Microstructure Evaluation of Fly Ash Geopolymers Alkali-Activated by Binary Composite Activators
Abstract
:1. Introduction
2. Materials and Characterization
2.1. Starting Materials and Preparation of Specimens
2.2. Preparation of Specimens
2.3. Characterizations
3. Results
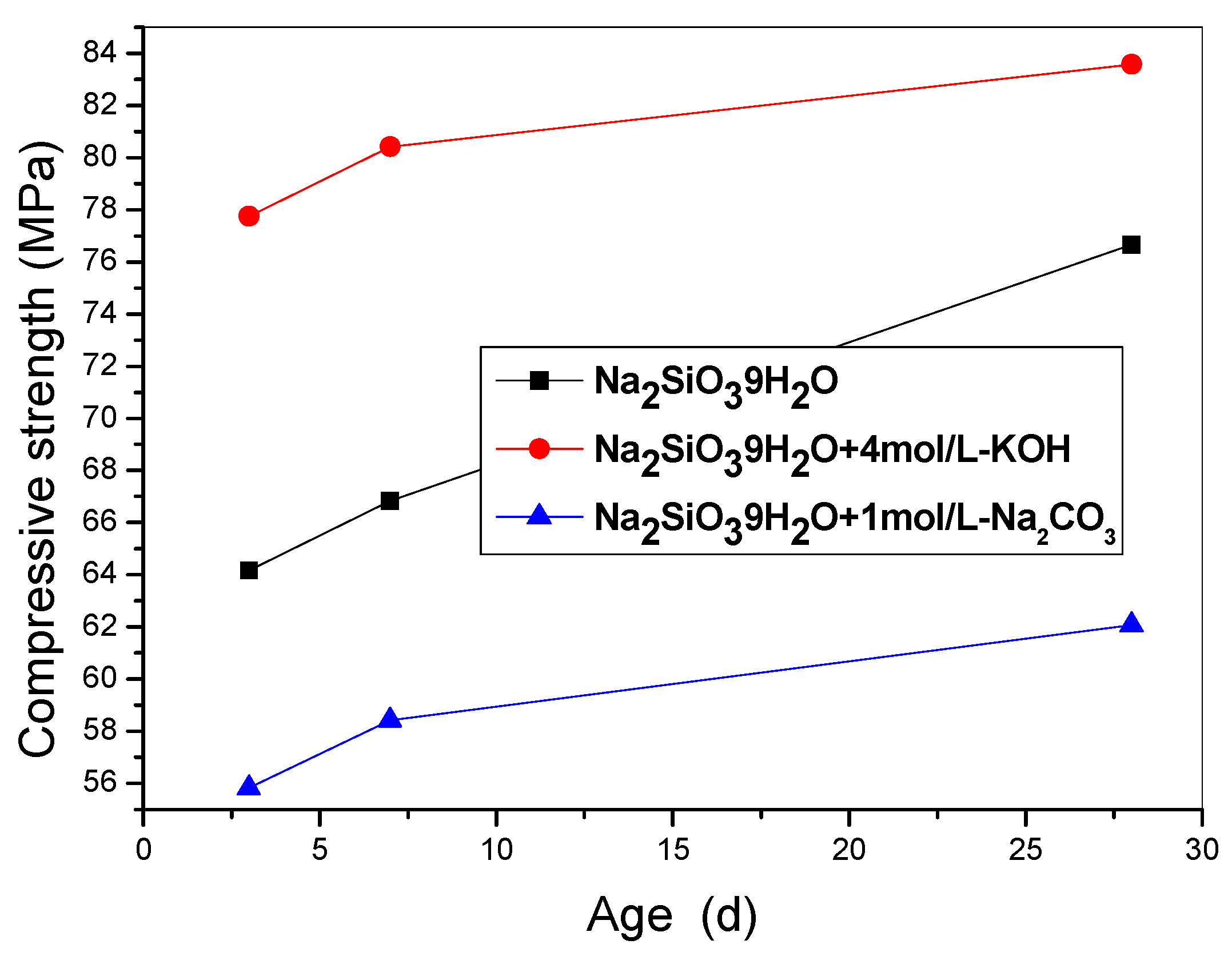
3.1. Mechanical Properties
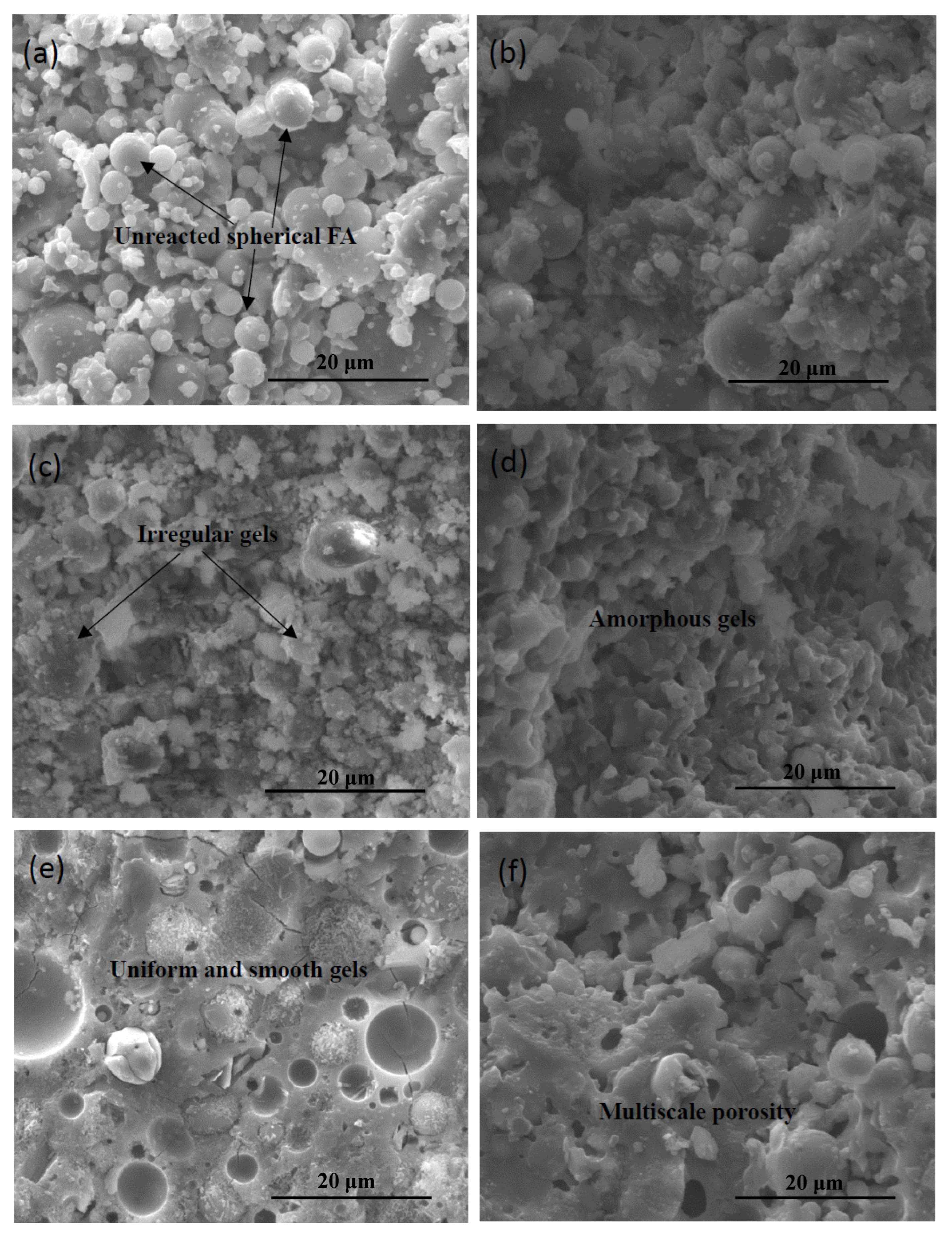
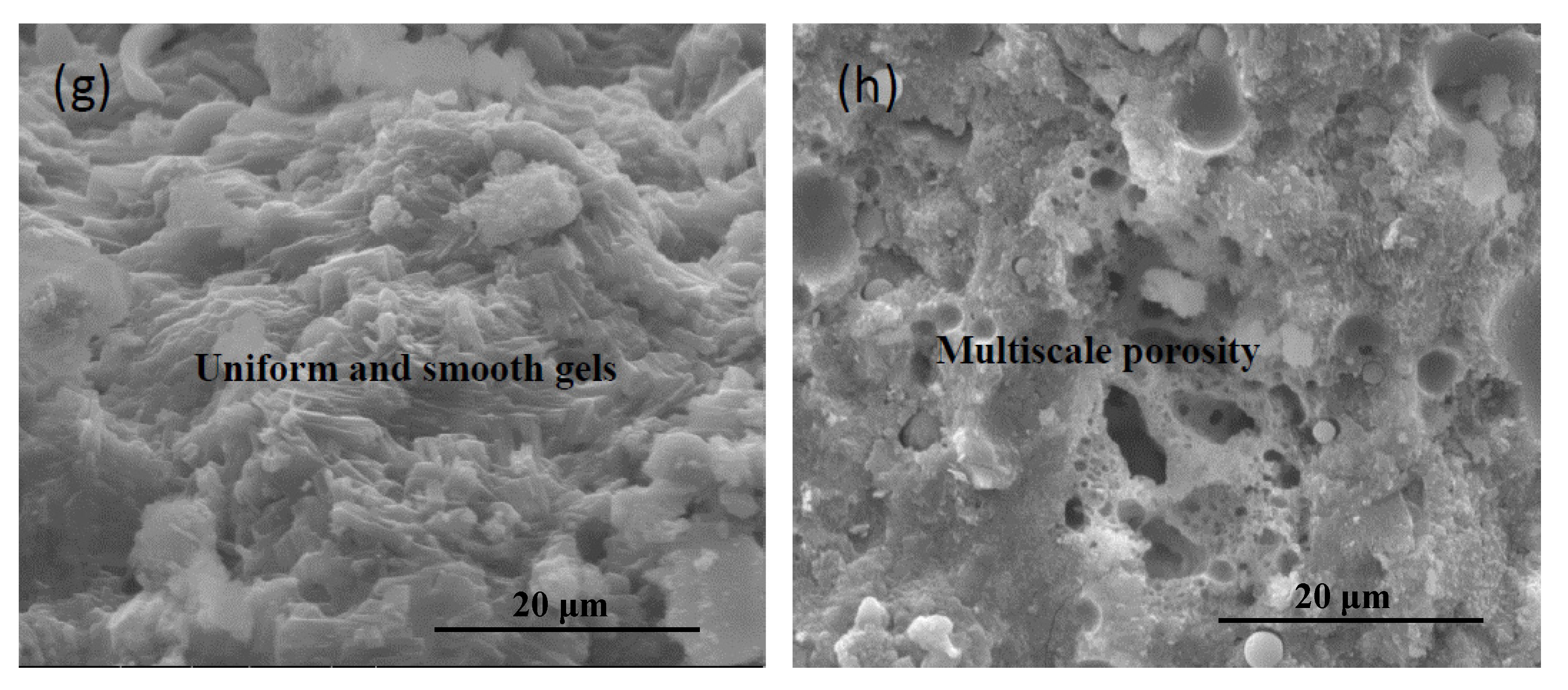
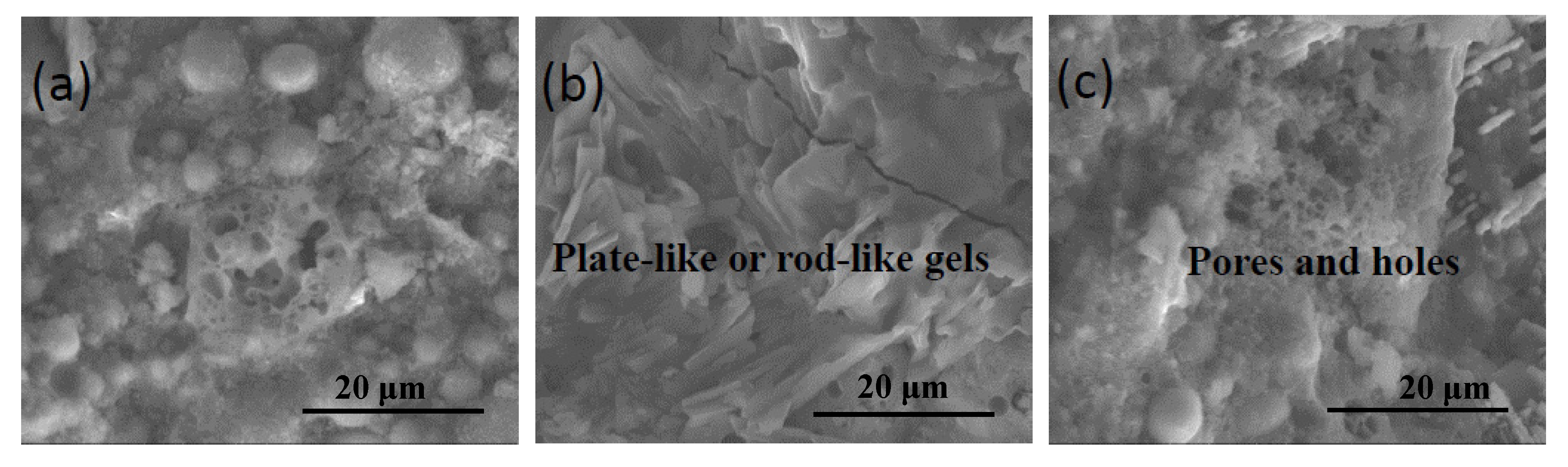
3.2. Morphology and Microstructure
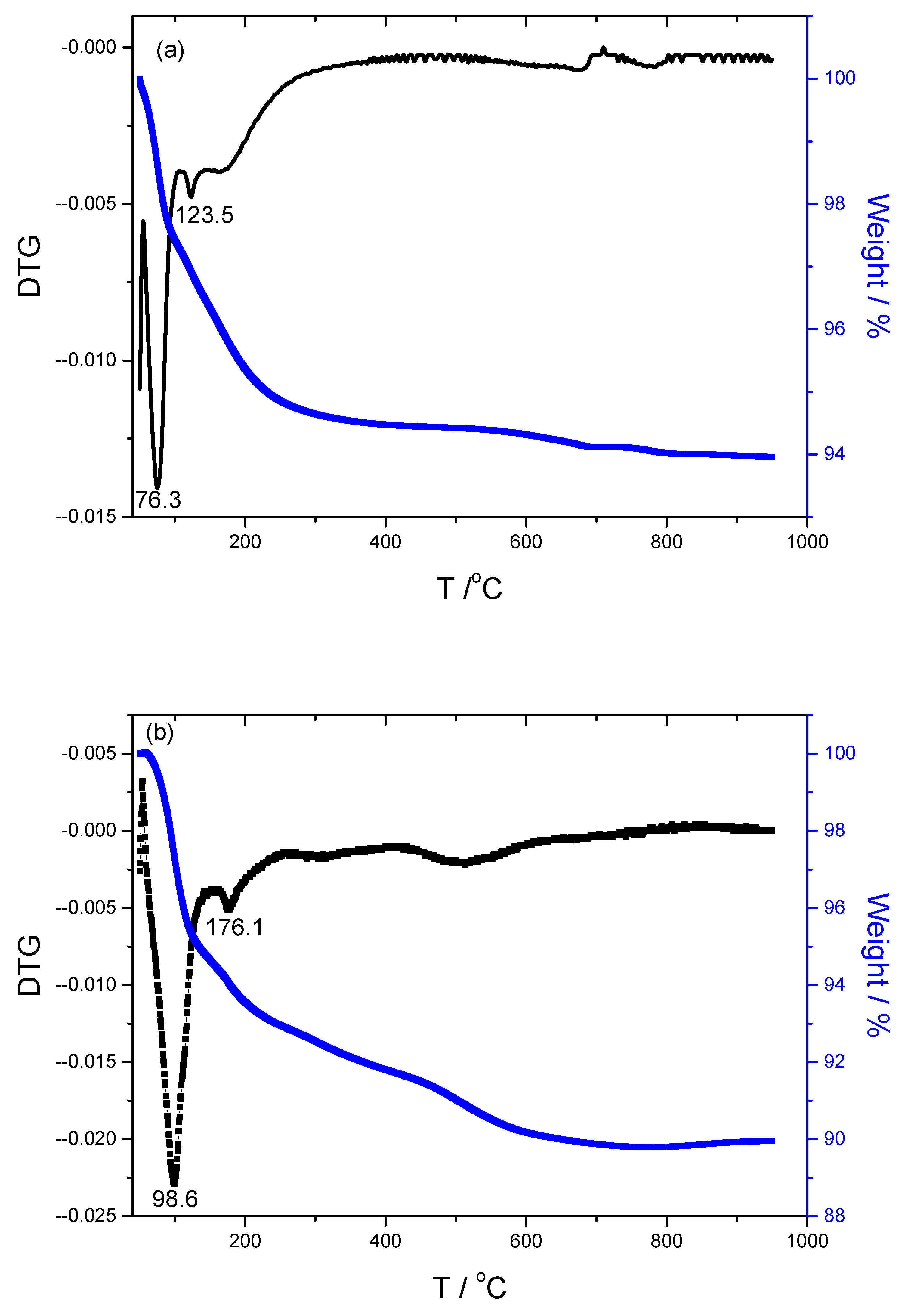
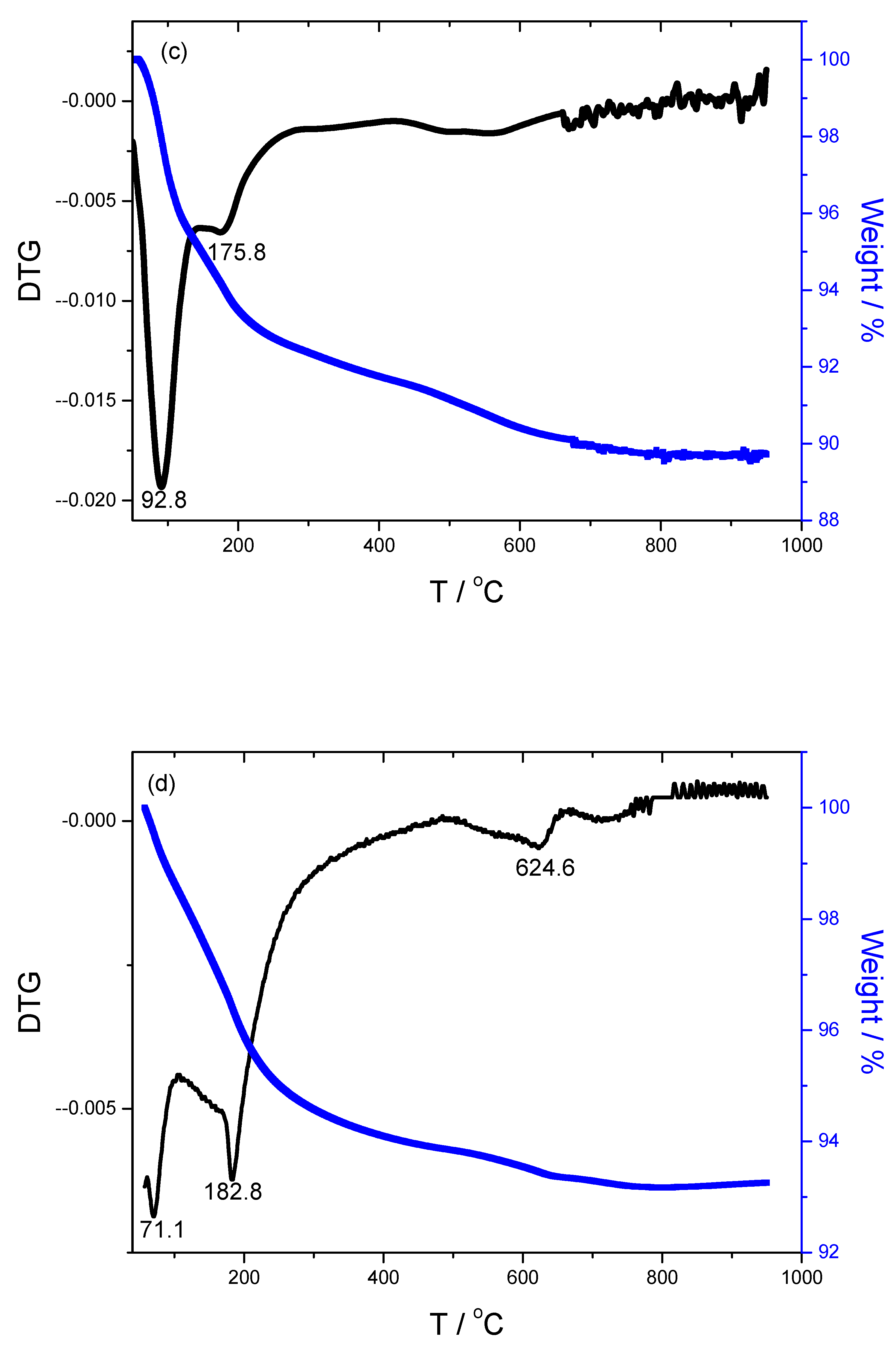
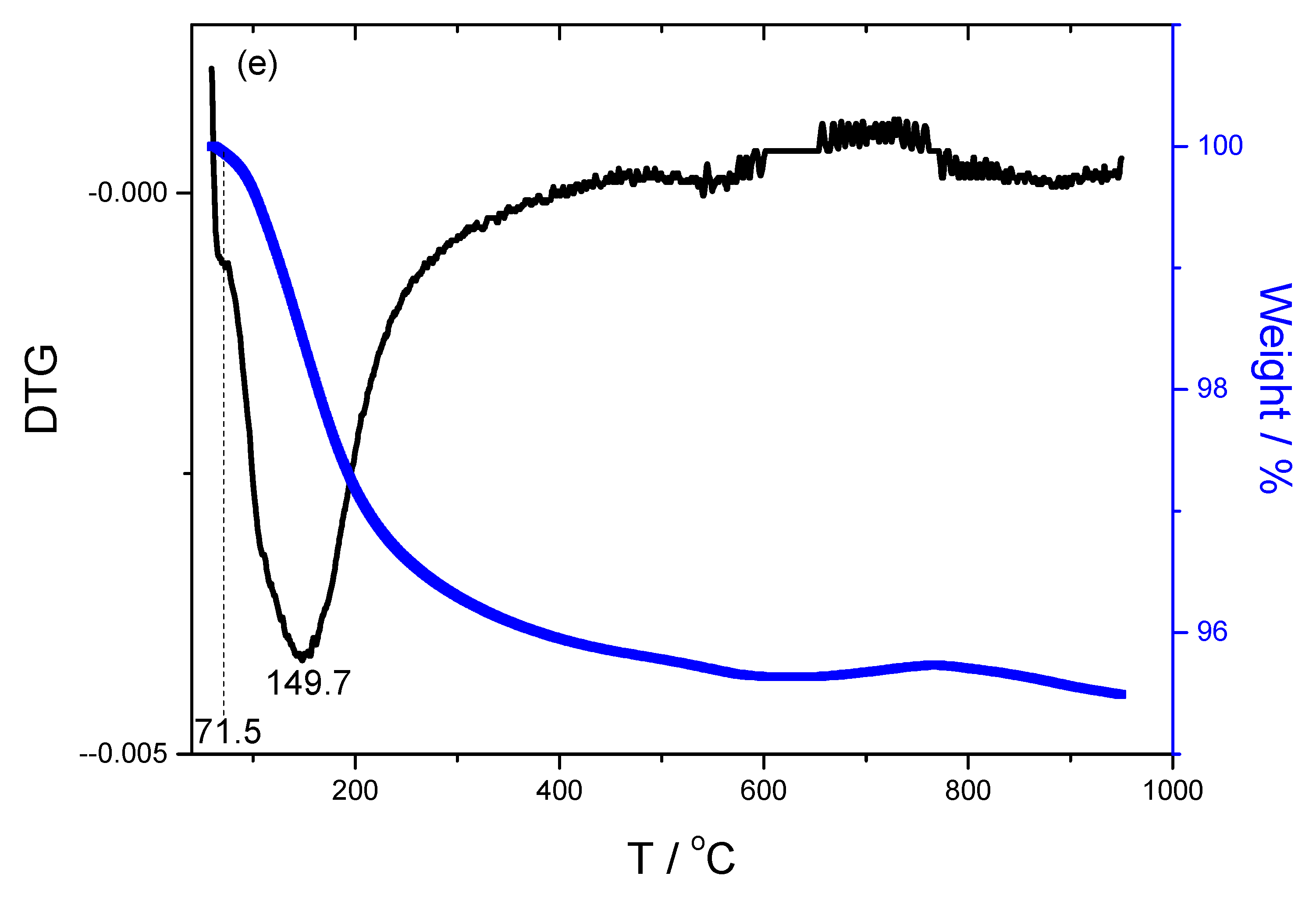
3.3. TG/DTG Analysis
3.4. XRD Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deventer, J.; Provis, J.; Duxson, P.; Lukey, G. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhang, Q.; Wen, Q.; Lu, X.; Xiao, K.; Ekberg, C.; Zhang, S. Application of geopolymers for treatment of industrial solid waste containing heavy metals: State-of-the-art review. J. Clean. Prod. 2023, 390, 136053. [Google Scholar] [CrossRef]
- Peng, X.; Li, H.; Hu, Y. Preparation of metakaolin-fly ash cenosphere based geopolymer matrices for passive fire protection. J. Mater. Res. Technol. 2023, 23, 604–610. [Google Scholar] [CrossRef]
- Harmal, A.; Khouchani, O.; El-Korchi, T.; Tao, M.; Walker, H.W. Bioinspired brick-and-mortar geopolymer composites with ultra-high toughness. Cem. Concr. Compos. 2023, 137, 104944. [Google Scholar] [CrossRef]
- Pławecka, K.; Bazan, P.; Lin, W.-T.; Korniejenko, K.; Sitarz, M.; Nykiel, M. Development of Geopolymers Based on Fly Ashes from Different Combustion Processes. Polymers 2022, 14, 1954. [Google Scholar] [CrossRef]
- Sarıdemir, M.; Çelikten, S. Effects of Ms modulus, Na concentration and fly ash content on properties of vapour-cured geopolymer mortars exposed to high temperatures. Constr. Build. Mater. 2023, 363, 129868. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Bradić, V. Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef]
- Cheng, T.-W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium water glass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Esaifan, M.; Khoury, H.; Aldabsheh, I.; Rahier, H.; Hourani, M.; Wastiels, J. Hydrated lime/potassium carbonate as alkaline activating mixture to produce kaolinitic clay based inorganic polymer. Appl. Clay Sci. 2016, 126, 278–286. [Google Scholar] [CrossRef]
- Wang, W.; Fan, C.; Wang, B.; Zhang, X.; Liu, Z. Workability, rheology, and geopolymerization of fly ash geopolymer: Role of alkali content, modulus, and water–binder ratio. Constr. Build. Mater. 2023, 367, 130357. [Google Scholar] [CrossRef]
- Yan, S.; Pan, D.; Dan, J.; Wang, J.; Yu, Y. Calcium carbide residue and Glauber’s salt as composite activators for fly ash-based geopolymer. Cem. Concr. Compos. 2023, 140, 105081. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; He, X.; Zeng, J.; Su, Y.; Wang, X.; Zhao, H.; Mao, C. Performances and microstructure of one-part fly ash geopolymer activated by calcium carbide slag and sodium metasilicate powder. Constr. Build. Mater. 2023, 367, 130303. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Gao, H.; Yuan, T.; Zhang, X. The effects of salt-loss soda residue and oxalate acid on property and structure of fly ash-based geopolymer. Constr. Build. Mater. 2023, 366, 130214. [Google Scholar] [CrossRef]
- Yliniemi, J.; Nugteren, H.; Illikainen, M.; Tiainen, M.; Weststrate, R.; Niinimäki, J. Lightweight aggregates produced by granulation of peat-wood fly ash with alkali activator. Int. J. Miner. Process. 2016, 149, 42–49. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Thaiwitcharoen, S.; Kaewpirom, S.; Rattanasak, U. Controlling ettringite formation in FBC fly ash geopolymer concrete. Cem. Concr. Compos. 2013, 41, 24–28. [Google Scholar] [CrossRef]
- Prudhomme, E.; Michaud, P.; Joussein, E.; Clacens, J.-M.; Rossignol, S. Role of alkaline cations and water content on geomaterial foams: Monitoring during formation. J. Non-Crystalline Solids 2011, 357, 1270–1278. [Google Scholar] [CrossRef]
- Guerrieri, M.; Sanjayan, J.G. Behavior of combined fly ash/slag-based geopolymers when exposed to high temperatures. Fire Mater. 2010, 34, 163–175. [Google Scholar] [CrossRef]
- Kürklü, G. The effect of high temperature on the design of blast furnace slag and coarse fly ash-based geopolymer mortar. Compos. Part B 2016, 92, 9–18. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- MarieSkofteland, B.; Ellesad, O.; Lillerud, K. Potassium merlinoite: Crystallization, structural and thermal properties. Microporous Mesoporous Mater. 2001, 43, 61–71. [Google Scholar] [CrossRef]
- Lee, W.; Deventer, J. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids and Surfaces A: Physicochem. Eng. Asp. 2002, 211, 49–66. [Google Scholar] [CrossRef]
- Skorina, T. Ion exchange in amorphous alkali-activated aluminosilicates: Potassium based geopolymers. Appl. Clay Sci. 2014, 87, 205–211. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Comparative study on flame retardancy of silica fume-based geopolymer activated by different activators. J. Alloys Compd. 2018, 743, 108–114. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; He, X.; Su, Y.; Zeng, J.; Xiong, L.; Zeng, L.; Yu, X.; Tan, H. Low-carbon wet-ground fly ash geopolymer activated by single calcium carbide slag. Constr. Build. Mater. 2022, 353, 129084. [Google Scholar] [CrossRef]
- Murri, A.N.; Medri, V.; Ruffini, A.; Papa, E.; Landi, E. Study of the chemical activation of hydroxyapatite rich ashes as raw materials for geopolymers. Ceram. Int. 2015, 41, 9734–9744. [Google Scholar] [CrossRef]
- Peng, Z.; Vance, K.; Dakhane, A.; Marzke, R.; Neithalath, N. Microstructural and 29Si MAS NMR spectroscopic evaluations of alkali cationic effects on fly ash activation. Cem. Concr. Compos. 2015, 57, 34–43. [Google Scholar] [CrossRef]
- Cioffi, R.; Maffucci, L.; Santoro, L. Optimization of geopolymer synthesis by calcination and polycondensation of a kaolinitic residue. Resour. Conserv. Recycl. 2003, 40, 27–38. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Huynh, T.-P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Rajan, H.S.; Kathirvel, P. Sustainable development of geopolymer binder using sodium silicate synthesized from agricultural waste. J. Clean. Prod. 2021, 286, 124959. [Google Scholar] [CrossRef]
- Nassar, A.K.; Kathirvel, P. Effective utilization of agricultural waste in synthesizing activator for sustainable geopolymer technology. Constr. Build. Mater. 2023, 362, 129681. [Google Scholar] [CrossRef]

| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | SO3 | TiO2 | Loss |
|---|---|---|---|---|---|---|---|---|
| Weight percent | 50.16 | 36.25 | 4.46 | 3.85 | 1.84 | 0.54 | 1.18 | 1.76 |
| Sample | Activator Composition | Compressive Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|
| Na2SiO3·9H2O | Na2CO3 | K2CO3 | NaOH | KOH | 3 d | 7 d | 28 d | |
| S1 | 15 wt% | 0 | 0 | 0 | 0 | 19.83 | 20.08 | 20.25 |
| S2 | 0 | 1 mol/L | 0 | 0 | 0 | 1.50 | 1.58 | 1.75 |
| S3 | 0 | 2 mol/L | 0 | 0 | 0 | 2.00 | 2.42 | 5.25 |
| S4 | 0 | 0 | 1 mol/L | 0 | 0 | 0.08 | 0.17 | 0.17 |
| S5 | 0 | 0 | 2 mol/L | 0 | 0 | 0.17 | 0.17 | 0.25 |
| S6 | 0 | 0 | 0 | 4 mol/L | 0 | 4.83 | 5.25 | 6.42 |
| S7 | 0 | 0 | 0 | 8 mol/L | 0 | 21.33 | 23.00 | 15.67 |
| S8 | 0 | 0 | 0 | 0 | 4 mol/L | 2.08 | 2.17 | 2.30 |
| S9 | 0 | 0 | 0 | 0 | 8 mol/L | 15.92 | 11.83 | 10.80 |
| S10 | 0 | 0 | 0 | 4 mol/L | 4 mol/L | 8.75 | 10.83 | 9.58 |
| S11 | 0 | 0 | 0 | 8 mol/L | 4 mol/L | 21.83 | 33.25 | 30.92 |
| S12 | 15 wt% | 1 mol/L | 0 | 0 | 0 | 29.1 | 31.25 | 32.50 |
| S13 | 15 wt% | 2 mol/L | 0 | 0 | 0 | 30.67 | 33.08 | 35.50 |
| S14 | 15 wt% | 0 | 1 mol/L | 0 | 0 | 33.67 | 34.82 | 39.17 |
| S15 | 15 wt% | 0 | 2 mol/L | 0 | 0 | 38.08 | 40.25 | 43.08 |
| S16 | 15 wt% | 0 | 0 | 4 mol/L | 0 | 9.63 | 10.42 | 10.67 |
| S17 | 15 wt% | 0 | 0 | 0 | 4 mol/L | 54.08 | 54.67 | 56.08 |
| S18 | 15 wt% | 0 | 0 | 0 | 8 mol/L | 51.71 | 53.93 | 55.17 |
| S19 | 0 | 1 mol/L | 0 | 4 mol/L | 0 | 17.50 | 17.92 | 18.25 |
| S20 | 0 | 2 mol/L | 0 | 4 mol/L | 0 | 20.17 | 21.08 | 21.92 |
| S21 | 0 | 0 | 1 mol/L | 0 | 4 mol/L | 4.92 | 4.92 | 5.17 |
| S22 | 0 | 0 | 2 mol/L | 0 | 4 mol/L | 4.25 | 4.50 | 4.68 |
| S23 | 0 | 2 mol/L | 2 mol/L | 0 | 0 | 0.17 | 0.17 | 0.25 |
| S24 | 0 | 2 mol/L | 0 | 0 | 4 mol/L | 5.25 | 5.42 | 5.50 |
| Specimens | <100 nm (%) | 100–200 nm (%) | >0.2 μm (%) | Median Pore Diameter (nm) | Porosity (%) | Total Intrusion Volume (mL/g) |
|---|---|---|---|---|---|---|
| S1 | 0.56 | 14.53 | 84.91 | 239.7 | 23.98 | 0.1961 |
| S15 | 27.87 | 14.18 | 57.95 | 104.8 | 21.36 | 0.1719 |
| S17 | 52.54 | 2.16 | 45.31 | 74.6 | 19.69 | 0.1578 |
| S25 | 53.67 | 6.94 | 39.39 | 67.1 | 19.52 | 0.1523 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, Y. Microstructure Evaluation of Fly Ash Geopolymers Alkali-Activated by Binary Composite Activators. Minerals 2023, 13, 910. https://doi.org/10.3390/min13070910
Zhao J, Wang Y. Microstructure Evaluation of Fly Ash Geopolymers Alkali-Activated by Binary Composite Activators. Minerals. 2023; 13(7):910. https://doi.org/10.3390/min13070910
Chicago/Turabian StyleZhao, Jiangping, and Yachao Wang. 2023. "Microstructure Evaluation of Fly Ash Geopolymers Alkali-Activated by Binary Composite Activators" Minerals 13, no. 7: 910. https://doi.org/10.3390/min13070910
APA StyleZhao, J., & Wang, Y. (2023). Microstructure Evaluation of Fly Ash Geopolymers Alkali-Activated by Binary Composite Activators. Minerals, 13(7), 910. https://doi.org/10.3390/min13070910






