Low-Temperature Fluorocarbonate Mineralization in Lower Devonian Rhynie Chert, UK
Abstract
1. Introduction

- (i)
- Measurement of the chemical composition of the mineral.
- (ii)
- Comparison with the composition of known fluorocarbonate mineralization and identification of the phase.
- (iii)
- Constraints on the temperature of formation.
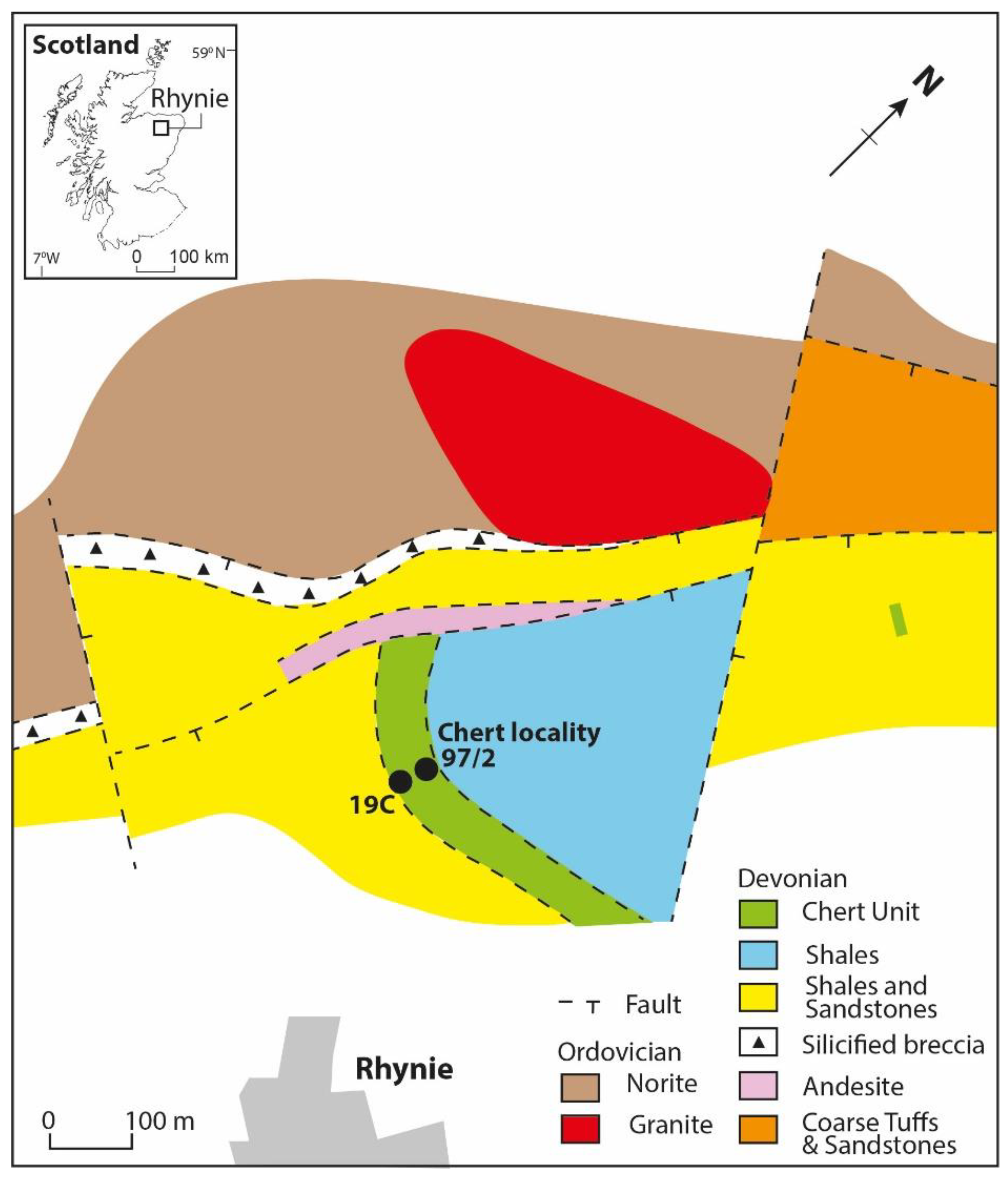
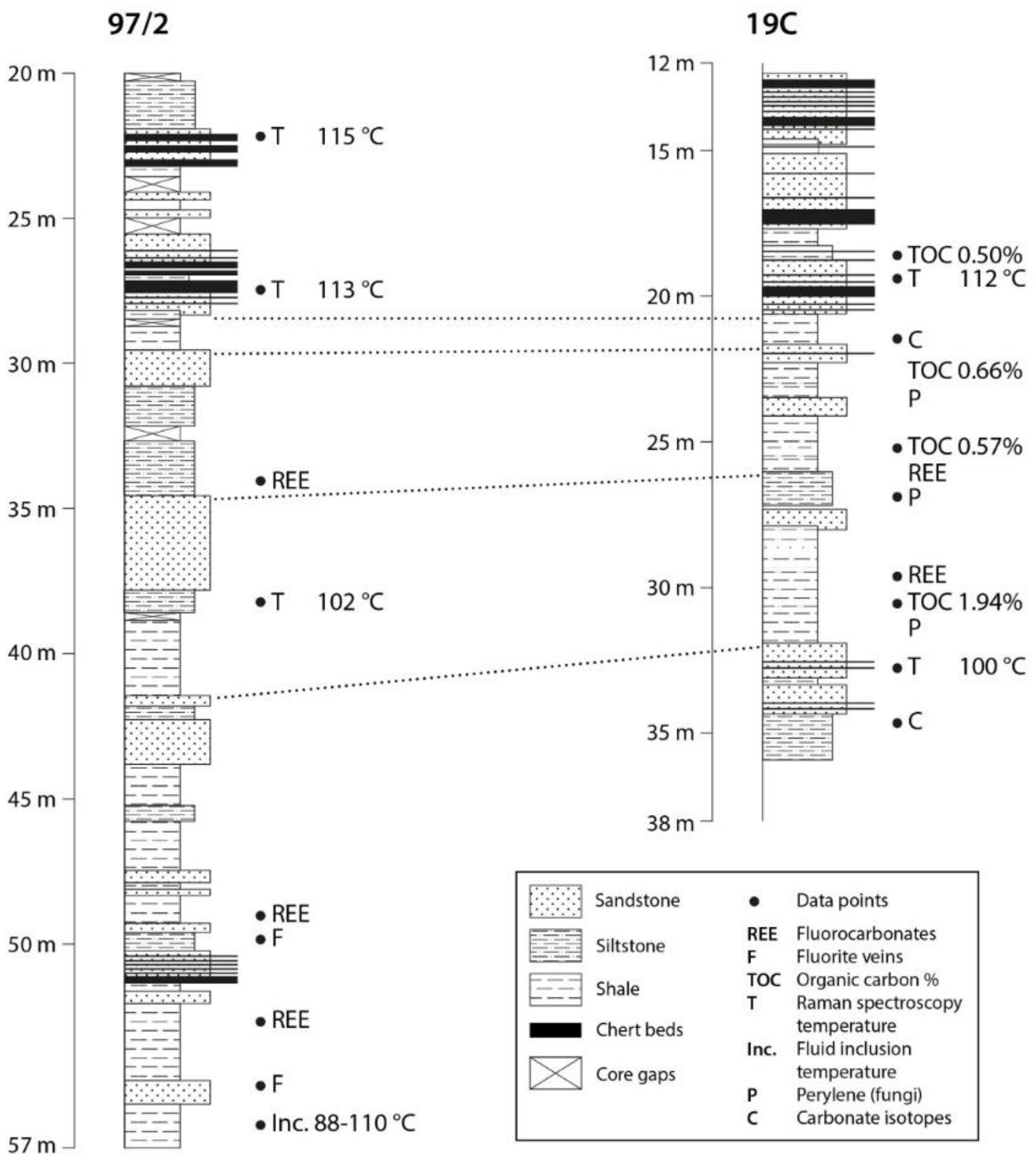
2. Materials and Methods
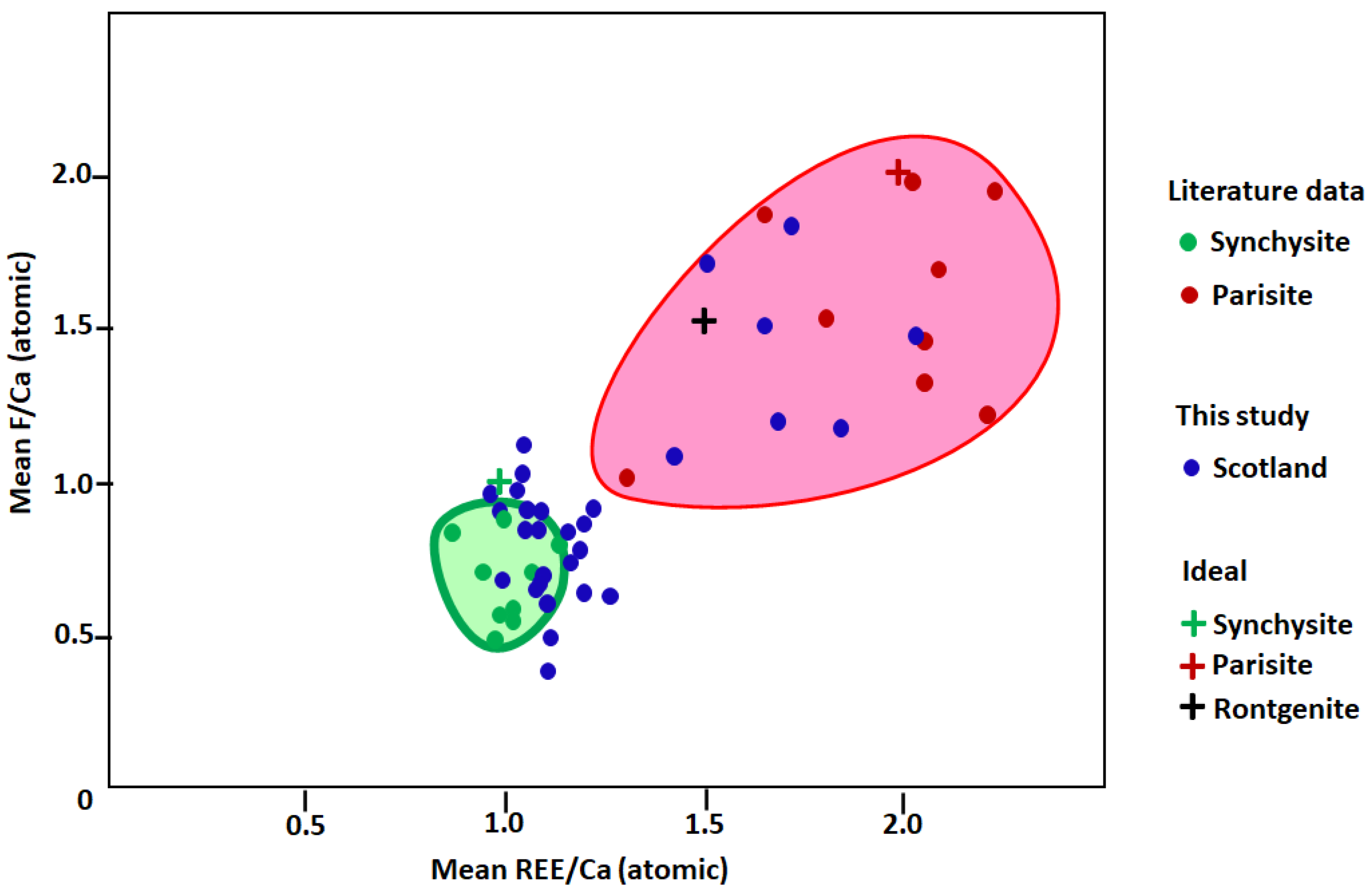
| Mineral | Location | Setting | Mineralization Temperature | Mean F/Ca (atomic) | Mean REE/Ca (atomic) | Reference |
|---|---|---|---|---|---|---|
| Synchysite | NE Vietnam | Hydrothermal base metal | ~400 ℃ | 0.50 | 0.97 | [10] |
| Synchysite | Olympic Dam, Australia | Breccia-hosted iron oxide | ~300 ℃ | 0.55 | 1.00 | [11] |
| Synchysite | South Hungary | Coal, intruded | <200 ℃ | 0.57 | 0.98 | [33] |
| Synchysite | Cinquevalli, Italy | Ag-Cu-quartz veins | 0.58 | 1.01 | [34] | |
| Synchysite | Rif, Morocco | Pelites | 420–450 ℃ | 0.71 | 1.06 | [35] |
| Synchysite | Anti-Atlas, Morocco | Syenite | >150–250 ℃ | 0.71 | 0.94 | [5] |
| Synchysite | Erzgebirge, Germany | Altered granite | 550–600 ℃ | 0.80 | 1.13 | [36] |
| Synchysite | Rozna, Czech Republic | U deposit | <100 ℃ | 0.84 | 0.86 | [37] |
| Synchysite | Jiangxi, China | Weathered granite | <100 ℃ | 0.87 | 0.99 | [14] |
| Parisite | Parana Basin, Brazil | Carbonatite | <375 ℃ | 1.02 | 1.30 | [6] |
| Parisite | Gujarat, India | Altered Carbonatite | 100–150 ℃ | 1.22 | 2.21 | [13] |
| Parisite | Bergslagen, Sweden | Metavolcanics, iron oxide ore | >400 ℃ | 1.32 | 2.05 | [12] |
| Parisite | Eastern Tibet | Carbonatite-syenite | 200–300 ℃ | 1.44 | [7] | |
| Parisite | Qinling, China | Carbonatite | <265 ℃ | 1.46 | 2.05 | [38] |
| Parisite | South Urals, Russia | Volcanic massive sulfide | 80–300 ℃ | 1.51 | 1.80 | [39] |
| Parisite | Wyoming, USA | Carbonatite | <250 ℃ | 1.70 | 2.09 | [8] |
| Parisite | Aar Massif, Switzerland | Granite, hydrothermal | ~300 ℃ | 1.87 | 1.63 | [40] |
| Parisite | Thor Lake, Canada | Syenite | <600 ℃ | 1.95 | 2.23 | [9] |
| Parisite | Maripí, Colombia | Emeralds in black shale | 290–360 ℃ | 1.97 | 2.02 | [41] |
| Fluoro-carbonate | Rhynie Chert | Black shale | 100–115 ℃ | 0.85 | 1.10 | This study |
3. Results
4. Discussion
4.1. Requirements for Fluorocarbonate Mineralization
4.2. Role of Granite
4.3. Identification of Fluorocarbonate Mineral
4.4. Temperature of Mineralization
4.5. Biological Contribution to REE Mineralization
4.6. Organic-Rich Sediments
5. Conclusions
- (i)
- Availability of REEs by bioweathering of monazite grains in the sequence, derived from underlying granite.
- (ii)
- Availability of fluorine, evident as fluorite, associated with hot spring activity also related to underlying granite.
- (iii)
- Precipitation in the alkaline environment of carbonate-bearing shales.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.P.; Henderson, P.; Peishan, Z. Reaction relationships in the Bayan obo Fe–REE–Nb deposit Inner Mongolia, China, implications for the relative stability of rare-earth element phosphates and fluorcarbonates. Contrib. Mineral. Petrol. 1999, 134, 294–310. [Google Scholar] [CrossRef]
- Louvel, M.; Etschmann, B.; Guan, Q.; Testemale, D.; Brugger, J. Carbonate complexation enhances hydrothermal transport of rare earth elements in alkaline fluids. Nat. Commun. 2022, 13, 1456. [Google Scholar] [CrossRef]
- Marien, C.; Dijkstra, A.; Wilkins, C. The hydrothermal alteration of carbonatite in the Fen Complex, Norway: Mineralogy, geochemistry, and implications for rare-earth element resource formation. Mineral. Mag. 2018, 82, S115–S131. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Benaouda, R.; Devey, C.W.; Badra, L.; Ennaciri, A. Light rare-earth element mineralization in hydrothermal veins related to the Jbel Boho alkaline igneous complex, AntiAtlas/Morocco: The role of fluid-carbonate interactions in the deposition of synchysite-(Ce). J. Geochem. Explor. 2017, 177, 28–44. [Google Scholar] [CrossRef]
- Manfredi, T.R.; Bastos Neto, A.C.; Pereira, V.P.; Barbanson, L.; Schuck, C. The parisite-(Ce) mineralization associated with the Fazenda Varela carbonatite (Correia Pinto, SC). Pesquisas em Geociências 2013, 40, 295–307. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Yang, Z.; Sun, X.; Zhu, Z.; Zhang, Q. Mineralogical and geochemical studies of brecciated ores in the Dalucao REE deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2015, 70, 613–636. [Google Scholar] [CrossRef]
- Andersen, A.K.; Clark, J.G.; Larson, P.B.; Donovan, J.J. REE fractionation, mineral speciation, and supergene enrichment of the Bear Lodge carbonatites, Wyoming, USA. Ore Geol. Rev. 2017, 89, 780–807. [Google Scholar] [CrossRef]
- Hoshino, M.; Watanabe, Y.; Murakami, H.; Kon, Y.; Tsunematsu, M. Formation process of zircon associated with REE-fluorocarbonate and niobium minerals in the Nechalacho REE deposit, Thor Lake, Canada. Resour. Geol. 2012, 63, 1–26. [Google Scholar] [CrossRef]
- Pham-Ngoc, C.; Ishiyama, D.; Tran, T.A.; Hoshino, M.; Taguchi, S. Characteristic features of REE and Pb–Zn–Ag mineralizations in the Na Son Deposit, Northeastern Vietnam. Resour. Geol. 2016, 66, 404–418. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Wade, B.P.; Gilbert, S.; Kamenetsky, V.S. Rare Earth Element Fluorocarbonate Minerals from the Olympic Dam Cu-U-Au-Ag Deposit, South Australia. Minerals 2017, 7, 202. [Google Scholar] [CrossRef]
- Majka, J.; Włodek, A.; Jonsson, E.; Högdahl, K. Contrasting coronas: Microscale fluid variation deduced from monazite breakdown products in altered metavolcanic rocks associated with the Grängesberg apatite-iron oxide ore, Bergslagen, Sweden. GFF 2022, 144, 89–96. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Viladkar, S.G.; Ripp, G.S.; Burtseva, M.V. Hydrothermal REE mineralization in the Amba Dongar carbonatite complex, Gujurat, India. Can. Mineral. 2009, 47, 1105–1116. [Google Scholar] [CrossRef]
- Ishihara, S.; Hua, R.; Hoshino, M.; Murakami, H. REE abundance and REE minerals in granitic rocks in the Nanling Range, Jiangxi Province, southern China, and generation of the REE-rich weathered crust deposits. Resour. Geol. 2008, 58, 355–372. [Google Scholar] [CrossRef]
- Rice, C.M.; Ashcroft, W.A.; Batten, D.J.; Boyce, A.J.; Caulfield, J.B.D.; Fallick, A.E.; Hole, M.J.; Jones, E.; Pearson, M.J.; Rogers, G.; et al. A Devonian auriferous hot spring system, Rhynie, Scotland. J. Geol. Soc. Lond. 1995, 152, 229–250. [Google Scholar] [CrossRef]
- Rice, C.M.; Trewin, N.H.; Anderson, L.I. Geological setting of the Early Devonian Rhynie cherts, Aberdeenshire, Scotland: An early terrestrial hot spring system. J. Geol. Soc. Lond. 2002, 159, 203–214. [Google Scholar] [CrossRef]
- Trewin, N.H.; Wilson, E. Correlation of the Early Devonian Rhynie chert beds between three boreholes at Rhynie, Aberdeenshire. Scott. J. Geol. 2004, 40, 73–81. [Google Scholar] [CrossRef]
- Baron, M.; Hillier, S.; Rice, C.M.; Czapnik, K.; Parnell, J. Fluids and hydrothermal alteration assemblages in a Devonian gold-bearing hot-spring system, Rhynie, Scotland. Trans. R. Soc. Edinb. Earth Sci. 2004, 94, 309–324. [Google Scholar] [CrossRef]
- Taylor, T.N.; Klavins, S.D.; Krings, M.; Taylor, E.L.; Kerp, H.; Hass, H. Fungi from the Rhynie Chert: A view from the dark side. Trans. R. Soc. Edinb. Earth Sci. 2004, 94, 457–473. [Google Scholar] [CrossRef]
- Strullu-Derrien, C.; Kenrick, P.; Pressel, S.; Duckett, J.G.; Rioult, J.-P.; Strullu, D.-G. Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: Novel insights into ancestral plant–fungus symbioses. New Phytol. 2014, 203, 964–979. [Google Scholar] [CrossRef]
- Krings, M.; Harper, C.J.; Taylor, E.L. Fungi and fungal interactions in the Rhynie chert: A review of the evidence, with the description of Perexiflasca tayloriana gen. et sp. nov. Philos. Trans. R. Soc. B 2018, 373, 20160500. [Google Scholar] [CrossRef] [PubMed]
- Keekan, K.K.; Jalondhara, J.C. Aspergillus niger PSSG8 mediated bioleaching of monazite for the recovery of rare earth and other metal constituents. Adv. Mater. Res. 2015, 1130, 238–242. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioeng. 2016, 113, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Corbett, M.K.; Eksteen, J.J.; Niu, X.-Z.; Croue, J.-P.; Watkin, E.L. Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: A study utilizing Western Australian monazite. Bioprocess Biosyst. Eng. 2017, 40, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Fathollahzadeh, H.; Eksteen, J.J.; Kaksonen, A.H.; Watkin, E.L.J. Role of microorganisms in bioleaching of rare earth elements from primary and secondary resources. Appl. Microbiol. Biotechnol. 2019, 103, 1043–1057. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A. Bioleaching of phosphate minerals using Aspergillus niger: Recovery of copper and rare earth elements. Metals 2020, 10, 978. [Google Scholar] [CrossRef]
- Kang, X.; Csetenyi, L.; Gadd, G.M. Colonization and bioweathering of monazite by Aspergillus niger: Solubilization and precipitation of rare earth elements. Environ. Microbiol. 2021, 23, 3970–3986. [Google Scholar] [CrossRef]
- Trewin, N.H.; Fayers, S.R. Macro to micro aspects of the plant preservation in the Early Devonian Rhynie cherts, Aberdeenshire, Scotland. Earth Environ. Sci. Trans. R. Soc. Edinb. 2015, 106, 67–80. [Google Scholar] [CrossRef]
- Marynowski, L.; Smolarek, J.; Bechtel, A.; Phillippe, M.; Kurkiewicz, S.; Simoneit, B.R.T. Perylene as an indicator of conifer fossil wood degradation by wood-degrading fungi. Org. Geochem. 2013, 59, 143–151. [Google Scholar] [CrossRef]
- Schito, A.; Romano, C.; Corrado, S.; Grigo, D.; Poe, B. Diagenetic thermal evolution of organic matter by Raman spectroscopy. Org. Geochem. 2017, 106, 57–67. [Google Scholar] [CrossRef]
- Schito, A.; Corrado, S. An automatic approach for characterization of the thermal maturity of dispersed organic matter Raman spectra at low diagenetic stages. Geol. Soc. Lond. Spec. Publ. 2020, 484, 107–119. [Google Scholar] [CrossRef]
- Barker, C.E.; Pawlewicz, M.J. The correlation of vitrinite reflectance with maximum temperature in humic organic matter. In Paleogeothermics; Lecture Notes in Earth Sciences; Buntebarth, G., Stegena, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 5, pp. 79–93. [Google Scholar]
- Szakáll, S.; Nagy, G.; Sajó, I.E. Synchysite-(Ce) from the Komló coal deposit, Mecsek Mts., South Hungary. Acta Mineral.-Petrogr. Abstr. Ser. 2003, 1, 100. [Google Scholar]
- Capitani, G. Synchysite-(Ce) from Cinquevalli (Trento, Italy): Stacking disorder and the polytypism of (Ca,REE)-Fluorcarbonates. Minerals 2020, 10, 77. [Google Scholar] [CrossRef]
- Janots, E.; Negro, F.; Brunet, F.; Goffé, B.; Engi, M.; Bouybaouène, M.L. Evolution of the REE mineralogy in HP-LT metapelites of the Sebtide complex, Rif, Morocco: Monazite stability and geochronology. Lithos 2006, 87, 214–234. [Google Scholar] [CrossRef]
- Förster, H.J. Synchysite-(Y)-synchysite-(Ce) solid solutions from Markersbach, Erzgebirge, Germany: REE and Th mobility during high-T alteration of highly fractionated aluminous A-type granites. Mineral. Petrol. 2001, 72, 259–280. [Google Scholar] [CrossRef]
- Kříbek, B.; Knésl, I.; Dobeš, P.; Veselovský, F.; Pořádek, P.; Škoda, R.; Čopjaková, R.; Leichmann, J.; Košek, F. The origin of synchysite-(Ce) and sources of rare-earth elements in the Rožná uranium deposit, Czech Republic. Minerals 2022, 12, 690. [Google Scholar] [CrossRef]
- Smith, M.; Kynicky, J.; Xu, C.; Song, W.; Spratt, J.; Jeffries, T.; Brtnicky, M.; Kopriva, A.; Cangelosi, D. The origin of secondary heavy rare earth element enrichment in carbonatites: Constraints from the evolution of the Huanglongpu district, China. Lithos 2018, 308–309, 65–82. [Google Scholar] [CrossRef]
- Ayupova, N.; Melekestseva, I.; Maslennikov, V.; Sadykov, S. Mineralogy and geochemistry of clastic sulfide ores from the Talgan VHMS deposit, South Urals, Russia: Signatures of diagnostic alteration. Ore Geol. Rev. 2022, 144, 104839. [Google Scholar] [CrossRef]
- Bucher, K.; Seelig, U. Bristen granite: A highly differentiated, fluorite-bearing A-type granite from the Aar massif, Central Alps, Switzerland. Swiss J. Geosci. 2018, 111, 317–340. [Google Scholar] [CrossRef]
- Zeug, M.; Nasdala, L.; Ende, M.; Habler, G.; Hauzenberger, C.; Chanmaung, C.; Škoda, R.; Topa, D.; Wildner, M.; Wirth, R. The parisite-(Ce) enigma: Challenges in the identification of fluorcarbonate minerals. Mineral. Petrol. 2021, 115, 1–19. [Google Scholar] [CrossRef]
- Trabucho-Alexandre, J. More gaps than shale: Erosion of mud and its effect on preserved geochemical and palaeobiological signals. Geol. Soc. Lond. Spec. Publ. 2014, 404, 251–270. [Google Scholar] [CrossRef]
- Parviainena, A.; Loukola-Ruskeeniemi, K. Environmental impact of mineralised black shales. Earth-Sci. Rev. 2019, 192, 65–90. [Google Scholar] [CrossRef]
- Bishop, B.A.; Shivakumar, K.R.; Alessi, D.S.; Robbins, L.J. Insights into the rare earth element potential of coal combustion by-products from western Canada. Environ. Sci. Adv. 2023, 2, 529–542. [Google Scholar] [CrossRef]
- Wang, Q.; Morse, J.W. Pyrite formation under conditions approximating those in anoxic sediments I. Pathway and morphology. Mar. Chem. 1996, 2, 99–121. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; Cleverley, J.S. Controls on the genesis of a high-fluoride thermal spring: Innot Hot Springs, north Queensland. Aust. J. Earth Sci. 2007, 54, 597–607. [Google Scholar] [CrossRef]
- Shitumbanuma, V.; Tembo, F.; Tembo, J.M.; Chilala, S.; Van Ranst, E. Dental fluorosis associated with drinking water from hot springs in Choma district in southern province, Zambia. Environ. Geochem. Health 2007, 29, 51–58. [Google Scholar] [CrossRef]
- Addison, M.J.; Rivett, M.O.; Phiri, O.L.; Milne, N.; Milne, V.; McMahon, A.D.; Macpherson, L.M.D.; Bagg, J.; Conway, D.I.; Phiri, P.; et al. ‘Hidden Hot Springs’ as a source of groundwater fluoride and severe dental fluorosis in Malawi. Water 2021, 13, 1106. [Google Scholar] [CrossRef]
- Chesworth, W.; Martínez Cortizas, A.; García-Rodeja, E. The redox–pH approach to the geochemistry of the Earth’s land surface, with application to peatlands. In Peatlands: Evolution and Records of Environmental and Climate Changes; Martini, I.P., Martínez Cortizas, A., Chesworth, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 175–195. [Google Scholar]
- Liu, X.; Wang, Q.; Zhang, Q.; Zhang, Y.; Li, Y. Genesis of REE minerals in the karstic bauxite in western Guangxi, China, and its constraints on the deposit formation conditions. Ore Geol. Rev. 2016, 75, 100–115. [Google Scholar] [CrossRef]
- Mackie, W. The heavier accessory minerals in the granites of Scotland. Trans. Edinb. Geol. Soc. 1925, 12, 22–40. [Google Scholar] [CrossRef]
- Plant, J.A.; Henney, P.J.; Simpson, P.R. The genesis of tin-uranium granites in the Scottish Caledonides: Implications for metallogenesis. Geol. J. 1990, 25, 431–442. [Google Scholar] [CrossRef]
- Ansberque, C.; Mark, C.; Caulfield, J.T.; Chew, D.M. Combined in-situ determination of halogen (F, Cl) content in igneous and detrital apatite by SEM-EDS and LA-Q-ICPMS: A potential new provenance tool. Chem. Geol. 2019, 524, 406–420. [Google Scholar] [CrossRef]
- Tindle, A.G.; Webb, P.C. Estimation of lithium contents in trioctahedral micas using microprobe data: Application to micas from granitic rocks. Eur. J. Mineral. 1990, 2, 595–610. [Google Scholar] [CrossRef]
- Gould, D. Geology of the Aboyne District. Memoir of the British Geological Survey, Sheet 66W (Scotland); The Stationery Office: London, UK, 2001. [Google Scholar]
- Smith, C.G.; Goodman, S.; Robertson, S. Geology of the Ballater District. Memoir of the British Geological Survey, Sheet 65E (Scotland); The Stationery Office: London, UK, 2002. [Google Scholar]
- Oliver, G.J.H.; Wilde, S.A.; Wan, Y. Geochronology and geodynamics of Scottish granitoids from the late Neoproterozoic break-up of Rodinia to Palaeozoic collision. J. Geol. Soc. Lond. 2008, 165, 661–674. [Google Scholar] [CrossRef]
- Mark, D.F.; Rice, C.M.; Fallick, A.E.; Trewin, N.H.; Lee, M.R.; Boyce, A.; Lee, J.K.W. 40Ar/39Ar dating of hydrothermal activity, biota and gold mineralization in the Rhynie hot-spring system, Aberdeenshire, Scotland. Geochim. Cosmochimca Acta 2011, 75, 555–569. [Google Scholar] [CrossRef]
- Hall, A.; Walsh, J.N. Zinnwaldite granite from Glen Gairn, Aberdeenshire. Scott. J. Geol. 1972, 8, 265–267. [Google Scholar] [CrossRef]
- Webb, P.C.; Tindle, A.G.; Ixer, R.A. W-Sn-Mo-Bi-Ag mineralization associated with zinnwaldite-bearing granite from Glen Gairn, Scotland. Trans. Inst. Min. Metall. (Sect. B Appl. Earth Sci.) 1992, 101, B59–B72. [Google Scholar]
- Parnell, J.; Akinsanpe, T.O.; Armstrong, J.G.; Boyce, A.J.; Still, J.W.; Bowden, S.A.; Clases, D.; Gonzalez de Vega, R.; Feldmann, J. Trace element geochemistry in the earliest terrestrial ecosystem, the Rhynie Chert. Geochem. Geophys. Geosyst. 2022, 23, e2022GC010647. [Google Scholar] [CrossRef]
- Colman, T.B.; Beer, K.E.; Cameron, D.G.; Kimbell, G.S. Molybdenum Mineralisation near Chapel of Garioch, Inverurie, Aberdeenshire; British Geological Survey Technical Report WF/89/3, Mineral Reconnaissance Programme Report 100; British Geological Survey: Keyworth, UK, 1989.
- Zhu, X.K.; O’Nions, R.K. Monazite chemical composition: Some implications for monazite geochronology. Contrib. Mineral. Petrol. 1999, 137, 351–363. [Google Scholar] [CrossRef]
- Bern, C.R.; Shah, A.K.; Benzel, W.M.; Lowers, H.A. The distribution and composition of REE-bearing minerals in placers of the Atlantic and Gulf coastal plains, USA. J. Geochem. Explor. 2016, 162, 50–61. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Slattery, A.D.; Ehrig, K.; Liu, W.Y. Nanoscale intergrowths in the bastnäsite–synchysite series record transition toward thermodynamic equilibrium. MRS Bull. 2022, 47, 250–257. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Kontonikas-Charos, A.; Slattery, A.; Cook, N.J.; Ehrig, K.; Wade, B.P. Short-range stacking disorder in mixed-layer compounds: A HAADF STEM study of bastnäsite-parisite intergrowths. Minerals 2017, 7, 227. [Google Scholar] [CrossRef]
- Fame, M.L.; Spotila, J.A.; Owen, L.A.; Dortch, J.N.; Shuster, D.L. Spatially heterogeneous post-Caledonian burial and exhumation across the Scottish Highlands. Lithosphere 2018, 10, 406–425. [Google Scholar] [CrossRef]
- Wellman, C.H. Palaeoecology and palaeophytogeography of the Rhynie chert plants: Evidence from integrated analysis of in situ and dispersed spores. Proc. R. Soc. Biol. Sci. 2004, 271, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, X.; He, H.; Li, J.; Ma, L.; Tan, W.; Zhong, Y.; Zhu, J.; Zhou, M.; Song, H. Microorganisms accelerate REE mineralization in supergene environments. Appl. Environ. Microbiol. 2022, 88, e00632-22. [Google Scholar] [CrossRef]
- Strzelecki, A.C.; Migdisov, A.; Boukhalfa, H.; Sauer, K.; McIntosh, K.G.; Currier, R.P.; Williams-Jones, A.E.; Guo, X. Fluocerite as a precursor to rare earth element fractionation in ore-forming systems. Nat. Geosci. 2022, 15, 327–333. [Google Scholar] [CrossRef]
- Fernando, Q.; Yanagihara, N.; Dyke, J.T.; Vemulapalli, K. Formation of Rare Earth Carbonates Using Supercritical Carbon Dioxide. U.S. Patent 5045289, 3 September 1991. [Google Scholar]
- Philp, R.P.; De Garmo, D. Geochemical characterization of the Devonian-Mississippian Woodford Shale from the McAlister Cemetery Quarry, Criner Hills Uplift, Ardmore Basin, Oklahoma. Mar. Pet. Geol. 2020, 112, 104078. [Google Scholar] [CrossRef]
- Powell, C.L.; Trewin, N.H.; Edwards, D. Palaeoecology and plant succession in a borehole through the Rhynie cherts, Lower Old Red Sandstone, Scotland. Geol. Soc. Lond. Spec. Publ. 2000, 180, 439–457. [Google Scholar] [CrossRef]
- Dean, W.E. The carbon cycle and biogeochemical dynamics in lake sediments. J. Paleolimnol. 1999, 21, 375–393. [Google Scholar] [CrossRef]
- Gierlowski-Kordesch, E.H. Lacustrine carbonates. Dev. Sedimentol. 2010, 61, 1–101. [Google Scholar]
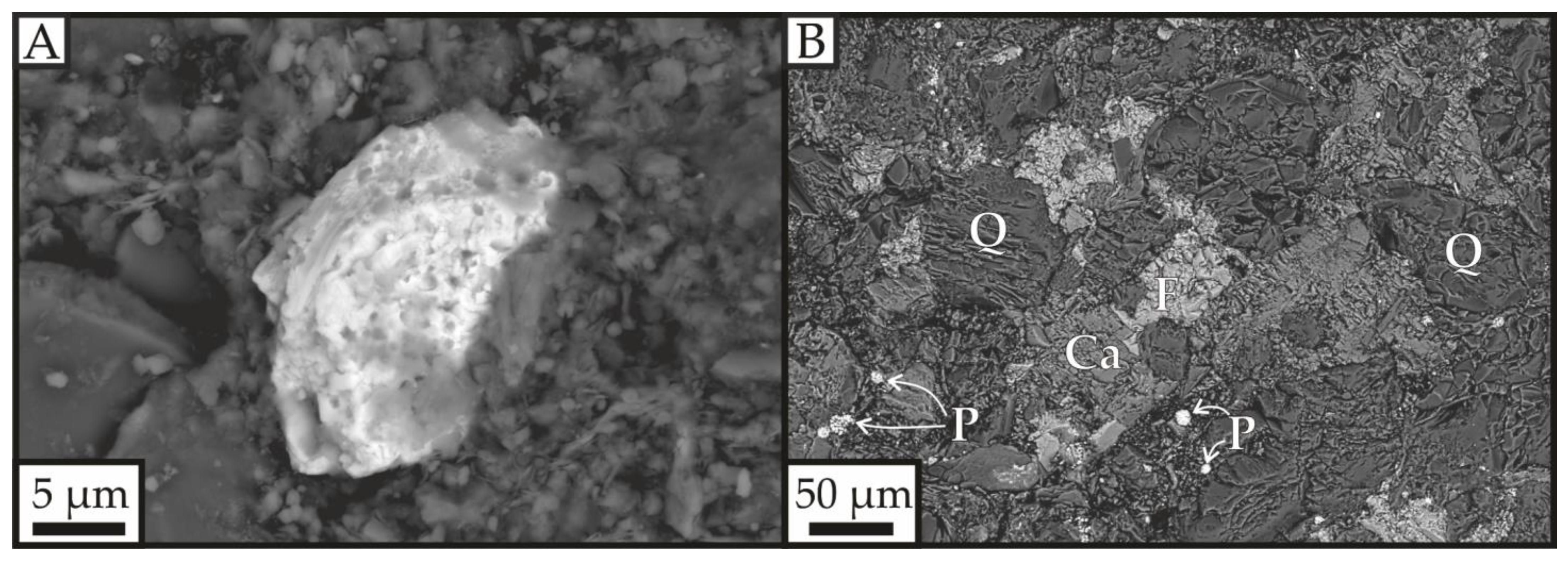
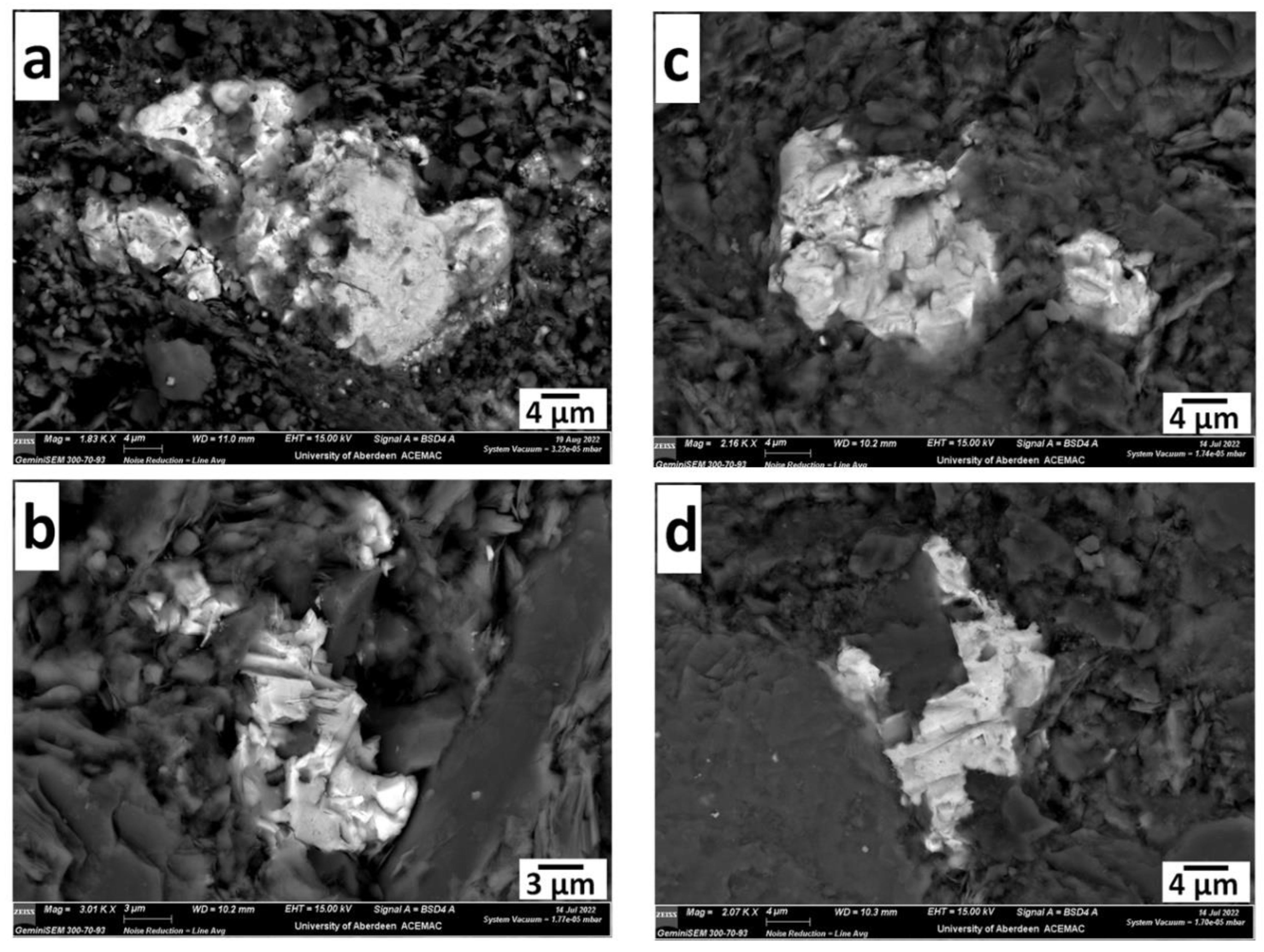

| 97/2 34.3 m | 97/2 49.5 m | 97/2 49.5 m | 97/2 49.5 m | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxide % | Site 11-1 | Site 11-2 | Site 11-3 | Site 11-4 | Site 11-7 | Site 9-2 | Site 9-7 | Site 22-1 | Site 22-4 | Site 22-6 | Site 25-1 | Site 25-2 |
| CaO | 16.25 | 12.33 | 15.73 | 15.85 | 15.99 | 14.50 | 14.45 | 14.76 | 12.69 | 15.29 | 15.42 | 16.39 |
| La2O3 | 13.23 | 14.57 | 12.88 | 13.73 | 13.43 | 11.34 | 12.24 | 15.42 | 12.67 | 11.76 | 11.06 | 11.19 |
| Ce2O3 | 24.97 | 26.32 | 24.27 | 25.85 | 26.71 | 23.55 | 23.93 | 25.18 | 24.51 | 22.73 | 24.16 | 23.68 |
| Pr2O3 | 2.72 | 2.20 | 2.46 | 2.94 | 2.94 | 2.70 | 2.53 | 2.50 | 2.73 | 2.54 | 2.96 | 2.48 |
| Nd2O3 | 9.23 | 9.61 | 8.99 | 9.62 | 10.25 | 10.18 | 9.86 | 8.04 | 10.22 | 9.13 | 9.76 | 10.11 |
| Sm2O3 | 1.33 | 1.18 | 1.40 | 1.42 | 1.76 | 1.72 | 1.46 | 1.51 | 1.25 | 1.54 | 1.76 | 1.87 |
| Gd2O3 | 0.68 | 0.00 | 0.00 | 0.66 | 0.69 | 0.80 | 0.80 | 1.01 | 0.84 | 0.89 | 1.18 | 0.99 |
| Y2O3 | 0.00 | 0.00 | 0.33 | 0.00 | 0.00 | 0.37 | 0.00 | 0.72 | 0.50 | 0.56 | 0.91 | 0.95 |
| CO2 | 29.72 | 23.00 | 29.49 | 29.64 | 29.99 | 26.89 | 28.11 | 29.92 | 25.75 | 29.45 | 28.04 | 27.73 |
| F | 3.36 | 7.13 | 2.65 | 3.88 | 4.20 | 4.13 | 3.06 | 3.08 | 4.64 | 1.98 | 4.24 | 4.99 |
| Total | 101.48 | 96.34 | 98.20 | 103.59 | 105.96 | 96.18 | 96.43 | 102.15 | 95.79 | 95.89 | 99.50 | 100.38 |
| −O≡F | 1.41 | 3.00 | 1.12 | 1.63 | 1.77 | 1.74 | 1.29 | 1.30 | 1.95 | 0.83 | 1.79 | 2.10 |
| TOTAL | 100.07 | 93.34 | 97.08 | 101.95 | 104.19 | 94.44 | 95.15 | 100.85 | 93.84 | 95.05 | 97.71 | 98.27 |
| Elemental % | ||||||||||||
| Ca | 0.92 | 0.79 | 0.91 | 0.89 | 0.88 | 0.88 | 0.87 | 0.84 | 0.79 | 0.90 | 0.90 | 0.95 |
| La | 0.26 | 0.32 | 0.26 | 0.27 | 0.26 | 0.24 | 0.25 | 0.30 | 0.27 | 0.24 | 0.22 | 0.22 |
| Ce | 0.48 | 0.58 | 0.48 | 0.50 | 0.50 | 0.49 | 0.49 | 0.49 | 0.52 | 0.46 | 0.48 | 0.47 |
| Pr | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.06 | 0.05 | 0.05 | 0.06 | 0.05 | 0.06 | 0.05 |
| Nd | 0.17 | 0.21 | 0.17 | 0.18 | 0.19 | 0.21 | 0.20 | 0.15 | 0.21 | 0.18 | 0.19 | 0.20 |
| Sm | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 |
| Gd | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Y | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 |
| REE total | 1.00 | 1.18 | 1.00 | 1.03 | 1.04 | 1.05 | 1.04 | 1.05 | 1.12 | 0.99 | 1.04 | 1.02 |
| C | 2.15 | 1.88 | 2.18 | 2.12 | 2.11 | 2.09 | 2.15 | 2.16 | 2.05 | 2.22 | 2.09 | 2.05 |
| F | 0.56 | 1.35 | 0.45 | 0.64 | 0.68 | 0.74 | 0.54 | 0.52 | 0.86 | 0.35 | 0.73 | 0.85 |
| F/Ca | 0.61 | 1.71 | 0.50 | 0.72 | 0.78 | 0.84 | 0.62 | 0.62 | 1.08 | 0.38 | 0.81 | 0.90 |
| REE/Ca | 1.09 | 1.49 | 1.09 | 1.16 | 1.19 | 1.19 | 1.19 | 1.26 | 1.42 | 1.10 | 1.15 | 1.07 |
| Identity | synchysite | Intermed. synchysite-parisite | synchysite | synchysite | synchysite | synchysite | synchysite | synchysite | Intermed. synchysite-parisite | synchysite | synchysite | synchysite |
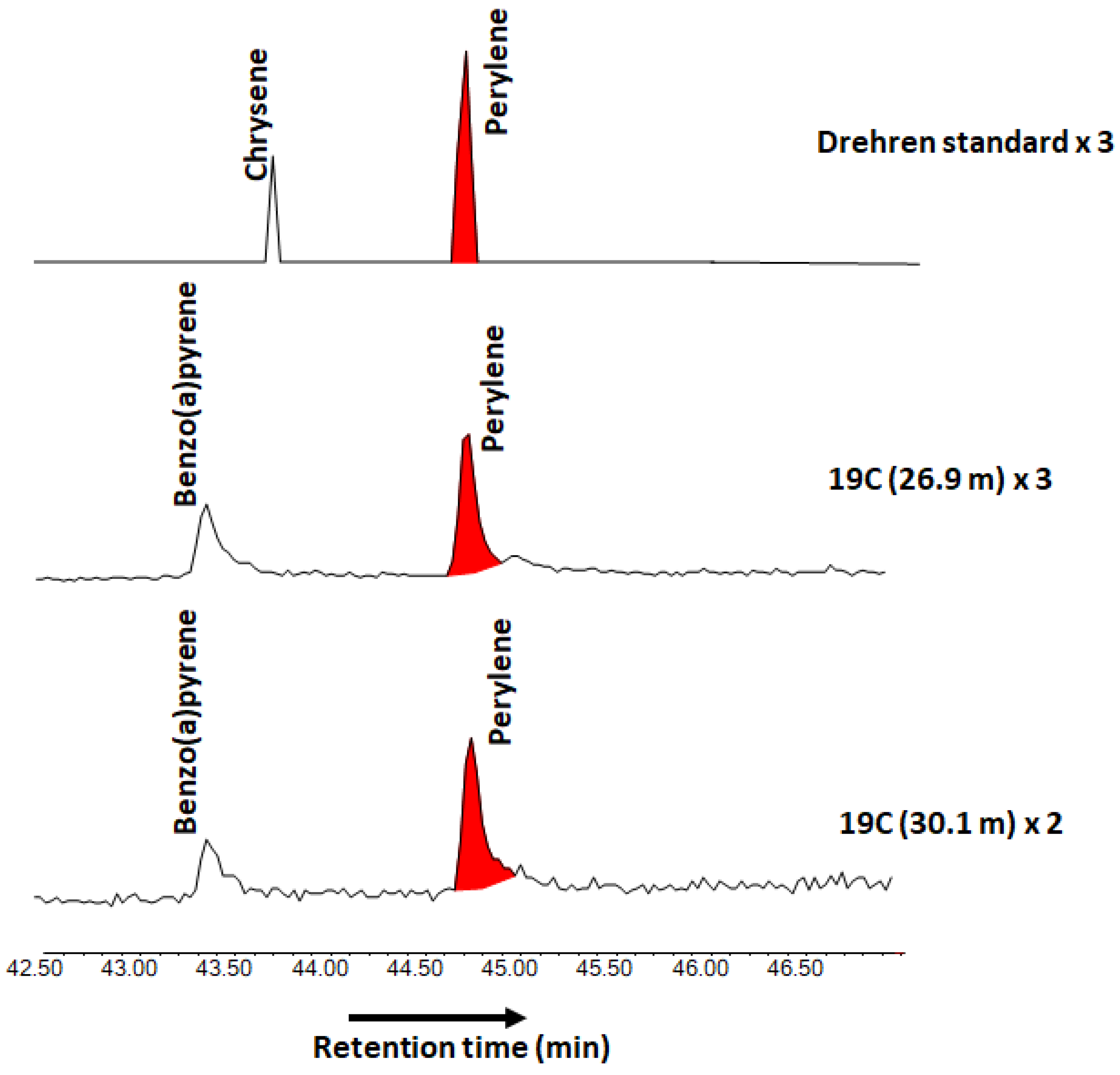
| Borehole | Depth (m) | Roeq% | sd | Temperature (°C) | sd | n |
|---|---|---|---|---|---|---|
| 97/2 | 30.00 | 0.73 | 0.11 | 112.8 | 18.9 | 16 |
| 22.30 | 0.74 | 0.10 | 114.7 | 17.7 | 7 | |
| 38.50 | 0.67 | 0.05 | 102.4 | 9.8 | 10 | |
| 19C | 19.60 | 0.73 | 0.11 | 112.1 | 20.7 | 10 |
| 33.55 | 0.66 | 0.06 | 100.4 | 10.8 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnell, J.; Akinsanpe, T.O.; Still, J.W.; Schito, A.; Bowden, S.A.; Muirhead, D.K.; Armstrong, J.G.T. Low-Temperature Fluorocarbonate Mineralization in Lower Devonian Rhynie Chert, UK. Minerals 2023, 13, 595. https://doi.org/10.3390/min13050595
Parnell J, Akinsanpe TO, Still JW, Schito A, Bowden SA, Muirhead DK, Armstrong JGT. Low-Temperature Fluorocarbonate Mineralization in Lower Devonian Rhynie Chert, UK. Minerals. 2023; 13(5):595. https://doi.org/10.3390/min13050595
Chicago/Turabian StyleParnell, John, Temitope O. Akinsanpe, John W. Still, Andrea Schito, Stephen A. Bowden, David K. Muirhead, and Joseph G. T. Armstrong. 2023. "Low-Temperature Fluorocarbonate Mineralization in Lower Devonian Rhynie Chert, UK" Minerals 13, no. 5: 595. https://doi.org/10.3390/min13050595
APA StyleParnell, J., Akinsanpe, T. O., Still, J. W., Schito, A., Bowden, S. A., Muirhead, D. K., & Armstrong, J. G. T. (2023). Low-Temperature Fluorocarbonate Mineralization in Lower Devonian Rhynie Chert, UK. Minerals, 13(5), 595. https://doi.org/10.3390/min13050595







