Abstract
Coal fly ash (CFA) is a highly versatile raw material that has the potential to yield multiple value-added products, including cenospheres, zeolites, carbon nanotubes, and fertiliser substrates. Despite its versatility, a majority of these components are often overlooked, and CFA is primarily used for construction. Conventional processing methods of CFA are known to pose significant environmental challenges, including the leaching of hazardous materials, emission of toxic gases, and the high energy consumption needed to extract the value-added components. Herein, we explore the potential of biometallurgical approaches as an eco-friendly alternative to conventional processing methods for the comprehensive utilisation of CFA. Our focus is on the application of different microorganisms to CFA, the domestication of microorganisms, preprocessing of CFA to facilitate effective biometallurgical processes, the use of bioreactors, and synthesis of nano silica particles. We also propose a novel method for extracting the value-added components from CFA using a preprocessing technique (i.e., washing cycle), combined with multiple interactions with biometallurgical processes. Adopting this approach, we not only enhance environmental stewardship but also improve the circular economic aspects of multi-component utilisation, while providing valuable insights for the development of sustainable techniques for utilising CFA.
1. Introduction
Coal remains an attractive source of power, particularly in developing countries and those with high electricity demand, such as India and China, owing to its economic benefits and operational convenience. Despite the environmental and social stresses associated with coal combustion, the ever-increasing demand for energy has led to a 1.2% increase in coal usage for power generation during the global economic and political crisis in 2021, resulting in all-time high coal production in 2022. Furthermore, high gas prices and an increase in nuclear power generation suggest that no downfall in coal demand is imminent [1]. By 2025, renewable energy resources will fulfil only 90% of the future energy demand, which suggests the dominance of coal power in the near future [1]. Coal combustion products (CCPs) (along with the generation of CO) are the main concerns of thermal power plants operating by burning coal. CCPs include bottom ash, fly ash, boiler slag, fluidised bed combustion ash, and other solid fine particles.
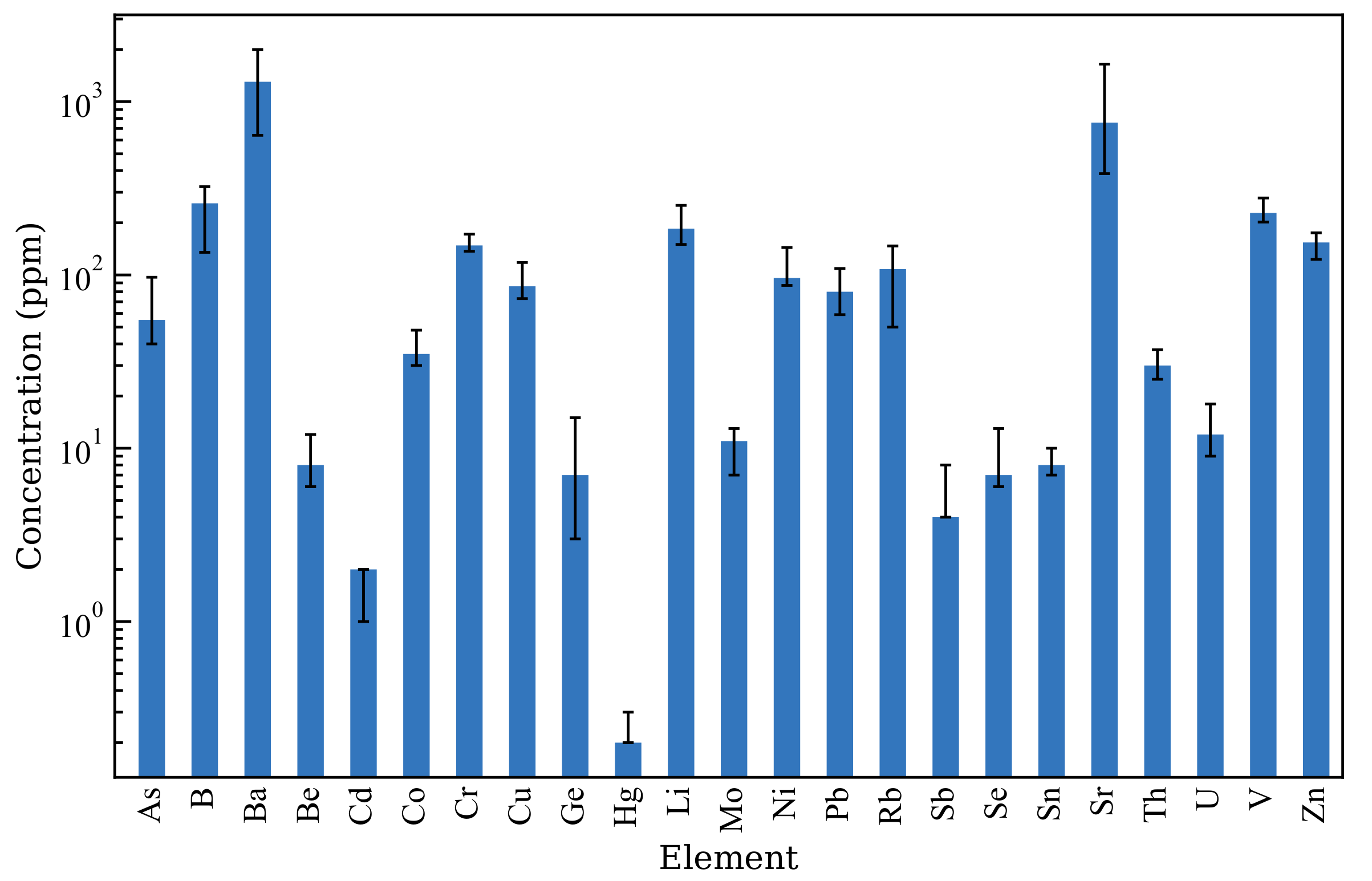
Among the types of ash generated, coal fly ash (CFA) constitutes 65%–90% of the total ash volume, with an annual generation of over 1 billion metric tonnes [2]. A total of 40% of the CFA produced worldwide is not utilised to end up in landfills, disposal ponds, and elsewhere, leading to environmental, social, and economic concerns [3,4]. The properties of CFA are significantly influenced by the properties of the parent coal and combustion conditions. Typically, CFA is a fine spherical material primarily composed of SiO and AlO, containing both crystalline and amorphous phases, with over 50% of the total composition being amorphous. The amorphous phase is more reactive to acids and alkaline solutions, whereas the crystalline phase requires high-temperature and high-pressure conditions [5,6]. Table 1 provides a description of the major chemical compositions of CFA worldwide, whereas Figure 1 describes the distribution of trace element concentrations in CFA according to Moreno et al. [7]. The variations in the chemical composition is influenced by the type of parent coal and boiler conditions at the thermal power plant [8]. The pH of CFA varies from 1.2 to 12.5, which is largely attributed to the Ca/S molar ratio of CFA. Additionally, other minor alkaline cations, such as Na, Mg, K, and Mn, also contribute to alkalinity [9,10,11]. Although the majority of CFA particles are spherical silica and alumina components, irregular silica and alumina particles, Ca crystals, and unburnt carbon are also present. CFA particles generally range from a few nanometres to 500 μm in diameter [12]. A summary of the physicochemical properties of the CFA is presented in Table 2. The diversity in the properties exhibited by CFA renders it a notably challenging material to characterise, as its properties can differ across regions and even within the same region.

Table 1.
Distribution of chemical composition of CFA by region. Adapted from Blissett and Rowson [13], Fomenko et al. [14], and du Toit et al. [15]. LOI—Loss on Ignition, USA—United States of America, S. Africa—South Africa, and ng—not given.
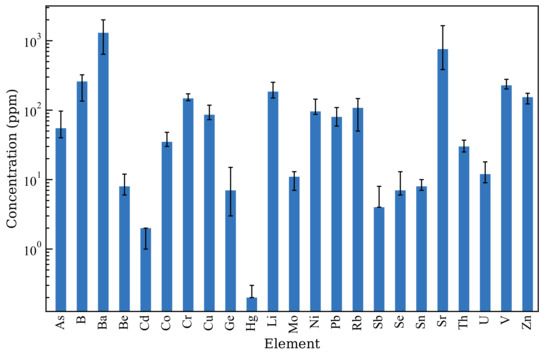
Figure 1.
Distribution of trace elements of European CFA samples. Adapted from Moreno et al. [7].

Table 2.
A brief summary of the chemical and physical properties of CFA. Adapted from Gollakota et al. [16].
The diverse properties of CFA offer numerous potential applications, such as construction materials [17,18,19], adsorbents [20,21,22], catalysts [23,24], ceramics [25,26,27], geopolymers [28,29,30], zeolites [31,32,33], aerogels [34,35], carbon nanotubes [36,37,38], soil stabilisers [39,40], and metal recovery [41,42]. Despite its widespread use in the construction industry, the economic value of CFA waste is not often fully realised.
The multi-component utilisation of CFA often involves high-energy separation processes such as calcination, high-concentration acid treatments, grinding, or milling, which arise from the heterogeneous nature of the CFA, which requires several continuous processes to effectively extract the value-added components, particularly second-generation components such as cenospheres [43,44,45], zeolites [13,46], carbon nanotubes [47,48], and fertiliser substrates [8,49].
CFA and Biometallurgy
The world is marching towards the concept of net zero for the emission of CO, which is presently acknowledged by research and industry. Fossil-fuel combustion, especially coal combustion, is a significant contributor to atmospheric CO emissions. However, achieving a net zero via the unsustainable combustion of fossil fuels is economically and politically unsustainable [50]. On the contrary, up to 17% of unburnt carbon (UC) aggregates into CFA because of the low NO burners that control the emissions of oxides of nitrogen [51,52,53]. As a result, achieving net zero emissions during the valorisation process of CFA is challenging owing to the presence of UC and the potential risk of emitting other greenhouse gases. Nevertheless, it is essential to optimise the valorisation process while adhering to net-zero and sustainable concepts. In light of this, biometallurgical processes, which involve microorganisms, are promising techniques with a higher chance of driving CFA valorisation towards the expected net-zero goal.
Biometallurgy is an eco-friendly process that involves the interaction between microorganisms and metal-bearing ores using biotechnology [54,55]. Biotechnology harnesses the natural weathering capabilities of microorganisms to develop commercially profitable metal extraction flowsheets by growing them alongside the metal-containing ores or wastes under suitable conditions. Bioleaching and biooxidation are two different mechanisms in biometallurgical processes. Bioleaching refers to the solubilisation of the target metal(s), whereas biooxidation refers to the pretreatment process of solubilising host minerals to recover the target metal(s) for subsequent processes [56]. Both processes use essentially the same principles and consortia of microorganisms for solubility. Biometallurgical studies typically use microorganisms belonging to bacteria, archaea, and fungi, because of their ability to grow under a wide range of environmental conditions.
While the application of microorganisms for processing low-grade sulphide ore has emerged as an alternative to conventional processes, the mainstream adoption of utilising microorganisms for non-sulphide minerals remains limited, although there have been studies on extracting elements from metal oxides such as laterite ores [57,58,59]. The capabilities of microorganisms under extreme and varying conditions, as discussed in detail by Dopson and Okibe [60], recent advances in synthetic biology, and the identification of various strains of microorganisms have provided researchers with a viable option to explore the possibility of using microorganisms for extensive biometallurgical processes [61,62]. Thus, this process is particularly attractive for the treatment of low-grade complex ores, such as CFA [55].
Biomining and bioremediation are two aspects of biometallurgy that are currently being highlighted by researchers, particularly in the treatment of CFA, which comprises hazardous materials in trace amounts. Biomining refers to the extraction of metals from metal sources, whereas bioremediation involves the removal or immobilisation of hazardous contaminants such as heavy metals and radioactive elements from sources [63].
Biomining of CFA involves the extraction of approximately 316 individual minerals and 188 mineral groups through bioleaching or biooxidation processes [64]. Bioleaching of CFA can be performed using one-step, two-step, or spent medium-step methods, depending on the specific requirements of the process [65]. The one-step process involves the simultaneous addition of both microorganisms and ore during the leaching process. In contrast, in the two-step process, microorganisms are added to the ore when biometabolite production begins [66]. The spent medium-step uses the leachants produced by the microorganisms in the absence of microorganisms for the bioleaching process. The one-step and two-step processes involve the direct bioleaching of microorganisms, whereas the spent medium step does not involve direct interaction and is therefore referred to as indirect bioleaching [65]. On the other hand, bioremediation of CFA is an essential component of its utilisation. CFA contains significant quantities of potentially toxic elements, such as Cd, Pb, Ni, Se, and Hg which can enter the food chain when contaminated with soil or groundwater [8]. Additionally, the presence of As in CFA and the formation of different chemical forms owing to their different oxidation states (i.e., −3, 0, +3, and +5) can be a significant threat to aquatic, terrestrial, and human life [67]. The advantage of bioremediation is that it solubilises hazardous arsenic components and retains them in solution, unlike pyrometallurgical processes which produce various flue gases (for example, AsO and AsO), which are harmful to livelihoods [68,69].
Biometallurgical processes have advantages, such as operation at low temperatures and atmospheric pressure (in general), low energy consumption, and a small carbon footprint compared to other metallurgical processes. On the other hand, prolonged operational times (in the order of months to years) to obtain economically valuable target products and to maintain the longevity and robustness of biological systems are among the primary concerns of biometallurgical processes [56]. Table 3 provides a detailed account of the major differences between the pyrometallurgy, conventional hydrometallurgy, and biometallurgical processes.

Table 3.
Advantages and challenges in major metallurgical processes compared to biometallurgy. After Mossali et al. [70].
Although biotechnology has made remarkable advances in various fields, including medicine, pharmaceuticals, agriculture, and biofuels, its application to coal [71,72,73] and coal-derived products has been relatively less explored. CFA waste, in particular, presents significant challenges owing to its heterogeneous chemical and physical nature, which makes it difficult to propose a robust workflow. Unlike electronic wastes such as printed circuit boards [74], which have a relatively more explicit distribution of elements, the natural distribution of elements in CFA is not always evident. This review delves into the historical evolution of biometallurgical methods for extracting elements from CFA, and sheds light on the challenges and possibilities within this field. By exploring the potential expansion of biometallurgy in the CFA domain, this paper presents a promising starting point for biometallurgy-incorporated multi-component utilisation of CFA.
2. Diversity of Microorganisms in CFA Biometallurgical Processes
The microorganisms utilised in biometallurgical processes for CFA can be categorised into two primary groups based on the processes they facilitate. The first group comprises those that aid in bioleaching, whereas the second group comprises those that carry out bioaccumulation or biosorption. Bioleaching is facilitated by chemolithoautotrophic bacteria that can survive in acidic environments with low pH and utilise inorganic materials, such as iron and sulphur, as energy sources, as well as CO as the carbon source. These microorganisms are commonly used to leach sulphidic minerals and ores. In contrast, heterotrophs that use organic carbon as the carbon source are typically employed for non-sulphide minerals, such as CFA [66,75]. However, various microorganisms and experimental parameters have been tested to evaluate the feasibility of using biometallurgical processes to recover value-added components from CFA.
2.1. Fungus
The first study to investigate the bioleaching of CFA used an Aspergillus niger (A. niger) strain (ATCC 9142), which produces citric acid as a leaching agent [76]. This strain converts sucrose into equal amounts of citric and oxalic acid to produce the necessary organic acids for leaching [77]. Studies by Singer et al. [76] and Torma and Singh [77] focused on Al extraction, and Jadhav and Hocheng [78] achieved almost complete removal of metals such as Mn, Mg, Cu, Zn, Na, Fe, Ca, B, K, Al, Se, Co, V, Ti, and Cd, except for Cr (93% ± 1.18), Ni (83% ± 0.32), As (78% ± 0.52), and Pb (70% ± 0.20), within 4 h under optimal conditions using the same fungal strain. However, A. niger strains cannot survive high concentrations of CFA, making scaling up for industrial purposes challenging [79]. Therefore, two other fungal strains, Fusarium oxysporum and Penicillium glabrum, were employed by Tacstan [79] and they were found to perform optimally at pH 5.0 and pH 6.0, respectively. F. oxysporum yielded high recoveries for Mo (100%), S (64.36%), Ni (50%), and Cu (33.33%), while P. glabrum yielded recoveries for Mo (100%), S (57.43%), Ni (25%), Si (24.66%), V (12.5%), Ti (5%), and Sr (3.2%). In addition to citric and oxalic acids, Cladosporium cladosporioides, a heterotrophic fungus, was found to bioleach V and Ni with 65.4% and 74.6% efficiencies, respectively, using malic acid production [80]. The two-step process of heterotrophic fungal bioleaching was found to be more effective than the one-step process because it allows fungal spores to germinate into mycelia and extract elements from CFA [79].
However, major concerns regarding heterotrophic fungal bioleaching are that it is typically performed in a neutral pH range, making contamination by other microorganisms likely. Moreover, bioleaching through fungal mycelia can lead to the adsorption or encapsulation of CFA particles, making subsequent processes such as zeolite synthesis impossible [75].
2.2. Bacteria
Although chemolithoautotrophic bacteria are generally not considered effective for non-sulphidic ores, they have been the subject of many studies investigating CFA bioleaching. These microorganisms perform bioleaching through both contact and non-contact mechanisms, whereby they oxidise ferrous (Fe) to ferric (Fe) and reduce sulphur (S, SO and HS or polysulphides) to sulphuric acid (HSO) [66]. Unlike heterotrophic fungi, a source of energy, such as pyrite, is essential to support the growth of chemolithoautotrophic bacteria. For instance, meso-acidophilic A. ferrooxidans produces H ions through pyrite hydrolysis/oxidation, which are then consumed by CFA to further contribute during dissolution. In contrast, chemolithotrophic heterotrophs, such as Typha latifolia, which use organic carbon as a carbon source, require additional carbon during the bioleaching process, which is supplemented to the bacterial strain during inoculation [81]. However, in one-step or spend medium-step processes, the additional carbon requirement is fulfilled by the presence of UC in the CFA.
The patent for the bioleaching method for CFA was acquired by Fass et al. [82] in 1994 using Thiobacillus thiooxidans strains adapted to a seawater-based culture medium containing 50% CFA to extract Al, Ti, and Co. The use of chemolithoautotrophic bacteria in CFA bioleaching is heavily affected by the alkaline nature of CFA particles, which inhibits the growth of microorganisms. However, the domestication process can enhance their growth; a detailed account of the domestication process can be found in Section 3. In the one-step process, the bacteria produce multiple acids and extracellular polysaccharides during the multiplication and bioleaching phases, which are adsorbed onto the surface of the CFA particles, especially for microorganisms with flagella, such as Bacillus amyloliquefaciens [83]. The bacteria then secrete acidic products such as citric, lactic, succinic, and acetic acids, which dissolve the surface of the CFA particles and break down the bonds of the amorphous components.
The bioleaching process using Thiobacillus thiooxidans occurs indirectly through the production of sulphuric acid, which is the primary cellular metabolite, with other metabolites having relatively insignificant effects on bioleaching. In a comparative study, stock sulphuric acid and T. thiooxidans resulted in similar extraction rates and levels of major metal components (i.e., Al and Fe) from CFA particles under the same leaching conditions (0.8 ≤ pH ≤ 5 and the same rate of pH decrease) [84]. However, after notable preprocessing procedures, such as acid treatment and washing with deionised water, Acidithiobacillus ferrooxidans, a bacterium that generates Fe(SO), performed 5–22 times higher in leaching experiments than the control suspension [85]. Unlike the use of sulphuric acid in a two-step process, this study used a spent medium step which enhanced the efficacy of the process by incorporating microbial cultures into the leaching environment to sustain the metabolic processes necessary for an effective leaching.
Bioleaching processes using chemolithotrophic bacteria are often performed in a two-step process owing to the alkaline nature of CFA. However, chemolithotrophic bacteria were able to tolerate a higher pulp density of CFA (40% (w/v)), and effective leaching was performed at 20% (w/v) during the study by Kermer et al. [86], showing its ability to perform in the one-step process as well. Furthermore, an increase in temperature led to increased bioleaching kinetics; however, the final yields of the elements were the same except for Fe. Reductive bioleaching under anaerobic conditions increased the recovery of Fe, Mg, Zn, Al, Ca, Si, and several REEs compared with aerobic conditions. The extraction of most of the considered elements reached values between 30 and 60%, with Cr, Mg, Mn, and Zn reaching 60%–70%. Bioleaching using gluconic acid-producing Acidomonas methanolica increased the mobilisation of several mineral ions, such as Al, Ca, Fe, and Mg, compared with stock HCl experiments. The extraction of element ions ranged from 30 to 60% for Ce, Sr, Ti, V, and Zr, and 50%–80% extraction for Ca.
The potential use of CFA in nutrient restoration, along with nitrogen-fixing blue-green algae, for agricultural purposes in an eco-friendly manner, was enabled by using cyanobacterial strains, including Nostoc muscorum, Anabaena variabilis, Tolypothrix tenuis, and Aulosira fertilissimia, which are commonly used as biofertilisers [87]. Under the optimal conditions, Nostoc muscorum accumulated 3.65 mg/g of Cr and 2.12 mg/g of Pb, while Anabaena variabilis yielded 0.313 mg/g of Cu, 2.01 mg/g of Pb, 1.21 mg/g of Cr, and 0.697 mg/g of Zn.
Assuming that CFA shrinks uniformly over time, researchers used a shrinking core model to evaluate the rate control factor of bioleaching [88]. The findings indicated that for all the metals considered in this study, the rate-controlling mechanism was diffusion through the ash layer. This observation may be due to the interference of biofilms formed by Pseudomonas species and organic complexing agents during bioleaching. As biofilm formation increases over time, the extraction of trace metals from the CFA surface diminishes and the penetration of organic acids produced by the bacteria decreases. A subsequent study by Rezaei et al. [89] reinforced that the rate-limiting step is the diffusion of reagents to the surface of the particles.
In addition to the bioleaching process, a bio-accumulation process was also investigated to recover the Ti-rich fraction from the leachate produced by the acid treatment of CFA using a specifically cultivated bacterium, Rhodococcus GIN-1, on magnetite particles to form a magnetite biosorbent, as described by Shabtai and Mukmenev [90].
The resulting leachate was subjected to stepwise precipitation (similar to Fass et al. [82]) using pH changes, and the precipitates at a pH of 2.05 were coupled to the bacterium strain. Three cycles of biomagnetic separation were performed, resulting in 70-fold enrichment of Ti from the initial precipitate. The aggregation of TiO around the bacterial cells during precipitation suggests a possible nucleation effect on the bacteria. The other suspensions were collected by adjusting the pH of the NHOH solution. This study effectively employed bacterial translocation from magnetite to Ti-containing materials, and almost 99% of the magnetite was recovered after operation and could be reused. This study offers a new perspective on the biometallurgical application of the bioaccumulation process for extracting industrially valuable Ti, which provides high-purity Ti in an environmentally friendly manner.
2.3. Yeast
The potential for utilising a single strain of Y. lipolytica for both bioleaching and bioaccumulation was investigated by Banker et al. [91]. This study revealed that Y. lipolytica can accumulate heavy metals through its cell wall, membrane, and cytoplasm, as well as perform bioleaching through the secretion of citric acid and two extracellular proteins. Notably, this approach achieved a Cu extraction rate of 59.41%, which was higher than that obtained using synthetic citric acid.
In another study, Park and Liang [92] explored the use of three microbial strains, namely, Candida bombicola, Phanerochaete chrysosporium, and Cryptococcus curvatus, for leaching REEs. Only C. bombicola was selected, owing to its superior mineral leaching efficiency. C. bombicola has the ability to thrive in low pH conditions and demonstrated the best performance among all the strains tested.
2.4. Using Mixed Culture of Microorganisms for the Bioleaching
The use of mixed culture of microorganisms in biotechnological processes has become an effective means to encounter dynamic changes in the process environments due to the interaction between microorganisms and non-sulphidic ores [93]. It is crucial to understand the role of each microorganism under bioleaching conditions to enhance the efficiency of the bioleaching process [94]. While considering the alkaline nature of CFA, it can hinder the performance of sulphur-oxidising bacteria, which are one of the commonly used microorganisms in bioleaching processes. As such, this may lead to a decreased production of sulphuric acid, which subsequently can affect the performance of the bioleaching process. To overcome this, pre-processing techniques can be used to reduce the alkaline nature of CFA, as discussed in Section 4. Alternatively, biosurfactant-producing microorganisms (i.e., microorganisms that produce surface-active biomolecules) such as Pseudomonas, Bacillus, Candida, Rhodococcus, and Corynebacterium can be used to facilitate the bioleaching process at higher pH levels owing to their better environmental compatibility and specific activity under extreme conditions [95,96]. This behaviour enables continuous bioleaching over a wide range of pH values and yields higher metal recovery during the process [97].
Another effective combination of microorganisms for bioleaching, as suggested by Mahmoud et al. [93], includes chemoautotrophic bacteria and heterotrophic microorganisms. Chemoautotrophs have the ability to decrease the pH of the medium or solution by oxidising sulphide minerals. The resulting biogenic acid, specifically HSO, can then interact with the CFA to counterbalance its alkalinity. Consequently, the CFA sample becomes more conducive to the activity of heterotrophic bacteria, which also produce acidity through the generation of organic acids. This synergistic effect can enhance the efficiency of the leaching process.
3. Domestication Process of Microorganisms
A key element in the domestication of microorganisms for industrial processes is the purposeful selection and breeding of microorganisms from diverse sources such as waste dumps, soil, plants, and waterways [98]. The collected microorganisms are then grown in the presence of increasing concentrations of materials to be leached; this process is known as domestication [66]. Despite the time-consuming nature of the domestication process, which can take several months to years, it has been widely adopted in most biometallurgical applications. A successful transition to industrial processes demands prompt adaptation of microorganisms, as it is a crucial parameter that governs the rate and feasibility of bioprocesses. Previous studies on sulphide mineral bioleaching have demonstrated that a gradual increase in the concentration of metal ions during the domestication process enhances the tolerance of microorganisms, leading to an increase in the rate and degree of bioleaching [99]. In addition to the metal ion concentration, domestication also improves the adaptability of microorganisms to pH variations, temperature fluctuations, and tolerance to toxic materials. The goal of domestication is to reduce or eliminate the lag phase during the bioleaching process, resulting in a pronounced performance of adapted microorganisms compared to their unadapted counterparts [100].
The process of domesticating microorganisms for use in biometallurgy of CFA is significantly affected by the characteristics of CFA. The high-pH environment created by CFA during the bioleaching process results in an alkaline solution that can hinder the growth of acidophilic bacteria. Additionally, easily dissolvable ions such as Ca, Mg, and K, which are found on the surface of CFA particles, dissolve into the solution and further increase its pH. Furthermore, the presence in the CFA of toxic elements such As, Cd, Cr, Cu, Hg, Pb, V, and Zn, inhibits the proliferation of microorganisms. Therefore, the adaptability of microorganisms prior to their use in CFA biometallurgical processes is essential for achieving notable outcomes.
Domestication has been shown to enhance the production of microbial secondary metabolites in both one-step and two-step processes. In particular, the domestication of microorganisms plays a crucial role in the one-step process of bioleaching because they directly interact with CFA. However, it is necessary to adapt the microorganisms in the bioaccumulation or biosorption processes, as they will also directly interact with CFA. Seidel and Zimmels [101] reported the inhibition of Thiobacillus thiooxidans growth due to an increase in pH, which resulted in a lag phase of approximately 5 to 6 days in the bioleaching process. Similar results were observed recently by Su et al. [100]. The study found that Acidithiobacillus thiooxidans required acclimatisation, particularly in alkaline-treated CFA, because of the pH increase in the first five days. The acclimatisation process delayed the stabilisation of Acidithiobacillus thiooxidans by three days in the alkali-treated CFA. The results of domestication on the adaptability of the microorganisms are evident by their ability to perform bioleaching at a relatively high concentration of 20% CFA slurry medium.
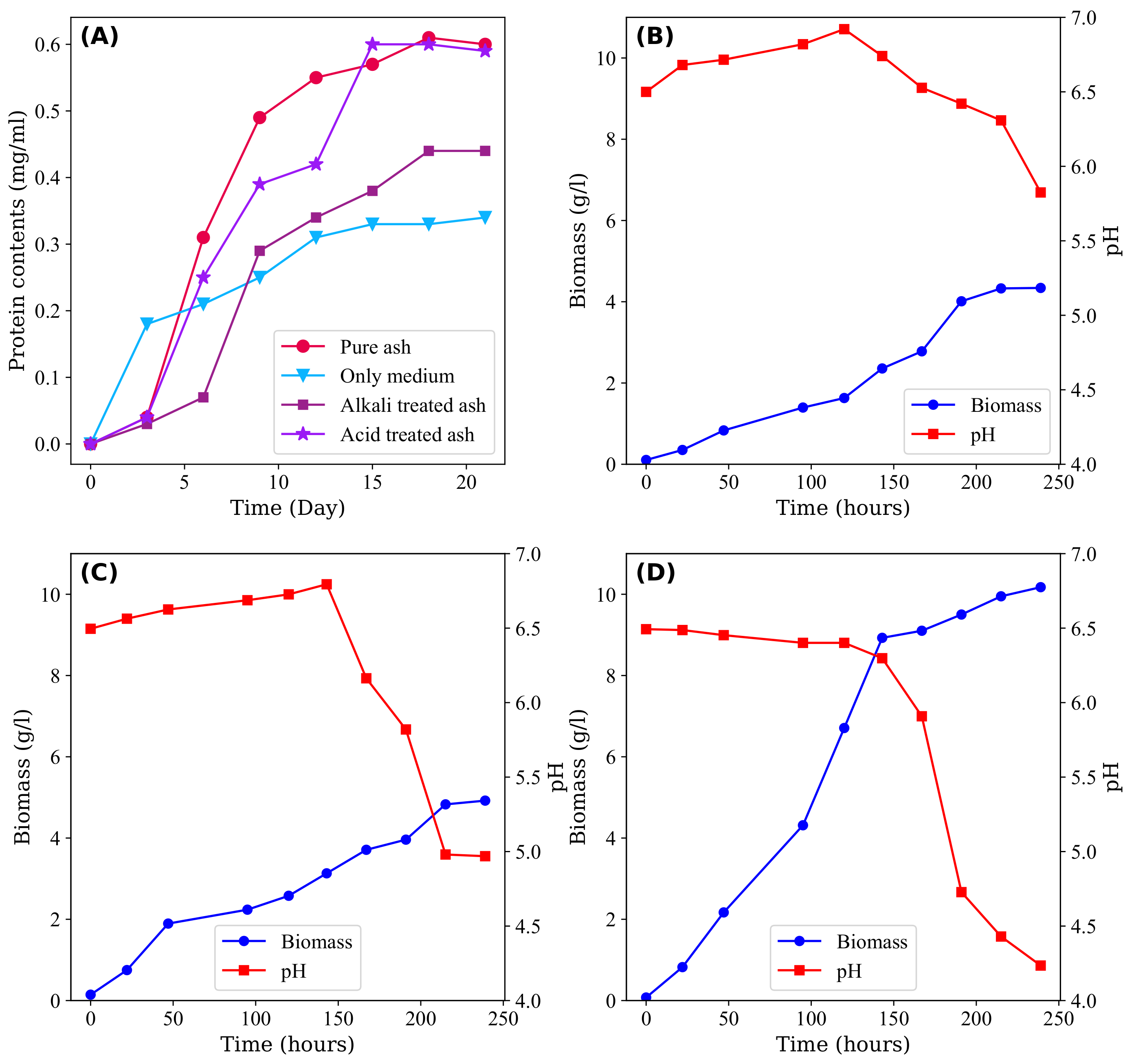
Figure 2A illustrates the protein levels of Acidithiobacillus thiooxidans under various conditions, including pure, alkali-treated, and acid-treated CFA, as well as a medium without CFA. The initial growth of microorganisms is impeded in the presence of CFA. However, after a 5-day acclimation period, bacterial growth increased and surpassed that of the solution without CFA. At the plateau stage, the amounts of bacterial protein reached 0.615, 0.445, and 0.608 mg/mL, respectively, which were greater than that of the solution without CFA. This behaviour can be largely attributed to the presence of S in CFA, which serves as an energy source for the growth of microorganisms [100].
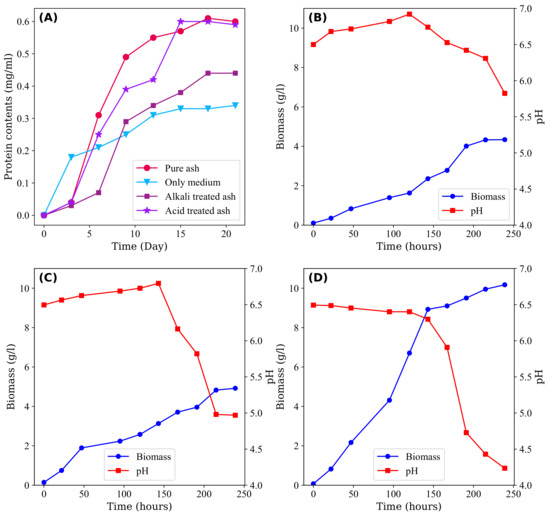
Figure 2.
(A) Protein content of Acidithiobacillus thiooxidans in various conditions: pure ash, alkali-treated ash, acid-treated ash, and culture medium after the domestication process [100]. (B) Changes in pH and biomass concentration during the growth of Bacillus barbaricus during the absence of CFA, (C) presence of CFA, and (D) presence of water washed CFA [102].
4. Preprocessing of CFA for the Biometallurgical Processes
The primary purpose of preprocessing is to facilitate successful interactions between CFA particles and microorganisms, enhance the growth of microorganisms, and increase the kinetics of the biometallurgical processes. Various pre-processing methods have been reported in the literature, including size reduction/separation, thermal treatment, alkaline/acid treatment, and washing with water, which can be used alone or in combination to achieve optimal results.
The effect of preprocessing on the biometallurgical process can be divided into two parts: leaching of the surface rim and grooving of the CFA core. The surface rim encompasses easily leachable alkaline components that impede the initial growth of microorganisms (see Section 3) [100,101]. These alkaline oxides dissolve in the medium during the bioleaching process and increase the pH of the system, subsequently controlling the growth of the microorganisms. Furthermore, dissolving the surface rim of CFA can enhance the leaching process by increasing the surface area of CFA particles and subsequently the interaction between microorganisms and CFA particles, facilitating the direct involvement of microorganisms with the core of the CFA [103].
4.1. Washing with Water
Washing with deionised water is an effective technique for removing easily dissolvable ions from the CFA surface rim. Studies have shown that washing with water can remove a significant amount of alkaline ions from the CFA surfaces. Jadhav and Hocheng [78] used 100 mL of distilled water and 1 g of CFA sample at 30 C for 24 h to remove 100% Na, 47% (±0.45) B, 38.07% (±0.12) Ca, 29.89% (±0.78) Mg, and 11.8% (±0.05) K through bioleaching.
The removal of alkaline components enhanced the production of acids by Bacillus barbaricus during the bioleaching process performed by Sen et al. [102]. Under optimal conditions (i.e., pH 6, temperature 37 C, 1% CFA, 240 mesh particle size, and 40% inoculum concentration), citric, oxalic, and silicic acid production for untreated CFA were 22.57%, 11.29%, and 9.56%, respectively, and those treated with water (CFA) were 28.54%, 16.89%, and 13.21%, respectively. These results show that washed CFA yielded better outcomes than unwashed CFA, allowing for higher acid production than untreated CFA. The alumina concentration in the washed CFA increased from 25.45% to 34.72% after 60 days, and the residual silica content was reduced from 62.14% to 40.71%. This result demonstrates the efficiency of washing CFA with water before bioleaching. The biometallurgical approach for extracting silica from CFA has limitations compared to the volumetric method, which involves acid treatment followed by alkali digestion under pressure. The volumetric method requires only 4 h to leach out 37.09% of silica, whereas the biometallurgical approach requires 60 days to leach out only 21.43% of silica [104]. Additionally, the results of bioleaching time reported by Jadhav and Hocheng [78] and Sen et al. [102] differed, which may be due to the quantity of CFA samples used for the study, as Jadhav and Hocheng [78] used 1 g, whereas Sen et al. [102] used 10 g. In addition to the initial CFA mass, factors such as pH, temperature, additional energy sources, and other parameters may also have contributed to the differences in these observations.
Figure 2 depicts the pH and biomass (total weight of microorganisms per unit volume of solution) trends under three distinct conditions: (B) the absence of CFA, (C) untreated CFA, and (D) CFA treated with water. Notably, at the end of the 250th hour, there was an observable trend in the amount of biomass (g/L of solution), where the highest amount of biomass was found in the water-treated CFA, followed by untreated CFA and the absence of CFA. This outcome is surprising given that the presence of CFA creates harsh conditions for microorganism growth due to the confinement and release of toxic materials, which contests the common hypothesis. One plausible explanation for the observed enhancement is the presence of sulphur in the form of SO, which may serve as a direct energy source for microbial growth. Furthermore, trace elements enriched in CFA may provide additional growth factors, as suggested by Su et al. [100]. Moreover, Sen et al. [102] found that the kinetics of treated CFA became faster than that of untreated CFA, as evidenced by the sigmoidal behaviour of the biomass in washed CFA compared with untreated CFA.
4.2. Grinding and Sieving
The exposed surface area of the particles heavily influences the probable interactions between microorganisms and CFA particles. This phenomenon is determined by the particle size and shape. Smaller particles have a higher specific surface area than larger particles, and are therefore more likely to interact with microorganisms and increase the efficiency of the bioprocess. The particle size distribution of CFA varies widely, ranging from a few nanometres to hundreds of micrometres [13]. The experimental results from Jekic et al. [85] showed that even with acid treatment, the extraction of elements from the CFA was not significant. This observation was mainly due to the larger particle size of the CFA in the experiment, with over 46% measuring 100 m or more. Therefore, most elements are encapsulated inside the core of the CFA. In light of this, using smaller particles in biometallurgical processes related to CFA is desirable [85,105].
To this end, grinding is often a go-to-process employed to obtain suitable particle sizes to effectively process the CFA particles. In some studies, researchers have collected a particular fraction of CFA for further analysis, which can be isolated through sieving. Different sizes of CFA have been preferred for biometallurgical processes by various researchers. For instance, Torma and Singh [77] ground their particles to a size of less than 45 m, whereas Park and Liang [92] used particles that passed through a 200 sieve (i.e., 74 m) to extract REEs. The selection of finer particles for REEs extraction is important because REEs commonly aggregate as fine particles in the CFA matrix. In another study, Sen et al. [102] found that the optimal particle size for the leaching process was a 240 sieve, that is, less than 53 m. Grinding before sieving has little effect on REE extraction but is explicitly beneficial for major elements such as Si and Al.
4.3. Thermal Treatment
Thermal treatment is a widely recognised technique for facilitating particle–microorganism interactions. Three types of thermal treatments associated with CFA preprocessing have been identified before biometallurgical processes: heating CFA particles alone, and heating them with acid or alkali components to intensify the reaction kinetics. In this section, we focus on the effects of heating CFA particles alone, with the other two treatments discussed in subsequent sections.
Thermal treatment of CFA is often performed at very high temperatures because it has already been exposed to temperatures ranging from 1000 C to 1600 C during coal combustion in thermal power plants [106,107,108]. Thermal pretreatment serves two primary purposes before bioprocesses: the recrystallisation of amorphous components of the CFA increases the efficiency of the bioleaching, and the transformation of low soluble components to readily soluble components [86]. Kermer et al. [86] examined CFA samples heated at 900, 1000, and 1057 C for 1, 4, and 9 h to investigate the mineralogical changes induced by thermal treatment. The results showed a significant decrease in the amorphous fraction of CFA, which was attributed to the partial recrystallisation of major mineral components such as quartz (SiO) (melting point of 1650 C). However, no significant changes were observed in the haematite (FeO), periclase (MgO), or rutile (TiO) phases. Complex components including gypsum (CaSO·2HO) and ettringite (3CaO·AlO·3CaSO·32HO) were broken down into simpler components, making their elements available for leaching. A possible mechanism for the dissociation of ettringite is presented in Equations (1)–(3): Notably, the study also observed the formation of gehlenite (CaAl[AlSiO]) complexes at approximately 800 C, which, upon interaction with SiO, resulted in the formation of anorthite (CaAlSiO) and wollastonite (CaSiO), as shown in Equations (4) and (5).
However, it is essential to acknowledge that thermal treatment at high temperatures for extended periods of time requires substantial energy and poses significant sustainability concerns. Despite the shown promise for subsequent bioleaching of heat-treated CFA operating at higher temperatures, it must be carefully monitored to mitigate adverse environmental impacts such as the generation of flue gases and high energy utilisation.
4.4. Acid Treatment
The primary objective of treating CFA with acid is to modify the crystal lattice and make the components present in the core of the CFA available for biometallurgical processes. Further, in the place of water washing processes, acid treatment can be used to remove the alkali components. However, prior to treatment with sulphuric acid, a comprehensive assessment of the presence of calcium is necessary, as it can lead to the formation of calcium sulphate on the cells of microorganisms and energy sources, such as sulphur particles. This precipitation can hamper cell growth, resulting in self-inhibition and hindrances to mass transfer [84]. Therefore, it is recommended to use nitric acid or hydrochloric acid for acid treatment of unwashed CFA. Seidel et al. [84] found that the growth of microorganisms in acid-treated CFA (with HCl) is faster than that in untreated CFA. However, the effectiveness of acid treatment may decrease with a larger particle size, thereby lowering the expected metal extraction.
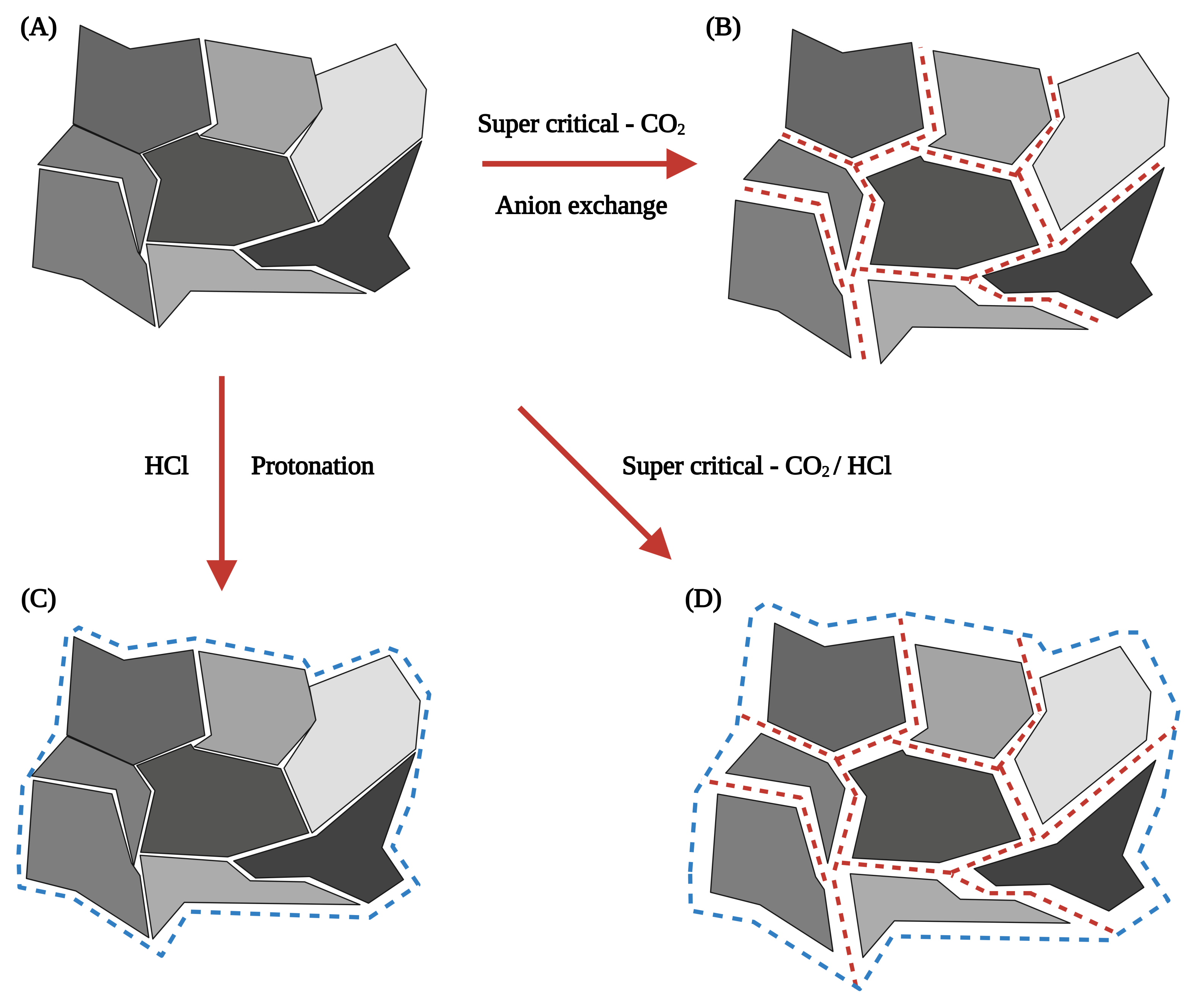
According to Kermer et al. [86], treating digested CFA with HCl (31%, 100 C, for 4 h) improved the leaching efficiency of Ca, Fe, Al, and Mg. The residue left after the HCl treatment comprised amorphous materials, quartz, and smaller amounts of diopside (CaMgSiO), mullite (AlSiO), and rutile. Additionally, supercritical-CO digestion, by redirecting the emitted CO from smokestacks to CFA, can cause anion exchange on the CFA surface. The strong diffusion and permeability properties of supercritical-CO can cause near-surface conversion of the crystal lattice of CFA, resulting in tension within the structure and encouraging proton attack. This process may play a role in achieving net-zero CO emissions using emitted CO for CFA treatment [109]. Further, the addition of acid enhances the process, as shown in Figure 3. However, acid treatment results in carbonification of CFA particles, which is not conducive to the extraction of REEs [100]. Preprocessing CFA with high-concentration acids for preliminary leaching is not recommended, as it is intended only to prepare CFA for subsequent bioleaching processes.
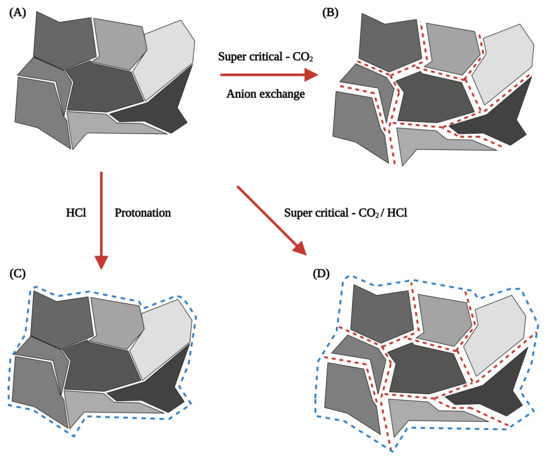
Figure 3.
Schematic diagram of individual effects of super critical-CO (A,B) and HCl (A–C), as well as their combined effect (A–D) on the mineral components of CFA [86].
4.5. Alkaline Treatment
The alkaline treatment of CFA has a notable effect on the structure of the particles. The core of the CFA, which contains Al, Si, and critical elements, is enclosed by strong and chemically stable Si-O-Si and Si-O-Al bonds. However, the addition of alkaline components, such as -OH bonds, to the CFA matrix causes a decrease in the degree of polymerisation of these stable bonds and subsequently increases the number of broken and unsaturated bonds [110]. This change in the surface charge distribution of CFA particles leads to the deterioration of the stable structure of the CFA core, enabling microorganisms to interact with CFA.
Additionally, alkaline treatment reduces the Si and Al contents in the CFA matrix (due to the desilication and dissolution of mullite), resulting in a highly porous structure, which is an excellent opportunity for the diffusion of other elements through bioleaching [100,111]. This effect is further demonstrated by the observed decrease in the particle size () of pure, acid-treated, and alkali-treated CFA particles, which were found to be 36.21, 24.62, and 16.76 m, respectively [100]. The reduction in particle size and the increase in irregular particles due to the grooves created by the alkaline treatment enhanced the adsorption kinetics and diffusion mechanism of CFA. Comparative experiments presented in Su et al. [100] revealed that hydrothermal alkaline treatment yielded higher concentrations of critical elements than hydrothermal acid treatment. Furthermore, the experiments related to treating the CFA with a NaOH solution clearly demonstrate the process of core deterioration in the CFA, thereby revealing its inner components for additional leaching [111]. However, there is a possibility of precipitation of desilication products, specifically Na[AlSiO]·NaX, where X represents different inorganic anions. This precipitation could potentially impact the continuous leaching process. To overcome this challenge, it is recommended to employ a high concentration of alkaline solution or follow it with subsequent acid leaching [111,112]. This process can be described by Equations (6)–(8). A commonly observed trend in the application of alkaline treatments is their use for extracting trace elements during bioleaching processes.
Seddiek et al. [80] conducted a stepwise roasting process of CFA to concentrate V and increase its accessibility for the bioleaching process by fungi called Cladosporium cladosporioides in a two-step process. The CFA was preheated to 500 C for 3 h and 90% wt. NaCO at 850 C and 20% wt. NaCO was then added at 1000 C for 2 h. An increase in temperature without NaCO inhibits the metal recovery, even though, with the removal of carbon and volatile components, the fungal growth is increased, whereas on the other hand, the dissolving of heavy metals into the solution will be inhibited by the toxic metals. On the other hand, alkaline treatment increases the efficiency of V bioleaching at 850 C using 90% wt. NaCO, whereas Ni was extracted from the raw CFA without any treatment. A plausible reason for this increased efficiency in V but not in Ni is that the addition of NaCO during heating leads to the formation of water-soluble sodium metavanadate (NaVO), which subsequently enhances V leaching.
Torma and Singh [77] calcined CFA with a CaO/SiO molar ratio of 2. The as-received CFA was resistant to 1- and 2-step leaching processes, and yielded only 5%–8% of Al. However, calcined CFA produced an extraction yield of 93.5 %. Fan et al. [113] roasted CFA using NaCO at 850 C for 2 h. This process transforms mullite and sillimanite (AlSiO) into a more extractable nepheline via the reactions presented in Equations (9)–(11). Figure 4 illustrates the morphological conversions of CFA with varying CFA/NaCO mass ratios. Increasing the amount of NaCO causes the rigid spherical structure of CFA to break down, resulting in irregular shapes. An increase in the amount of NaCO destroys the rigid spherical physical body of the CFA particles and makes them irregular. This modification significantly enhances the surface area of the CFA particles. Nevertheless, excessive NaCO can cause CFA particles to aggregate once again, resulting in large and ineffective irregular particles. Bioleaching experiments were then performed using meso-acidophilic Acidithiobacillus ferrooxidans on activated CFA samples as a one-step process. Pyrite was added as an energy source to provide Fe and sulphur. A. ferrooxidans produces H ions through the bioleaching of pyrite, which is consumed by the CFA during dissolution.

Figure 4.
Scanning electron microscopy (SEM) images of (A) CFA and CFA particles activated under CFA/NaCO mass ratios (B) 1:0.19, (C) 1:0.22, and (D) 1:0.25 [113].
5. Utilisation of Bioreactors
The use of bioreactors is crucial in the fields of biotechnology and bioengineering because they provide controlled environments for the growth of living cells and organisms. Bioreactors have been used in a variety of biotechnological processes, including the production of vaccines, enzymes, and biofuels. However, potential applications of bioreactors extend beyond the production of biologically derived products. These devices or systems enable the precise control of a range of parameters, including temperature, pH, pulp density, nutrient levels, dissolved oxygen, stirring rate, etc. The ability to control these parameters facilitates the optimisation of cellular growth and metabolic activity, leading to a robust process, thereby increasing the yield and quality of the products. Furthermore, bioreactors establish synergy between mixed cultures of microorganisms, leading to improved biotechnological processes. Interested readers can find more detailed discussions on bioreactor technology from other sources, such as [55]. The use of bioreactors in the field of mineral processing has been industrialised since 1986, with the extraction of Au from sulphide sources in the Fairview mine in South Africa [93]. This success paved the way for further industrial and laboratory applications of bioreactors, particularly in the recovery of elements from low-grade mineral ores and secondary resources, such as electronic circuit boards and combustion residuals.
The use of bioreactors in the biometallurgical process of CFA has not been well established and rarely experimented. To ensure consistent output in the face of the heterogeneous nature of CFA, it is crucial to establish a robust working environment. The dynamic changes caused by mineral leaching at different stages and their pH-dependent precipitation ability pose a concern that must be closely monitored and managed. It is also important to create a favourable environment for microorganisms to carry out bioleaching and regulate the supply of energy sources, particularly during one-step or spent medium-step processes. Given these complexities, tight control over these parameters is necessary, and the use of bioreactors may be a viable option to achieve this control. Muravyov et al. [114] conducted batch processing of bioleaching in laboratory reactors with a capacity of 2.5 L, using acidophilic chemolithotrophic microbial communities to produce sulphuric acid. The parameters employed were a stirring rate of 500 rpm, aeration rate of 4 L/min, and an operating temperature of 28 (±1) or 45 (±1) C. Optimal conditions for bioleaching were found to be a pulp density of 10%, an initial pH of 2.0, and a 10:1 ratio of acid-soluble waste to elemental sulphur. The yields of Sc, Y, and La recovered after 10 days of bioleaching at 45 C were 52.0%, 52.6%, and 59.5%, respectively.
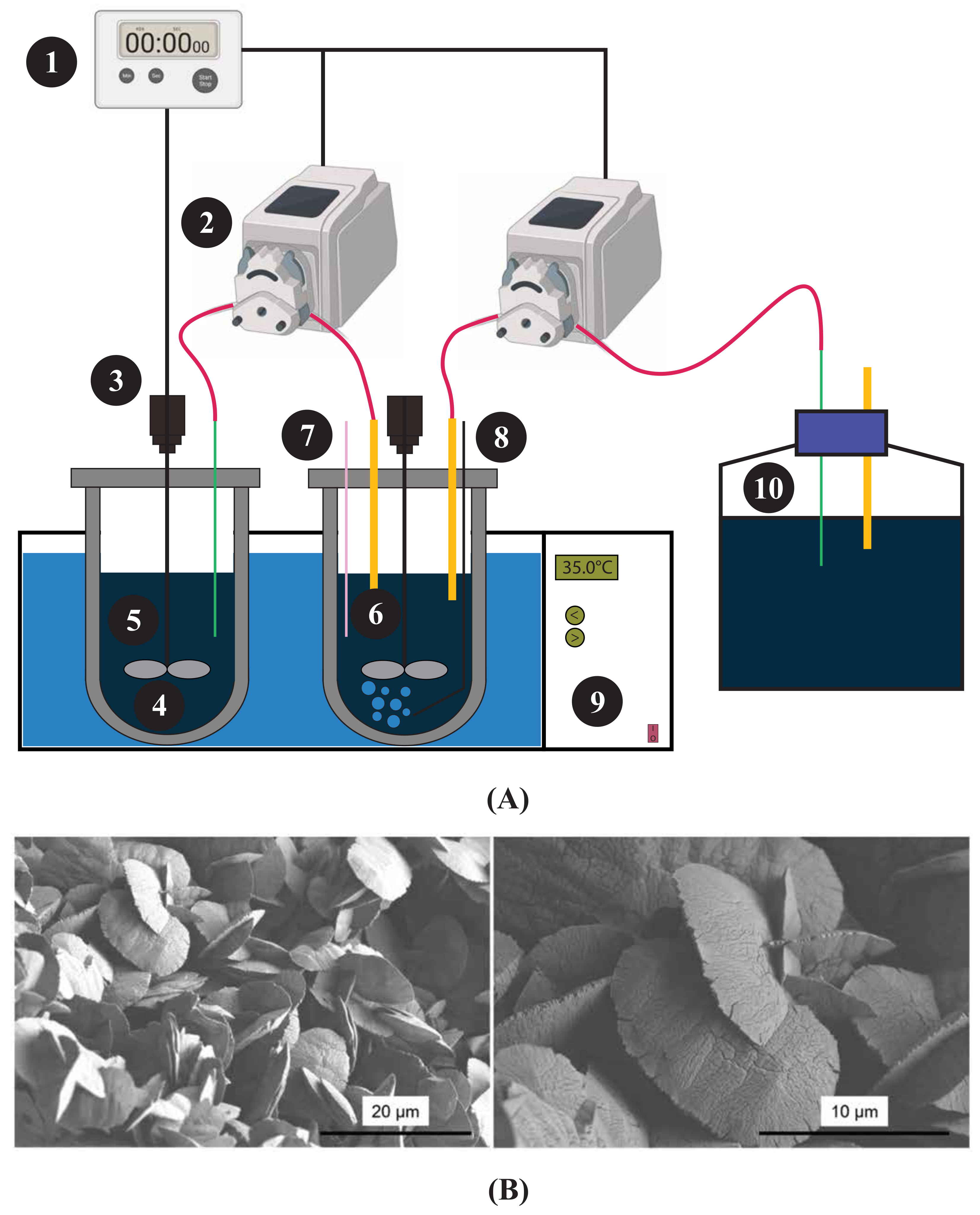
Zhang et al. [115] performed a two-step bioleaching process using a custom-made automatic bioreactor setup, which accommodated the Acidithiobacillus ferrooxidans. A schematic of the designed bioreactor is presented in Figure 5A, and the bioreactor is capable of providing a daily input of pure pyrite of 15 g, resident time up to 5 days, with an effluent concentration of Fe(SO) of 10 g/L, and a pH of approximately 1.3. The resulting solution was then used for leaching for almost 72 h, yielding approximately 4 ppm or 13%–14% yield of the total REEs. Longer-duration tests yielded approximately 40%–60% of the individual REEs. Precipitation was designed using Visual Minteq calculations and modelling, resulting in a final REEs precipitation product containing 36.7% REEs. Figure 5B shows the precipitates of REEs as small flakes of solids about 10 to 15 m in size. Most REEs are associated with the aluminosilicate phase of CFA, making them inaccessible through conventional bioleaching processes [116]. Therefore, the use of bioreactors in the recovery of REEs is a promising step towards achieving a high yield, as the critical parameters can be changed and adjusted for optimal performance of microorganisms.
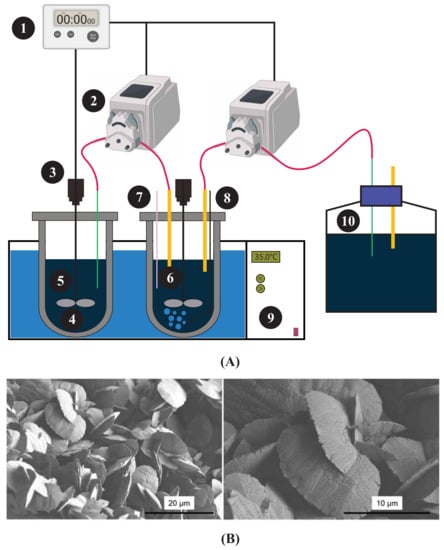
Figure 5.
(A) Schematic diagram of a custom-made automatic bioreactor from the study of Zhang et al. [115]. Here 1—timer, 2—pumps, 3—motors, 4—shaft and impeller, 5—feeding vessel, 6—bioleaching vessel, 7—pH/Eh portal, 8—glass gas sparger, 9—water bath, and 10—leachate reservoir. Adapted from Zhang et al. [115] (B) Scanning electron microscopy (SEM) images of the REE precipitate [115].
The appropriate bioreactor configuration for acidophiles is the stirring tank reactor model, as highlighted by Deive and Sanroman [117]. Additionally, the pozzolanic properties of CFA and its sedimentation characteristics must be carefully assessed, as these factors are detrimental to bioreactor performance. Stirring through a shaft and impeller in the stir tank configuration can help ensure a homogeneous solution throughout the process, which could potentially eliminate CFA cementation. The efficiency of bioreactor operations can be further enhanced by developing a series of in-line bioreactors, each with different operating conditions, for extracting multiple elements at each stage. This continuous flow of CFA through multiple reactors enables the stepwise dissolution of specific elements and their extraction and retrieval, significantly improving the extraction efficiency and proper utilisation of CFA resources. However, sedimentation of CFA particles in conveying tubes can be a practical issue in creating a series of in-line bioreactor processes that requires additional precautions in CFA-associated bioreactor processes.
6. Synthesis of Silica Nanoparticles
The synthesis of silica nanoparticles within the CFA domain has been overlooked, despite the vast abundance of silica in CFA. The economic significance of silica nanoparticles, due to their small size and high surface area, has garnered immense attention in various industries as catalysts and catalyst supports, in biomedical applications, ceramics, resins, molecular sieves, pigments, electronic substrates, precursors in photonic and solar cells for solar devices, thin-film substrates, electrical and thermal insulators, drug delivery in medicine, filters for exhaust gases, and adsorbents [118,119]. Currently, the production of silica nanoparticles from sodium silicate involves acidification, which is presently carried out through using acids or ion exchange [120]. However, efficient extraction of silica nanoparticles from CFA may require different processing conditions because of the amorphous and crystalline forms of silica-bearing minerals [119].
Khan et al. [121] discovered that the mesophilic fungus, Fusarium oxysporum, could produce highly crystalline, protein-capped, and water-soluble silica nanoparticles with quasi-surface morphology extracellularly within 24 h under ambient conditions. The fungus secretes proteins and enzymes during CFA leaching, and natural protein capping caused by fungal metabolism and metabolic energy involvement during the reaction may be responsible for the leaching of nanosized silica. Furthermore, they discovered photoluminescence at room temperature, which could be useful for biomedical applications. The synthesis of porous or hollow silica is possible by changing the reaction conditions, which is useful because of its high drug-loading capacity. Khan et al. [121] found that only 40% of the yield was silica nanoparticles, and the other 50% was carbon, which could be removed using flotation. The byproducts of this process can be used to synthesise aluminosilicate compounds, such as zeolites.
The only other source in the literature for the synthesis of silica nanoparticles from CFA was found in the review Yadav and Fulekar [122]. Their previous study found that they could synthesise porous silica nanosheets 80–120 nm in size using the B. circulans MTCC 6811 supernatant and fly-ash-extracted sodium silicate. The optimum ratio was 4:1 of the supernatant to the extracted sodium silicate by volume. Fulekar and Yadav synthesised spherical aggregated clusters of silica nanosheets, 40–80 nm in size by incubating Fusarium oxysporum supernatant and sodium silicate at an optimised ratio of 3:2 in an incubator shaker at 28 C for 48–72 h. Here, Si was leached from the sillimanite and mullite of CFA by hydrolytic enzymes of F. oxysporum. The Si leached from the CFA formed water-soluble silica nanoparticles, which were further dried, and the silica powder was obtained using a rotary evaporator.
7. Washing Cycle and Biometallurgy
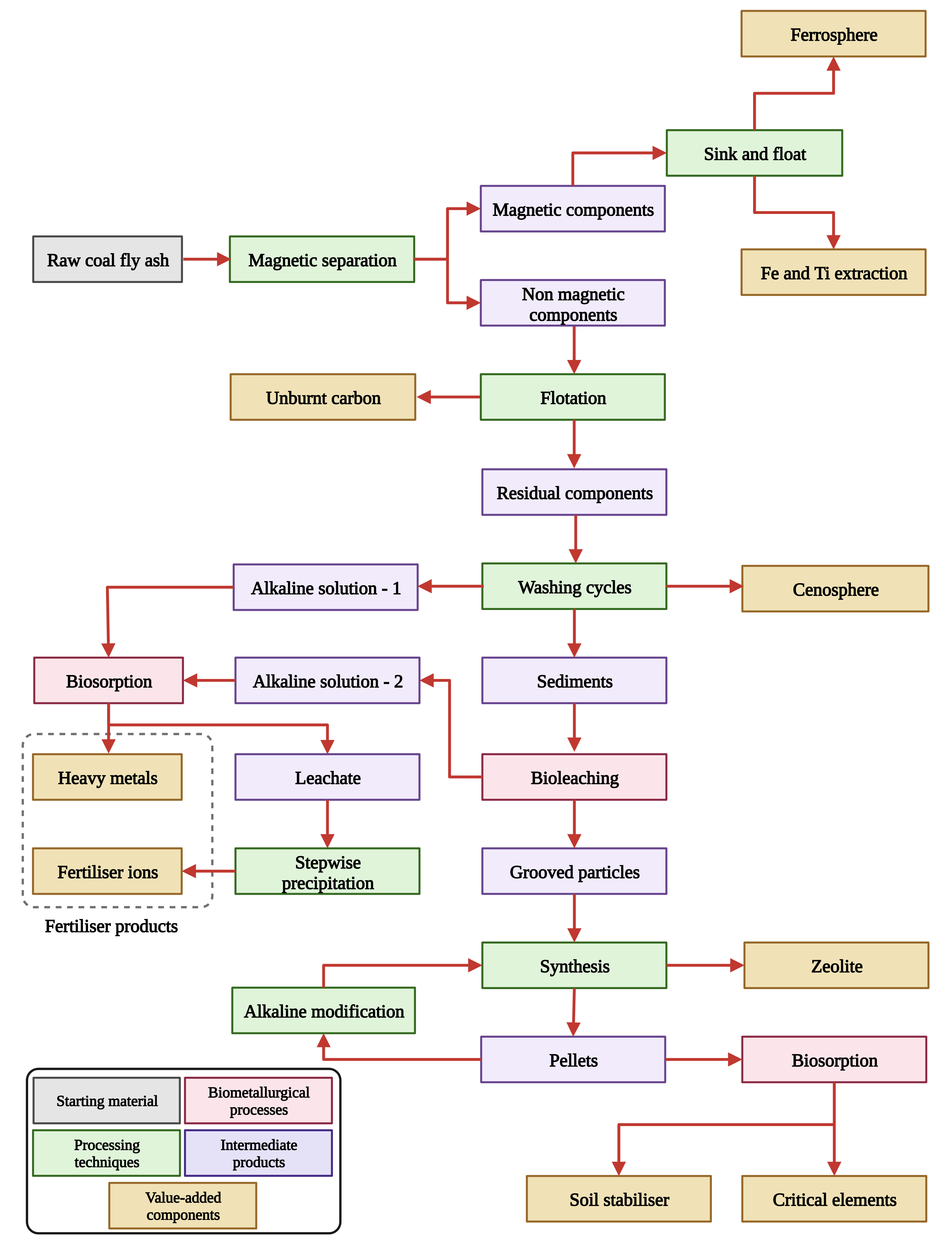
Washing with water is a simple and effective technique that laboratories and industries can easily adopt with minimal costs. A scientific approach to washing with deionised water was studied by the authors and named the process the “washing cycle”. Here, the raw CFA is continuously stirred for 15 min and then allowed to settle for another 15 min at a constant temperature. The rate of kinetics can be increased by increasing the temperature; however, it is recommended to perform washing cycles at room temperature, considering the energy requirements. This process removes loosely attached alkaline ions and other trace materials from the surface rim of the CFA, which is evinced by an increase in the pH and electrical conductivity of the leachate. These measurements acted as indicators of the dissolution efficiency of easily soluble ions from the surface rim. The number of washing cycles can be optimised to achieve the maximum dissolution of alkaline ions, as indicated by the low conductivity and pH of the solution after multiple washing cycles. This process not only prepares CFA for subsequent processes, such as zeolite synthesis or bioleaching, but also disintegrates components based on their density and ability to dissolve in deionised water. The floating top layer of the washing cycle mostly consists of cenospheres (hollow spheres), whereas the leachate contains easily soluble alkaline ions such as Ca, Mg, Na, and K. The bottom layer, which is mainly composed of Al and Si, is ideal for zeolite synthesis and is an adsorbent for wastewater treatment. This demonstrates the possibility of considering the washing cycle as a pivotal point in constructing a circular economic path for CFA waste that is presently underutilised or dumped in ash ponds.
In this section, we investigate the potential integration of eco-friendly biometallurgical techniques into the previously proposed flowsheet for CFA processing, with the aim of enhancing environmental sustainability and valorisation. The biometallurgical process can be incorporated into the existing flowsheet at three different stages: (1) immediately after the washing cycle to the leachate alkaline solution, (2) as a replacement for acid leaching and the resultant leachate alkaline solution, and (3) after the synthesis of zeolites to pellet residues. Figure 6 presents a revised flowsheet that includes the possible biometallurgical processes.
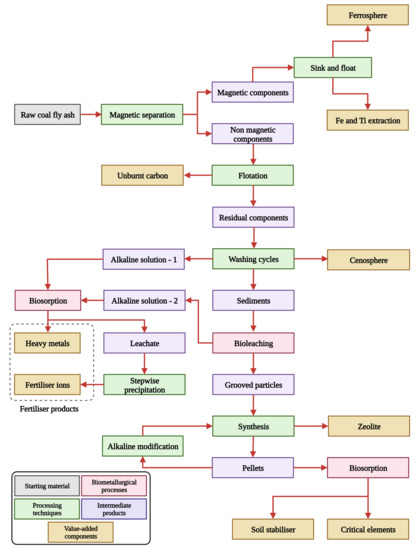
Figure 6.
A prospective flowsheet for the multi-component utilisation of the CFA achieved by replacing high-energy combustion methods of processing with the biometallurgical processes.
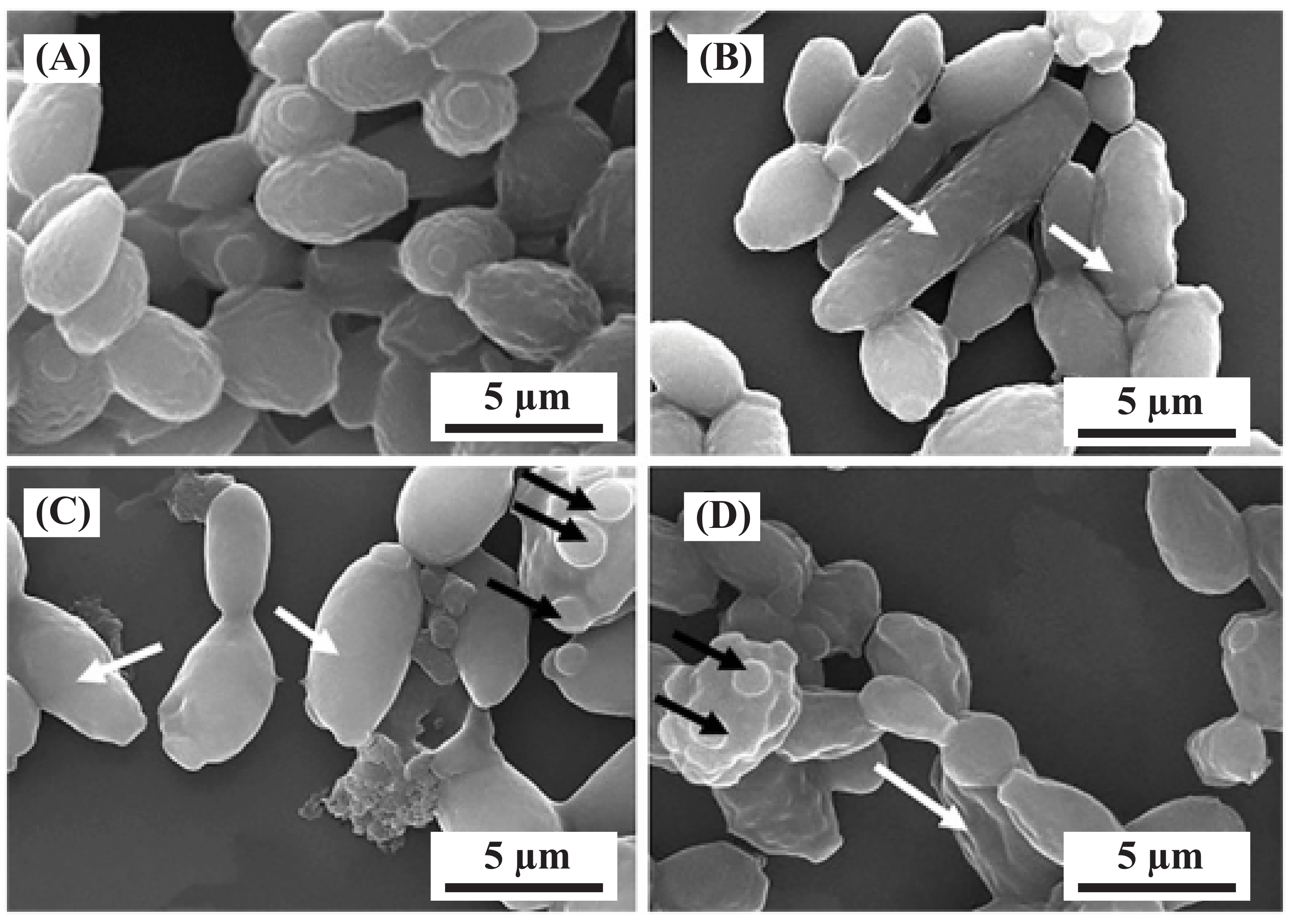
The alkaline solution obtained from the washing cycle contains both macro and micro nutrients, including P, K, Ca, Mg, S, Fe, Zn, Mn, Cu, Co, B, and Mo, which is a significant number of heavy metals. Banker et al.’s [91] study found that Yarrowia lipolytica can bioaccumulate heavy metals in the cell wall, membrane, and cytoplasm, with a Cu extraction of 59.41%. This suggests that it may be possible to bioaccumulate Fe, Zn, Mn, Cu, Co, Mo, and Pb through the addition of appropriate microorganisms into the alkaline solution. Figure 7 shows the morphological changes resulting from heavy-metal biosorption by Y. lipolytica. The remaining solution after the biosorption process can be precipitated using a stepwise pH change and the resulting ions can be used as fertiliser products.
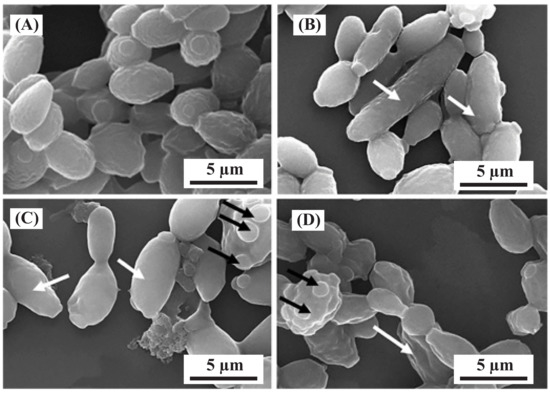
Figure 7.
The changes in the morphology of Y. lipolytica NCIM 3589 after exposure to various metals. Scanning electron microscopy (SEM) images of cells grown in the presence of (A) no metal and different metals at a concentration of 0.5 mM for (B) zinc, (C) cobalt, and (D) nickel. Elongated cells are indicated by white arrows, while cells with multipolar buds are indicated by black arrows [123].
In the revised flowsheet, the grinding process has been intentionally omitted to avoid particle breakdown and to simplify the subsequent processes. Additionally, grinding particles can increase the overall energy consumption of the process. On the other hand, conveyance of CFA during subsequent processes may also introduce micro-cracks due to abrasion; this could have a beneficial effect on the biometallurgical processes. Bioleaching can be used as an alternative to conventional acid treatment for non-selective leaching of sediments from washing cycles, making the process environmentally friendly. The resulting leachate, i.e., alkaline solution-1, will have additional components that cannot leach during the washing cycles. The leachate can be subjected to biosorption and stepwise precipitation processes to recover heavy metals and fertiliser ions, respectively.
The solid residue from the bioleaching process consisted of grooved CFA particles that mainly encompassed Al, Si, and other critical elements. The subsequent alkaline treatment process for these grooved CFA particles extracts Al and Si, which can then be used for zeolite synthesis. Additionally, the extraction process can be intensified with microwave or ultrasonication assistance [124]. After the separation of the Al- and Si-rich solutions, the remaining pellets, enriched with critical elements including REEs, can undergo microbe-mediated surface adsorption or biosorption. This process offers a sustainable and viable approach for selective adsorption of REEs. Microorganisms with high-density surface-accessible functional groups such as carboxylates and phosphates can facilitate high-capacity REE adsorption during growth [125]. Recent studies have explored biosorption as a potential solution for the selective recovery of REEs from coal leachates and CFA. A study by Park et al. [126] compared the use of the Escherichia coli strain (genetically engineered for cell surface display of lanthanide binding tags) and Arthrobacter nicotianae (a native bacterium that exhibits a high REE adsorption capacity) on coal leachate and CFA. The results showed that CFA had a lower REE recovery efficiency than coal leachate due to its higher non-REE content, with most of it encapsulated inside the strong core of CFA. Additionally, the low-pH wash step (i.e., acid leaching or even water washing) between adsorption and desorption yielded higher concentrations of REEs among the total metals (80% and 50% for the coal leachate and CFA, respectively). However, the present flowsheet rectified the problem associated with the higher non-REE content by removing the most of the other elements in the previous steps (i.e., washing cycle and bioleaching). In a nutshell, the loosely attached alkaline components on the surface of the rim will be gathered and precipitated through the washing cycle and biosorption process, respectively. The resulting sediment will then undergo bioleaching to improve the availability of the CFA core for subsequent processes. Therefore, a better result along with faster reaction kinetics compared with other similar experiments can be expected in the proposed flowsheet. Nevertheless, it is essential to optimise the proposed biometallurgical methods by considering influential parameters such as temperature, pulp density, stirring rate, dissolved oxygen, and energy sources. In particular, when utilising bioreactors, optimising these influential parameters and implementing process control measures will greatly enhance the overall yield. Conducting a thorough study of these parameters will be instrumental in achieving a higher scalability for this plausible flowsheet.
The updated flowsheet aims to achieve a circular economy by utilising sustainable methods to valorise the CFA. Instead of hazardous acid treatment, biometallurgical approaches have been used to improve the recovery of value-added components. Selective adsorption and precipitation steps were introduced, and possible integration was critically evaluated. However, it is important to address the limitations of these methods. CFA is a material with multiple complexities and uncertainties, making the processing flowsheet subject to change based on specific requirements. For the proposed biometallurgical processes, the use of stirring tank bioreactors is advantageous because of their high level of control over operational parameters. However, the investment costs associated with these processes are higher than those of conventional methods but can offset the recovery of additional valuable components.
8. Conclusions and Future Prospects
In this study, we reviewed and presented the application of biometallurgical processes in the multi-component utilisation of CFA to recover multiple valuable elements in an environmentally benign manner. The challenges associated with the application of these processes to CFA require a thorough understanding of the extent to which the separation process can be enhanced. Thus, in this study, we explored the interplay between biometallurgy and CFA, identifying critical factors such as the selection of microorganisms, domestication processes, preprocessing of CFA, and usage of bioreactors and their influence on the bio metallurgical processes. Furthermore, we investigated the possibility of incorporating biometallurgy into the CFA preprocessing technique, specifically, the washing cycle, and examined the potential for multi-component utilisation of CFA while adhering to the principles of the circular economy and sustainability. Our illuminating takeaways from this study are as follows:
- The addition of biometallurgical processes at different stages of the washing cycle flowsheet has been found to significantly enhance the separation of selective metal and metal groups, thereby enabling the effective recovery of heavy metals and fertiliser ions.
- Bioleaching of sediment particles after the washing cycle can improve zeolite synthesis by introducing grooves on the CFA substrate, which are effective in subsequent synthesis processes, such as alkaline-assisted hydrothermal synthesis, offering a promising avenue for the production of reproducible pure zeolites.
- Pellets generated at the end of the flowsheet have been shown to contain critical elements, including REEs, and can be extracted using biosorption, which could prove to be an alternate source for the production of advanced materials.
- The use of biometallurgical processes in flowsheets significantly improves their environmental benignity, enabling the replacement of energy-intensive processes such as acid leaching and grinding with more sustainable alternatives.
Author Contributions
Conceptualisation, W.A.M.F. and S.P.; formal analysis, B.K. and W.A.M.F.; writing—original draft preparation, B.K.; writing—review and editing, W.A.M.F., S.P., C.J., D.A. and D.A.S.A.; visualisation, B.K.; supervision, W.A.M.F., C.J., D.A. and D.A.S.A.; project administration, C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful to C.A.N Fernando for his valuable insights in this study and we are also thankful to the officials from the Norochcholai Power Station, Puttalam for providing the CFA samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. Global Coal Consumption, 2020–2023; IEA: Paris, France, 2022. [Google Scholar]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Valeev, D.; Kunilova, I.; Alpatov, A.; Varnavskaya, A.; Ju, D. Magnetite and carbon extraction from coal fly ash using magnetic separation and flotation methods. Minerals 2019, 9, 320. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.; Sarker, P.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The recycling of coal fly ash: A review on sustainable developments and economic considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Kelmers, A.; Canon, R.; Egan, B.; Felker, L.; Gilliam, T.; Jones, G.; Owen, G.; Seeley, F.; Watson, J. Chemistry of the direct acid leach, calsinter, and pressure digestion-acid leach methods for the recovery of alumina from fly ash. Resour. Conserv. 1982, 9, 271–279. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicová, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements—A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching behaviour of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef]
- Kolbe, J.L.; Lee, L.S.; Jafvert, C.T.; Murarka, I.P. Use of alkaline coal ash for reclamation of a former strip mine. In Proceedings of the World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011; pp. 1–15. [Google Scholar]
- Ward, C.R.; French, D.; Jankowski, J.; Dubikova, M.; Li, Z.; Riley, K.W. Element mobility from fresh and long-stored acidic fly ashes associated with an Australian power station. Int. J. Coal Geol. 2009, 80, 224–236. [Google Scholar] [CrossRef]
- Wang, N.; Sun, X.; Zhao, Q.; Yang, Y.; Wang, P. Leachability and adverse effects of coal fly ash: A review. J. Hazard. Mater. 2020, 396, 122725. [Google Scholar] [CrossRef]
- Blissett, R.; Rowson, N. A review of the multi-component utilisation of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Kushnerova, O.A.; Akimochkina, G.V.; Kukhtetskiy, S.V.; Anshits, A.G. Separation of nonmagnetic fine narrow fractions of PM10 from coal fly ash and their characteristics and mineral precursors. Energy Fuels 2019, 33, 3584–3593. [Google Scholar] [CrossRef]
- du Toit, G.; van der Merwe, E.M.; Kruger, R.A.; McDonald, J.M.; Kearsley, E.P. Characterisation of the hydration products of a chemically and mechanically activated high coal fly ash hybrid cement. Minerals 2022, 12, 157. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Volli, V.; Shu, C.M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, B.; Zhang, Q.; Wen, Q.; Wang, S.; Xiao, K.; Zhang, S. Recycling of Coal Fly Ash in Building Materials: A Review. Minerals 2023, 13, 25. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Su, Y.; He, X.; Tan, H.; Yang, W.; Strnadel, B. Eco-friendly treatment of low-calcium coal fly ash for high pozzolanic reactivity: A step towards waste utilization in sustainable building material. J. Clean. Prod. 2019, 238, 117962. [Google Scholar] [CrossRef]
- Kelechi, S.; Adamu, M.; Uche, O.; Okokpujie, I.; Ibrahim, Y.E.; Obianyo, I. A comprehensive review on coal fly ash and its application in the construction industry. Cogent Eng. 2022, 9, 2114201. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, A.; De, A.; Singh, P. Coal fly ash: An emerging material for water remediation. Int. J. Coal Sci. Technol. 2022, 9, 1–32. [Google Scholar] [CrossRef]
- Visa, M.; Isac, L.; Duta, A. Fly ash adsorbents for multi-cation wastewater treatment. Appl. Surf. Sci. 2012, 258, 6345–6352. [Google Scholar] [CrossRef]
- Asl, S.M.H.; Ghadi, A.; Baei, M.S.; Javadian, H.; Maghsudi, M.; Kazemian, H. Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications: A review. Fuel 2018, 217, 320–342. [Google Scholar]
- Pavlović, S.M.; Marinković, D.M.; Kostić, M.D.; Janković-Častvan, I.M.; Mojović, L.V.; Stanković, M.V.; Veljković, V.B. A CaO/zeolite-based catalyst obtained from waste chicken eggshell and coal fly ash for biodiesel production. Fuel 2020, 267, 117171. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Chen, J. Utilization of coal fly ash for the production of glass-ceramics with unique performances: A brief review. J. Mater. Sci. Technol. 2014, 30, 1208–1212. [Google Scholar] [CrossRef]
- Little, M.; Adell, V.; Boccaccini, A.; Cheeseman, C. Production of novel ceramic materials from coal fly ash and metal finishing wastes. Resour. Conserv. Recycl. 2008, 52, 1329–1335. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Mericboyu, A. Comparison of the properties of glass, glass–ceramic and ceramic materials produced from coal fly ash. J. Hazard. Mater. 2008, 153, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ho, S.W.; Li, T.; Maneerung, T.; Wang, C.H. Techno-economic analysis of geopolymer production from the coal fly ash with high iron oxide and calcium oxide contents. J. Hazard. Mater. 2019, 361, 237–244. [Google Scholar] [CrossRef]
- Nyale, S.M.; Babajide, O.O.; Birch, G.D.; Böke, N.; Petrik, L.F. Synthesis and characterization of coal fly ash-based foamed geopolymer. Procedia Environ. Sci. 2013, 18, 722–730. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Paprocki, A.; Ferret, L.S.; Azevedo, C.M.; Pires, M. Synthesis of zeolite Na-P1 under mild conditions using Brazilian coal fly ash and its application in wastewater treatment. Fuel 2015, 139, 59–67. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.t.; Alastuey, A.; Hernández, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Koshy, N.; Singh, D. Fly ash zeolites for water treatment applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Wu, X.; Fan, M.; Mclaughlin, J.F.; Shen, X.; Tan, G. A novel low-cost method of silica aerogel fabrication using fly ash and trona ore with ambient pressure drying technique. Powder Technol. 2018, 323, 310–322. [Google Scholar] [CrossRef]
- Kang, A.H.; Shang, K.; Ye, D.D.; Wang, Y.T.; Wang, H.; Zhu, Z.M.; Liao, W.; Xu, S.M.; Wang, Y.Z.; Schiraldi, D.A. Rejuvenated fly ash in poly (vinyl alcohol)-based composite aerogels with high fire safety and smoke suppression. Chem. Eng. J. 2017, 327, 992–999. [Google Scholar] [CrossRef]
- Dunens, O.M.; MacKenzie, K.J.; Harris, A.T. Synthesis of multiwalled carbon nanotubes on fly ash derived catalysts. Environ. Sci. Technol. 2009, 43, 7889–7894. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Al-Ghamdi, A.A.; Memic, A.; Habib, S.S.; Khan, Z.H. Formation of carbon nanotubes from carbon-rich fly ash: Growth parameters and mechanism. Mater. Manuf. Process. 2016, 31, 146–156. [Google Scholar] [CrossRef]
- Alam, J.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.; Tavker, N.; Choudhary, N.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M.; Hamid, A.A. Recent advances in methods for the recovery of carbon nanominerals and polyaromatic hydrocarbons from coal fly ash and their emerging applications. Crystals 2021, 11, 88. [Google Scholar] [CrossRef]
- Rivera, J.F.; Orobio, A.; Cristelo, N.; de Gutierrez, R.M. Fly ash-based geopolymer as A4 type soil stabiliser. Transp. Geotech. 2020, 25, 100409. [Google Scholar] [CrossRef]
- Nalbantoğlu, Z. Effectiveness of class C fly ash as an expansive soil stabilizer. Constr. Build. Mater. 2004, 18, 377–381. [Google Scholar] [CrossRef]
- Wu, M.; Qi, C.; Chen, Q.; Liu, H. Evaluating the metal recovery potential of coal fly ash based on sequential extraction and machine learning. Environ. Res. 2023, 224, 115546. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A. Formation mechanism and applications of cenospheres: A review. J. Mater. Sci. 2020, 55, 4539–4557. [Google Scholar] [CrossRef]
- Shende, D.Z.; Wasewar, K.L.; Wadatkar, S.S. Target-Specific Applications of Fly Ash Cenosphere as Smart Material. Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Cham, Switzerland, 2021; pp. 3349–3369. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Singh, R.; Volli, V.; Lohani, L.; Purkait, M.K. Polymeric ultrafiltration membranes modified with fly ash based carbon nanotubes for thermal stability and protein separation. Case Stud. Chem. Environ. Eng. 2021, 4, 100155. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Reddy, B.R.; Zhou, J. Preparation of coal-based carbon nanotubes using catalytical pyrolysis: A brief review. Fuel Process. Technol. 2022, 229, 107171. [Google Scholar] [CrossRef]
- Jala, S.; Goyal, D. Fly ash as a soil ameliorant for improving crop production—A review. Bioresour. Technol. 2006, 97, 1136–1147. [Google Scholar] [CrossRef]
- Fankhauser, S.; Smith, S.M.; Allen, M.; Axelsson, K.; Hale, T.; Hepburn, C.; Kendall, J.M.; Khosla, R.; Lezaun, J.; Mitchell-Larson, E.; et al. The meaning of net zero and how to get it right. Nat. Clim. Chang. 2022, 12, 15–21. [Google Scholar] [CrossRef]
- Jha, V.K.; Nagae, M.; Matsuda, M.; Miyake, M. Zeolite formation from coal fly ash and heavy metal ion removal characteristics of thus-obtained Zeolite X in multi-metal systems. J. Environ. Manag. 2009, 90, 2507–2514. [Google Scholar] [CrossRef]
- Temuujin, J.v.; Van Riessen, A. Effect of fly ash preliminary calcination on the properties of geopolymer. J. Hazard. Mater. 2009, 164, 634–639. [Google Scholar] [CrossRef]
- Miyake, M.; Kimura, Y.; Ohashi, T.; Matsuda, M. Preparation of activated carbon–zeolite composite materials from coal fly ash. Microporous Mesoporous Mater. 2008, 112, 170–177. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. Biomining; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining—Biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ghassa, S.; Noaparast, M.; Shafaei, S.Z.; Abdollahi, H.; Gharib, F.; Magdouli, S. Optimization of pyrite bio-oxidation to produce ferric reagent for sphalerite leaching. J. Hazardous Toxic Radioact. Waste 2022, 26, 04021035. [Google Scholar] [CrossRef]
- Saleh, D.K.; Abdollahi, H.; Noaparast, M.; Nosratabad, A.F.; Tuovinen, O.H. Dissolution of Al from metakaolin with carboxylic acids produced by Aspergillus niger, Penicillium bilaji, Pseudomonas putida, and Pseudomonas koreensis. Hydrometallurgy 2019, 186, 235–243. [Google Scholar] [CrossRef]
- Schippers, A.; Hedrich, S.; Vasters, J.; Drobe, M.; Sand, W.; Willscher, S. Biomining: Metal recovery from ores with microorganisms. Geobiotechnol. I Met. Relat. Issues 2014, 141, 1–47. [Google Scholar]
- Dopson, M.; Okibe, N. Biomining Microorganisms: Diversity and Modus Operandi. In Biomining Technologies: Extracting and Recovering Metals from Ores and Wastes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 89–110. [Google Scholar]
- Gumulya, Y.; Boxall, N.J.; Khaleque, H.N.; Santala, V.; Carlson, R.P.; Kaksonen, A.H. In a quest for engineering acidophiles for biomining applications: Challenges and opportunities. Genes 2018, 9, 116. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Deng, X.; Bohu, T.; Zea, L.; Khaleque, H.N.; Gumulya, Y.; Boxall, N.J.; Morris, C.; Cheng, K.Y. Prospective directions for biohydrometallurgy. Hydrometallurgy 2020, 195, 105376. [Google Scholar] [CrossRef]
- Zhuang, W.Q.; Fitts, J.P.; Ajo-Franklin, C.M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Asghari, I.; Mousavi, S.; Amiri, F.; Tavassoli, S. Bioleaching of spent refinery catalysts: A review. J. Ind. Eng. Chem. 2013, 19, 1069–1081. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, J.S.; Singh, R.P.; Singh, N.; Yunus, M. Arsenic hazards in coal fly ash and its fate in Indian scenario. Resour. Conserv. Recycl. 2011, 55, 819–835. [Google Scholar] [CrossRef]
- Johnson, D.B.; Roberto, F.F. Evolution and Current Status of Mineral Bioprocessing Technologies. In Biomining Technologies: Extracting and Recovering Metals from Ores and Wastes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–13. [Google Scholar]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C. Formation mechanism of arsenic-containing dust in the flue gas cleaning process of flash copper pyrometallurgy: A quantitative identification of arsenic speciation. Chem. Eng. J. 2021, 423, 130193. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Mishra, S.; Akcil, A.; Panda, S.; Erust, C. Biodesulphurization of Turkish lignite by Leptospirillum ferriphilum: Effect of ferrous iron, Span-80 and ultrasonication. Hydrometallurgy 2018, 176, 166–175. [Google Scholar] [CrossRef]
- Mishra, S.; Akcil, A.; Panda, S.; Tuncuk, A. Effect of Span-80 and ultrasonication on biodesulphurization of lignite by Rhodococcus erythropolis: Lab to semi-pilot scale tests. Miner. Eng. 2018, 119, 183–190. [Google Scholar] [CrossRef]
- Mishra, S.; Akcil, A.; Panda, S.; Agcasulu, I. Laboratory and semipilot bioreactor feasibility tests for desulphurization of Turkish lignite using Leptospirillum ferriphilum. Energy Fuels 2018, 32, 2869–2877. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S.; Agcasulu, I. A Review on Chemical versus Microbial Leaching of Electronic Wastes with Emphasis on Base Metals Dissolution. Minerals 2021, 11, 1255. [Google Scholar] [CrossRef]
- Jain, N.; Sharma, D. Biohydrometallurgy for nonsulfidic minerals—A review. Geomicrobiol. J. 2004, 21, 135–144. [Google Scholar] [CrossRef]
- Singer, A.; Navrot, J.; Shapira, R. Extraction of aluminum from fly-ash by commercial and microbiologically-produced citric acid. Eur. J. Appl. Microbiol. Biotechnol. 1982, 16, 228–230. [Google Scholar] [CrossRef]
- Torma, A.E.; Singh, A.K. Acidolysis of coal fly ash by Aspergillus niger. Fuel 1993, 72, 1625–1630. [Google Scholar] [CrossRef]
- Jadhav, U.U.; Hocheng, H. Analysis of metal bioleaching from thermal power plant fly ash by Aspergillus niger 34770 culture supernatant and reduction of phytotoxicity during the process. Appl. Biochem. Biotechnol. 2015, 175, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Taştan, B.E. Clean up fly ash from coal burning plants by new isolated fungi Fusarium oxysporum and Penicillium glabrum. J. Environ. Manag. 2017, 200, 46–52. [Google Scholar] [CrossRef]
- Seddiek, H.A.; Shetaia, Y.M.; Mahamound, K.F.; El-Aassy, I.E.; Hussien, S.S. Bioleaching of Egyptian Fly Ash Using Cladosporium cladosporioides. Ann. Biol. 2021, 37, 18–22. [Google Scholar]
- Tiwari, S.; Kumari, B.; Singh, S. Microbe-induced changes in metal extractability from fly ash. Chemosphere 2008, 71, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.; Geva, J.; Shalita, Z.; White, M.; Lezion, R.; Fleming, J. Bioleaching Method for the Extraction of Metals from Coal Fly Ash Using Thiobacillus. US Patent 5,278,069, 1994. [Google Scholar]
- Guo, Y.; Teng, Q.; Yang, Z.; Sun, B.; Liu, S. Investigation on bio-desilication process of fly ash based on a self-screened strain of Bacillus amyloliquefaciens and its metabolites. J. Biotechnol. 2021, 341, 146–154. [Google Scholar] [CrossRef]
- Seidel, A.; Zimmels, Y.; Armon, R. Mechanism of bioleaching of coal fly ash by Thiobacillus thiooxidans. Chem. Eng. J. 2001, 83, 123–130. [Google Scholar] [CrossRef]
- Jekić, J.S.; Beškoski, V.P.; Gojgić-Cvijović, G.; Grbavčić, M.; Vrvić, M.M. Bacterially generated Fe2(SO4)3 from pyrite, as a leaching agent for heavy metals from lignite ash. J. Serbian Chem. Soc. 2007, 72, 615–619. [Google Scholar] [CrossRef]
- Kermer, R.; Hedrich, S.; Bellenberg, S.; Brett, B.; Schrader, D.; Schoenherr, P.; Koepcke, M.; Siewert, K.; Guenther, N.; Gehrke, T.; et al. Lignite ash: Waste material or potential resource-Investigation of metal recovery and utilization options. Hydrometallurgy 2017, 168, 141–152. [Google Scholar] [CrossRef]
- Kaur, R.; Goyal, D. Heavy metal accumulation from coal fly ash by cyanobacterial biofertilizers. Part. Sci. Technol. 2018, 36, 513–516. [Google Scholar] [CrossRef]
- Pangayao, D.; Promentilla, M.A.; Gallardo, S.; van Hullebusch, E. Bioleaching kinetics of trace metals from coal ash using Pseudomonas spp. In Proceedings of the MATEC Web of Conferences, Makati, Philippines, 21–22 November 2018; EDP Sciences: Les Ulis, France, 2019; Volume 268, p. 01010. [Google Scholar]
- Rezaei, H.; Shafaei, S.Z.; Abdollahi, H.; Ghassa, S.; Boroumand, Z.; Nosratabad, A.F. Spent-medium leaching of germanium, vanadium and lithium from coal fly ash with biogenic carboxylic acids and comparison with chemical leaching. Hydrometallurgy 2023, 217, 106038. [Google Scholar] [CrossRef]
- Shabtai, Y.; Mukmenev, I. A combined chemical-biotechnological treatment of coal fly ash (CFA). J. Biotechnol. 1996, 51, 209–217. [Google Scholar] [CrossRef]
- Bankar, A.; Winey, M.; Prakash, D.; Kumar, A.R.; Gosavi, S.; Kapadnis, B.; Zinjarde, S. Bioleaching of fly ash by the tropical marine yeast, Yarrowia lipolytica NCIM 3589. Appl. Biochem. Biotechnol. 2012, 168, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Liang, Y. Bioleaching of trace elements and rare earth elements from coal fly ash. Int. J. Coal Sci. Technol. 2019, 6, 74–83. [Google Scholar] [CrossRef]
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.; Contamine, F.; d’Hugues, P. A review of sulfide minerals microbially assisted leaching in stirred tank reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Akcil, A.; Ciftci, H.; Deveci, H. Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner. Eng. 2007, 20, 310–318. [Google Scholar] [CrossRef]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef]
- Karwowska, E.; Wojtkowska, M.; Andrzejewska, D. The influence of metal speciation in combustion waste on the efficiency of Cu, Pb, Zn, Cd, Ni and Cr bioleaching in a mixed culture of sulfur-oxidizing and biosurfactant-producing bacteria. J. Hazard. Mater. 2015, 299, 35–41. [Google Scholar] [CrossRef]
- Steensels, J.; Gallone, B.; Voordeckers, K.; Verstrepen, K.J. Domestication of industrial microbes. Curr. Biol. 2019, 29, R381–R393. [Google Scholar] [CrossRef]
- Elzeky, M.; Attia, Y. Effect of bacterial adaptation on kinetics and mechanisms of bioleaching ferrous sulfides. Chem. Eng. J. Biochem. Eng. J. 1995, 56, B115–B124. [Google Scholar] [CrossRef]
- Su, H.; Tan, F.; Lin, J. An integrated approach combines hydrothermal chemical and biological treatment to enhance recycle of rare metals from coal fly ash. Chem. Eng. J. 2020, 395, 124640. [Google Scholar] [CrossRef]
- Seidel, A.; Zimmels, Y. Mechanism and kinetics of aluminum and iron leaching from coal fly ash by sulfuric acid. Chem. Eng. Sci. 1998, 53, 3835–3852. [Google Scholar] [CrossRef]
- Sen, S.K.; Das, M.M.; Bandyopadhyay, P.; Dash, R.; Raut, S. Green process using hot spring bacterium to concentrate alumina in coal fly ash. Ecol. Eng. 2016, 88, 10–19. [Google Scholar] [CrossRef]
- Meer, I.; Nazir, R. Removal techniques for heavy metals from fly ash. J. Mater. Cycles Waste Manag. 2018, 20, 703–722. [Google Scholar] [CrossRef]
- Katyal, N.; Sharma, J.; Dhawan, A.; Ali, M.; Mohan, K. Development of rapid method for the estimation of reactive silica in fly ash. Cem. Concr. Res. 2008, 38, 104–106. [Google Scholar] [CrossRef]
- Pangayao, D.; Gallardo, S.; Promentilla, M.A.; Van Hullebusch, E. Bioleaching of trace metals from coal ash using local isolate from coal ash ponds. In Proceedings of the MATEC Web of Conferences, Semarang, Indonesia, 15–16 November 2017; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 03031. [Google Scholar]
- Rompalski, P.; Róg, L. Effect of the temperature of different combustion zones in the boiler grate on changes in physical and chemical parameters of bituminous coal and slags. J. Sustain. Min. 2016, 15, 73–83. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, L.; Pu, J.; Zhao, J. Melting characteristics of coal ash and properties of fly ash to understand the slag formation in the shell gasifier. ACS Omega 2021, 6, 16066–16075. [Google Scholar] [CrossRef]
- Bicer, A. Effect of production temperature on thermal and mechanical properties of polystyrene—Fly ash composites. Adv. Compos. Lett. 2020, 29, 2633366X20917988. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Y.; Wang, T.; Wang, J.; Romero, C.E. Mineralization characteristics of coal fly ash in the transition from non-supercritical CO2 to supercritical CO2. Fuel 2022, 318, 123636. [Google Scholar] [CrossRef]
- Iyer, R. The surface chemistry of leaching coal fly ash. J. Hazard. Mater. 2002, 93, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Shoppert, A.; Loginova, I.; Valeev, D. Kinetics study of al extraction from desilicated coal fly ash by NaOH at atmospheric pressure. Materials 2021, 14, 7700. [Google Scholar] [CrossRef]
- Shoppert, A.; Valeev, D.; Loginova, I.; Chaikin, L. Complete extraction of amorphous aluminosilicate from coal fly ash by alkali leaching under atmospheric pressure. Metals 2020, 10, 1684. [Google Scholar] [CrossRef]
- Fan, X.l.; Lv, S.q.; Xia, J.l.; Nie, Z.y.; Zhang, D.r.; Pan, X.; Liu, L.z.; Wen, W.; Zheng, L.; Zhao, Y.d. Extraction of Al and Ce from coal fly ash by biogenic Fe3+ and H2SO4. Chem. Eng. J. 2019, 370, 1407–1424. [Google Scholar] [CrossRef]
- Muravyov, M.; Bulaev, A.; Melamud, V.; Kondrat’eva, T. Leaching of rare earth elements from coal ashes using acidophilic chemolithotrophic microbial communities. Microbiology 2015, 84, 194–201. [Google Scholar] [CrossRef]
- Zhang, Z.; Allen, L.; Podder, P.; Free, M.L.; Sarswat, P.K. Recovery and enhanced upgrading of rare earth elements from coal-based resources: Bioleaching and precipitation. Minerals 2021, 11, 484. [Google Scholar] [CrossRef]
- Lin, R.; Stuckman, M.; Howard, B.H.; Bank, T.L.; Roth, E.A.; Macala, M.K.; Lopano, C.; Soong, Y.; Granite, E.J. Application of sequential extraction and hydrothermal treatment for characterization and enrichment of rare earth elements from coal fly ash. Fuel 2018, 232, 124–133. [Google Scholar] [CrossRef]
- Deive, F.; Sanromán, M. Bioreactor development for the cultivation of extremophilic microorganisms. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 403–432. [Google Scholar]
- Aphane, M.E.; Doucet, F.J.; Kruger, R.A.; Petrik, L.; van der Merwe, E.M. Preparation of sodium silicate solutions and silica nanoparticles from South African coal fly ash. Waste Biomass Valorization 2020, 11, 4403–4417. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M. Green synthesis and characterization of amorphous silica nanoparticles from fly ash. Mater. Today Proc. 2019, 18, 4351–4359. [Google Scholar] [CrossRef]
- Bai, G.; Teng, W.; Wang, X.; Zhang, H.; Xu, P. Processing and kinetics studies on the alumina enrichment of coal fly ash by fractionating silicon dioxide as nano particles. Fuel Process. Technol. 2010, 91, 175–184. [Google Scholar] [CrossRef]
- Khan, S.A.; Uddin, I.; Moeez, S.; Ahmad, A. Fungus-mediated preferential bioleaching of waste material such as fly-ash as a means of producing extracellular, protein capped, fluorescent and water soluble silica nanoparticles. PLoS ONE 2014, 9, e107597. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Fulekar, M.H. Advances in methods for recovery of ferrous, alumina, and silica nanoparticles from fly ash waste. Ceramics 2020, 3, 384–420. [Google Scholar] [CrossRef]
- Kolhe, N.; Damle, E.; Pradhan, A.; Zinjarde, S. A comprehensive assessment of Yarrowia lipolytica and its interactions with metals: Current updates and future prospective. Biotechnol. Adv. 2022, 59, 107967. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Moriwaki, H.; Yamamoto, H. Interactions of microorganisms with rare earth ions and their utilization for separation and environmental technology. Appl. Microbiol. Biotechnol. 2013, 97, 1–8. [Google Scholar] [CrossRef]
- Park, J.; Saratale, G.D.; Cho, S.K.; Bae, S. Synergistic effect of Cu loading on Fe sites of fly ash for enhanced catalytic reduction of nitrophenol. Sci. Total. Environ. 2020, 705, 134544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).