The Immobility of Uranium (U) in Metamorphic Fluids Explained by the Predominance of Aqueous U(IV)
Abstract
1. Introduction
2. Thermodynamic Modeling Methods and Limitation
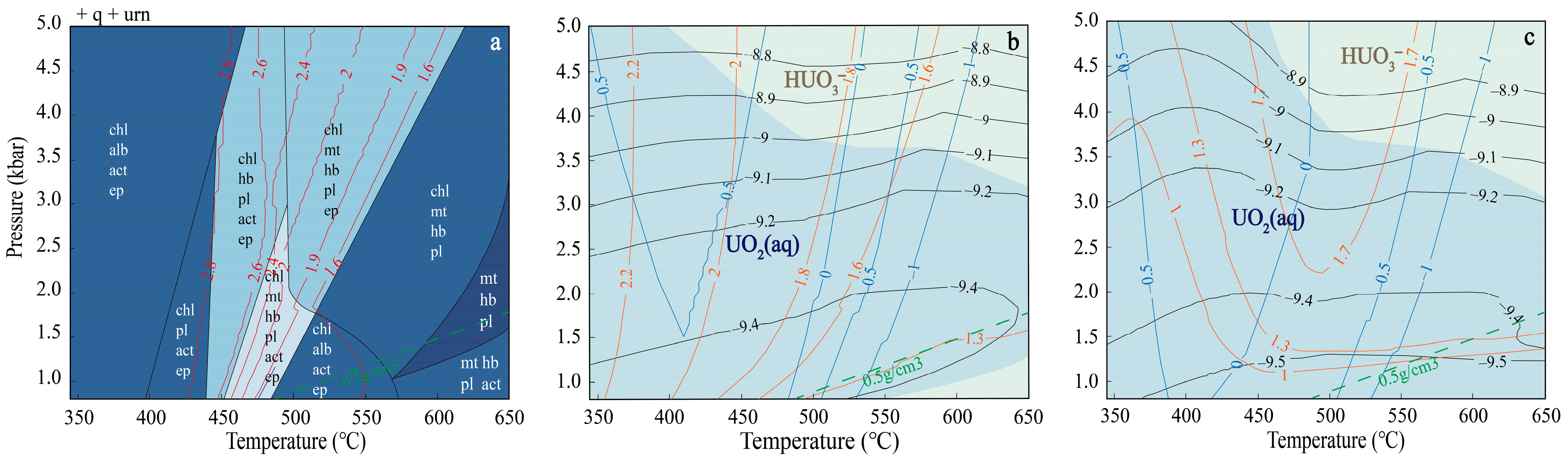
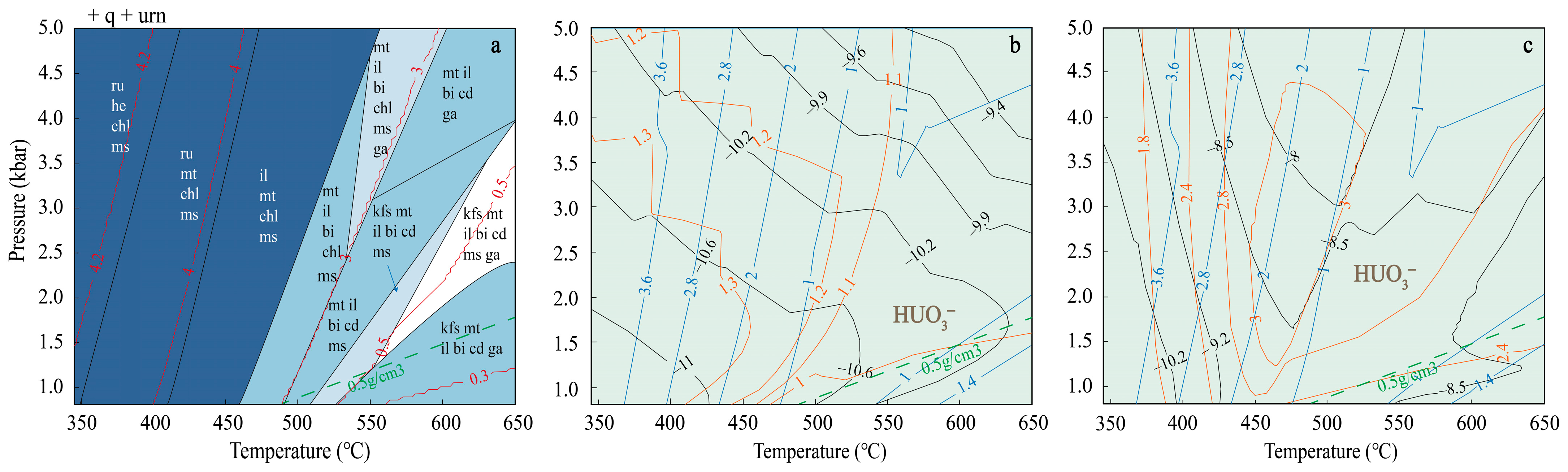
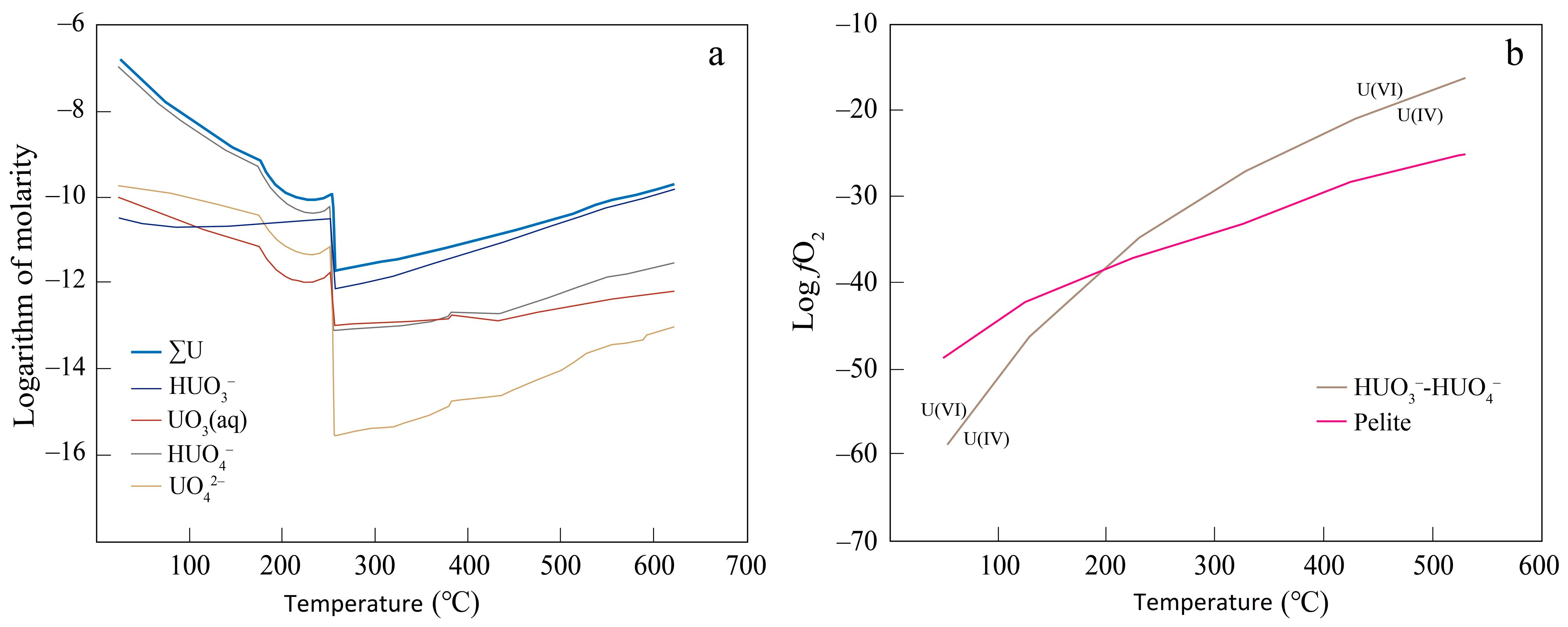
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Aqueous Species | |||
|---|---|---|---|
| H2O | NaHSiO3(aq) | Al3+ | HFeO2− |
| H+ | NaAl4(aq) | AlOH2+ | FeCl+ |
| OH− | NaAlO2(aq) | Al(OH)2+ | Fe3+ |
| H2(aq) | Mg2+ | Al(OH)3(aq) | FeOH+ |
| O2(aq) | MgOH+ | Al(OH)4− | FeOH2+ |
| H3SiO4− | MgCl− | AlO2− | Fe(OH)3(aq) |
| H4SiO4(aq) | MgCl2(aq) | AlOOH(aq) | Fe(OH)4− |
| SiO2(aq) | Mg(HSiO3)+ | Fe2+ | FeO+ |
| Cl− | Ca2+ | FeOH+ | HFeO2(aq) |
| Na+ | CaOH+ | Fe(OH)2(aq) | FeCl2+ |
| NaOH(aq) | CaCl+ | Fe(OH)3− | FeCl4− |
| NaCl(aq) | CaCl2 | FeO(aq) | FeCl3(aq) |
| U3+ | UOH2+ | UO2OH+ | HUO3− |
| UO2+ | UO2+ | (UO2)2(OH)22+ | UCl3+ |
| UO2− | UO+ | UO2Cl+ | UO2OH(aq) |
| U4+ | HUO2(aq) | HUO2+ | UO2Cl2− |
| UO22+ | U3+ | UO2(aq) | UO2Cl2(aq) |
| UO3(aq) | HUO4− | UO42– | (UO2)2OH3+ |
| Aqueous Species | |||
|---|---|---|---|
| H2O | NaHSiO3(aq) | Al3+ | HFeO2− |
| H+ | NaAl4(aq) | AlOH2+ | FeCl+ |
| OH− | NaAlO2(aq) | Al(OH)2+ | Fe3+ |
| H2(aq) | Mg2+ | Al(OH)3(aq) | FeOH+ |
| O2(aq) | MgOH+ | Al(OH)4− | FeOH2+ |
| H3SiO4− | MgCl− | AlO2− | Fe(OH)3(aq) |
| H4SiO4(aq) | MgCl2(aq) | AlOOH(aq) | Fe(OH)4− |
| SiO2(aq) | Mg(HSiO3)+ | Fe2+ | FeO+ |
| Cl− | Ca2+ | FeOH+ | HFeO2(aq) |
| Na+ | CaOH+ | Fe(OH)2(aq) | FeCl2+ |
| NaOH(aq) | CaCl+ | Fe(OH)3− | FeCl4− |
| NaCl(aq) | CaCl2 | FeO(aq) | FeCl3(aq) |
| U3+ | UOH2+ | UO2OH+ | HUO3− |
| UO2+ | UO2+ | (UO2)2(OH)22+ | UCl3+ |
| UO2− | UO+ | UO2Cl+ | UO2OH(aq) |
| U4+ | HUO2(aq) | HUO2+ | UO2Cl2− |
| UO22+ | U3+ | UO2(aq) | (UO2)2OH3+ |
| UO3(aq) | HUO4− | UO42− | KAlO2(aq) |
| UO2Cl2(aq) | K+ | KOH(aq) | KCl(aq) |
| KAl(OH)4(aq) | |||
| Minerals | Formula |
|---|---|
| Magnetite (Mt) Hematite (Hem) Albite (Alb) Quartz (Qt) Sillimanite (Sil) Kyanite (Ky) Andalusite (And) | Fe3O4 Fe2O3 NaAlSi3O8 SiO2 Al2SiO5 Al2SiO5 Al2SiO5 |
| Minerals | Formula |
|---|---|
| Magnetite (Mt) Hematite (Hem) K-Feldspar (Kfs) Quartz (Qt) Sillimanite (Sil) Kyanite (Ky) Sanidine (Sa) Andalusite (And) | Fe3O4 Fe2O3 KAlSi3O8 SiO2 Al2SiO5 Al2SiO5 KAlSi3O8 Al2SiO5 |
| Mineral | End Member | Formula | Activity Model |
|---|---|---|---|
| Chlorite | Daphnite | Fe5Al2Si3O10 | Symmetrical Wdaphnite-WAl-free chlorite = 14.5 Wdaphnite-Wamesite = 13.5 Wdaphnite-Wclinochlore = 2.5 WAl-free chlorite-Wamesite = 20 WAl-free chlorite-Wclinochlore = 18 Wamesite-Wclinochlore = 18 |
| Al-free chlorite | Mg6Si4O10 | ||
| Amesite | Mg4Al4Si2O10 | ||
| Clinochlore | Mg5Al2Si3O10 | ||
| Actinolite | Tremolite | Ca2Mg5Si8O22 | Ideal |
| Ferroactinolite | Ca2Fe5Si8O22 | ||
| Hornblende | Tremolite | Ca2Mg5Si8O22 | Symmetrical WTremolite-Wglaucophane = 65 Wtremolite-Wpargasite = 33 Wtremolite-Wtschermakite = 20 Wtremolite-Wferroactinolite = 10 Wglaucophane-Wpargasite = 50 Wglaucophane-Wtschermakite = 25 Wglaucophane-Wferroactinolite = 39.3 Wpargasite-Wtschermakite = −38.5 Wpargasite-Wferroactinolite = −1.9 Wtschermakite-Wferroactinolite = 12.5 |
| Tschermakite | Ca2Mg3Al4Si6O22 | ||
| Pargasite | NaCa2Mg4Al3Si6O22 | ||
| Glaucophane | Na2Mg3Al2Si8O22 | ||
| Ferroactinolite | Ca2Mg5Si8O22 | ||
| Plagioclase | Anorthite | CaAl2Si2O8 | Symmetrical Wabh-Wan = 3.1 |
| albite | NaAlSi3O8 | ||
| Epidote | Clinozoisite epidote Fe-epidote | Ca2Al3Si3O12 Ca2FeAl2Si3O12Ca2Fe2AlSi3O12 | Symmetrical Wclinozoisite-Wepidote = 0 Wclinozoisite-WFe-epidote = 15.4 Wepidote-WFe-epidote = 3 |
| Mineral | End Member | Formula | Activity Model |
|---|---|---|---|
| Biotite | annite | KFe3AlSi3O10 | Symmetrical Wannite-Wphlogopite = 9 Wannite-Weastonite = −1 Wannite-Wobi = 6 Wannite-Wtbi = 10 Wannite-Wfbi = 8 Wphlogopite-Weastonite = 10 Wphlogopite-Wobi = 3 Weastonite-Wobi = 10 |
| phlogopite | KMg3AlSi3O10 | ||
| eastonite | KMg2Al3Si2O10 | ||
| obi | KFeMg2AlSi3O10 | ||
| tbi | KMg2TiAlSi3O12 | ||
| fbi | KMg2Al2FeSi2O10 | ||
| Chlorite | daphnite | Fe5Al2Si3O10 | Symmetrical Wdaphnite-WAl-free chl = 14.5 Wdaphnite-Wamesite = 13.5 Wdaphnite-Wchlinoclore = 2.5 WAl-free chlorite-Wamesite = 20 WAl-free chlorite-Wchlinoclore = 18 Wamesite-Wchlinoclore = 18 |
| Al-free chlorite | Mg6Si4O10 | ||
| amesite | Mg4Al4Si2O10 | ||
| chlinoclore | Mg5Al2Si3O10 | ||
| Cordierite | Fe-cordierite | Fe2Al4Si5O18 | Ideal |
| hydro cordierite | Mg2Al4Si5O17 | ||
| cordierite | Mg2Al4Si5O18 | ||
| Garnet | almandine | Fe3Al2Si3O12 | Symetrical Wamandine-Wpyrope = 2.5 Wamandine-Wkho = 23 |
| pyrope | Mg3Al2Si3O12 | ||
| kho | Mg3Fe2Si3O12 | ||
| Muscovite | muscovite | KAl3Si3O10 | Ideal |
| celadonite | KMgAlSi4O10 | ||
| Fe-celadonite | KFeAlSi4O10 | ||
| Staurolite | Fe-staurolite | Fe4Al18Si8O48H2 | Symetrical WFe-staurolite-WMg-staurolite = −8 |
| Mg-staurolite | Mg4Al18Si8O48H2 | ||
| Chloritoid | Fe-ctd | FeAl2SiO5 | WFe-ctd-WMg-ctd = −8 |
| Mg-ctd | MgAl2SiO5 | ||
| ctdo | MgFe2SiO5 |
References
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547−569. [Google Scholar] [CrossRef]
- Bonnetti, C.; Liu, X.; Zhaobin, Y.; Cuney, M.; Michels, R.; Malartre, F.; Mercadier, J.; Cai, J. Coupled uranium mineralisation and bacterial sulphate reduction for the genesis of the Baxingtu sandstone-hosted U deposit, SW Songliao Basin, NE China. Ore Geol. Rev. 2017, 82, 108–129. [Google Scholar] [CrossRef]
- Cui, T.; Yang, J.; Samson, I.M. Tectonic deformation and fluid flow: Implications for the formation of unconformity-related uranium deposits. Econ. Geol. 2012, 107, 147–163. [Google Scholar] [CrossRef]
- Ruzicka, V. Monometallic and polymetallic deposits associated with the sub-Athabasca unconformity in Saskatchewan. Geol. Surv. Can. Paper 1989, 1, 67−79. [Google Scholar]
- Cuney, M.; Mercadier, J.; Bonnetti, C. Classification of sandstone-related uranium deposits. J. Earth Sci. 2022, 33, 236−256. [Google Scholar] [CrossRef]
- Taylor, B.E.; Slack, J.F. Tourmalines from appalachian-caledonian massive sulfide deposits; textural, chemical, and isotopic relationships. Econ. Geol. 1984, 79, 1703−1726. [Google Scholar] [CrossRef]
- Lottermoser, B.; Plimer, I. Chemical variation in tourmalines, Umberatana, South Australia. Neues Jahrb. Für Mineral. Mon. 1987, 7, 314−326. [Google Scholar]
- Gallagher, V.; Kennan, P. Tourmaline on the margin of the Leinster Granite, southeast Ireland: Petrogenetic implications. Irish J. Earth Sci. 1992, 11, 131−150. [Google Scholar]
- Slack, J.F.; Coad, P.R. Multiple hydrothermal and metamorphic events in the Kidd Creek volcanogenic massive sulphide deposit, Timmins, Ontario: Evidence from tourmalines and chlorites. Can. J. Earth Sci. 1989, 26, 694−715. [Google Scholar] [CrossRef]
- Zheng, J.; Shen, P.; Feng, W. Hydrothermal apatite record of ore-forming processes in the Hatu orogenic gold deposit, West Junggar, Northwest China. Contrib. Miner. Petrol. 2022, 177, 27. [Google Scholar] [CrossRef]
- Hazarika, P.; Mishra, B.; Pruseth, K.L. Diverse tourmaline compositions from orogenic gold deposits in the Hutti-Maski greenstone belt, India: Implications for sources of ore-forming fluids. Econ. Geol. 2015, 110, 337−353. [Google Scholar] [CrossRef]
- Sciuba, M.; Beaudoin, G.; Makvandi, S. Chemical composition of tourmaline in orogenic gold deposits. Miner. Deposita 2021, 56, 537−560. [Google Scholar] [CrossRef]
- McGloin, M.V.; Tomkins, A.G.; Webb, G.P.; Spiers, K.; MacRae, C.M.; Paterson, D.; Ryan, C.G. Release of uranium from highly radiogenic zircon through metamictization: The source of orogenic uranium ores. Geology 2016, 44, 15−18. [Google Scholar] [CrossRef]
- White, R.; Powell, R.; Holland, T. Calculation of partial melting equilibria in the system Na2O–CaO–K2O–FeO–MgO–Al2O3–SiO2–H2O (NCKFMASH). J. Metamorph. Geol. 2001, 19, 139−153. [Google Scholar] [CrossRef]
- Haack, U.; Heinrichs, H.; Boness, M.; Schneider, A. Loss of metals from pelites during regional metamorphism. Contrib. Miner. Petrol. 1984, 85, 116−132. [Google Scholar] [CrossRef]
- Barker, A.; Coogan, L.; Gillis, K. Insights into the behaviour of sulphur in mid-ocean ridge axial hydrothermal systems from the composition of the sheeted dyke complex at Pito Deep. Chem. Geol. 2010, 275, 105−115. [Google Scholar] [CrossRef]
- Shock, E.L.; Sassani, D.C.; Betz, H. Uranium in geologic fluids: Estimates of standard partial molal properties, oxidation potentials, and hydrolysis constants at high temperatures and pressures. Geochim. Cosmochim. Acta 1997, 61, 4245−4266. [Google Scholar] [CrossRef]
- Huang, F.; Sverjensky, D.A. Extended deep earth water model for predicting major element mantle metasomatism. Geochim. Cosmochim. Acta 2019, 254, 192−230. [Google Scholar] [CrossRef]
- Holland, T.; Powell, R. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 1998, 16, 309−343. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Baker, T.; Dubé, B.; Groves, D.I.; Hart, C.J.; Gosselin, P. Distribution, character, and genesis of gold deposits in metamorphic terran. Econ. Geol. 2005, 407–475. [Google Scholar] [CrossRef]
- Zhong, R.; Brugger, J.; Chen, Y.; Li, W. Contrasting regimes of Cu, Zn and Pb transport in ore-forming hydrothermal fluids. Chem. Geol. 2015, 395, 154−164. [Google Scholar] [CrossRef]
- Tanger, J.C.; Helgeson, H.C. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures; revised equations of state for the standard partial molal properties of ions and electrolytes. Am. J. Sci. 1988, 288, 19−98. [Google Scholar] [CrossRef]
- Tomkins, A.G. Windows of metamorphic sulfur liberation in the crust: Implications for gold deposit genesis. Geochim. Cosmochim. Acta 2010, 74, 3246−3259. [Google Scholar] [CrossRef]
- Sverjensky, D.A.; Harrison, B.; Azzolini, D. Water in the deep Earth: The dielectric constant and the solubilities of quartz and corundum to 60 kb and 1200 °C. Geochim. Cosmochim. Acta 2014, 129, 125−145. [Google Scholar] [CrossRef]
- Zhong, R.; Li, Y.; Etschmann, B.; Brugger, J.; Yu, C.; Cui, H. HighPGibbs, a practical tool for fluid-rock thermodynamic simulation in deep Earth and its application on calculating nitrogen speciation in subduction zone fluids. Geochem. Geophys. Geosyst. 2020, 21, e2020GC008973. [Google Scholar] [CrossRef]
- Galvez, M.E.; Connolly, J.A.; Manning, C.E. Implications for metal and volatile cycles from the pH of subduction zone fluids. Nature 2016, 539, 420−424. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.A.; Galvez, M.E. Electrolytic fluid speciation by Gibbs energy minimization and implications for subduction zone mass transfer. Earth Planet. Sci. Lett. 2018, 501, 90−102. [Google Scholar] [CrossRef]
- Shock, E.L.; Helgeson, H.C. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000 °C. Geochim. Cosmochim. Acta 1988, 52, 2009−2036. [Google Scholar] [CrossRef]
- Giles, D.; Nutman, A.P. SHRIMP U–Pb monazite dating of 1600–1580 Ma amphibolite facies metamorphism in the southeastern Mt Isa Block, Australia. Aust. J. Earth Sci. 2002, 49, 455−465. [Google Scholar] [CrossRef]
- Bingen, B.; Demaiffe, D.; Hertogen, J. Redistribution of rare earth elements, thorium, and uranium over accessory minerals in the course of amphibolite to granulite facies metamorphism: The role of apatite and monazite in orthogneisses from southwestern Norway. Geochim. Cosmochim. Acta 1996, 60, 1341−1354. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Chew, D.; Kenny, G.; Henrichs, I.; Mulligan, D. The trace element composition of apatite and its application to detrital provenance studies. Earth Sci. Rev. 2020, 201, 103044. [Google Scholar] [CrossRef]
- Pyle, J.M.; Spear, F.S.; Rudnick, R.L.; Mcdonough, W.F. Monazite–xenotime–garnet equilibrium in metapelites and a new monazite–garnet thermometer. J. Petrol. 2001, 42, 2083−2107. [Google Scholar] [CrossRef]
- Mercadier, J.; Annesley, I.; McKechnie, C.; Bogdan, T.; Creighton, S. Magmatic and metamorphic uraninite mineralization in the western margin of the Trans-Hudson orogen (Saskatchewan, Canada): A uranium source for unconformity-related uranium deposits? Econ. Geol. 2013, 108, 1037−1065. [Google Scholar] [CrossRef]
- Diener, J.; Powell, R.; White, R.; Holland, T. A new thermodynamic model for clino-and orthoamphiboles in the system Na2O–CaO–FeO–MgO–Al2O3–SiO2–H2O–O. J. Metamorph. Geol. 2007, 25, 631−656. [Google Scholar] [CrossRef]
- Wei, C.; Duan, Z. Phase relations in metabasic rocks: Constraints from the results of experiments, phase modeling and ACF analysis. Geol. Soc. Lond. Spec. Pub. 2019, 474, 25−45. [Google Scholar] [CrossRef]
- Zhong, R.; Brugger, J.; Tomkins, A.G.; Chen, Y.; Li, W. Fate of gold and base metals during metamorphic devolatilization of a pelite. Geochim. Cosmochim. Acta 2015, 171, 338−352. [Google Scholar] [CrossRef]
- Cuney, M. The extreme diversity of uranium deposits. Miner. Deposita 2009, 44, 3−9. [Google Scholar] [CrossRef]
- Richard, A.; Rozsypal, C.; Mercadier, J.; Banks, D.A.; Cuney, M.; Boiron, M.-C.; Cathelineau, M. Giant uranium deposits formed from exceptionally uranium-rich acidic brines. Nat. Geosci. 2012, 5, 142−146. [Google Scholar] [CrossRef]
- Komninou, A.; Sverjensky, D. Geochemical modeling of the formation of an unconformity-type uranium deposit. Econ. Geol. 1996, 91, 590−606. [Google Scholar] [CrossRef]
- Timofeev, A.; Migdisov, A.A.; Williams-Jones, A.E.; Roback, R.; Nelson, A.T.; Xu, H. Uranium transport in acidic brines under reducing conditions. Nat. Commun. 2018, 9, 1469. [Google Scholar] [CrossRef]
- Evans, K.A.; Tomkins, A.G. Metamorphic fluids in orogenic settings. Elements 2020, 16, 381−387. [Google Scholar] [CrossRef]
- Hoeve, J.; Sibbald, T.I. On the genesis of Rabbit Lake and other unconformity-type uranium deposits in northern Saskatchewan, Canada. Econ. Geol. 1978, 73, 1450−1473. [Google Scholar] [CrossRef]
- Hostetler, P.; Garrels, R. Transportation and precipitation of uranium and vanadium at low temperatures, with special reference to sandstone-type uranium deposits. Econ. Geol. 1962, 57, 137−167. [Google Scholar] [CrossRef]
- Cui, H.; Zhong, R.; Xie, Y.; Wang, X.; Chen, H. Melt–fluid and fluid–fluid immiscibility in a Na2SO4–SiO2–H2O system and implications for the formation of rare earth deposits. Acta Geol. Sin-Engl. 2021, 95, 1604−1610. [Google Scholar]
- Dale, J.; Powell, R.; White, R.; Elmer, F.; Holland, T. A thermodynamic model for Ca–Na clinoamphiboles in Na2O–CaO–FeO–MgO–Al2O3–SiO2–H2O–O for petrological calculations. J. Metamorph. Geol. 2005, 23, 771−791. [Google Scholar] [CrossRef]
- White, R.; Powell, R.; Holland, T.; Worley, B. The effect of TiO2 and Fe2O3 on metapelitic assemblages at greenschist and amphibolite facies conditions: Mineral equilibria calculations in the system K2O–FeO–MgO–Al2O3–SiO2–H2O–TiO2–Fe2O3. J. Metamorph. Geol. 2000, 18, 497−511. [Google Scholar] [CrossRef]
| Altered Oceanic Basalt | Average Pelite | Oxidized Pelite | |
|---|---|---|---|
| SiO2 | 51.98 | 71.11 | 59.8 |
| Al2O3 | 14.80 | 14.38 | 19.6 |
| MgO | 7.65 | 2.31 | 1.00 |
| FeO | 9.98 | 7.18 | 0.60 |
| Fe2O3 | 1.98 | 0.75 | 7.30 |
| CaO | 10.48 | - | - |
| Na2O | 2.29 | - | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhong, R.; Yu, C.; Cui, H. The Immobility of Uranium (U) in Metamorphic Fluids Explained by the Predominance of Aqueous U(IV). Minerals 2023, 13, 427. https://doi.org/10.3390/min13030427
Zhang M, Zhong R, Yu C, Cui H. The Immobility of Uranium (U) in Metamorphic Fluids Explained by the Predominance of Aqueous U(IV). Minerals. 2023; 13(3):427. https://doi.org/10.3390/min13030427
Chicago/Turabian StyleZhang, Min, Richen Zhong, Chang Yu, and Hao Cui. 2023. "The Immobility of Uranium (U) in Metamorphic Fluids Explained by the Predominance of Aqueous U(IV)" Minerals 13, no. 3: 427. https://doi.org/10.3390/min13030427
APA StyleZhang, M., Zhong, R., Yu, C., & Cui, H. (2023). The Immobility of Uranium (U) in Metamorphic Fluids Explained by the Predominance of Aqueous U(IV). Minerals, 13(3), 427. https://doi.org/10.3390/min13030427





