Genesis of the Supergiant Shuangjianzishan Ag–Pb–Zn Deposit in the Southern Great Xing’an Range, NE China: Constraints from Geochronology, Isotope Geochemistry, and Fluid Inclusion
Abstract
1. Introduction
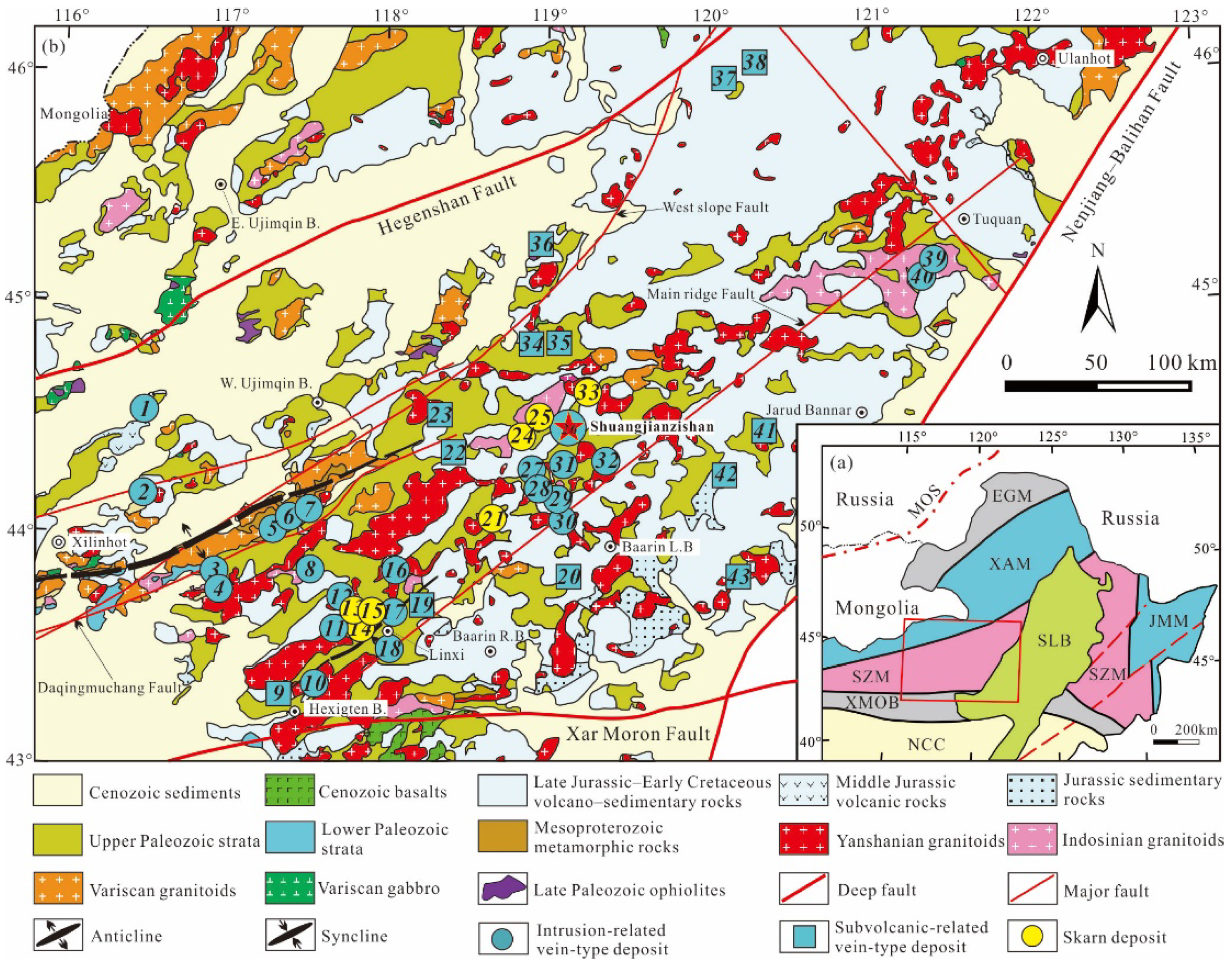
2. Regional Geology
3. Ore District and Deposit Geology
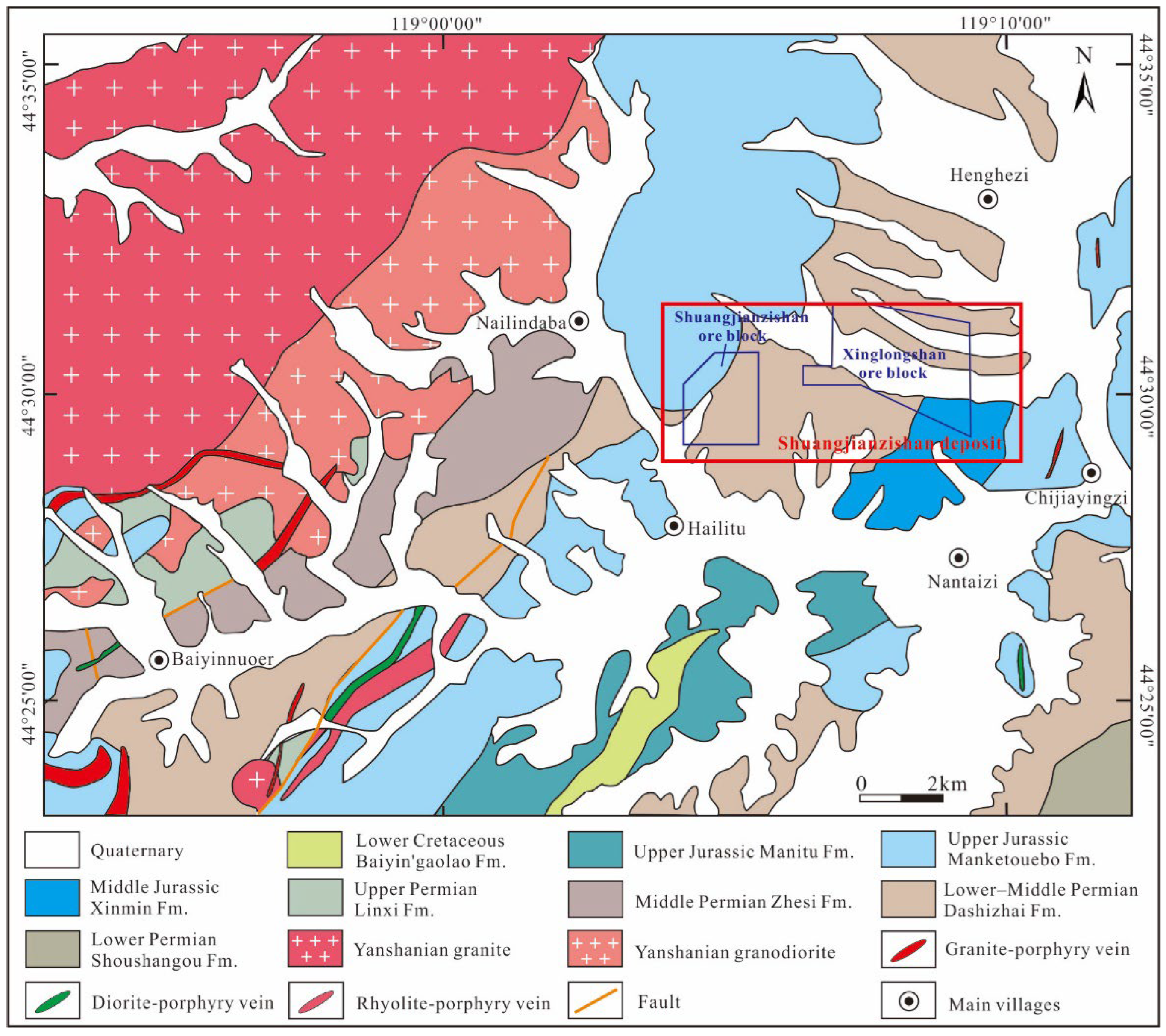
3.1. Ore District Geology
3.2. Ore Deposit Geology
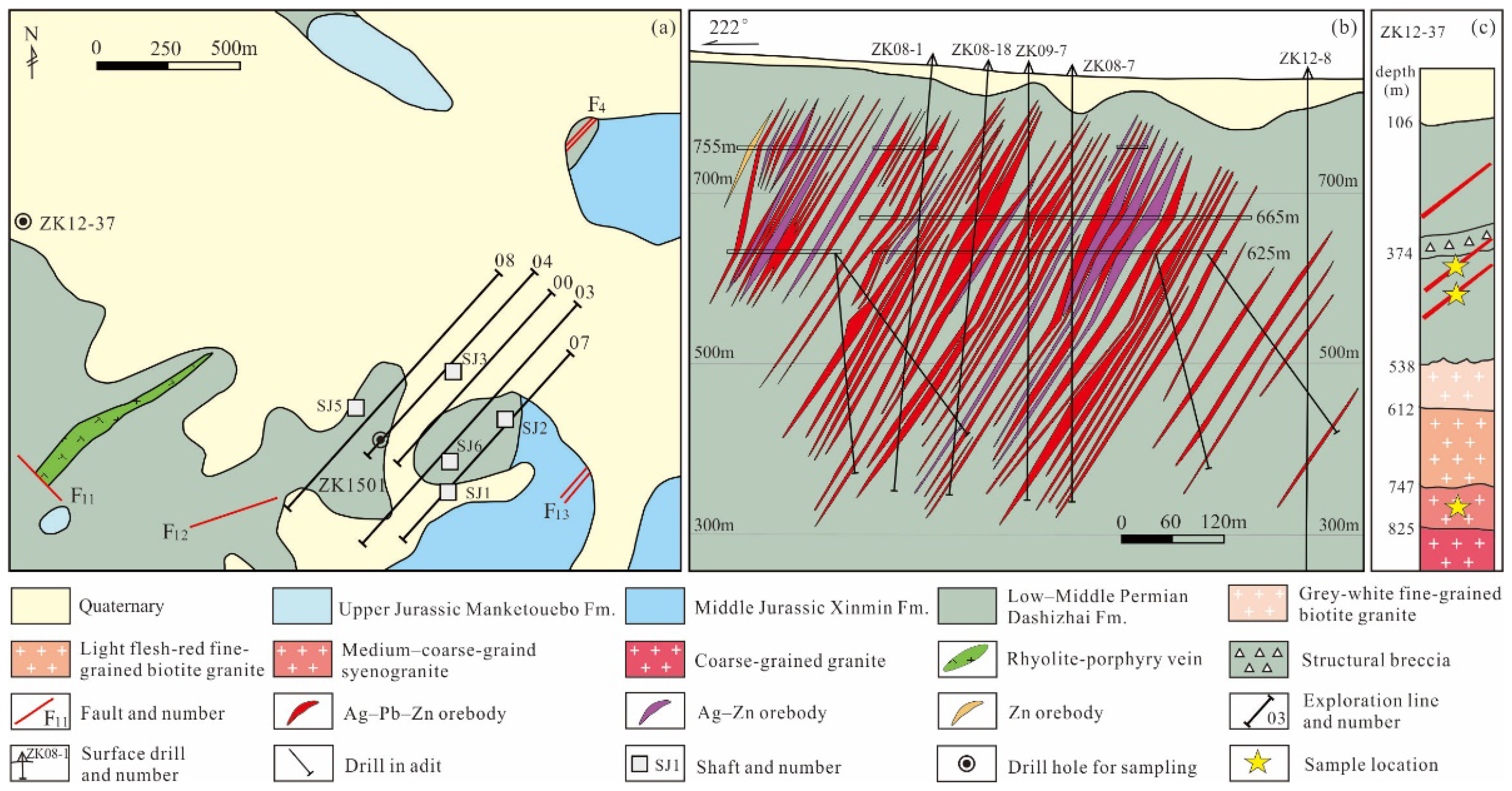
3.2.1. Xinglongshan Ore Block
- (1)
- The NW-trending Ag–Pb–Zn (Cu) ore vein group: The orebodies in the ore vein group distributed in the central and eastern parts of the ore block are controlled by a NW-trending thick and large fractured zone. The ore-hosting rocks are slate and silty slate of the Lower–Middle Permian Dashizhai Formation (Figure 3b). The overall strike of the ore-vein group is 300°–310°, inclined towards the southwest, with dip angles of 50°–65° and a length > 2000 m and a width > 1200 m. The ore-vein group is mainly composed of Ag–Pb–Zn orebodies and Ag–Zn orebodies, with a small amount of Zn orebodies and Ag–Cu–Pb–Zn orebodies (Figure 3b). The individual orebodies occur as veins, usually having a length of 100–800 m and a thickness of 1–10 m, with a maximum thickness exceeding 100 m (Figure 3b). The ore structure is mainly of fine-vein, stockwork, disseminated, and dense disseminated (Figure 4a–d). The major wall-rock alteration includes silicification and chloritization, with minor sericitization (Figure 4a–d). These altered minerals mainly occur in the form of fine veins and stockworks within a thick NW-trending alteration zone, with minor veins and crumbs of quartz. The grade of the Ag–Pb–Zn orebodies is relatively low, with average grades of 98 g/t Ag, 1.6% Zn, and 0.6% Pb, respectively [23]. The Ag–Cu–Pb–Zn orebodies usually occur at the top of the ore-vein group, with a controlled length greater than 300 m and a thickness of 0.6–2.8 m. The Ag–Cu–Pb–Zn orebodies have average grades of 263 g/t Ag, 0.7% Cu, 2.2% Zn, and 0.9% Pb, respectively [23].
- (2)
- The NNW-trending Ag–Pb–Zn orebodies: These orebodies, mainly distributed in the central and eastern parts of the ore block, are controlled by NNW-trending faults. The overall trend of these orebodies is approximately 310°, with a thickness of 3–15 m and a length of > 400 m. The ore structure is mainly of massive and vein (Figure 4e,f). The wall-rock alteration is characterized by silicification and carbonation, with minor chloritization, and quartz and calcite mainly occur as fine veins within the orebodies and within a range of no more than two meters nearby (Figure 4e,f). The NW-trending Ag–Pb–Zn orebodies have average grades of 400 g/t Ag (some up to 10,000 g/t), 2.8% Zn, and 4.3% Pb, respectively [23].
- (3)
- The NNE-trending Ag–Pb–Zn orebodies: These orebodies, distributed in the eastern part of the ore block, are composed of five parallel orebodies. They are controlled by NNE-trending faults, which dip to NWW at > 65°. The ore structure is mainly of massive and vein (Figure 4g,h). The types and distribution characteristics of wall-rock alteration are similar to those of the NNW-trending orebodies. These orebodies have a thickness of 2–6 m and a control length > 600 m, with average grades of 400 g/t Ag, 2.5% Zn, and 3.2% Pb, respectively [23].
3.2.2. Shuangjianzishan Ore Block

3.2.3. Ore Mineralogy and Textures of the Xinglongshan Ore Block

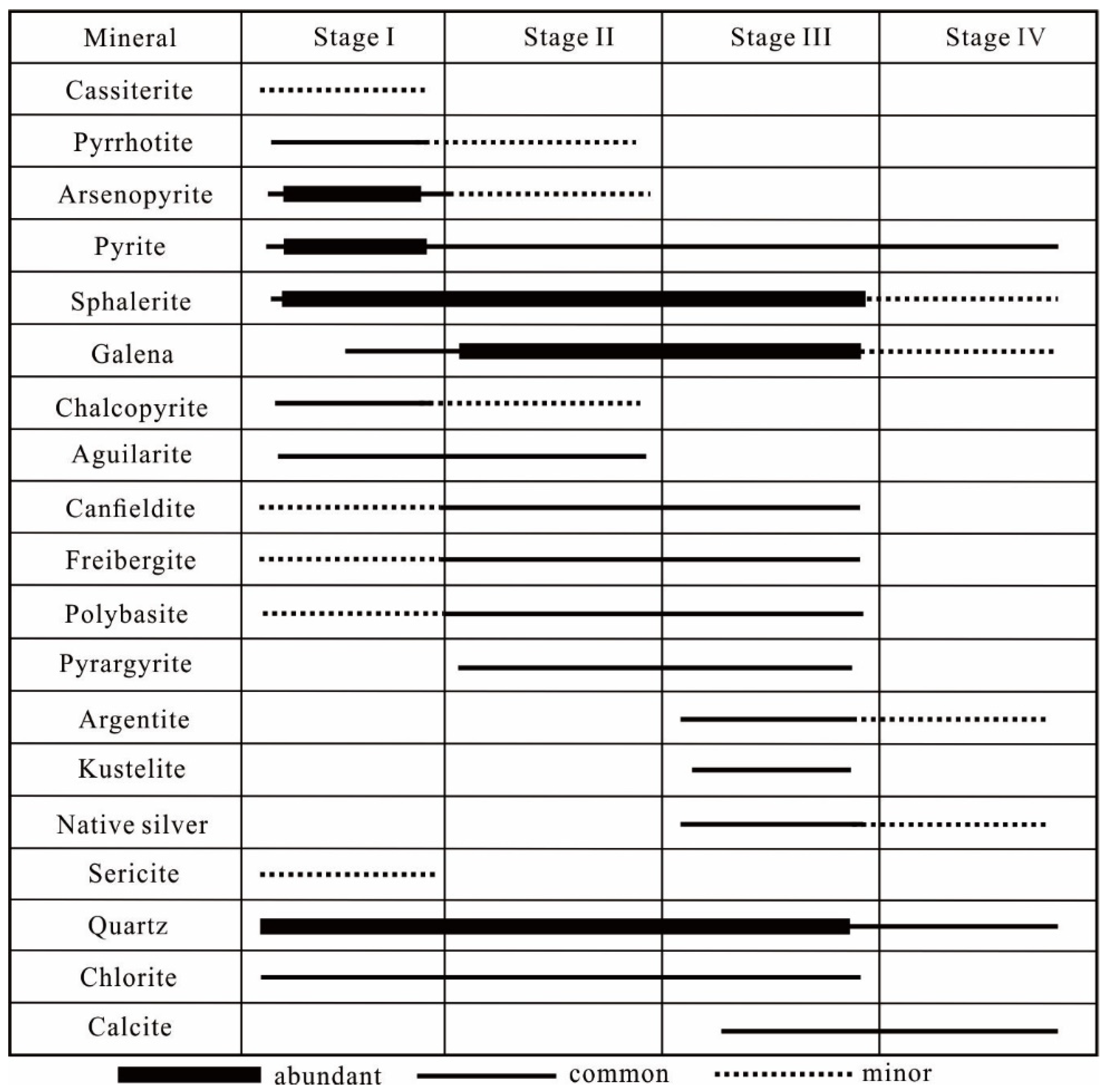
3.2.4. Mineralization Stages
4. Sampling and Analytical Methods
4.1. Sampling
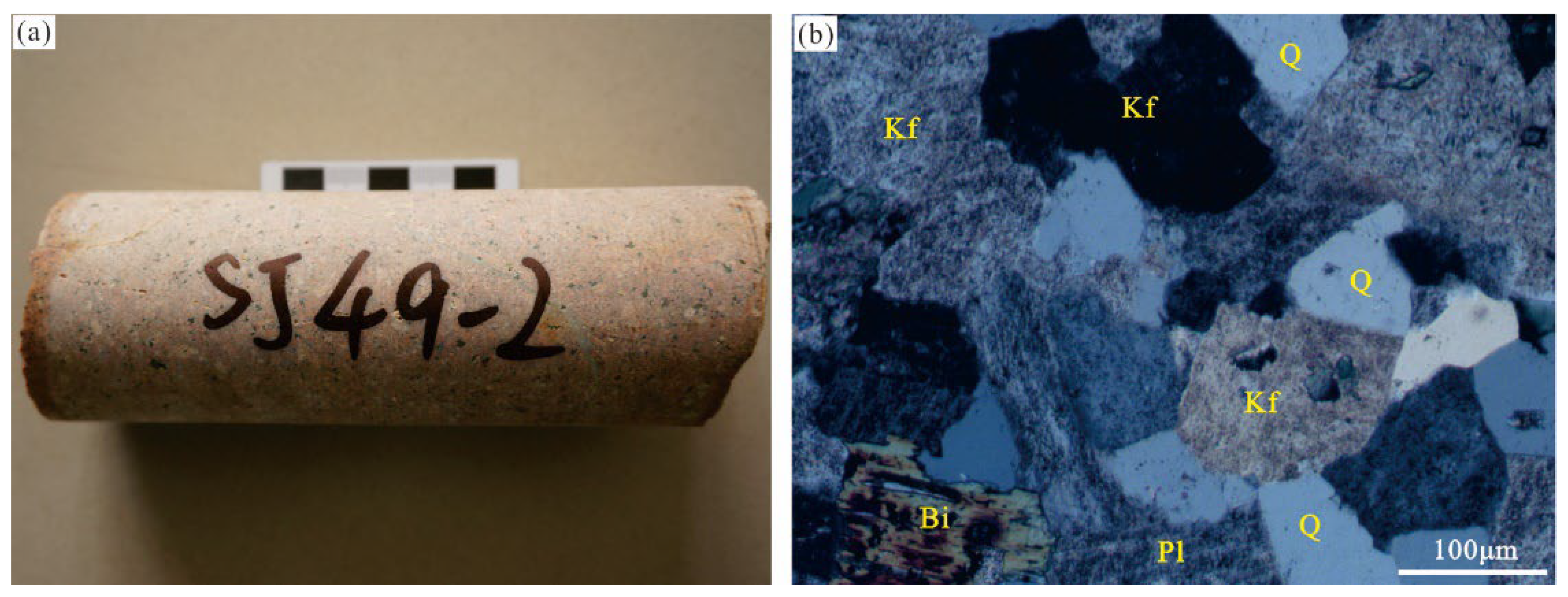
4.2. Syenogranite Zircon U–Pb Dating
4.3. Fluid Inclusion Microthermometry and Laser Raman Spectroscopy
4.4. H–O–C–Pb Isotope Analyses
4.4.1. H–O–C Isotope Analyses
4.4.2. Pb Isotope Analyses
5. Results
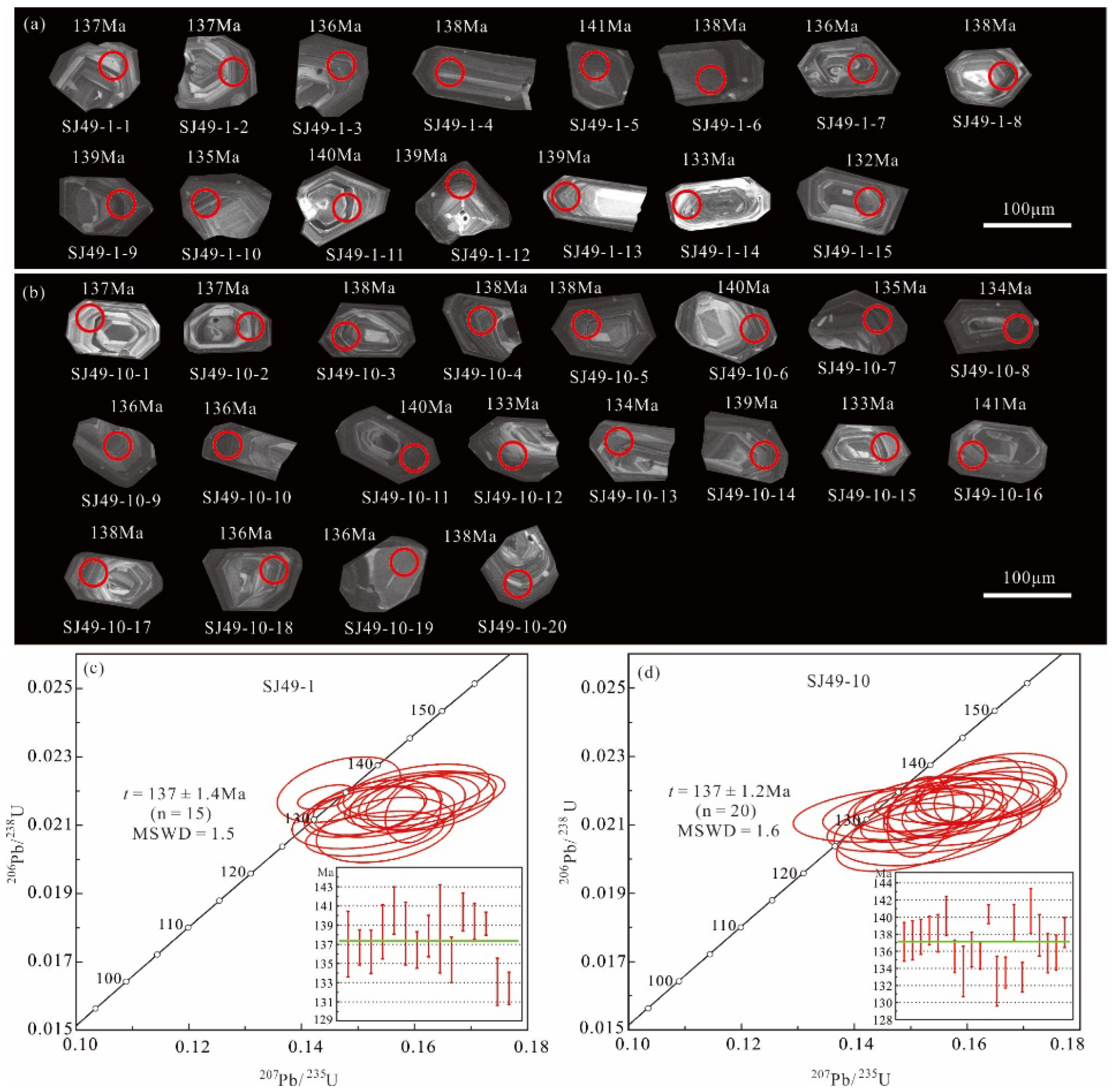
5.1. Syenogranite Zircon U–Pb Dating
5.2. Fluid Inclusion Data
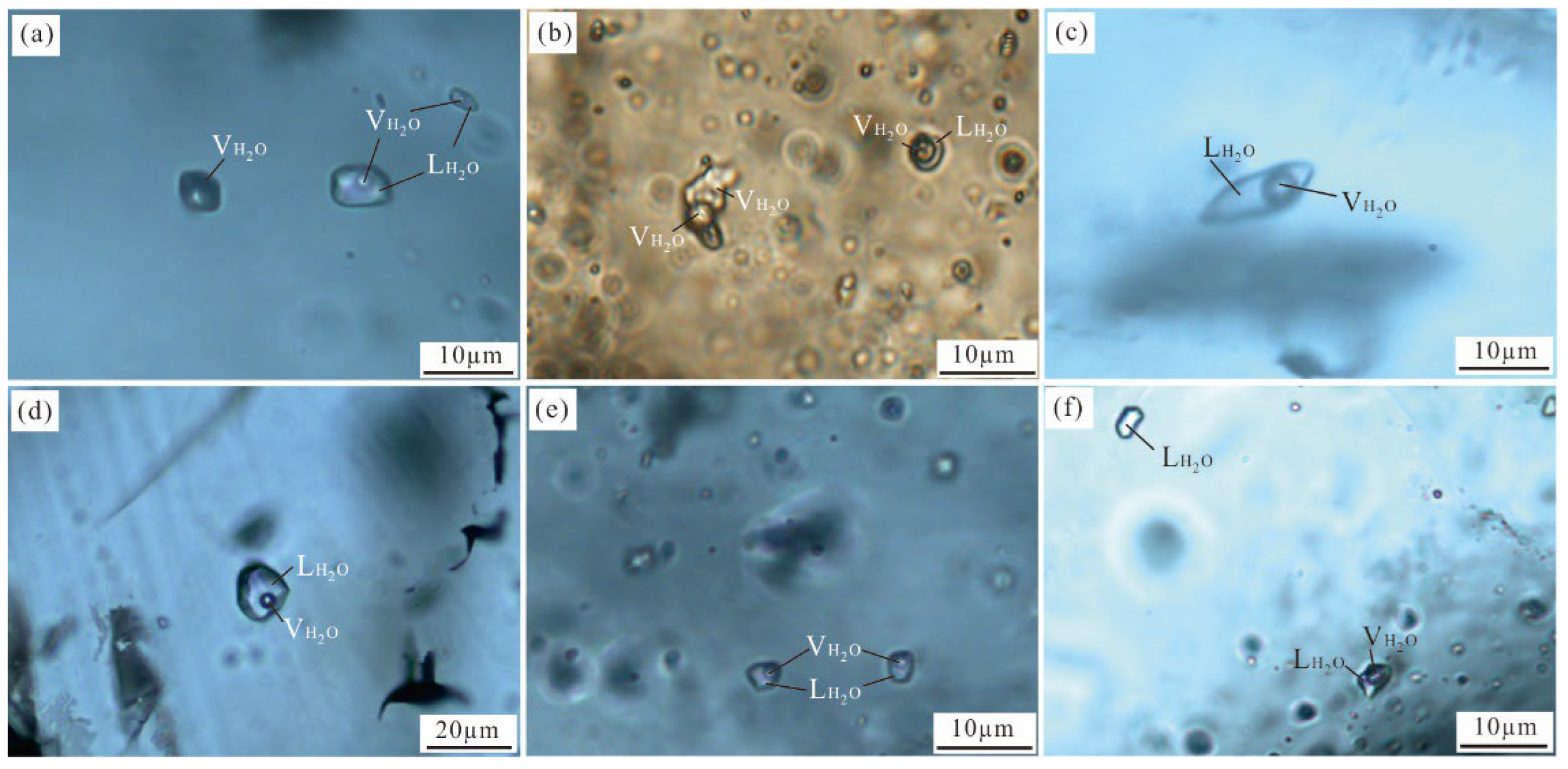
5.2.1. Petrography
- (1)
- Liquid-rich inclusions (WL-type): Within the examined quartz and calcite samples from all stages, these fluid inclusions are prevalent, constituting approximately 85% of the total number of inclusions. They exhibit elliptical, elongated columnar, and irregular shapes, with long axes ranging from 5 to 35 μm. Notably, the bubbles within them occupy 5%–45% of the total volume at room temperature (Figure 9a–f). When subjected to heating, these fluid inclusions underwent homogenization, transitioning into a liquid phase.
- (2)
- Gas-rich inclusions (WG-type): Exclusively found in quartz from stage I, these inclusions make up approximately 5% of the total number of fluid inclusions, with long axes ranging from 45 to 60 μm. WG-type inclusions are typically oval or circular in shape, with bubbles occupying 55%–70% of their total volume (Figure 9b). Upon heating, these inclusions homogenized into a vapor phase.
- (3)
- Pure gas inclusions (G-type): Predominantly present in quartz from stage I, these inclusions measure 5–10 μm in size and display irregular or round shapes (Figure 9a). They account for 3% of the total number of fluid inclusions and remain in a gaseous phase at room temperature, undergoing no phase change when heated.
- (4)
- Pure liquid inclusions (L-type): Primarily hosted within quartz and calcite from stages III and IV, these inclusions exhibit irregular and negative crystal shapes and have a size of 5–10 μm (Figure 9f). They constitute approximately 7% of the total number of fluid inclusions and remain in a liquid phase at room temperature. Upon heating, the L-type inclusions do not undergo any phase change.
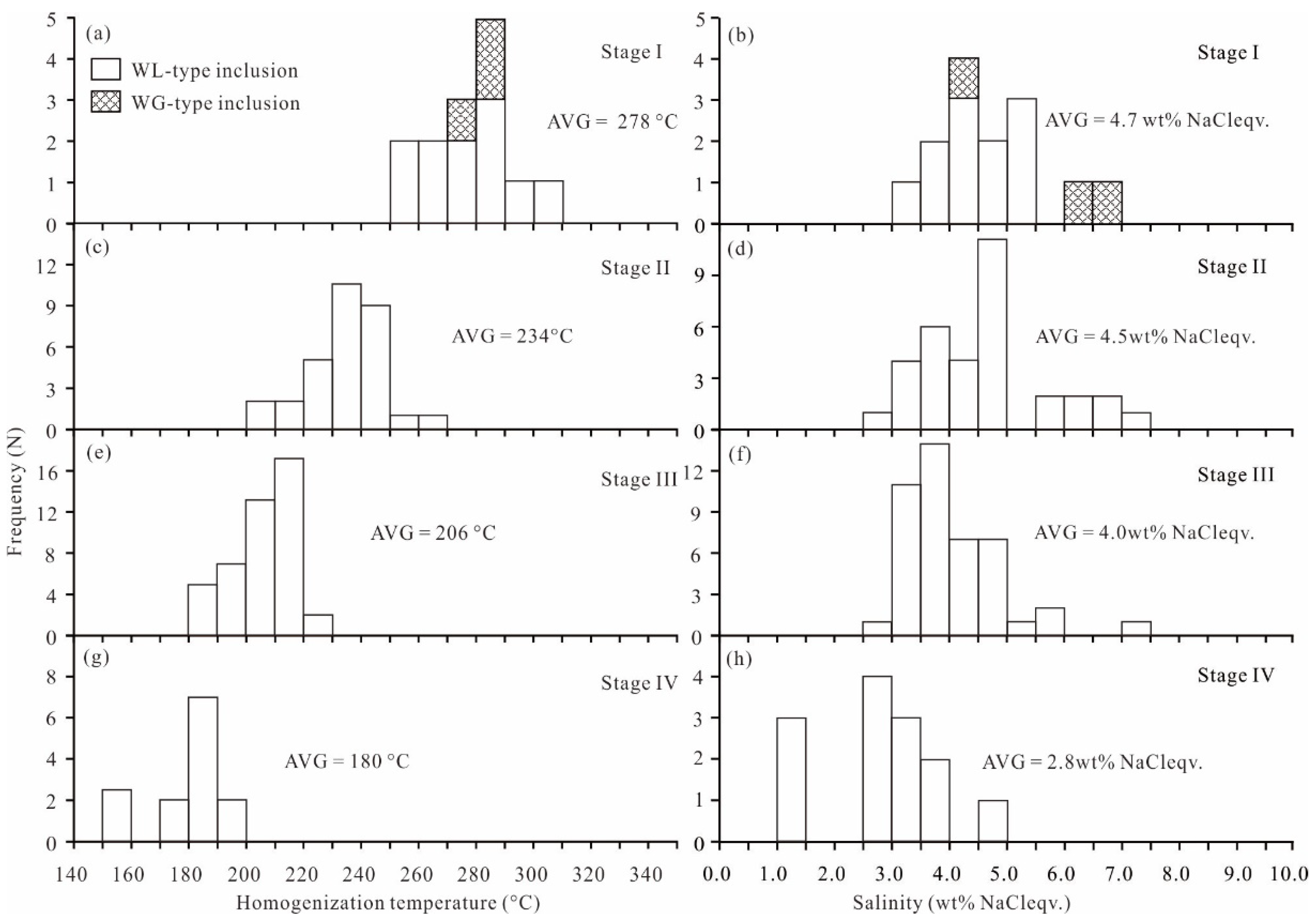
5.2.2. Microthermometry
- (1)
- FIs in quartz of stage I (sample SJ14): Three FIAs have been recognized. The homogenization temperature of two WL-type FIAs varies from 253 °C to 302 °C (Figure 10a), and the final ice melting temperature is −3.2 °C to −1.9 °C, corresponding to salinities of 3.4–5.3 wt% NaCl eqv. (Figure 10b), and the density of the fluid is 0.74–0.83 g/cm3. The homogenization temperature of one WG-type FIA varies from 279 °C to 289 °C, and the final ice melting temperature is −4.1 °C to −2.4 °C, corresponding to salinities of 4.0–6.6 wt% NaCl eqv., and the density of the fluid is 0.79–0.80 g/cm3.
- (2)
- FIs in quartz of stage II (samples SJ6 and SJ31): Three FIAs have been recognized. The homogenization temperature of these FIAs varies from 203 °C to 268 °C (Figure 10c), and the final ice melting temperature ranges from −4.5 °C to −1.6 °C, corresponding to salinities of 2.6–7.2 wt% NaCl eqv. (Figure 10d), and the fluid density is 0.80–0.88 g/cm3.
- (3)
- FIs in quartz and calcite of stage III (samples SJ11, SJ12, and SJ13-1): Three FIAs have been recognized. The homogenization temperature of two WL-type FIAs in quartz varies from 200 °C to 222 °C (Figure 10e), and the final ice melting temperature ranges from −3.5 °C to −1.8 °C, corresponding to salinities of 3.1–5.7 wt% NaCl eqv. (Figure 10f), and the fluid density is 0.86–0.91 g/cm3. The homogenization temperature of one WL-type FIA in calcite varies from 184 °C to 199 °C, and the final ice melting temperature ranges from −4.4 °C to −1.7 °C, corresponding to salinities of 2.9–7.0 wt% NaCl eqv., and the fluid density is 0.89–0.92 g/cm3.
- (4)
- FIs in quartz and calcite of stage IV (samples SJ8 and SJ45): Two FIAs have been recognized. The homogenization temperature of one WL-type FIA in quartz varies from 185 °C to 198 °C (Figure 10g), and the final ice melting temperature ranges from −2.2 °C to −1.5 °C, corresponding to salinities of 2.6–3.7 wt% NaCl eqv. (Figure 10h), and the fluid density is 0.89–0.91 g/cm3. The homogenization temperature of one WL-type FIA in calcite varies from 153 °C to 187 °C, and the final ice melting temperature ranges from −2.9 °C to −0.7 °C, corresponding to salinities of 1.2–4.8 wt% NaCl eqv., and the fluid density is 0.90–0.93 g/cm3.
5.2.3. Laser Raman Spectra

5.3. Isotope Data
5.3.1. Hydrogen and Oxygen Isotopes
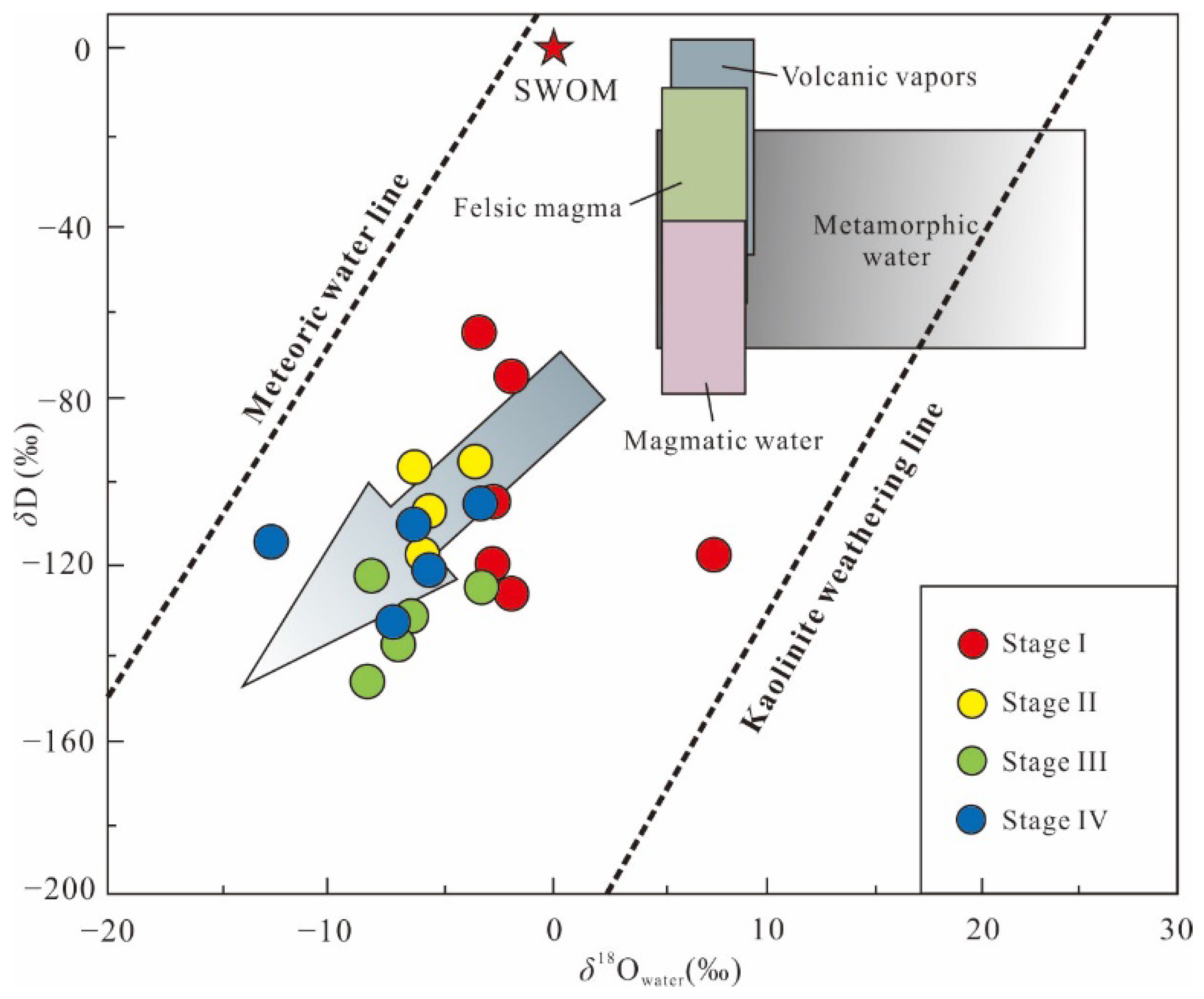
5.3.2. Carbon Isotope
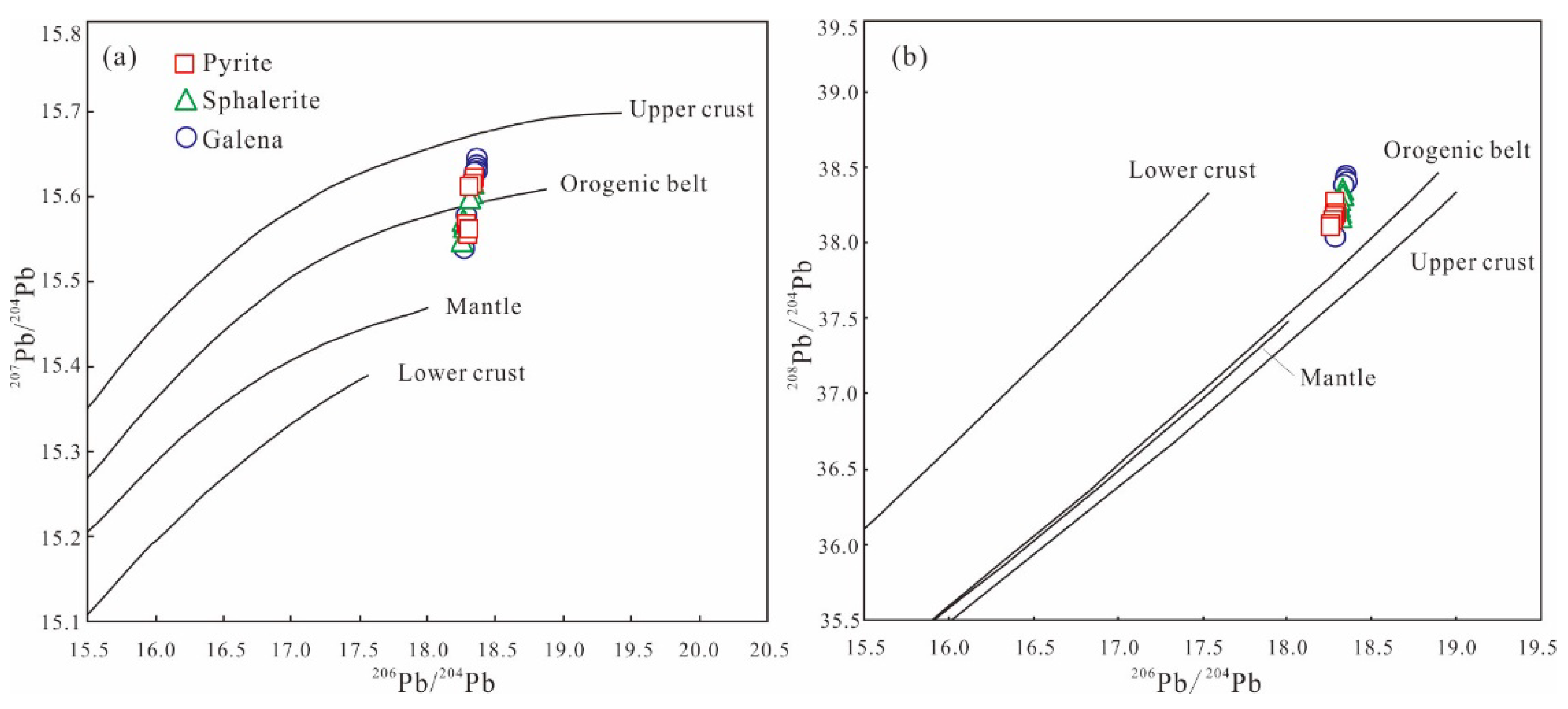
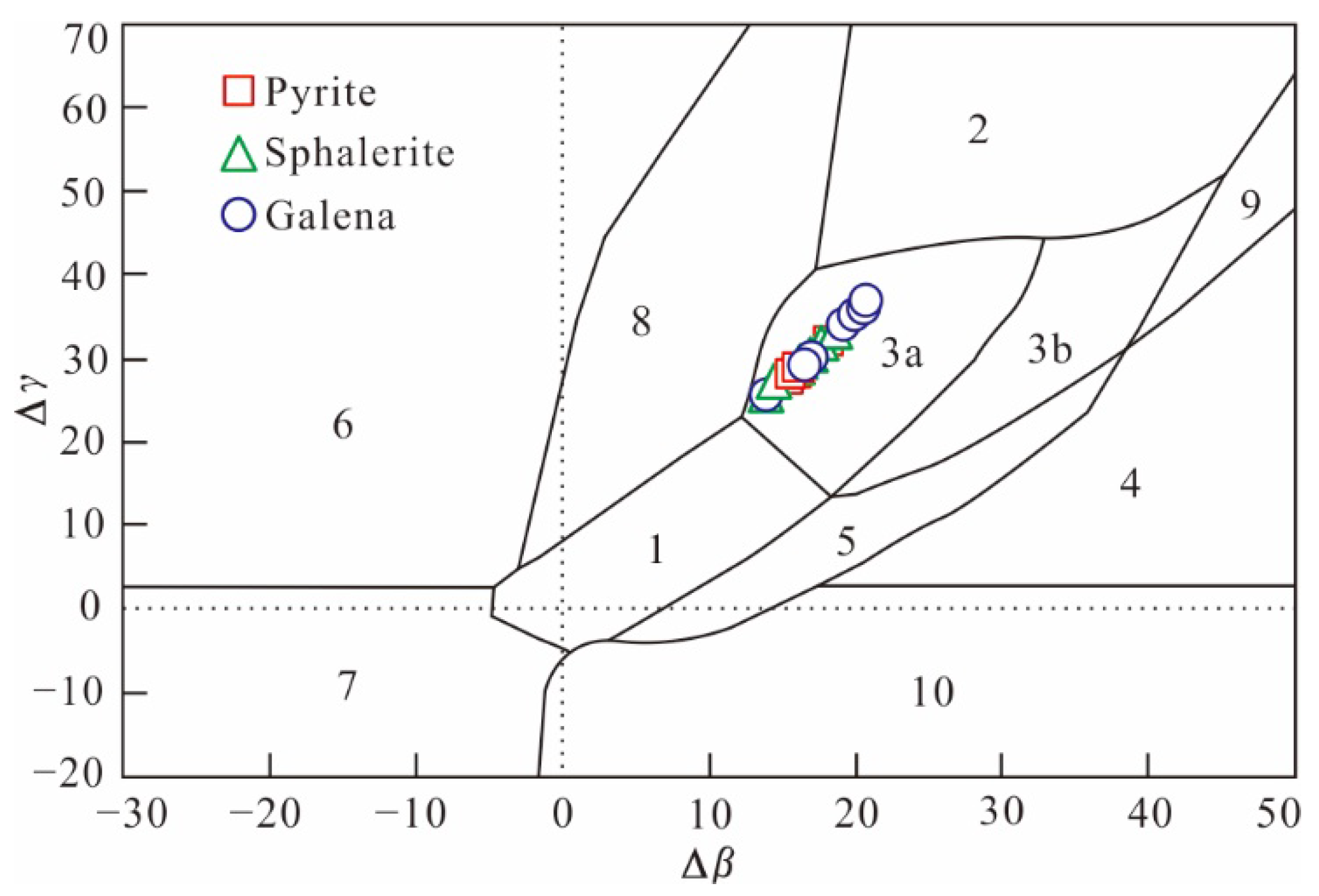
5.3.3. Lead Isotope
6. Discussion
6.1. Timing of the Syenogranite and Mineralization
6.2. Source and Evolution of Ore-Forming Fluids
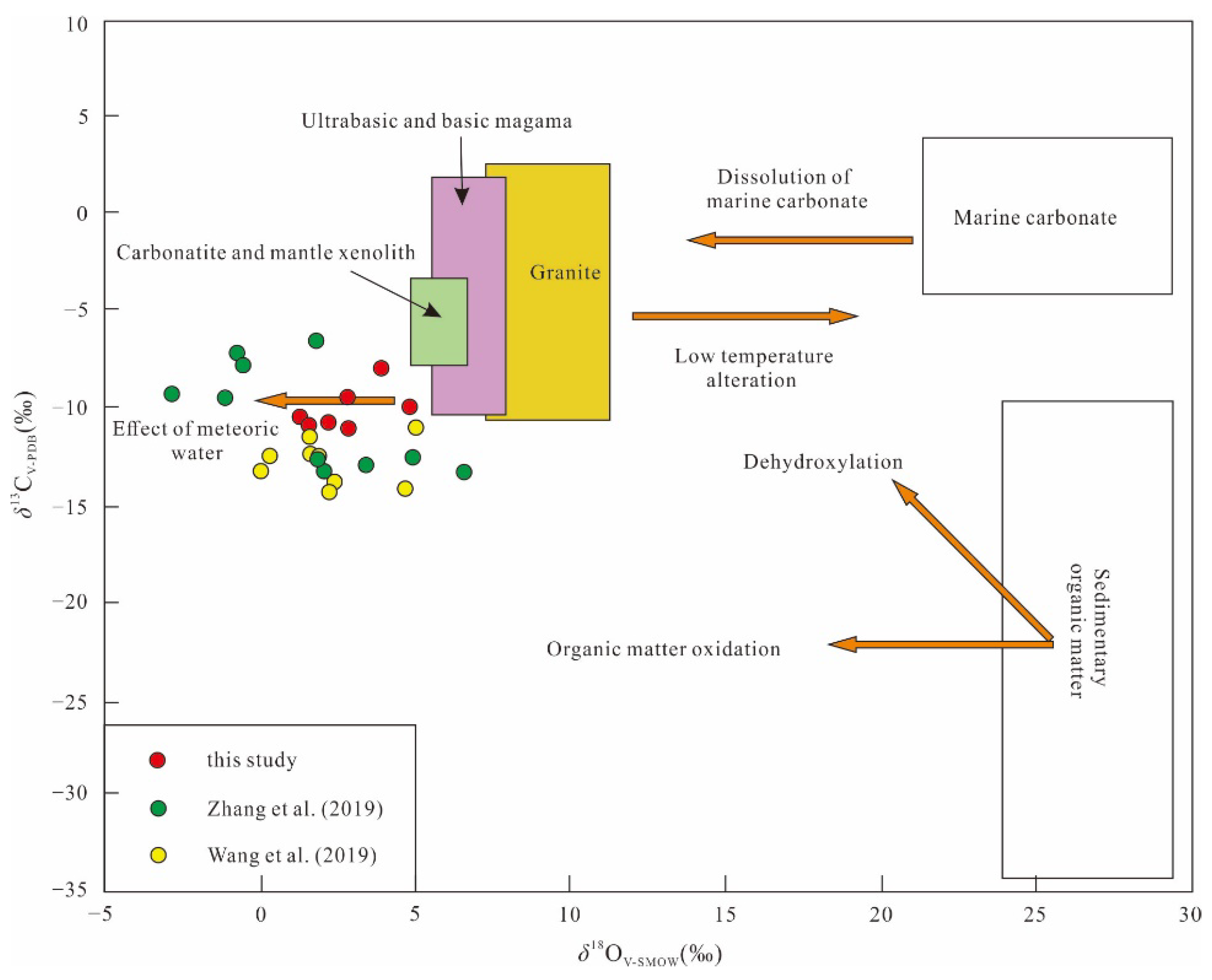

6.3. Sources of Ore-Forming Materials
6.4. Mechanism of Mineral Deposition
6.5. Ore Deposit Type and Metallogenic Model
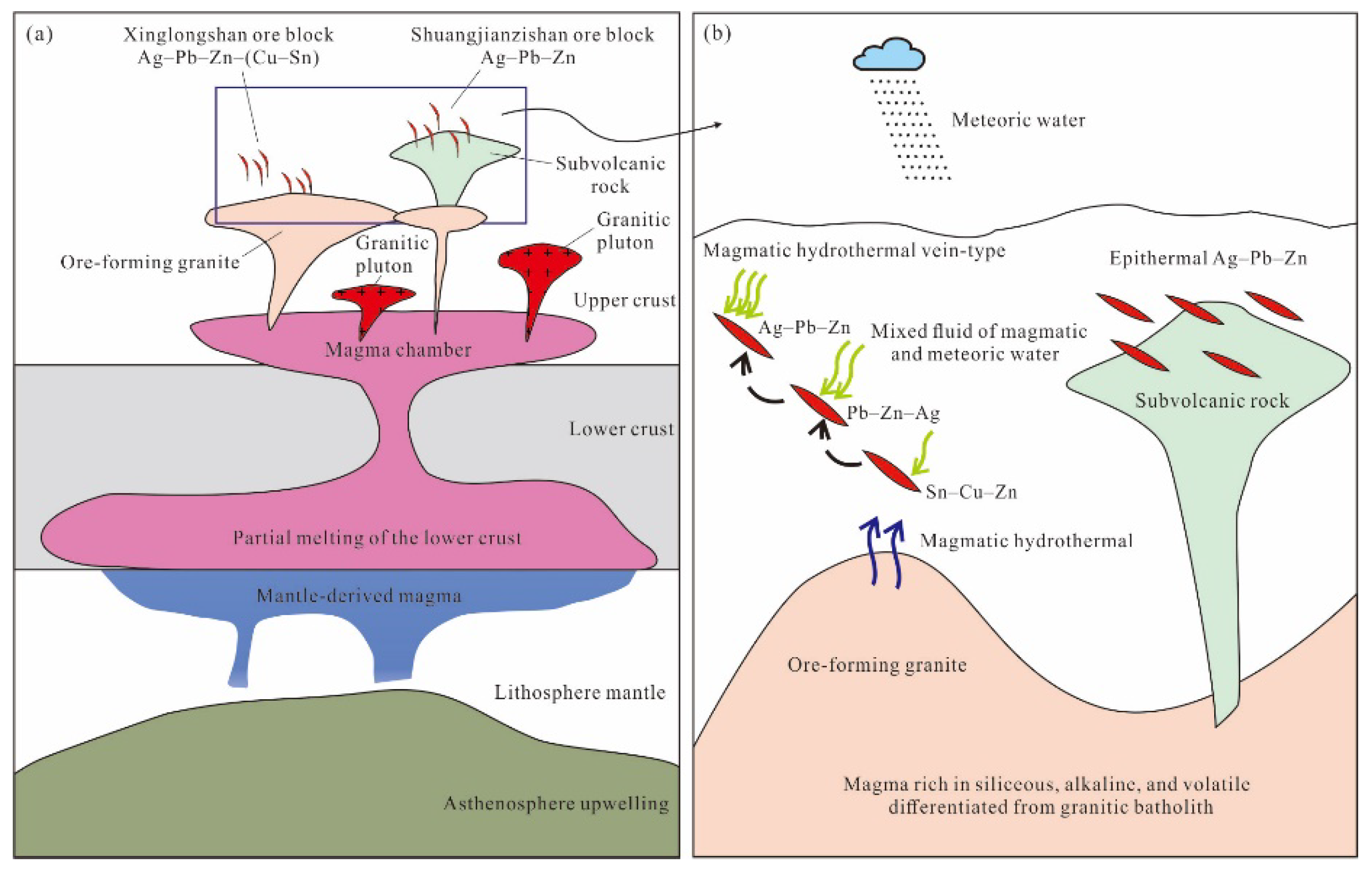
7. Conclusions
- (1)
- The concealed syenogranite genetically related to the mineralization of the Xinglongshan ore block formed at approximately 137 Ma.
- (2)
- The ore-forming fluid of the Xinglongshan ore block originated from a mixture of magmatic and meteoric water. With the evolution of ore-forming fluid, the amount of meteoric water increased gradually. The ore-forming fluid is characterized by medium–low temperature and low salinity and has an affinity of H2O–NaCl ± CH4 ± C6H6 in composition.
- (3)
- The ore-forming material dominantly came from the Early Cretaceous granitic magma. Fluid mixing was the main mechanism for mineral precipitation.
- (4)
- The Xinglongshan ore block belongs to a magmatic-hydrothermal vein-type deposit related to the Early Cretaceous syenogranite, and the Shuangjianzishan ore block belongs to an intermediate sulfidation epithermal deposit related to subvolcanic rocks. The Ag–Pb–Zn mineralization at Shuangjianzishan was genetically closely related to the Early Cretaceous volcanic–intrusive complex.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulson, B.L. Differences in lead isotope composition in the stratiform McArthur zinc–lead–silver deposit. Miner. Deposita 1975, 10, 277–286. [Google Scholar] [CrossRef]
- Beaudoin, G.; Sangster, D.F. A descriptive model for silver–lead–zinc veins in clastic metasedimentary terranes. Econ. Geol. 1992, 87, 1005–1021. [Google Scholar] [CrossRef]
- Leach, D.L.; Bradley, D.C.; Huston, D.; Pisarevsky, S.A.; Taylor, R.D.; Gardoll, S.J. Sediment-hosted lead–zinc deposits in Earth history. Econ. Geol. 2010, 105, 593–625. [Google Scholar] [CrossRef]
- Wei, C.; Ye, L.; Huang, Z.L.; Gao, W.; Hu, Y.S.; Li, Z.L.; Zhang, J.W. Ore Genesis and Geodynamic Setting of Laochang Ag–Pb–Zn–Cu Deposit, Southern Sanjiang Tethys Metallogenic Belt, China: Constraints from Whole Rock Geochemistry, Trace Elements in Sphalerite, Zircon U–Pb Dating and Pb Isotopes. Minerals 2018, 8, 516. [Google Scholar] [CrossRef]
- Yu, P.P.; Zheng, Y.; Wang, C.M. Trace elemental and sulfur–lead isotopic variations in metamorphosed volcanogenic massive sulfide (VMS) mineralization systems: An example from the Keketale Pb–Zn (–Ag) deposit, NW China. Ore Geol. Rev. 2020, 125, 103685. [Google Scholar] [CrossRef]
- Sun, C.; Yang, X.Y.; Zhang, H.S.; Ji, W.H.; Chen, B.; Dong, Z.C.; Faisal, M.; Xi, D.H. Tracing the formation and modification of the Keketale VMS-type Pb–Zn deposit, Altai Mountains: Insights from ore deposit geology, geochronology, and magnetite geochemistry. Ore Geol. Rev. 2022, 144, 104852. [Google Scholar] [CrossRef]
- Cooke, D.R.; Bull, S.W.; Large, R.R.; McGoldrick, P.J. The importance of oxidized brines for the formation of Australian Proterozoic stratiform sediment-hosted Pb–Zn (SEDEX) deposits. Econ. Geol. 2000, 95, 1–18. [Google Scholar] [CrossRef]
- Large, R.R.; Bull, S.W.; McGoldrick, P.J.; Walters, S.; Derrick, G.M.; Carr, G.R. Stratiform and strata-bound Zn–Pb–Ag deposits in Proterozoic sedimentary basins, northern Australia. Econ. Geol. 2005, 100, 931–963. [Google Scholar]
- Sangster, D.F. Evidence that Broken Hill-type Pb–Zn deposits are metamorphosed SEDEX deposits. Miner. Deposita 2020, 55, 1263–1270. [Google Scholar] [CrossRef]
- Box, S.E.; Bookstrom, A.A.; Anderson, R.G. Origins of mineral deposits, Belt-Purcell basin, United States and Canada: An introduction. Econ. Geol. 2010, 107, 1081–1088. [Google Scholar] [CrossRef]
- Newberry, R.J.; Einaudi, M.T.; Eastman, H.S. Zoning and genesis of the Darwin Pb–Zn–Ag skarn deposit, California: A reinterpretation based on new data. Econ. Geol. 1991, 86, 960–982. [Google Scholar] [CrossRef]
- Roache, T.J.; Williams, P.J.; Richmond, J.M.; Chapman, L.H. Vein and skarn formation at the Cannington Ag–Pb–Zn deposit, Northeastern Australia. Can. Mineral. 2009, 43, 241–262. [Google Scholar] [CrossRef][Green Version]
- Megaw, P.K.; Ruiz, J.; Titley, S.R. High-temperature, carbonate-hosted Ag–Pb–Zn (Cu) deposits of northern Mexico. Econ. Geol. 1988, 83, 1856–1885. [Google Scholar] [CrossRef]
- Yang, F.; Wu, G.; Li, R.H.; Zhang, T.; Chen, G.Z.; Xu, Y.M.; Li, Y.L.; Li, T.G.; Liu, R.H.; Chen, Y.J. Age, fluid inclusion, and H–O–S–Pb isotope geochemistry of the Baiyinchagan Sn–Ag–polymetallic deposit in the southern Great Xing’an Range, NE China. Ore Geol. Rev. 2022, 150, 105194. [Google Scholar] [CrossRef]
- Ouyang, H.G.; Mao, J.W.; Santosh, M.; Zhou, J.; Zhou, Z.H.; Wu, Y.; Hou, L. Geodynamic setting of Mesozoic magmatism in NE China and surrounding regions: Perspectives from spatio–temporal distribution patterns of ore deposits. J. Asian Earth Sci. 2013, 78, 222–236. [Google Scholar] [CrossRef]
- Camprubí, A.; Albinson, T. Epithermal deposits in México—Update of current knowledge, and an empirical reclassification. Geol. Soc. Am. Spec. Pap. 2007, 422, 377–415. [Google Scholar]
- Wang, L.; Qin, K.Z.; Song, G.X.; Li, G.M. A review of intermediate sulfidation epithermal deposits and subclassification. Ore Geol. Rev. 2019, 107, 434–456. [Google Scholar] [CrossRef]
- Zhai, D.G.; Williams-Jones, A.E.; Liu, J.J.; Selby, D.; Voudouris, P.C.; Tombros, S.; Li, K.; Li, P.L.; Sun, H.J. The genesis of the giant Shuangjianzishan epithermal Ag–Pb–Zn deposit, Inner Mongolia, northeastern China. Econ. Geol. 2020, 115, 101–128. [Google Scholar] [CrossRef]
- Macario, P.R. Metallogenesis of the Penasquito Polymetallic Deposit: A Contribution to the Understanding of the Magmatic Ore System. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 2016; pp. 1–310. [Google Scholar]
- Wang, J.B.; Wang, Y.W.; Wang, L.J.; Uemoto, T. Tin–polymetallic mineralization in the southern part of the Da Hinggan Mountains, China. Resour. Geol. 2001, 51, 283–291. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Mao, J.W.; Lyckberg, P. Geochronology and isotopic geochemistry of the A-type granites from the Huanggang Sn–Fe deposit, southern Great Hinggan Range, NE China: Implication for their origin and tectonic setting. J. Asian Earth Sci. 2012, 49, 272–286. [Google Scholar] [CrossRef]
- Ouyang, H.G.; Mao, J.W.; Zhou, Z.H.; Su, H.M. Late Mesozoic metallogeny and intracontinental magmatism, southern Great Xing’an Range, northeastern China. Gondwana Res. 2015, 27, 1153–1172. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, F.; Zhao, S.J.; Fan, M.J.; Guo, X.G. SHRIMP U–Pb Zircon Ages, Geochemistry and Sr–Nd–Hf Isotope Systematics of the Zalute Intrusive Suite in the Southern Great Xing’an Range, NE China: Petrogenesis and Geodynamical Implications. Minerals 2020, 10, 927. [Google Scholar] [CrossRef]
- Mao, J.W.; Zhou, Z.H.; Wu, G.; Jiang, S.H.; Liu, C.L.; Li, H.M.; Ouyang, H.G.; Liu, J. Metallogenic regularity and minerogenetic series of ore deposits in Inner Mongolia and adjacent areas. Miner. Depos. 2013, 32, 715–729, (In Chinese with English Abstract). [Google Scholar]
- Ouyang, H.G.; Mao, J.W.; Santosh, M.; Wu, Y.; Hou, L.; Wang, X.F. The Early Cretaceous Weilasituo Zn–Cu–Ag vein deposit in the southern Great Xing’an Range, northeast China: Fluid inclusions, H, O, S, Pb isotope geochemistry and genetic implications. Ore Geol. Rev. 2014, 56, 503–515. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Z.X.; Chen, G.Z.; Yi, H.N.; Yang, F.; Lü, X.; Shi, J.P.; Dou, H.B.; Wu, G. Age, Fluid Inclusion, and H–O–S–Pb Isotope Geochemistry of the Superlarge Huaaobaote Ag–Pb–Zn Deposit in the Southern Great Xing’an Range, NE China. Minerals 2023, 13, 939. [Google Scholar] [CrossRef]
- Wu, G.; Liu, R.L.; Chen, G.Z.; Li, T.G.; Li, R.H.; Li, Y.L.; Yang, F.; Zhang, T. Mineralization of the Weilasituo rare metal–tin–polymetallic ore deposit in Inner Mongolia: Insights from fractional crystallization of granitic magmas. Acta Petrol. Sin. 2021, 37, 637–664, (In Chinese with English Abstract). [Google Scholar]
- Wang, F.X.; Bagas, L.; Jiang, S.H.; Liu, Y.F. Geological, geochemical, and geochronological characteristics of Weilasituo Sn–polymetal deposit, Inner Mongolia, China. Ore Geol. Rev. 2017, 80, 1206–1229. [Google Scholar] [CrossRef]
- Chen, G.Z.; Wu, G.; Li, T.G.; Liu, R.L.; Li, R.H.; Li, Y.L.; Yang, F. Mineralization of the Daolundaba Cu–Sn–W–Ag deposit in the southern Great Xing’an Range, China: Constraints from geochronology, geochemistry, and Hf isotope. Ore Geol. Rev. 2021, 133, 104–117. [Google Scholar] [CrossRef]
- Chen, G.Z.; Wu, G.; Yang, F.; Zhang, T.; Li, T.G.; Liu, R.L.; Li, R.H.; Li, Y.L.; Wu, L.W.; Zhang, P.C. Ages, H–O–C–S–Pb isotopes, and fluid inclusion study of the Daolundaba Cu–Sn–W–Ag deposit in Inner Mongolia, NE China. Ore Geol. Rev. 2022, 150, 105171. [Google Scholar] [CrossRef]
- Yang, F.; Wu, G.; Li, R.H.; Zhang, T.; Chen, G.Z.; Chen, Y.J. Petrogenesis of the Alubaogeshan intrusion in the Maodeng–Xiaogushan area, southern Great Xing’an Range, NE China: Implications for magma evolution and tin–polymetallic mineralization. J. Asian Earth Sci. 2022, 238, 105395. [Google Scholar] [CrossRef]
- Yang, F.; Wu, G.; Chen, G.Z.; Li, S.H.; Li, Y.L.; Zhang, T.; Chen, Y.J. Petrogenesis and implications for tin mineralization of the Beidashan granitic pluton, southern Great Xing’an Range, NE China: Constraints from whole-rock and accessory mineral geochemistry. J. Asian Earth Sci. 2023, 259, 105883. [Google Scholar] [CrossRef]
- Kuang, Y.S.; Zheng, G.R.; Lu, M.J.; Liu, Y.L.; Zhang, S.J.; Li, R.Y.; Cheng, W.J. Basic characteristics of Shuangjianzishan silver polymetallic deposit in Chifeng City, Inner Mongolia. Miner. Depos. 2014, 33, 847–856, (In Chinese with English Abstract). [Google Scholar]
- Large, R.R.; Bull, S.W.; Selley, D.; Yang, J.W.; Cooke, D.R.; Garven, G.; McGoldrick, P.J. Controls on the Formation of Giant Stratiform Sediment-Hosted Zn–Pb–Ag Deposits: With Particular Reference to the North Australian Proterozoic. In Giant Ore Deposits: Characteristics, Genesis and Exploration; Centre for Special Ore Deposit and Exploration (CODES) Special Publication; Cooke, D.R., Pongratz, J., Eds.; University of Tasmania: Hobart, Australia, 2002; Volume 4, pp. 107–149. [Google Scholar]
- Xu, Z.G.; Chen, Y.C.; Wang, D.H.; Chen, Z.H.; Li, H.M. Scheme of the Classification of the Minerogenetic Units in China; Geological Publishing House: Beijing, China, 2008; pp. 1–128. (In Chinese) [Google Scholar]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Wang, F.X.; Bagas, L.; Jiang, S.H.; Zhang, F.X.; Liu, Y.F.; Chong, X.X. Geochronology and ore genesis of the Shuangjianzishan Ag–polymetallic deposit, Inner Mongolia, China. Ore Geol. Rev. 2019, 107, 1020–1045. [Google Scholar] [CrossRef]
- Liu, C.H.; Leon, B.; Wang, F.X. Isotopic analysis of the superlarge Shuangjianzishan Pb–Zn–Ag deposit in Inner Mongolia, China: Constraints on magmatism, metallogenesis, and tectonic setting. Ore Geol. Rev. 2016, 75, 252–267. [Google Scholar] [CrossRef]
- Wu, G.B.; Liu, J.M.; Zeng, Q.D.; Sun, H.S.; Liu, M.T. The metallogenic age of Shuangjianzishan Ag–Pb–Zn deposit of Great Hinggan Range, Inner Mongolia. Acta Miner. Sin. 2013, 33, 619, (In Chinese with English Abstract). [Google Scholar]
- Zhang, H.Y.; Zhai, D.G.; Liu, J.J.; Li, P.L.; Li, K.; Sun, H.J. Fluid inclusion and stable (H–O–C) isotope studies of the giant Shuangjianzishan epithermal Ag–Pb–Zn deposit, Inner Mongolia, NE China. Ore Geol. Rev. 2019, 115, 103–170. [Google Scholar] [CrossRef]
- Xue, J.X.; Shi, Y.; Liu, Z.H.; Xue, L.F. Closure of the Eastern Paleo-Asian Ocean: Evidence from Permian–Triassic Volcanic Rocks in the Northern Margin of the North China Craton. Minerals 2023, 13, 606. [Google Scholar] [CrossRef]
- Wang, T.; Guo, L.; Zheng, Y.D.; Donskaya, T.; Gladkochub, D.; Zeng, L.S.; Li, J.B.; Wang, Y.B.; Mazukabzov, A. Timing and processes of late Mesozoic mid–lower-crustal extension in continental NE Asia and implications for the tectonic setting of the destruction of the North China Craton: Mainly constrained by zircon U–Pb ages from metamorphic core complexes. Lithos 2012, 154, 315–345. [Google Scholar] [CrossRef]
- Chen, B.; Jahn, B.M.; Tian, W. Evolution of the Solonker suture zone: Constraints from zircon U–Pb ages, Hf isotopic ratios and whole-rock Sr–Nd isotope compositions of subduction- and collision-related magmas and forearc sediments. J. Asian Earth Sci. 2009, 34, 245–257. [Google Scholar] [CrossRef]
- Lu, L.; Qin, Y.; Zhang, K.J.; Han, C.Y.; Wei, T.; Li, F.Z.; Qu, Z.H. Provenance and tectonic settings of the late Paleozoic sandstones in central Inner Mongolia, NE China: Constraints on the evolution of the southeastern Central Asian Orogenic Belt. Gondwana Res. 2019, 77, 111–135. [Google Scholar] [CrossRef]
- Xu, W.L.; Pei, F.P.; Wang, F.; Meng, E.; Ji, W.Q.; Yang, D.B.; Wang, W. Spatialtemporal relationships of Mesozoic volcanic rocks in NE China: Constraints on tectonic overprinting and transformations between multiple tectonic regimes. J. Asian Earth Sci. 2013, 74, 167–193. [Google Scholar] [CrossRef]
- Zhou, J.B.; Li, L. The Mesozoic accretionary complex in Northeast China: Evidence for the accretion history of Paleo-Pacific subduction. J. Asian Earth Sci. 2017, 145, 91–100. [Google Scholar] [CrossRef]
- Dong, P.P.; Li, Y.J.; Xie, Y.; Wang, J.F.; Li, H.Y. Petrogenesis of the Late Carboniferous Trondhjemite in Central Inner Mongolia in North China and Constraints of Intra-Oceanic Subduction in the Southern Paleo-Asian Ocean. Minerals 2022, 12, 1212. [Google Scholar] [CrossRef]
- Zhang, J.H.; Gao, S.; Ge, W.C.; Wu, F.Y.; Yang, J.H.; Wilde, S.A.; Li, M. Geochronology of the Mesozoic volcanic rocks in the Great Xing’an Range, northeastern China: Implications for subduction-induced delamination. Chem. Geol. 2010, 276, 144–165. [Google Scholar] [CrossRef]
- Zhai, D.G.; Liu, J.J.; Zhang, H.Y.; Tombros, S.; Zhang, A.L. A magmatic-hydrothermal origin for Ag–Pb–Zn vein formation at the Bianjiadayuan deposit, Inner Mongolia, NE China: Evidences from fluid inclusion, stable (C–H–O) and noble gas isotope studies. Ore Geol. Rev. 2018, 101, 1–16. [Google Scholar] [CrossRef]
- Griffin, W.L.; Belousova, E.A.; Shee, S.R.; Pearson, N.J.; O’reilly, S.Y. Archean crustal evolution in the northern Yilgarn Craton: U–Pb and Hf-isotope evidence from detrital zircons. Precambrian Res. 2004, 131, 231–282. [Google Scholar] [CrossRef]
- Frei, D.; Gerdes, A. Precise and accurate in situ U–Pb dating of zircon with high sample throughput by automated LA–SF–ICP–MS. Chem. Geol. 2009, 261, 261–270. [Google Scholar] [CrossRef]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U–Pb zircon geochronology. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Black, L.P.; Gulson, B.L. The age of the mud tank carbonatite, strangways range, northern territory. Geol. Geophys. 1978, 3, 227–232. [Google Scholar]
- Ludwig, K.R. User’s Manual for Isoplot/Ex, Version 3.0, A Geochronological Toolkit for Microsoft Excel; Berkeley Geochronology Center: Berkeley, CA, USA, 2003; pp. 1–74. [Google Scholar]
- Andersen, T. Correction of common lead in U–Pb analyses that do not report 204Pb. Chem. Geol. 2002, 192, 59–79. [Google Scholar] [CrossRef]
- Bodnar, R.J. Revised equation and table for determining the freezing-point depression of H2O–NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Brown, P.E. FLINCOR: A microcomputer program for the reduction and investigation of fluid-inclusion data. Am. Mineral. 1989, 74, 1390–1393. [Google Scholar]
- Clayton, R.N.; Mayeda, T.K. The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim. Cosmochim. Acta 1963, 27, 43–52. [Google Scholar] [CrossRef]
- Coleman, M.L.; Shepherd, T.J.; Durham, J.J.; Rouse, J.E.; Moore, G.R. Reduction of water with zinc for hydrogen isotope analysis. Anal. Chem. 1982, 54, 993–995. [Google Scholar] [CrossRef]
- Clayton, R.N.; Mayeda, T.K.; Oneil, J.R. Oxygen isotope: Exchange between quartz and water. J. Geophys. Res.-Earth 1972, 77, 3057–3067. [Google Scholar] [CrossRef]
- Todt, W.; Cliff, R.A.; Hanser, A.; Hofmann, A.W. Re-calibration of NBS lead standards using a 202Pb–205Pb double spike. Terra Abstr. 1993, 5, 396. [Google Scholar]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1994; pp. 1–199. [Google Scholar]
- Sheppard, S.M.F. Characterization and isotopic variations in natural-waters. Rev. Mineral. Geochem. 1986, 16, 165–183. [Google Scholar]
- Chen, Y.W.; Mao, C.X.; Zhu, B.Q. Lead isotopic composition and genesis of Phanerozoic metal deposit in China. Geochemistry 1982, 1, 137–158. [Google Scholar] [CrossRef]
- Cui, M. The Geochemical Characteristics and Diagenesis of Ore-Forming Diorite-Porphyrite from Shuangjianzishan Ag Polymetallic Deposit in Inner Mongolia. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2015. (In Chinese with English Abstract). [Google Scholar]
- Ouyang, H.G.; Li, R.H.; Zhou, Z.H. The Jurassic mineralization of the Shuangjianzishan Ag-polymetallic deposit and its significance in prospecting: Evidence from geochronology. Acta Geol. Sin. 2016, 90, 1835–1845, (In Chinese with English Abstract). [Google Scholar]
- Wang, F.X. Magmatic Activity and Silver Polymetallic Mineralization in the Shuangjianzishan Silver Polymetallic Deposit and Its Surrounding Areas in Inner Mongolia. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2018; pp. 1–189, (In Chinese with English Abstract). [Google Scholar]
- Wu, Y.H.; Han, J.T.; Liu, Y.H.; Ma, G.Q.; Han, F.X.; Yang, Y.C.; Liu, L.J.; Guo, L.; Guan, Y.; Zhang, Y.H.; et al. Metallogenic model of the Shuangjianzishan Ag–Pb–Zn district, Northeast China: Revealed from integrated geophysical investigation. Geosci. Front. 2022, 13, 101321. [Google Scholar] [CrossRef]
- Wang, F.X.; Sun, H.J.; Pei, R.F.; Liu, Y.F.; Liu, C.H.; Jiang, S.H. The geologic features and genesis of Shuangjianzishan silver–polymetallic deposit, Balinzuo Qi, Inner Mongolia. Geol. Rev. 2016, 62, 1241–1256, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y.F.; Jiang, S.H.; Bagas, L. The genesis of metal zonation in the Weilasituo and Bairendaba Ag–Zn–Pb–Cu–(Sn–W) deposits in the shallow part of a porphyry Sn–W–Rb system, Inner Mongolia, China. Ore Geol. Rev. 2016, 75, 150–173. [Google Scholar] [CrossRef]
- Yang, F.; Sun, J.G.; Wang, Y.; Fu, J.Y.; Na, F.C.; Fan, Z.Y.; Hu, Z.Z. Geology, Geochronology and Geochemistry of Weilasituo Sn-Polymetallic Deposit in Inner Mongolia, China. Minerals 2019, 9, 104. [Google Scholar] [CrossRef]
- Rye, R.O.; Ohmoto, H. Sulfur and carbon isotopes and ore genesis: A review. Econ. Geol. 1974, 69, 826–842. [Google Scholar] [CrossRef]
- Wilkinson, J.J.; Jenkin, G.R.T.; Fallick, A.E.; Foster, R.P. Oxygen and hydrogen isotopic evolution of Variscan crustal fluids, south Cornwall, UK. Chem. Geol. 1995, 123, 239–254. [Google Scholar] [CrossRef]
- Ohmoto, H. Formation of volcanogenic massive sulfide deposits: The Kuroko perspective. Ore Geol. Rev. 1996, 10, 135–177. [Google Scholar] [CrossRef]
- Taylor, H.P., Jr.; Frechen, J.; Degens, E.T. Oxygen and carbon isotope studies of carbonatites from the Laacher See District, West Germany and the Alnö District, Sweden. Geochim. Cosmochim. Acta 1967, 31, 407–430. [Google Scholar] [CrossRef]
- Hoefs, J. Isotope Fractionation Processes of Selected Elements. In Stable Isotope Geochemistry, 9th ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 1–528. [Google Scholar]
- Faure, G. Origin of Igneous Rocks: The Isotopic Evidence; Springer Science & Business Media: Cham, Switzerland, 2013; pp. 1–398. [Google Scholar]
- Townley, B.K.; Godwin, C.I. Isotope characterization of lead in galena from ore deposits of the Aysen Region, southern Chile. Miner. Depos. 2001, 36, 45–57. [Google Scholar] [CrossRef]
- Kamenov, G.D.; Macfarlane, A.W.; Riciputi, L.R. Sources of lead in the San Cristobal, Pulacayo, and Potosi mining districts, Bolivia, and a revaluation of regional ore lead isotope provinces. Econ. Geol. 2002, 97, 573–592. [Google Scholar]
- Marcoux, E.; Grancea, I.; Lupulescu, M. Lead isotope signatures of epithermal and porphyry-type ore deposits from the Romanian Carpathian Mountains. Miner. Depos. 2002, 37, 173–184. [Google Scholar] [CrossRef]
- Chiaradia, M.; Fontboté, L.; Paladines, A. Metal sources in mineral deposits and crustal rocks of Ecuador (1 N–4 S): A lead isotope synthesis. Econo. Geol. 2004, 99, 1085–1106. [Google Scholar]
- Zartman, R.E.; Doe, B.R. Plumbotectonics: The model. Tectonophysics 1981, 75, 135–162. [Google Scholar] [CrossRef]
- Zhu, B.Q. Tri-dimension spacial topological diagrams of ore lead isotopes and their application to the division of geochemical provinces and mineralizations. Geochimica 1993, 21, 209–216, (In Chinese with English Abstract). [Google Scholar]
- Richards, J.P. Magmatic to hydrothermal metal fluxes in convergent and collided margins. Ore Geol. Rev. 2011, 40, 1–26. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and their bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Stefansson, A.; Seward, T.M. Experimental determination of the stability and stoichiometry of sulphide complexes of silver (I) in hydrothermal solutions to 400 degrees. Geochim. Cosmochim. Acta 2003, 67, 1395–1413. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A. Experimental Constraints on the Transport and Deposition of Metals in Ore-Forming Hydrothermal Systems. In Building Exploration Capability for the 21st Century; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2014; pp. 77–95. [Google Scholar]
- Zhong, R.C.; Brugger, J.; Chen, Y.J.; Li, W.B. Contrasting regimes of Cu, Zn and Pb transport in ore-forming hydrothermal fluids. Chem. Geol. 2015, 395, 154–164. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The hydrothermal geochemistry of tungsten in granitoid environments: I. Relative solubilities of ferberite and scheelite as a function of T, P, pH, and m (NaCl). Econ. Geol. 2000, 95, 143–182. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer Science & Business Media B.V.: Perth, Australia, 2009; pp. 1–1250. [Google Scholar]
- Seward, T.M.; Williams-Jones, A.E.; Migdisov, A.A. The Chemistry of Metal Transport and Deposition by Ore-Forming Hydrothermal Fluids. In Treatise on Geochemistry; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 29–57. [Google Scholar]
- Korges, M.; Weis, P.; Lüders, V.; Laurent, O. Depressurization and boiling of a single magmatic fluid as a mechanism for tin–tungsten deposit formation. Geology 2017, 46, 75–78. [Google Scholar] [CrossRef]
- Jiang, S.H.; Chen, C.L.; Bagas, L.; Liu, Y.; Han, N.; Kang, H.; Wang, Z.H. Two mineralization events in the Baiyinnuoer Zn–Pb deposit in Inner Mongolia, China: Evidence from field observations, S–Pb isotopic compositions and U–Pb zircon ages. J. Asian Earth Sci. 2017, 144, 339–367. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Zhou, Z.H.; Ouyang, H.G.; Chen, B.Q.; Liu, W.J.; Yang, F. Zircon U–Pb age and geochemistry of quartz syenite porphyry in Shuangjianzishan Ag–Pb–Zn (Sn) deposit, Inner Mongolia, and their geological implications. Miner. Depos. 2022, 41, 324–344, (In Chinese with English Abstract). [Google Scholar]
- Chinchilla, D.; Ortega, L.; Piña, R.; Merinero, R.; Moncada, D.; Bodnar, R.J.; Quesada, C.; Valverde, C.; Lunar, R. The Patricia Zn–Pb–Ag epithermal ore deposit: An uncommon type of mineralization in northeastern Chile. Ore Geol. Rev. 2016, 73, 104–126. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
| Sample | Orebody No./Lithology | Position | Stage | Mineral | Analysis |
|---|---|---|---|---|---|
| SJ49-1 | Syenogranite | Drill No. ZK12-37 at 780 m deep | Zrn | U–Pb dating | |
| SJ49-10 | Syenogranite | Drill No. ZK12-37 at 1071 m deep | Zrn | U–Pb dating | |
| SJ14 | No. 2 Pb–Zn–Cu orebody | 625-m level | I | Qtz | FIs study |
| SJ6 | Disseminated Pb–Zn–Ag orebody | 625-m level | II | Qtz | FIs study |
| SJ31 | No. 2 Pb–Zn–Ag orebody | Drill No. ZK1501 at 1207 m deep | II | Qtz | FIs study |
| SJ11 | No. 1-3 Pb–Zn–Ag orebody | 625-m level | III | Qtz | FIs study |
| SJ12 | No. 2 Pb–Zn–Ag orebody | 625-m level | III | Qtz | FIs study |
| SJ13-1 | Pb–Zn–Ag–Au orebody | 625-m level | III | Cal | FIs study |
| SJ8 | No. 2 Pb–Zn–Ag mineralized orebody | 625-m level | IV | Qtz | FIs study |
| SJ45 | No. 2-16 Pb–Zn–Ag mineralized body | 665-m level | IV | Cal | FIs study |
| 2-3m-3 | No. 2-3 Pb–Zn–Cu orebody | 625-m level | I | Qtz | H–O isotopes |
| 8-16m-1 | No. 8-16 Pb–Zn–Cu orebody | 665-m level | I | Qtz | H–O isotopes |
| 1m-3 | No. 1 Pb–Zn–Cu orebody | 625-m level | I | Qtz | H–O isotopes |
| 8-16m-3 | No. 8-16 Pb–Zn–Cu orebody | 665-m level | I | Qtz | H–O isotopes |
| 8-16m-4 | No. 8-16 Pb–Zn–Cu orebody | 665-m level | I | Qtz | H–O isotopes |
| 2-3m-7 | No. 2-3 Pb–Zn–Cu orebody | 625-m level | I | Qtz | H–O isotopes |
| 2-3m-6 | No. 2-3 Pb–Zn–Ag orebody | 625-m level | II | Qtz | H–O isotopes |
| 1-5-1m-2 | No. 1-5-1 Pb–Zn–Ag–Au orebody | 625-m level | II | Qtz | H–O isotopes |
| 5#KD-2-1 | No. 2 Pb–Zn–Ag orebody | 625-m level | II | Qtz | H–O isotopes |
| 5#KD-2-2 | No. 2 Pb–Zn–Ag orebody | 625-m level | II | Qtz | H–O isotopes |
| ZD4-1-1m-1 | No. 1-1 Pb–Zn–Ag orebody | 665-m level | III | Cal | H–O–C isotopes |
| ZD4-1-1m-2 | No. 1-1 Pb–Zn–Ag orebody | 665-m level | III | Cal | H–O–C isotopes |
| ZD4-1-1m-3 | No. 1-1 Pb–Zn–Ag orebody | 665-m level | III | Cal | H–O–C isotopes |
| ZD4-1-1m-4 | No. 1-1 Pb–Zn–Ag orebody | 665-m level | III | Qtz | H–O isotopes |
| ZD4-1-1m-4 | No. 1-1 Pb–Zn–Ag orebody | 665-m level | III | Cal | H–O–C isotopes |
| 5#KD-1 | No. 1-1 Pb–Zn–Ag mineralized body | 665-m level | IV | Qtz | H–O isotopes |
| 8-12m-1 | No. 8-12 Pb–Zn–Ag mineralized body | 665-m level | IV | Cal | H–O–C isotopes |
| 8-12m-2 | No. 8-12 Pb–Zn–Ag mineralized body | 665-m level | IV | Qtz | H–O isotopes |
| 8-12m-2 | No. 8-12 Pb–Zn–Ag mineralized body | 665-m level | IV | Cal | H–O–C isotopes |
| 8-12m-3 | No. 8-12 Pb–Zn–Ag mineralized body | 665-m level | IV | Cal | H–O–C isotopes |
| 2-3m-1 | No. 2-3 Pb–Zn–Cu orebody | 625-m level | I | Gn, Sp, Py | Pb isotope |
| 2m-3 | No. 2-3 Pb–Zn–Cu orebody | 625-m level | I | Gn, Sp, Py | Pb isotope |
| 1m-1 | No. 1 Pb–Zn–Cu orebody | 625-m level | I | Gn, Sp, Py | Pb isotope |
| ZD5-1-1m-1 | No. 1-1 Pb–Zn–Ag orebody | 625-m level | II | Gn, Sp | Pb isotope |
| ZD4-1-1m-1 | No. 1-1 Pb–Zn–Ag orebody | 665-m level | III | Gn, Sp, Py | Pb isotope |
| 8-16m-2 | No. 8-16 Pb–Zn–Ag mineralized body | 665-m level | IV | Gn, Sp, Py | Pb isotope |
| 8-12m-1 | No. 8-12 Pb–Zn–Ag mineralized body | 665-m level | IV | Gn, Sp, Py | Pb isotope |
| Spot No. | Isotopic Ratios | Age (Ma) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 207Pb/206Pb | 1σ | 207Pb/235U | 1σ | 206Pb/238U | 1σ | 238U/206Pb | 1σ | 206Pb/238U | 1σ | |
| Sample SJ49-1 | ||||||||||
| SJ49-1-5 | 0.05207 | 0.00419 | 0.15345 | 0.00467 | 0.02148 | 0.00034 | 145 | 11 | 137 | 3 |
| SJ49-1-6 | 0.05151 | 0.00219 | 0.15133 | 0.00427 | 0.02143 | 0.00029 | 143 | 6 | 137 | 2 |
| SJ49-1-7 | 0.05404 | 0.00366 | 0.15977 | 0.00201 | 0.02136 | 0.00036 | 151 | 11 | 136 | 2 |
| SJ49-1-8 | 0.05460 | 0.00363 | 0.16211 | 0.00490 | 0.02168 | 0.00035 | 153 | 9 | 138 | 3 |
| SJ49-1-9 | 0.04866 | 0.00210 | 0.14663 | 0.00447 | 0.02204 | 0.00039 | 139 | 6 | 141 | 2 |
| SJ49-1-10 | 0.05433 | 0.00359 | 0.16148 | 0.00411 | 0.02166 | 0.00032 | 152 | 10 | 138 | 3 |
| SJ49-1-11 | 0.05142 | 0.00149 | 0.15133 | 0.00442 | 0.02139 | 0.00030 | 143 | 4 | 136 | 2 |
| SJ49-1-12 | 0.05311 | 0.00273 | 0.15878 | 0.00418 | 0.02162 | 0.00034 | 150 | 7 | 138 | 2 |
| SJ49-1-13 | 0.05426 | 0.00331 | 0.16184 | 0.00580 | 0.02173 | 0.00033 | 152 | 9 | 139 | 5 |
| SJ49-1-14 | 0.05297 | 0.00267 | 0.15271 | 0.00588 | 0.02122 | 0.00037 | 144 | 6 | 135 | 2 |
| SJ49-1-15 | 0.05326 | 0.00183 | 0.16031 | 0.00529 | 0.02201 | 0.00032 | 151 | 5 | 140 | 2 |
| Sample SJ49-10 | ||||||||||
| SJ49-10-1 | 0.05360 | 0.00234 | 0.15744 | 0.00366 | 0.02150 | 0.00036 | 354 | 98 | 149 | 6 |
| SJ49-10-2 | 0.05298 | 0.00273 | 0.15447 | 0.00351 | 0.02152 | 0.00036 | 328 | 117 | 146 | 7 |
| SJ49-10-3 | 0.05103 | 0.00178 | 0.15186 | 0.00530 | 0.02159 | 0.00033 | 243 | 77 | 144 | 5 |
| SJ49-10-4 | 0.05306 | 0.00211 | 0.16012 | 0.00674 | 0.02171 | 0.00027 | 332 | 91 | 151 | 6 |
| SJ49-10-5 | 0.05211 | 0.00203 | 0.15586 | 0.00647 | 0.02165 | 0.00035 | 300 | 89 | 147 | 6 |
| SJ49-10-6 | 0.05381 | 0.00304 | 0.16331 | 0.00512 | 0.02198 | 0.00037 | 365 | 128 | 154 | 8 |
| SJ49-10-7 | 0.05146 | 0.00127 | 0.15120 | 0.00409 | 0.02123 | 0.00030 | 261 | 53 | 143 | 4 |
| SJ49-10-8 | 0.05294 | 0.00370 | 0.15308 | 0.00681 | 0.02095 | 0.00047 | 328 | 155 | 145 | 9 |
| SJ49-10-9 | 0.05087 | 0.00207 | 0.15079 | 0.00669 | 0.02135 | 0.00032 | 235 | 94 | 143 | 6 |
| SJ49-10-10 | 0.05095 | 0.00659 | 0.15175 | 0.00241 | 0.02123 | 0.00048 | 239 | 283 | 144 | 21 |
| SJ49-10-11 | 0.05309 | 0.00244 | 0.16196 | 0.00318 | 0.02201 | 0.00039 | 332 | 101 | 152 | 7 |
| SJ49-10-12 | 0.05295 | 0.00226 | 0.15167 | 0.00605 | 0.02077 | 0.00046 | 328 | 94 | 143 | 5 |
| SJ49-10-13 | 0.05161 | 0.00190 | 0.14800 | 0.00525 | 0.02093 | 0.00029 | 333 | 81 | 140 | 5 |
| SJ49-10-14 | 0.05390 | 0.00150 | 0.16319 | 0.00505 | 0.02185 | 0.00034 | 369 | 63 | 154 | 4 |
| SJ49-10-15 | 0.05386 | 0.00215 | 0.15455 | 0.00616 | 0.02084 | 0.00027 | 365 | 91 | 146 | 5 |
| SJ49-10-16 | 0.05468 | 0.00306 | 0.16463 | 0.00531 | 0.02207 | 0.00042 | 398 | 94 | 155 | 7 |
| SJ49-10-17 | 0.05391 | 0.00209 | 0.16044 | 0.00622 | 0.02162 | 0.00038 | 369 | 89 | 151 | 5 |
| SJ49-10-18 | 0.05461 | 0.00307 | 0.15926 | 0.00573 | 0.02129 | 0.00036 | 395 | 126 | 150 | 8 |
| SJ49-10-19 | 0.05024 | 0.00245 | 0.14607 | 0.00689 | 0.02130 | 0.00033 | 206 | 118 | 138 | 6 |
| SJ49-10-20 | 0.05368 | 0.00154 | 0.16087 | 0.00483 | 0.02167 | 0.00028 | 367 | 60 | 152 | 4 |
| Type | Host Mineral | FIA No. | No. | Size (μm) | V (vol.%) | Tm (ice) (°C) | Th (°C) | Salinity (wt% NaCl Eqv.) | Density (g/cm3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Range | Mean | Range | |||||||
| Stage I: sphalerite–arsenopyrite–pyrite–chalcopyrite–quartz stage (sample SJ14) | ||||||||||
| WL | Quartz | 1 | 6 | 5–30 | 5–40 | −3.2 to −1.9 | 278–302 | 288 | 3.4–5.3 | 0.74–0.79 |
| WL | Quartz | 2 | 5 | 10–30 | 10–35 | −3.2 to −2.2 | 253–274 | 263 | 3.7–5.3 | 0.80–0.83 |
| WG | Quartz | 3 | 3 | 45–60 | 55–70 | −4.1 to −2.4 | 279–289 | 284 | 4.0–6.6 | 0.79–0.80 |
| Stage II: sphalerite–galena–pyrite–silver-bearing minerals–quartz stage (samples SJ6 and SJ31) | ||||||||||
| WL | Quartz | 4 | 11 | 5–30 | 10–25 | −4.5 to −2.5 | 224–248 | 238 | 3.9–7.2 | 0.84–0.88 |
| WL | Quartz | 5 | 16 | 10–35 | 5–45 | −4.2 to −1.6 | 229–268 | 238 | 3.2–6.7 | 0.83–0.87 |
| WL | Quartz | 6 | 6 | 10–30 | 5–20 | −2.3 to −1.8 | 203–221 | 220 | 2.6–3.9 | 0.80–0.88 |
| Stage III: sphalerite–galena–silver-bearing minerals–quartz–calcite stage (samples SJ11, SJ12, and SJ13-1) | ||||||||||
| WL | Quartz | 7 | 20 | 10–35 | 5–20 | −3.5 to −1.8 | 200–222 | 212 | 3.1–5.7 | 0.86–0.91 |
| WL | Quartz | 8 | 12 | 10–30 | 10–20 | −2.8 to −1.9 | 200–216 | 208 | 3.2–4.6 | 0.87–0.90 |
| WL | Calcite | 9 | 12 | 10–30 | 10–20 | −4.4 to −1.7 | 184–199 | 192 | 2.9–7.0 | 0.89–0.92 |
| Stage IV: weakly mineralized quartz–calcite stage (samples SJ8 and SJ45) | ||||||||||
| WL | Quartz | 10 | 4 | 15–20 | 5–20 | −2.2 to −1.5 | 185–198 | 191 | 2.6–3.7 | 0.89–0.91 |
| WL | Calcite | 11 | 9 | 10–35 | 10–20 | −2.9 to −0.7 | 153–187 | 176 | 1.2–4.8 | 0.90–0.93 |
| Sample No. | Mineral | Stage | Th (°C) | δ18Oquartz (V-SMOW) | δ18Owater (V-SMOW) | δDV-SMOW | δ13CV-PDB |
|---|---|---|---|---|---|---|---|
| 2-3m-3 | Quartz | I | 278 | 4.1 | −3.6 | −65 | |
| 8-16m-1 | Quartz | I | 278 | 5.8 | −1.9 | −75 | |
| 1m-3 | Quartz | I | 278 | 5.8 | −1.9 | −126 | |
| 8-16m-3 | Quartz | I | 278 | 5.1 | −2.6 | −106 | |
| 8-16m-4 | Quartz | I | 278 | 5.1 | −2.6 | −120 | |
| 2-3m-7 | Quartz | I | 278 | 15.1 | 7.4 | −117 | |
| 2-3m-6 | Quartz | II | 234 | 4.1 | −5.6 | −97 | |
| 5#KD-2-1 | Quartz | II | 234 | 4.4 | −5.3 | −118 | |
| 5#KD-2-2 | Quartz | II | 234 | 4.5 | −5.2 | −103 | |
| 1-5-1m-2 | Quartz | II | 234 | 6.1 | −3.6 | −94 | |
| ZD4-1-1m-1 | Calcite | III | 206 | 2.5 | −7.0 | −133 | −11.0 |
| ZD4-1-1m-2 | Calcite | III | 206 | 1.9 | −7.6 | −138 | −10.7 |
| ZD4-1-1m-3 | Calcite | III | 206 | 1.0 | −8.5 | −145 | −10.5 |
| ZD4-1-1m-4 | Quartz | III | 206 | 7.4 | −3.9 | −123 | |
| ZD4-1-1m-4 | Calcite | III | 206 | 1.3 | −8.2 | −121 | −10.9 |
| 5#KD-1 | Quartz | IV | 180 | 7.9 | −5.2 | −106 | |
| 8-12m-1 | Calcite | IV | 180 | 4.5 | −6.5 | −123 | −9.9 |
| 8-12m-2 | Quartz | IV | 180 | −0.8 | −13.9 | −116 | |
| 8-12m-2 | Calcite | IV | 180 | 3.6 | −7.4 | −112 | −7.9 |
| 8-12m-3 | Calcite | IV | 180 | 2.5 | −8.5 | −134 | −9.4 |
| Sample No. | Mineral | Stage | 206Pb/204Pb | 207Pb/204Pb | 208Pb/204Pb | t (Ma) | μ | ω | κ | Δα | Δβ | Δγ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-3m-1 | I | Galena | 18.308 | 15.569 | 38.229 | 137 | 9.31 | 35.5 | 3.69 | 65.2 | 15.9 | 26.1 |
| 2-3m-1 | I | Sphalerite | 18.285 | 15.536 | 38.130 | 137 | 9.29 | 35.1 | 3.65 | 63.9 | 13.7 | 23.5 |
| 2-3m-1 | I | Pyrite | 18.303 | 15.564 | 38.205 | 137 | 9.31 | 35.4 | 3.67 | 64.9 | 15.6 | 25.5 |
| 2m-3 | I | Galena | 18.361 | 15.634 | 38.448 | 137 | 9.37 | 36.3 | 3.75 | 68.3 | 20.1 | 32.0 |
| 2m-3 | I | Sphalerite | 18.290 | 15.545 | 38.179 | 137 | 9.29 | 35.3 | 3.67 | 64.2 | 14.3 | 24.8 |
| 2m-3 | I | Pyrite | 18.308 | 15.566 | 38.240 | 137 | 9.31 | 35.5 | 3.69 | 65.2 | 15.7 | 26.4 |
| 1m-1 | I | Galena | 18.355 | 15.626 | 38.419 | 137 | 9.36 | 36.2 | 3.75 | 67.9 | 19.6 | 31.2 |
| 1m-1 | I | Sphalerite | 18.323 | 15.585 | 38.261 | 137 | 9.33 | 35.6 | 3.69 | 66.1 | 16.9 | 27.0 |
| 1m-1 | I | Pyrite | 18.302 | 15.561 | 38.211 | 137 | 9.31 | 35.4 | 3.68 | 64.8 | 15.4 | 25.6 |
| ZD5-1-1m-1 | II | Galena | 18.358 | 15.630 | 38.438 | 137 | 9.36 | 36.3 | 3.75 | 68.1 | 19.9 | 31.7 |
| ZD5-1-1m-1 | II | Sphalerite | 18.311 | 15.567 | 38.234 | 137 | 9.31 | 35.5 | 3.69 | 65.4 | 15.8 | 26.2 |
| ZD4-1-1m-1 | III | Galena | 18.278 | 15.530 | 38.107 | 137 | 9.28 | 35.0 | 3.65 | 63.5 | 13.3 | 22.8 |
| ZD4-1-1m-1 | III | Sphalerite | 18.310 | 15.570 | 38.234 | 137 | 9.31 | 35.5 | 3.69 | 65.3 | 16.0 | 26.2 |
| ZD4-1-1m-1 | III | Pyrite | 18.299 | 15.555 | 38.199 | 137 | 9.30 | 35.3 | 3.68 | 64.7 | 15.0 | 25.3 |
| 8-16m-2 | IV | Galena | 18.311 | 15.569 | 38.239 | 137 | 9.31 | 35.5 | 3.69 | 65.4 | 15.9 | 26.4 |
| 8-16m-2 | IV | Sphalerite | 18.293 | 15.571 | 38.234 | 137 | 9.30 | 35.5 | 3.69 | 64.3 | 16.0 | 26.2 |
| 8-16m-2 | IV | Pyrite | 18.332 | 15.597 | 38.319 | 137 | 9.34 | 35.8 | 3.71 | 66.6 | 17.7 | 28.5 |
| 8-12m-1 | IV | Galena | 18.346 | 15.616 | 38.389 | 137 | 9.35 | 36.1 | 3.74 | 67.4 | 19.0 | 30.4 |
| 8-12m-1 | IV | Sphalerite | 18.329 | 15.598 | 38.331 | 137 | 9.33 | 35.9 | 3.72 | 66.4 | 17.8 | 28.9 |
| 8-12m-1 | IV | Pyrite | 18.307 | 15.564 | 38.229 | 137 | 9.31 | 35.5 | 3.69 | 65.1 | 15.6 | 26.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Wu, G.; Chen, G.; Yang, F.; Zhang, T.; Jiang, B.; Liu, W. Genesis of the Supergiant Shuangjianzishan Ag–Pb–Zn Deposit in the Southern Great Xing’an Range, NE China: Constraints from Geochronology, Isotope Geochemistry, and Fluid Inclusion. Minerals 2024, 14, 60. https://doi.org/10.3390/min14010060
Shi J, Wu G, Chen G, Yang F, Zhang T, Jiang B, Liu W. Genesis of the Supergiant Shuangjianzishan Ag–Pb–Zn Deposit in the Southern Great Xing’an Range, NE China: Constraints from Geochronology, Isotope Geochemistry, and Fluid Inclusion. Minerals. 2024; 14(1):60. https://doi.org/10.3390/min14010060
Chicago/Turabian StyleShi, Jiangpeng, Guang Wu, Gongzheng Chen, Fei Yang, Tong Zhang, Biao Jiang, and Wenyuan Liu. 2024. "Genesis of the Supergiant Shuangjianzishan Ag–Pb–Zn Deposit in the Southern Great Xing’an Range, NE China: Constraints from Geochronology, Isotope Geochemistry, and Fluid Inclusion" Minerals 14, no. 1: 60. https://doi.org/10.3390/min14010060
APA StyleShi, J., Wu, G., Chen, G., Yang, F., Zhang, T., Jiang, B., & Liu, W. (2024). Genesis of the Supergiant Shuangjianzishan Ag–Pb–Zn Deposit in the Southern Great Xing’an Range, NE China: Constraints from Geochronology, Isotope Geochemistry, and Fluid Inclusion. Minerals, 14(1), 60. https://doi.org/10.3390/min14010060