The Effect of the Addition of Eggshell Residues in Mass Formulation for Ceramic Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Preparation
2.3. Characterisation of Samples after Sintering Treatment
2.3.1. Linear Retraction (RL)
2.3.2. Water Absorption (AA)
2.3.3. Apparent Porosity (AP)
2.3.4. Three-Point Bending Failure Stress (TRF)
3. Results and Discussions
3.1. Characterisation of Raw Materials
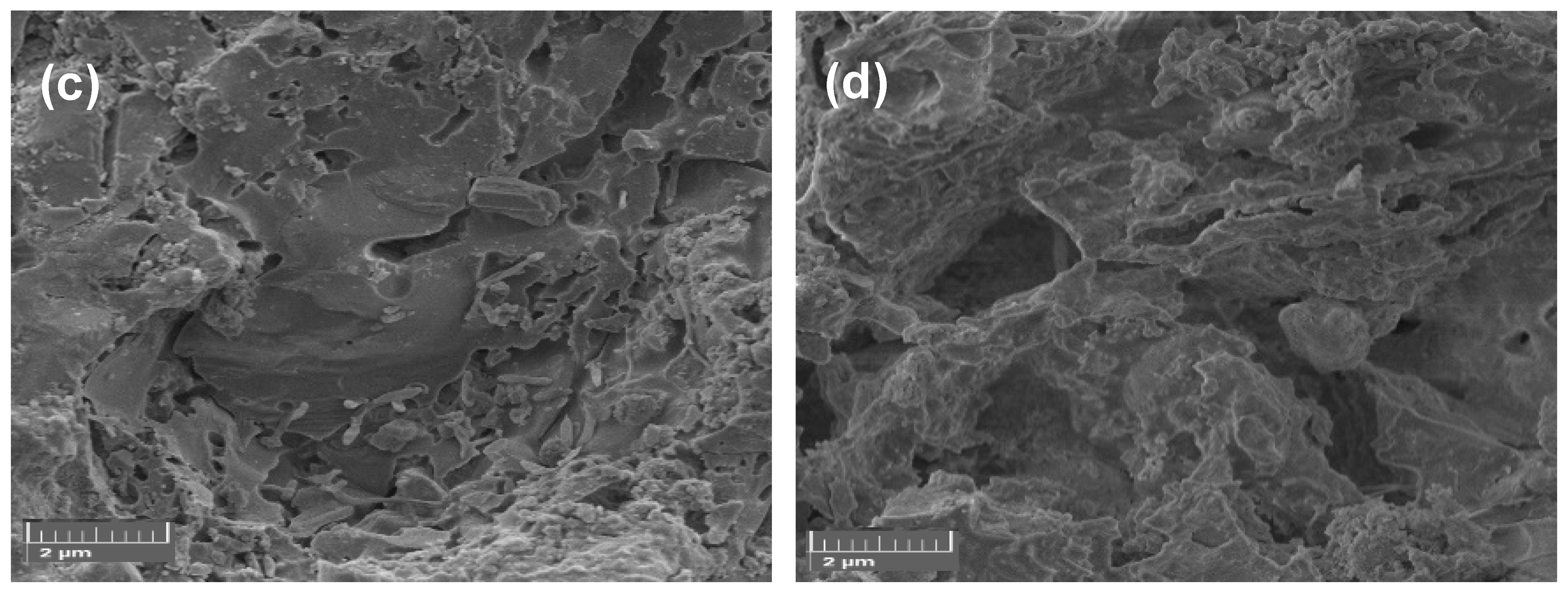
3.2. Mineralogical Phases and Physical-Mechanical Properties of Sintered Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maraveas, C. Production of Sustainable Construction Materials Using Agro-Wastes. Materials 2020, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Sathiparan, N. Utilization prospects of eggshell powder in sustainable construction material—A review. Constr. Build. Mater. 2021, 293, 123465. [Google Scholar] [CrossRef]
- Engelberth, A.S. Evaluating economic potential of food waste valorization: Onward to a diverse feedstock biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 26, 100385. [Google Scholar] [CrossRef]
- Pauzi, N.I.M.; Aziz, M.A.A.M.A.; Ismail, M.S.; Omar, H. Waste soil improvement using egg shell powder and lime at open dumping area. Int. J. Civ. Eng. Technol. 2019, 10, 268–277. [Google Scholar]
- Vandeginste, V. Food waste eggshell valorization through development of new composites: A review. Sustain. Mater. Technol. 2021, 29, e00317. [Google Scholar] [CrossRef]
- Palaniyappan, S.; Annamalai, V.E.; Ashwinkumaran, S.; Thenmuhil, D.; Veeman, D. Utilização de resíduos da indústria abrasiva como material substituto para a produção de tijolo refractário. J. Construir. Eng. 2022, 45, 103606. [Google Scholar]
- Meda, S.R.; Sharma, S.K.; Tyagi, G.D. Utilização de lodo residual como material de construção—Uma revisão. Mate. Hoje Proc. 2021, 46, 4195–4262. [Google Scholar]
- Vilarinho, I.S.; Filippi, E.; Seabra, M.P. Development of eco-ceramic wall tiles with bio-CaCO3 from eggshells waste. Open Ceram. 2022, 9, 100220. [Google Scholar] [CrossRef]
- Kaling, T.; Rohit, G.; Manish, K. Utilização de lama de esgoto corrigida com cinzas volantes como tijolo para material de construção sustentável com ênfase especial no efeito dimensional. J. Limpo. Prod. 2020, 275, 123942. [Google Scholar]
- Stempkowska, A. Características de parâmetros térmicos e algumas propriedades físicas de mineral tipo eutético: Feldspatos Petalite-Alkali. Materiais 2021, 14, 7321. [Google Scholar] [CrossRef]
- Stempkowska, A. Caracterização do sistema de fluxo: Silicato de lítio-alumínio (Li)-feldspatos alcalinos (Na,K)-magnésio (Mg) e silicatos de cálcio (Ca). Materiais 2021, 14, 7386. [Google Scholar] [CrossRef] [PubMed]
- Izak, P.; Delikhovskyi, Y.; Olszyna, A. Uso de Rejeitos Solidificados Pós-Flotação da Produção de Cobre para Produção de Telha Cerâmica. Materiais 2023, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.N.; Odiong, I.C.; Akpabio, E.E. The Effects of Eggshell Ash on Strength Properties of Cement-Stabilized Lateritic. Int. J. Sustain. Constr. Eng. Technol. 2012, 3, 2180–3242. [Google Scholar]
- Bhagavatheswaran, E.S.; Das, A.; Rastin, H.; Saeidi, H.; Jafari, S.H.; Vahabi, H.; Najafi, F.; Khonakdar, H.A.; Formela, K.; Jouyandeh, M.; et al. The Taste of Waste: The Edge of Eggshell over Calcium Carbonate in Acrylonitrile Butadiene Rubber. J. Polym. Environ. 2019, 27, 2478–2489. [Google Scholar] [CrossRef]
- Rashad, A.M.; Khafaga, S.A.; Gharieb, M. Valorização de cinzas volantes como aditivo para cimento de geopolímero de escória de forno elétrico a arco. Const. Build. Mater. 2021, 294, 123570. [Google Scholar] [CrossRef]
- Teo, P.T.; Zakaria, S.K.; Salleh, S.Z.; Taib, M.A.A.; Mohd Sharif, N.; Abu Seman, A.; Mohamed, J.J.; Yusoff, M.; Yusoff, A.H.; Mohamad, M.; et al. Avaliação das opções de reciclagem de resíduos de escória de aço de forno elétrico (EAF) em produtos verdes com valor agregado: Uma revisão. Metais 2020, 10, 1347. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Zanelli, C.; Conte, S.; Molinari, C.; Soldati, R.; Dondi, M. Waste recycling in ceramic tiles: A technological outlook. Resour. Conserv. Recycl. 2020, 168, 105289. [Google Scholar] [CrossRef]
- Biffi, G. O Grês Porcelanato: Manual de Fabricação e Técnicas de Emprego; Gruppo Editoriale: São Paulo, Brazil; Faenza, Italy, 2006. [Google Scholar]
- Barba, A. Materias Primas Para la Fabricación de Soportes de Baldosas Cerámicas; Instituto de Tecnología Cerámica: São Paulo, Brazil, 1997. [Google Scholar]
- SOARES, Roberto Arruda Lima. Efeito da Adição de Carbonatos em Formulação de Massa para Revestimento Cerâmico Utilizando Matérias-Primas do Piauí. 2010. 146 f. Tese (Doutorado em Processamento de Materiais a Partir do Pó; Polímeros e Compósitos; Processamento de Materiais a Part)—Universidade Federal do Rio Grande do Norte, Natal. 2010. Available online: https://oasisbr.ibict.br/vufind/Record/UFRN_25b35af2da39b92a685396ea44f47335 (accessed on 5 July 2023).
- ABNT NBR 15270:2017; Componentes Cerâmicos—Blocos e Tijolos Para Alvenaria: Métodos de Ensaios. Associação Brasileira de Normas e Técnicas: Rio de Janeiro, Brazil, 2017.
- NBR 13816; Placas Cerâmicas para Revestimento: Terminologia. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 1997.
- NBR 13818; Placas Cerâmicas Para Revestimento—Especificações e Métodos de Ensaios. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 1997.
- NBR 15463; Placas Cerâmicas Para Revestimento—Porcelanato. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2013.
- NBR 7181; Solo—Análise Granulométrica. Associação Brasileira de Normas Técnicas-ABNT: Rio de Janeiro, Brazil, 2017.
- ASTM D790-02; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2002. Available online: http://www.astm.org (accessed on 5 July 2023).
- ISO. SI 314; Ceramic Tiles: Definitions, Classification, Characteristics and Marking. ISO: Geneva, Switzerland, 2018.
- da Silva, V.J.; de Almeida, E.P.; Gonçalves, W.P.; da Nóbrega, R.B.; Neves, G.D.A.; Lira, H.D.L.; Menezes, R.R.; Santana, L.N.D.L. Mineralogical and dielectric properties of mullite and cordierite ceramics produced using wastes. Ceram. Int. 2019, 45, 4692–4699. [Google Scholar] [CrossRef]
- Silva, R.H.L.; Neves, G.A.; Ferreira, H.C.; Santana, L.N.L.; Nóbrega, A.C.V.; Menezes, R.R. Use of diopside in ceramic masses for sanitary ware. Cerâmica 2019, 65, 1–12. [Google Scholar] [CrossRef]
- de Medeiros, P.S.S.; Lira, H.D.L.; Rodriguez, M.A.; Menezes, R.R.; Neves, G.D.A.; Santana, L.N.D.L. Incorporation of quartzite waste in mixtures used to prepare sanitary ware. J. Mater. Res. Technol. 2019, 8, 2148–2156. [Google Scholar] [CrossRef]
- Babisk, M.P.; Amaral, L.F.; Ribeiro, L.D.S.; Vieira, C.M.F.; Prado, U.S.D.; Gadioli, M.C.B.; Oliveira, M.S.; da Luz, F.S.; Monteiro, S.N.; Filho, F.D.C.G. Evaluation and application of sintered red mud and its incorporated clay ceramics as materials for building construction. J. Mater. Res. Technol. 2020, 9, 2186–2195. [Google Scholar] [CrossRef]
- Alekseev, K.; Mymrin, V.; Avanci, M.A.; Klitzke, W.; Magalhães, W.L.; Silva, P.R.; Catai, R.E.; Silva, D.A.; Ferraz, F.A. Environmentally clean construction materials from hazardous bauxite waste red mud and spent foundry sand. Constr. Build. Mater. 2019, 229, 116860. [Google Scholar] [CrossRef]
- Da Costa, F.P.; da Silva Morais, C.R.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- Da Costa, F.P.; da Silva Morais, C.R.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Guedes, D.G.; da Costa, F.P.; Rodrigues, A.M.; Neves, G.d.A.; Menezes, R.R.; Santana, L.N.d.L. Sustainable Ceramic Materials Manufactured from Ceramic Formulations Containing Quartzite and Scheelite Tailings. Sustainability 2020, 12, 9417. [Google Scholar] [CrossRef]
- Figueiredo, J.M.R.; Fernandes, I.M.M.; Silva, V.J.; Neves, G.A.; Ferreira, H.C.; Santana, L.N.L. Influence of composition and processing variables of clay-based formulations—Use in refractory materials. Ceramica 2018, 64, 10–19. [Google Scholar] [CrossRef]
- Palakurthy, S.; Samudrala, R.K. In Vitro bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Mater. Sci. Eng. C 2018, 98, 109–117. [Google Scholar] [CrossRef]
- Hossain, S.S.; Ranjan, V.; Pyare, R.; Roy, P. Study the effect of physico-mechanical characteristics of ceramic tiles after addition of river silts and wollastonite derived from wastes. Constr. Build. Mater. 2019, 209, 315–325. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Rodrigues, A.M.; Sampaio, D.V.; Silva, L.D.; Cunha, T.R.; Zanotto, E.D.; Pizani, P.S. The origin of the unusual DSC peaks of supercooled barium disilicate liquid. Crystengcomm 2019, 21, 2768–2778. [Google Scholar] [CrossRef]
- Silva, D.; Sampaio, D.; Silva, J.; Rodrigues, A.; Pena, R.; Moulton, B.; Pizani, P.; Rino, J.; Silva, R. Synthesis of PbO·SiO2 glass by CO2 laser melting method. J. Non-Cryst. Solids 2019, 522, 119572. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.; Ahn, B. Efeitos do Whisker de Carbonato de Cálcio na Porosidade, Resistência e Absorção de Água de Materiais à Base de Cimento. Materiais 2021, 14, 4945. [Google Scholar] [CrossRef] [PubMed]
- Tangboriboon, N.; Selarak, P.; Shamunee, P.; Sirivat, A. Reação de carbonato durante a sinterização de tijolos de magnésia-espinélio e seus efeitos na porosidade e mecanismos de obtenção de materiais de baixa porosidade. Materials 2021, 14, 3952. [Google Scholar] [CrossRef]
- Lobo Junior, R.P. Influência da adição de carbonato de cálcio nas propriedades queimadas de uma massa cerâmica contendo alumina e carboneto de silício. J. Eur. Ceram. Soc. 2020, 40, 553–559. [Google Scholar]
- Dwivedi, S.P.; Sharma, S.; Mishra, R.K. Characterization of waste eggshells and CaCO3 reinforced AA2014 green metal matrix composites: A green approach in the synthesis of composites. Int. J. Precis. Eng. Manuf. 2016, 17, 1383–1393. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Eggshell waste as catalyst: A review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Selarak, P.; Shamunee, P. Bio-Filler verde inovador e carbonato de cálcio Bio-Flux de cascas de ovo para preparação de porcelana dura-macia. Chem. Technol. Ind. J. 2016, 6, 109. [Google Scholar]
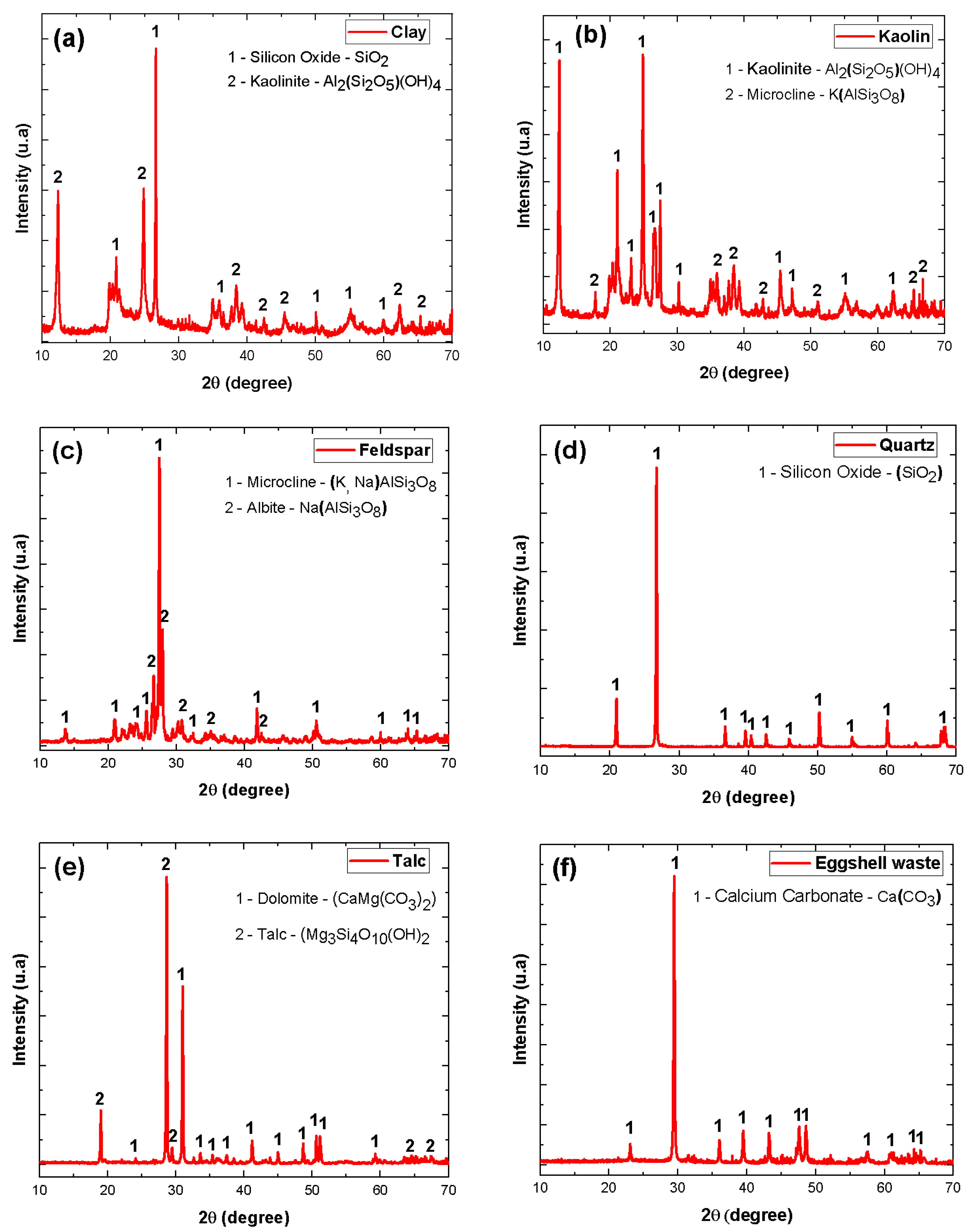
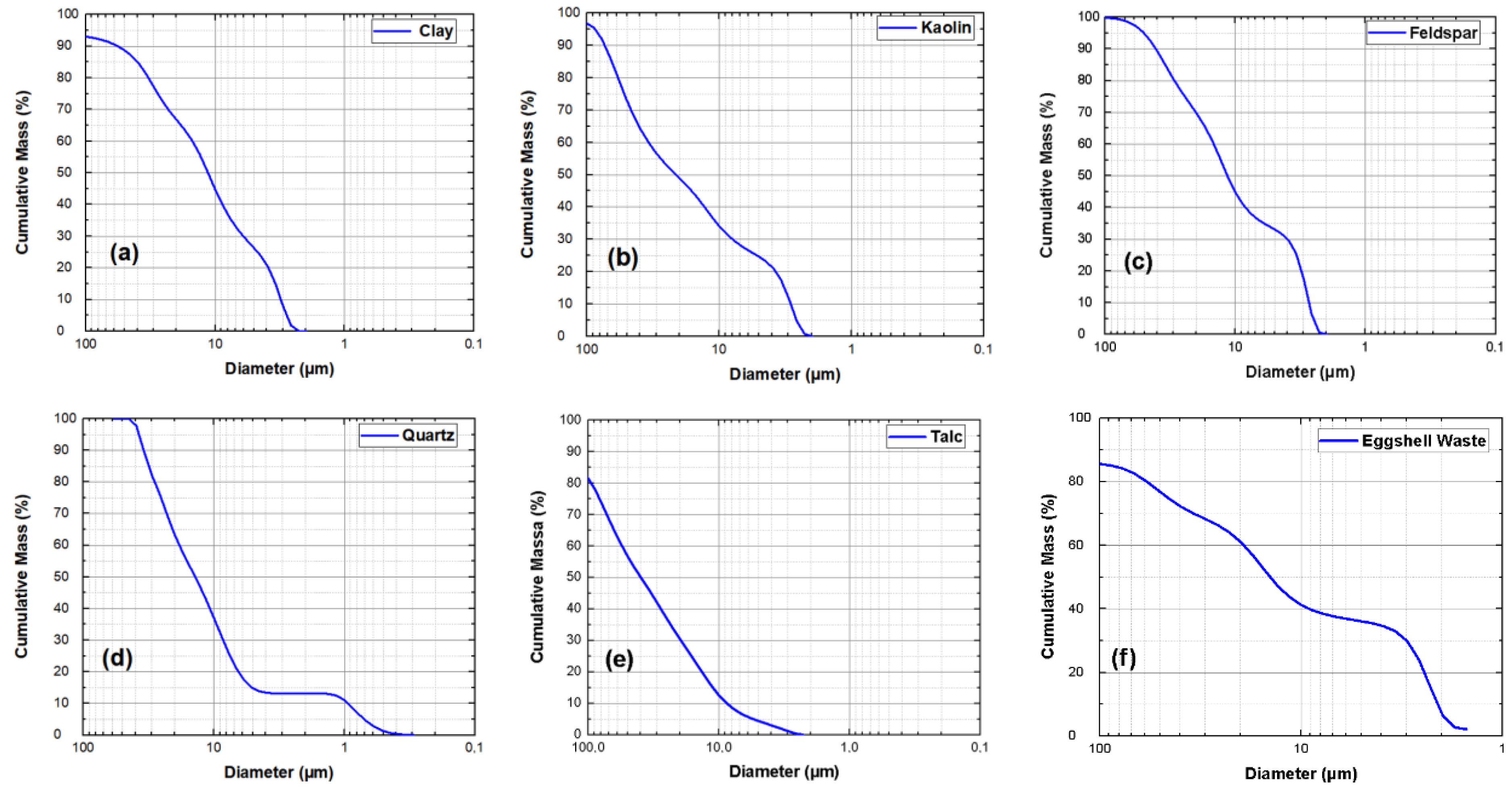

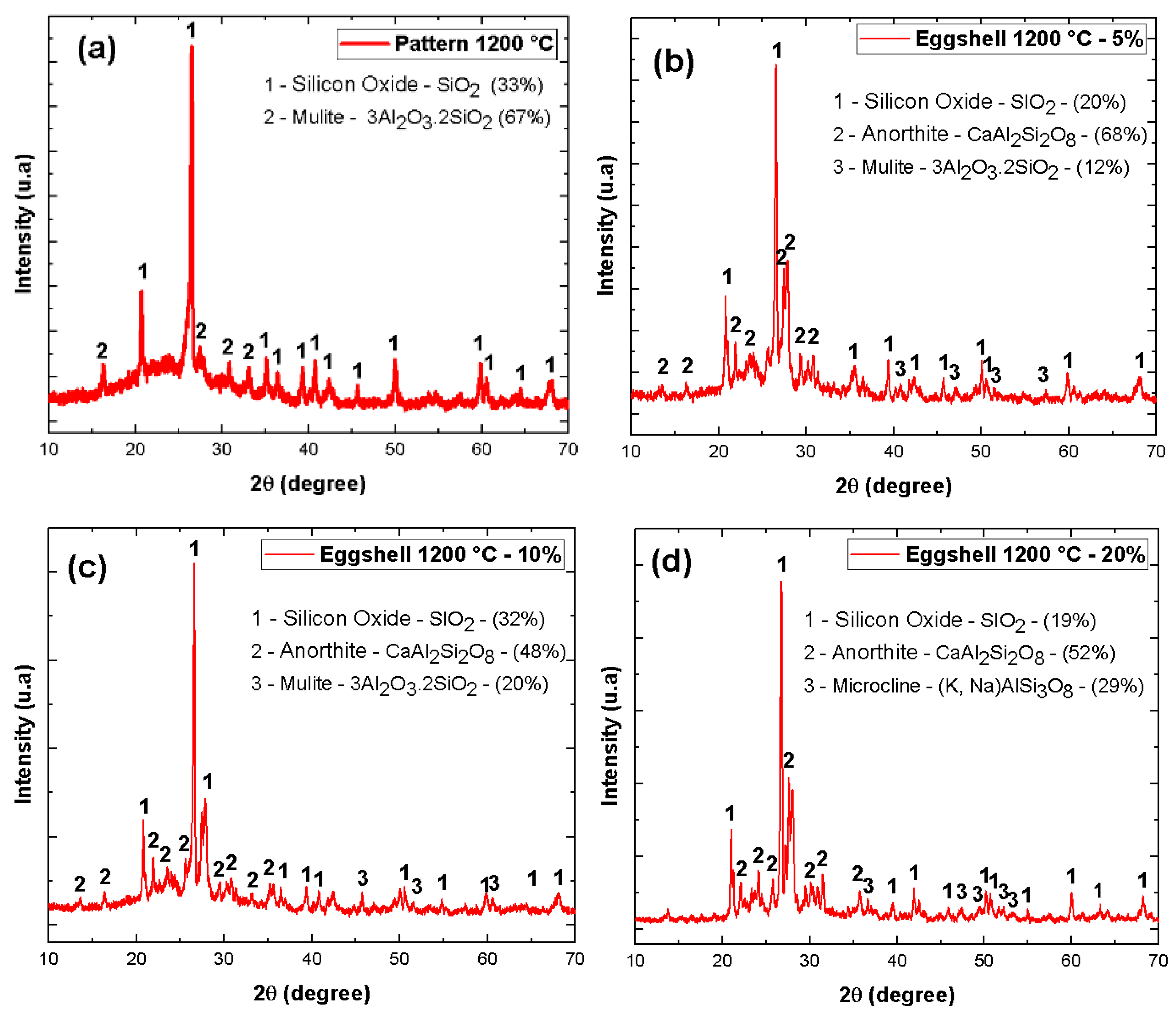
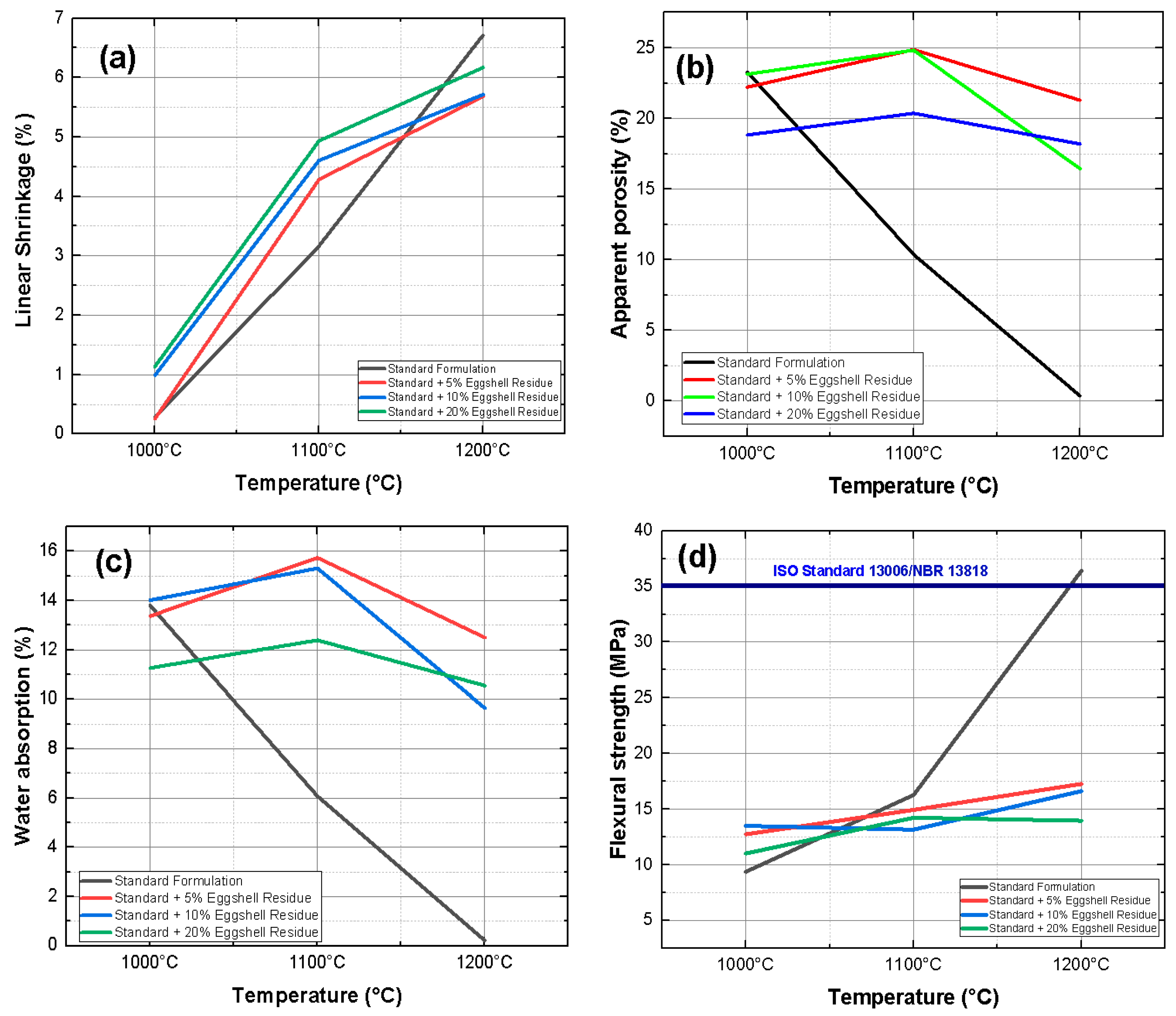

| Material | Feature |
|---|---|
| Feldspar * | Mainly containing the phases: Microcline—(K, Na) AlSi3O8 and Albite—Na(AlSi3O8) |
| Clay * | Mainly containing the phases: Silicon Oxide—SiO2 and Kaolinite—Al2(Si2O5)(OH)4 |
| Kaolin * | Mainly containing the phases: Kaolinite—Al2(Si2O5)(OH)4 and Microcline—K(AlSi3O8) |
| Quartz * | Mainly containing the phases: Silicon Oxide—(SiO2) |
| Talc ** | Mainly containing the phases: Dolomite—CaMg(C3)2 and Talc—Mg3Si4O10(OH)2 |
| Eggshell waste *** | Mainly containing the phases: Calcium Carbonate—Ca(CO3) |
| Raw Material | Composition (wt %) |
|---|---|
| Feldspar | 45 |
| Clay | 30 |
| Kaolin | 15 |
| Quartz | 7 |
| Talc | 3 |
| Raw Material | Composition (wt %) | ||
|---|---|---|---|
| Standard Formulation | 95 | 90 | 80 |
| Eggshell waste | 5 | 10 | 20 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | TiO2 | MgO |
|---|---|---|---|---|---|---|---|---|
| Clay | 49.50 | 46.44 | 2.01 | 0.11 | - | 0.63 | 1.30 | - |
| Kaolin | 50.44 | 46.15 | 0.39 | - | - | 3.03 | - | - |
| Quartz | 99.88 | - | - | - | - | 0.12 | - | - |
| Talc | 39.55 | - | 1.15 | 22.06 | - | - | - | 37.13 |
| Feldspar | 65.06 | 20.57 | 0.11 | - | 5.80 | 8.41 | - | - |
| Eggshell waste | 0.75 | - | - | 98.36 | - | 0.08 | - | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avelino, F.P.; Soares, R.A.L.; Peña-Garcia, R.R.; Lobo, A.O. The Effect of the Addition of Eggshell Residues in Mass Formulation for Ceramic Coating. Minerals 2023, 13, 1123. https://doi.org/10.3390/min13091123
Avelino FP, Soares RAL, Peña-Garcia RR, Lobo AO. The Effect of the Addition of Eggshell Residues in Mass Formulation for Ceramic Coating. Minerals. 2023; 13(9):1123. https://doi.org/10.3390/min13091123
Chicago/Turabian StyleAvelino, Flávio Pessoa, Roberto Arruda Lima Soares, Ramón Raudel Peña-Garcia, and Anderson O. Lobo. 2023. "The Effect of the Addition of Eggshell Residues in Mass Formulation for Ceramic Coating" Minerals 13, no. 9: 1123. https://doi.org/10.3390/min13091123
APA StyleAvelino, F. P., Soares, R. A. L., Peña-Garcia, R. R., & Lobo, A. O. (2023). The Effect of the Addition of Eggshell Residues in Mass Formulation for Ceramic Coating. Minerals, 13(9), 1123. https://doi.org/10.3390/min13091123







