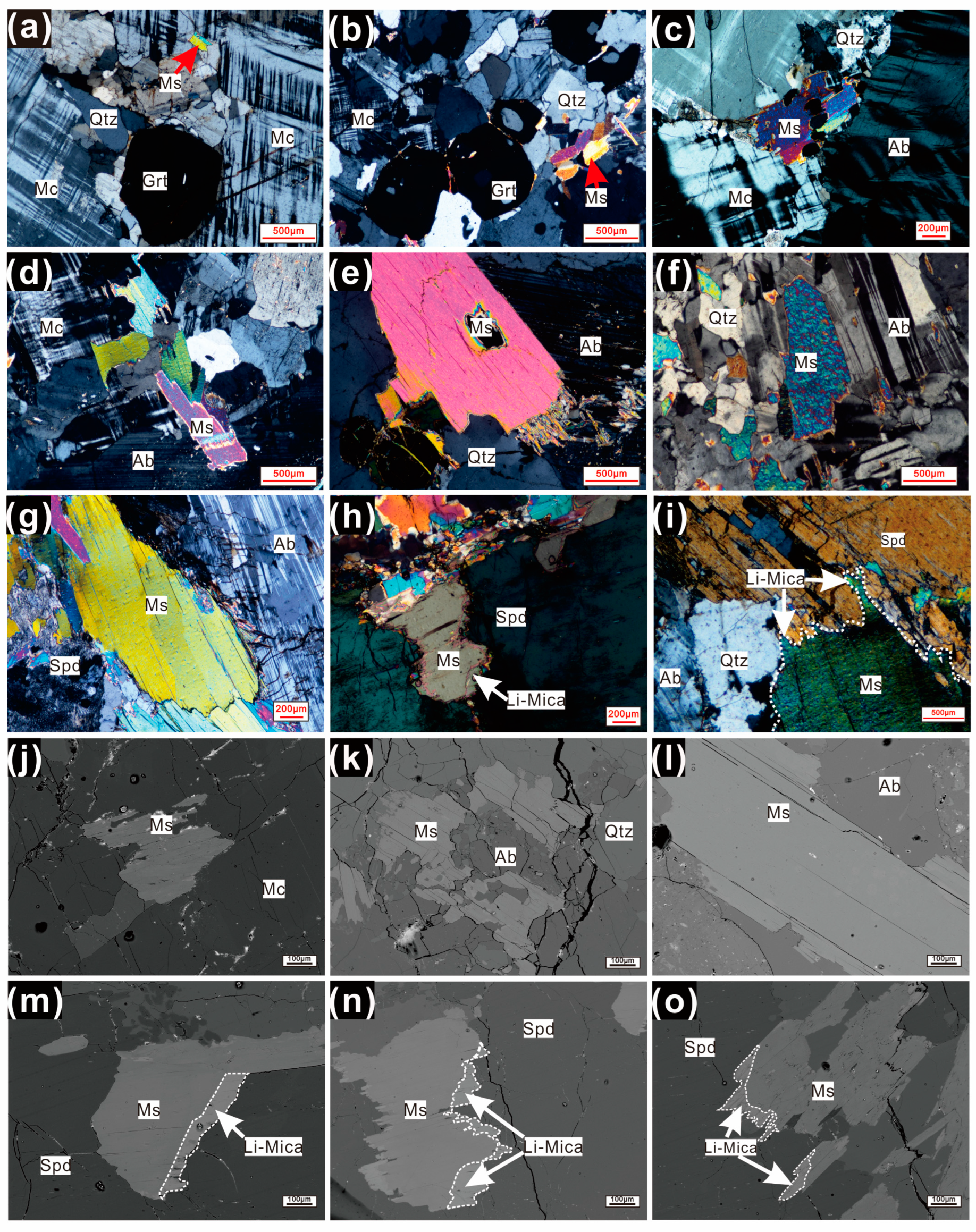
3.2. Mineralogical Characteristics of Micas
4. Analytical Methods
4.1. Whole-Rock Analysis
4.2. EPMA Analysis
4.3. LA-ICPMS Analysis
5. Results
5.1. Whole-Rock Geochemistry
5.2. Major and Trace Element Compositions of Muscovite
6. Discussion
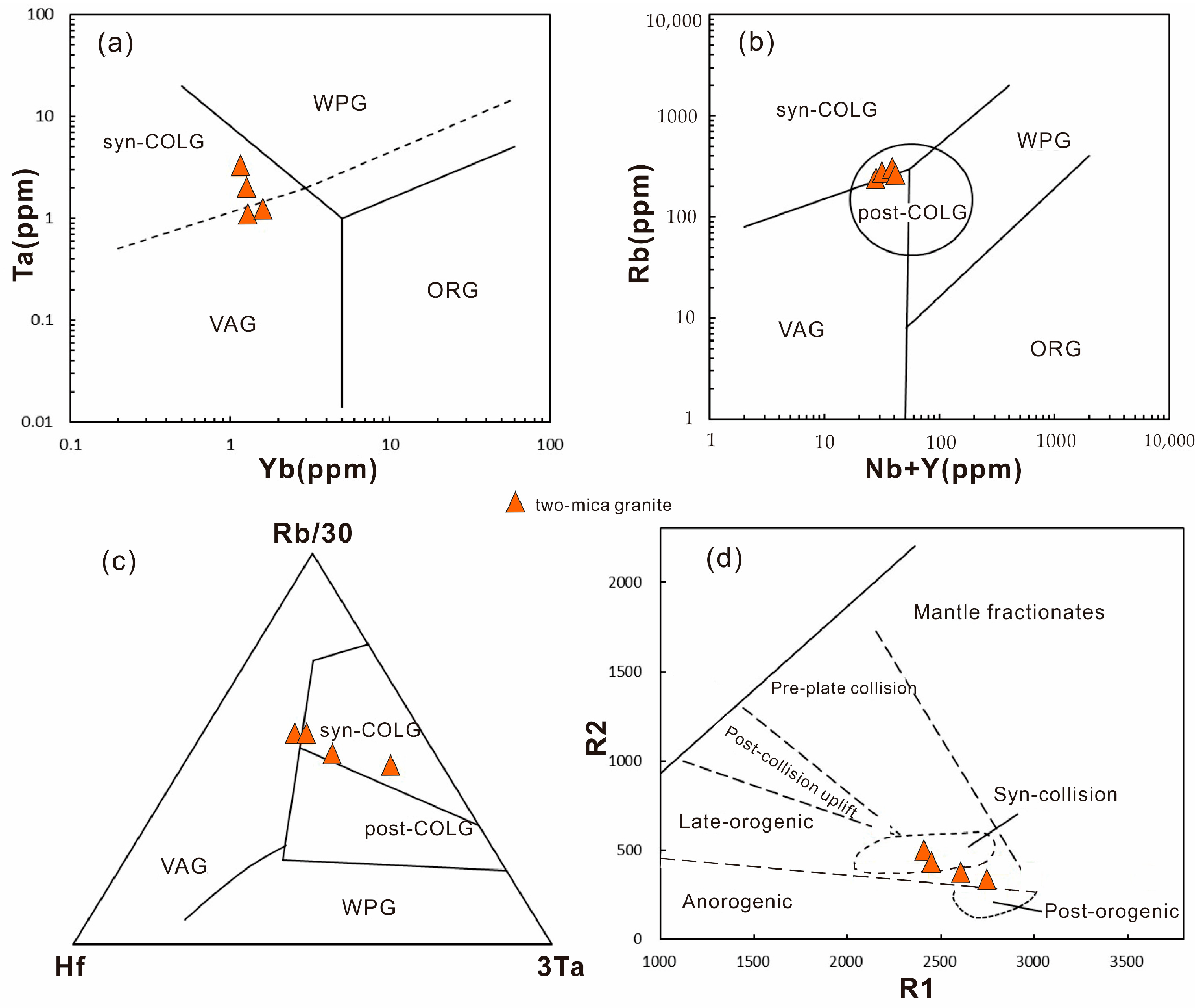
6.1. Tectonic Environment
6.2. Indications of Mica for the Evolution of Pegmatite
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.S.; Yuan, Q.H. The global supply situation of lithium ore and suggestions on resources security in China. China Min. Mag. 2019, 28, 1–6, (In Chinese with English Abstract). [Google Scholar]
- Wen, H.J.; Luo, C.G.; Du, S.J.; Yu, W.X.; Gu, H.N.; Ling, K.Y.; Cui, Y.; Li, Y.; Yang, J.H. Carbonate-hosted clay-type lithium deposit and its prospecting significance. Chin. Sci. Bull. 2020, 65, 53–59, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, D.H. Study on critical mineral resources: Significance of research, determination of types, attributes of resources, progress of prospecting, problems of utilization, and direction of exploitation. Acta Geol. Sin. 2019, 93, 1189–1209, (In Chinese with English Abstract). [Google Scholar]
- Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J. From “strategic” tungsten to “green” neodymium: A century of critical metals at a glance. Ore Geol. Rev. 2015, 64, 455–458. [Google Scholar] [CrossRef]
- Shearer, C.K.; Papike, J.J.; Jolliff, B.L. Petrogenetic links among granites and pegmatites in the Harney Peak rare element granite-pegmatite system, Black Hills, South Dakota. Can. Mineral. 1992, 30, 785–809. [Google Scholar]
- London, D.; Kontak, D.J. Granitic pegmatites: Scientific wonders and economic bonanzas. Elements 2012, 8, 257–261. [Google Scholar] [CrossRef]
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Han, L.; Pan, J.Y.; Ni, P.; Chen, H. Cassiterite deposition induced by cooling of a single-phase magmatic fluid: Evidence from SEM-CL and fluid inclusion LA-ICP-MS analysis. Geochim. Cosmochim. Acta 2023, 342, 108–127. [Google Scholar] [CrossRef]
- Ni, P.; Pan, J.Y.; Han, L.; Cui, J.M.; Gao, Y.; Fan, M.S.; Li, W.S.; Chi, Z.; Zhang, K.H.; Cheng, Z.L.; et al. Tungsten and tin deposits in South China: Temporal and spatial distribution, metallogenic models and prospecting directions. Ore Geol. Rev. 2023, 157, 105453. [Google Scholar] [CrossRef]
- Li, W.S.; Ni, P.; Wang, G.G.; Yang, Y.L.; Pan, J.Y.; Wang, X.L.; Chen, L.L.; Fan, M.S. A possible linkage between highly fractionated granitoids and associated W mineralization in the Mesozoic Yaogangxian granitic intrusion, Nanling region, South China. J. Asian Earth Sci. 2020, 193, 104314. [Google Scholar] [CrossRef]
- Liu, X.H.; Chen, X.Y.; He, L.H.; Zhao, Z.W. Study on extraction of lithium from salt lake brine by men brane electrolysis. Desalination 2015, 376, 35–40. [Google Scholar] [CrossRef]
- Zheng, M.P.; Zhang, Y.S.; Liu, X.F.; Qi, W.; Kong, F.J.; Nie, Z.; Jia, Q.X.; Pu, L.Z.; Hou, X.H.; Wang, H.L.; et al. Progress and prospects of salt lake research in China. Acta Geol. Sin. 2016, 90, 2123–2165, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, D.H.; Chen, Y.C.; Jiang, B.; Huang, F.; Wang, Y.; Li, H.Q.; Hou, K.J. Preliminary study on the Triassic continental mineralization system in China. Earth Sci. Front. 2020, 27, 45–59, (In Chinese with English Abstract). [Google Scholar]
- Selway, J.B.; Breaks, F.W.; Tindle, A.G. A review of rare-element (Li-Cs-Ta) pegmatite exploration techniques for the Superior Province, Canada, and large worldwide tantalum deposits. Explor. Min. Geol. 2005, 14, 1–30. [Google Scholar] [CrossRef]
- Černý, P. Petrogenesis of granitic pegmatites. In granitic pegmatites in science and industry. Mineral. Assoc. Can. Short Course 1982, 8, 293–327. [Google Scholar]
- Černý, P.; Anderson, A.J.; Tomascak, P.B.; Chapman, R. Geochemical and morphological features of beryl from the Bikita granitic pegmatite, Zimbabwe. Can. Min. 2003, 41, 1003–1011. [Google Scholar] [CrossRef]
- Romer, R.; Pichanvant, M. Rare metal (Sn, W, Ta-Nb, Li) granites and pegmatites. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020; pp. 840–846. [Google Scholar]
- Li, J.K.; Wang, D.H.; Chen, Y.C. The ore-forming mechanism of the Jiajika pegmatite-type rare metal deposit in western Sichuan province: Evidence form isotope dating. Acta Geol. Sin. Engl. Ed. 2013, 87, 91–101. [Google Scholar]
- Fei, G.C.; Menuge, J.F.; Chen, C.S.; Yang, Y.L.; Deng, Y.; Li, Y.G.; Zheng, L. Evolution of pegmatite ore-forming fluid: The Lijiagou spodumene pegmatites in the Songpan-Garze fold belt, southwestern Sichuan province, China. Ore Geol. Rev. 2021, 139, 104441. [Google Scholar] [CrossRef]
- Deng, J.Y.; Li, J.K.; Zhang, D.H.; Chou, I.M.; Yan, Q.G.; Xiong, X. Origin of pegmatitic melts from granitic magmas in the formation of the Jiajika lithium deposit in the eastern Tibetan Plateau. Asian Earth Sci. 2022, 229, 105147. [Google Scholar] [CrossRef]
- Wang, R.C.; Xie, L.; Zhu, Z.Y.; Hu, H. Micas: Important indicators of granite-pegmatite-related rare-metal mineralization. Acta Petrol. Sin. 2019, 35, 69–75, (In Chinese with English Abstract). [Google Scholar]
- Jolliff, B.L.; Papike, J.J.; Shearer, C.K. Fractionation trends in mica and tourmaline as indicators of pegmatite internal evolution: Bob Ingersoll pegmatite, Black Hills, South Dakota. Geochim. Et Cosmochim. Acta 1987, 51, 519–543. [Google Scholar] [CrossRef]
- Roda, E.; Pesquera, A.; Velasco, F. Micas of the muscovite-lepidolite series from the Fregeneda pegmatites (Salamanca, Spain). Mineral. Petrol. 1995, 55, 145–157. [Google Scholar] [CrossRef]
- Lichtervelde, M.V.; Grégoire, M.; Linnen, R.L.; Béziat, D.; Salvi, S. Trace element geochemistry by laser ablation ICP-MS of micas associated with Ta mineralization in the Tanco pegmatite, Manitoba, Canada. Contrib. Mineral. Petrol. 2008, 155, 791–806. [Google Scholar] [CrossRef]
- Anderson, M.; Lentz, D.; McFarlane, C.; Falck, H. A geological, geochemical and textural study of an LCT pegmatite: Implications for the magmatic versus metasomatic origin of Nb-Ta mineralization in the Moose II pegmatite, NWT, Canada. J. Geosci. 2013, 58, 299–320. [Google Scholar] [CrossRef]
- Kaeter, D.; Barros, R.; Menuge, J.F.; Chew, D.M. The magmatic–hydrothermal transition in rare-element pegmatites from Southeast Ireland: LA-ICP-MS chemical mapping of muscovite and columbite-tantalite. Geochim. Cosmochim. Acta 2018, 240, 98–130. [Google Scholar] [CrossRef]
- Fei, G.C.; Li, B.; Yang, J.; Chen, X.; Luo, W.; Li, Y.; Tang, W.; Gu, C.; Zhong, W.; Yang, G. Geology, Fluid Inclusion Characteristics and H-O-C Isotopes of Large Lijiagou pegmatite spodumene deposit in Songpan-Garze fold belt, Eastern Tibet: Implications for ore Genesis. Resour. Geol. 2018, 68, 37–50. [Google Scholar] [CrossRef]
- Luo, W.; Yang, B.; Gu, C.H.; Li, J.; Pang, L.W. The Geological Characteristics and ore indicators of Lijiagou Super-large Spodumene Deposit in Jinchuan County, Western Sichuan. Sichuan Nonferrous Met. 2021, 2, 16–18+21, (In Chinese with English Abstract). [Google Scholar]
- Deng, Y.; Fei, G.C.; Li, J.; Tang, W.C.; Zhong, W.; Yang, G.B. Study of C-H-O isotopes and geochronology of the Lijiagou pegmatite spodumene deposit in Sichuan Province. J. Mineral. Petrol. 2018, 38, 40–47, (In Chinese with English Abstract). [Google Scholar]
- Yuan, Y.B.; Chen, B.; Shang, L.B.; Sendula, E.; Chou, M.I. Lithium enrichment of the magmatic-hydrothermal fluid in albite-spodumene pegmatite from Lijiagou, Eastern Tibetan Plateau: Evidence from fluid inclusions. Ore Geol. Rev. 2023, 162, 105685. [Google Scholar] [CrossRef]
- Fei, G.C.; Menuge, J.F.; Li, Y.Q.; Yang, J.Y.; Deng, Y.; Chen, C.S.; Yang, Y.F.; Yang, Z.; Qin, L.Y.; Zheng, L.; et al. Petrogenesis of the Lijiagou spodumene pegmatites in Songpan-garze fold belt, west Sichuan, China: Evidence from geochemistry, zircon, cassiterite and coltan U-Pb geochronology and Hf isotopic compositions. Lithos 2020, 364, 105555. [Google Scholar] [CrossRef]
- Xu, J.B.; Fei, G.C.; Qin, L.Y.; Yang, J.Y.; Zheng, L.; Tang, W.C. LA-MC-ICP-MS U-Pb dating of cassiterite from the Lijiagou pegmatite-type rare-metal deposit in the Ke’eryin orefield, Sichuan province and its geological implication. Geol. Explor. 2020, 56, 346–358, (In Chinese with English Abstract). [Google Scholar]
- Weislogel, A.L.; Graham, S.A.; Chang, E.Z.; Wooden, J.L.; Gehrels, G.E. Detrital zircon provenance from three turbidite depocenters of the Middle-Upper Triassic Songpan-Ganzi complex, central China: Record of collisional tectonics, erosional exhumation, and sediment production. Geol. Soc. Am. Bull. 2010, 122, 2041–2062. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Du, J.X.; Liang, F.H. Structure and metamorphism of Markam gneiss dome from the eastern Tibetan plateau and its implications for crustal thickening, metamorphism, and exhumation. Geochem. Geophys. Geosyst. 2019, 20, 24–45. [Google Scholar] [CrossRef]
- De Sigoyer, J.; Vanderhaeghe, O.; Duchêne, S.; Billerot, A. Generation and emplacement of Triassic granitoids within the Songpan Ganze accretionary-orogenic wedge in a context of slab retreat accommodated by tear faulting, Eastern Tibetan Plateau, China. Asian Earth Sci. 2014, 88, 192–216. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Fu, X.F.; Wang, R.C.; Li, G.W.; Zheng, Y.L.; Zhao, Z.B.; Lian, D.Y. Generation of lithium-bearing pegmatite deposits within the Songpan-Ganze orogenic belt. East Tibet. Lithos 2020, 354–355, 105281. [Google Scholar] [CrossRef]
- Deschamps, F.; Duchêne, S.; Sigoyer, J.; Bosse, V.; Benoit, M.; Vanderhaeghe, O. Coeval mantle-derived and crust-derived magmas forming two neighbouring plutons in the Songpan Garze accretionary orogenic wedge (SW China). J. Petrol. 2018, 58, 2221–2256. [Google Scholar] [CrossRef]
- Li, J.K. Mineralizing Mechanism and Continental Geodynamics of Typical Pegmatite Deposits in Western Sichuan, China. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2006; 61p. [Google Scholar]
- Gu, C.H.; Huang, W.J.; Zhou, J.R.; Li, J.; Luo, W.; Jiang, J. The Reserve Estimation and Detailed Investigation Report of the Dangba Spodumene Pegmatite in Markam Country, Sichuan; Unpublished Reports; Geochemistry Exploration Team of the Sichuan Bureau of Geology and Mineral Resources Exploration: Deyang, China, 2013; pp. 41–44. (In Chinese) [Google Scholar]
- Li, J.K.; Wang, D.H.; Fu, X.F. 40Ar/39Ar ages of the Ke’eryin pegmatite type rare metal deposit, Western Sichuan, and its tectonic significances. Acta Geol. Sin. 2006, 80, 843–848, (In Chinese with English Abstract). [Google Scholar]
- Fei, G.C.; Li, T.R.; Menuge, J.F.; Hui, Z.Q.; Yuan, Y.W.; Zhu, H.P.; Tan, H.; Cai, Y.H.; Tang, W.C.; Yang, G.B.; et al. Petrogenesis of aplites in the Ke’eryin rare metal ore field in the Songpan-Garze Fold Belt, Eastern Tibet: Evidence from mineralogy, geochemistry, geochronology and Hf-Nd isotopes. Lithos 2023, 438, 107017. [Google Scholar] [CrossRef]
- Che, X.D.; Wu, F.Y.; Wang, R.C.; Gerdes, A.; Ji, W.Q.; Zhao, Z.H.; Yang, J.H.; Zhu, Z.Y. In situ U-Pb isotopic dating of columbite–tantalite by LA–ICP–MS. Ore Geol. Rev. 2015, 665, 978–989. [Google Scholar] [CrossRef]
- Yan, Q.H.; Qiu, Z.W.; Wang, H.; Wang, M.; Wei, X.P.; Li, P.; Zhang, R.Q.; Li, C.Y.; Liu, J.P. Age of the Dahongliutan rare metal pegmatite deposit, West Kunlun, Xinjiang (NW China): Constraints from LA–ICP–MS U–Pb dating of columbite-(Fe) and cassiterite. Ore Geol. Rev. 2018, 100, 561–573. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Lehmann, B.; Seltmann, R.; Sun, W.D.; Li, C.Y. Cassiterite U–Pb geochronology constrains magmatic-hydrothermal evolution in complex evolved granite systems: The classic Erzgebirge tin province (Saxony and Bohemia). Geology 2017, 45, 1095–1098. [Google Scholar] [CrossRef]
- Tan, X.J.; Wang, Z.M. General high-pressure closed acidic decomposition method of rock samples for trace element determination using-inductively-coupled-plasma-mass-spectrometry. J. Anal. Chem. 2020, 75, 1295–1303. [Google Scholar]
- Li, H.; Gan, J.; He, Z.W.; Gan, Y.; Wang, B.; Li, Y.; Jiang, W. Petrogenesis and Geodynamic Significance of the Early Triassic Nanpo Adakitic Pluton of the Luang Prabang-Loei Tectonic Belt (Northwestern Laos) in the East Tethys Domain: Constraints from Zircon U-Pb-Hf Isotope Analyses and Whole-Rock Geochemistry. Minerals 2023, 13, 821. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Li, J.K.; Zhang, D.H.; Wang, D.H.; Zhang, W.H. Liquid Immiscibility of Fluorine-rich Granite Magma and Its Diagenesis and Metallogeny. Geol. Rev. 2008, 54, 175–183, (In Chinese with English Abstract). [Google Scholar]
- Webster, J.D.; Thomas, R.; Rhede, D.; Förster, H.J.; Seltmann, R. Melt inclusions in quartz from an evolved peraluminous pegmatite: Geochemical evidence for strong tin enrichment in fluorine-rich and phosphorus-rich residual liquids. Geochim. Cosmochim. Acta 1997, 61, 2589–2604. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Naming materials in the magma/igneous rock system. Earth-Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Maniar, P.D.; Piccoli, P.M. Tectonic discrimination of granitoids. Geol. Soc. Am. Bull. 1989, 101, 635–643. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of eocene calc-alkaline volcanic rocks from the Kastamonu area, Northern Turkey. Contrib. Mineral. Petr. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Wright, J.B. A simple alkalinity ratio and its application to questions of non-orogenic granite genesis. Geol. Mag. 1969, 106, 370–384. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Fan, G.; Li, G.W.; Shen, G.F.; Xu, J.S.; Dai, J. Luanshiweiite: A New Member of Lepidolite Series. Acta Mieralogica Sin. 2013, 33, 713–721, (In Chinese with English Abstract). [Google Scholar]
- Qu, K.; Sima, X.Z.; Li, G.W.; Fan, G.; Shen, G.F.; Liu, X.; Xiao, Z.B.; Guo, H.; Qiu, L.F.; Wang, Y.J. Fluorluanshiweiite, KLiAl1.5□0.5(Si3.5Al0.5)O10F2, a New Mineral of the Mica Group from the Nanyangshan LCT Pegmatite Deposit, North Qinling Orogen, China. Minerals 2020, 10, 93. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace element discrimination diagrams for the tectonic interpretation of granitic rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Harris, N.B.W.; Pearce, J.A.; Tindle, A.G. Geochemical characteristics of collision-zone magmatism. Geol. Soc. Lond. Spec. Publ 1986, 19, 67–81. [Google Scholar] [CrossRef]
- Batchelor, R.A.; Bowden, P. Petrogenetic Interpretation of Granitoid Rock Series Using Multicationic Parameters. Chem. Geol. 1985, 48, 43–55. [Google Scholar] [CrossRef]
- Hao, X.F.; Fu, X.F.; Liang, B.; Yuan, L.P.; Pan, M.; Tang, Y. Formation ages of granite and X03 pegmatite vein in Jiajika, western Sichuan, and their geological significance. Miner. Depos. 2015, 34, 1199–1208, (In Chinese with English Abstract). [Google Scholar]
- Xu, Z.Q.; Hou, L.W.; Wang, Z.X.; Fu, X.F.; Huang, M.H. Orogenic Process of the Songpan-Ganzi Orogenic Belt; Geological Publishing House: Beijing, China, 1992; pp. 1–202. (In Chinese) [Google Scholar]
- Černý, P.; Meintzer, R.E.; Anderson, A.J. Extreme fractionation in rare-element granitic pegmatites: Selected examples of data of mechanisms. Can. Mineral. 1985, 23, 381–421. [Google Scholar]
- London, D. Magmatic-hydrothermal transition in the Tanco rare-metal pegmatite: Evidence from fluid inclusions and phase-equilibrium experiments. Am. Mineral. 1986, 71, 376–395. [Google Scholar]
- London, D. Internal differentiation of rare-element pegmatites: Effects of boron, phosphorus, and fluorine. Geochim. Cosmochim. Acta 1987, 51, 403–420. [Google Scholar] [CrossRef]
- Thomas, R.; Webster, J.D.; Heinrich, W. Melt inclusions in pegmatite quartz: Complete miscibility between silicate melts and hydrous fluids at low pressure. Contrib. Mineral. Petrol. 2000, 139, 394–401. [Google Scholar] [CrossRef]
- Burnham, C.W.; Ohmoto, H. Late stage processes of felsic magmatism. Min. Geol. Spec. Issue 1980, 8, 1–11. [Google Scholar]
- Wise, M.A. Trace element chemistry of lithium-rich micasfrom rare-element granitic pegmatites. Mineral. Petrol. 1995, 55, 203–221. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.; Torres-Ruiz, J. From granite to highly evolved pegmatite: A case study of thePinilla de Fermoselle granite-pegmatite system (Zamora, Spain). Lithos 2012, 153, 192–207. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Keller, P.; Pesquera, A. Micas of the muscovite-lepidolite series from Karibib pegmatites, Namibia. Mineral. Mag. 2007, 71, 41–62. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Müller, A.; Roda-Robles, E. Extreme fractionation in a granite–pegmatite system documented by quartz chemistry: The case study of Tres Arroyos (Central Iberian Zone, Spain). Lithos 2017, 286–287, 162–174. [Google Scholar] [CrossRef]
- Icenhower, J.; London, D. An experimental study of element partitioning among biotite, muscovite, and coexisting peraluminous silicic melt at 200 MPa (H2O). Am. Miner. 1995, 80, 1229–1251. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Hertogen, J.; Dewaele, S.; André, L.; Muchez, P. Alkali metal and rare earth element evolution of rock-forming minerals from the Gatumba area pegmatites (Rwanda): Quantitative assessment of crystal-melt fractionation in the regional zonation of pegmatite groups. Geochim. Cosmochim. Acta 2014, 132, 349–374. [Google Scholar] [CrossRef]
- Ballouard, C.; Poujol, M.; Boulvais, P.; Branquet, Y.; Tartèse, R.; Vigneresse, J.L. Nb-Ta fractionation in peraluminous granites: A marker of the magmatic-hydrothermal transition. Geology 2016, 44, 231–234. [Google Scholar] [CrossRef]
- Irber, W. The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu, Sr/Eu, Y/Ho, and Zr/Hf of evolving peraluminous granite suites. Geochim. Cosmochim. Acta 1999, 63, 489–508. [Google Scholar] [CrossRef]
- Shaw, D.M. A review of K-Rb fractionation trends by covariance analysis. Geochim. Cosmochim. Acta 1968, 32, 573–601. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.Y.; Li, J.K.; Li, P.; Xiong, X.; Yang, H.; Zhou, F.C. Indication of mica minerals for magmatic-hydrothermal evolution of Renli rare metal pegmatite deposit. Miner. Depos. 2019, 38, 1039–1052, (In Chinese with English Abstract). [Google Scholar]
- Yin, R.; Huang, X.L.; Xu, Y.G.; Wang, R.C.; Wang, H.; Yuan, C.; Ma, Q.; Sun, X.M.; Chen, L.L. Mineralogical constraints on the magmatic-hydrothermal evolution of rare-elements deposits in the Bailongshan granitic pegmatites, Xinjiang, NW China. Lithos 2020, 352–353, 105208. [Google Scholar] [CrossRef]
- Mu, S.T.; Shao, Y.J.; Song, Z.Y.; Zhou, H.X.; Liu, Q.Q.; Xiong, Y.Q. Geochemical characteristics and indication of mica in Renli—Chuanziyuan pegmatite type rare metal deposit in Hunan Province. J. Cent. South Univ. 2021, 52, 2973–2989. [Google Scholar]
- Linnen, R.L.; Keppler, H. Columbite solubility in granitic melts: Consequences for the enrichment and fractionation of Nb and Ta in the Earth’s crust. Contrib. Mineral. Pet. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- López-moro, F.J.; García, F.; Llorens, T.; Luis, J.; Contreras, S.; Fernández, A.; Candelas, M.; Benito, M. Ta and Sn concentration by muscovite fractionation and degassing in a lens-like granite body: The case study of the Penouta rare-metal albite granite (NW Spain). Ore Geol. Rev. 2017, 82, 10–30. [Google Scholar] [CrossRef]


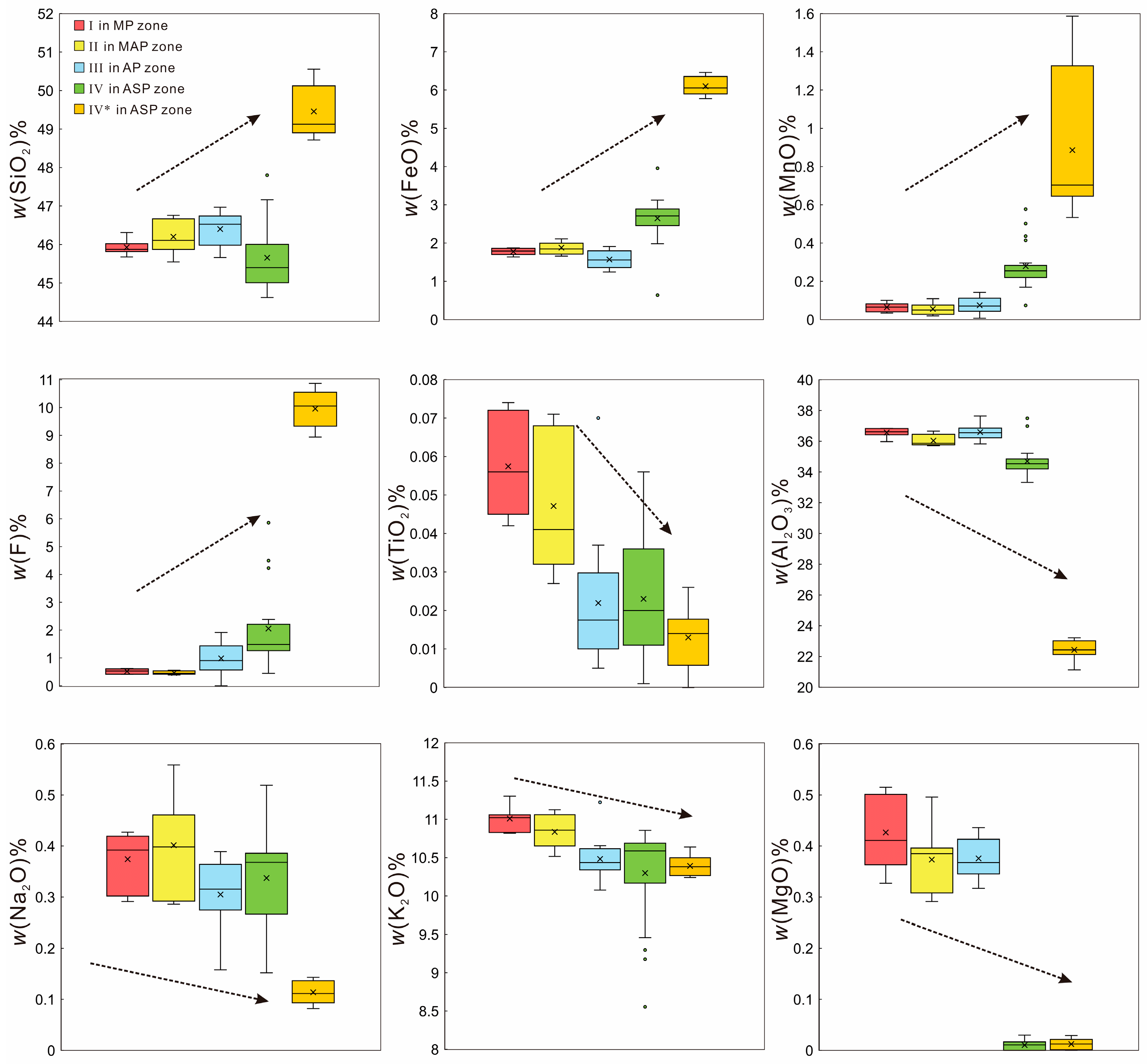

| (Ke’eryin Pluton) Two-Mica Granite | (Barren Pegmatite) MP–MAP–AP | (Ore-Bearing Pegmatite) ASP | ||||
|---|---|---|---|---|---|---|
| Min–Max | Avg | Min–Max | Avg | Min–Max | Avg | |
| SiO2 (wt%) | 68.06–73.61 | 72.09 | 73.14–77.52 | 74.8 | 68.68–75.29 | 71.70 |
| TiO2 (wt%) | 0.05–0.36 | 0.16 | - | - | 0.01–0.04 | 0.02 |
| Al2O3 (wt%) | 14.37–15.97 | 15.15 | 12.08–15.11 | 14.09 | 13.43–16.91 | 14.57 |
| Fe2O3 (wt%) | 0.16–2.59 | 0.66 | 0.31–0.7 | 0.45 | 0.27–0.62 | 0.45 |
| MnO (wt%) | 0.02–0.22 | 0.06 | 0.02–0.37 | 0.15 | 0.07–0.36 | 0.15 |
| MgO (wt%) | 0.03–0.70 | 0.25 | 0.01–0.04 | 0.03 | 0.01–0.2 | 0.09 |
| CaO (wt%) | 0.95–2.62 | 1.39 | 0.34–0.6 | 0.49 | 0.2–0.4 | 0.32 |
| Na2O (wt%) | 2.50–3.59 | 3.13 | 2.66–7.17 | 3.86 | 1.98–5.32 | 4.06 |
| K2O (wt%) | 4.03–6.01 | 5.16 | 1.03–6.17 | 3.86 | 0.97–3.27 | 2.60 |
| P2O5 (wt%) | 0.11–0.33 | 0.17 | 0.07–0.35 | 0.20 | 0.22–0.78 | 0.47 |
| LOI | 0.32–1.17 | 0.68 | 0.33–1.09 | 0.74 | 0.5–0.69 | 0.59 |
| Na2O + K2O | 7.83–8.56 | 8.30 | 8.21–8.83 | 8.58 | 5.24–7.4 | 6.66 |
| Na2O/K2O | 0.42–0.79 | 0.62 | 0.43–6.94 | 2.78 | 0.61–5.19 | 2.04 |
| DI | 81.32–92.65 | 89.71 | 94.71–97.52 | 95.71 | 88.84–94.64 | 92.57 |
| σ43 | 2.00–2.90 | 2.37 | 2.22–2.44 | 2.31 | 0.85–2.05 | 1.56 |
| AR | 2.73–3.43 | 3.04 | 3.19–5.92 | 4.21 | 2.03–3.36 | 2.73 |
| R1 | 2174–2817 | 2505.70 | 2097–2790 | 2423.30 | 2367–3616 | 2862.80 |
| R2 | 406–633 | 466.67 | 277–367 | 334.67 | 321–381 | 343.17 |
| A/NK | 1.71–1.96 | 1.83 | 1.37–1.84 | 1.65 | 1.8–2.88 | 2.24 |
| A/CNK | 1.42–1.74 | 1.57 | 1.32–1.72 | 1.56 | 1.73–2.69 | 2.13 |
| (Ke’eryin Pluton) Two-Mica Granite | (Barren Pegmatite) MP–MAP–AP | (Ore-Bearing Pegmatite) ASP | ||||
|---|---|---|---|---|---|---|
| Min–Max | Avg | Min–Max | Avg | Min–Max | Avg | |
| Li (ppm) | 93–211 | 149.00 | 36–446 | 228.00 | 9353–11,176 | 10,264.5 |
| Be (ppm) | 4.31–6.46 | 5.19 | 9.27–160.53 | 59.06 | 184.99–399.64 | 292.32 |
| Sn (ppm) | 5.21–16.5 | 9.00 | 12.6–295.5 | 120.45 | 258.55–266.27 | 262.41 |
| Cs (ppm) | 13.5–19.4 | 16.60 | 40.6–83.2 | 58.70 | 148.8–191.1 | 169.95 |
| Ga (ppm) | 16.9–22.4 | 19.38 | 13.5–27.5 | 21.80 | 12.3–19.5 | 15.9 |
| Se (ppm) | 0.03–0.66 | 0.19 | 0.03–0.06 | 0.04 | 0–0.03 | 0.03 |
| Rb (ppm) | 251–289 | 270.75 | 320–982 | 738.33 | 496–1120 | 808 |
| Ba (ppm) | 158–1669 | 1004.25 | 5.92–24.53 | 13.37 | 0.69–2.69 | 1.69 |
| Th (ppm) | 10.2–20.8 | 17.60 | 0.39–1.9 | 0.94 | 0.08–2.31 | 1.2 |
| U (ppm) | 2.74–8.01 | 5.04 | 0.51–31.4 | 10.85 | 5.53–14.83 | 10.18 |
| Ta (ppm) | 1.08–3.08 | 1.82 | 3.83–11.26 | 8.41 | 57.39–87.82 | 72.61 |
| Nb (ppm) | 10.67–20.6 | 15.97 | 11.73–49.67 | 32.87 | 141.31–185.48 | 163.4 |
| Sr (ppm) | 60.2–335 | 224.51 | 9.62–52.52 | 25.32 | 0.95–1.68 | 1.32 |
| Zr (ppm) | 58.3–164 | 123.66 | 3.8–25.52 | 12.94 | 5.76–16.12 | 10.94 |
| Hf (ppm) | 2.32–4.32 | 3.69 | 0.15–1.4 | 0.65 | 0.47–1.73 | 1.1 |
| Yb (ppm) | 1.14–1.56 | 1.32 | 0.01–0.22 | 0.14 | 0.01 | 0.01 |
| Y (ppm) | 17.1–18.2 | 17.77 | 0.1–1.49 | 0.84 | 0.15–0.24 | 0.2 |
| K/Rb | 154.47–198.69 | 166.90 | 26.71–56.08 | 39.96 | 14.30–24.15 | 19.27 |
| Zr/Hf | 25.13–38.68 | 32.61 | 18.22–24.58 | 21.96 | 9.34–12.25 | 10.80 |
| Nb/Ta | 6.69–12.31 | 9.51 | 3.06–4.41 | 3.71 | 1.61–3.23 | 2.42 |
| La (ppm) | 16.9–54.9 | 38.88 | 0.05–0.64 | 0.42 | 0.11–0.22 | 0.16 |
| Ce (ppm) | 34.5–102 | 71.05 | 0.09–1.22 | 0.83 | 0.13–0.32 | 0.23 |
| Pr (ppm) | 4.21–12.5 | 8.68 | 0.01–0.13 | 0.08 | 0.01 | 0.01 |
| Nd (ppm) | 17.1–50.4 | 33.55 | 0.01–0.42 | 0.26 | 0.01 | 0.01 |
| Sm (ppm) | 4.38–8.91 | 6.21 | 0–0.19 | 0.10 | - | - |
| Eu (ppm) | 0.55–2.1 | 1.44 | 0.01–0.02 | 0.02 | - | - |
| Gd (ppm) | 3.68–6.05 | 5.03 | 0.01–0.18 | 0.09 | 0.01 | 0.01 |
| Tb (ppm) | 0.6–0.79 | 0.68 | 0–0.04 | 0.02 | - | - |
| Dy (ppm) | 2.34–3.36 | 3.05 | 0.01–0.25 | 0.13 | 0.01–0.03 | 0.02 |
| Ho (ppm) | 0.6–0.62 | 0.61 | 0–0.04 | 0.02 | - | - |
| Er (ppm) | 1.49–1.78 | 1.64 | 0.01–0.11 | 0.07 | 0.01–0.02 | 0.01 |
| Tm (ppm) | 0.2–0.25 | 0.23 | 0–0.02 | 0.02 | - | - |
| Yb (ppm) | 1.14–1.56 | 1.32 | 0–0.22 | 0.14 | 0.01 | 0.01 |
| Lu (ppm) | 0.17–0.23 | 0.21 | 0–0.04 | 0.02 | - | - |
| ∑REE | 87.99–244.76 | 172.58 | 0.19–3.43 | 2.21 | 0.36–0.61 | 0.48 |
| ∑LREE | 77.64–230.81 | 159.80 | 0.17–2.57 | 1.71 | 0.26–0.57 | 0.42 |
| ∑HREE | 10.35–13.97 | 12.77 | 0.03–0.86 | 0.50 | 0.1–0.04 | 0.07 |
| LREE/HREE | 7.5–16.55 | 12.22 | 2.97–6.7 | 4.51 | 2.78–13.86 | 8.32 |
| LaN/YbN | 10.63–30.29 | 20.88 | 2.04–7.61 | 3.92 | 5.25–11.02 | 8.14 |
| δEu | 0.41–0.97 | 0.73 | 0.34–6.41 | 2.48 | 0.18–0.57 | 0.37 |
| δCe | 0.82–0.98 | 0.92 | 0.99–1.17 | 1.06 | 0.86–1 | 0.93 |
| Type | Value | wt% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | Na2O | K2O | F | |||
| I | Avg | 45.93 | 0.06 | 36.57 | 1.78 | 0.06 | 0.43 | 0.37 | 11.01 | 0.52 | |
| Min | 45.93 | 0.04 | 35.98 | 1.71 | 0.03 | 0.33 | 0.29 | 10.82 | 0.41 | ||
| Max | 46.31 | 0.07 | 36.83 | 1.87 | 0.1 | 0.51 | 0.43 | 11.3 | 0.62 | ||
| II | Avg | 46.21 | 0.05 | 36.04 | 1.88 | 0.05 | 0.37 | 0.4 | 10.84 | 0.46 | |
| Min | 45.55 | 0.03 | 35.72 | 1.66 | 0.02 | 0.29 | 0.28 | 10.52 | 0.38 | ||
| Max | 46.76 | 0.07 | 36.66 | 2.11 | 0.11 | 0.49 | 0.56 | 11.13 | 0.56 | ||
| III | Avg | 46.4 | 0.02 | 36.59 | 1.57 | 0.07 | 0.38 | 0.31 | 10.48 | 0.98 | |
| Min | 45.66 | 0 | 35.83 | 1.24 | 0 | 0.32 | 0.16 | 10.08 | 0 | ||
| Max | 46.97 | 0.07 | 37.64 | 1.91 | 0.14 | 0.44 | 0.39 | 11.22 | 1.91 | ||
| IV | Avg | 45.66 | 0.02 | 34.7 | 2.65 | 0.28 | 0.01 | 0.34 | 10.29 | 2.05 | |
| Min | 44.62 | 0 | 33.32 | 0.64 | 0.07 | 0 | 0.15 | 8.55 | 0.44 | ||
| Max | 47.8 | 0.06 | 37.49 | 3.96 | 0.58 | 0.03 | 0.52 | 10.86 | 5.86 | ||
| IV * | Avg | 49.46 | 0.01 | 22.43 | 6.1 | 0.88 | 0.01 | 0.11 | 10.39 | 9.96 | |
| Min | 48.72 | 0 | 21.14 | 5.79 | 0.53 | 0 | 0.08 | 10.24 | 8.94 | ||
| Max | 50.55 | 0.03 | 23.21 | 6.46 | 1.59 | 0.03 | 0.14 | 10.64 | 10.87 | ||
| Type | Value | ppm | K/Rb | Nb/Ta | |||||||
| Li | Be | B | Rb | Cs | Nb | Ta | Sn | ||||
| I | Avg | 299.17 | 14.64 | 118.32 | 1163.6 | 54.72 | 191.98 | 23.27 | 194.47 | 80.05 | 8.37 |
| Min | 273.74 | 11.04 | 109.21 | 980.68 | 45.44 | 167.09 | 20.30 | 167.27 | 68.74 | 6.13 | |
| Max | 326.07 | 18.79 | 126.19 | 1354.68 | 67.11 | 210.79 | 27.25 | 228.38 | 95.64 | 9.9 | |
| II | Avg | 941.99 | 22.75 | 121.44 | 2205.11 | 97.57 | 265.62 | 53.26 | 417.93 | 41.11 | 5.24 |
| Min | 869.61 | 21.66 | 113.03 | 2088.68 | 93.17 | 246.51 | 32.85 | 385.96 | 39.35 | 4.52 | |
| Max | 1027.76 | 24.24 | 131.18 | 2297.73 | 101.57 | 288.50 | 63.02 | 453.43 | 42.02 | 7.92 | |
| III | Avg | 1961.49 | 20.2 | 173.01 | 5673.41 | 657.63 | 111.01 | 71.00 | 510.16 | 15.52 | 1.69 |
| Min | 1603.77 | 18.09 | 118.07 | 3922.32 | 325.81 | 65.40 | 30.26 | 401.81 | 13.64 | 1.12 | |
| Max | 2466.98 | 23.2 | 211.24 | 6343.63 | 866.97 | 176.10 | 115.69 | 723.67 | 21.57 | 2.63 | |
| IV | Avg | 4469.80 | 24.63 | 187.10 | 5630.59 | 693.80 | 141.55 | 44.37 | 540.22 | 15.77 | 3.35 |
| Min | 2444.11 | 18.71 | 132.22 | 4735.45 | 225.95 | 14.85 | 10.07 | 453.21 | 13.08 | 1.06 | |
| Max | 7317.67 | 33.71 | 223.94 | 7051.88 | 1265.15 | 239.92 | 157.16 | 635.85 | 18.93 | 7.95 | |
| IV * | Avg | 24,480.34 | 16.33 | 22.19 | 8733.76 | 1708.60 | 121.87 | 47.15 | 171.07 | 10.34 | 4.25 |
| Min | 23,241.02 | 13.05 | 15.21 | 7780.91 | 1240.91 | 38.37 | 5.47 | 134.69 | 9.76 | 2.02 | |
| Max | 25,646.55 | 21.65 | 28.47 | 9303.18 | 1974.36 | 222.22 | 103.78 | 212.3 | 11.64 | 6.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, C.; Lai, X.; Yang, Y.; Gu, Y.; Cai, Y. Whole-Rock Geochemistry and Mica Compositions in Lijiagou Pegmatite Spodumene Deposit, Western Sichuan, China. Minerals 2024, 14, 69. https://doi.org/10.3390/min14010069
Chen X, Chen C, Lai X, Yang Y, Gu Y, Cai Y. Whole-Rock Geochemistry and Mica Compositions in Lijiagou Pegmatite Spodumene Deposit, Western Sichuan, China. Minerals. 2024; 14(1):69. https://doi.org/10.3390/min14010069
Chicago/Turabian StyleChen, Xiaojie, Cuihua Chen, Xiang Lai, Yulong Yang, Ying Gu, and Yunhua Cai. 2024. "Whole-Rock Geochemistry and Mica Compositions in Lijiagou Pegmatite Spodumene Deposit, Western Sichuan, China" Minerals 14, no. 1: 69. https://doi.org/10.3390/min14010069
APA StyleChen, X., Chen, C., Lai, X., Yang, Y., Gu, Y., & Cai, Y. (2024). Whole-Rock Geochemistry and Mica Compositions in Lijiagou Pegmatite Spodumene Deposit, Western Sichuan, China. Minerals, 14(1), 69. https://doi.org/10.3390/min14010069




