Porcelain Ceramic Tile Manufactured with the Addition of Hydroxyapatite in Ceramic Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Preparation
2.3. Characterization Samples after Sintering Treatment
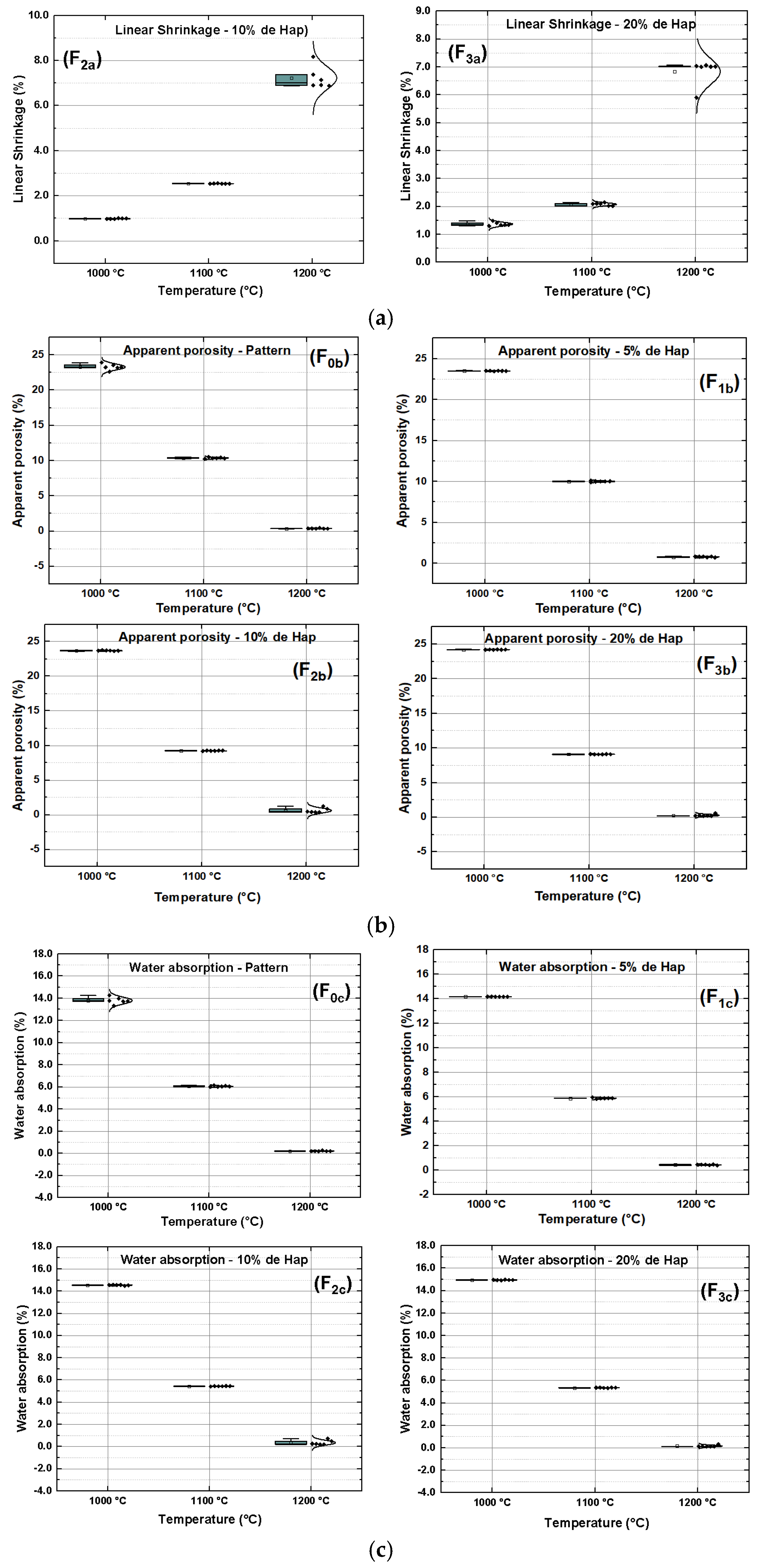
2.3.1. Linear Retraction (LR)
2.3.2. Water Absorption (WA)
2.3.3. Apparent Porosity (AP)
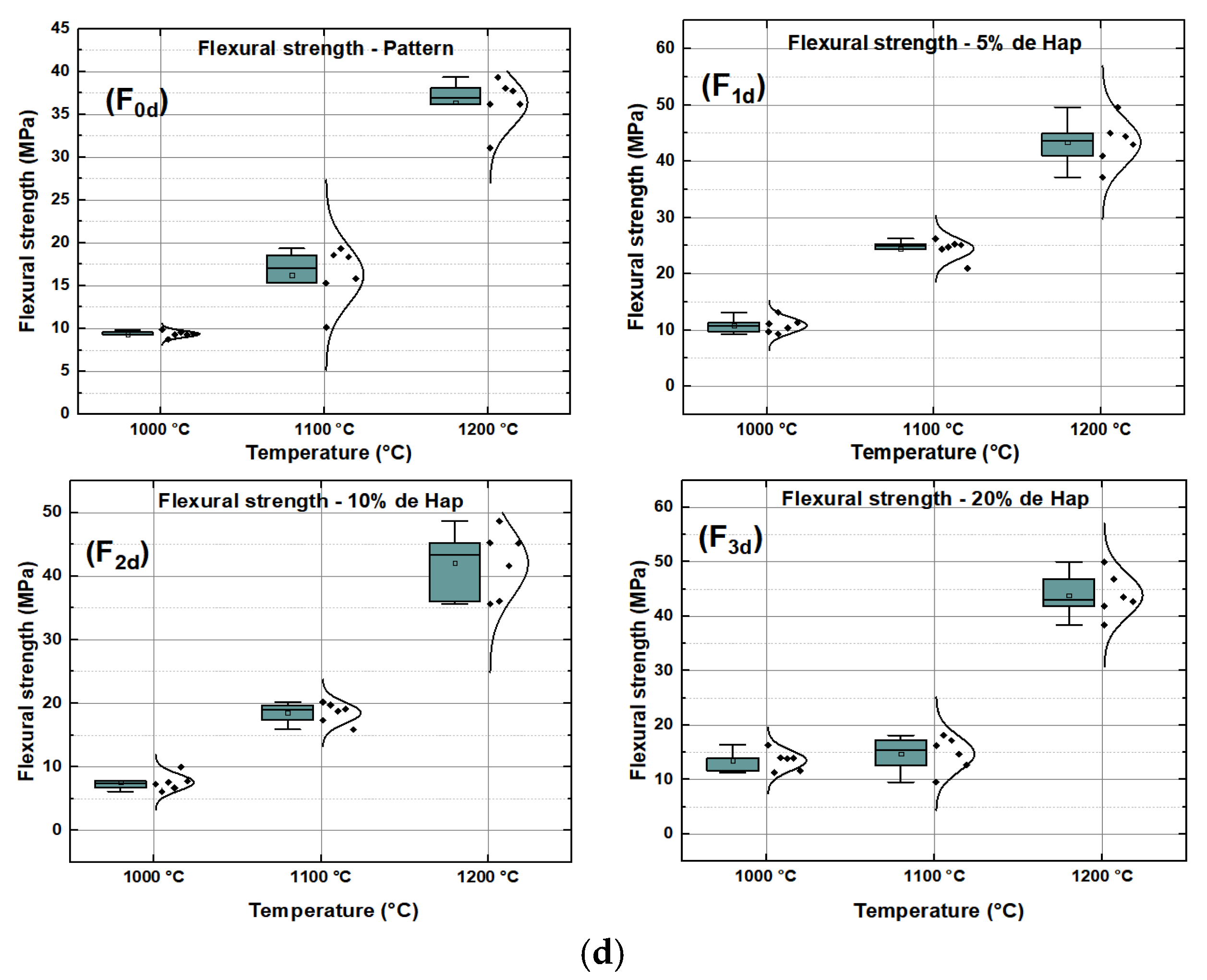
2.3.4. Three-Point Bending Failure Stress (TRF)
3. Results and Discussions
3.1. Characterization of Raw Materials
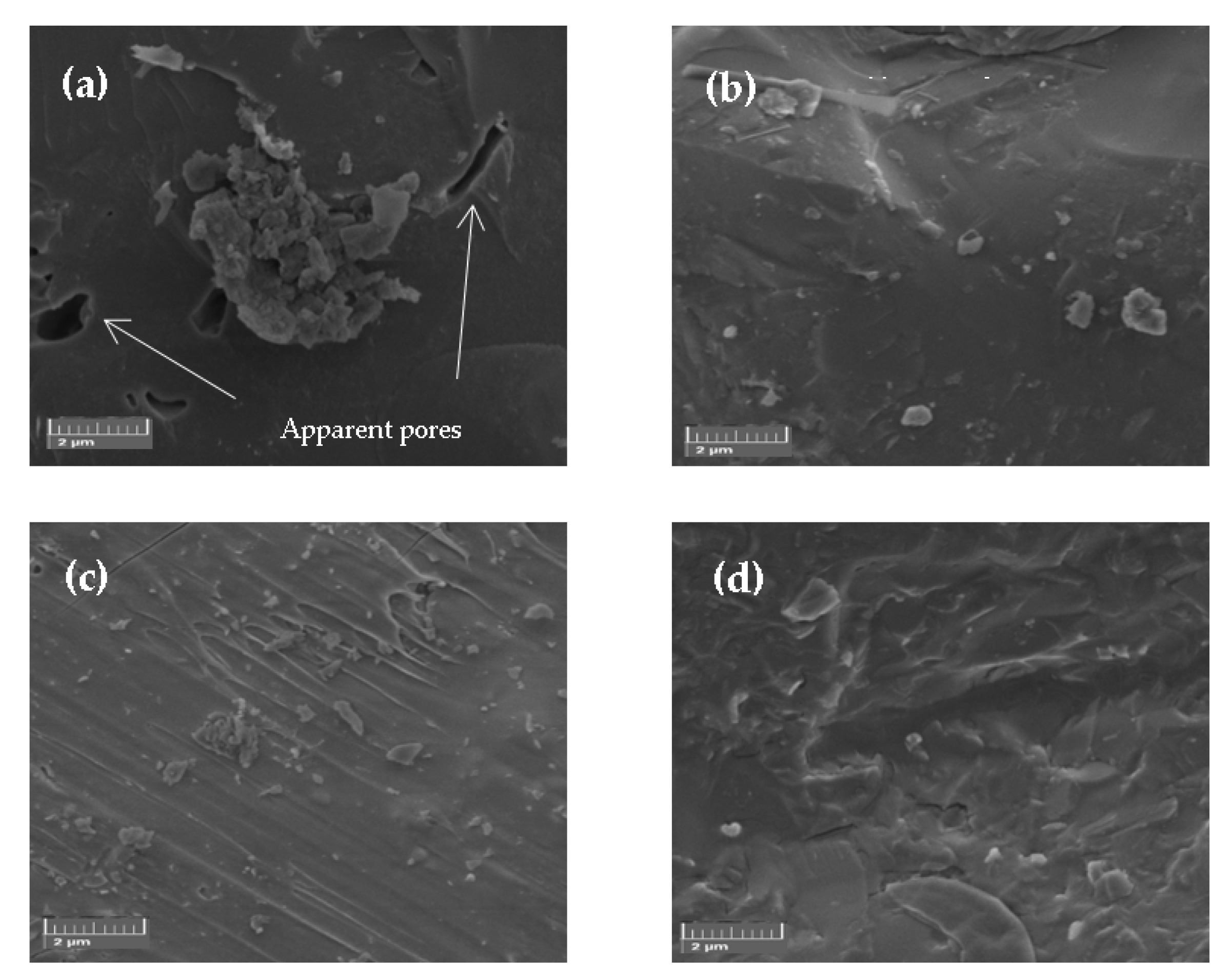
3.2. Mineralogical Phases and Physical–Mechanical Properties of Sintered Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, J.C.O. Uso de matérias-primas sintéticas em esmaltes cerâmicos industriais: Uma revisão. J. Ceram. Sci. Technol. 2020, 10, 100–115. [Google Scholar]
- Vilarinho, I.S.; Filippi, E.; Seabra, M.P. Development of Eco-Ceramic Wall Tiles with bio-CaCO3 from Eggshells Waste. OpenCeram. 2022, 9, 100200. [Google Scholar] [CrossRef]
- De Medeiros, P.S.S.; Lira, H.D.L.; Rodriguez, M.A.; Menezes, R.R.; Neves, G.D.A.; Santana, L.N.D.L. Incorporation of quartzite waste in mixtures used to prepare sanitary ware. J. Mater. Res. Technol. 2019, 8, 2148–2156. [Google Scholar] [CrossRef]
- Babisk, M.P.; Amaral, L.F.; da Silva Ribeiro, L.; Vieira, C.M.F.; Prado, U.S.; Gadioli, M.C.B.; Oliveira, M.S.; da Luz, F.S.; Monteiro, S.N.; da Costa Garcia Filho, F. Evaluation and application of sintered red mud and its incorporated clay ceramics as materials for building construction. J. Mater. Res. Technol. 2020, 9, 2186–2195. [Google Scholar] [CrossRef]
- Alekseev, K.; Mymrin, V.; Avanci, M.A.; Klitzke, W.; Magalhães, W.L.E.; Silva, P.R.; Catai, R.E.; Silva, D.A.; Ferraz, F.A. Environmentally clean construction materials from hazardous bauxite waste red mud and spent foundry sand. Constr. Build. Mater. 2019, 229, 116860. [Google Scholar] [CrossRef]
- da Costa, F.P.; da Silva Morais, C.R.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- Da Costa, F.P.; da Silva Morais, C.R.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Guedes, D.G.; da Costa, F.P.; Rodrigues, A.M.; de Araújo Neves, G.; Menezes, R.R.; de Lima Santana, L.N. Sustainable ceramic materials manufactured from ceramic formulations containing quartzite and scheelite tailings. Sustainability 2020, 12, 9417. [Google Scholar] [CrossRef]
- Uribe, R.; Uvillús, A.; Fernández, L.; Bonilla, O.; Jara, A.; González, G. Deposição Eletroquímica de Hidroxiapatita em Aço Inoxidável Revestido com Tântalo/Nitreto de Tântalo Usando Fluido Corporal Simulado como Meio Eletrolítico. Coatings 2022, 12, 440. [Google Scholar] [CrossRef]
- Silva, R.H.L.; Neves, G.A.; Ferreira, H.C.; Santana, L.N.L.; Nóbrega, A.C.V.; Menezes, R.R. Use of diopside in ceramic masses for sanitary ware. Cerâmica 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Da Silva, V.J.; de Almeida, E.P.; Gonçalves, W.P.; da Nóbrega, R.B.; de Araújo Neves, G.; de Lucena Lira, H.; Menezes, R.R.; de Lima Santana, L.N. Mineralogical and dielectric properties of mullite and cordierite ceramics produced using wastes. Ceram. Int. 2019, 45, 4692–4699. [Google Scholar] [CrossRef]
- Palakurthy, S.; Venu Gopal Reddy, K.; Samudrala, R.K.; Abdul Azeem, P. In vitro bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Mater. Sci. Eng. C 2019, 98, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.K.S.; Ranjan, V.; Pyare, R.; Roy, P.K. Study the effect of physicomechanical characteristics of ceramic tiles after addition of river silts and wollastonite derived from wastes. Construct. Build. Mater. 2019, 209, 315–325. [Google Scholar] [CrossRef]
- Kinnunen, P.; Ismailov, A.; Solismaa, S.; Sreenivasan, H.; Räisänen, M.L.; Levänen, E.; Illikainen, M. Recycling mine tailings in chemically bonded ceramics—A review. J. Clean. Prod. 2018, 174, 634–649. [Google Scholar] [CrossRef]
- Cengiz, O.; Kara, A. Effect of alkaline-earth oxides on firing behaviour of monoporosa wall tile bodies. J. Ceram. Process. Res. 2018, 19, 189–197. [Google Scholar]
- Lottermoser, B.; Lottermoser, B.G. Introduction to Mine Wastes. In Mine Wastes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–41. [Google Scholar]
- Jones, H.; Boger, D.V. Sustainability and waste management in the resource industries. Ind. Eng. Chem. Res. 2012, 51, 10057–10065. [Google Scholar] [CrossRef]
- Hughes, D.J.; Shimmield, T.M.; Black, K.D.; Howe, J.A. Ecological impacts of large-scale disposal of mining waste in the deep sea. Sci. Rep. 2015, 5, 9985. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Zanin, H.; Rosa, C.; Eliaz, N.; Maio, P.; Marciano, F.; Lobo, A. Deposição assistida de nano-hidroxiapatita em andaimes de óxido de nanotubos de carbono esfoliados. Nanoscale 2015, 7, 10218–10232. [Google Scholar] [CrossRef]
- Grinet, M.A.; Zanin, H.; Granato, A.E.C.; Porcionatto, M.; Marciano, F.R.; Lobo, A.O. Preparação rápida de scaffolds de nanotubos de carbono alinhados verticalmente com nanohidroxiapatita. J. Mater. Química B 2014, 2, 1196–1204. [Google Scholar] [CrossRef]
- Rodrigues, B.V.; Leite, N.C.; das Neves Cavalcanti, B.; da Silva, N.S.; Marciano, F.R.; Corat, E.J.; Webster, T.J.; Lobo, A.O. Óxido de grafeno/nanotubos de carbono de paredes múltiplas como scaffolds nanofeatured para a deposição assistida de nanohidroxiapatita: Caracterização e avaliação biológica. Int. J. Nanome. 2016, 11, 2569. [Google Scholar]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutierrez, M.C.; del Monte, F. Arquiteturas macroporosas tridimensionais e aerogéis construídos com nanotubos de carbono e/ou grafeno: Síntese e aplicações. Química Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Barbosa, M.C.R.; Messmer, N.R.; Brazil, T.R.; Marciano, F.; Lobo, A.O. The effect of ultrasonic irradiation on the crystallinity of nano-hydroxyapatite produced via the wet chemical method. Mater. Sci. Eng. C 2013, 33, 2020–2625. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.O. Grês Porcelanato: Manual de Fabricação e Técnicas de Emprego; Gruppo Editoriale: São Paulo, Brazil, 2006. [Google Scholar]
- Barba, A. Materias Primas Para la Fabricación de Soportes de Baldosas Cerámicas; Instituto de Tecnología Cerámica: São Paulo, Brazil, 1997. [Google Scholar]
- ABNT NBR 15270:2017; Componentes Cerâmicos—Blocos e Tijolos Para Alvenaria: Métodos de Ensaios. Associação Brasileira de Normas e Técnicas: São Paulo, Brazil, 2017.
- NBR 13816; Associação Brasileira de Normas Técnicas. Placas Cerâmicas Para Revestimento: Terminologia. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 1997.
- NBR 13818; Associação Brasileira de Normas Técnicas. Placas Cerâmicas PARA Revestimento—Especificações e Métodos de Ensaios. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 1997.
- NBR 15463; Associação Brasileira de Normas Técnicas. Placas Cerâmicas Para Revestimento—Porcelanato. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 2013.
- NBR 7181; Associação Brasileira de Normas Técnicas-Abnt. Solo—Análise Granulométrica. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 2017.
- ASTM D790; American Society for Testing and Materials. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 2002.
- ISO 13006:2019; ABNT. Placas Cerâmicas—Definições, Classificações, Características e Marcação. ABNT: Rio de Janeiro, Brazil, 2019.
- Mahmoudi, S.; Bennour, A.; Meguebli, A.; Srasra, E.; Zargouni, F. Characterization and traditional ceramic application of clays from the Douiret region in South Tunisia. Appl. Clay Sci. 2016, 127–128, 78–87. [Google Scholar] [CrossRef]
- De Aza, A.H.; Turrillas, X.; Rodriguez, M.A.; Duran, T.; Pena, P. Time-resolved powder neutron diffraction study of the phase transformation sequence of kaolinite to Mullite. J. Eur. Ceram. Soc. 2014, 34, 1409–1421. [Google Scholar] [CrossRef]
- Menezes, R.R.; de A Neves, G.; Ferreira, H.C. State of the art about the use of wastes as alternative to ceramic raw materials. Rev. Bras. De Eng. Agrícola E Ambient. 2002, 6, 303–313. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tuan, W.H. The processing of kaolin powder compact. Ceram. Int. 2001, 27, 795–800. [Google Scholar] [CrossRef]
- Brindley, G.W.; Nakahira, M. The kaolinite-mullite reaction series: I, a survey of outstanding problems. J. Am. Ceram. Soc. 1959, 42, 311–314. [Google Scholar] [CrossRef]
- McConville, C.J.; Lee, W.E. Microstructural evolution in fred kaolinite. Brit. Ceram. Trans. 1998, 97, 162–168. [Google Scholar]
- Soares, R.A.L. Efeitos da Adição de Carbonatos em Formulações de Massa Para Revestimento Cerâmico Utilizando Matérias-Primas do Piauí; Tese de Doutorado—Universidade Federal do Rio Grande do Norte: Natal, Brazil, 2010. [Google Scholar]
- Chen, C.Y.; Lan, G.S.; Tuan, W.H. Microstructural evolution of Mullite during the sintering of kaolin powder compact. Ceram. Int. 2000, 26, 715–720. [Google Scholar] [CrossRef]
- Özkan, İ. Ceramic properties of a Turkish clay in the Aydın region. J. Ceram. Proc. Res. 2014, 15, 44–47. [Google Scholar]
- Issaoui, M.; Limousy, L.; Lebeau, B.; Bouaziz, J.; Fourati, M. Design and characterization of flat membrane supports elaborated from kaolin and aluminum powders. Comptes Rendus Chim. 2016, 19, 496–504. [Google Scholar] [CrossRef]
- Santos, P.S. Ciência e Tecnologia de Argilas, 2nd ed.; Edgard Blücher: São Paulo, Brazil, 1989. [Google Scholar]
- Liu, D.M.; Troczynski, T.; Tseng, W.J. Water-based sol–gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721. [Google Scholar] [PubMed]
- Nour, W.; Mostafa, A.; Ibrahim, D. Recycled wastes as precursor for synthesizing wollastonite. Ceram. Int. 2008, 34, 101–105. [Google Scholar] [CrossRef]
- Chin, C.L.; Ahmad, Z.A.; Sow, S.S. Relationship between the thermal behaviour of the clays and their mineralogical and chemical composition: Example of Ipoh, Kuala Rompin and Mersing (Malaysia). Appl. Clay Sci. 2017, 143, 327–335. [Google Scholar] [CrossRef]
- JFigueiredo, M.R.; Fernandes, I.M.M.; Silva, V.J.; Neves, G.A.; Ferreira, H.C.; Santana, L.N.L. Influence of composition and processing variables of clay-based formulations—Use in refractory materials. Ceramica 2018, 64, 10–19. [Google Scholar]
- Ismail, H.; Shamsudin, R.; Hamid, M.A.A. Effect of autoclaving and sintering on the formation of β-wollastonite. Mater. Sci. Eng. C 2016, 58, 1077–1081. [Google Scholar] [CrossRef]
- Mendonça, A.M.G.D.; Cartaxo, J.M.; Menezes, R.R.; Santana, L.N.L.; Neves, G.A.; Ferreira, H.C. Moisture expansion of ceramic tiles produced using kaolin and granite wastes. Ceramica 2012, 58, 216–224. [Google Scholar] [CrossRef]
- Ozturk, Z.B. Effect of addition of Avanos’s (Nevsehir) clays on the physical and microstructure properties of ceramic tile. J. Aust. Ceram. Soc. 2016, 53, 101–107. [Google Scholar] [CrossRef]
- Marino, L.F.B.; Boschi, A.O. A Expansão Térmica dos Revestimentos Cerâmicos. Parte VI: Comparação Entre os Efeitos das Adições de Calcita, Dolomita e Talco. Ceram. Ind. 2000, 5, 21–23. [Google Scholar]
- Teixeira, S.R.; Souza, A.E.; Carvalho, C.L.; Reynoso, V.C.S.; Romero, M.; Rincon, J.M. Characterization of a wollastonite glass-ceramic material prepared using sugar cane bagasse ash (SCBA) as one of the raw materials. Mater. Char. 2014, 98, 209–214. [Google Scholar] [CrossRef]
- Varela, M.L.; Formiga, F.L.; Dutra, R.P.S.; Nascimento, R.M.D.; Paskocimas, C.A. Influence of kaolin waste addition on tech-nological properties of a standard stoneware formulation produced in industrial scale. Ceramica 2009, 55, 209–215. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Rodrigues, A.M.; Pizani, P.S.; Sampaio, D.V.; Zanotto, E.D. A Raman investigation of the structural evolution of supercooled liquid barium disilicate during crystallization. Int. J. Appl. Glas. Sci. 2018, 9, 510–517. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Silva, L.D.; Zhang, R.; Soares, V.O. Structural effects on glass stability and crystallization. Crystengcomm 2018, 20, 2278–2283. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Rodrigues, A.M.; Sampaio, D.V.; Silva, L.D.; Cunha, T.R.; Zanotto, E.D.; Pizani, P.S. The origin of the unusual DSC peaks of supercooled barium disilicate liquid. Crystengcomm 2019, 21, 2768–2778. [Google Scholar] [CrossRef]
- Silva, D.; Sampaio, D.; Silva, J.; Rodrigues, A.; Pena, R.; Moulton, B.; Pizani, P.; Rino, J.; Silva, R. Synthesis of PbO·SiO2 glass by CO2 laser melting method. J. Non-Cryst. Solids 2019, 522, 119572. [Google Scholar] [CrossRef]
- Ozturk, Z.B. Microstructural characterization of Mullite and anorthite-based Porcelain tile using regional clay. J. Ceram. Process. Res. 2016, 17, 555–559. [Google Scholar]
- Fernández-Pradas, J.; Serra, P.; Morenza, J.; De Aza, P. Pulsed laser deposition of pseudowollastonite coatings. Biomaterials 2002, 23, 2057–2061. [Google Scholar] [CrossRef]
- Wenk, H.-R. Polymorphism of wollastonite. Contrib. Miner. Pet. 1969, 22, 238–247. [Google Scholar] [CrossRef]
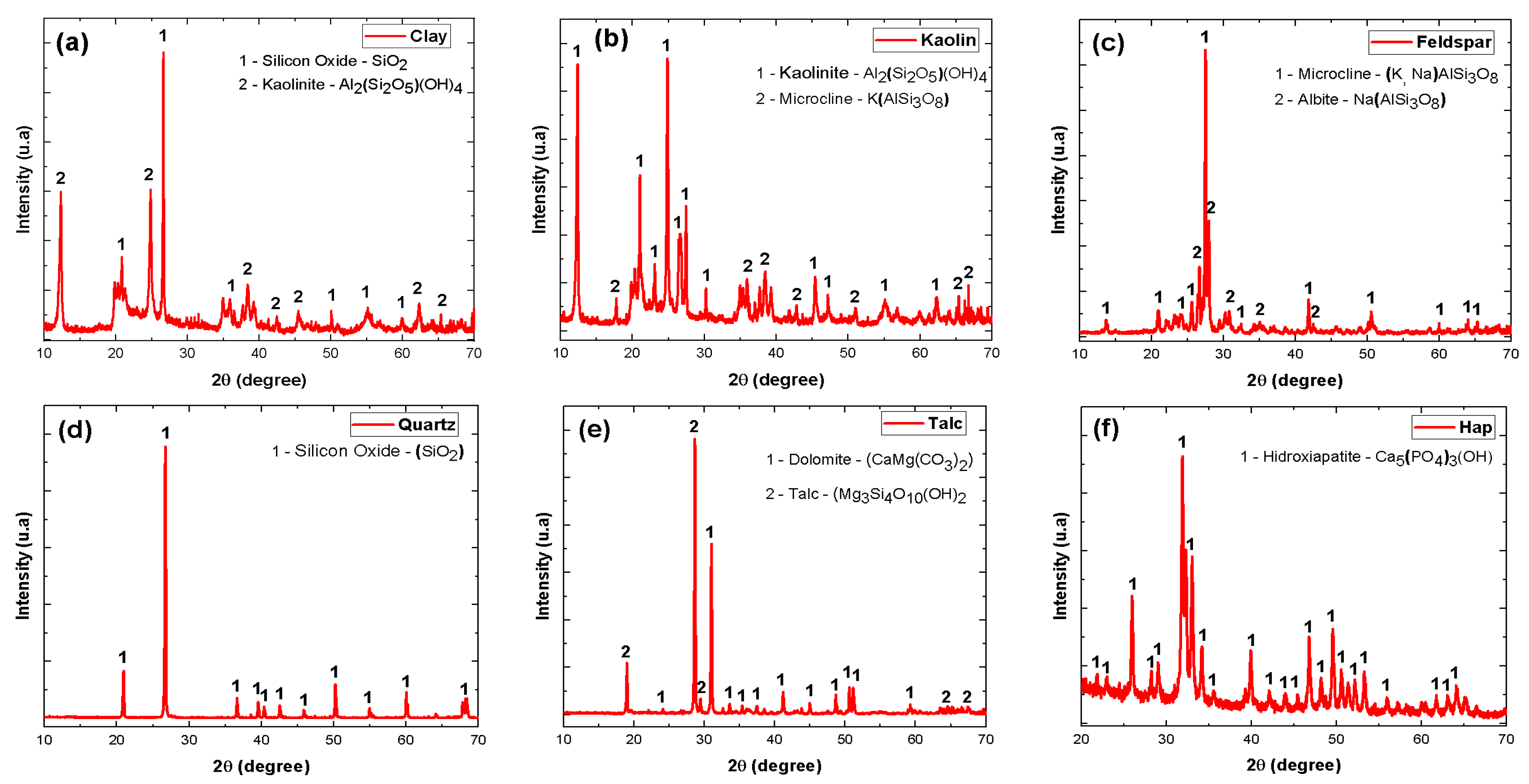

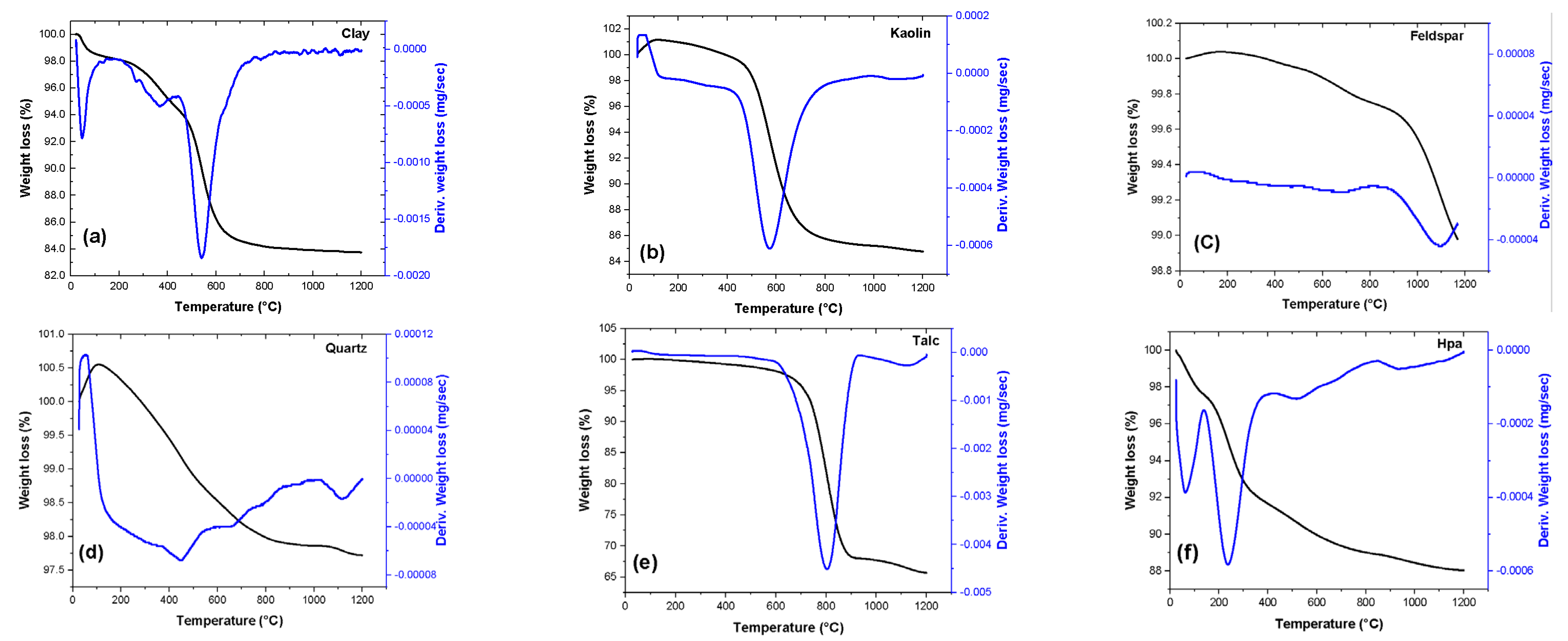
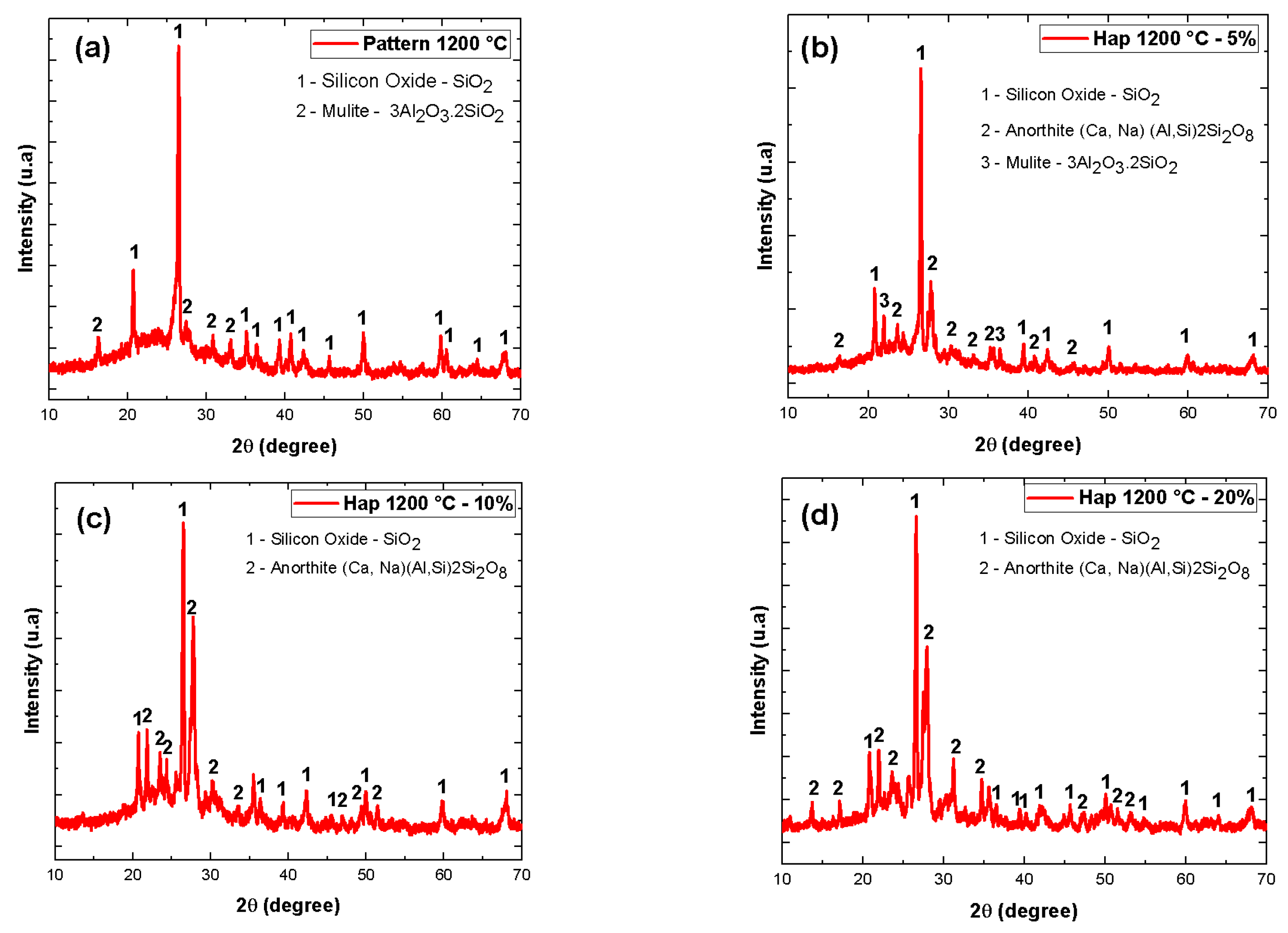
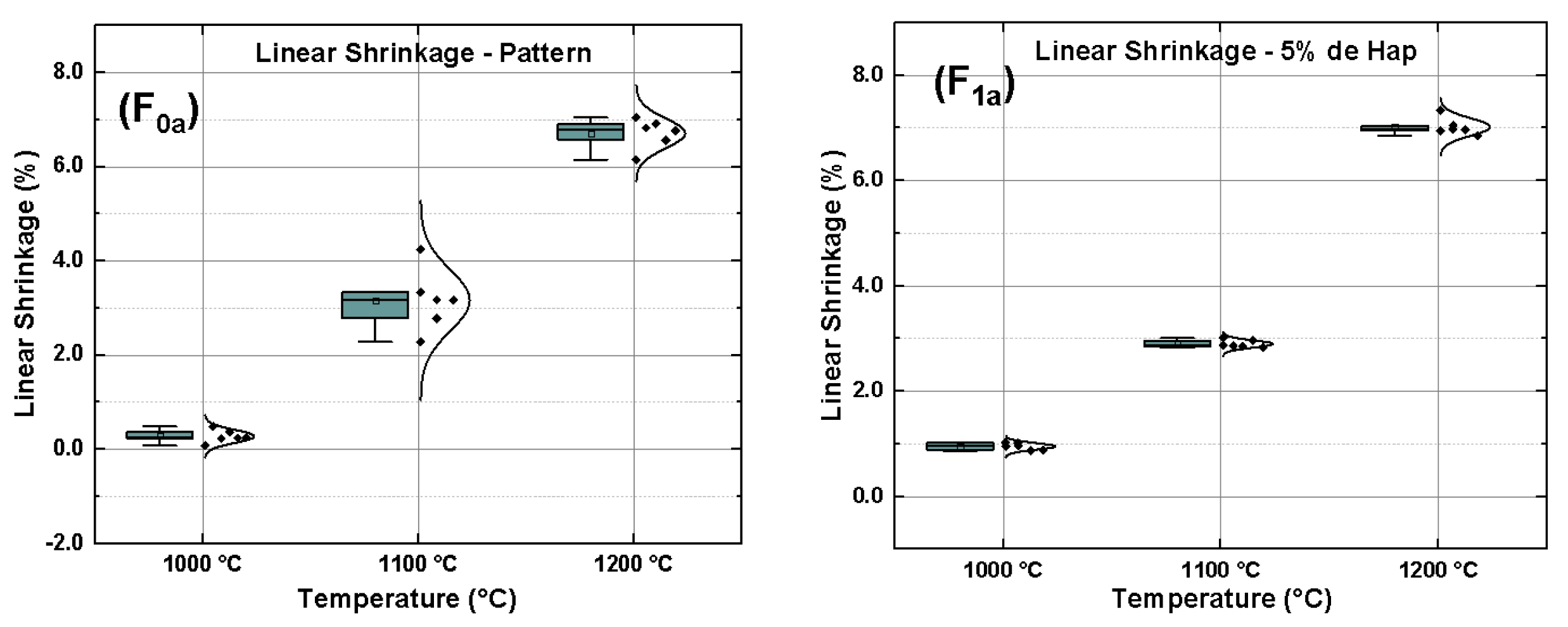
| Material | Feature |
|---|---|
| Feldspar * | Containing essentially the phases: microcline—(K, Na)AlSi3O8 and albite—Na(AlSi3O8) |
| Light burning clay * | Containing essentially the phases: silicon oxide—SiO2 and kaolinite—Al2(Si2O5)(OH)4 |
| Kaolin * | Containing essentially the phases: kaolinite—Al2(Si2O5)(OH)4 and microcline—K(AlSi3O8) |
| Quartz * | Containing essentially the phase: silicon oxide—(Si2) |
| Talc ** | Containing essentially the phases: dolomite—CaMg(C3)2 and talc—Mg3Si4O10(OH)2 |
| Hydroxyapatite *** | Essentially containing the phase: hydroxyapatite—Ca5(PO4)3(OH) |
| Raw Material | Composition (wt%) |
|---|---|
| Feldspar | 45 |
| Clay | 30 |
| Kaolin | 15 |
| Quartz | 7 |
| Talc | 3 |
| Raw Material | Composition (wt%) | ||
|---|---|---|---|
| Standard formulation | 95 | 90 | 80 |
| Hydroxyapatite (5%, 10%, and 20%) | 5 | 10 | 20 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | TiO2 | MgO | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Clay | 49.50 | 46.44 | 2.01 | 0.11 | - | 0.63 | 1.30 | - | - |
| Kaolin | 50.44 | 46.15 | 0.39 | - | - | 3.03 | - | - | - |
| Quartz | 99.88 | - | - | - | - | 0.12 | - | - | - |
| Talc | 39.55 | - | 1.15 | 22.06 | - | - | - | 37.13 | - |
| Feldspar | 65.06 | 20.57 | 0.11 | - | 5.80 | 8.41 | - | - | - |
| Hydroxyapatite | - | 0.20 | - | 62.41 | - | - | - | 0.42 | 36.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avelino, F.P.; de Araujo, W.M.P.; Lima Soares, R.A.; Peña-Garcia, R.; Lobo, A.O. Porcelain Ceramic Tile Manufactured with the Addition of Hydroxyapatite in Ceramic Formulations. Minerals 2023, 13, 1120. https://doi.org/10.3390/min13091120
Avelino FP, de Araujo WMP, Lima Soares RA, Peña-Garcia R, Lobo AO. Porcelain Ceramic Tile Manufactured with the Addition of Hydroxyapatite in Ceramic Formulations. Minerals. 2023; 13(9):1120. https://doi.org/10.3390/min13091120
Chicago/Turabian StyleAvelino, Flávio Pessoa, Wendel Melo Prudêncio de Araujo, Roberto Arruda Lima Soares, Ramon Peña-Garcia, and Anderson O. Lobo. 2023. "Porcelain Ceramic Tile Manufactured with the Addition of Hydroxyapatite in Ceramic Formulations" Minerals 13, no. 9: 1120. https://doi.org/10.3390/min13091120
APA StyleAvelino, F. P., de Araujo, W. M. P., Lima Soares, R. A., Peña-Garcia, R., & Lobo, A. O. (2023). Porcelain Ceramic Tile Manufactured with the Addition of Hydroxyapatite in Ceramic Formulations. Minerals, 13(9), 1120. https://doi.org/10.3390/min13091120







