Origin of Amphibole-Biotite-Fluorite-Rich Enclaves from Gabal El-Ineigi Fluorite-Bearing Granite, Central Eastern Desert of Egypt: Insights into Fluoride–Calcium and Silicate Liquid Immiscibility
Abstract
1. Introduction
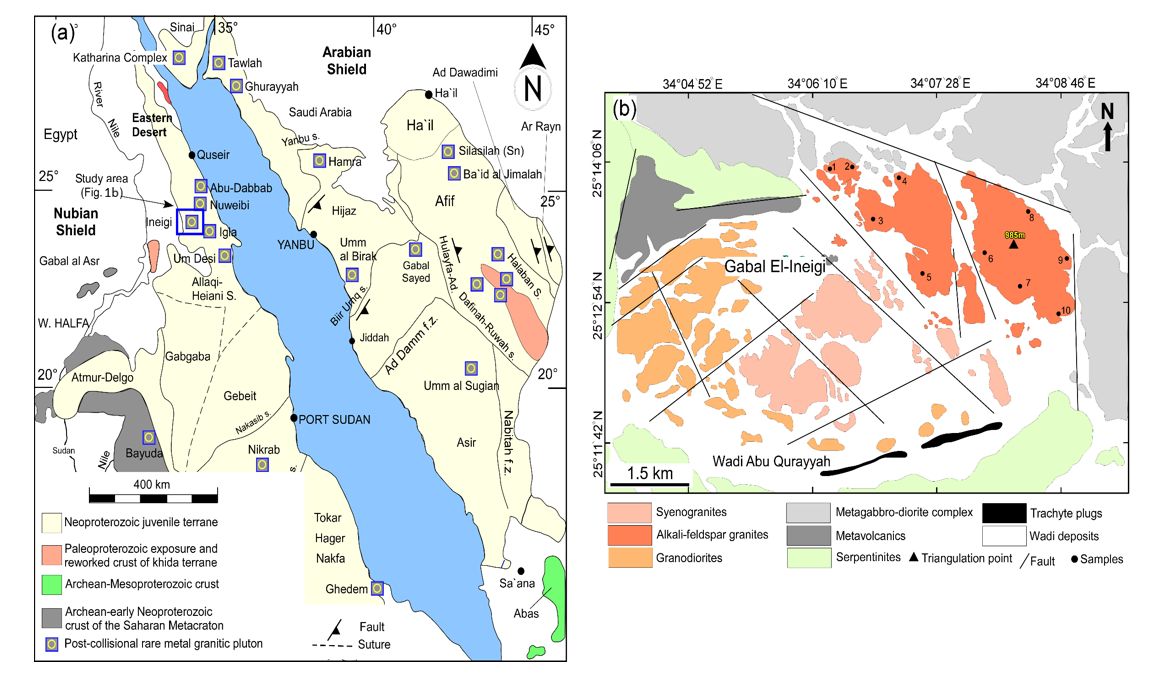
2. Geological Setting
3. Analytical Methods
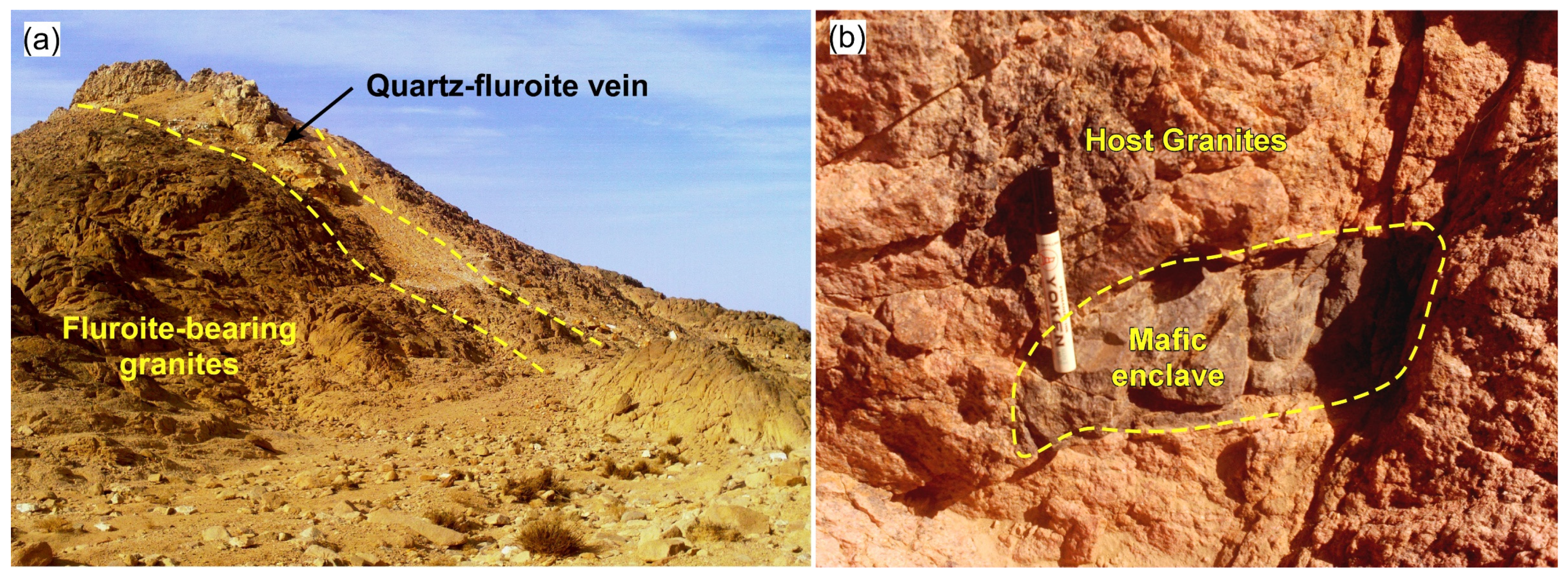
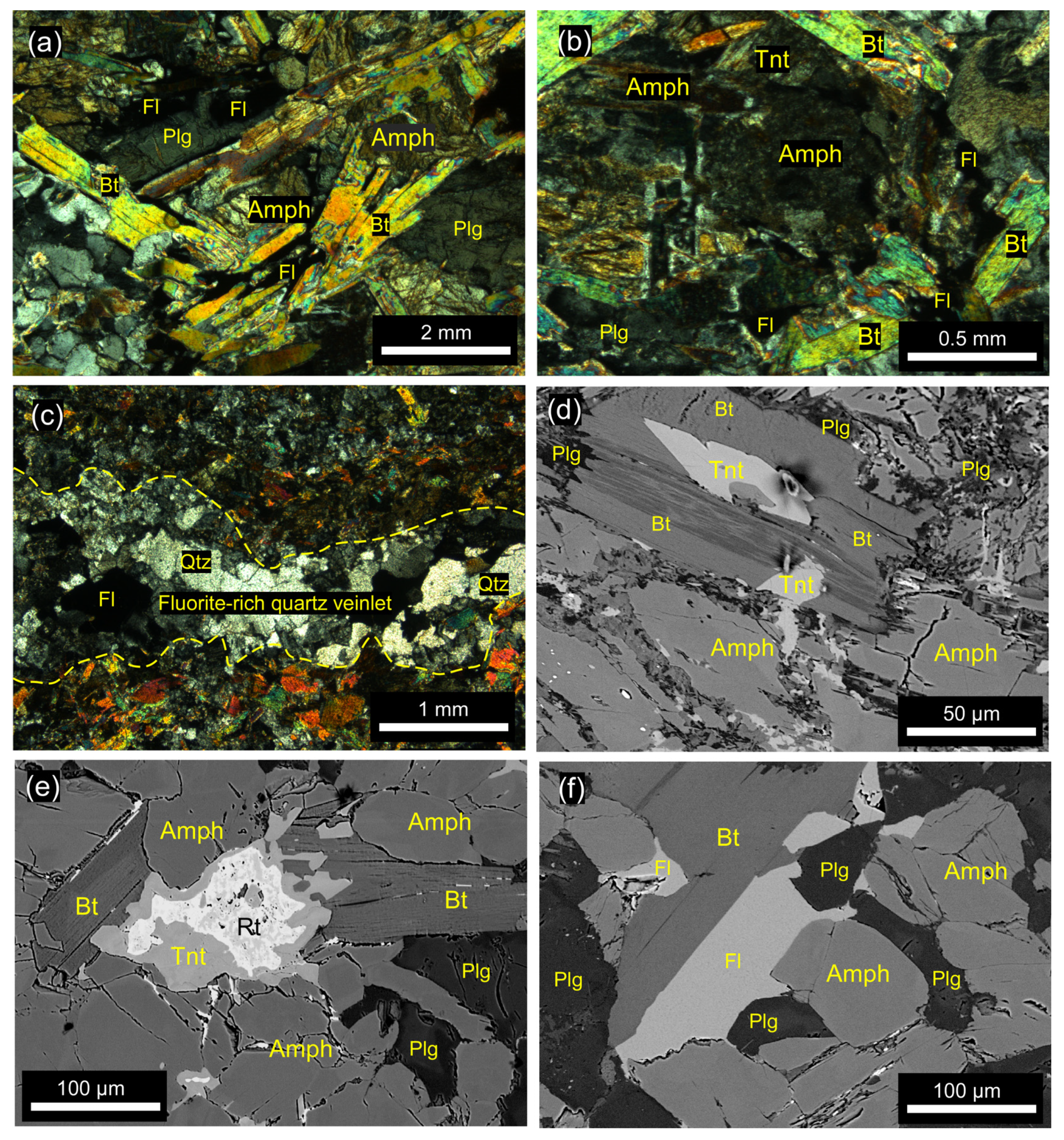
4. Petrography of the Host Rock and Enclaves
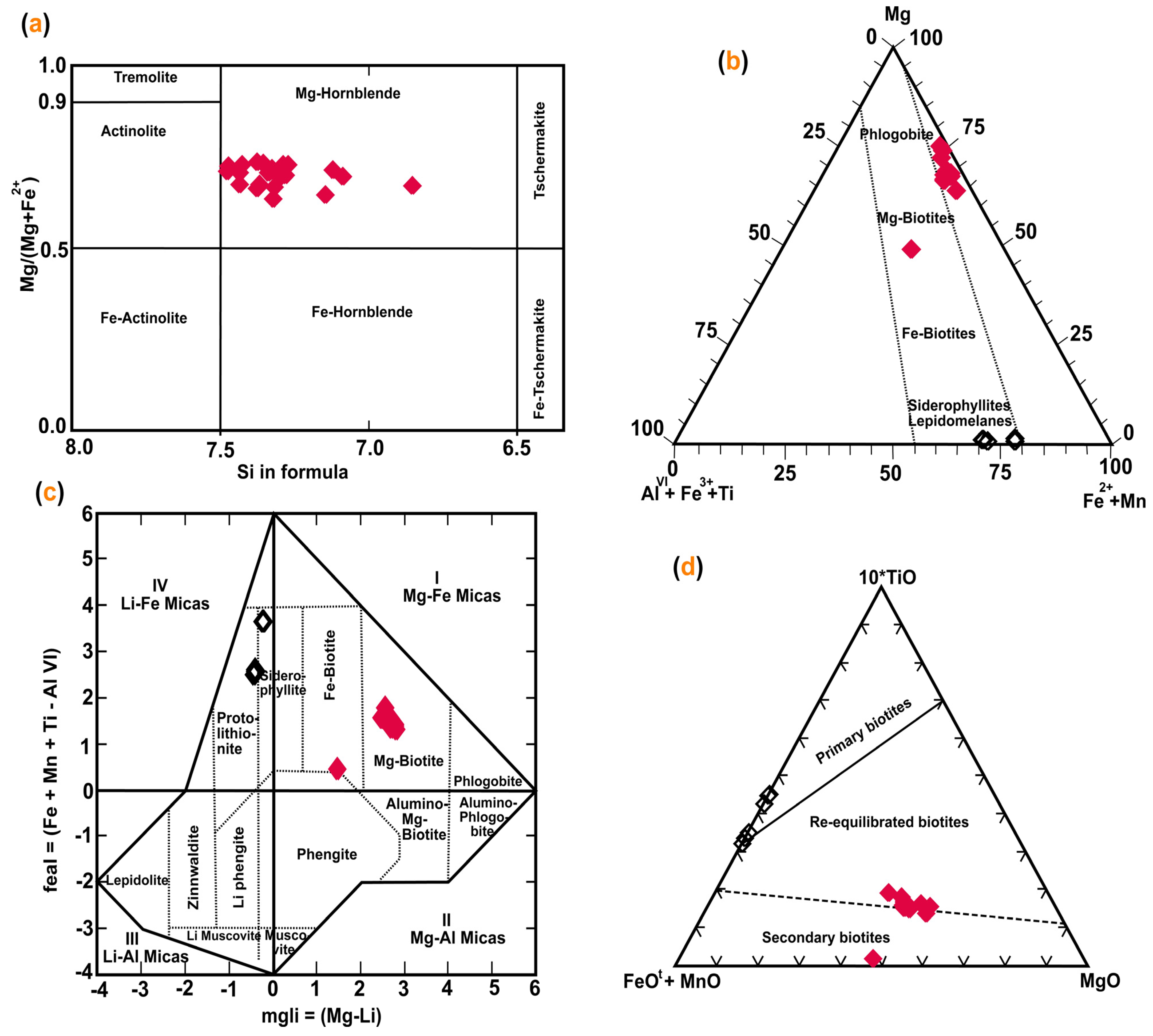
5. Mineral Chemistry
| Spot# | Am1 | Am2 | Am3 | Am4 | Am5 | Am6 | Am7 | Am8 | Am9 | Am10 | Am11 | Am12 | Am13 | Am14 | Am15 | Am16 | Am17 | Am18 | Am19 | Am20 | Am21 | Am22 | Am23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 48.01 | 49.94 | 49.84 | 49.85 | 50.26 | 51.29 | 46.34 | 50.04 | 50.14 | 50.56 | 50.77 | 50.04 | 48.80 | 49.63 | 50.45 | 50.11 | 48.11 | 50.07 | 48.49 | 51.30 | 51.66 | 49.97 | 50.96 |
| TiO2 | 0.23 | 0.63 | 0.49 | 0.68 | 0.73 | 0.29 | 0.79 | 0.39 | 0.63 | 0.43 | 0.25 | 0.40 | 0.15 | 0.13 | 0.18 | 0.21 | 0.23 | 0.16 | 0.56 | 0.20 | 0.33 | 0.21 | 0.25 |
| Al2O3 | 6.42 | 4.61 | 5.06 | 4.73 | 4.46 | 3.74 | 7.98 | 4.79 | 5.01 | 4.47 | 4.55 | 5.03 | 4.48 | 4.66 | 3.84 | 4.09 | 5.73 | 4.14 | 5.78 | 3.67 | 3.37 | 4.65 | 3.96 |
| FeO | 12.84 | 12.49 | 12.11 | 12.08 | 12.02 | 11.80 | 13.12 | 11.67 | 11.60 | 11.34 | 11.36 | 11.90 | 14.54 | 14.73 | 14.00 | 14.80 | 15.17 | 14.34 | 12.91 | 12.54 | 12.38 | 12.62 | 12.28 |
| MnO | 0.29 | 0.30 | 0.29 | 0.27 | 0.24 | 0.25 | 0.23 | 0.25 | 0.27 | 0.28 | 0.24 | 0.25 | 0.52 | 0.51 | 0.52 | 0.52 | 0.53 | 0.52 | 0.30 | 0.36 | 0.37 | 0.35 | 0.34 |
| MgO | 14.16 | 14.83 | 14.89 | 14.99 | 15.12 | 15.47 | 13.53 | 15.27 | 15.36 | 15.72 | 15.65 | 15.43 | 13.24 | 13.47 | 14.00 | 13.63 | 12.73 | 13.92 | 14.50 | 15.50 | 15.57 | 14.84 | 15.18 |
| CaO | 12.40 | 12.54 | 12.67 | 12.40 | 12.56 | 12.77 | 12.63 | 12.66 | 12.48 | 12.73 | 12.70 | 12.71 | 12.70 | 12.27 | 12.29 | 12.22 | 12.23 | 12.39 | 12.37 | 12.67 | 12.59 | 12.57 | 12.62 |
| Na2O | 1.15 | 1.18 | 1.14 | 1.06 | 1.06 | 0.86 | 1.48 | 0.99 | 1.16 | 1.04 | 0.86 | 0.99 | 0.78 | 0.70 | 0.91 | 1.04 | 0.98 | 0.87 | 1.24 | 0.80 | 0.94 | 0.90 | 0.95 |
| K2O | 0.81 | 0.24 | 0.27 | 0.22 | 0.23 | 0.18 | 0.57 | 0.30 | 0.20 | 0.20 | 0.27 | 0.44 | 0.49 | 0.57 | 0.19 | 0.16 | 0.53 | 0.30 | 0.30 | 0.25 | 0.14 | 0.38 | 0.21 |
| F | 0.71 | 0.82 | 0.80 | 0.83 | 0.68 | 0.66 | 0.71 | 0.86 | 0.67 | 0.64 | 0.69 | 0.77 | 0.60 | 0.82 | 0.71 | 0.79 | 0.91 | 0.86 | 0.81 | 0.76 | 0.82 | 0.82 | 0.87 |
| Cl | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l | u.d.l |
| Cr2O3 | 0.25 | 0.37 | 0.36 | 0.38 | 0.21 | 0.14 | 0.28 | 0.12 | 0.09 | 0.06 | 0.12 | 0.01 | 0.15 | 0.10 | 0.07 | 0.07 | 0.44 | 0.06 | 0.16 | 0.10 | 0.10 | 0.10 | 0.10 |
| NiO | 0.04 | 0.02 | 0.01 | 0.01 | 0.03 | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0.03 | 0.04 | 0.03 | 0.04 | 0.05 | 0.02 | 0.01 | 0.07 | 0.04 | 0.04 | 0.05 | 0.05 | 0.04 |
| Total | 97.31 | 97.97 | 97.93 | 97.48 | 97.58 | 97.46 | 97.67 | 97.35 | 97.61 | 97.51 | 97.50 | 98.02 | 96.47 | 97.63 | 97.21 | 97.65 | 97.60 | 97.70 | 97.46 | 98.19 | 98.31 | 97.44 | 97.75 |
| Si | 7.09 | 7.30 | 7.28 | 7.29 | 7.34 | 7.48 | 6.85 | 7.32 | 7.29 | 7.35 | 7.38 | 7.27 | 7.32 | 7.32 | 7.43 | 7.38 | 7.15 | 7.37 | 7.12 | 7.43 | 7.47 | 7.32 | 7.43 |
| Al iv | 0.91 | 0.70 | 0.72 | 0.71 | 0.66 | 0.52 | 1.15 | 0.68 | 0.71 | 0.65 | 0.62 | 0.73 | 0.68 | 0.68 | 0.57 | 0.62 | 0.85 | 0.63 | 0.88 | 0.57 | 0.53 | 0.68 | 0.57 |
| Al vi | 0.20 | 0.09 | 0.15 | 0.10 | 0.11 | 0.12 | 0.24 | 0.15 | 0.15 | 0.12 | 0.16 | 0.13 | 0.11 | 0.13 | 0.10 | 0.09 | 0.15 | 0.09 | 0.12 | 0.05 | 0.05 | 0.13 | 0.12 |
| Ti | 0.03 | 0.07 | 0.05 | 0.07 | 0.08 | 0.03 | 0.09 | 0.04 | 0.07 | 0.05 | 0.03 | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.06 | 0.02 | 0.04 | 0.02 | 0.03 |
| Cr | 0.03 | 0.04 | 0.04 | 0.04 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.01 | 0.01 | 0.05 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| Fe3+ | 0.23 | 0.12 | 0.09 | 0.19 | 0.11 | 0.05 | 0.16 | 0.12 | 0.17 | 0.13 | 0.15 | 0.19 | 0.12 | 0.33 | 0.24 | 0.30 | 0.33 | 0.29 | 0.32 | 0.26 | 0.21 | 0.22 | 0.13 |
| Fe2+ | 1.36 | 1.40 | 1.39 | 1.29 | 1.36 | 1.39 | 1.46 | 1.31 | 1.24 | 1.25 | 1.23 | 1.26 | 1.71 | 1.49 | 1.48 | 1.52 | 1.56 | 1.47 | 1.26 | 1.26 | 1.29 | 1.33 | 1.37 |
| Mn | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.07 | 0.06 | 0.07 | 0.07 | 0.07 | 0.06 | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 |
| Mg | 3.12 | 3.23 | 3.24 | 3.27 | 3.29 | 3.36 | 2.98 | 3.33 | 3.33 | 3.41 | 3.39 | 3.34 | 2.96 | 2.96 | 3.08 | 2.99 | 2.82 | 3.05 | 3.17 | 3.35 | 3.36 | 3.24 | 3.30 |
| Ni | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 |
| Ca | 1.96 | 1.96 | 1.98 | 1.94 | 1.96 | 2.00 | 2.00 | 1.99 | 1.94 | 1.98 | 1.98 | 1.98 | 2.04 | 1.94 | 1.94 | 1.93 | 1.95 | 1.95 | 1.95 | 1.97 | 1.95 | 1.97 | 1.97 |
| Na | 0.33 | 0.34 | 0.32 | 0.30 | 0.30 | 0.24 | 0.42 | 0.28 | 0.33 | 0.29 | 0.24 | 0.28 | 0.23 | 0.20 | 0.26 | 0.30 | 0.28 | 0.25 | 0.35 | 0.23 | 0.26 | 0.26 | 0.27 |
| K | 0.15 | 0.04 | 0.05 | 0.04 | 0.04 | 0.03 | 0.11 | 0.06 | 0.04 | 0.04 | 0.05 | 0.08 | 0.09 | 0.11 | 0.04 | 0.03 | 0.10 | 0.06 | 0.06 | 0.05 | 0.03 | 0.07 | 0.04 |
| F | 0.33 | 0.38 | 0.37 | 0.38 | 0.31 | 0.31 | 0.33 | 0.40 | 0.31 | 0.30 | 0.32 | 0.36 | 0.29 | 0.38 | 0.33 | 0.37 | 0.43 | 0.40 | 0.38 | 0.35 | 0.38 | 0.38 | 0.40 |
| Cl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| OH * | 1.67 | 1.62 | 1.63 | 1.62 | 1.69 | 1.69 | 1.67 | 1.60 | 1.69 | 1.70 | 1.68 | 1.65 | 1.71 | 1.62 | 1.67 | 1.63 | 1.57 | 1.60 | 1.62 | 1.65 | 1.62 | 1.62 | 1.60 |
| Total | 17.44 | 17.34 | 17.36 | 17.28 | 17.30 | 17.27 | 17.53 | 17.32 | 17.31 | 17.32 | 17.27 | 17.34 | 17.36 | 17.24 | 17.24 | 17.25 | 17.33 | 17.26 | 17.35 | 17.24 | 17.24 | 17.30 | 17.28 |
| Mg/Mg + Fe2) | 0.70 | 0.70 | 0.70 | 0.72 | 0.71 | 0.71 | 0.67 | 0.72 | 0.73 | 0.73 | 0.73 | 0.73 | 0.63 | 0.67 | 0.68 | 0.66 | 0.64 | 0.68 | 0.72 | 0.73 | 0.72 | 0.71 | 0.71 |
| T (°C) | 794 | 752 | 761 | 752 | 747 | 724 | 843 | 754 | 759 | 750 | 744 | 762 | - | 731 | 714 | 719 | 762 | 724 | 781 | 722 | 715 | 746 | 728 |
| (σest) | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | - | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| Spot# | Bo1 | Bo2 | Bo3 | Bo4 | Bo5 | Bo6 | Bo7 | Bo8 | Bo9 | Bo10 | Bo11 | Bo12 | Bo13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 38.79 | 38.57 | 38.96 | 39.81 | 40.18 | 39.73 | 40.39 | 40.22 | 39.04 | 40.60 | 40.78 | 40.74 | 40.72 |
| TiO2 | 0.59 | 0.61 | 0.55 | 0.05 | 0.57 | 0.66 | 0.52 | 0.58 | 0.72 | 0.54 | 0.54 | 0.47 | 0.57 |
| Al2O3 | 14.68 | 14.44 | 14.26 | 18.89 | 12.54 | 12.67 | 12.62 | 12.58 | 13.34 | 12.54 | 12.50 | 12.55 | 13.00 |
| FeO | 12.73 | 12.59 | 12.34 | 12.69 | 12.79 | 13.08 | 12.29 | 12.74 | 14.31 | 12.79 | 10.49 | 11.00 | 11.23 |
| MnO | 0.17 | 0.14 | 0.16 | 0.15 | 0.30 | 0.26 | 0.29 | 0.31 | 0.34 | 0.31 | 0.14 | 0.17 | 0.18 |
| MgO | 16.77 | 16.70 | 16.93 | 11.75 | 16.79 | 16.87 | 17.32 | 17.13 | 16.03 | 17.05 | 18.91 | 18.65 | 18.27 |
| CaO | 0.17 | 0.18 | 0.17 | 1.73 | 0.01 | 0.03 | 0.05 | u.d.l | 0.02 | u.d.l | 0.08 | 0.04 | 0.05 |
| Na2O | 0.26 | 0.14 | 0.10 | 2.43 | 0.05 | 0.05 | 0.06 | 0.05 | 0.06 | 0.04 | 0.14 | 0.07 | 0.12 |
| K2O | 9.72 | 9.74 | 9.95 | 0.43 | 8.55 | 8.77 | 8.28 | 8.59 | 9.07 | 8.85 | 9.01 | 9.19 | 8.74 |
| F | 2.52 | 2.48 | 2.71 | 2.35 | 2.17 | 2.46 | 2.55 | 2.34 | 2.11 | 2.71 | 3.32 | 3.00 | 2.90 |
| Cl | 0.03 | 0.02 | 0.01 | 0.02 | 0.03 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.00 | 0.02 |
| Cr2O3 | 0.08 | 0.10 | 0.09 | 0.12 | 0.11 | 0.09 | 0.07 | 0.11 | 0.18 | 0.09 | 0.08 | 0.06 | 0.11 |
| Li2O * | 1.58 | 1.52 | 1.63 | 1.87 | 1.98 | 1.85 | 2.04 | 1.99 | 1.65 | 2.10 | 2.15 | 2.14 | 2.14 |
| H2O * | 2.89 | 2.88 | 2.79 | 2.96 | 3.02 | 2.88 | 2.87 | 2.97 | 3.04 | 2.82 | 2.56 | 2.72 | 2.77 |
| Subtotal | 100.97 | 100.09 | 100.64 | 95.25 | 99.08 | 99.39 | 99.39 | 99.61 | 99.92 | 100.45 | 100.72 | 100.80 | 100.81 |
| O=F,Cl | 1.07 | 1.05 | 1.14 | 0.99 | 0.92 | 1.04 | 1.08 | 0.99 | 0.89 | 1.14 | 1.40 | 1.26 | 1.23 |
| Total | 99.90 | 99.04 | 99.50 | 94.26 | 98.16 | 98.35 | 98.31 | 98.62 | 99.03 | 99.30 | 99.32 | 99.54 | 99.58 |
| Si | 5.69 | 5.70 | 5.73 | 5.85 | 5.93 | 5.88 | 5.93 | 5.91 | 5.79 | 5.93 | 5.91 | 5.90 | 5.89 |
| Aliv | 2.32 | 2.30 | 2.27 | 2.15 | 2.07 | 2.12 | 2.07 | 2.09 | 2.21 | 2.07 | 2.10 | 2.10 | 2.12 |
| Sum (Z) | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Alvi | 0.22 | 0.22 | 0.20 | 1.12 | 0.12 | 0.09 | 0.12 | 0.09 | 0.12 | 0.09 | 0.04 | 0.04 | 0.10 |
| Ti | 0.07 | 0.07 | 0.06 | 0.01 | 0.06 | 0.07 | 0.06 | 0.07 | 0.08 | 0.06 | 0.06 | 0.05 | 0.06 |
| Cr | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| Fe | 1.56 | 1.56 | 1.52 | 1.56 | 1.58 | 1.62 | 1.51 | 1.57 | 1.78 | 1.56 | 1.27 | 1.33 | 1.36 |
| Mn | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 | 0.02 | 0.02 | 0.02 |
| Mg | 3.66 | 3.68 | 3.71 | 2.57 | 3.70 | 3.72 | 3.79 | 3.75 | 3.55 | 3.71 | 4.08 | 4.03 | 3.94 |
| Li * | 0.93 | 0.90 | 0.96 | 1.11 | 1.18 | 1.10 | 1.21 | 1.18 | 0.99 | 1.23 | 1.25 | 1.25 | 1.24 |
| Sum (Y) | 6.47 | 6.45 | 6.48 | 6.40 | 6.68 | 6.65 | 6.73 | 6.70 | 6.57 | 6.71 | 6.73 | 6.73 | 6.73 |
| Ca | 0.03 | 0.03 | 0.03 | 0.27 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 |
| Na | 0.07 | 0.04 | 0.03 | 0.69 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.04 | 0.02 | 0.03 |
| K | 1.82 | 1.84 | 1.87 | 0.08 | 1.61 | 1.66 | 1.55 | 1.61 | 1.72 | 1.65 | 1.66 | 1.70 | 1.61 |
| Sum (X) | 1.92 | 1.90 | 1.92 | 1.04 | 1.63 | 1.67 | 1.58 | 1.62 | 1.74 | 1.66 | 1.72 | 1.73 | 1.65 |
| OH * | 2.82 | 2.84 | 2.74 | 2.90 | 2.98 | 2.85 | 2.81 | 2.91 | 3.01 | 2.75 | 2.48 | 2.63 | 2.67 |
| F | 1.17 | 1.16 | 1.26 | 1.09 | 1.01 | 1.15 | 1.19 | 1.09 | 0.99 | 1.25 | 1.52 | 1.37 | 1.33 |
| Cl | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sum | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| TOTAL | 20.39 | 20.36 | 20.40 | 19.44 | 20.31 | 20.32 | 20.30 | 20.33 | 20.31 | 20.37 | 20.44 | 20.46 | 20.38 |
| Al total | 2.54 | 2.52 | 2.47 | 3.27 | 2.18 | 2.21 | 2.18 | 2.18 | 2.33 | 2.16 | 2.13 | 2.14 | 2.21 |
| Fe/Fe + Mg | 0.30 | 0.30 | 0.29 | 0.38 | 0.30 | 0.30 | 0.29 | 0.29 | 0.33 | 0.30 | 0.24 | 0.25 | 0.26 |
| Mg/Fe | 2.35 | 2.36 | 2.45 | 1.65 | 2.34 | 2.30 | 2.51 | 2.40 | 2.00 | 2.38 | 3.21 | 3.02 | 2.90 |
| mgli ** | 2.73 | 2.78 | 2.75 | 1.47 | 2.52 | 2.62 | 2.59 | 2.58 | 2.56 | 2.48 | 2.83 | 2.78 | 2.69 |
| feal ** | 1.43 | 1.42 | 1.40 | 0.46 | 1.57 | 1.63 | 1.49 | 1.58 | 1.77 | 1.57 | 1.31 | 1.36 | 1.34 |
6. Whole Rock Geochemistry
7. Discussion
7.1. Testing of Magmatic Processes and Various Models
7.2. Evidence for Liquid Immiscibility
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sami, M.; El Monsef, M.A.; Abart, R.; Toksoy-Köksal, F.; Abdelfadil, K.M. Unraveling the Genesis of Highly Fractionated Rare-Metal Granites in the Nubian Shield via the Rare-Earth Elements Tetrad Effect, Sr–Nd Isotope Systematics, and Mineral Chemistry. ACS Earth Space Chem. 2022, 6, 2368–2384. [Google Scholar] [CrossRef]
- Siegel, K.; Vasyukova, O.V.; Williams-Jones, A.E. Magmatic evolution and controls on rare metal-enrichment of the Strange Lake A-type peralkaline granitic pluton, Québec-Labrador. Lithos 2018, 308, 34–52. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E.; Collins, P. Lithogeochemical vectors for hydrothermal processes in the Strange Lake peralkaline granitic REE-Zr-Nb deposit. Econ. Geol. 2016, 111, 1241–1276. [Google Scholar] [CrossRef]
- Mahdy, N.M.; El Kalioubi, B.A.; Wohlgemuth-Ueberwasser, C.C.; Shalaby, M.H.; El-Afandy, A.H. Petrogenesis of U- and Mo-bearing A2-type granite of the Gattar batholith in the Arabian Nubian Shield, Northeastern Desert, Egypt: Evidence for the favorability of host rocks for the origin of associated ore deposits. Ore Geol. Rev. 2015, 71, 57–81. [Google Scholar] [CrossRef]
- Mahdy, N.M.; Ntaflos, T.; Pease, V.; Sami, M.; Slobodník, M.; Abu Steet, A.A.; Abdelfadil, K.M.; Fathy, D. Combined zircon U-Pb dating and chemical Th–U–total Pb chronology of monazite and thorite, Abu Diab A-type granite, Central Eastern Desert of Egypt: Constraints on the timing and magmatic-hydrothermal evolution of rare metal granitic magmatism in the Arabian Nubian Shield. Geochemistry 2020, 80, 125669. [Google Scholar] [CrossRef]
- Abd El Monsef, M.; Sami, M.; Toksoy-Köksal, F.; Abart, R.; Ondrejka, M.; Abdelfadil, K.M. Role of Magmatism and Related-Exsolved Fluids during Ta-Nb-Sn Concentration in the Central Eastern Desert of Egypt: Evidences from Mineral Chemistry and Fluid Inclusions. J. Earth Sci. 2023. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, C.; Tan, K.; Duan, X.; Shi, K.; Sui, Q.; Feng, P.; Sami, M.; Ahmed, M.S.; Zi, F. Two Distinct Fractional Crystallization Mechanisms of A-Type Granites in the Nanling Range, South China: A Case Study of the Jiuyishan Complex Massif and Xianghualing Intrusive Stocks. Minerals 2023, 13, 605. [Google Scholar] [CrossRef]
- Vasyukova, O.; Williams-Jones, A.E. Fluoride–silicate melt immiscibility and its role in REE ore formation: Evidence from the Strange Lake rare metal deposit, Québec-Labrador, Canada. Geochim. Cosmochim. Acta 2014, 139, 110–130. [Google Scholar] [CrossRef]
- Vasyukova, O.; Williams-Jones, A. The evolution of immiscible silicate and fluoride melts: Implications for REE ore-genesis. Geochim. Cosmochim. Acta 2016, 172, 205–224. [Google Scholar] [CrossRef]
- Thompson, A.B.; Aerts, M.; Hack, A.C. Liquid immiscibility in silicate melts and related systems. Rev. Miner. Geochem. 2007, 65, 99–127. [Google Scholar] [CrossRef]
- Veksler, I.V. Liquid immiscibility and its role at the magmatic–hydrothermal transition: A summary of experimental studies. Chem. Geol. 2004, 210, 7–31. [Google Scholar] [CrossRef]
- Roedder, E.; Weiblen, P.W. High-silica glass inclusions in olivine of Luna-24 samples. Geophys. Res. Lett. 1977, 4, 485–488. [Google Scholar] [CrossRef]
- Philpott, A.R. Immiscibility between critically undersaturated basic and oversaturated felsic magmas. In Transactions American Geophysical Union; Spilhaus, A.F., Ed.; American Geophysical Union: Washington, DC, USA, 1970; p. 438. [Google Scholar]
- Tornos, F.; Velasco, F.; Hanchar, J.M. Iron-rich melts, magmatic magnetite, and superheated hydrothermal systems: The El Laco deposit, Chile. Geology 2016, 44, 427–430. [Google Scholar] [CrossRef]
- Hou, T.; Charlier, B.; Holtz, F.; Veksler, I.; Zhang, Z.; Thomas, R.; Namur, O. Immiscible hydrous Fe–Ca–P melt and the origin of iron oxide-apatite ore deposits. Nat. Commun. 2018, 9, 1415. [Google Scholar] [CrossRef]
- Thomas, R.; Kamenetsky, V.S.; Davidson, P. Laser Raman spectroscopic measurements of water in unexposed glass inclusions. Am. Mineral. 2006, 91, 467–470. [Google Scholar] [CrossRef]
- Naumov, V.; Kamenetsky, V. Silicate and salt melts in the genesis of the industrial’noe tin deposit: Evidence from inclusions in minerals. Geochem. Int. 2006, 44, 1181–1190. [Google Scholar] [CrossRef]
- Holness, M.B.; Stripp, G.; Humphreys, M.; Veksler, I.V.; Nielsen, T.F.; Tegner, C. Silicate liquid immiscibility within the crystal mush: Late-stage magmatic microstructures in the Skaergaard intrusion, East Greenland. J. Pet. 2011, 52, 175–222. [Google Scholar] [CrossRef]
- Jakobsen, J.K.; Veksler, I.V.; Tegner, C.; Brooks, C.K. Crystallization of the Skaergaard intrusion from an emulsion of immiscible iron-and silica-rich liquids: Evidence from melt inclusions in plagioclase. J. Pet. 2011, 52, 345–373. [Google Scholar] [CrossRef]
- Yang, L.; van Hinsberg, V.J. Liquid immiscibility in the CaF2-granite system and trace element partitioning between the immiscible liquids. Chem. Geol. 2019, 511, 28–41. [Google Scholar] [CrossRef]
- Borisov, A.; Veksler, I.V. Immiscible silicate liquids: K and Fe distribution as a test for chemical equilibrium and insight into the kinetics of magma unmixing. Contrib. Mineral. Petrol. 2021, 176, 1–11. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Charlier, B.; Zhitova, L.; Sharygin, V.; Davidson, P.; Feig, S. Magma chamber–scale liquid immiscibility in the Siberian Traps represented by melt pools in native iron. Geology 2013, 41, 1091–1094. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Kamenetsky, V.S.; Zaitsev, A.N.; Kamenetsky, M.B. Silicate–natrocarbonatite liquid immiscibility in 1917 eruption combeite–wollastonite nephelinite, Oldoinyo Lengai Volcano, Tanzania: Melt inclusion study. Lithos 2012, 152, 23–39. [Google Scholar] [CrossRef]
- Potter, N.J.; Kamenetsky, V.S.; Simonetti, A.; Goemann, K. Different types of liquid immiscibility in carbonatite magmas: A case study of the Oldoinyo Lengai 1993 lava and melt inclusions. Chem. Geol. 2017, 455, 376–384. [Google Scholar] [CrossRef]
- Panina, L.; Motorina, I. Liquid immiscibility in deep-seated magmas and the generation of carbonatite melts. Geochem. Int. 2008, 46, 448–464. [Google Scholar] [CrossRef]
- Zelenski, M.; Kamenetsky, V.; Mavrogenes, J.; Gurenko, A.; Danyushevsky, L. Silicate-sulfide liquid immiscibility in modern arc basalt (Tolbachik volcano, Kamchatka): Part I. Occurrence and compositions of sulfide melts. Chem. Geol. 2018, 478, 102–111. [Google Scholar] [CrossRef]
- Webster, J.D. The exsolution of magmatic hydrosaline chloride liquids. Chem. Geol. 2004, 210, 33–48. [Google Scholar] [CrossRef]
- Klemme, S. Evidence for fluoride melts in Earth’s mantle formed by liquid immiscibility. Geology 2004, 32, 441–444. [Google Scholar] [CrossRef]
- Stern, R.J.; Johnson, P. Continental lithosphere of the Arabian Plate: A geologic, petrologic, and geophysical synthesis. Earth Sci. Rev. 2010, 101, 29–67. [Google Scholar] [CrossRef]
- Boukar, M.; Njom, B.; Yannah, M.; Moundi, A.; Fathy, D.; Abou, T.; Temdjim, R.; Paul-Desiré, N.; Sami, M. Local structural markers of the Batouri gold-bearing shear zone in Southeast Cameroon. Geopersia 2023. [Google Scholar] [CrossRef]
- Sami, M.; Adam, M.M.; Lv, X.; Lasheen, E.S.R.; Ene, A.; Zakaly, H.M.; Alarifi, S.S.; Mahdy, N.M.; Abdel Rahman, A.R.A.; Saeed, A. Petrogenesis and Tectonic Implications of the Cryogenian I-Type Granodiorites from Gabgaba Terrane (NE Sudan). Minerals 2023, 13, 331. [Google Scholar] [CrossRef]
- Sami, M.; Azer, M.; Abdel-Karim, A.-A. Post-collisional Ferani volcanics from north Arabian-Nubian Shield (south Sinai, Egypt): Petrogenesis and implication for Ediacaran (607-593 Ma) geodynamic evolution. J. Geol. 2023. [Google Scholar] [CrossRef]
- Abdelfadil, K.M.; Saleh, G.M.; Putiš, M.; Sami, M. Mantle source characteristics of the late Neoproterozoic post-collisional gabbroic intrusion of Wadi Abu Hadieda, north Arabian-Nubian Shield, Egypt. J. Afr. Earth Sci. 2022, 194, 104607. [Google Scholar] [CrossRef]
- El-Bialy, M.Z.; Eliwa, H.A.; Mahdy, N.M.; Murata, M.; El-Gameel, K.H.; Sehsah, H.; Omar, M.; Kato, Y.; Fujinaga, K.; Andresen, A.; et al. U-Pb zircon geochronology and geochemical constraints on the Ediacaran continental arc and post-collision Granites of Wadi Hawashiya, North Eastern Desert, Egypt: Insights into the ~600 Ma crust-forming Event in the northernmost part of Arabian-Nubian Shield. Precambrian Res. 2020, 345, 105777. [Google Scholar] [CrossRef]
- Weissman, A.; Kessel, R.; Navon, O.; Stein, M. The petrogenesis of calc-alkaline granites from the Elat massif, Northern Arabian–Nubian shield. Precambrian Res. 2013, 236, 252–264. [Google Scholar] [CrossRef]
- Kessel, R.; Stein, M.; Navon, O. Petrogenesis of Late Neoproterozoic dikes in the Northern Arabian–Nubian Shield: Implications for the origin of A-type granites. Precambrian Res. 1998, 92, 195–213. [Google Scholar] [CrossRef]
- Mushkin, A.; Navon, O.; Halicz, L.; Hartmann, G.; Stein, M. The petrogenesis of A-type magmas from the Amram Massif, southern Israel. J. Pet. 2003, 44, 815–832. [Google Scholar] [CrossRef]
- Melcher, F.; Graupner, T.; Gäbler, H.-E.; Sitnikova, M.; Henjes-Kunst, F.; Oberthür, T.; Gerdes, A.; Dewaele, S. Tantalum–(niobium–tin) mineralisation in African pegmatites and rare metal granites: Constraints from Ta–Nb oxide mineralogy, geochemistry and U–Pb geochronology. Ore Geol. Rev. 2015, 64, 667–719. [Google Scholar] [CrossRef]
- Gahlan, H.A.; Azer, M.K.; Al-Hashim, M.H.; Heikal, M.T.S. Highly evolved rare-metal bearing granite overprinted by alkali metasomatism in the Arabian Shield: A case study from the Jabal Tawlah granites. J. Afr. Earth Sci. 2022, 192, 104556. [Google Scholar] [CrossRef]
- Mahdy, N.M. Textural and chemical characteristics of zircon, monazite, and thorite, Wadi Al-Baroud area, Eastern Desert of Egypt: Implication for rare metal pegmatite genesis. Ore Geol. Rev. 2021, 136, 104225. [Google Scholar] [CrossRef]
- Sami, M.; Ntaflos, T.; Farahat, E.S.; Mohamed, H.A.; Ahmed, A.F.; Hauzenberger, C. Mineralogical, geochemical and Sr-Nd isotopes characteristics of fluorite-bearing granites in the Northern Arabian-Nubian Shield, Egypt: Constraints on petrogenesis and evolution of their associated rare metal mineralization. Ore Geol. Rev. 2017, 88, 1–22. [Google Scholar] [CrossRef]
- Eliwa, H.; Deevsalar, R.; Mahdy, N.; Kumar, S.; El-Gameel, K.; Zafar, T.; Khalaf, I.; Murata, M.; Ozawa, H.; Andresen, A. Field, textural, geochemical, and isotopic constraints on the origin and evolution of the magmatic microgranular enclaves from the Gharib Granitoid Complex, North Eastern Desert, Egypt. Precambrian Res. 2021, 365, 106380. [Google Scholar] [CrossRef]
- Pouchou, J.-L.; Pichoir, F. Quantitative Analysis of Homogeneous or Stratified Microvolumes Applying the Model “PAP”. In Electron Probe Quantitation; Heinrich, K.F.J., Newbury, D.E., Eds.; Springer US: Boston, MA, USA, 1991; pp. 31–75. [Google Scholar]
- Ali, S.; Abart, R.; Sayyed, M.I.; Hauzenberger, C.A.; Sami, M. Petrogenesis of the Wadi El-Faliq Gabbroic Intrusion in the Central Eastern Desert of Egypt: Implications for Neoproterozoic Post-Collisional Magmatism Associated with the Najd Fault System. Minerals 2023, 13, 10. [Google Scholar] [CrossRef]
- Leake, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.C.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of Amphiboles; Report of the Subcommittee on Amphiboles of the International Mineralogical Association Commission on New Minerals and Mineral Names. Miner. Mag. 1997, 61, 295–310. [Google Scholar] [CrossRef]
- Ridolfi, F.; Renzulli, A.; Puerini, M. Stability and chemical equilibrium of amphibole in calc-alkaline magmas: An overview, new thermobarometric formulations and application to subduction-related volcanoes. Contrib. Mineral. Petrol. 2010, 160, 45–66. [Google Scholar] [CrossRef]
- Munoz, J.L. F-OH and Cl-OH exchange in micas with applications to hydrothermal ore deposits. Rev. Mineral. Geochem. 1984, 13, 469–493. [Google Scholar]
- Scott, K. Phyllosilicate and rutile compositions as indicators of Sn specialization in some southeastern Australian granites. Miner. Depos. 1988, 23, 159–165. [Google Scholar] [CrossRef]
- Azadbakht, Z.; Lentz, D.R.; McFarlane, C.R.; Whalen, J.B. Using magmatic biotite chemistry to differentiate barren and mineralized Silurian–Devonian granitoids of New Brunswick, Canada. Contrib. Mineral. Petrol. 2020, 175, 69. [Google Scholar] [CrossRef]
- Foster, M. Interpretation of Composition of Trioctaheral Micas; Professional Papers. 354-B; US Geological Survey: Reston, VI, USA, 1960; pp. 1–49. [Google Scholar]
- Tischendorf, G.; Gottesmann, B.; Förster, H.-J.; Trumbull, R.B. On Li-bearing micas: Estimating Li from electron microprobe analyses and an improved diagram for graphical representation. Miner. Mag. 1997, 61, 809–834. [Google Scholar] [CrossRef]
- Nachit, H.; Ibhi, A.; Abia, E.H.; Ben Ohoud, M. Discrimination between primary magmatic biotites, reequilibrated biotites and neoformed biotites. Comptes Rendus Geosci. 2005, 337, 1415–1420. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.-C.; Chen, J.; Zhu, J.-C. Mineralogical evidence for magmatic and hydrothermal processes in the Qitianling oxidized tin-bearing granite (Hunan, South China): EMP and (MC)-LA-ICPMS investigations of three types of titanite. Chem. Geol. 2010, 276, 53–68. [Google Scholar] [CrossRef]
- Pan, L.-C.; Hu, R.-Z.; Bi, X.-W.; Li, C.; Wang, X.-S.; Zhu, J.-J. Titanite major and trace element compositions as petrogenetic and metallogenic indicators of Mo ore deposits: Examples from four granite plutons in the southern Yidun arc, SW China. Am. Mineral. J. Earth Planet. Mater. 2018, 103, 1417–1434. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. Some Recent Advances in the Mineralogy and Geochemistry of Nb and Ta in Rare-Element Granitic Pegmatites. Bull. De Mineral. 1985, 108, 499–532. [Google Scholar] [CrossRef]
- Breiter, K.; Škoda, R.; Uher, P. Nb-Ta-Ti-W-Sn-oxide minerals as indicators of a peraluminous P- and F-rich granitic system evolution: Podlesí, Czech Republic. Mineral. Petrol. 2007, 91, 225–248. [Google Scholar] [CrossRef]
- Tindle, A.G.; Webb, P.C. Estimation of lithium contents in trioctahedral micas using microprobe data: Application to micas from granitic rocks. Eur. J. Mineral. 1990, 2, 595–610. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; IUGS Subcommission on the Systematics of Igneous Rocks. A chemical classification of volcanic rocks based on the total alkali-silica diagram. J. Pet. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Naming materials in the magma/igneous rock system. Earth Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Maniar, P.D.; Piccoli, P.M. Tectonic discrimination of granitoids. Geol. Soc. Am. Bull. 1989, 101, 635–643. [Google Scholar] [CrossRef]
- Frost, B.R.; Barnes, C.G.; Collins, W.J.; Arculus, R.J.; Ellis, D.J.; Frost, C.D. A geochemical classification for granitic rocks. J. Pet. 2001, 42, 2033–2048. [Google Scholar] [CrossRef]
- Irber, W. The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu∗, Sr/Eu, Y/Ho, and Zr/Hf of evolving peraluminous granite suites. Geochim. Cosmochim. Acta 1999, 63, 489–508. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Dou, J.; Huang, X.; Chen, F. Successive magma mixing in deep-seated magma chambers recorded in zircon from mafic microgranular enclaves in the Triassic Mishuling granitic pluton, Western Qinling, Central China. J. Asian Earth Sci. 2021, 207, 104656. [Google Scholar] [CrossRef]
- Poli, G.; Christofides, G.; Koroneos, A.; Trajanova, M.; Zupančič, N. Multiple processes in the genesis of the Pohorje igneous complex: Evidence from petrology and geochemistry. Lithos 2020, 364–365, 105512. [Google Scholar] [CrossRef]
- Barnes, C.; Werts, K.; Memeti, V.; Paterson, S.; Bremer, R. A tale of five enclaves: Mineral perspectives on origins of mafic enclaves in the Tuolumne Intrusive Complex. Geosphere 2021, 17, 352–374. [Google Scholar] [CrossRef]
- Sami, M.; Ntaflos, T.; Farahat, E.S.; Mohamed, H.A.; Hauzenberger, C.; Ahmed, A.F. Petrogenesis and geodynamic implications of Ediacaran highly fractionated A-type granitoids in the north Arabian-Nubian Shield (Egypt): Constraints from whole-rock geochemistry and Sr-Nd isotopes. Lithos 2018, 304–307, 329–346. [Google Scholar] [CrossRef]
- Perugini, D.; Poli, G.; Christofides, G.; Eleftheriadis, G. Magma mixing in the Sithonia Plutonic Complex, Greece: Evidence from mafic microgranular enclaves. Mineral. Petrol. 2003, 78, 173–200. [Google Scholar] [CrossRef]
- Kocak, K.; Zedef, V.; Kansun, G. Magma mixing/mingling in the Eocene Horoz (Nigde) granitoids, Central southern Turkey: Evidence from mafic microgranular enclaves. Mineral. Petrol. 2011, 103, 149–167. [Google Scholar] [CrossRef]
- Zafar, T.; Rehman, H.U.; Mahar, M.A.; Alam, M.; Oyebamiji, A.; Rehman, S.U.; Leng, C.-B. A critical review on petrogenetic, metallogenic and geodynamic implications of granitic rocks exposed in north and east China: New insights from apatite geochemistry. J. Geodyn. 2020, 136, 101723. [Google Scholar] [CrossRef]
- Shellnutt, J.G.; Jahn, B.M.; Dostal, J. Elemental and Sr–Nd isotope geochemistry of microgranular enclaves from peralkaline A-type granitic plutons of the Emeishan large igneous province, SW China. Lithos 2010, 119, 34–46. [Google Scholar] [CrossRef]
- Zhou, J.-S.; Yang, Z.-S.; Hou, Z.-Q.; Wang, Q. Amphibole-rich cumulate xenoliths in the Zhazhalong intrusive suite, Gangdese arc: Implications for the role of amphibole fractionation during magma evolution. Am. Mineral. 2020, 105, 262–275. [Google Scholar] [CrossRef]
- Chen, Y.; Price, R.; White, A. Inclusions in three S-type granites from southeastern Australia. J. Pet. 1989, 30, 1181–1218. [Google Scholar] [CrossRef]
- Montel, J.; Didier, J.; Pichavant, M. Origin of surmicaceous enclaves in intrusive granites. In Enclaves and Granite Petrology; Didier, J., Barbarin, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 509–528. [Google Scholar]
- Mushkin, A.; Stein, M.; Halicz, L.; Navon, O. The Daly gap: Low-pressure fractionation and heat-loss from cooling magma chamber. Geochim. Cosmochim. Acta 2002, 66, A539. [Google Scholar]
- Dorais, M.J.; Lira, R.; Chen, Y.; Tingey, D. Origin of biotite-apatite-rich enclaves, Achala batholith, Argentina. Contrib. Mineral. Petrol. 1997, 130, 31–46. [Google Scholar] [CrossRef]
- Chazot, G.; Bertrand, H. Genesis of silicic magmas during tertiary continental rifting in Yemen. Lithos 1995, 36, 69–83. [Google Scholar] [CrossRef]
- Veksler, I.V.; Dorfman, A.M.; Rhede, D.; Wirth, R.; Borisov, A.A.; Dingwell, D.B. Liquid unmixing kinetics and the extent of immiscibility in the system K2O–CaO–FeO–Al2O3–SiO2. Chem. Geol. 2008, 256, 119–130. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B. Magmatic fluids immiscible with silicate melts: Examples from inclusions in phenocrysts and glasses, and implications for magma evolution and metal transport. Geofluids 2010, 10, 293–311. [Google Scholar]
- Peretyazhko, I.; Savina, E. Fluid and magmatic processes in the formation of the Ary-Bulak ongonite massif (eastern Transbaikalia). Russ. Geol. Geophys. 2010, 51, 1110–1125. [Google Scholar] [CrossRef]
- Veksler, I.V.; Dorfman, A.M.; Kamenetsky, M.; Dulski, P.; Dingwell, D.B. Partitioning of lanthanides and Y between immiscible silicate and fluoride melts, fluorite and cryolite and the origin of the lanthanide tetrad effect in igneous rocks. Geochim. Cosmochim. Acta 2005, 69, 2847–2860. [Google Scholar] [CrossRef]
- Alferyeva, Y.O.; Gramenitskii, E.; Shchekina, T. Experimental study of phase relations in a lithium-bearing fluorine-rich haplogranite and nepheline syenite system. Geochem. Int. 2011, 49, 676–690. [Google Scholar] [CrossRef]
- Veksler, I.V.; Dorfman, A.M.; Dulski, P.; Kamenetsky, V.S.; Danyushevsky, L.V.; Jeffries, T.; Dingwell, D.B. Partitioning of elements between silicate melt and immiscible fluoride, chloride, carbonate, phosphate and sulfate melts, with implications to the origin of natrocarbonatite. Geochim. Cosmochim. Acta 2012, 79, 20–40. [Google Scholar] [CrossRef]
- Peretyazhko, I.; Savina, E.; Suk, N.; Kotelnikov, A.; Sapozhnikov, A.; Shendrik, R.Y. Evolution of the fluoride–calcium melt composition according to experimental data and fluorite formation in rhyolites. Petrology 2020, 28, 221–245. [Google Scholar] [CrossRef]
- Dolejš, D. Evidence for fluoride melts in Earth’s mantle formed by liquid immiscibility: Comment and Reply: COMMENT. Geology 2005, 33, e76. [Google Scholar] [CrossRef]
- Marshall, A.; Hinton, R.; MacDonald, R. Phenocrystic fluorite in peralkaline rhyolites, Olkaria, Kenya rift valley. Miner. Mag. 1998, 62, 477–486. [Google Scholar] [CrossRef]
- Bucher, K.; Seelig, U. Bristen granite: A highly differentiated, fluorite-bearing A-type granite from the Aar massif, Central Alps, Switzerland. Swiss J. Geosci. 2018, 111, 317–340. [Google Scholar] [CrossRef]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Bao, Z.W.; Qiao, Y.L. A peculiar composite M- and W-type REE tetrad effect: Evidence from the Shuiquangou alkaline syenite complex, Hebei Province, China. Chin. Sci. Bull. 2010, 55, 2684–2696. [Google Scholar] [CrossRef]
- Mclennan, S.M. Rare-Earth Element Geochemistry and the Tetrad Effect. Geochim. Cosmochim. Acta 1994, 58, 2025–2033. [Google Scholar] [CrossRef]
- Monecke, T.; Dulski, P.; Kempe, U. Origin of convex tetrads in rare earth element patterns of hydrothermally altered siliceous igneous rocks from the Zinnwald Sn–W deposit, Germany. Geochim. Cosmochim. Acta 2007, 71, 335–353. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Zhang, H. The lanthanide tetrad effect in apatite from the Altay No. 3 pegmatite, Xingjiang, China: An intrinsic feature of the pegmatite magma. Chem. Geol. 2005, 214, 61–77. [Google Scholar] [CrossRef]
- Cao, M.-J.; Zhou, Q.-F.; Qin, K.-Z.; Tang, D.-M.; Evans, N.J. The tetrad effect and geochemistry of apatite from the Altay Koktokay No. 3 pegmatite, Xinjiang, China: Implications for pegmatite petrogenesis. Mineral. Petrol. 2013, 107, 985–1005. [Google Scholar] [CrossRef]
- King, P.L.; White, A.J.R.; Chappell, B.W.; Allen, C.M. Characterization and origin of aluminous A-type granites from the Lachlan Fold Belt, Southeastern Australia. J. Pet. 1997, 38, 371–391. [Google Scholar] [CrossRef]
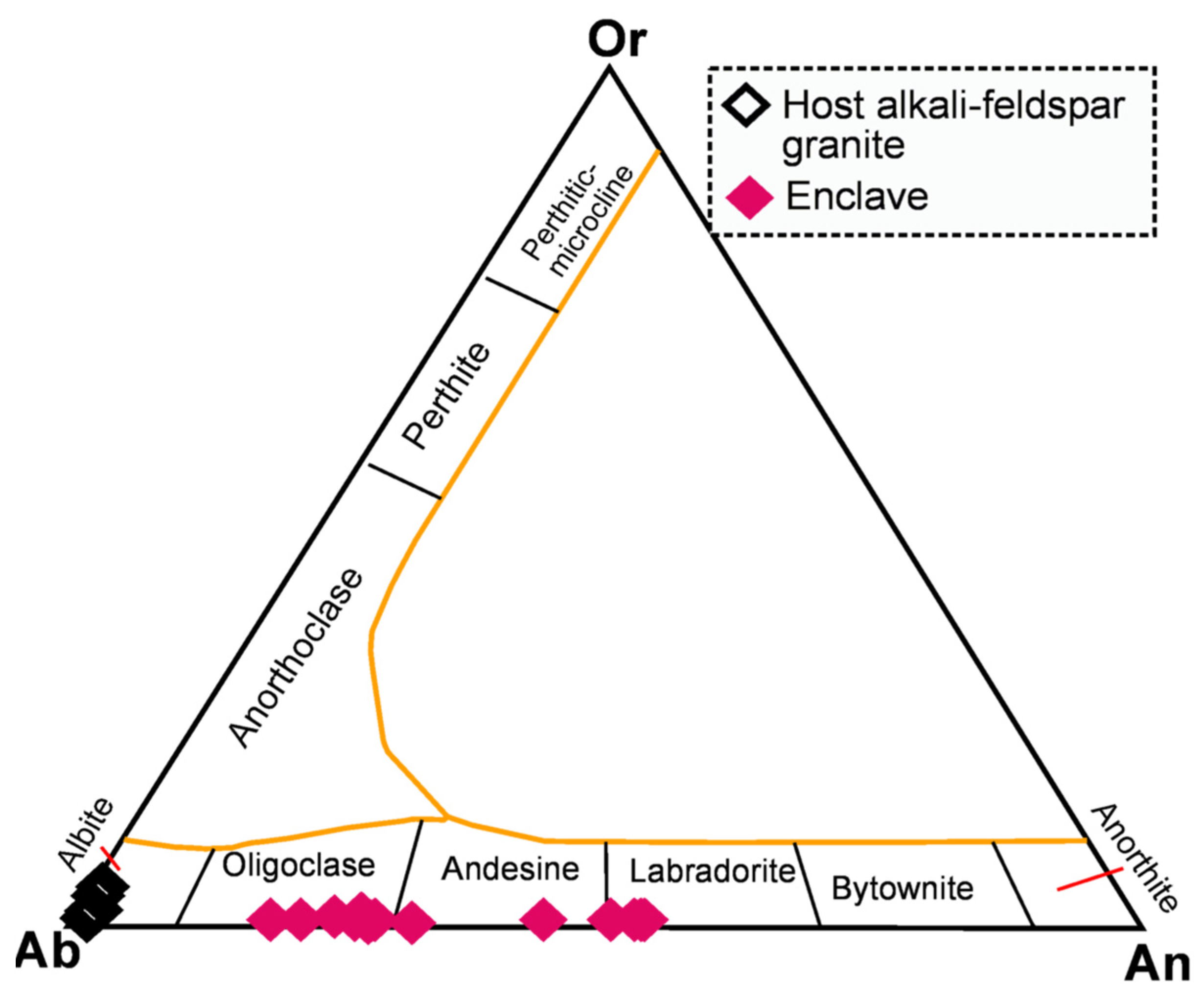
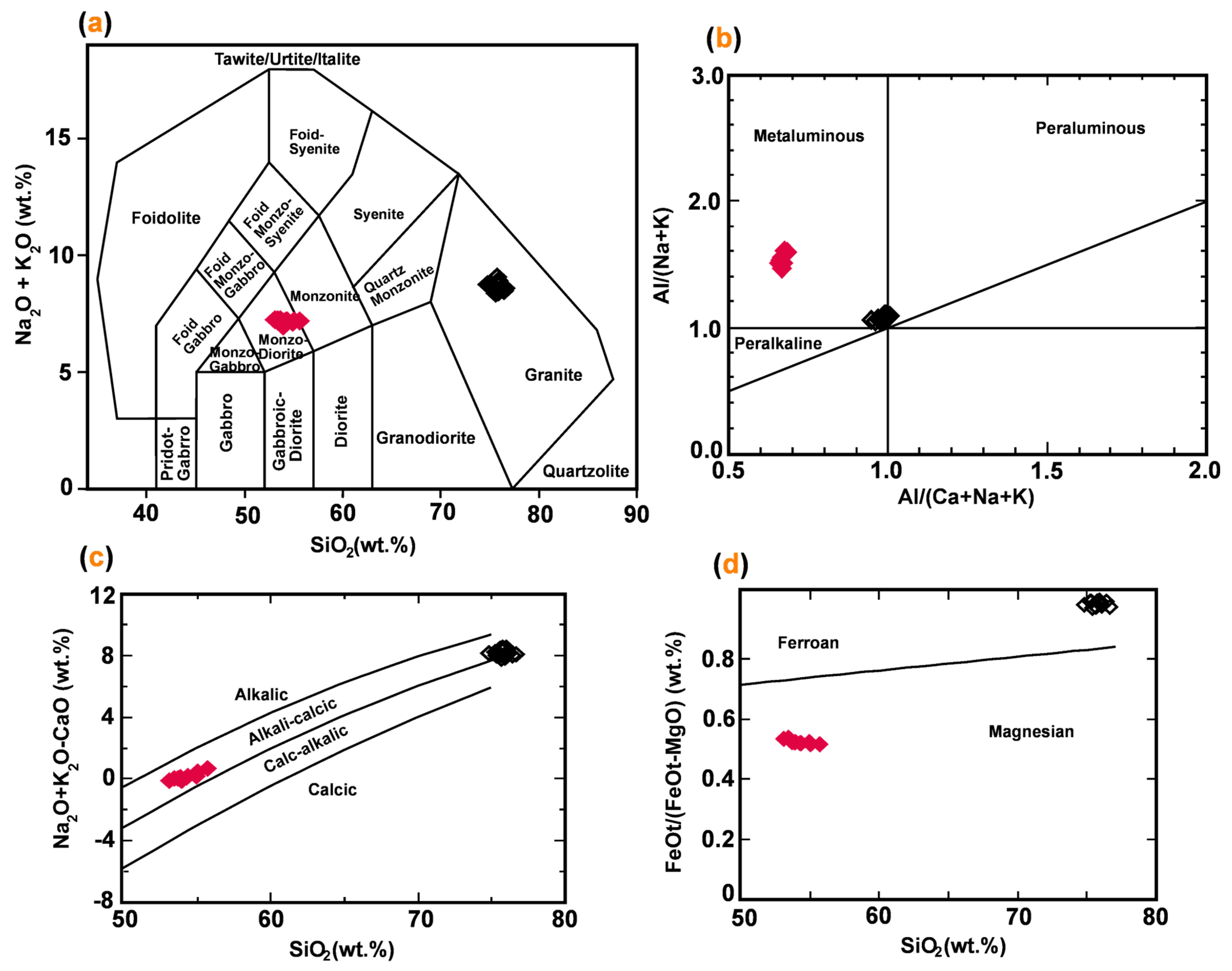


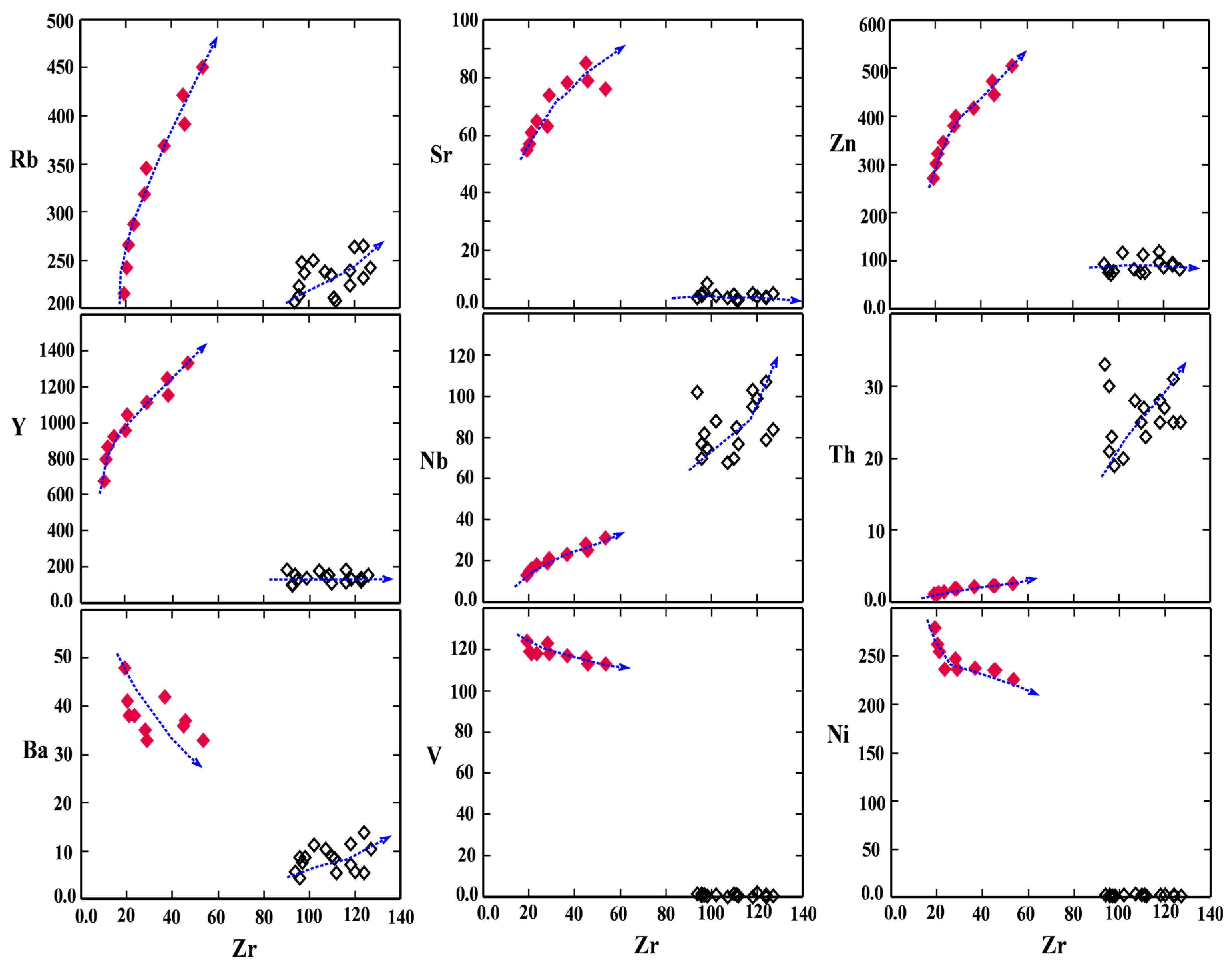
| Spot# | Pl1 | Pl2 | Pl3 | Pl4 | Pl5 | Pl6 | Pl7 | Pl8 | Pl9 | Pl10 | Pl11 | Pl12 | Pl13 | Pl14 | Pl15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 70.95 | 60.24 | 56.06 | 54.89 | 55.51 | 54.92 | 54.78 | 57.18 | 54.63 | 54.81 | 62.05 | 61.22 | 62.6 | 63.43 | 61.36 |
| TiO2 | 0.002 | 0.004 | 0.003 | 0.004 | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.011 | 0.001 | 0.011 | 0.001 | 0.001 | 0.006 |
| Al2O3 | 17.68 | 24.56 | 22.37 | 28.09 | 27.73 | 28.28 | 28.31 | 26.83 | 28.27 | 28.28 | 23.98 | 24.54 | 23.7 | 22.74 | 24.67 |
| FeO | 1.2 | 0.25 | 2.91 | 0.12 | 0.1 | 0.03 | 0.06 | 0.11 | 0.08 | 0.09 | 0.29 | 0.16 | 0.18 | 0.18 | 0.28 |
| CaO | 3.79 | 6.4 | 4.79 | 10.7 | 10.4 | 10.87 | 10.99 | 8.97 | 11.04 | 10.9 | 5.06 | 5.46 | 4.44 | 3.88 | 6.02 |
| Na2O | 5.67 | 7.59 | 6.87 | 5.33 | 5.62 | 5.41 | 5.23 | 6.3 | 5.34 | 5.31 | 8.66 | 8.35 | 9.03 | 9.39 | 8.4 |
| K2O | 0.14 | 0.07 | 0.06 | 0.1 | 0.12 | 0.1 | 0.1 | 0.11 | 0.12 | 0.09 | 0.15 | 0.1 | 0.09 | 0.09 | 0.08 |
| MgO | 0.94 | 0.02 | 2.47 | 0.02 | 0.04 | 0.05 | 0.04 | 0.11 | 0.13 | 0.14 | 0.02 | 0.07 | 0.03 | 0.02 | 0.01 |
| Total | 100.37 | 99.13 | 95.52 | 99.26 | 99.53 | 99.66 | 99.49 | 99.61 | 99.62 | 99.63 | 100.21 | 99.91 | 100.08 | 99.73 | 100.82 |
| Chemical formula based on 32 oxygen atoms | |||||||||||||||
| Si | 12.26 | 10.81 | 10.58 | 9.97 | 10.04 | 9.94 | 9.93 | 10.29 | 9.9 | 9.92 | 10.99 | 10.88 | 11.08 | 11.24 | 10.83 |
| Ti | 0 | 0.001 | 0 | 0.001 | 0 | 0 | 0 | 0 | 0 | 0.001 | 0 | 0.001 | 0 | 0 | 0.001 |
| Al | 3.6 | 5.19 | 4.97 | 6.01 | 5.91 | 6.03 | 6.04 | 5.69 | 6.04 | 6.03 | 5.01 | 5.14 | 4.94 | 4.75 | 5.13 |
| Fe(ii) | 0.17 | 0.04 | 0.46 | 0.02 | 0.02 | 0 | 0.01 | 0.02 | 0.01 | 0.01 | 0.04 | 0.02 | 0.03 | 0.03 | 0.04 |
| Ca | 0.7 | 1.23 | 0.97 | 2.08 | 2.02 | 2.11 | 2.13 | 1.73 | 2.14 | 2.11 | 0.96 | 1.04 | 0.84 | 0.74 | 1.14 |
| Na | 1.9 | 2.64 | 2.51 | 1.88 | 1.97 | 1.9 | 1.84 | 2.2 | 1.88 | 1.86 | 2.97 | 2.88 | 3.1 | 3.23 | 2.88 |
| K | 0.03 | 0.02 | 0.01 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 |
| Mg | 0.24 | 0.01 | 0.69 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.04 | 0.04 | 0.01 | 0.02 | 0.01 | 0.01 | 0 |
| Total | 18.91 | 19.93 | 20.2 | 19.98 | 20 | 20.01 | 19.98 | 19.98 | 20.03 | 20 | 20.01 | 20 | 20.01 | 20.01 | 20.04 |
| End members | |||||||||||||||
| An | 27 | 32 | 28 | 52 | 50 | 52 | 53 | 44 | 53 | 53 | 24 | 26 | 21 | 18 | 28 |
| Ab | 72 | 68 | 72 | 47 | 49 | 47 | 46 | 56 | 46 | 47 | 75 | 73 | 78 | 81 | 71 |
| Or | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Sample | IE1 | IE2 | IE3 | IE4 | IE5 | IE6 | IE7 | IE8 | IE9 | IE10 |
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 53.88 | 54.36 | 53.46 | 55.04 | 53.67 | 54.27 | 53.08 | 53.94 | 54.89 | 55.68 |
| TiO2 | 0.34 | 0.29 | 0.37 | 0.26 | 0.35 | 0.31 | 0.39 | 0.36 | 0.30 | 0.25 |
| Al2O3 | 15.26 | 14.95 | 15.69 | 14.89 | 15.42 | 15.11 | 15.72 | 15.22 | 15.07 | 14.76 |
| FeO | 6.47 | 6.30 | 6.88 | 6.17 | 6.52 | 6.32 | 6.96 | 6.41 | 6.26 | 6.02 |
| MnO | 0.14 | 0.13 | 0.15 | 0.12 | 0.14 | 0.13 | 0.15 | 0.14 | 0.13 | 0.12 |
| MgO | 5.88 | 5.80 | 5.94 | 5.77 | 5.91 | 5.83 | 6.03 | 5.82 | 5.71 | 5.65 |
| CaO | 7.14 | 7.01 | 7.23 | 6.78 | 7.21 | 7.09 | 7.40 | 7.07 | 7.00 | 6.58 |
| Na2O | 3.64 | 3.70 | 3.56 | 3.75 | 3.61 | 3.68 | 3.42 | 3.49 | 3.81 | 4.08 |
| K2O | 3.59 | 3.52 | 3.68 | 3.46 | 3.64 | 3.53 | 3.84 | 3.50 | 3.36 | 3.15 |
| P2O5 | 0.04 | 0.03 | 0.05 | 0.03 | 0.04 | 0.04 | 0.06 | 0.04 | 0.03 | 0.03 |
| F | 2.17 | 2.28 | 2.23 | 3.06 | 2.31 | 2.38 | 2.09 | 2.25 | 2.49 | 2.66 |
| LOI | 1.31 | 1.25 | 1.39 | 1.19 | 1.34 | 1.29 | 1.51 | 1.68 | 0.98 | 0.86 |
| Sum | 99.86 | 99.62 | 100.63 | 100.52 | 100.16 | 99.98 | 100.65 | 99.92 | 100.03 | 99.84 |
| Ba | 37.83 | 42.09 | 41.13 | 35.88 | 38.12 | 33.36 | 47.77 | 34.67 | 37.40 | 33.13 |
| Co | 39.30 | 38.41 | 42.49 | 36.78 | 39.49 | 38.91 | 42.65 | 39.10 | 37.10 | 34.44 |
| Cr | 460.60 | 581.08 | 560.66 | 361.80 | 361.73 | 364.32 | 480.57 | 421.58 | 472.00 | 316.13 |
| Cu | 3.63 | 3.18 | 4.46 | 3.12 | 4.00 | 3.37 | 4.92 | 3.74 | 4.10 | 3.54 |
| Ga | 17.40 | 18.30 | 16.90 | 18.40 | 17.10 | 17.70 | 17.00 | 17.50 | 18.10 | 18.50 |
| Hf | 0.68 | 0.99 | 0.91 | 0.60 | 0.91 | 0.60 | 0.72 | 0.80 | 1.05 | 0.84 |
| Mo | 0.98 | 0.80 | 1.26 | 0.40 | 1.07 | 0.89 | 0.97 | 1.18 | 0.70 | 0.61 |
| Nb | 17.55 | 23.04 | 15.28 | 28.40 | 15.96 | 20.57 | 12.84 | 19.04 | 25.18 | 30.77 |
| Ni | 236.18 | 237.81 | 261.90 | 235.17 | 255.45 | 235.62 | 278.89 | 247.24 | 235.00 | 226.24 |
| Pb | 7.20 | 10.35 | 5.85 | 13.30 | 6.37 | 8.76 | 5.10 | 8.08 | 11.88 | 12.50 |
| Rb | 287.25 | 369.00 | 242.45 | 421.00 | 266.00 | 345.10 | 215.40 | 317.60 | 391.40 | 450.45 |
| Sn | 8.18 | 10.62 | 6.05 | 13.40 | 8.12 | 10.46 | 5.04 | 9.28 | 12.83 | 14.91 |
| Sr | 64.95 | 77.76 | 57.40 | 84.70 | 61.18 | 74.12 | 55.44 | 63.12 | 79.33 | 75.92 |
| Ta | 1.98 | 2.27 | 1.89 | 3.65 | 1.96 | 2.12 | 1.66 | 2.07 | 3.45 | 3.98 |
| Th | 1.45 | 2.15 | 1.22 | 2.39 | 1.36 | 1.91 | 1.19 | 1.88 | 2.29 | 2.63 |
| U | 0.99 | 1.11 | 0.89 | 1.13 | 0.95 | 1.05 | 0.86 | 1.02 | 1.06 | 1.16 |
| V | 117.60 | 117.41 | 119.31 | 115.58 | 117.98 | 117.81 | 124.49 | 123.13 | 113.00 | 113.12 |
| W | 19.70 | 20.20 | 18.43 | 21.11 | 18.82 | 20.10 | 16.41 | 18.72 | 20.00 | 24.24 |
| Y | 922.50 | 1112.40 | 796.25 | 1245.00 | 863.80 | 1044.65 | 675.60 | 956.80 | 1149.50 | 1332.45 |
| Zn | 346.50 | 417.60 | 302.25 | 474.00 | 322.70 | 399.50 | 271.20 | 380.80 | 445.55 | 505.05 |
| Zr | 23.25 | 36.90 | 20.15 | 45.00 | 21.00 | 28.90 | 19.20 | 28.00 | 45.60 | 53.55 |
| Li | 498.00 | - | 423.80 | - | 450.10 | - | 381.00 | 536.80 | - | - |
| Cs | 11.06 | - | 9.37 | - | 9.88 | - | 8.29 | 11.94 | - | - |
| Sc | 14.94 | - | 12.32 | - | 13.43 | - | 10.54 | 16.25 | - | - |
| La | 7.22 | - | 6.07 | - | 6.78 | - | 5.48 | 8.00 | - | - |
| Ce | 24.23 | - | 20.35 | - | 22.36 | - | 18.15 | 26.44 | - | - |
| Pr | 4.51 | - | 3.61 | - | 3.99 | - | 3.24 | 5.01 | - | - |
| Nd | 34.46 | - | 28.83 | - | 31.73 | - | 25.99 | 37.12 | - | - |
| Sm | 17.99 | - | 15.01 | - | 16.57 | - | 13.65 | 19.40 | - | - |
| Eu | 0.43 | - | 0.38 | - | 0.44 | - | 0.40 | 0.44 | - | - |
| Gd | 36.21 | - | 30.02 | - | 32.82 | - | 26.72 | 39.04 | - | - |
| Tb | 7.86 | - | 6.51 | - | 7.19 | - | 5.74 | 8.57 | - | - |
| Dy | 71.90 | - | 57.19 | - | 64.46 | - | 51.86 | 77.39 | - | - |
| Ho | 17.24 | - | 14.20 | - | 15.55 | - | 12.80 | 18.63 | - | - |
| Er | 51.63 | - | 42.82 | - | 46.59 | - | 38.91 | 55.46 | - | - |
| Tm | 7.86 | - | 6.40 | - | 7.15 | - | 5.73 | 8.34 | - | - |
| Yb | 57.85 | - | 49.34 | - | 53.52 | - | 45.08 | 62.19 | - | - |
| Lu | 10.37 | - | 8.81 | - | 9.57 | - | 8.05 | 11.10 | - | - |
| Sample# | ASI | A/NK | Fe# | Mg# | ΣREE | ΣHREE | ΣLREE | La/YbN | Y/Ho | TE1 | TE3 | TE4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IE1 | 0.67 | 1.55 | 0.52 | 47.61 | 350 | 261 | 88 | 0.089 | 53.50 | 0.91 | 1.05 | 0.91 |
| IE2 | 0.66 | 1.51 | 0.52 | 47.93 | - | - | - | - | - | - | - | - |
| IE3 | 0.68 | 1.59 | 0.54 | 46.33 | 290 | 215 | 74 | 0.088 | 56.08 | 0.89 | 1.03 | 0.90 |
| IE4 | 0.67 | 1.50 | 0.52 | 48.32 | - | - | - | - | - | - | - | - |
| IE5 | 0.67 | 1.56 | 0.52 | 47.55 | 319 | 237 | 81 | 0.091 | 55.56 | 0.89 | 1.05 | 0.91 |
| IE6 | 0.67 | 1.53 | 0.52 | 47.98 | - | - | - | - | - | - | - | - |
| IE7 | 0.68 | 1.61 | 0.54 | 46.42 | 262 | 195 | 67 | 0.087 | 52.77 | 0.89 | 1.03 | 0.89 |
| IE8 | 0.68 | 1.60 | 0.52 | 47.59 | 377 | 281 | 96 | 0.092 | 51.36 | 0.92 | 1.06 | 0.90 |
| IE9 | 0.67 | 1.52 | 0.52 | 47.70 | - | - | - | - | - | - | - | - |
| IE10 | 0.67 | 1.46 | 0.52 | 48.41 | - | - | - | - | - | - | - | - |
| I2 * | 1.00 | 1.08 | 0.99 | 1.22 | 150 | 73 | 77 | 0.42 | 30.64 | 1.12 | 1.38 | 1.03 |
| I3 * | 1.01 | 1.08 | 0.98 | 2.47 | - | - | - | - | - | - | - | - |
| I4 * | 1.00 | 1.09 | 0.98 | 1.90 | - | - | - | - | - | - | - | - |
| I7 * | 0.98 | 1.07 | 0.98 | 2.44 | - | - | - | - | - | - | - | - |
| I8 * | 0.97 | 1.06 | 0.99 | 1.14 | - | - | - | - | - | - | - | - |
| I15 * | 0.99 | 1.06 | 0.98 | 1.67 | - | - | - | - | - | - | - | - |
| I16 * | 0.98 | 1.05 | 0.97 | 3.13 | - | - | - | - | - | - | - | - |
| I17 * | 0.99 | 1.06 | 0.97 | 2.53 | 96 | 59 | 38 | 0.19 | 36.67 | 1.23 | 1.18 | 1.07 |
| I18 * | 1.00 | 1.08 | 0.97 | 3.08 | - | - | - | - | - | - | - | - |
| I19 * | 0.99 | 1.06 | 0.98 | 2.33 | 127 | 71 | 56 | 0.26 | 38.05 | 1.13 | 1.18 | 1.06 |
| I20 * | 1.00 | 1.09 | 0.98 | 2.35 | - | - | - | - | - | - | - | - |
| I21 * | 0.95 | 1.06 | 0.99 | 1.30 | 138 | 84 | 54 | 0.21 | 40.65 | 1.10 | 1.21 | 1.07 |
| I22 * | 0.98 | 1.07 | 0.99 | 1.12 | - | - | - | - | - | - | - | - |
| I26 * | 0.99 | 1.09 | 0.97 | 2.74 | - | - | - | - | - | - | - | - |
| I31 * | 0.99 | 1.09 | 0.99 | 0.81 | - | - | - | - | - | - | - | - |
| I46 * | 0.96 | 1.05 | 0.99 | 1.23 | 119 | 59 | 60 | 0.43 | 36.88 | 1.06 | 1.15 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Dokouny, H.A.; Mahdy, N.M.; El Hadek, H.H.; Sami, M.; Abart, R.; Ahmed, M.S.; Zafar, T.; Sanislav, I.V. Origin of Amphibole-Biotite-Fluorite-Rich Enclaves from Gabal El-Ineigi Fluorite-Bearing Granite, Central Eastern Desert of Egypt: Insights into Fluoride–Calcium and Silicate Liquid Immiscibility. Minerals 2023, 13, 670. https://doi.org/10.3390/min13050670
El-Dokouny HA, Mahdy NM, El Hadek HH, Sami M, Abart R, Ahmed MS, Zafar T, Sanislav IV. Origin of Amphibole-Biotite-Fluorite-Rich Enclaves from Gabal El-Ineigi Fluorite-Bearing Granite, Central Eastern Desert of Egypt: Insights into Fluoride–Calcium and Silicate Liquid Immiscibility. Minerals. 2023; 13(5):670. https://doi.org/10.3390/min13050670
Chicago/Turabian StyleEl-Dokouny, Hanaa A., Nasser M. Mahdy, Hany H. El Hadek, Mabrouk Sami, Rainer Abart, Mohamed S. Ahmed, Tehseen Zafar, and Ioan V. Sanislav. 2023. "Origin of Amphibole-Biotite-Fluorite-Rich Enclaves from Gabal El-Ineigi Fluorite-Bearing Granite, Central Eastern Desert of Egypt: Insights into Fluoride–Calcium and Silicate Liquid Immiscibility" Minerals 13, no. 5: 670. https://doi.org/10.3390/min13050670
APA StyleEl-Dokouny, H. A., Mahdy, N. M., El Hadek, H. H., Sami, M., Abart, R., Ahmed, M. S., Zafar, T., & Sanislav, I. V. (2023). Origin of Amphibole-Biotite-Fluorite-Rich Enclaves from Gabal El-Ineigi Fluorite-Bearing Granite, Central Eastern Desert of Egypt: Insights into Fluoride–Calcium and Silicate Liquid Immiscibility. Minerals, 13(5), 670. https://doi.org/10.3390/min13050670








