Abstract
The primary coexisting mineral with galena is sphalerite. Hence, it is critical to selectively separate galena from sphalerite by flotation. In this work, thiourea and related derivatives as potential flotation collectors for separating galena from sphalerite were investigated. Thiourea and its related derivatives were found to be effective selective collectors in batch flotation studies of a single mineral, with 1,1-diphenylthiourea (11DTA) emerging as the best choice. Galena has superior floatability compared to sphalerite in the presence of 11DTA, and the recovery difference between the two minerals at pH 8 (where the 11DTA concentration is 5 × 10−6 mol/L) is around 38%. This was revealed in batch flotation studies using artificial mixed minerals. Moreover, the findings from the measurements of adsorption amount, FTIR, zeta potential and XPS revealed that 11DTA has a strong adsorption on galena yet a relatively weak adsorption on sphalerite. Additionally, DFT calculations demonstrated that sphalerite exhibits stronger hydrophilicity than galena, and 11DTA possessed a better affinity for galena.
1. Introduction
Lead is a strategic mineral resource which is mainly derived from sulfide deposits [1]. Galena (PbS) is the most abundant source of lead, but it often coexists with pyrite (FeS2), sphalerite (ZnS) and other minerals [2]. Among these minerals, the flotation separation of galena and sphalerite is a persistent research hotspot in mineral processing because of their similar surface properties [3]. The traditional differential flotation adds ZnSO4 to deactivate sphalerite, but it has some demerits, such as increasing activation difficulty, enlarging the pollution risk of heavy metals and increasing processing costs [4]. Flotation collectors play an important role in the flotation separation of galena and sphalerite, and an efficient collector with a tailored molecular structure is crucial to regulate collection selectivity in a mixed flotation system. Currently, a selective collector with an outstanding molecular structure towards a galena/sphalerite mixed flotation system is highly desired.
It has been proven that even subtle changes in collector molecular structures (mainly bonding groups and hydrophobic groups) could potentially lead to significant change in collecting performance [5]. The species, structures and number of bonding groups and hydrophobic groups could lead to changes in the geometrical sizes, electronic effects and spatial effects of collector molecules, thereby affecting final flotation separation effects [6,7,8]. As a consequence, structure–activity relationships are of significant research value and could be employed as an effective tool for exploring the molecular structures of efficient collectors [9].
Thiourea and related derivatives exhibit excellent performance in the flotation beneficiation of galena against sphalerite [10], and 1,3-diphenylthiourea (13DTA, collector 1) was proven to be a selective collector in our prior investigation [11]. For the molecular structure of 13DTA, a sulfur atom is the main chelating site, but amine groups also play a role in the frontier molecular orbital. As important functional group for constructing ligands, the primary amine group shows better properties than the secondary amine group [12]. It could be hypothesized that replacing the secondary amine group in the molecular structure of 13DTA with a primary amine group, 1-phenylthiourea (1PTA, collector 2), a better selectivity collector, is obtained. Furthermore, the isomeride 11DTA (collector 3, with a secondary amine group) is obtained from the molecular structure of 13DTA by the transposition of the phenyl group. In this work, to develop selective collectors and test the hypotheses in Figure 1, the selective collecting abilities and adsorption behaviors of 13DTA, 1PTA and 11DTA on galena and sphalerite were investigated.
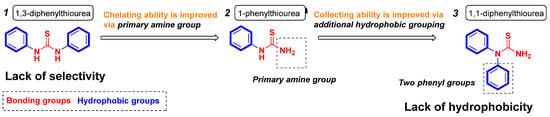
Figure 1.
Molecular structures and functional groups of 1,3-diphenylthiourea (13DTA), 1-phenylthiourea (1PTA) and 1,1-diphenylthiourea (11DTA).
2. Experimental
2.1. Minerals and Reagents
The galena and sphalerite minerals with a high degree of purity came from Hunan Province, China. The purities of the galena and sphalerite samples were over 98% and 100%, respectively. Two ores were used for the batch flotation tests and other measurements. The artificial mixed mineral ore was prepared by mixing high-purity galena and sphalerite samples at a ratio of 1:1. After crushing, grinding and sieving, minerals with particle sizes ranging from 37 to 74 μm were used for the flotation tests. Then, minerals used for measuring zeta potential were further ground to a size of approximately 5 μm.
1,3-diphenylthiourea (13DTA), 1-phenylthiourea (1PTA), 1,1-diphenylthiourea (11DTA) and terpineol with an analytical grade used for flotation tests were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Solutions of HCl and NaOH were utilized to adjust the pH value of the slurry, each at a certain concentration. Deionized water (DI water) was applied for the entire experiment.
2.2. Batch Flotation Tests
An XFG flotation machine with an impeller speed of 1992 rpm and a 40 mL plexiglass cell were employed in the batch flotation tests of single and artificial mixed minerals. In each experiment, firstly, a 2.0 g pure galena or sphalerite sample or artificial mixed mineral sample (1.0 g galena and 1.0 g sphalerite) was mixed with 40 mL DI water in a batch flotation cell and agitated for 1 min. On top of that, diluted HCl or NaOH solution was used to adjust the pH value of the slurry. Particularly, the pH value was not adjusted in the variety of collector experiments where the pH value was approximately 7.2 for galena and 7.6 for sphalerite. After 3 min of agitation, the collector was added in succession to the slurry and agitated for 3 min. The flotation lasted for 3 min, and the concentrate was scraped. Finally, the concentrate and tailings were dried in an oven at a constant temperature and weighed to calculate the recovery. The average of three replicates was reported as the final data.
2.3. Adsorption Amount Measurements
The adsorption amounts of 13DTA, 1PTA and 11DTA on galena and sphalerite were arrived at as follows: In each measurement, the pure mineral sample (2 g galena or sphalerite; −5 μm) and a certain volume of DI water were combined in a 100 mL beaker. The suspension’s pH value was then adjusted with diluted NaOH or HCl solution. Subsequently, the collector at a specific concentration was added to the beaker. The total volume of the suspension was set as 50 mL. After being shaken for 30 min, the residual concentration of the collector in the supernatant was determined by a UV–vis spectrophotometer (UV-2600, Shimadzu, Kyoto, Japan). The concentration can be calculated with ultraviolet absorbance according to the Beer–Lambert law, and the final result was the average of 3 parallel tests.
The amount of the collector was calculated by Equation (1).
where Q is the adsorption amount of the collector (mol/g); C0 is the concentration of the collector before adsorption (mol/L); Cr is the concentration of the collector after adsorption (mol/L); V is the volume of the solution (L); and m is the weight of mineral (g).
2.4. FTIR Measurements
The Fourier-transform infrared (FTIR) spectra measurements were made using a Nicolet iS50 (Thermo Fisher Scientific, Waltham, MA, USA). The FTIR spectra of 11DTA and the pure minerals in the absence and presence of 11DTA were obtained with a range from 4000 cm−1 to 400 cm−1 and a measurement resolution of 2 cm−1. Each mineral sample (2 g galena or sphalerite; −5 μm) was mixed with 40 mL DI water, and 11DTA was added to the suspension at pH value 8. After the suspension was stirred for 45 min at 25 °C, the mineral sample was obtained by filtration, using DI water to wash 3 times and drying for 1 day in an oven at a constant temperature. The measurements used the KBr pellet method, and 1 mg of the sample was mixed with 100 mg of KBr.
2.5. Zeta Potential Measurements
The zeta potentials of both galena and sphalerite with and without 11DTA were measured at a constant temperature of 25 °C using a Malvern Zeta Sizer Nano Series (Zetasizer Nano ZS90, Malvern Panalytical, Almelo, Netherlands). In each measurement, the pure mineral sample (sizes of approximately 5 μm; 30 mg) was added to 150 mL of KNO3 electrolyte solution (0.2 g/L) in a beaker (200 mL), and the suspension was set at a given pH value using a pH regulator (1% HCl or NaOH solution). Subsequently, 11DTA at an optimal concentration was added to the suspension for 5 min of conditioning. Finally, before the supernatant was extracted to measure the zeta potential, the suspension stood for 5 min. Each experiment was carried out at least three times, and we reported the average results and the standard deviation herein.
2.6. XPS Measurements
XPS spectrums of galena and sphalerite with and without 11DTA adsorption were performed using a Thermo Scientific K-Alpha spectrometer (Thermo Scientific Co., Waltham, MA, USA). The excitation source adopted an Al K-Alpha ray (hv = 1486.6 eV), the working voltage was 12.5 kV, the filament current was 16 mA and the signal was accumulated for 10 cycles. The analysis chamber vacuum degree was 8 × l0−10 Pa. The test passing energy was set to 100 eV, and the XPS spectra were calibrated against the standard C 1s peak at 284.80 eV with the Avantage software. In each measurement, the treatment of the test sample (particle sizes ranging from 37 to 74 μm) was the same as the flotation of pure minerals. After filtration, the residue was dried for 1 day in an oven at 35 °C and collected for XPS measurements.
2.7. Density Functional Theory Calculations
Spin-polarized density functional theory (DFT) calculations were performed using the Cambridge Serial Total Energy Package (CASTEP) code [13]. The electron exchange-correlation potential was conducted using the Perdew–Burke–Ernzerhof (PBE) functional of generalized gradient approximation (GGA), and ultrasoft pseudopotentials were employed [14]. To simulate the adsorption configurations for the collectors, the oriented crystallographic (100) plane and (110) plane of galena and sphalerite, as suggested from the XRD characterization, were constructed using a periodic slab with a vacuum region of 15 Å along with the z-axis. Dispersion correction for DFT (DFT-D) was used to describe the van der Waals interactions. The Brillouin zone integration was sampled with a 2 × 2 × 1 Monkhorst Pack mesh k-point for the surface model and an energy cutoff of 500 eV was applied for the plane-wave basis set. The convergence tolerances were set to be 1 × 10−5 eV per atom for energy, 1 × 10−3 Å for maximum displacement and 0.03 eV/Å for maximum force. The binding energy () of the collector on the mineral surface was calculated as follows:
where is the total energy of the mineral slab adsorbed with the collector, is the energy of the mineral slab with a clean surface and is the energy of the adsorbed collector. The value of binding energy shows the stability of the adsorption system. Increasingly negative interaction energy values indicate a more favorable interaction between the mineral surface and collector. In addition, in order to simulate the solvation effect, the adsorption configurations with or without water were considered comprehensively.
3. Results and Discussion
3.1. Batch Flotation Tests
To compare the collecting selectivity of the three thiourea derivatives and verify the hypotheses, micro-flotation tests of single minerals (galena and sphalerite) were carried out firstly. The results are presented in Figure 2. In general, the three collectors performed obvious collecting selectivity towards galena and sphalerite, and the flotation recoveries of both galena and sphalerite were improved as the collector concentration increased, which was in accordance with the results reported by Ozun and Jia [15,16]. This fully proves that thiourea and related derivatives could act as selective collectors for the flotation of a galena/sphalerite system. Moreover, by meticulously comparing the flotation recovery differences generated by the three different collectors, 11DTA emerged as the optimized candidate because of the maximal flotation recovery difference (the biggest flotation recovery differences of galena and sphalerite by 13DTA, 1PTA and 11DTA were 13.4%, 20.7% and 23.9%, respectively).
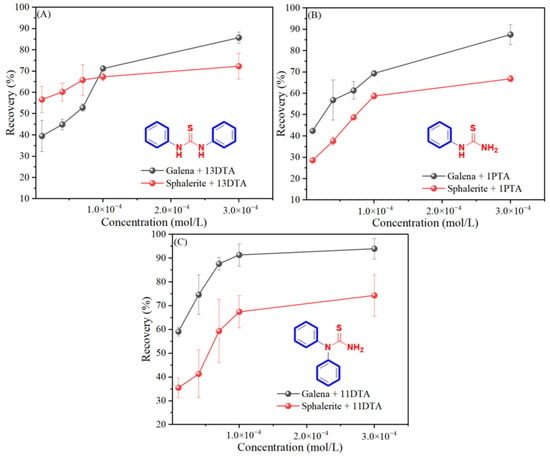
Figure 2.
Flotation recovery of galena and sphalerite as a function of concentration of 13DTA, 1PTA and 11DTA, respectively. (A) 13DTA as flotation collector; (B) 1PTA as flotation collector; (C) 11DTA as flotation collector.
The flotation recoveries of galena and sphalerite at different pH values in the presence of 1 × 10−4 mol/L 11DTA are shown in Figure 3. Generally, this research demonstrated that the recovery of these two minerals decreased with pH value increments. For sphalerite flotation, the recovery of sphalerite did not surpass 57% in the entire examined pH value range. However, the recovery of galena collected by 11DTA stood as high as 82% within a pH value range of 3–10. Moreover, by meticulously comparing the flotation recovery differences at various pH values, a relatively great flotation recovery difference was achieved between galena and sphalerite at pH value 8, at which the flotation recovery of galena and sphalerite was 92.2% and 48.1%, respectively. Therefore, a solution pH value of 8 was the optimal separation pH value and was employed in all follow-up flotation tests.
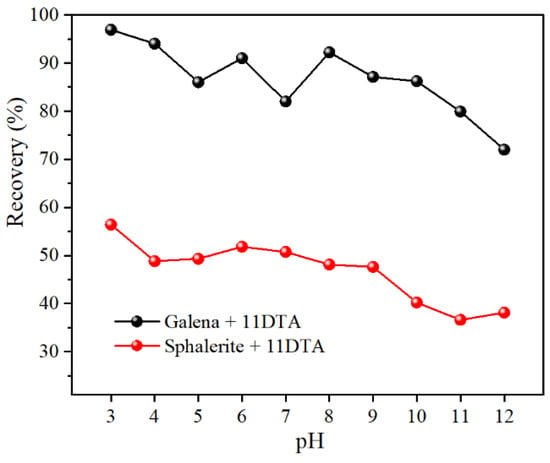
Figure 3.
Flotation recovery of galena and sphalerite as a function of pH value (11DTA concentration of 1 × 10−4 mol/L).
Galena and sphalerite are more likely to produce different flotation behaviors when mixed up [17]. To verify the collecting selectivity of 11DTA towards galena and sphalerite, batch flotation tests of artificial mixed minerals were carried out at pH value 8. Figure 4 presents the flotation recovery of the artificial mixed minerals as a function of 11DTA concentration. The flotation recovery of galena remained above 77% in the entire studied range of 11DTA concentration, and the flotation recovery of galena gradually improved with the decrease of 11DTA concentration. However, the flotation recovery of sphalerite was considerably decreased with the decrease of 11DTA concentration, which demonstrated that the collecting selectivity of 11DTA gradually weakened with the increase of the concentration. When the concentration of 11DTA was 5 × 10−6 mol/L, the recovery of galena was approximately 92.2% and the recovery of sphalerite was only about 54.2%, indicating the significant collecting selectivity of 11DTA towards galena and sphalerite.
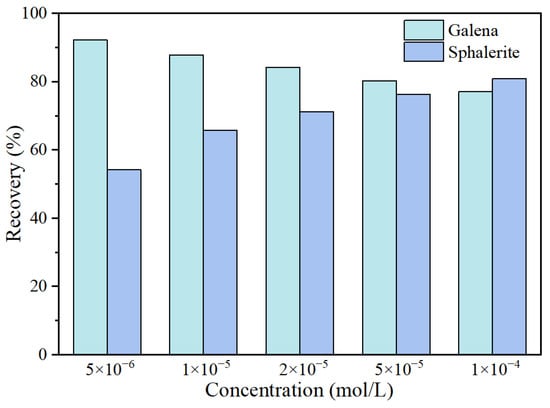
Figure 4.
Flotation recovery of artificial mixed mineral as a function of 11DTA concentration.
3.2. Investigation of Pb–Zn Separation Mechanism
3.2.1. Adsorption Behavior Analyses
To study the adsorption behaviors of the different collectors on the surface of galena and sphalerite and verify the hypotheses, the amounts of the three collectors adsorbed on galena and sphalerite were measured at the optimal flotation concentration at pH value 8. As Figure 5 shows, the amount of 11DTA adsorbed on galena (6.8 × 10−6 mol/g) was highest among the three thiourea derivatives, indicating a stronger affinity between 11DTA and galena. Moreover, 11DTA preferred to be adsorbed on galena instead of sphalerite. It was noted that 11DTA was the optimized candidate because of the maximum adsorption amount difference (the biggest adsorption amount difference of galena and sphalerite by 13DTA, 1PTA and 11DTA was 0.2 × 10−6 mol/g, 1.8 × 10−6 mol/g and 2.9 × 10−6 mol/g, respectively), by meticulously comparing the adsorption amount differences generated by the three different collectors.
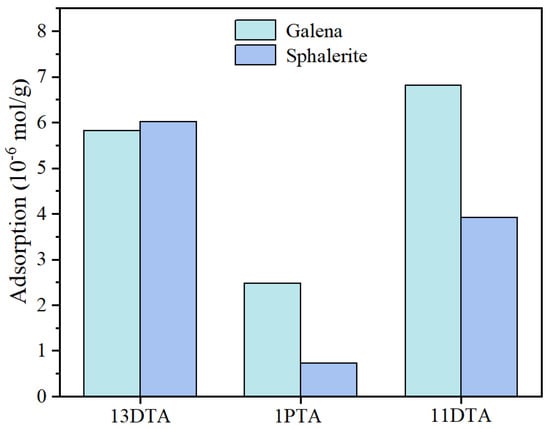
Figure 5.
Adsorption amounts of 13DTA, 1PTA and 11DTA, respectively, on galena and sphalerite.
The infrared spectra of galena and sphalerite both before and after interaction with 11DTA were measured and the results are displayed in Figure 6. As observed from the spectrum of 11DTA, the characteristic peaks at 3451 and 3273 cm−1 were related to the asymmetrical stretching vibration of the N–H group and symmetrical stretching vibration of the N-H group, respectively [18]. The peaks at 3060 and 3030 cm−1 were related to the stretching vibration of aromatic C–H [19]. The peaks at 1593 and 1075 cm−1 corresponded to the stretching vibration of C–N and C=S, respectively [20]. The analyses of the FTIR results were in accordance with the molecular structure of 11DTA. After treatment with 11DTA, the peaks at 1593, 818 and 625 cm−1, belonging to the stretching vibrations of C–N and aromatic C–H of 11DTA, appeared on the spectrum of galena, indicating that the 11DTA tightly attached on the galena surface. Meanwhile, a new peak which belonged to C–N–Pb appeared at 1642 cm−1 after it interacted with galena. However, the C=S stretching vibration from 11DTA was almost absent and a new peak which belonged to C–S–Pb appeared at 405 cm−1 after it interacted with galena [21].
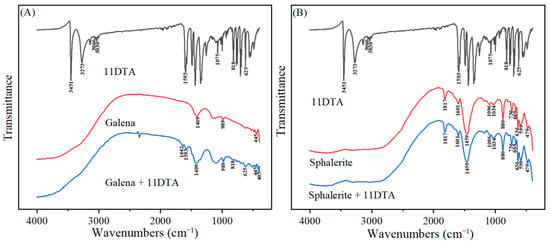
Figure 6.
FTIR spectra of galena (A) and sphalerite (B) before and after 11DTA treatment.
After 11DTA treatment, the spectrum of the sphalerite did not undergo any observable change, implying that 11DTA was not widely adsorbed on the sphalerite surface. The results are presented in Figure 6B. They demonstrate that no new peaks appeared on the sphalerite surface after the 11DTA interaction beyond its original peaks.
The above FTIR spectra show that the adsorption of 11DTA on the galena surface was mostly dependent on the C=S and C–N groups, which could react with lead species and form C–S–Pb and C–N–Pb structures on the surface of galena.
3.2.2. Zeta Potential Analyses
To investigate thoroughly the mechanism of flotation separation and verify the collecting selectivity of 11DTA, zeta potential measurements were carried out. The changes in dynamic potential on the surfaces of galena and sphalerite at specific pH values with or without 11DTA were measured and are presented in Figure 7.
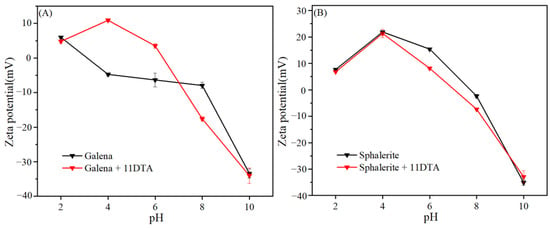
Figure 7.
Zeta potentials of galena (A) and sphalerite (B) before and after the addition of 11DTA at different pH values.
As shown in Figure 7A, the zeta potential curves of galena changed obviously after the galena interaction with 11DTA. However, the zeta potential curves of sphalerite exhibited a slight negative shift after sphalerite interaction with 11DTA, with the results shown in Figure 7B. Due to primary amine group of 11DTA being converted into quaternary ammonium salt under acidic conditions [22], the zeta potential of galena shifted dramatically in a positive direction at pH values of 4 and 6. In particular, the zeta potential of sphalerite decreased by 5 mV, with little difference before and after the addition of 11DTA, which revealed the weak adsorption of 11DTA on sphalerite at pH value 8 (the optimal separation pH value). However, the zeta potential of galena shifted dramatically in a negative direction (decreased by 10 mV), indicating a strong interaction between the 11DTA and galena at pH value 8.
3.2.3. XPS Analyses
In order to further investigate the mechanism of 11DTA on the surface of galena and sphalerite, XPS detections were carried out to evaluate the different adsorption performances of 11DTA towards galena and sphalerite. In Figure 8, the results of the galena and sphalerite XPS survey spectra in the absence and presence of 11DTA, respectively, are shown. As shown in Figure 8A, before interacting with 11DTA, the C 1s peak at 284.8 eV was caused by the contamination of carbon. In addition, the peaks at 532.4, 161.0 and 137.5 eV are related to O 1s, S 2p and Pb 4f, respectively. In the XPS spectrum of galena treated with 11DTA, a small new N 1s peak, namely, the amino signal produced by 11DTA, existed at 400.5 eV, which meant that the 11DTA was attached to the galena. However, apart from the peaks of Zn 2p, O 1s, C 1s and S 2p from the sphalerite itself, the XPS spectrum of sphalerite revealed no N peak after 11DTA treatment, which means that the adsorption of 11DTA on the sphalerite surface was negligible. In general, this result is consistent with FTIR measurements, namely, the better flotation recovery of galena than sphalerite and stronger adsorption of 11DTA on the galena surface rather than on the sphalerite surface.
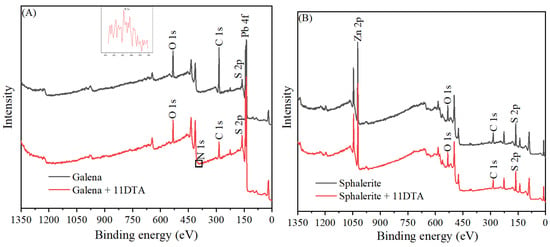
Figure 8.
XPS spectra of galena (A) and sphalerite (B) before and after conditioning with 11DTA.
3.2.4. Theoretical Calculation Analyses
The molecular orbital theory, proposed by Fukui in 1952, has been successful in predicting chemical behaviors for an enormous number of molecules. The highest occupation molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) play a prominent role in governing the chemical reactions of organics [23,24]. The energy gap between HOMO and LUMO is an important stability index, e.g., a large HOMO–LUMO gap implies a high stability for the molecule in chemical reactions [25,26]. Therefore, the molecule frontier orbital energies of thiourea-based collectors were calculated to predict their chemical characteristics, and the results are given in Figure 9.
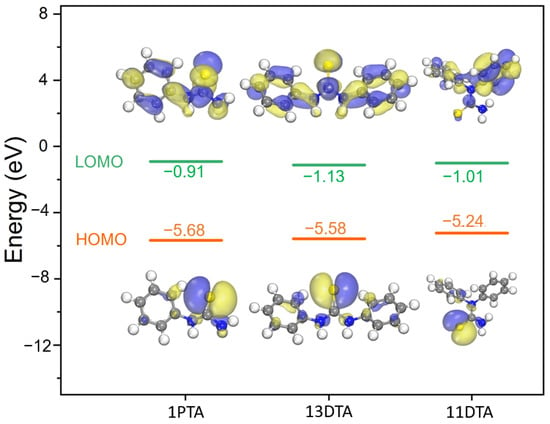
Figure 9.
Isosurfaces of the frontier orbitals of thiourea-based collectors.
As shown in Figure 9, the HOMO isosurfaces of 13DTA, 1PTA and 11DTA were all located in the S=C group with energies of −5.68 eV, −5.58 eV and −5.24 eV, respectively. Obviously, the HOMO–LUMO gap of 11DTA was the lowest among the collectors, reflecting that 11DTA exhibited a higher chemical reactivity than 13DTA and 1PTA based on the frontier molecular orbital theory. Thus, 11DTA was selected for an in-depth study.
To clarify the role of the selective adsorption of 11DTA on galena and sphalerite surfaces, DFT calculations were performed to mimic the adsorption configurations of 11DTA on galena and sphalerite surfaces with or without water.
As shown in Figure 10A,D,G, sphalerite possessed a higher affinity for water than galena with a shorter bond distance (2.188 Å) and more negative binding energy (−2.98 eV). Without water, 11DTA was still more inclined to adsorb on a sphalerite surface than galena through a bridged interaction, with a more negative binding energy (−0.69 eV). When considering the solvation effect, 11DTA seemed to be able to adsorb on both sphalerite and galena surfaces from the perspective of adsorption configurations (Figure 10C,F). However, the binding energy between 11DTA and sphalerite is positive (Figure 10G), which means that it could be difficult for 11DTA to adsorb on a sphalerite surface by breaking the hydration film. The above results demonstrated that sphalerite exhibits stronger hydrophilicity than galena, unveiling the reason why 11DTA possessed a better floatability for galena, because the flotation process was carried out in solution conditions.
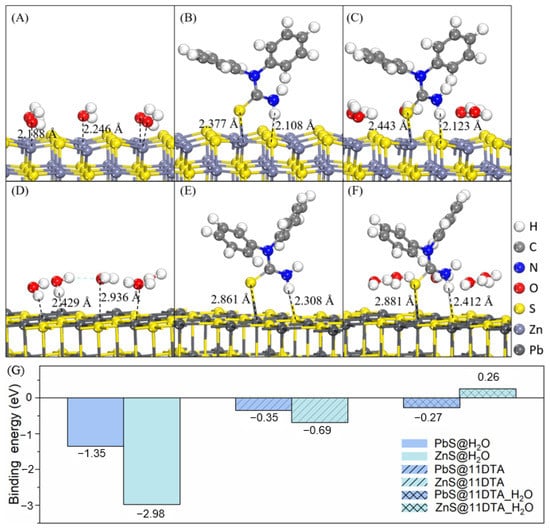
Figure 10.
Adsorption configurations of water and 11DTA on sphalerite and galena surfaces with or without water. (A) ZnS@H2O; (B) ZnS@11DTA; (C) ZnS@11DTA_H2O; (D) PbS@H2O; (E) PbS@11DTA; (F) PbS@11DTA_H2O; (G) Binding energy.
4. Conclusions
This research investigated the collecting performance and adsorption mechanism of 11DTA as a collector via flotation experiments and surface detection. The main conclusions of this work are summarized and listed as follows:
- (1)
- Flotation experiments revealed that 11DTA exhibited stronger collecting ability and superior selectivity for galena over sphalerite compared to other aryl thiourea collectors (13DTA and 1PTA), which can prove amine groups play an imperative role in frontier molecular orbitals.
- (2)
- The adsorption amount of 11DTA on the galena surface, the newly emerged adsorption peaks in the 11DTA-treated galena FTIR spectra, the negatively shifted zeta potential of galena and the XPS spectra of 11DTA-treated galena all suggested that the 11DTA collector could selectively adsorb onto the surface of galena and improve its hydrophobicity significantly. These measurement methods also revealed that the adsorption of 11DTA on sphalerite was much weaker than that on galena.
- (3)
- The results of the DFT calculations further confirmed that 11DTA exhibited a higher chemical reactivity than 13DTA and 1PTA based on the frontier molecular orbital theory and 11DTA possessed a superior floatability for galena.
Author Contributions
Conceptualization, W.S.; methodology, Z.G. and R.Z.; software, B.L.; investigation, B.L.; resources, W.S.; writing—original draft preparation, B.L.; writing—review and editing, R.Z. and J.C; supervision, Z.G. and J.C.; project administration, J.C.; funding acquisition, R.Z. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (52004336, 52274287, 52204300); and the Science and Technology Innovation Program of Hunan Province (2022RC1039).
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chen, W.; Chen, F.; Zhang, Z.; Tian, X.; Bu, X.; Feng, Q. Investigations on the depressant effect of sodium alginate on galena flotation in different sulfide ore collector systems. Miner. Eng. 2021, 160, 106705. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, M.; Liu, R.; Sun, W. A review on the electrochemistry of galena flotation. Miner. Eng. 2020, 150, 106272. [Google Scholar] [CrossRef]
- Long, X.; Chen, Y.; Chen, J.; Xu, Z.; Liu, Q.; Du, Z. The effect of water molecules on the thiol collector interaction on the galena (PbS) and sphalerite (ZnS) surfaces: A DFT study. Appl. Surf. Sci. 2016, 389, 103–111. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Q. Reexamining the functions of zinc sulfate as a selective depressant in differential sulfide flotation—The role of coagulation. J. Colloid Interface Sci. 2006, 301, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, D.; Farinato, R. Evolution of flotation chemistry and chemicals: A century of innovations and the lingering challenges. Miner. Eng. 2016, 96–97, 2–14. [Google Scholar] [CrossRef]
- Wang, D.Z. Flotation Reagents: Applied Surface Chemistry on Minerals Flotation and Energy Resources Beneficiation; Springer: Singapore, 2016. [Google Scholar]
- Cao, F.; Sun, C.-Y.; Wang, H.-J.; Chen, F.-W. Effect of alkyl structure on the flotation performance of xanthate collectors. J. Univ. Sci. Technol. Beijing 2014, 36, 1589–1594. [Google Scholar] [CrossRef]
- Lu, D.; Wang, Y. Optimisation of fine auriferous pyrite recovery using anionic and non-ionic collectors. Miner. Process. Extr. Metall. 2017, 127, 127–132. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Zhong, H.; Xia, L.-Y.; Wang, S.; Dai, T.G. Effect of N-substituents on performance of thiourea collectors by density functional theory calculations. Trans. Nonferrous Met. Soc. China 2010, 20, 695–701. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Cheng, S.; Liu, S.; Cao, J.; Chen, P. Selective flotation separation of galena from sphalerite via chelation collectors with different nitrogen functional groups. Appl. Surf. Sci. 2021, 568, 150956. [Google Scholar] [CrossRef]
- Fliedel, C.; Ghisolfi, A.; Braunstein, P. Functional Short-Bite Ligands: Synthesis, Coordination Chemistry, and Applications of N-Functionalized Bis (diaryl/dialkylphosphino) amine-type Ligands. Chem. Rev. 2016, 116, 9237–9304. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Özün, S.; Ergen, G. Determination of Optimum Parameters for Flotation of Galena: Effect of Chain Length and Chain Structure of Xanthates on Flotation Recovery. ACS Omega 2019, 4, 1516–1524. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, X.; Huang, K.; Wang, S.; Cao, Z.; Zhong, H. Synthesis, flotation performance and adsorption mechanism of 3-(ethylamino)-N-phenyl-3-thioxopropanamide onto galena/sphalerite surfaces. J. Ind. Eng. Chem. 2019, 77, 416–425. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. A self-assembly mixed collector system and the mechanism for the flotation separation of spodumene from feldspar and quartz. Miner. Eng. 2021, 171, 107082. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S.; Chakraborty, A.; Maiti, D.K. In(OTf)3 catalysed simple one-pot synthesis of α-amino phosphonates. J. Mol. Catal. A Chem. 2004, 210, 53–57. [Google Scholar] [CrossRef]
- Duan, H.; Huang, X.; Cao, X.; Cao, Z.; Zhong, H.; Zeng, J.; Zhou, H.; Xue, J.; Liu, Y. Investigating the flotation performance and interfacial adsorption mechanism of N-benzoyl-N′,N′-diethyl thiourea on chalcopyrite and pyrite. Miner. Eng. 2021, 172, 107178. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Liu, G.; Zhong, H.; Wang, Y.; Zeng, H. Hetero-difunctional reagent with superior flotation performance to chalcopyrite and the associated surface interaction mechanism. Langmuir 2019, 35, 4353–4363. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xiong, W.; Zhu, Y.; Zhang, X.; Zheng, Y. Depression behaviors of N-thiourea-maleamic acid and its adsorption mechanism on galena in Mo-Pb flotation separation. Int. J. Min. Sci. Technol. 2022, 32, 181–189. [Google Scholar] [CrossRef]
- Sung, W.; Avazbaeva, Z.; Kim, D. Salt Promotes Protonation of Amine Groups at Air/Water Interface. J. Phys. Chem. Lett. 2017, 8, 3601–3606. [Google Scholar] [CrossRef] [PubMed]
- Roonasi, P.; Yang, X.; Holmgren, A. Competition between sodium oleate and sodium silicate for a silicate/oleate modified magnetite surface studied by in situ ATR-FTIR spectroscopy. J. Colloid Interface Sci. 2010, 343, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, S.; Minato, T. Frontier orbital theory. Chem. Educ. 1992, 40, 450–454. [Google Scholar]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Zhou, Z.; Parr, R.G. Activation hardness: New index for describing the orientation of electrophilic aromatic substitution. J. Am. Chem. Soc. 1990, 112, 5720–5724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).