Figure 1.
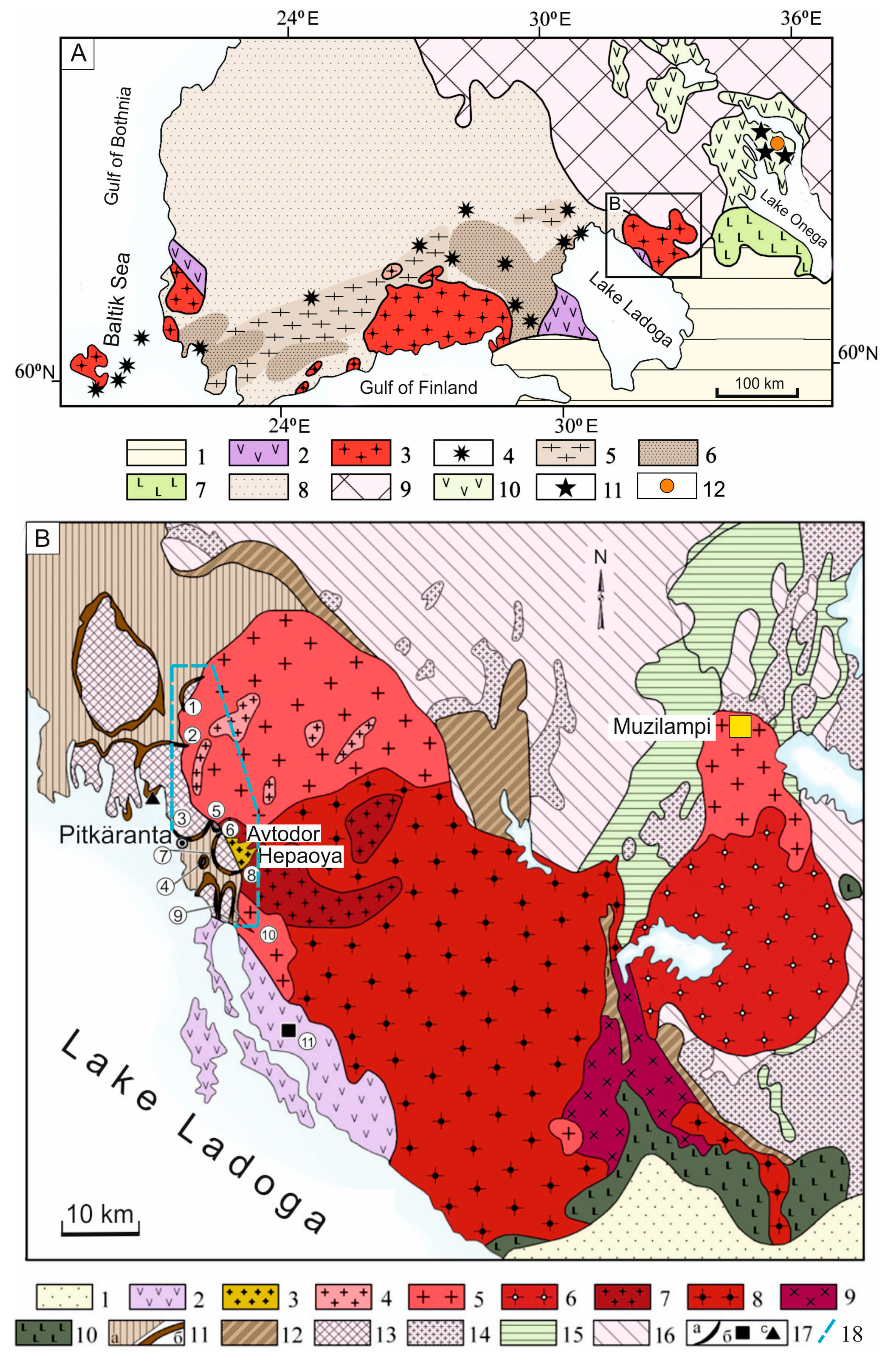
Geological structure of SARGB and its position in the Ladoga-Dalecarlian anorthosite-rapakivi granite pluton belt, Fennoscandian Shield. After: [
16,
17] modified. (
A) Ladoga-Dalecarlian anorthosite-rapakivi granite pluton belt (1660–1530 Ma). 1—East European Platform cover; 2—Jotnian grabens (volcanic-sedimentary rocks and diabase dikes); 3—anorthosite-rapakivi granite plutons; 4–6—Late Svecofennian (post-orogenic) igneous complexes: 4—shoshonitic series intrusives (1815–1770 Ma), 5—Potassium S-granite zones (1814–1806 Ma), 6—HT/LP granulite-facies metamorphic zones; 7—Vepsian depressions (volcanic-sedimentary and sedimentary complexes of the Petrozavodsk and Shoksha suites, 1760 Ma gabbro-dolerite sills); 8—Early Svecofennian volcanic-sedimentary and igneous complexes; 9—Archean rocks of the Karelian Craton; 10—Early Proterozoic depressions (Sumian, Jatulian, Ludicovian) of the Karelian Craton; 11—kimberlitic Kimozero (1.92 Ga) [
18]. (
B) Geological structure of the SARGB and the distribution of deposits in the PMD, modified after [
16,
17]. 1—platform cover; 2—Jotnian volcanic-sedimentary rocks (Salmi suite); 3–10—SARGB rocks: 3—topaz-bearing granites (Li–F granites), 4—fine-grained porphyraceous biotite granites, 5—coarse-grained biotite granites, 6—coarse-grained biotite-hornblende granites, 7—ovoid biotite-hornblende rapakivi granites with fine-grained matrix, 8—vyborgites and pyterlites, 9—coarse-grained biotite-hornblende quartz syenites, 10—mafic and intermediate rocks (anorthosite, norite, ferrodiorite, monzonite); 11–12—PR
1 supracrustal rocks: 11—from the Svecokarelian Folded Region (a—Ladoga series, b—Sortavala series), 12—from the Karelian Craton; 13—AR
2-PR
1 remobilized Archean gneiss–granite cupolas; 14–16—Karelian Craton complexes (AR
2): 14—granites and migmatite-granites, 15—greenstone belts, 16—тonalite-trondhjemite-granodiorite-association; 17—ore deposits and occurrences: a—Sn-Be-base metal deposits PMD, b—Karhu U-base metal deposit, c—Kuivaniemi Mo-occurrence in quartz-feldspathic metasomatic rocks. 18—border of
Figure 2. Deposits of the PMD (numbers in a white circle on the scheme): 1–4—skarn-propylitic-Sn-base metal (1—Kulismajoki, 2—Kitelä, 3—Staroye Rudnoye Pole, 4— Heposelka), 5–10—skarn-greisen Sn–Be and Sn-Be-base metal: (5—Novoye Rudnoye Pole, 6—Hopunvaara, 7—Lupikko, 8—South Lupikko, 9—Ristiniemi, 10—Uuksa), 11—Karhu (U).
Figure 1.
Geological structure of SARGB and its position in the Ladoga-Dalecarlian anorthosite-rapakivi granite pluton belt, Fennoscandian Shield. After: [
16,
17] modified. (
A) Ladoga-Dalecarlian anorthosite-rapakivi granite pluton belt (1660–1530 Ma). 1—East European Platform cover; 2—Jotnian grabens (volcanic-sedimentary rocks and diabase dikes); 3—anorthosite-rapakivi granite plutons; 4–6—Late Svecofennian (post-orogenic) igneous complexes: 4—shoshonitic series intrusives (1815–1770 Ma), 5—Potassium S-granite zones (1814–1806 Ma), 6—HT/LP granulite-facies metamorphic zones; 7—Vepsian depressions (volcanic-sedimentary and sedimentary complexes of the Petrozavodsk and Shoksha suites, 1760 Ma gabbro-dolerite sills); 8—Early Svecofennian volcanic-sedimentary and igneous complexes; 9—Archean rocks of the Karelian Craton; 10—Early Proterozoic depressions (Sumian, Jatulian, Ludicovian) of the Karelian Craton; 11—kimberlitic Kimozero (1.92 Ga) [
18]. (
B) Geological structure of the SARGB and the distribution of deposits in the PMD, modified after [
16,
17]. 1—platform cover; 2—Jotnian volcanic-sedimentary rocks (Salmi suite); 3–10—SARGB rocks: 3—topaz-bearing granites (Li–F granites), 4—fine-grained porphyraceous biotite granites, 5—coarse-grained biotite granites, 6—coarse-grained biotite-hornblende granites, 7—ovoid biotite-hornblende rapakivi granites with fine-grained matrix, 8—vyborgites and pyterlites, 9—coarse-grained biotite-hornblende quartz syenites, 10—mafic and intermediate rocks (anorthosite, norite, ferrodiorite, monzonite); 11–12—PR
1 supracrustal rocks: 11—from the Svecokarelian Folded Region (a—Ladoga series, b—Sortavala series), 12—from the Karelian Craton; 13—AR
2-PR
1 remobilized Archean gneiss–granite cupolas; 14–16—Karelian Craton complexes (AR
2): 14—granites and migmatite-granites, 15—greenstone belts, 16—тonalite-trondhjemite-granodiorite-association; 17—ore deposits and occurrences: a—Sn-Be-base metal deposits PMD, b—Karhu U-base metal deposit, c—Kuivaniemi Mo-occurrence in quartz-feldspathic metasomatic rocks. 18—border of
Figure 2. Deposits of the PMD (numbers in a white circle on the scheme): 1–4—skarn-propylitic-Sn-base metal (1—Kulismajoki, 2—Kitelä, 3—Staroye Rudnoye Pole, 4— Heposelka), 5–10—skarn-greisen Sn–Be and Sn-Be-base metal: (5—Novoye Rudnoye Pole, 6—Hopunvaara, 7—Lupikko, 8—South Lupikko, 9—Ristiniemi, 10—Uuksa), 11—Karhu (U).
![Minerals 13 00648 g001 Minerals 13 00648 g001]()
Figure 2.
Scheme showing the geological structure of the PMD, modified after [
13,
16]. 1—Salmi suite: a—sandstones, conglomerates, b—basalts, dolerites; 2–5—rapakivi granites: 2—leucogranites and lithium-fluorine granites, 3—fine–grained granites, 4—medium-grained porphyritic biotite granites, 5a—granite–porphyry, 5b—porphyritic amphibole–biotite granites; 6—pegmatites; 7—synorogenic plagiogranites, granodiorites; 8—remobilized Archean gneissose granitic domes (1—Pitkäranta, 2—Vinberg, 3—Lupikko, 4—Uuksa, 5—Ristiniemi, 6—Heposelka, 7—Juläristi, 8—Pusunsaari, 9—Kulismajoki); 9—Ladoga series: biotite–quartz, quartz–feldspathic–biotite and graphite–bearing schists; 10—Pitkäranta suite: Amphibolites, amphibole, graphite and graphite–bearing schists, dolomitic and calcitic marbles and skarns after them; 11—skarns, greisenized skarns and low-temperature metasomatic rocks after them with Fe–Cu–Zn–Sn and rare–metal mineralization; 12—tectonic dislocations; 13—projection onto the modern erosion surface of the boundary of the sharp bend of the top of the Salmi massif (it delineates the skarn zone with Fe–Cu–Zn–Sn mineralization).
Figure 2.
Scheme showing the geological structure of the PMD, modified after [
13,
16]. 1—Salmi suite: a—sandstones, conglomerates, b—basalts, dolerites; 2–5—rapakivi granites: 2—leucogranites and lithium-fluorine granites, 3—fine–grained granites, 4—medium-grained porphyritic biotite granites, 5a—granite–porphyry, 5b—porphyritic amphibole–biotite granites; 6—pegmatites; 7—synorogenic plagiogranites, granodiorites; 8—remobilized Archean gneissose granitic domes (1—Pitkäranta, 2—Vinberg, 3—Lupikko, 4—Uuksa, 5—Ristiniemi, 6—Heposelka, 7—Juläristi, 8—Pusunsaari, 9—Kulismajoki); 9—Ladoga series: biotite–quartz, quartz–feldspathic–biotite and graphite–bearing schists; 10—Pitkäranta suite: Amphibolites, amphibole, graphite and graphite–bearing schists, dolomitic and calcitic marbles and skarns after them; 11—skarns, greisenized skarns and low-temperature metasomatic rocks after them with Fe–Cu–Zn–Sn and rare–metal mineralization; 12—tectonic dislocations; 13—projection onto the modern erosion surface of the boundary of the sharp bend of the top of the Salmi massif (it delineates the skarn zone with Fe–Cu–Zn–Sn mineralization).
![Minerals 13 00648 g002 Minerals 13 00648 g002]()
Figure 3.
Fluorite mineralization in Li-F granites at Avtodor occurrence. The arrow shows the vertical direction upwards.
Figure 3.
Fluorite mineralization in Li-F granites at Avtodor occurrence. The arrow shows the vertical direction upwards.
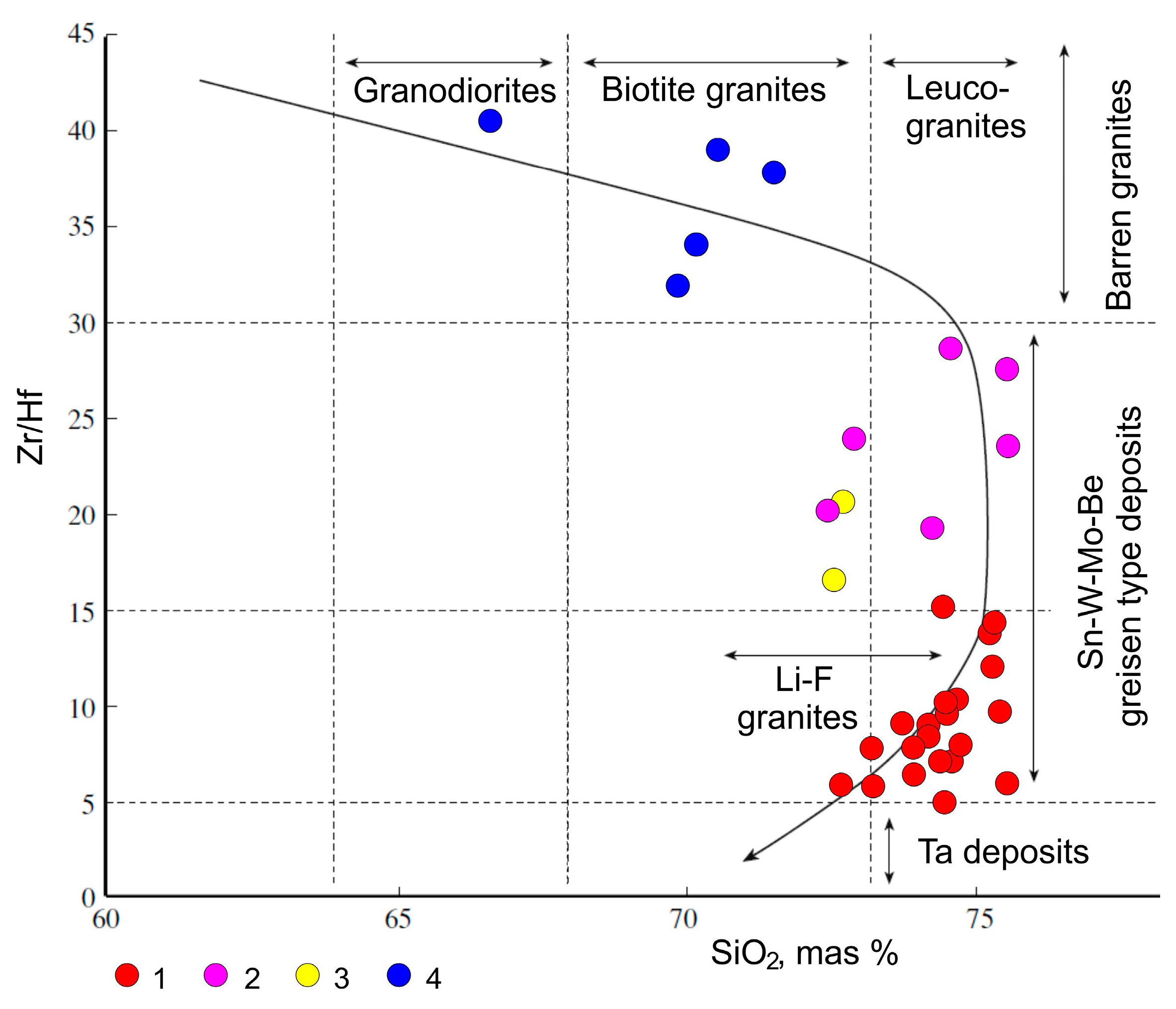
Figure 4.
SARGB granites on the classification-forest diagram Zr/Hf-SiO
2; partially used data [
31]; based on [
32]. 1—rare-metal siderophyllitic and Li–F granites; 2—leucogranites and biotite granites; 3—granite-porphyry; 4—biotite-amphibole granites.
Figure 4.
SARGB granites on the classification-forest diagram Zr/Hf-SiO
2; partially used data [
31]; based on [
32]. 1—rare-metal siderophyllitic and Li–F granites; 2—leucogranites and biotite granites; 3—granite-porphyry; 4—biotite-amphibole granites.
Figure 5.
BSE images. Zavaritskite mineralization in W. Lupikko ores. (a) Zavaritskite in serpentine. (b) Zavaritskite and fluorite in serpentinized diopside. (c) Zavaritskite in diopside. (d) Zavaritskite intergrown with chalcopyrite. For more detail, see text. Di—diopside, Ccp—chalcopyrite, Flr—fluorite, Phl—phlogopite, Py—pyrite, Sp—sphalerite, Srp—serpentine, Zav—zavaritskite BiOF.
Figure 5.
BSE images. Zavaritskite mineralization in W. Lupikko ores. (a) Zavaritskite in serpentine. (b) Zavaritskite and fluorite in serpentinized diopside. (c) Zavaritskite in diopside. (d) Zavaritskite intergrown with chalcopyrite. For more detail, see text. Di—diopside, Ccp—chalcopyrite, Flr—fluorite, Phl—phlogopite, Py—pyrite, Sp—sphalerite, Srp—serpentine, Zav—zavaritskite BiOF.
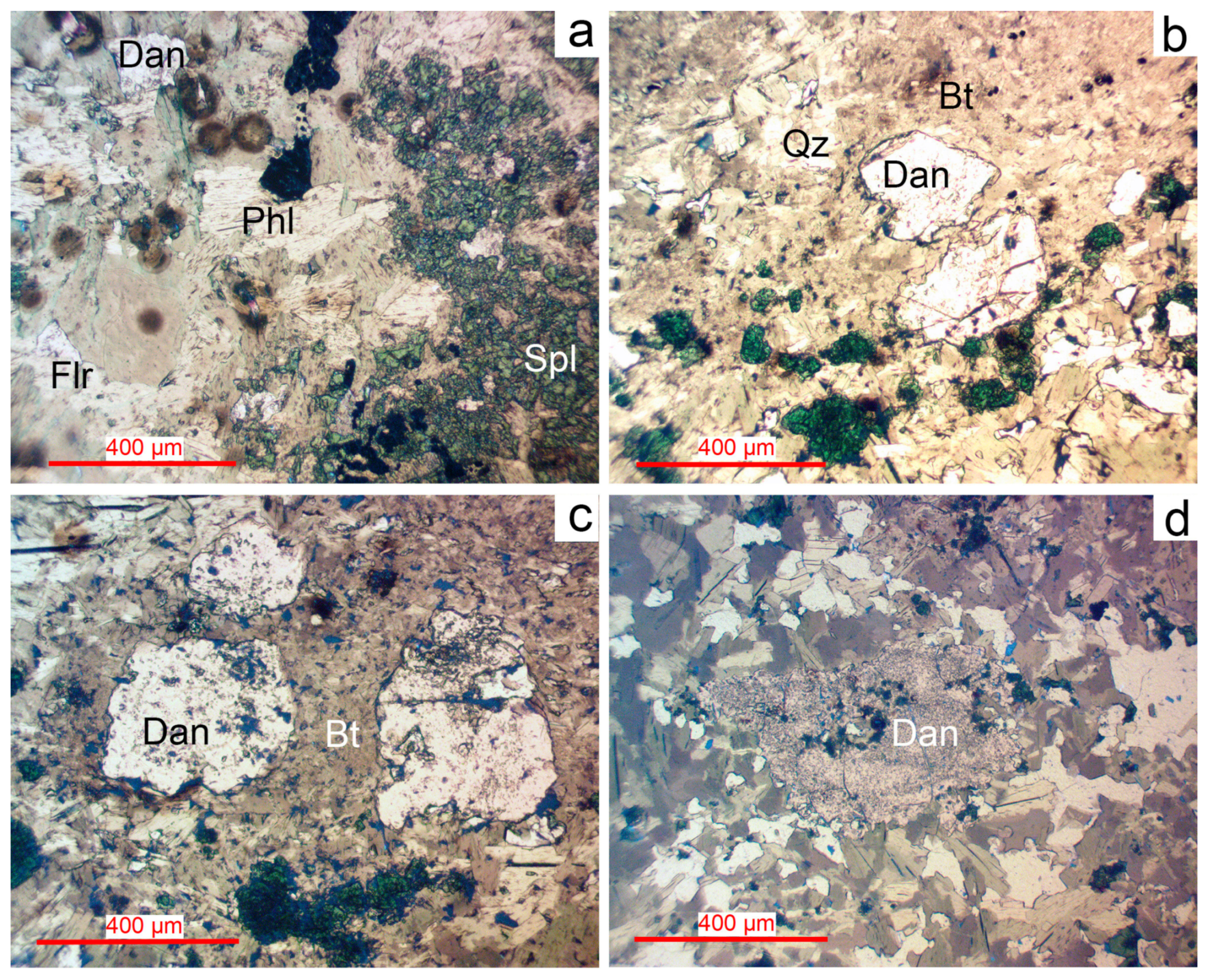
Figure 6.
Photomicrographs of danalite in transmitted light; W. Lupikko greisenized skarns. (a) Danalite in association with phlogopite and fluorite. (b) Danalite in association with biotite, quartz and Zn-spinel. (c) Danalite in biotite. (d) Danalite in a fine-grained aggregate of biotite and fluorite. For more detail, see text. Bt—biotite, Dan—danalite Be3(Fe,Mn,Zn)4(SiO4)3S, Flr—fluorite, Phl—phlogopite, Qz—quartz, Spl—spinel (ZnO 22%–25%).
Figure 6.
Photomicrographs of danalite in transmitted light; W. Lupikko greisenized skarns. (a) Danalite in association with phlogopite and fluorite. (b) Danalite in association with biotite, quartz and Zn-spinel. (c) Danalite in biotite. (d) Danalite in a fine-grained aggregate of biotite and fluorite. For more detail, see text. Bt—biotite, Dan—danalite Be3(Fe,Mn,Zn)4(SiO4)3S, Flr—fluorite, Phl—phlogopite, Qz—quartz, Spl—spinel (ZnO 22%–25%).
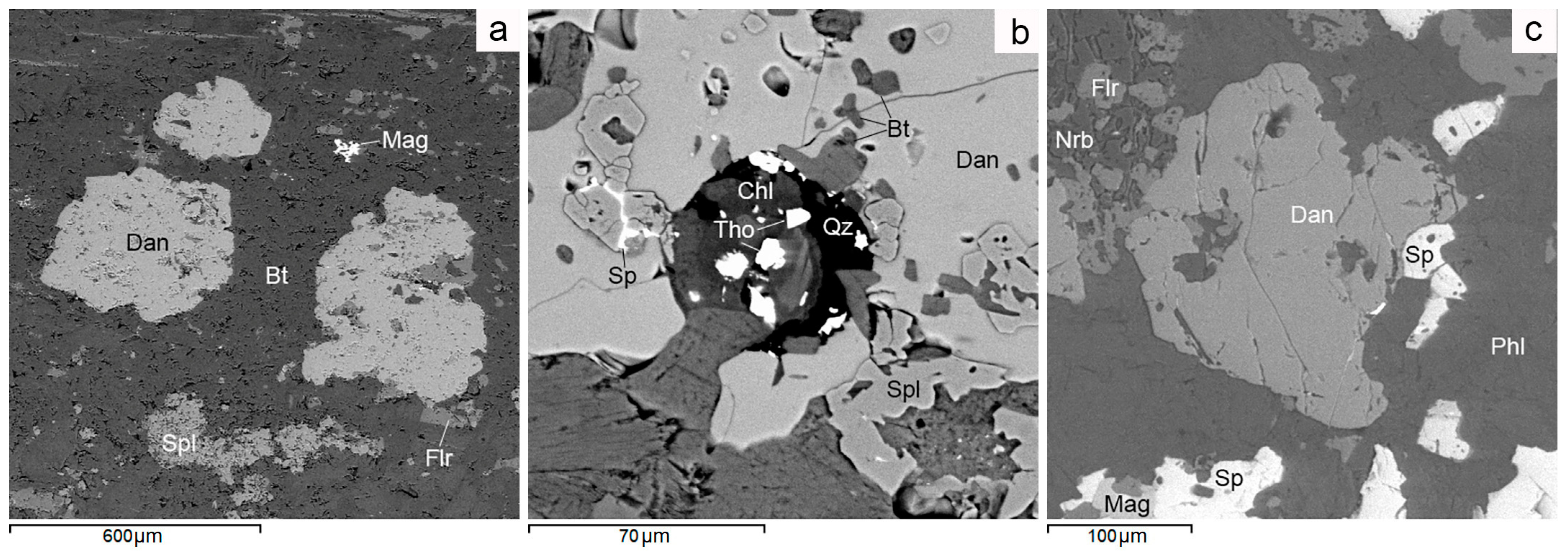
Figure 7.
BSE images. Danalite mineralization in W. Lupikko ores. (a) Danalite in biotite, containing inclusions of magnetite, fluorite and Zn spinel. (b) Danalite with microinclusions of biotite, Zn spinel and sphalerite. (c) Danalite is corroded by phlogopite and norbergite. For more detail, see text. Bt—biotite, Chl—chlorite, Dan—danalite Be3(Fe,Mn,Zn)4(SiO4)3S, Flr—fluorite, Mag—magnetite, Nrb—norbergite, Phl—phlogopite, Qz—quartz, Sp—sphalerite, Spl—spinel (ZnO 22%–25%), Tho—thorianite.
Figure 7.
BSE images. Danalite mineralization in W. Lupikko ores. (a) Danalite in biotite, containing inclusions of magnetite, fluorite and Zn spinel. (b) Danalite with microinclusions of biotite, Zn spinel and sphalerite. (c) Danalite is corroded by phlogopite and norbergite. For more detail, see text. Bt—biotite, Chl—chlorite, Dan—danalite Be3(Fe,Mn,Zn)4(SiO4)3S, Flr—fluorite, Mag—magnetite, Nrb—norbergite, Phl—phlogopite, Qz—quartz, Sp—sphalerite, Spl—spinel (ZnO 22%–25%), Tho—thorianite.
Figure 8.
Bertrandite mineralization in Bek ores: (a,b)—transmitted light photomicrographs; (c,d)—BSE images; for more detail, see text. Btd—bertrandite Be4Si2O7(OH)2, Chl—chlorite, Mag—magnetite, Qz—quartz, Sp—sphalerite.
Figure 8.
Bertrandite mineralization in Bek ores: (a,b)—transmitted light photomicrographs; (c,d)—BSE images; for more detail, see text. Btd—bertrandite Be4Si2O7(OH)2, Chl—chlorite, Mag—magnetite, Qz—quartz, Sp—sphalerite.
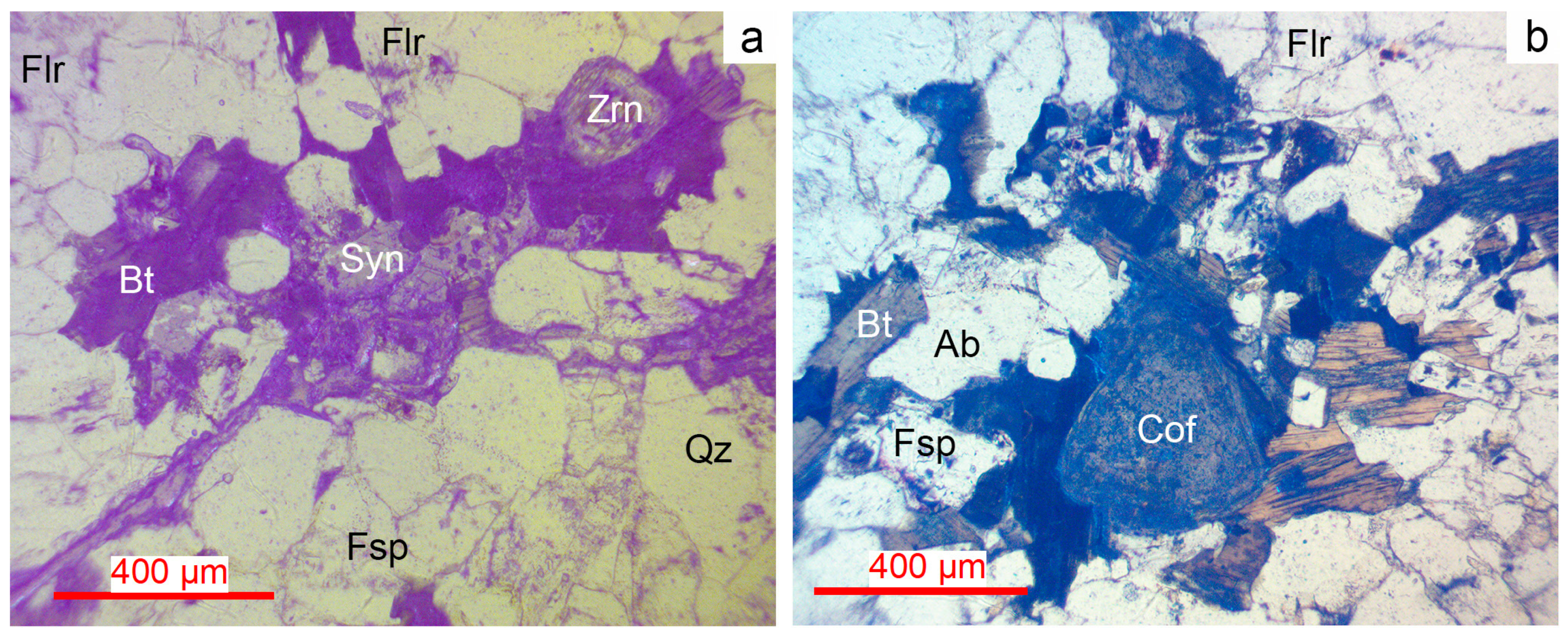
Figure 9.
Microphotographs of synchysite, zircon, coffinite and fluorite in transmitted light in Muzilampi granites. (a) Synchysite and zircon in a biotite-fluorite aggregate. (b) Coffinite in albite-fluorite-biotite aggregate. For more detail, see text. Bt—biotite, Cof—coffinite, Flr—fluorite, Qz—quartz, Fsp—feldspar, Syn—synchysite, Zrn—zircon.
Figure 9.
Microphotographs of synchysite, zircon, coffinite and fluorite in transmitted light in Muzilampi granites. (a) Synchysite and zircon in a biotite-fluorite aggregate. (b) Coffinite in albite-fluorite-biotite aggregate. For more detail, see text. Bt—biotite, Cof—coffinite, Flr—fluorite, Qz—quartz, Fsp—feldspar, Syn—synchysite, Zrn—zircon.
Figure 10.
BSE images. Rare-earth mineralization in Muzilampi granites. (a) Bastnäsite, euxenite and zircon in biotite. (b) Intergrowths of pyrochlore with bastnäsite and columbite in biotite. (c) Coffinite in association with biotite, albite and fluorite. (d) Intergrowths of håleniusite with parisite in association with biotite and albite. (e) Intergrowths of håleniusite, parisite and fluorite association with biotite and quartz. (f) Biotite with microinclusions of bastnäsite. (g) Fluorite with parisite microinclusions. (h) Fluorite with microinclusions of bastnäsite and parisite. (i) Fluorite-1 with fluorite-2 rim. For more detail, see text. Ab—albite, Bsn—bastnäsite, Bt—biotite, Cof—coffinite, Eux—euxenite, Flr—fluorite, Fsp—feldspar, Hal—håleniusite, Pcl—pyrochlore, Pst—parisite, Qz—quartz, Zrn—zircon.
Figure 10.
BSE images. Rare-earth mineralization in Muzilampi granites. (a) Bastnäsite, euxenite and zircon in biotite. (b) Intergrowths of pyrochlore with bastnäsite and columbite in biotite. (c) Coffinite in association with biotite, albite and fluorite. (d) Intergrowths of håleniusite with parisite in association with biotite and albite. (e) Intergrowths of håleniusite, parisite and fluorite association with biotite and quartz. (f) Biotite with microinclusions of bastnäsite. (g) Fluorite with parisite microinclusions. (h) Fluorite with microinclusions of bastnäsite and parisite. (i) Fluorite-1 with fluorite-2 rim. For more detail, see text. Ab—albite, Bsn—bastnäsite, Bt—biotite, Cof—coffinite, Eux—euxenite, Flr—fluorite, Fsp—feldspar, Hal—håleniusite, Pcl—pyrochlore, Pst—parisite, Qz—quartz, Zrn—zircon.
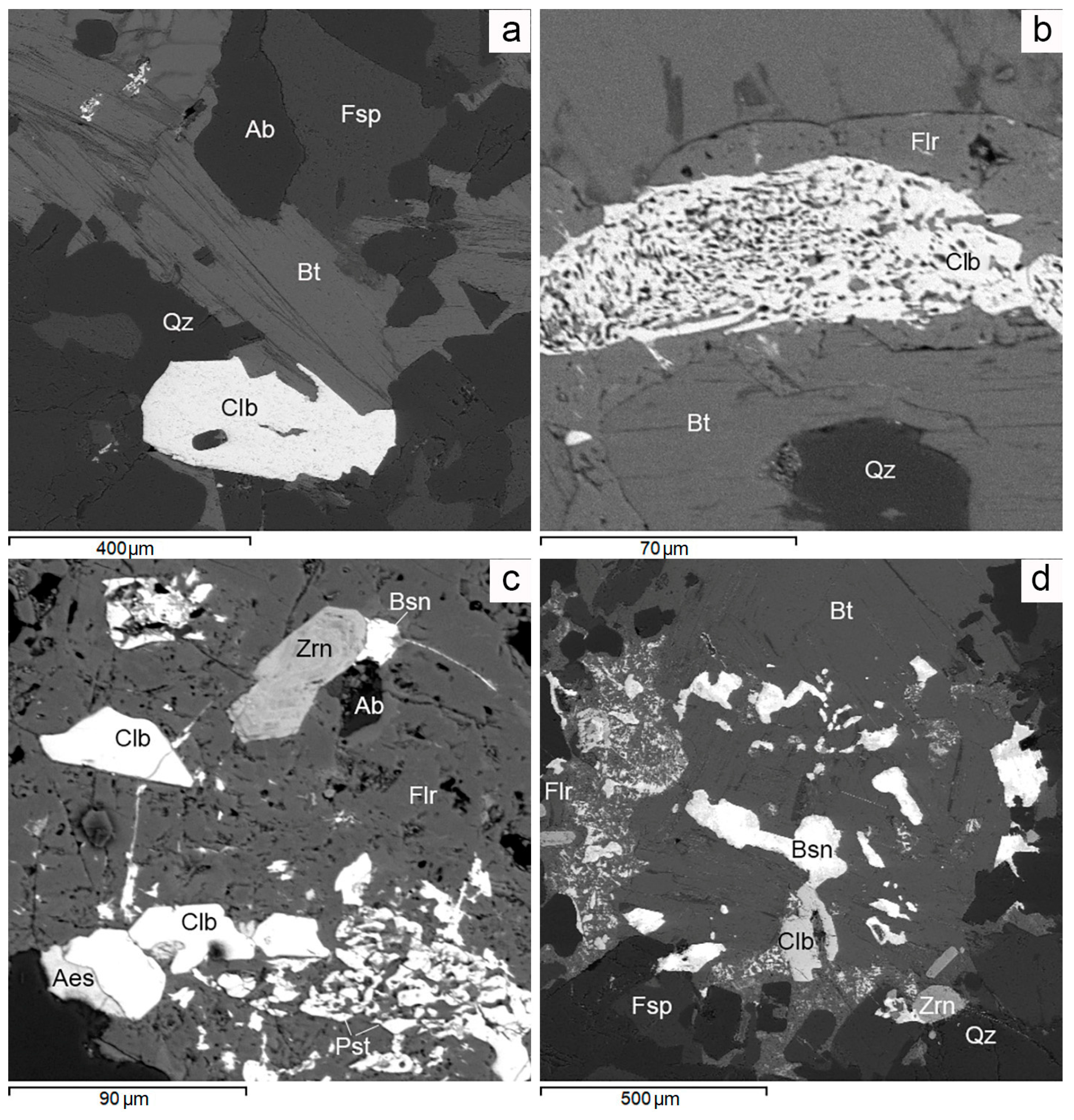
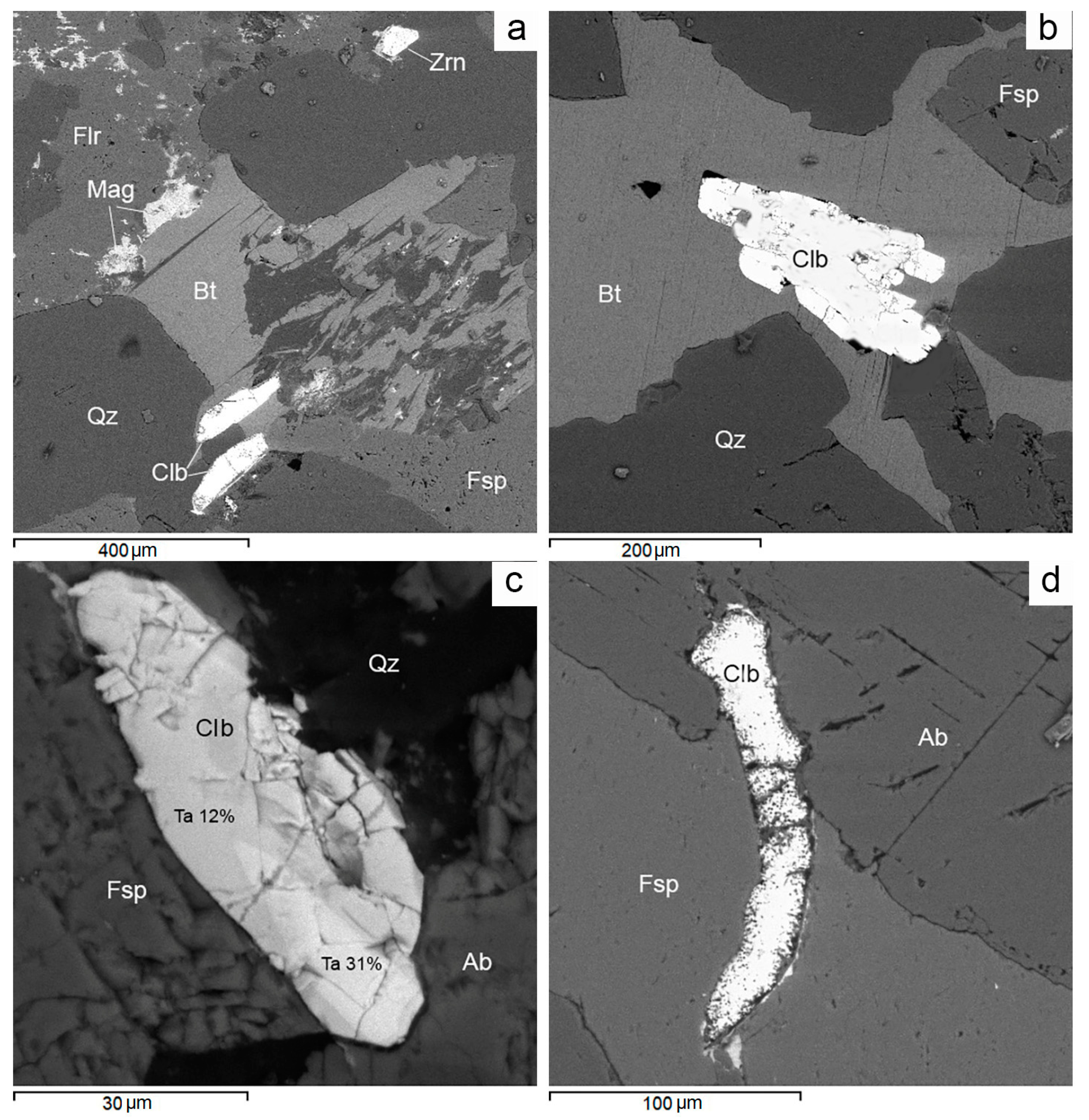
Figure 11.
BSE images. Ferricolumbite mineralization in Muzilampi granites. (a) Columbite in association with biotite and quartz. (b) Columbite with a spongy structure filled with fluorite microinclusions. (c) Columbite intergrown with aeschynite in fluorite. (d) Columbite intergrown with bastnäsite in biotite. For more detail, see text. Ab—albite, Aes—aeschynite, Bsn—bastnäsite, Bt—biotite, Clb—columbite, Flr—fluorite, Fsp—feldspar, Pst—parisite, Qz—quartz, Zrn—zircon.
Figure 11.
BSE images. Ferricolumbite mineralization in Muzilampi granites. (a) Columbite in association with biotite and quartz. (b) Columbite with a spongy structure filled with fluorite microinclusions. (c) Columbite intergrown with aeschynite in fluorite. (d) Columbite intergrown with bastnäsite in biotite. For more detail, see text. Ab—albite, Aes—aeschynite, Bsn—bastnäsite, Bt—biotite, Clb—columbite, Flr—fluorite, Fsp—feldspar, Pst—parisite, Qz—quartz, Zrn—zircon.
Figure 12.
BSE images. Rare-earth mineralization in Hepaoja granites. (a) Microinclusions of bastnäsite in fluorite. (b) Parisite in association with muscovite and quartz. (c) Kaolinite and aeschynite in biotite. (d) Intergrowths of monazite with magnetite. (e) Intergrowths of columbite and bastnäsite in fluorite. (f) Zircon in association with biotite, albite and quartz. For more detail, see text. Ab—albite, Aes—aeschynite, Bsn—bastnäsite, Bt—biotite, Chl—chlorite, Flr—fluorite, Fsp—feldspar, Kln—kaolinite, Clb—columbite, Mag—magnetite, Mnz—monazite, Ms—muscovite, Qz—quartz, Zrn—zircon.
Figure 12.
BSE images. Rare-earth mineralization in Hepaoja granites. (a) Microinclusions of bastnäsite in fluorite. (b) Parisite in association with muscovite and quartz. (c) Kaolinite and aeschynite in biotite. (d) Intergrowths of monazite with magnetite. (e) Intergrowths of columbite and bastnäsite in fluorite. (f) Zircon in association with biotite, albite and quartz. For more detail, see text. Ab—albite, Aes—aeschynite, Bsn—bastnäsite, Bt—biotite, Chl—chlorite, Flr—fluorite, Fsp—feldspar, Kln—kaolinite, Clb—columbite, Mag—magnetite, Mnz—monazite, Ms—muscovite, Qz—quartz, Zrn—zircon.
Figure 13.
BSE images. Ferricolumbite mineralization in Hepaoja granites. (a) Columbite in association with quartz and biotite. (b) Intergrowths of prismatic crystals of columbite in biotite. (c) Zonal columbite crystal. (d) Veinlet-like grain of columbite. For more detail, see text. Ab—albite, Bt—biotite, Clb—columbite, Flr—fluorite, Fsp—feldspar, Mag—magnetite, Qz—quartz, Zrn—zircon.
Figure 13.
BSE images. Ferricolumbite mineralization in Hepaoja granites. (a) Columbite in association with quartz and biotite. (b) Intergrowths of prismatic crystals of columbite in biotite. (c) Zonal columbite crystal. (d) Veinlet-like grain of columbite. For more detail, see text. Ab—albite, Bt—biotite, Clb—columbite, Flr—fluorite, Fsp—feldspar, Mag—magnetite, Qz—quartz, Zrn—zircon.
Figure 14.
Endocontact breccia of Li-F granites at Avtodor occurrence. (a) Siderophyllitic granites and amphibole schists occur as fragments; matrix consists of topaz-protolithionitic granites; orange patch—fluorite filled with micron-sized synchysite inclusions (b), BSE image.
Figure 14.
Endocontact breccia of Li-F granites at Avtodor occurrence. (a) Siderophyllitic granites and amphibole schists occur as fragments; matrix consists of topaz-protolithionitic granites; orange patch—fluorite filled with micron-sized synchysite inclusions (b), BSE image.
Figure 15.
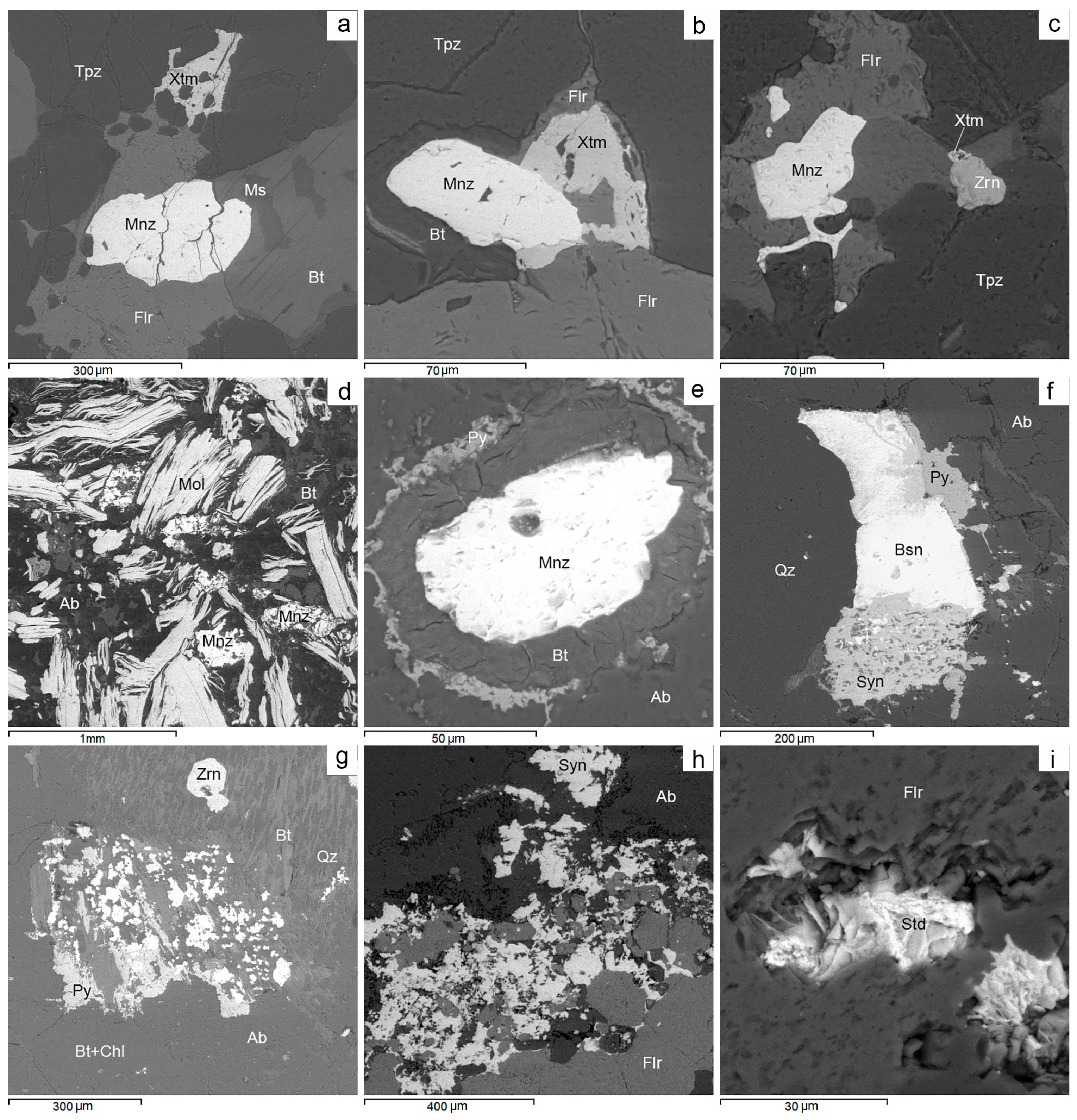
BSE images. Rare-earth mineralization in Avtodor granites. (a) Monazite and xenotime in association with topaz, fluorite and biotite. (b) Intergrowth of monazite and xenotime crystals in topaz. (c) Veinlet-like grain of monazite in fluorite. (d) Molybdenite and monazite in association with albite and biotite. (e) Monazite with biotite and pyrite rims. (f) Intergrowth of bastnäsite with synchysite and pyrite. (g) Microinclusions of bastnäsite and synchysite in fluorite. (h) Microinclusions of bastnäsite and synchysite in fluorite. (i) Stetindite in fluorite. For more detail, see text. Ab—albite, Bsn—bastnäsite, Bt—biotite, Chl—chlorite, Flr—fluorite, Mnz—monazite, Mol—molybdenite, Ms—muscovite, Py—pyrite, Qz—quartz, Std—stetindite, Tpz—topaz, Xtm—xenotime, Zrn—zircon.
Figure 15.
BSE images. Rare-earth mineralization in Avtodor granites. (a) Monazite and xenotime in association with topaz, fluorite and biotite. (b) Intergrowth of monazite and xenotime crystals in topaz. (c) Veinlet-like grain of monazite in fluorite. (d) Molybdenite and monazite in association with albite and biotite. (e) Monazite with biotite and pyrite rims. (f) Intergrowth of bastnäsite with synchysite and pyrite. (g) Microinclusions of bastnäsite and synchysite in fluorite. (h) Microinclusions of bastnäsite and synchysite in fluorite. (i) Stetindite in fluorite. For more detail, see text. Ab—albite, Bsn—bastnäsite, Bt—biotite, Chl—chlorite, Flr—fluorite, Mnz—monazite, Mol—molybdenite, Ms—muscovite, Py—pyrite, Qz—quartz, Std—stetindite, Tpz—topaz, Xtm—xenotime, Zrn—zircon.
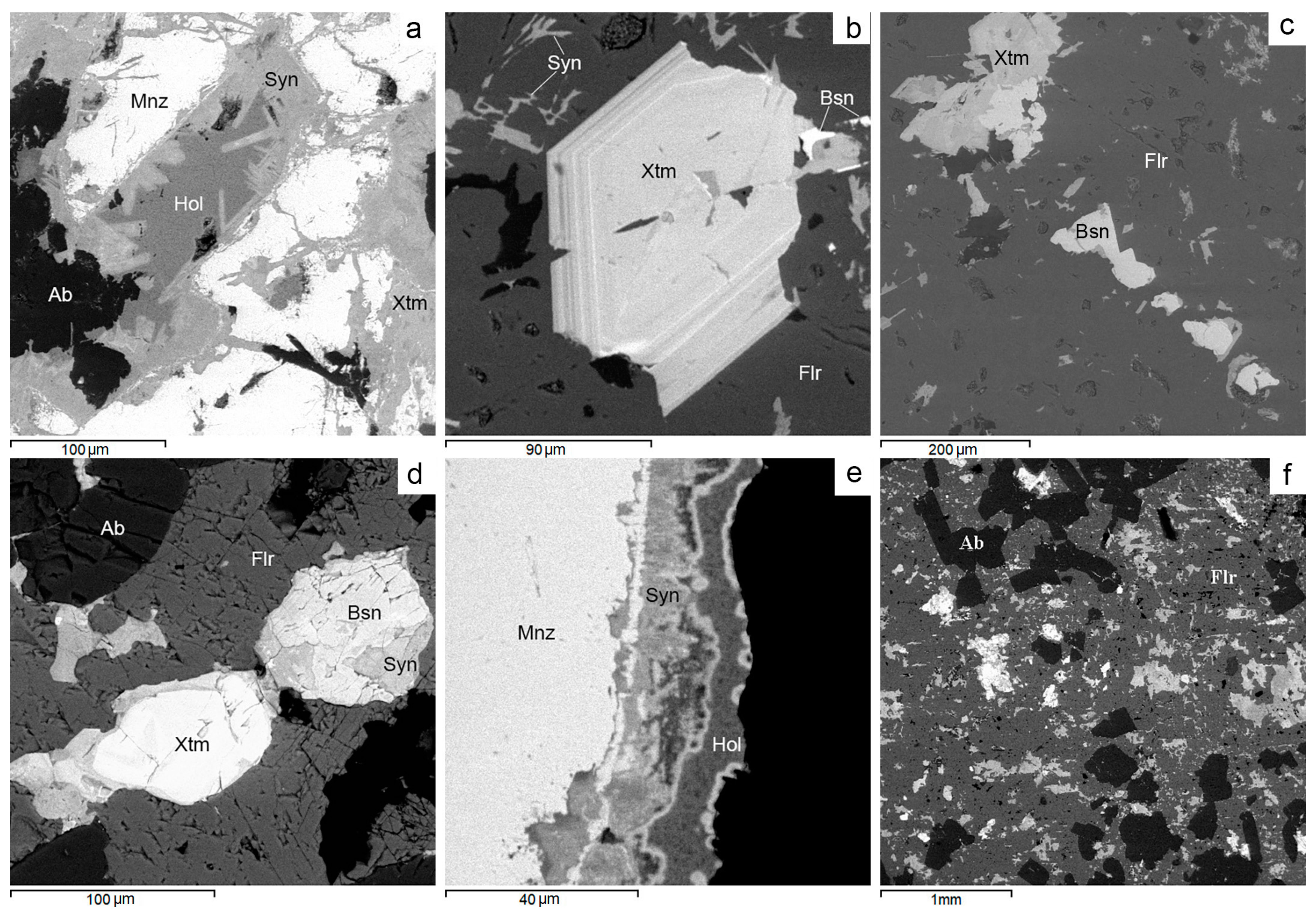
Figure 16.
BSE images. Rare-earth mineralization in Avtodor granites. (a) Micromiarole with synhysite and hollandite in monazite. (b) Zonal xenotime crystal in fluorite. (c) Intergrowths of xenotime and bastnäsite in fluorite. (d) Intergrowths of bastnäsite, synchysite and xenotime in fluorite. (e) Monazite with synhysite and hollandite rims. (f) Bastnäsite and synhysite in an albite-fluorite aggregate. For more detail, see text. Ab—albite, Bsn—bastnäsite, Flr—fluorite, Hol—hollandite, Mnz—monazite, Qz—quartz, Syn—synchysite, Xtm—xenotime.
Figure 16.
BSE images. Rare-earth mineralization in Avtodor granites. (a) Micromiarole with synhysite and hollandite in monazite. (b) Zonal xenotime crystal in fluorite. (c) Intergrowths of xenotime and bastnäsite in fluorite. (d) Intergrowths of bastnäsite, synchysite and xenotime in fluorite. (e) Monazite with synhysite and hollandite rims. (f) Bastnäsite and synhysite in an albite-fluorite aggregate. For more detail, see text. Ab—albite, Bsn—bastnäsite, Flr—fluorite, Hol—hollandite, Mnz—monazite, Qz—quartz, Syn—synchysite, Xtm—xenotime.
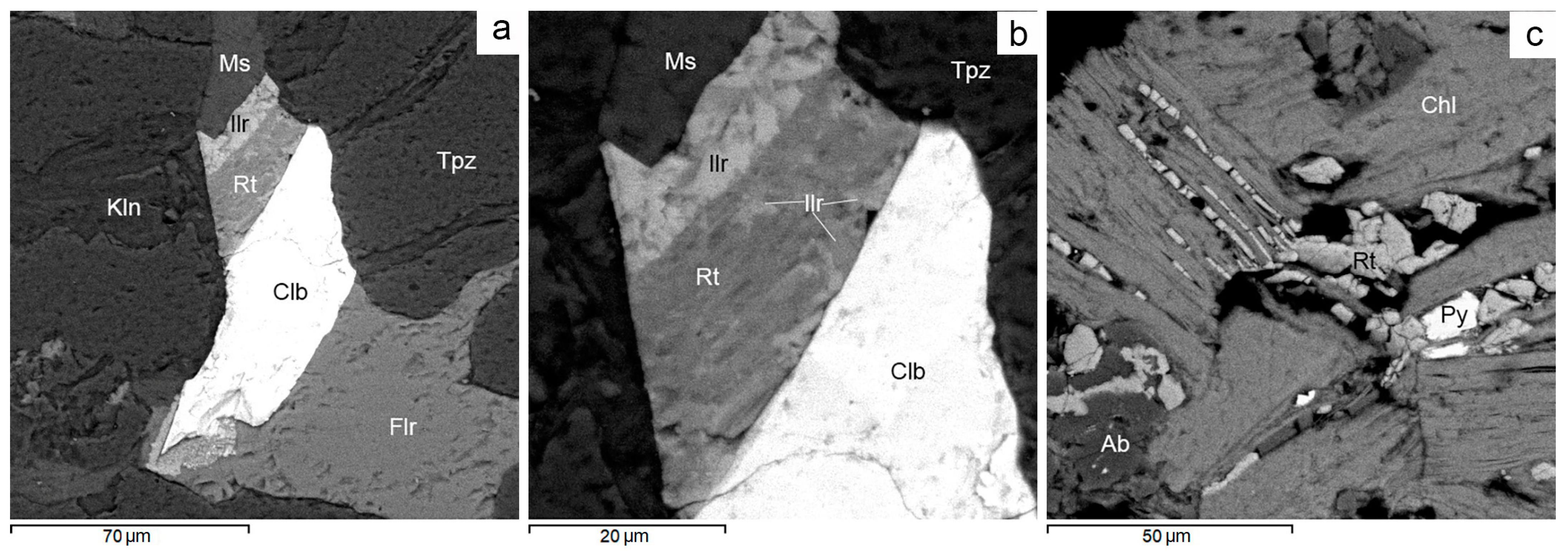
Figure 17.
BSE images. Ferricolumbite mineralization in Avtodor granites. (a) Zonal veinlet-like grain of fluorite, columbite, ilmenorutile, rutile and muscovite in topaz. (b) Sprouts of ilmerutile in rutile and rutile in ilmenorutile. (c) Nb-bearing rutile and pyrite in chlorite. For more detail, see text. Ab—albite, Chl—chlorite, Clb—columbite, Flr—fluorite, Fsp—feldspar, Ilr—ilmenorutile, Kln—kaolinite, Ms—muscovite, Py—pyrite, Rt—rutile, Tpz—topaz.
Figure 17.
BSE images. Ferricolumbite mineralization in Avtodor granites. (a) Zonal veinlet-like grain of fluorite, columbite, ilmenorutile, rutile and muscovite in topaz. (b) Sprouts of ilmerutile in rutile and rutile in ilmenorutile. (c) Nb-bearing rutile and pyrite in chlorite. For more detail, see text. Ab—albite, Chl—chlorite, Clb—columbite, Flr—fluorite, Fsp—feldspar, Ilr—ilmenorutile, Kln—kaolinite, Ms—muscovite, Py—pyrite, Rt—rutile, Tpz—topaz.
Figure 18.
Fe2+, Mn2+, Zn2+ ratio in helvite group minerals W. Lupikko and Gerberz-I.
Figure 18.
Fe2+, Mn2+, Zn2+ ratio in helvite group minerals W. Lupikko and Gerberz-I.
Figure 19.
Compositions of the aeschynite, euxenite, and samarskite analyzed on the CV1–CV2 three-group diagram of [
41].
Figure 19.
Compositions of the aeschynite, euxenite, and samarskite analyzed on the CV1–CV2 three-group diagram of [
41].
Figure 20.
Columbite-tantalite-group minerals at Musilampi, Hepaoja, and Avtodor occurrences on a classification diagram [
7,
68].
Figure 20.
Columbite-tantalite-group minerals at Musilampi, Hepaoja, and Avtodor occurrences on a classification diagram [
7,
68].
Table 1.
Representative electron microanalyses and atomic proportions of zavaritskite.
Table 1.
Representative electron microanalyses and atomic proportions of zavaritskite.
| wt.% | Lu1 | Lu2 | Lu3 | Lu4 | Lu5 | Lu6 | Lu7 |
|---|
| Bi2O3 | 95.90 | 95.83 | 95.10 | 81.57 | 90.31 | 94.19 | 87.98 |
| TeO2 | bd | bd | bd | 12.13 | bd | bd | bd |
| PbO | bd | bd | bd | bd | 4.73 | bd | 6.02 |
| F | 7.18 | 7.16 | 7.35 | 9.52 | 7.58 | 9.92 | 8.76 |
| Total | 103.08 | 102.99 | 102.44 | 103.22 | 102.62 | 104.11 | 102.76 |
| -O=F2 | 3.02 | 3.01 | 3.09 | 4.01 | 3.19 | 4.18 | 3.69 |
| Total | 100.06 | 99.98 | 99.35 | 99.21 | 99.43 | 99.93 | 99.07 |
| Chemical formula |
| Bi | 0.984 | 0.985 | 0.976 | 0.724 | 0.930 | 0.890 | 0.878 |
| Te | | | | 0.157 | | | |
| Pb | | | | | 0.051 | | 0.063 |
| O | 1.024 | 1.047 | 1.002 | 0.882 | 0.968 | 0.761 | 0.844 |
| F | 0.904 | 0.902 | 0.925 | 1.037 | 0.957 | 1.149 | 1.072 |
Table 2.
Representative electron microanalyses and atomic proportions of aeschynite. (Mu1, Mu4, Mu5) and euxenite (Mu2, Mu3, Mu6, Mu7) occurrence Muzilampi.
Table 2.
Representative electron microanalyses and atomic proportions of aeschynite. (Mu1, Mu4, Mu5) and euxenite (Mu2, Mu3, Mu6, Mu7) occurrence Muzilampi.
| wt.% | Mu1 | Mu2 | Mu3 | Mu4 | Mu5 | Mu6 | Mu7 |
|---|
| CaO | 3.06 | 2.20 | 2.02 | 1.91 | 2.70 | 1.89 | 1.92 |
| TiO2 | 7.64 | 13.00 | 11.71 | 11.51 | 12.30 | 11.21 | 12.57 |
| FeO | 7.16 | 4.45 | 6.94 | 4.42 | 6.17 | 7.14 | 8.33 |
| Y2O3 | bd | 8.35 | 7.62 | 8.52 | 7.90 | 8.82 | 6.24 |
| Nb2O5 | 36.15 | 40.94 | 43.38 | 38.84 | 42.79 | 40.99 | 42.12 |
| Ta2O5 | 23.20 | 10.44 | 8.75 | 9.76 | 8.80 | 9.38 | 9.33 |
| Ce2O3 | 3.72 | 3.37 | 2.05 | 3.14 | 4.80 | 3.04 | 3.05 |
| Nd2O3 | 2.34 | bd | 1.92 | 1.43 | bd | bd | 0.48 |
| WO3 | bd | 6.03 | 6.08 | 6.21 | 4.61 | 6.04 | 4.77 |
| ThO2 | 8.65 | 4.76 | 2.24 | 3.49 | 2.51 | 3.43 | 4.38 |
| UO3 | 6.95 | 5.53 | 7.33 | 9.66 | 6.38 | 7.29 | 6.49 |
| Total | 98.87 | 99.09 | 100.04 | 100.08 | 98.96 | 99.24 | 99.67 |
| Chemical formula |
| Ca | 0.227 | 0.151 | 0.135 | 0.133 | 0.180 | 0.128 | 0.128 |
| Ti | 0.398 | 0.628 | 0.552 | 0.563 | 0.577 | 0.536 | 0.588 |
| Fe | 0.415 | 0.239 | 0.364 | 0.241 | 0.322 | 0.380 | 0.433 |
| Y | | 0.286 | 0.254 | 0.294 | 0.263 | 0.298 | 0.206 |
| Nb | 1.132 | 1.189 | 1.229 | 1.141 | 1.207 | 1.177 | 1.184 |
| Ta | 0.437 | 0.182 | 0.149 | 0.172 | 0.149 | 0.162 | 0.158 |
| Ce | 0.095 | 0.079 | 0.047 | 0.084 | 0.110 | 0.071 | 0.069 |
| Nd | 0.058 | | 0.043 | 0.033 | | | 0.011 |
| W | | 0.100 | 0.099 | 0.105 | 0.074 | 0.100 | 0.077 |
| Th | 0.137 | 0.070 | 0.032 | 0.052 | 0.036 | 0.050 | 0.062 |
| U | 0.101 | 0.074 | 0.097 | 0.132 | 0.083 | 0.097 | 0.085 |
| O | 6.066 | 6.209 | 6.119 | 6.082 | 6.025 | 6.075 | 6.046 |
| CV1 | −1.421 | 0.608 | −0.005 | 0.528 | 0.324 | −0.232 | −0.356 |
| CV2 | −0.284 | −1.031 | −1.738 | −1.361 | −1.494 | −1.867 | −1.565 |
Table 3.
Representative electron microanalyses and atomic proportions of columbite occurrence Muzilampi.
Table 3.
Representative electron microanalyses and atomic proportions of columbite occurrence Muzilampi.
| wt.% | Mu1 | Mu2 | Mu3 | Mu4 | Mu5 | Mu6 | Mu7 | Mu8 | Mu9 | Mu10 | Mu11 | Mu12 | Mu13 | Mu14 | Mu15 | Mu16 |
|---|
| Ta2O5 | bd | bd | 1.52 | 2.01 | 2.32 | 2.83 | 3.21 | 3.45 | 4.03 | 4.30 | 4.31 | 4.93 | 5.25 | 6.06 | 6.17 | 14.55 |
| Nb2O5 | 76.18 | 76.41 | 72.82 | 73.01 | 72.28 | 73.84 | 73.04 | 72.91 | 73.11 | 68.79 | 74.66 | 71.81 | 74.56 | 67.61 | 70.95 | 59.28 |
| TiO2 | 1.71 | 1.73 | 2.50 | 1.46 | 2.06 | 1.41 | 1.59 | 1.47 | 1.07 | 2.17 | 1.63 | 1.48 | 0.82 | 1.68 | 1.52 | 0.70 |
| WO3 | bd | bd | 2.64 | 3.16 | 2.45 | 1.26 | 0.74 | bd | 2.36 | 1.98 | bd | 1.66 | bd | 2.16 | 2.56 | 4.12 |
| Y2O3 | bd | bd | 1.71 | 2.10 | bd | bd | bd | bd | bd | bd | bd | bd | bd | bd | bd | 2.57 |
| Yb2O3 | bd | bd | bd | bd | bd | bd | bd | bd | bd | 2.16 | bd | bd | bd | bd | bd | bd |
| FeO | 19.41 | 18.69 | 17.05 | 18.13 | 18.24 | 18.44 | 19.11 | 19.18 | 17.53 | 17.88 | 17.66 | 18.80 | 17.32 | 18.24 | 17.56 | 17.18 |
| MnO | 2.27 | 2.52 | 1.30 | 1.46 | 1.99 | 2.23 | 1.75 | 2.99 | 1.47 | 1.99 | 1.74 | 1.95 | 1.78 | 1.99 | 2.32 | 0.89 |
| Total | 99.57 | 99.36 | 99.55 | 101.34 | 99.33 | 100 | 99.44 | 100 | 99.57 | 99.27 | 100 | 100.63 | 99.72 | 97.95 | 101.10 | 99.28 |
| Chemical formula |
| Ta | | | 0.024 | 0.031 | 0.036 | 0.044 | 0.049 | 0.053 | 0.064 | 0.068 | 0.067 | 0.077 | 0.083 | 0.097 | 0.97 | 0.243 |
| Nb | 1.918 | 1.932 | 1.893 | 1.869 | 1.870 | 1.897 | 1.878 | 1.846 | 1.926 | 1.804 | 1.933 | 1.848 | 1.954 | 1.792 | 1.843 | 1.646 |
| Ti | 0.072 | 0.073 | 0.108 | 0.062 | 0.088 | 0.060 | 0.068 | 0.061 | 0.046 | 0.094 | 0.07 | 0.063 | 0.035 | 0.074 | 0.066 | 0.033 |
| W | | | 0.039 | 0.046 | 0.036 | 0.019 | 0.049 | | 0.035 | 0.030 | | 0.024 | | 0.033 | 0.038 | 0.066 |
| Y | | | 0.053 | 0.063 | | | | | | | | | | | | 0.084 |
| Yb | | | | | | | | | | 0.039 | | | | | | |
| Fe | 0.904 | 0.875 | 0.820 | 0.859 | 0.872 | 0.876 | 0.909 | 0.898 | 0.854 | 0.867 | 0.845 | 0.895 | 0.840 | 0.904 | 0.843 | 0.882 |
| Mn | 0.107 | 0.119 | 0.064 | 0.071 | 0.097 | 0.107 | 0.084 | 0.142 | 0.073 | 0.098 | 0.084 | 0.094 | 0.087 | 0.099 | 0.113 | 0.046 |
| O | 5.950 | 5.970 | 6.089 | 6.036 | 6.018 | 6.013 | 5.980 | 5.910 | 6.099 | 5.982 | 6.069 | 6.00 | 6.089 | 5.973 | 6.052 | 6.041 |
| Ta/(Ta + Nb) | 0 | 0 | 0.013 | 0.016 | 0.019 | 0.023 | 0.025 | 0.028 | 0.032 | 0.036 | 0.034 | 0.040 | 0.041 | 0.051 | 0.050 | 0.129 |
| Mn/(Mn + Fe) | 0.106 | 0.120 | 0.072 | 0.076 | 0.100 | 0.109 | 0.085 | 0.137 | 0.079 | 0.102 | 0.090 | 0.095 | 0.094 | 0.099 | 0.118 | 0.050 |
Table 4.
Representative electron microanalyses and atomic proportions of the bastnäsite occurrence of Hepaoja.
Table 4.
Representative electron microanalyses and atomic proportions of the bastnäsite occurrence of Hepaoja.
| wt.% | H1 | H2 | H3 | H4 | H5 | H6 |
|---|
| CaO | bd | 1.36 | bd | 1.73 | 3.54 | 2.41 |
| Y2O3 | bd | bd | bd | bd | 1.31 | 4.66 |
| La2O3 | 354.80 | 37.75 | 16.73 | 20.22 | 13.34 | 12.21 |
| Ce2O3 | 8.46 | 14.82 | 40.72 | 36.95 | 36.82 | 35.41 |
| Pr2O3 | 7.51 | 3.78 | 3.39 | 3.56 | 4.26 | 4.00 |
| Nd2O3 | 18.36 | 14.48 | 14.91 | 11.85 | 14.87 | 14.76 |
| Pm2O3 | bd | 2.41 | bd | bd | bd | bd |
| Eu2O3 | bd | 2.42 | bd | bd | bd | bd |
| Gd2O3 | 3.13 | 2.04 | bd | bd | bd | bd |
| ThO2 | 1.45 | bd | bd | bd | 2.20 | 4.21 |
| CO2* | 19.92 | 18.87 | 20.05 | 21.24 | 19.80 | 18.67 |
| F | 9.27 | 5.93 | 7.25 | 7.69 | 6.66 | 6.33 |
| Total | 103.90 | 102.50 | 103.05 | 103.24 | 102.80 | 102.66 |
| -O=F2 | 3.90 | 2.50 | 3.05 | 3.24 | 2.80 | 2.66 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Chemical formula |
| Ca | | 0.055 | | 0.065 | 0.138 | 0.096 |
| Y | | | | | 0.025 | 0.092 |
| La | 0.472 | 0.524 | 0.225 | 0.262 | 0.179 | 0.168 |
| Ce | 0.111 | 0.204 | 0.545 | 0.476 | 0.490 | 0.484 |
| Pr | 0.099 | 0.052 | 0.045 | 0.046 | 0.056 | 0.054 |
| Nd | 0.234 | 0.195 | 0.194 | 0.149 | 0.193 | 0.197 |
| Gd | 0.037 | 0.032 | | | | |
| Th | 0.024 | 0.031 | | | 0.036 | 0.071 |
| | | 0.025 | | | | |
| CO3 | 0.971 | 0.970 | 1.000 | 1.020 | 0.983 | 0.952 |
| F | 1.047 | 0.706 | 0.838 | 0.855 | 0.766 | 0.748 |
Table 5.
Representative electron microanalyses and atomic proportions of the columbite occurrence of Hepaoja.
Table 5.
Representative electron microanalyses and atomic proportions of the columbite occurrence of Hepaoja.
| wt. % | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|
| Ta2O5 | 3.40 | 4.60 | 5.93 | 7.07 | 7.44 | 8.01 | 11.49 | 12.11 | 12.56 | 12.60 | 12.94 | 14.82 | 17.07 | 18.04 | 21.75 | 25.00 | 38.16 |
| Nb2O5 | 72.78 | 73.49 | 70.38 | 69.04 | 69.63 | 68.38 | 61.32 | 65.25 | 65.86 | 64.46 | 63.60 | 63.60 | 60.58 | 58.47 | 55.27 | 50.33 | 38.08 |
| TiO2 | 1.46 | 1.67 | 1.17 | 1.07 | 1.58 | 1.57 | 1.68 | 0.65 | bd | 0.61 | bd | 0.71 | 0.84 | 1.18 | 1.41 | 1.28 | 2.33 |
| WO3 | 1.64 | bd | 1.88 | 2.39 | bd | 1.75 | 6.24 | 1.81 | 2.81 | 2.56 | 2.62 | 2.52 | 2.35 | bd | 3.03 | 5.68 | 4.40 |
| Sc2O3 | bd | bd | 0.38 | bd | bd | bd | bd | bd | bd | bd | 0.40 | bd | bd | bd | 0.41 | bd | bd |
| FeO | 18.58 | 18.38 | 17.88 | 18.52 | 19.39 | 19.03 | 17.69 | 18.11 | 17.39 | 17.88 | 18.39 | 16.73 | 16.92 | 17.06 | 16.46 | 15.93 | 15.38 |
| MnO | 2.14 | 1.86 | 2.38 | 1.93 | 1.96 | 1.26 | 1.59 | 2.08 | 1.38 | 1.89 | 2.06 | 1.63 | 2.35 | 2.17 | 1.68 | 1.78 | 1.64 |
| Chemical formula |
| Ta | 0.052 | 0.071 | 0.093 | 0.112 | 0.115 | 0.126 | 0.188 | 0.195 | 0.207 | 0.205 | 0.209 | 0.245 | 0.282 | 0.299 | 0.368 | 0.435 | 0.696 |
| Nb | 1.873 | 1.892 | 1.832 | 1.812 | 1.798 | 1.794 | 1.669 | 1.748 | 1.800 | 1.739 | 1.711 | 1.748 | 1.667 | 1.614 | 1.553 | 1.458 | 1.155 |
| Ti | 0.063 | 0.071 | 0.050 | 0.047 | 0.068 | 0.068 | 0.076 | 0.029 | | 0.027 | | 0.033 | 0.038 | 0.054 | 0.066 | 0.062 | 0.118 |
| W | 0.024 | | 0.028 | 0.036 | | 0.026 | 0.097 | 0.028 | 0.044 | 0.040 | 0.040 | 0.040 | 0.037 | | 0.049 | 0.094 | 0.077 |
| Sc | | | 0.019 | | | | | | | | 0.021 | | | | 0.023 | | |
| Fe | 0.885 | 0.876 | 0.860 | 0.889 | 0.926 | 0.923 | 0.891 | 0.897 | 0.879 | 0.892 | 0.915 | 0.851 | 0.862 | 0.871 | 0.855 | 0.854 | 0.862 |
| Mn | 0.104 | 0.089 | 0.116 | 0.095 | 0.095 | 0.062 | 0.081 | 0.104 | 0.071 | 0.096 | 0.104 | 0.084 | 0.116 | 0.113 | 0.089 | 0.096 | 0.093 |
| O | 6.000 | 5.944 | 6.001 | 5.996 | 5.940 | 5.999 | 6.057 | 6.001 | 6.100 | 6.022 | 5.971 | 6.104 | 6.038 | 5.868 | 6.060 | 6.089 | 6.050 |
| Ta/(Ta + Nb) | 0.027 | 0.036 | 0.048 | 0.058 | 0.060 | 0.066 | 0.101 | 0.100 | 0.103 | 0.105 | 0.109 | 0.123 | 0.145 | 0.156 | 0.192 | 0.230 | 0.376 |
| Mn/(Mn + Fe) | 0.105 | 0.092 | 0.119 | 0.097 | 0.093 | 0.063 | 0.083 | 0.104 | 0.075 | 0.097 | 0.102 | 0.90 | 0.119 | 0.115 | 0.094 | 0.101 | 0.097 |
Table 6.
Representative electron microanalyses and atomic proportions of the monazite occurrence of Avtodor.
Table 6.
Representative electron microanalyses and atomic proportions of the monazite occurrence of Avtodor.
| Wt.% | Av1 | Av2 | Av3 | Av4 | Av5 | Av6 | Av7 | Av8 | Av9 | Av10 |
|---|
| La2O3 | 18.80 | 12.53 | 11.12 | 22.46 | 10.67 | 13.84 | 13.64 | 12.77 | 13.00 | 14.59 |
| Ce2O3 | 35.46 | 35.25 | 27.66 | 37.52 | 34.58 | 35.61 | 37.55 | 39.23 | 34.40 | 34.55 |
| Pr2O3 | bd | bd | bd | bd | 4.01 | 3.08 | bd | bd | bd | 2.80 |
| Nd2O3 | 12.35 | 13.23 | 22.00 | 8.66 | 16.19 | 10.55 | 9.86 | 10.93 | 14.58 | 12.37 |
| ThO2 | bd | 5.60 | 6,60 | bd | 3.87 | 10.02 | 8.55 | 5.28 | 4.37 | 6.24 |
| P2O5 | 32.05 | 33.38 | 32.86 | 30.78 | 30.29 | 27.28 | 29.34 | 32.33 | 32.29 | 29.32 |
| Total | 98.66 | 99.99 | 100.23 | 99.41 | 99.60 | 100.37 | 98.94 | 100.54 | 98.64 | 99.87 |
| Chemical formula |
| La | 0.269 | 0.183 | 0.157 | 0.320 | 0.164 | 0.211 | 0.201 | 0.170 | 0.173 | 0.216 |
| Ce | 0.504 | 0.503 | 0.398 | 0.540 | 0.506 | 0.538 | 0.560 | 0.530 | 0.451 | 0.509 |
| Pr | | | | | 0.060 | 0.046 | | | | 0.041 |
| Nd | 0.172 | 0.183 | 0.304 | 0.120 | 0.230 | 0.155 | 0.140 | 0.140 | 0.191 | 0.178 |
| Th | | 0.046 | 0.063 | | 0.030 | 0.094 | 0.080 | 0.050 | 0.040 | 0.057 |
| P | 1.055 | 1.085 | 1.078 | 1.020 | 1.010 | 0.956 | 1.001 | 1.110 | 1.100 | 0.999 |
| O | 4.055 | 4.108 | 4.052 | 4.020 | 4.025 | 4.003 | 4.014 | 4.135 | 4.053 | 4.005 |
Table 7.
Representative electron microanalyses and atomic proportions of the columbite (Av1, Av2), ilmenorutile (Av3-Av8) and rutile (Av9) occurrence Avtodor.
Table 7.
Representative electron microanalyses and atomic proportions of the columbite (Av1, Av2), ilmenorutile (Av3-Av8) and rutile (Av9) occurrence Avtodor.
| wt. % | Av1 | Av2 | Av3 | Av4 | Av5 | Av6 | Av7 | Av8 | Av9 |
|---|
| TiO2 | 1.79 | 3.13 | 31.06 | 68.27 | 61.52 | 74.10 | 71.55 | 78.14 | 95.24 |
| FeO | 13.94 | 13.53 | 9.52 | 6.90 | 7.20 | 5.48 | 5.76 | 5.08 | 2.10 |
| MnO | 4.58 | 5.35 | 3.68 | bd | bd | bd | bd | bd | bd |
| Nb2O5 | 61.35 | 65.26 | 39.45 | 19.65 | 18.02 | 15.84 | 14.24 | 11.36 | 2.21 |
| Ta2O5 | 14.79 | 8.66 | 9.33 | 4.61 | 12.08 | 4.21 | 5.98 | 4.99 | bd |
| WO3 | 4.29 | 2.80 | 6.68 | bd | 1.34 | bd | 2.86 | bd | bd |
| Sc2O3 | bd | 0.47 | bd | bd | bd | bd | bd | bd | bd |
| Total | 100.74 | 99.20 | 99.73 | 99.43 | 100.16 | 99.64 | 100.37 | 99.57 | 99.55 |
| Chemical formula |
| Ti | 0.081 | 0.138 | 0.413 | 0.764 | 0.722 | 0.812 | 0.798 | 0.846 | 0.963 |
| Fe | 0.704 | 0.664 | 0.141 | 0.086 | 0.094 | 0.067 | 0.071 | 0.061 | 0.024 |
| Mn | 0.235 | 0.266 | 0.550 | | | | | | |
| Nb | 1.673 | 1.729 | 0.315 | 0.132 | 0.127 | 0.104 | 0.095 | 0.074 | 0.013 |
| Ta | 0.243 | 0.161 | 0.450 | 0.019 | 0.051 | 0.017 | 0.024 | 0.019 | |
| W | 0.067 | 0.042 | 0.031 | | 0.005 | | 0.011 | | |
| O | 6.092 | 6.057 | | | | | | | |
| Ta/(Ta + Nb) | 0.127 | 0.085 | | | | | | | |
| Mn/(Mn + Fe) | 0.250 | 0.286 | | | | | | | |
Table 8.
Trace elements composition of biotite occurrence Muzilampi (LA ICP MS).
Table 8.
Trace elements composition of biotite occurrence Muzilampi (LA ICP MS).
| ppm | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Be | 22.35 | 44.7 | 22.35 | bd | 18.63 | bd |
| Sc | 39.89 | 44.22 | 45.86 | 46.68 | 40.12 | 49.25 |
| Zn | 264 | 253.2 | 390.3 | 593.5 | 131 | 199.4 |
| Ga | 125 | 166.3 | 192.2 | 222.9 | 103.5 | 137.3 |
| Ge | 29.49 | 26.22 | 30.95 | 32.04 | 19.66 | 39.32 |
| As | 35.91 | 39.57 | 58.26 | 32.98 | 38.47 | 64.12 |
| Rb | 2497 | 3756 | 5805 | 5037 | 1811 | 2932 |
| Sr | 183.5 | 134.9 | 125.9 | 135.8 | 182.3 | 335.9 |
| Y | 1442 | 941 | 1258 | 346.4 | 198.9 | 639.2 |
| Zr | 378.4 | 681.2 | 349.4 | 143.2 | 454.1 | 574 |
| Nb | 2517 | 5117 | 7779 | 7487 | 2645 | 3087 |
| Cd | bd | 23.57 | bd | 47.13 | 28.28 | bd |
| In | 10.17 | 7.557 | 8.196 | 7.128 | 6.628 | 9.077 |
| Sn | 43.37 | 64.32 | 68.69 | 98.09 | 25.61 | 48.02 |
| Ba | 692.4 | 1005 | 1110 | 1341 | 882.2 | 977.7 |
| Hf | 20.15 | 33.58 | 20.15 | 20.15 | 16.79 | 6.716 |
| Ta | 307.6 | 611.6 | 908.9 | 939.3 | 323.9 | 517.7 |
| Pb | 121.7 | 125.5 | 212.8 | 101.7 | 82.91 | 90.81 |
| Th | 43.5 | 48 | 79.5 | 25.5 | 36 | 29.25 |
| U | 46.41 | 65.25 | 33.82 | 16.42 | 17.47 | 8.84 |
| REE | 4756.85 | 2829.52 | 4016.57 | 1272.6 | 477.93 | 1786.21 |