Zn(NH3)2Cl2, a Mineral-like Anthropogenic Phase with Ammine Complexes from the Burned Dumps of the Chelyabinsk Coal Basin, South Urals, Russia: Crystal Structure, Spectroscopy and Thermal Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Occurrence
2.2. Chemical Composition
2.3. Raman Spectroscopy
2.4. Infrared Spectroscopy
2.5. Single-Crystal X-ray Diffraction
2.6. High-Temperature Powder X-ray Diffraction
2.7. Structural Complexity
3. Results
3.1. Chemical Composition
3.2. Raman Spectroscopy
3.3. Infrared Spectroscopy
3.4. Single-Crystal X-ray Diffraction
3.5. High-Temperature Powder X-ray Diffraction
3.6. Structural Complexity
4. Discussion
5. Conclusions
- (i).
- A detailed crystal chemical description of the anthropogenic Zn(NH3)2Cl2 (“amminite”) was carried out for the first time. It was found that this anthropogenic phase, formed under unique conditions of burned coal dumps, is identical to synthetic Zn(NH3)2Cl2.
- (ii).
- High-temperature powder and single crystals studies of the anthropogenic Zn(NH3)2Cl2 are in a good agreement with each other and show that, upon heating, Zn(NH3)2Cl2 is stable up to ca. 150 °C. This fact agrees well with the data on the temperature of its formation.
- (iii).
- The Raman and IR spectroscopic characteristics of this phase are close to those reported for ammineite, CuCl2(NH3)2, where the peaks corresponding to ammine (NH30) groups are clearly distinguishable.
- (iv).
- The thermal expansion of the anthropogenic mineral-like phase Zn(NH3)2Cl2 is anisotropic and is determined by the system of hydrogen bonds. The geometrical parameters of the ZnN2Cl2 tetrahedra do not change upon heating.
- (v).
- Apparently, the source of ammonia for the formation of Zn(NH3)2Cl2 in the burned coal dumps of the ChCB was a special organic substance contained in the coal-bearing dump material. However, it is not known whether this is directly related to coal.
- (vi).
- The formation of Zn(NH3)2Cl2 in nature is possible under very specific conditions, such as in the interactions of zinc-bearing minerals with organic matter containing ammine (NH30) groups (for example, with guano).
- (vii).
- The structural complexity of Zn(NH3)2Cl2 is relatively low. At the same time, the role of H-bonds in the values of the structural complexity of Zn(NH3)2Cl2 can be estimated to be very significant.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chesnokov, B.V.; Bazhenova, L.F.; Bushmakin, A.F.; Vilisov, V.A.; Lotova, E.V.; Michal, T.A.; Nishanbaev, T.P.; Shcherbakova, E.P. New minerals of burned spoil-heaps of the Chelyabinsk coal basin (Communication No. 2). In New Data on the Mineralogy of Endogenous Deposits and Technogenesis Zones of the Urals; Ural Branch of RAS: Sverdlovsk, Russia, 1991; pp. 3–36. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Shcherbakova, E.P.; Nishanbaev, T.P. Minerals of Burnt Dumps of the Chelyabinsk Coal Basin; Ural Branch of RAS: Miass, Russia, 2008; pp. 1–139. (In Russian) [Google Scholar]
- Parafiniuk, J.; Kruszewski, Ł. Ammonium minerals from burning coal-dumps of the Upper Silesian Coal Basin (Poland). Geol. Quart. 2009, 53, 341–356. [Google Scholar]
- Zolotarev, A.A.; Zhitova, E.S.; Krzhizhanovskaya, M.G.; Rassomakhin, M.A.; Shilovskikh, V.V.; Krivovichev, S.V. Crystal Chemistry and High-Temperature Behaviour of Ammonium Phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the Burned Dumps of the Chelyabinsk Coal Basin. Minerals 2019, 9, 486. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Sergeeva, A.V.; Nuzhdaev, A.A.; Krzhizhanovskaya, M.G.; Chubarov, V.M. Tschermigite from thermal fields of Southern Kamchatka: High-temperature transformation and peculiarities of IR-spectrum. Zapiski RMO (Proc. Russian Mineral. Soc.) 2019, 148, 100–116. (In Russian) [Google Scholar]
- Bojar, H.P.; Walter, F.; Baumgartner, J.; Färber, G. Ammineite, CuCl2(NH3)2, a new species containing an ammine complex: Mineral data and crystal structure. Can. Mineral. 2010, 48, 1359–1371. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Zubkova, N.V.; Mohn, G.; Pekov, I.V.; Pushcharovsky, D.Y.; Zadov, A.E. Chanabayaite, Cu2Cl(N3C2H2)2(NH3,Cl,H2O,□)4, a new mineral containing triazolate anion. Zapiski RMO (Proc. Russian Mineral. Soc.) 2015, 144, 36–47. (In Russian) [Google Scholar]
- Chukanov, N.V.; Zubkova, N.V.; Möhn, G.; Pekov, I.V.; Belakovskiy, D.I.; Van, K.V.; Britvin, S.N.; Pushcharovsky, D.Y. Triazolite, NaCu2(N3C2H2)2(NH3)2Cl3·4H2O, a new mineral species containing 1,2,4-triazolate anion, from a guano deposit at Pabellón de Pica, Iquique Province, Chile. Mineral. Mag. 2018, 82, 1007–1014. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Britvin, S.N.; Möhn, G.; Pekov, I.V.; Zubkova, N.V.; Nestola, F.; Kasatkin, A.V.; Dini, M. Shilovite, natural copper (II) tetrammine nitrate, a new mineral species. Mineral. Mag. 2015, 79, 613–623. [Google Scholar] [CrossRef]
- Bojar, H.; Walter, F.; Baumgartner, J. Joanneumite, Cu(C3N3O3H2)2(NH3)2, a new mineral from Pabellón de Pica, Chile and the crystal structure of its synthetic analogue. Mineral. Mag. 2017, 81, 155–166. [Google Scholar] [CrossRef]
- Von Zelewsky, A. Stereochemistry of Coordination Compounds; Wiley: Chichester, UK, 1996; pp. 1–266. [Google Scholar]
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994; pp. 1–411. [Google Scholar]
- Christensen, C.H.; Sørensen, R.Z.; Johannessen, T.; Quaade, U.J.; Honkala, K.; Elmøe, T.D.; Køhler, R.; Nørskov, J.K. Metal ammine complexes for hydrogen storage. J. Mater. Chem. 2005, 15, 4106–4108. [Google Scholar] [CrossRef]
- Ivšić, T.; Bi, D.W.; Magrez, A. New refinement of the crystal structure of Zn(NH3)2Cl2 at 100 K. Acta Crystallogr. E Crystallogr. Commun. 2019, 75, 1386–1388. [Google Scholar] [CrossRef]
- Gardner, P.J.; Pang, P.; Preston, S. Thermodynamics of the dissociation of ZnCl2(NH3)2 by modified entrainment. Thermochim. Acta 1989, 138, 371–374. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Firsova, V.A.; Filatov, S.K. Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (Theta to Tensor-TTT). Glass Phys. Chem. 2013, 39, 347–350. [Google Scholar] [CrossRef]
- CRYSALISPRO Software System; Version 1.171.39.44; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analisis program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Schuck, G.; Iwata, A.; Sasaki, A.; Himeda, A.; Konaka, H.; Muroyama, N. PDXL structure analysis wizard. Acta Crystallogr. A Found. Crystallogr. 2011, A67, C799–C800. [Google Scholar] [CrossRef]
- BRUKER-AXS Topas V4.2 General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker-AXS: Karlsruhe, Germany, 2009.
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Lindqvist, O. The Crystal Structure of Diamminedichlorozinc(II), ZnCl2(NH3)2. A New Refinement. Acta Chem. Scand. 1981, 35, 727–728. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I.V.; Kuporev, I.V.; et al. Structural and Chemical Complexity of Minerals: An Update. Mineral. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Košek, F.; Nẽmec, I.; Jehlička, J. Raman study of several Cu-bearing minerals from the guano deposit at Pabellón de Pica, Tarapaca region, Chile. J. Raman Spectrosc. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Hall, J.R.; Hirons, D.A. Infrared and Raman spectra of Magnus’ Green salt, [Pt(NH3)4] [PtCl4], and its Deuterate. Inorg. Chim. Acta 1979, 34, L277–L279. [Google Scholar] [CrossRef]
- Frezotti, T.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands, 2014; 1726p. [Google Scholar]
- Wang, C.; Zhan, H.; Lu, X.; Jing, R.; Zhang, H.; Yang, L.; Li, X.; Yue, F.; Zhou, D.; Xia, Q. A recyclable cobalt(iii)–ammonia complex catalyst for catalytic epoxidation of olefins with air as the oxidant. New J. Chem. 2021, 45, 2147–2156. [Google Scholar] [CrossRef]
- MacGillavry, C.H.; Bijvoet, J.M.Z. Die Kristallstruktur von Zn(NH3)2Cl2 und Zn(NH3)2Br2. Z. Kristallogr. 1936, 94, 249–255. [Google Scholar] [CrossRef]
- Parafiniuk, J.; Hatert, F. New IMA CNMNC guidelines on combustion products from burning coal dumps. Eur. J. Mineral. 2020, 32, 215–217. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Belakovskiy, D.I.; Britvin, S.N.; Stergiou, V.; Voudouris, P.; Magganas, A. Katerinopoulosite, (NH4)2Zn(SO4)2·6H2O, a new mineral from the Esperanza mine, Lavrion, Greece. Eur. J. Mineral. 2018, 30, 821–826. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Lykova, I.S.; Belakovskiy, D.I.; Vigasina, M.F.; Sidorov, E.G.; Britvin, S.N.; Pushcharovsky, D.Y. New zinc and potassium chlorides from fumaroles of the Tolbachik volcano, Kamchatka, Russia: Mineral data and crystal chemistry. I. Mellizinkalite, K3Zn2Cl7. Eur. J. Mineral. 2015, 27, 247–253. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Britvin, S.N.; Vigasina, M.F.; Sidorov, E.G.; Pushcharovsky, D.Y. New zinc and potassium chlorides from fumaroles of the Tolbachik volcano, Kamchatka, Russia: Mineral data and crystal chemistry. II. Flinteite, K2ZnCl4. Eur. J. Mineral. 2015, 27, 581–588. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Lukina, E.A.; Zaitsev, A.N.; Shilovskikh, V.V. Belousovite, KZn(SO4)Cl, a new sulfate mineral from the Tolbachik volcano with apophyllite sheet-topology. Mineral. Mag. 2018, 82, 1079–1088. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Pautov, L.A.; Yapaskurt, V.O.; Chukanov, N.V.; Lykova, I.S.; Britvin, S.N.; Sidorov, E.G.; Pushcharovsky, D.Y. Chubarovite, KZn2(BO3)Cl2, a new mineral species from the Tolbachik volcano, Kamchatka, Russia. Can. Mineral. 2015, 53, 273–284. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Britvin, S.N.; Yapaskurt, V.O.; Chukanov, N.V.; Lykova, I.S.; Sidorov, E.G.; Pushcharovsky, D.Y. New zinc and potassium chlorides from fumaroles of the Tolbachik volcano, Kamchatka, Russia: Mineral data and crystal chemistry. III. Cryobostryxite, KZnCl3·2H2O. Eur. J. Mineral. 2015, 27, 805–812. [Google Scholar] [CrossRef]
- Shuvalov, R.R.; Vergasova, L.P.; Semenova, T.F.; Filatov, S.K.; Krivovichev, S.V.; Siidra, O.I.; Rudashevsky, N.S. Prewittite, KPb1.5Cu6Zn(SeO3)2O2Cl10, a new mineral from Tolbachik fumaroles, Kamchatka peninsula, Russia: Description and crystal structure. Am. Mineral. 2013, 98, 463–469. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Filatov, S.K.; Semenova, T.F.; Filosofova, T.M. Sofiite Zn2(SeO3)Cl2—A new mineral from volcanic sublimates. Zapiski RMO (Proc. Russian Mineral. Soc.) 1989, 118, 65–69. (In Russian) [Google Scholar]
- Schmetzer, K.; Schnorrer-Köhler, G.; Medenbach, O. Wülfingite, epsilon-Zn(OH)2, and simonkolleite, Zn5(OH)8Cl2·H2O, two new minerals from Richelsdorf, Hesse, FRG. Neues Jahrb. Mineral. Monatshefte 1985, 151, 145–154. [Google Scholar]
- Okrugin, V.M.; Kudaeva, S.S.; Karimova, O.V.; Yakubovich, O.V.; Belakovskiy, D.I.; Chukanov, N.V.; Zolotarev, A.A.; Gurzhiy, V.V.; Zinovieva, N.G.; Shiryaev, A.A.; et al. The new mineral novograblenovite, (NH4,K)MgCl3·6H2O from the Tolbachik volcano, Kamchatka, Russia: Mineral description and crystal structure. Mineral. Mag. 2019, 83, 223–231. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Sheveleva, R.M.; Zolotarev, A.A.; Krivovichev, S.V.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Nazarova, M.A. The crystal structure of magnesian halotrichite, (Fe,Mg)Al2(SO4)4·22H2O: Hydrogen bonding, geometrical parameters and structural complexity. J. Geosci. 2023, 68, 111–134. [Google Scholar] [CrossRef]






| Crystal System | Orthorhombic |
| Space group | Imma |
| a, Å | 7.7399(6) |
| b, Å | 8.0551(5) |
| c, Å | 8.4767(8) |
| Volume, Å3 | 528.49(7) |
| Z | 4 |
| Dcalc, g/cm−3 | 2.141 |
| μ, mm−1 | 5.494 |
| F(000) | 336.0 |
| Crystal size, mm | 0.16 × 0.12 × 0.08 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection, ° | 6.978 to 67.308 |
| Index ranges | −9 ≤ h ≤ 10, −8 ≤ k ≤ 12, −12 ≤ l ≤ 10 |
| Reflections collected | 1668 |
| Independent reflections | 497 [Rint = 0.0420, Rsigma = 0.0425] |
| Data/restraints/parameters | 497/0/24 |
| Goodness-of-fit on F2 | 1.018 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0388, wR2 = 0.0994 |
| Final R indexes [all data] | R1 = 0.0486, wR2 = 0.1050 |
| Largest diff. peak/hole/e Å−3 | 1.01/−0.63 |
| Atom | x | y | z | U(eq) |
|---|---|---|---|---|
| Zn1 | ½ | ¼ | 0.61307(7) | 0.0237(2) |
| Cl1 | ½ | 0.47901(11) | 0.76901(12) | 0.0271(3) |
| N1 | 0.2843(5) | ¼ | 0.4814(5) | 0.0289(7) |
| H1A | 0.270(7) | 0.322(6) | 0.4140(5) | 0.056(14) |
| H1B | 0.189(10) | ¼ | 0.526(9) | 0.060(20) |
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| Zn1 | 0.0245(4) | 0.0247(3) | 0.0219(4) | 0 | 0 | 0 |
| Cl1 | 0.0282(5) | 0.0249(4) | 0.0281(5) | −0.0046(3) | 0 | 0 |
| N1 | 0.0272(17) | 0.0361(17) | 0.0234(17) | 0 | −0.0014(14) | 0 |
| Atom | Atom | Length | Atom | Atom | Atom | Angle |
|---|---|---|---|---|---|---|
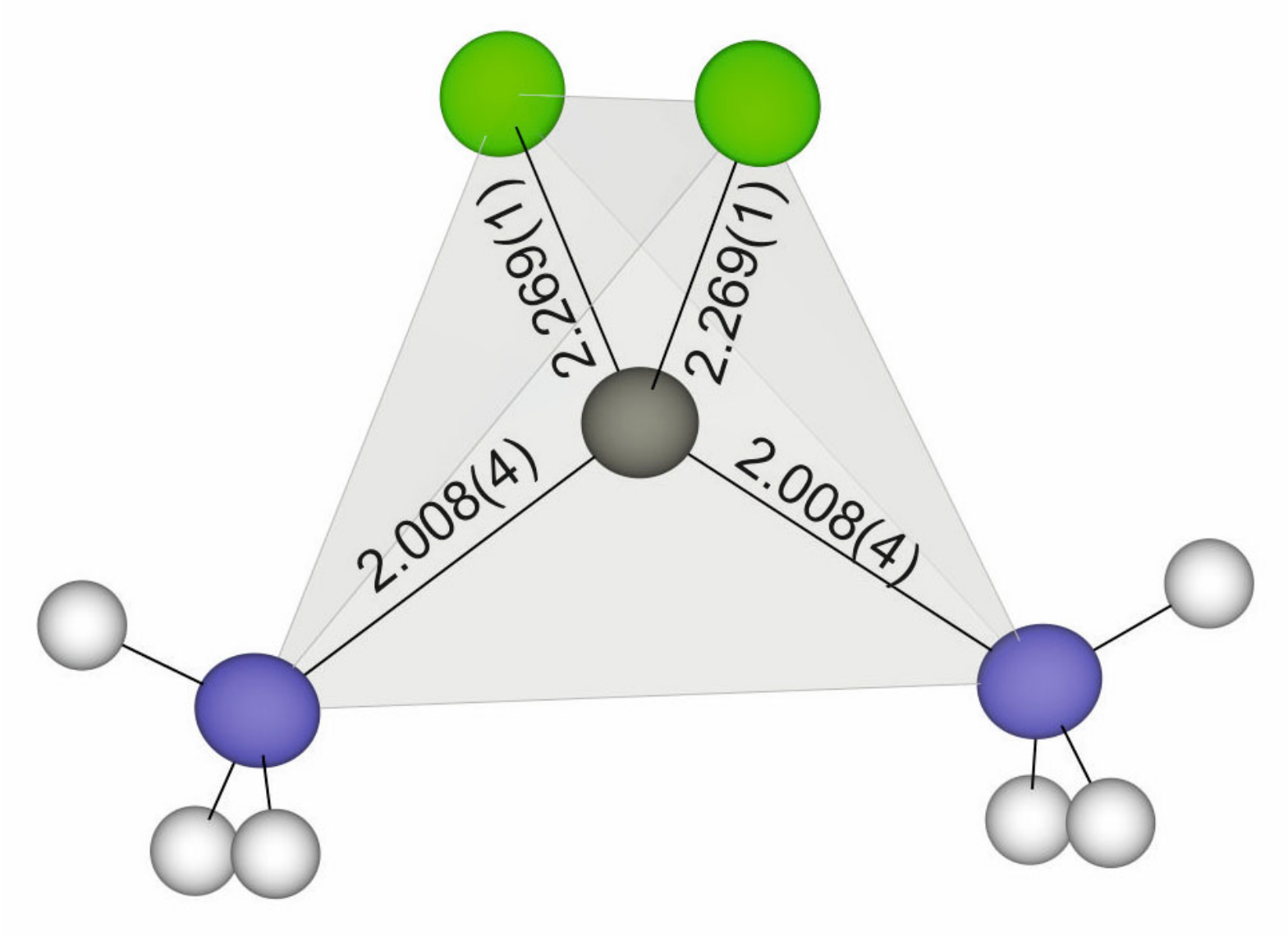
| Zn1 | Cl1 | 2.2694(10) | Cl1 | Zn1 | Cl1 1 | 108.75(5) |
| Zn1 | Cl1 1 | 2.2695(10) | N1 | Zn1 | Cl1 | 108.89(6) |
| Zn1 | N1 | 2.008(4) | N1 1 | Zn1 | Cl1 1 | 108.89(6) |
| Zn1 | N1 1 | 2.008(4) | N1 | Zn1 | Cl1 1 | 108.89(6) |
| N1 1 | Zn1 | Cl1 | 108.89(6) | |||
| N1 | Zn1 | N1 1 | 112.5(2) |
| D-H (Å) | H...A (Å) | D...A (Å) | <(DHA)˚ | D-H...A |
|---|---|---|---|---|
| −173 °C | ||||
| 0.74(5) | 2.83(6) | 3.458(3) | 143(6) | N1-H1A...Cl1 1 |
| 0.74(5) | 2.99(6) | 3.549(3) | 134(6) | N1-H1A...Cl1 2 |
| 0.91(8) | 2.83(6) | 3.548(4) | 136.9(19) | N1-H1B...Cl1 3 |
| 0.91(8) | 2.83(6) | 3.548(4) | 136.9(19) | N1-H1B...Cl1 4 |
| −123 °C | ||||
| 0.77(7) | 2.81(7) | 3.464(3) | 144(7) | N1-H1A...Cl1 1 |
| 0.77(7) | 3.00(8) | 3.564(3) | 132(7) | N1-H1A...Cl1 2 |
| 0.82(10) | 2.90(8) | 3.559(4) | 138(2) | N1-H1B...Cl1 3 |
| 0.82(10) | 2.90(8) | 3.559(4) | 138(2) | N1-H1B...Cl1 4 |
| −73 °C | ||||
| 0.83(5) | 2.85(5) | 3.472(3) | 133(5) | N1-H1A...Cl1 1 |
| 0.83(5) | 2.90(5) | 3.585(3) | 141(5) | N1-H1A...Cl1 2 |
| 0.83(8) | 2.93(7) | 3.567(4) | 136(3) | N1-H1B...Cl1 3 |
| 0.83(8) | 2.93(7) | 3.567(4) | 136(3) | N1-H1B...Cl1 4 |
| −23 °C | ||||
| 0.84(5) | 2.78(5) | 3.486(4) | 142(5) | N1-H1A...Cl1 1 |
| 0.84(5) | 2.99(5) | 3.598(3) | 131(5) | N1-H1A...Cl1 2 |
| 0.81(10) | 2.95(8) | 3.573(4) | 135(3) | N1-H1B...Cl1 3 |
| 0.81(10) | 2.95(8) | 3.573(4) | 135(3) | N1-H1B...Cl1 4 |
| 27 °C | ||||
| 0.79(6) | 2.83(7) | 3.493(4) | 142(6) | N1-H1A...Cl1 1 |
| 0.79(6) | 3.03(7) | 3.615(4) | 132(6) | N1-H1A...Cl1 2 |
| 0.77(9) | 3.00(7) | 3.586(5) | 135(4) | N1-H1B...Cl1 3 |
| 0.77(9) | 3.00(7) | 3.586(5) | 135(4) | N1-H1B...Cl1 4 |
| 77 °C | ||||
| 0.81(7) | 2.85(7) | 3.502(6) | 139(6) | N1-H1A...Cl1 1 |
| 0.81(7) | 3.01(7) | 3.634(4) | 136(6) | N1-H1A...Cl1 2 |
| 0.73(14) | 3.01(11) | 3.594(6) | 139(4) | N1-H1B...Cl1 3 |
| 0.73(14) | 3.01(11) | 3.594(6) | 139(4) | N1-H1B...Cl1 4 |
| a | b | c | V | |
|---|---|---|---|---|
| anthropogenic (our data) | ||||
| −173 °C | 7.6965(8) | 8.0158(7) | 8.4556(10) | 521.66(9) |
| −123 °C | 7.7181(6) | 8.0365(5) | 8.4696(7) | 525.34(7) |
| −73 °C | 7.7399(6) | 8.0551(5) | 8.4767(8) | 528.49(7) |
| −23 °C | 7.7679(7) | 8.0769(6) | 8.4933(9) | 532.87(8) |
| 27 °C | 7.7917(9) | 8.0974(8) | 8.5162(11) | 537.31(11) |
| 77 °C | 7.8080(9) | 8.1187(7) | 8.5325(11) | 540.88(10) |
| synthetic [14] | ||||
| −173 °C | 7.7077(2) | 8.0226(2) | 8.4526(3) | 522.67(3) |
| Constituent 1 | wt. % 2 | a.p.f.u. 3 |
|---|---|---|
| Zn | 38.66 | 1.01 |
| Cl | 41.46 | 2 |
| Ncalc | 16.37 | 2 |
| Hcalc | 3.51 | 6 |
| Total | 100 |
| T, °C | αa | αb | αc | αV | αV = αa+ αb+ αc | αmax/αmin |
|---|---|---|---|---|---|---|
| Based on single-crystal X-ray diffraction data | ||||||
| −120 | 59.7(1.8) | 51.1(5) | 36.5(2.8) | 147.3(3.8) | 147.32 | 1.64 |
| −70 | 59.5(1.8) | 51.0(5) | 36.4(2.7) | 146.9(3.8) | 146.89 | 1.63 |
| −20 | 59.3(1.8) | 50.9(5) | 36.4(2.7) | 146.5(3.8) | 146.56 | 1.63 |
| 30 | 59.1(1.8) | 50.7(5) | 36.3(2.7) | 146.2(3.7) | 146.13 | 1.63 |
| 70 | 59.0(1.8) | 50.6(5) | 36.2(2.7) | 145.9(3.7) | 145.83 | 1.63 |
| Based on powder X-ray diffraction data | ||||||
| 30 | 62.9(1.1) | 52.2(1) | 38.2(5) | 153.3(1.8) | 153.3 | 1.65 |
| 70 | 62.7(1.1) | 52.1(1) | 38.1(5) | 152.9(1.7) | 152.9 | 1.65 |
| 100 | 62.6(1.1) | 52.0(1) | 38.1(5) | 152.7(1.7) | 152.7 | 1.65 |
| 120 | 62.6(1.1) | 52.0(1) | 38.0 (5) | 152.5(1.7) | 152.6 | 1.65 |
| X | n | p0 | p1 | R2 |
|---|---|---|---|---|
| Based on single-crystal X-ray diffraction data (from −173 to 77 °C) | ||||
| a | 1 | 7.7758(14) | 0.461(14) | 1.00000 |
| b | 1 | 8.08645(39) | 0.4109(40) | 1.00000 |
| c | 1 | 8.5055(23) | 0.309(23) | 1.00000 |
| V | 1 | 534.83(20) | 78.0(2) | 1.00000 |
| Based on powder X-ray diffraction data (from 30 to 130 °C) | ||||
| a | 1 | 7.77892(79) | 0.4903(91) | 1.00000 |
| b | 1 | 8.08340(76) | 0.4226(88) | 1.00000 |
| c | 1 | 8.50151(38) | 0.3248(44) | 1.00000 |
| V | 1 | 534.554(82) | 82.73(96) | 1.00000 |
| Ig, Bits/Atom | IG,Total, Bits/Cell | IG(no H), Bits/Atom | IG,Total(no H), Bits/Cell | |
|---|---|---|---|---|
| Zn(NH3)2Cl2 | 2.187 | 48.107 | 1.522 | 15.219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotarev, A.A.; Avdontceva, M.S.; Sheveleva, R.M.; Pekov, I.V.; Vlasenko, N.S.; Bocharov, V.N.; Krzhizhanovskaya, M.G.; Zolotarev, A.A.; Rassomakhin, M.A.; Krivovichev, S.V. Zn(NH3)2Cl2, a Mineral-like Anthropogenic Phase with Ammine Complexes from the Burned Dumps of the Chelyabinsk Coal Basin, South Urals, Russia: Crystal Structure, Spectroscopy and Thermal Evolution. Minerals 2023, 13, 1109. https://doi.org/10.3390/min13081109
Zolotarev AA, Avdontceva MS, Sheveleva RM, Pekov IV, Vlasenko NS, Bocharov VN, Krzhizhanovskaya MG, Zolotarev AA, Rassomakhin MA, Krivovichev SV. Zn(NH3)2Cl2, a Mineral-like Anthropogenic Phase with Ammine Complexes from the Burned Dumps of the Chelyabinsk Coal Basin, South Urals, Russia: Crystal Structure, Spectroscopy and Thermal Evolution. Minerals. 2023; 13(8):1109. https://doi.org/10.3390/min13081109
Chicago/Turabian StyleZolotarev, Andrey A., Margarita S. Avdontceva, Rezeda M. Sheveleva, Igor V. Pekov, Natalia S. Vlasenko, Vladimir N. Bocharov, Maria G. Krzhizhanovskaya, Anatoly A. Zolotarev, Mikhail A. Rassomakhin, and Sergey V. Krivovichev. 2023. "Zn(NH3)2Cl2, a Mineral-like Anthropogenic Phase with Ammine Complexes from the Burned Dumps of the Chelyabinsk Coal Basin, South Urals, Russia: Crystal Structure, Spectroscopy and Thermal Evolution" Minerals 13, no. 8: 1109. https://doi.org/10.3390/min13081109
APA StyleZolotarev, A. A., Avdontceva, M. S., Sheveleva, R. M., Pekov, I. V., Vlasenko, N. S., Bocharov, V. N., Krzhizhanovskaya, M. G., Zolotarev, A. A., Rassomakhin, M. A., & Krivovichev, S. V. (2023). Zn(NH3)2Cl2, a Mineral-like Anthropogenic Phase with Ammine Complexes from the Burned Dumps of the Chelyabinsk Coal Basin, South Urals, Russia: Crystal Structure, Spectroscopy and Thermal Evolution. Minerals, 13(8), 1109. https://doi.org/10.3390/min13081109









