Evaluation of As-Received Green Liquor Dregs and Biomass Ash Residues from a Pulp and Paper Industry as Raw Materials for Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
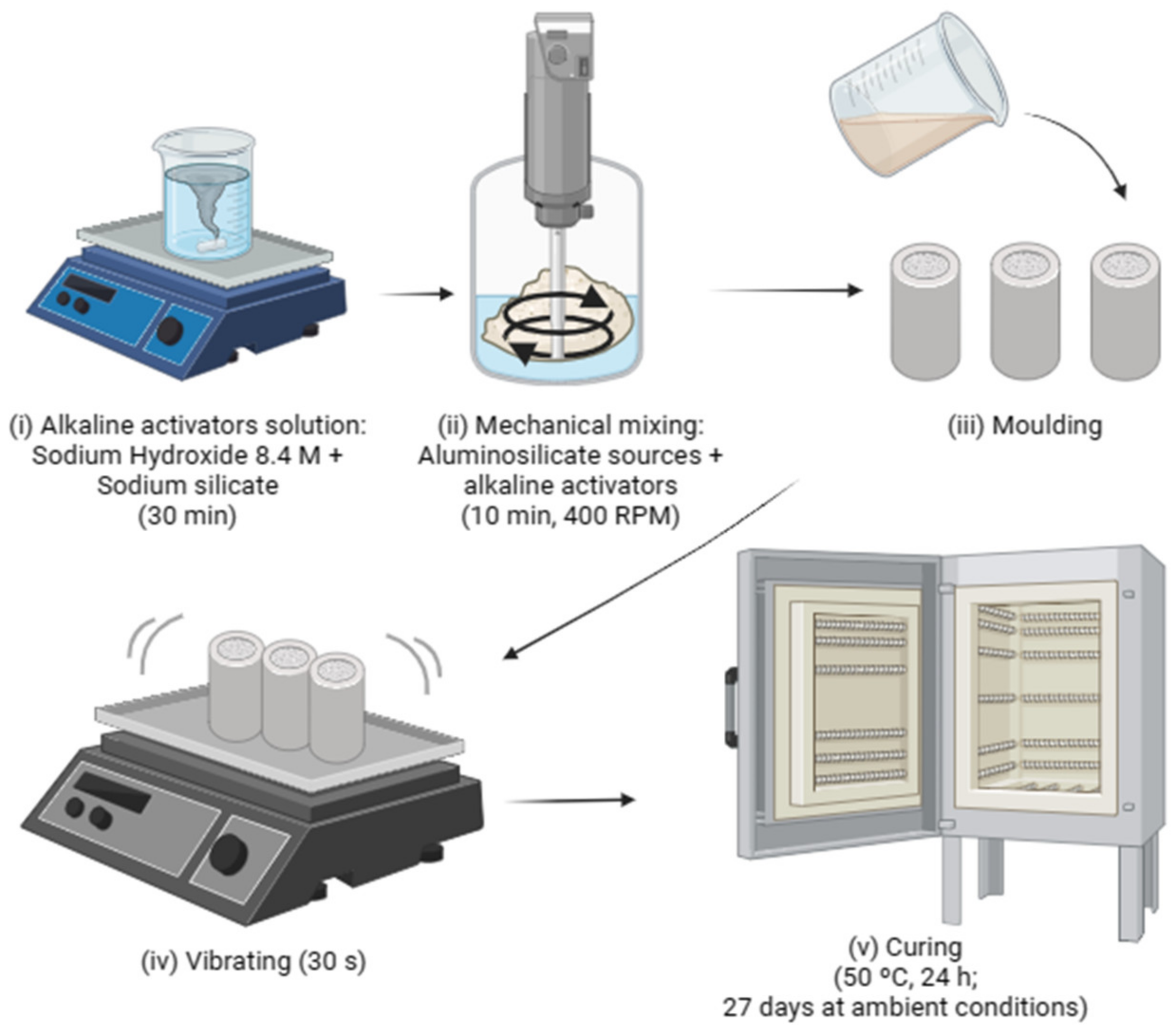
2.2. Samples Processing
2.3. Characterization
2.4. Experimental Plan
3. Results and Discussion
3.1. Chemical and Mineralogical Composition of Materials
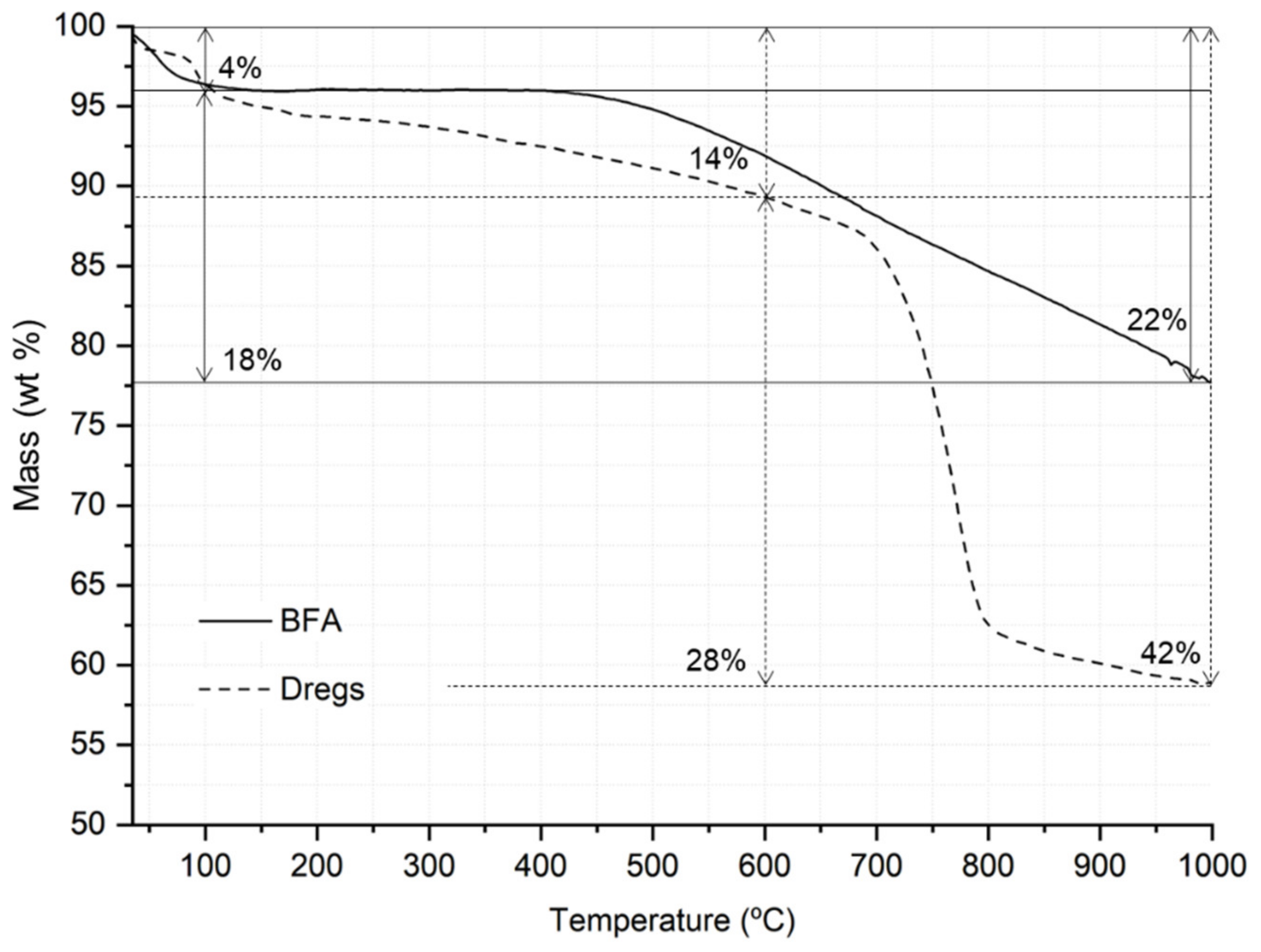
3.2. Thermal Characterization
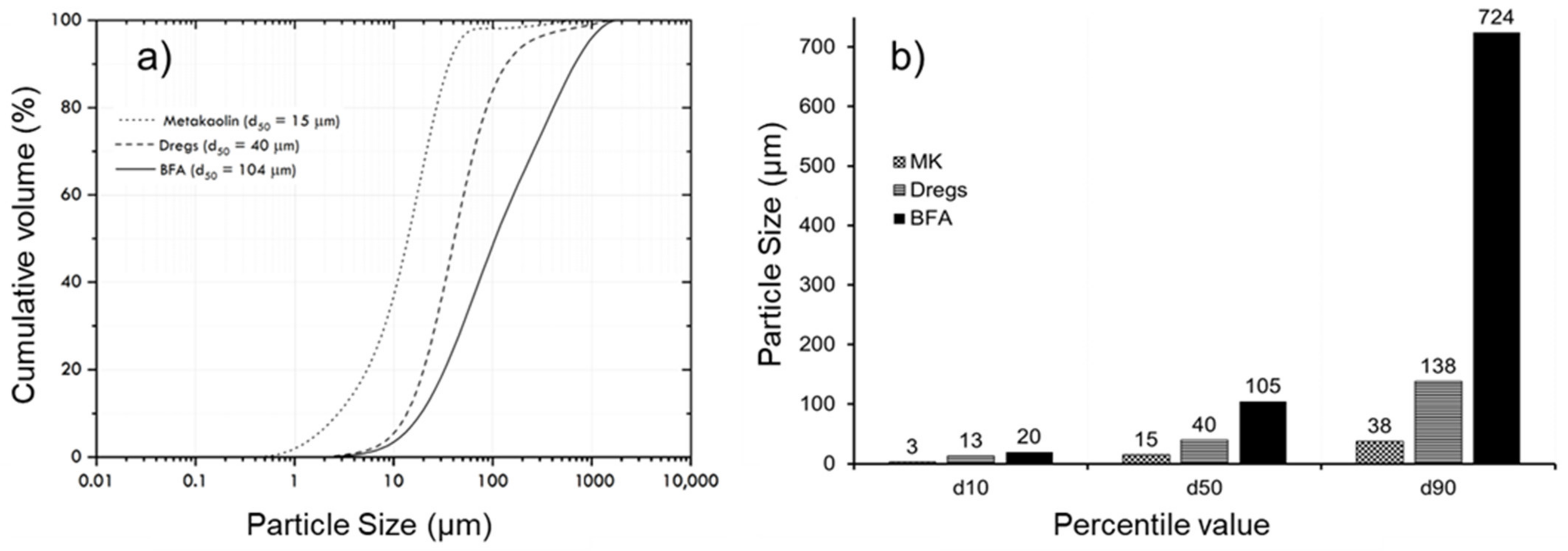
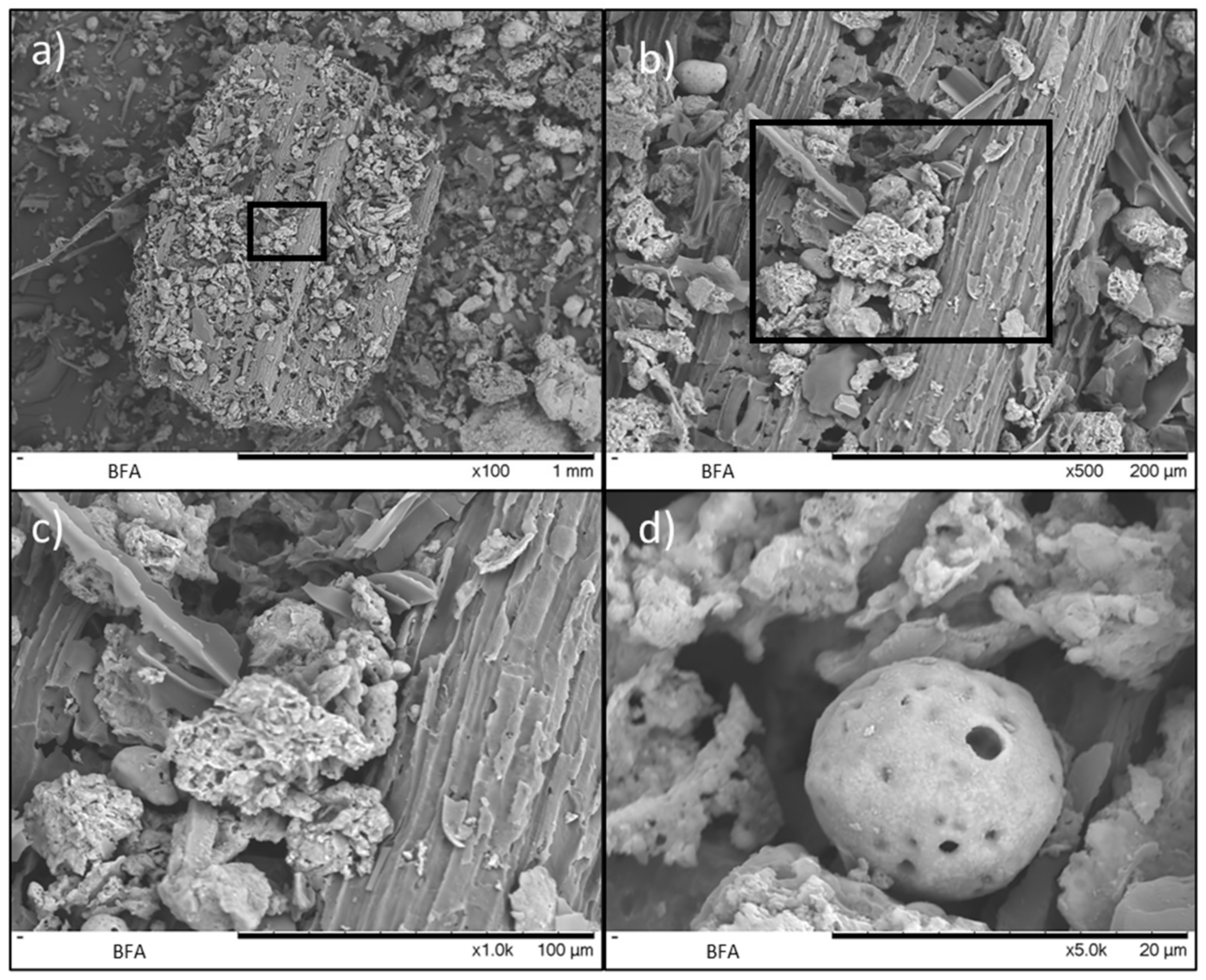
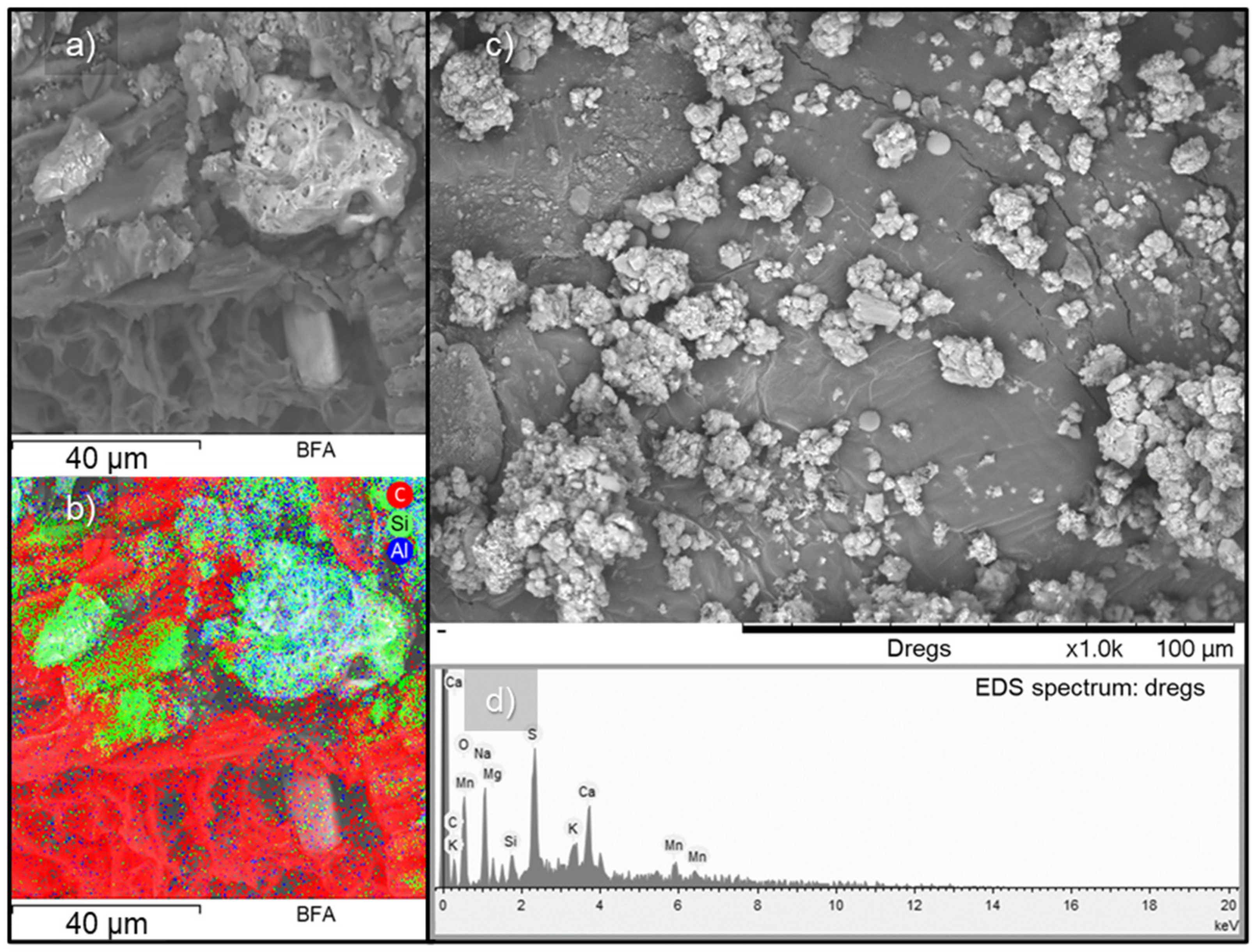
3.3. Morphological Characterization: Particle Size, SEM, and EDS
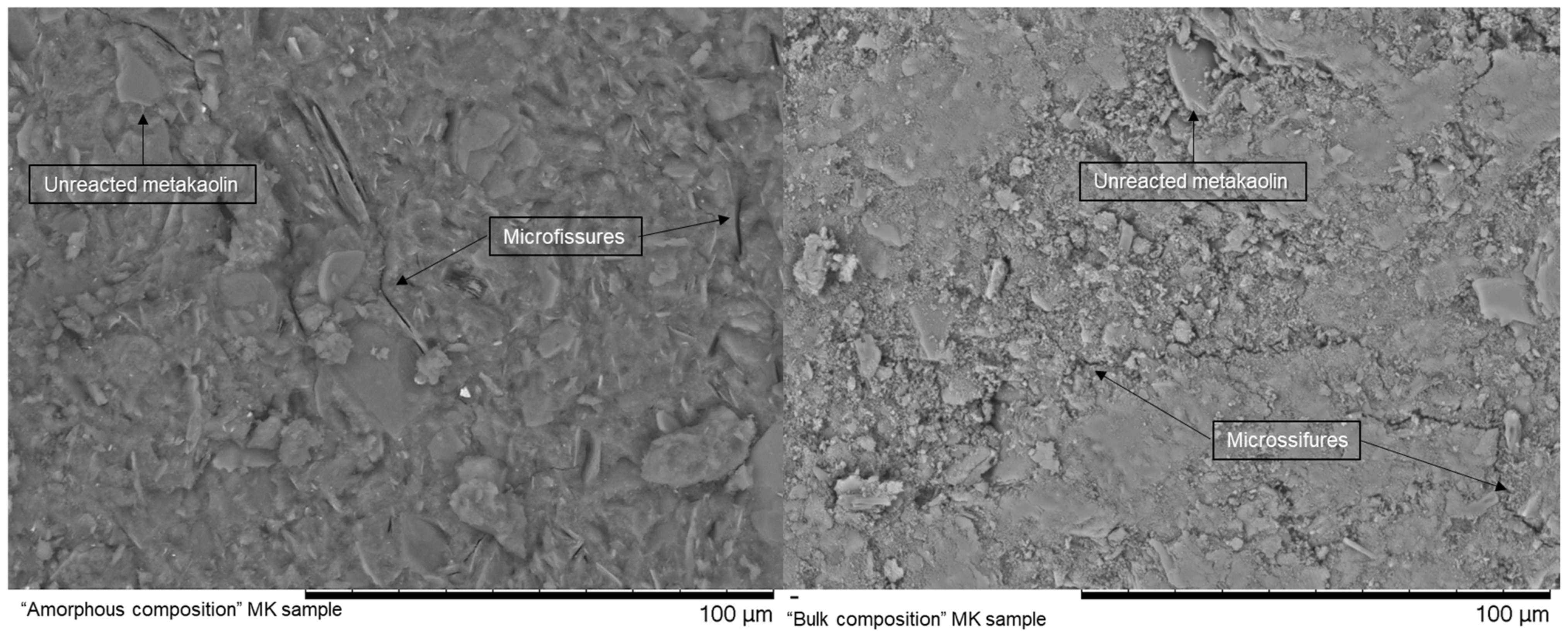
3.4. Compressive Strength: Amorphous vs. Bulk Composition
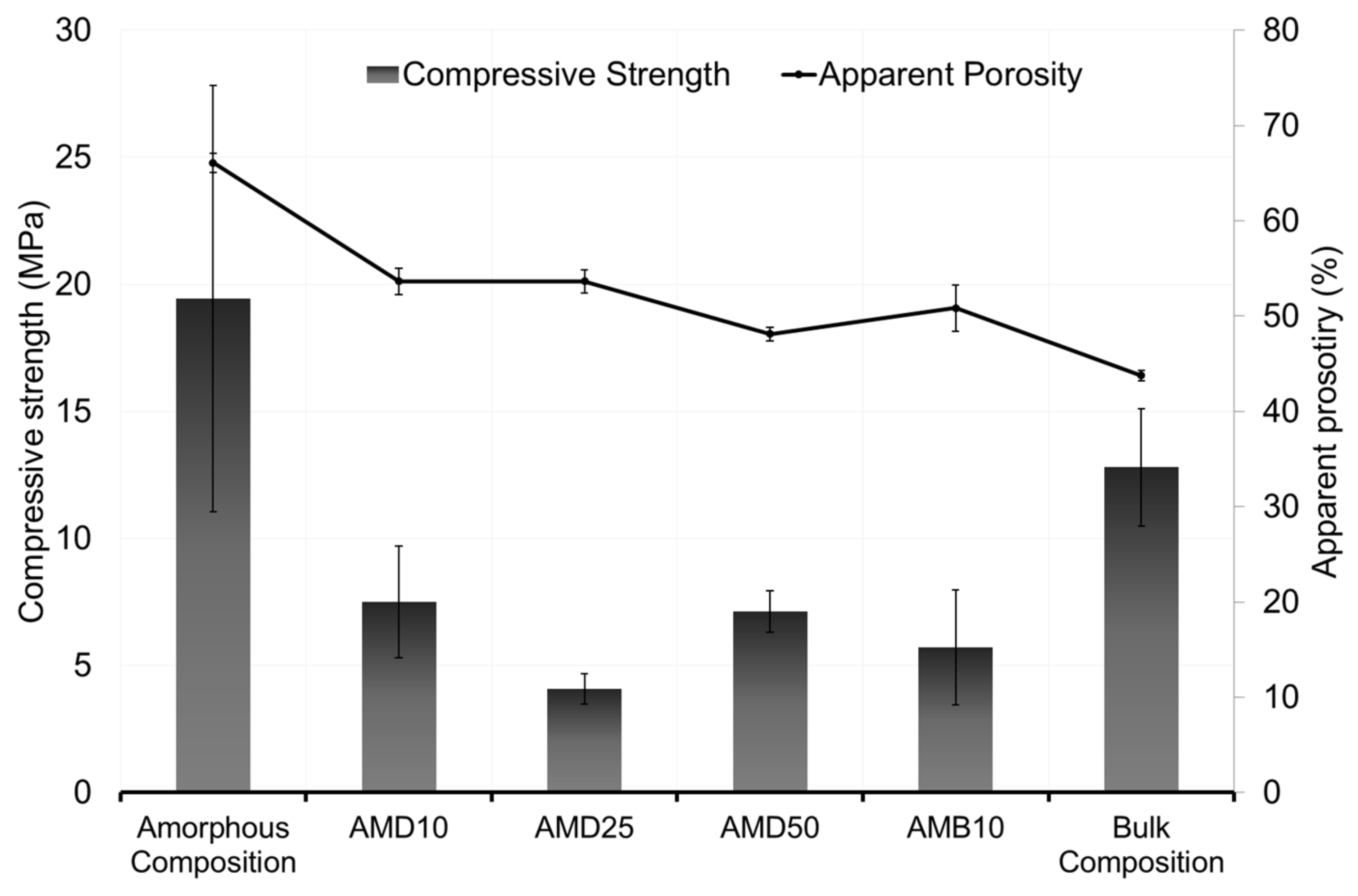
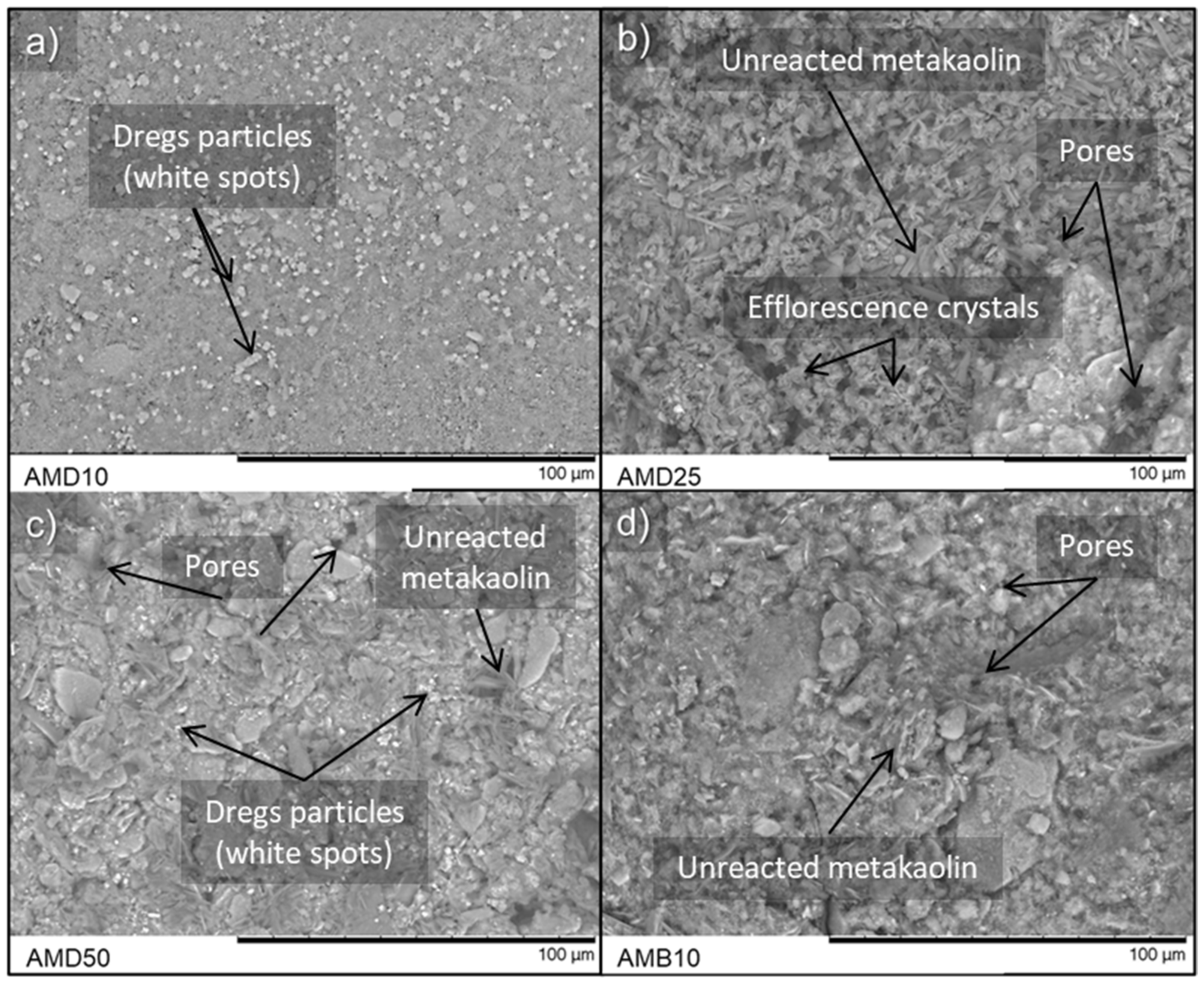
3.5. Waste Incorporation
4. Conclusions
- An approach based on the supposedly more reactive content of the materials was used to improve the results. Metakaolin was used as a benchmark initial material and the approach has improved its mechanical properties, even though it promoted too rapid a reaction and high shrinkage rates, with consequent high compressive strength development at an early age, but a decrease in the values after the total time of curing of 28 days due to the cracking of the matrix.
- Dregs are essentially inert, and biomass fly ash has a wide range of particle size distribution and high levels of impurities. These characteristics lead to the degradation of the geopolymeric gel and retard the reaction rate due to the filling effect of the residues’ particles, the mismatch derived from the range of their size distribution, and the presence of impurities such as organic matter possibly made of lignin and unburned wood.
- Overall, the wastes harmed the compressive strength of the geopolymeric pastes based on metakaolin with higher or lower levels of efflorescence development that does not seem to be directly related to the mechanical behavior; however, by calculating the mixture ratios with the amorphous composition of the raw materials, it was possible to incorporate the waste materials into the geopolymeric matrix while obtaining properties equal to or superior to the ones obtained with mixture ratios calculated from the total composition.
- The incorporation of dregs and BFA led to a reduction of the compressive strength, from 19.4 MPa in the benchmark metakaolin sample to 7.1 Mpa for 50 wt% of dregs (AMD50) and 5.1 Mpa for 10 wt% of BFA (AMB10). The apparent porosity of all the samples remained close to 50%, while the water absorption was close to 40%.
5. Recommendations for Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandeep; Gupta, G.K.; Liu, H.; Shukla, P. Pulp and Paper Industry–Based Pollutants, Their Health Hazards and Environmental Risks. Curr. Opin. Environ. Sci. Health 2019, 12, 48–56. [Google Scholar] [CrossRef]
- Simão, L.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O.R.K. Wastes from Pulp and Paper Mills—A Review of Generation and Recycling Alternatives. Cerâmica 2018, 64, 443–453. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Senff, L.; Labrincha, J.A. Upcycling Unexplored Dregs and Biomass Fly Ash from the Paper and Pulp Industry in the Production of Eco-Friendly Geopolymer Mortars: A Preliminary Assessment. Constr. Build. Mater. 2018, 184, 464–472. [Google Scholar] [CrossRef]
- Saeli, M.; Micale, R.; Seabra, M.P.; Labrincha, J.A.; La Scalia, G. Selection of Novel Geopolymeric Mortars for Sustainable Construction Applications Using Fuzzy Topsis Approach. Sustainability 2020, 12, 5987. [Google Scholar] [CrossRef]
- Simão, L.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O.R.K. Characterization of Pulp and Paper Mill Waste for the Production of Waste-Based Cement. Rev. Int. De Contam. Ambient. 2019, 35, 237–246. [Google Scholar] [CrossRef]
- Simão, L.; De Rossi, A.; Hotza, D.; Ribeiro, M.J.; Novais, R.M.; Klegues Montedo, O.R.; Raupp-Pereira, F. Zeolites-Containing Geopolymers Obtained from Biomass Fly Ash: Influence of Temperature, Composition, and Porosity. J. Am. Ceram. Soc. 2021, 104, 803–815. [Google Scholar] [CrossRef]
- Habert, G.; Ouellet-Plamondon, C. Recent Update on the Environmental Impact of Geopolymers. RILEM Tech. Lett. 2016, 1, 17–23. [Google Scholar] [CrossRef]
- La Scalia, G.; Saeli, M.; Adelfio, L.; Micale, R. From Lab to Industry: Scaling up Green Geopolymeric Mortars Manufacturing towards Circular Economy. J. Clean. Prod. 2021, 316, 128164. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Han, Z.C.; He, P.Y.; Chen, H. Geopolymer-Based Catalysts for Cost-Effective Environmental Governance: A Review Based on Source Control and End-of-Pipe Treatment. J. Clean. Prod. 2020, 263, 121–556. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Peralta, R.M.; Peralta, R.A.; Rodríguez-Castellón, E.; de Fatima Peralta Muniz Moreira, R. Adding Value to Aluminosilicate Solid Wastes to Produce Adsorbents, Catalysts and Filtration Membranes for Water and Wastewater Treatment. J. Mater. Sci. 2021, 56, 1039–1063. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, L.; Liu, T.; Hao, L.; Li, Y.; Liu, P.; Zhu, T. A Review of Low-Carbon Technologies and Projects for the Global Cement Industry. J. Environ. Sci. 2024, 136, 682–697. [Google Scholar] [CrossRef]
- Lanjewar, B.A.; Chippagiri, R.; Dakwale, V.A.; Ralegaonkar, R.V. Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder. Energies 2023, 16, 969. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, B.; Zhou, H.; Ma, Y.; Jiang, X. A State-of-the-Art Review of Crushed Urban Waste Glass Used in OPC and AAMs (Geopolymer): Progress and Challenges. Clean. Mater. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Acar, M.C.; Çelik, A.İ.; Kayabaşı, R.; Şener, A.; Özdöner, N.; Özkılıç, Y.O. Production of Perlite-Based-Aerated Geopolymer Using Hydrogen Peroxide as Eco-Friendly Material for Energy-Efficient Buildings. J. Mater. Res. Technol. 2023, 24, 81–99. [Google Scholar] [CrossRef]
- Meskhi, B.; Beskopylny, A.N.; Stel’makh, S.A.; Shcherban’, E.M.; Mailyan, L.R.; Shilov, A.A.; El’shaeva, D.; Shilova, K.; Karalar, M.; Aksoylu, C.; et al. Analytical Review of Geopolymer Concrete: Retrospective and Current Issues. Materials 2023, 16, 3792. [Google Scholar] [CrossRef] [PubMed]
- Sandanayake, M.; Law, D.; Sargent, P. A New Framework for Assessing the Environmental Impacts of Circular Economy Friendly Soil Waste-Based Geopolymer Cements. Build. Environ. 2022, 210, 108702. [Google Scholar] [CrossRef]
- Morsy, A.M.; Ragheb, A.M.; Shalan, A.H.; Mohamed, O.H. Mechanical Characteristics of GGBFS/FA-Based Geopolymer Concrete and Its Environmental Impact. Pract. Period. Struct. Des. Constr. 2022, 27, 04022017. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Zhang, Y.; Ma, J.; Xiao, R.; Guo, F.; Bai, Y.; Huang, B. Influence of Size Effect on the Properties of Slag and Waste Glass-Based Geopolymer Paste. J. Clean. Prod. 2023, 383, 135428. [Google Scholar] [CrossRef]
- Çelik, A.İ.; Tunç, U.; Bahrami, A.; Karalar, M.; Othuman Mydin, M.A.; Alomayri, T.; Özkılıç, Y.O. Use of Waste Glass Powder toward More Sustainable Geopolymer Concrete. J. Mater. Res. Technol. 2023, 24, 8533–8546. [Google Scholar] [CrossRef]
- Tochetto, G.A.; Simão, L.; de Oliveira, D.; Hotza, D.; Immich, A.P.S. Porous Geopolymers as Dye Adsorbents: Review and Perspectives. J. Clean. Prod. 2022, 374, 133982. [Google Scholar] [CrossRef]
- Luukkonen, T.; Yliniemi, J.; Sreenivasan, H.; Ohenoja, K.; Finnilä, M.; Franchin, G.; Colombo, P. Ag- or Cu-Modified Geopolymer Filters for Water Treatment Manufactured by 3D Printing, Direct Foaming, or Granulation. Sci. Rep. 2020, 10, 7233. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Yang, S.; He, Y.; Qin, Z.; Cui, X. Prepared Self-Growing Supported Nickel Catalyst by Recovering Ni (II) from Metal Wastewater Using Geopolymer Microspheres. J. Hazard. Mater. 2020, 389, 121919. [Google Scholar] [CrossRef] [PubMed]
- Davidovits, J. Geopolymers: Ceramic-like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Habert, G.; De Lacaillerie, J.D.E.; Roussel, N. An Environmental Evaluation of Geopolymer Based Concrete Production: Reviewing Current Research Trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Havukainen, J.; Nuortila-Jokinen, J.; Horttanainen, M. Environmental and Economic Perspective of Waste-Derived Activators on Alkali-Activated Mortars. J. Clean. Prod. 2021, 280, 124651. [Google Scholar] [CrossRef]
- Komkova, A.; Habert, G. Optimal Supply Chain Networks for Waste Materials Used in Alkali-Activated Concrete Fostering Circular Economy. Resour. Conserv. Recycl. 2023, 193, 106949. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Komkova, A.; Havukainen, J.; Habert, G.; Horttanainen, M. Identifying Optimal Precursors for Geopolymer Composite Mix Design for Different Regional Settings: A Multi-Objective Optimization Study. Recycling 2023, 8, 32. [Google Scholar] [CrossRef]
- De Rossi, A.; Simão, L.; Ribeiro, M.J.; Hotza, D.; Moreira, R.F.P.M. Study of Cure Conditions Effect on the Properties of Wood Biomass Fly Ash Geopolymers. J. Mater. Res. Technol. 2020, 9, 7518–7528. [Google Scholar] [CrossRef]
- Maschowski, C.; Kruspan, P.; Garra, P.; Talib Arif, A.; Trouvé, G.; Gieré, R. Physicochemical and Mineralogical Characterization of Biomass Ash from Different Power Plants in the Upper Rhine Region. Fuel 2019, 258, 116020. [Google Scholar] [CrossRef]
- Cherian, C.; Siddiqua, S. Pulp and Paper Mill Fly Ash: A Review. Sustainability 2019, 11, 4394. [Google Scholar] [CrossRef]
- dos Santos, V.R.; Dezena Cabrelon, M.; de Sousa Trichês, E.; Quinteiro, E. Green Liquor Dregs and Slaker Grits Residues Characterization of a Pulp and Paper Mill for Future Application on Ceramic Products. J. Clean. Prod. 2019, 240, 118220. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. App. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Chemistry & Applications, 2nd ed.; Davidovits, J., Ed.; Institut Géopolymère: Saint-Quentin, France, 2008; ISBN 9782954453118. [Google Scholar]
- Chen-Tan, N.W.; Van Riessen, A.; Ly, C.V.; Southam, D.C. Determining the Reactivity of a Fly Ash for Production of Geopolymer. J. Am. Ceram. Soc. 2009, 92, 881–887. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative Kinetic and Structural Analysis of Geopolymers. Part 1. The Activation of Metakaolin with Sodium Hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Williams, R.P.; Van Riessen, A. Determination of the Reactive Component of Fly Ashes for Geopolymer Production Using XRF and XRD. Fuel 2010, 89, 3683–3692. [Google Scholar] [CrossRef]
- Toniolo, N.; Boccaccini, A.R. Fly Ash-Based Geopolymers Containing Added Silicate Waste. A Review. Ceram. Int. 2017, 43, 14545–14551. [Google Scholar] [CrossRef]
- Martínez-García, R.; Jagadesh, P.; Zaid, O.; Șerbănoiu, A.A.; Fraile-Fernández, F.J.; de Prado-Gil, J.; Qaidi, S.M.A.; Grădinaru, C.M. The Present State of the Use of Waste Wood Ash as an Eco-Efficient Construction Material: A Review. Materials 2022, 15, 5349. [Google Scholar] [CrossRef] [PubMed]
- Simão, L.; Fernandes, E.; Hotza, D.; Ribeiro, M.J.; Montedo, O.R.K.; Raupp-Pereira, F. Controlling Efflorescence in Geopolymers: A New Approach. Case Stud. Constr. Mater. 2021, 15, e00740. [Google Scholar] [CrossRef]
- Allahverdi, A.; Najafi Kani, E.; Shaverdi, B. Carbonation Versus Efflorescence in Alkali-Activated Blast-Furnace Slag in Relation with Chemical Composition of Activator. Int. J. Civ. Eng. 2017, 15, 565–573. [Google Scholar] [CrossRef]
- Bernal, S.A.; Mejía De Gutiérrez, R.; Provis, J.L. Engineering and Durability Properties of Concretes Based on Alkali-Activated Granulated Blast Furnace Slag/Metakaolin Blends. Constr. Build. Mater. 2012, 33, 99–108. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Role of Alkali Concentration on Reaction Kinetics of Fly Ash Geopolymerization. J. Non. Cryst. Solids 2019, 505, 241–251. [Google Scholar] [CrossRef]
- Longhi, M.A.; Walkley, B.; Rodríguez, E.D.; Kirchheim, A.P.; Zhang, Z.; Wang, H. New Selective Dissolution Process to Quantify Reaction Extent and Product Stability in Metakaolin-Based Geopolymers. Compos. Part B Eng. 2019, 176, 107172. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, Properties and Applications of Highly Porous Geopolymers: A Review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]




| Oxides (wt%) | Metakaolin | BFA | Dregs |
|---|---|---|---|
| SiO2 | 57.1 | 45.1 | 1.7 |
| Al2O3 | 32.6 | 12.1 | 0.7 |
| Na2O | <0.1 | 0.2 | 6.9 |
| K2O | 2.1 | 2.3 | 0.4 |
| CaO | <0.1 | 1.6 | 41.7 |
| MgO | 0.6 | 1.0 | 2.5 |
| Fe2O3 | 2.3 | 3.5 | 0.5 |
| TiO2 | 1.5 | 0.8 | 0.1 |
| MnO | - | 0.2 | 0.9 |
| P2O5 | - | 1.2 | 0.4 |
| SO3 | <0.1 | - | 2.9 |
| LOI | 3.0 | 31.7 | 40.0 |
| SiO2/Al2O3 (mol) (mol) | 2.98 | 6.34 | 4.13 |
| Phase (wt%) | Metakaolin | BFA | Dregs |
|---|---|---|---|
| Muscovite | 39.0 | 0 | 0 |
| Quartz | 9.0 | 28.0 | 0 |
| Calcite | - | - | 64.0 |
| Amorphous | 52.0 | 72.0 | 36.0 |
| Amorphous wt% | Metakaolin | BFA |
|---|---|---|
| SiO2 | 29.0 | 17.0 |
| Al2O3 | 20.0 | 12.0 |
| SiO2/Al2O3 (mol) | 2.49 | 2.41 |
| Property | Amorphous Composition | Bulk |
|---|---|---|
| Apparent porosity (%) | 66.1 (1.0) | 43.8 (0.5) |
| Water absorption (%) | 60.8 (1.3) | 32.2 (0.6) |
| Bulk density (g/cm³) | 1.09 (0.01) | 1.36 (0.04) |
| Sample | Compressive Strength (28 Days, MPa) | Apparent Porosity (%) |
| AMB75D25 | 1.1 (0.4) | 57.4 (0.4) |
| Sample | Water Absorption (%) | Bulk Density (g/cm³) |
| AMB75D25 | 81.0 (1.6) | 0.71 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleutério, R.V.; Simão, L.; Lemes, P.; Hotza, D. Evaluation of As-Received Green Liquor Dregs and Biomass Ash Residues from a Pulp and Paper Industry as Raw Materials for Geopolymers. Minerals 2023, 13, 1158. https://doi.org/10.3390/min13091158
Eleutério RV, Simão L, Lemes P, Hotza D. Evaluation of As-Received Green Liquor Dregs and Biomass Ash Residues from a Pulp and Paper Industry as Raw Materials for Geopolymers. Minerals. 2023; 13(9):1158. https://doi.org/10.3390/min13091158
Chicago/Turabian StyleEleutério, Rafael Vidal, Lisandro Simão, Priscila Lemes, and Dachamir Hotza. 2023. "Evaluation of As-Received Green Liquor Dregs and Biomass Ash Residues from a Pulp and Paper Industry as Raw Materials for Geopolymers" Minerals 13, no. 9: 1158. https://doi.org/10.3390/min13091158
APA StyleEleutério, R. V., Simão, L., Lemes, P., & Hotza, D. (2023). Evaluation of As-Received Green Liquor Dregs and Biomass Ash Residues from a Pulp and Paper Industry as Raw Materials for Geopolymers. Minerals, 13(9), 1158. https://doi.org/10.3390/min13091158








