1. Introduction
With excellent physical and chemical properties of magnetics, light, and electricity, mid-heavy rare earth elements are widely applied in high-end magnetic materials, crystal materials, laser optical fibers, and other national defense and strategic emerging industries [
1,
2,
3]. Abundant mid-heavy rare earths are found in ion-adsorption rare earth ore, which are adsorbed mostly on the surface of clay minerals, mainly kaolinite, in the ion state or hydrated ion state [
4]. Generally, the rare earth ions adsorbed on the surface of clay minerals are desorbed into the leach solution with electrolyte solutions in the industry, realizing the leaching of rare earths [
5,
6,
7]. Xiao Y. F. et al. [
8,
9] found that when the ion-adsorption rare earth ore was leached using a 0.2 mol/L magnesium sulfate solution with the pH of 5.7, the leaching rate of rare earths was above 90%, while that of aluminum was approximately 50%. However, aluminum in the rare earth ore is often leached with rare earth elements simultaneously. Since the aluminum in the leach solution affects the chemical consumption and product purity in subsequent impurity removal, precipitation, or extraction processes, aluminum is considered a major impurity in the extraction and enrichment process of ion-adsorption rare earth ore [
10,
11,
12,
13].
To reduce the impact of aluminum on the extraction and enrichment process of ion-adsorption rare earth ore, extensive studies have been conducted by researchers to inhibit the leaching of aluminum during leaching. In previous work, a certain amount of aluminum leaching inhibitor is used to avoid the leaching of aluminum. For instance, Chi R. A. et al. [
14] found that after adding 0.032 mol/L ammonium formate to the 0.1 mol/L ammonium sulfate solution and controlling the pH at 5–8, the leaching rate of aluminum is reduced to 37.8% from 97% before the addition of ammonium formate. Qiu T. S. et al. [
13,
15] proposed adding 0.013 mol/L organic compound inhibitor LG-01 containing hydroxyl and carboxyl groups to 4 wt.% ammonium sulfate solution to generate a complex with aluminum ion (Al
3+), which can be retained in the leaching tailings, so that the Al
3+ content in the leach solution is reduced by 92%. A study conducted by Qiu T. S. et al. [
12] revealed that following the addition of 0.15 wt.% sulfosalicylic acid to the 3 wt.% ammonium sulfate solution with a pH of 5, Al
3+ binds to sulfosalicylic acid and is then adsorbed on the surface of clay minerals, so its concentration in the leach solution declines from 273.23 mg/L to 47.19 mg/L. The solution injection technique in the leaching process is also optimized to avoid contact between the leaching agent and the humic layer with high aluminum content, thereby reducing the leaching of aluminum. For example, Zhang Z. Y. et al. [
16] put forward the idea to inject a leaching agent directly into the fully weathered layer during the in situ leaching process to prevent it from flowing through the humus layer and laterite layer, so as to reduce the leaching of aluminum. However, none of the aforementioned studies investigated in depth the occurrences of aluminum in ion-adsorption rare earth ore or the occurrence transition during the leaching process.
The rare earths in ion-adsorption rare earth ore exist mainly in the ion-exchangeable and colloidal states [
17,
18], while the occurrences of aluminum are more complicated. The occurrences of aluminum in soil includes mainly water-soluble aluminum (Sol-Al), exchangeable aluminum (Ex-Al), hydroxyl-adsorbed aluminum (Hy-Al), iron oxide bonded aluminum, interlayer aluminum, amorphous aluminum, silicate aluminum, and mineral aluminum [
19,
20,
21]. An acidic inorganic salt leaching agent is usually used in the leaching process of ion-adsorption rare earth ore, and the leaching process may involve aluminum in three forms: Sol-Al, Ex-Al, and Hy-Al, which, however, have been rarely reported. For instance, Yang X. L. et al. [
22] found that after the addition of 0.05% acetate to 2 wt.% compound ammonium salt solution (the molar ratio of ammonium nitrate/ammonium sulfate is 4:1), the pH of the leach solution becomes 4.8–5 due to the hydrolysis of acetate, at which time, the Al
3+ in the solution is hydrolyzed into hydroxyl-adsorbed aluminum and the leaching rate of aluminum is reduced by 94.8%. Chi R. A. et al. [
23] discovered that when the 4 wt.% ammonium sulfate is used in leaching and the pH of the leaching agent is controlled at 2, a large amount of aluminum in those forms, except for Ex-Al, is leached. In summary, it is necessary to deeply study the occurrence transition of aluminum under different concentrations and pH of leaching agent in the leaching process of ion-adsorption rare earth ore to reveal the leaching mechanism of aluminum.
This paper focuses on the study of the leaching behavior of rare earths and aluminum in the column leaching of ion-adsorption rare earth ore under different pH and concentrations of magnesium sulfate leaching agent, as well as the occurrence transition of aluminum under different liquid-to-ore ratios, which reveals the leaching mechanism of aluminum and provides theoretical support for the selective leaching of ion-adsorption rare earth ore.
2. Materials and Methods
2.1. Materials
The ion-adsorption rare earth ore samples were obtained from Jianghua, Hunan Province, China, which belongs to the granitic rare earth deposit. The rare earth ore samples were air-dried at 100 °C for 12 h, and 0.15–0.2 mm ores were then screened out. The chemical composition of rare earth ore was analyzed (
Table 1).
The magnesium sulfate heptahydrate and potassium chloride used in this work were analytical reagents purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), the hydrochloric acid and sulfuric acid were superior pure reagents from Beijing Reagent Co., Ltd. (Beijing, China), and the deionized water was prepared by a self-made water purification system.
2.2. Column Leaching Test
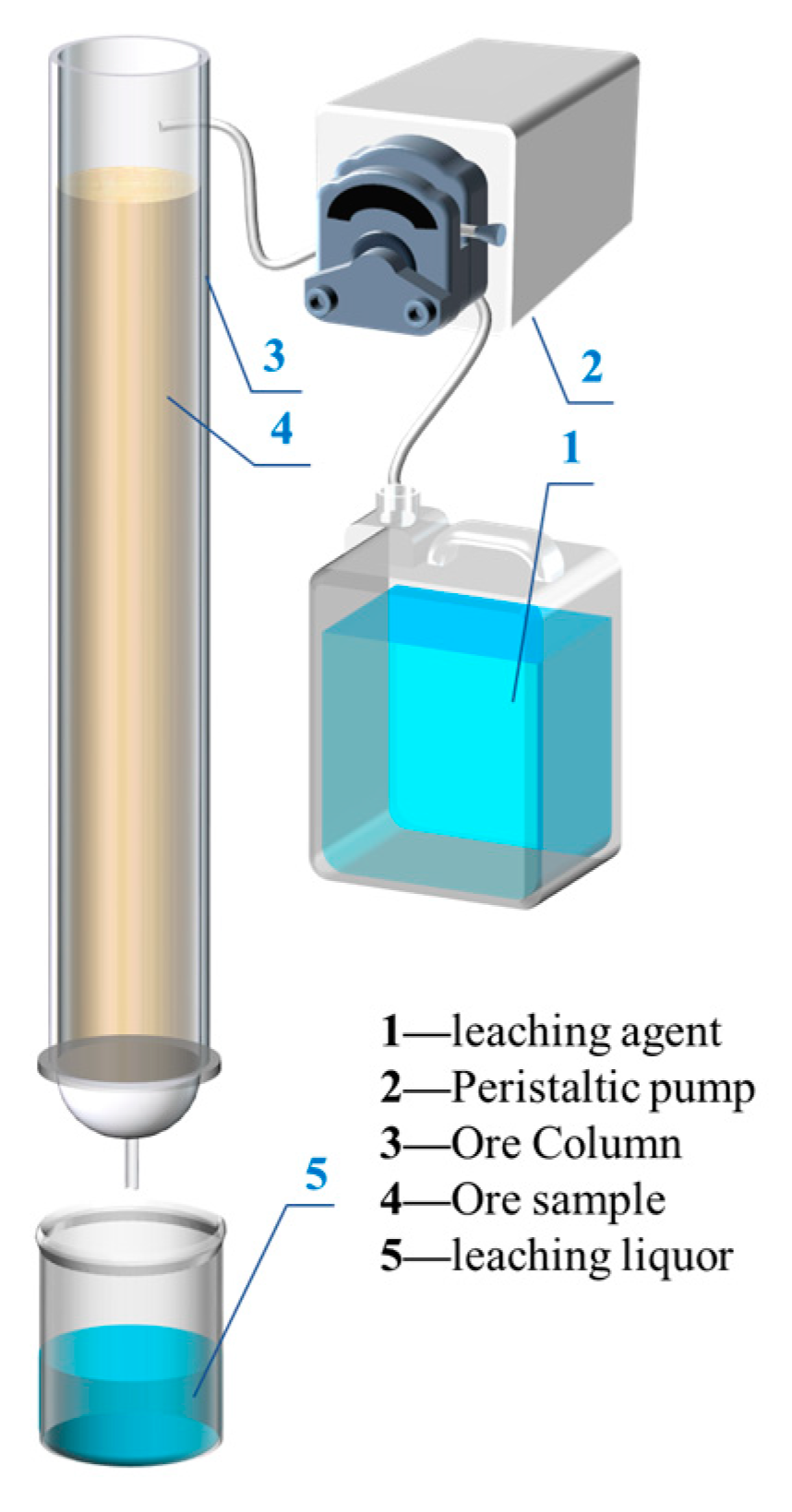
The leaching test was carried out in a leaching system (
Figure 1) consisting mainly of a leaching column with Φ = 4.1 cm, a peristaltic pump (310D, Baoding Senz Pump Co., Ltd.,Baoding, China), and an automatic fraction collector (BSZ-30, Shanghai Huxi Instrument Factory Co., Ltd.,Shanghai, China) as follows:
(1) Filter paper and 2 cm quartz sand were added into the leaching column first to prevent the ore samples from flowing out with the leach solution. Secondly, 230 g of rare earth ore was evenly loaded in layers to a height of 18 cm. Finally, 2 cm quartz sand and filter paper were added to the top of the ore samples to facilitate uniform liquid distribution.
(2) Then, 0.5 wt.%, 2 wt.%, and 4 wt.% magnesium sulfate solutions were prepared using magnesium sulfate heptahydrate, and the pH was adjusted to 2 or 5 with sulfuric acid.
(3) The liquid-to-ore ratio was controlled at a certain level, and the aforementioned leach solution was injected into the leaching column with a peristaltic pump at 0.2 mL/min. After that, the leaching column was divided into upper, middle, and lower parts by the height of 6 cm, and the samples were taken separately.
2.3. Sequential Extraction
To investigate the occurrence transition of aluminum during column leaching, the leached ore samples from different parts of the leaching column were subjected to sequential extraction method. Specifically, the Sol-Al was extracted by stirring the ore samples with water three times. Then, 1 mol/L KCl was applied for stirring the water-leached ore samples three times to extract the Ex-Al. Finally, the KCl-leached ore samples were stirred with 0.2 mol/L HCl three times, to extract the Hy-Al. The specific steps of grading extraction are shown in
Table 2.
2.4. Analysis Method
An inductively coupled plasma atomic emission spectrometer (ICP-AES, Agilent 5800, Agilent Technologies, Santa Clara, CA, USA) was employed to analyze the concentrations of magnesium, aluminum, and rare earth ions in solution samples, and a pH meter (pH meter, Mettler Toledo FE20, METTLER TOLEDO Technology (China) Co. Ltd, Shanghai, China) was used to analyze the pH of solution samples. The testing error was less than 3%.
3. Results and Discussion
3.1. Leaching of Rare Earths and Aluminum
With magnesium sulfate as the leaching agent, the leaching of rare earths and aluminum was explored at different concentrations of magnesium sulfate (0.5 wt.%, 2 wt.%, and 4 wt.%).
3.1.1. Magnesium Sulfate Leaching Agent with pH of 5
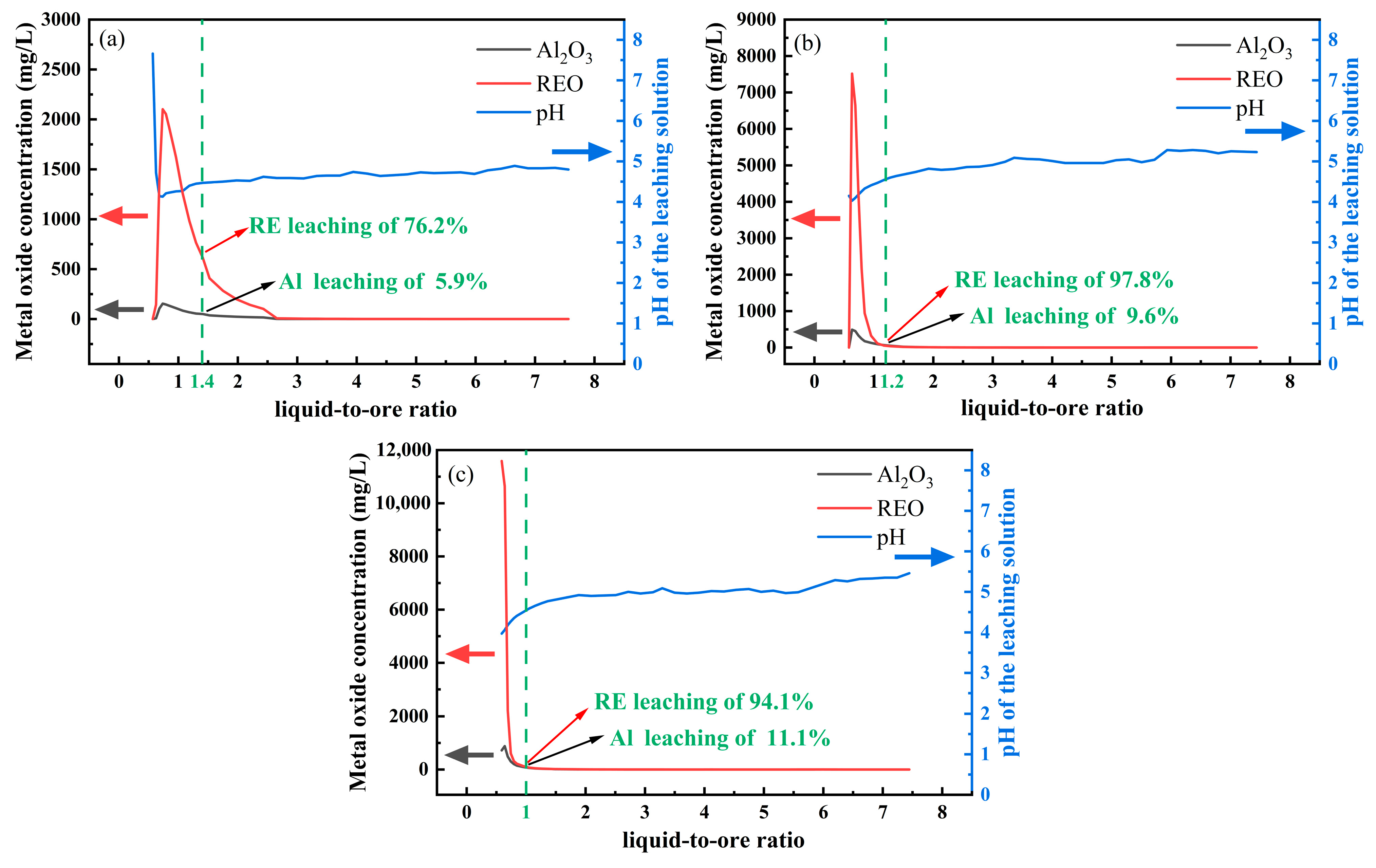
Figure 2a shows the pH change of the leach solution and the leaching curves of rare earths and aluminum under the condition that the concentration of magnesium sulfate was 0.5 wt.% and the pH of the leaching agent was 5.
When the liquid-to-ore ratio increased to 0.57, the leach solution gradually moved to the lower part of the ore column, and the area where ion exchange occurred in the ore column gradually expanded from top to bottom, but no liquid was collected. When the liquid-to-ore ratio increased from 0.57 to 0.74, the rare earths and aluminum were continuously exchanged and leached, the concentration of Al2O3 in the leach solution rose to 154.78 mg/L, and the concentration of rare earth oxide (REO) rose sharply to 2102.15 mg/L, while the pH of the leach solution dropped from 7.5 to approximately 4.1, which was within the scope of the buffering of soil pH. When the liquid-to-ore ratio increased from 0.74 to 2.65, the concentrations of Al2O3 and REO in the leach solution reduced to 1.45 mg/L and 7.46 mg/L, respectively, whereas the pH of the leach solution slowly elevated to 4.59, which may be related to the hydrolysis of aluminum. When the liquid-to-ore ratio was greater than 2.65, the concentrations of Al2O3 and REO in the leach solution were lower than 1 mg/L, and the pH was basically stabilized at 4.6–4.8.
The pH of the magnesium sulfate leaching agent was controlled at 5. When the concentrations of magnesium sulfate increased to 2 wt.% and 4 wt.% (
Figure 2b,c), the trends of REO and Al
2O
3 concentrations in the leach solution were basically the same as those when the magnesium sulfate concentration was 0.5 wt.%. However, the liquid-to-ore ratios of the leach solution dropped to approximately 0.63 when the REO and Al
2O
3 concentrations reached the peak (REO: 7.52 g/L and 11.59 g/L, Al
2O
3: 492.18 mg/L and 897.19 mg/L). According to Fick’s law, the probability and intensity of the ion exchange of magnesium ions (Mg
2+) with rare earth ions (RE
3+) and Al
3+ increase with the rising concentration of magnesium sulfate, so as the concentration difference between the liquid phase body and the solid phase surface increases, this accelerates the diffusion of Mg
2+ and results in the concentration peak shift and increase.
In summary, when the pH of the magnesium sulfate leaching agent was 5, the concentrations of REO and Al2O3 in the leach solution increased first and then decreased, while the pH change of the leach solution showed the opposite law, which may be related to the occurrence transition of aluminum.
3.1.2. Magnesium Sulfate Leaching Agent with pH of 2
With 0.5 wt.% magnesium sulfate solution as the leaching agent, where the pH was controlled at 2, the leaching behavior of rare earths and aluminum in the column leaching was investigated, and the results are shown in
Figure 3a. When the liquid-to-ore ratio reached 0.56, the leach solution started to flow out from the bottom of the leaching column. When the liquid-to-ore ratio rose to 0.72 from 0.56, the rare earths and aluminum were continuously exchanged and leached, the concentrations of REO and Al
2O
3 in the leach solution were elevated to 2525 mg/L and 183 mg/L, respectively, and the pH fell from 6.36 to 4.17, which was within the scope of the buffering of soil pH. When the liquid-to-ore ratio increased from 0.72 to 1.25, the concentrations of REO and Al
2O
3 in the leach solution reduced slowly to 905 mg/L and 73 mg/L, respectively, and the pH increased from 4.17 to 4.37, which may be related to the hydrolysis of aluminum. When the liquid-to-ore ratio increased from 1.25 to 1.9, the ion-exchangeable rare earths were gradually leached completely, and the concentration of REO in the leach solution continued to drop to 10 mg/L, while the concentration of Al
2O
3 increased to 500 mg/L and the pH decreased to 3.94, where the other forms of aluminum except for Ex-Al may be leached out. When the liquid-to-ore ratio kept rising, the concentration of REO in the leach solution remained basically unchanged (<6 mg/L), the concentration of Al
2O
3 remained unchanged (458–519 mg/L) first and then gradually decreased to 50 mg/L as the liquid-to-ore ratio was greater than 2.7, and the pH continued to decrease until it reached the same as that of the leaching agent.
The pH of the magnesium sulfate leaching agent was controlled at 2. When the concentration of magnesium sulfate increased to 2 wt.% and 4 wt.% (
Figure 3b,c), the concentrations of REO and Al
2O
3 in the leach solution exhibited the same trend as those when the magnesium sulfate concentration was 0.5 wt.%, but the peak concentrations of REO in the leach solution rose to 7.72 g/L and 11.26 g/L, respectively, and the peak concentrations of Al
2O
3 were elevated to 507 mg/L and 574 mg/L, respectively. The liquid-to-ore ratios at the peak concentrations were all reduced to approximately 0.63, which is consistent with the results of He Z. Y. et al. [
25,
26].
Comparing the leaching rates of rare earths and aluminum in different pH of leaching agents (
Figure 2 and
Figure 3), at a liquid-to-ore ratio of 1.4 and magnesium sulfate concentration of 0.5 wt.%, the leaching rate of rare earths increased from 76.2% to 91.0% as pH decreased from 5 to 2, while the leaching rate of aluminum increased from 5.9% to 7.7%. However, at a liquid-to-solid ratio of 1 and magnesium sulfate concentration of 4 wt.%, the leaching rate of rare earths increased from 94.1% to 101.4%, and the leaching rate of aluminum increased from 11.1% to 26.4%. When the magnesium sulfate concentration was low, decreasing the pH of the leaching agent significantly increased the leaching rate of rare earths but had no significant effect on the leaching rate of aluminum. When the magnesium sulfate concentration was high, decreasing the pH of the leaching agent slightly increased the leaching rate of rare earths but significantly increased the leaching rate of aluminum. Therefore, it is necessary to further study the occurrence transition and migration of aluminum during the leaching process to regulate the leaching behavior of aluminum and improve the leaching of rare earths while reducing the leaching of aluminum.
3.2. Occurrence Transition and Migration of Aluminum
On the basis of the leaching behavior of rare earths and aluminum mentioned above, it can be observed that the concentration of REO in the leach solution initially increased and then decreased with an increase in the liquid-to-ore ratio. On the other hand, impurity of aluminum exhibited different changes with the pH of the leaching agent, and the impact of pH on the leaching rate of aluminum varied under different magnesium sulfate concentrations. Therefore, a sequential extraction method was employed to analyze the occurrence of aluminum in the leaching tailings in order to clarify the effects of magnesium sulfate concentration and pH on the occurrence transformation and migration of aluminum in the rare earth ore.
3.2.1. Magnesium Sulfate Leaching Agent with pH of 5
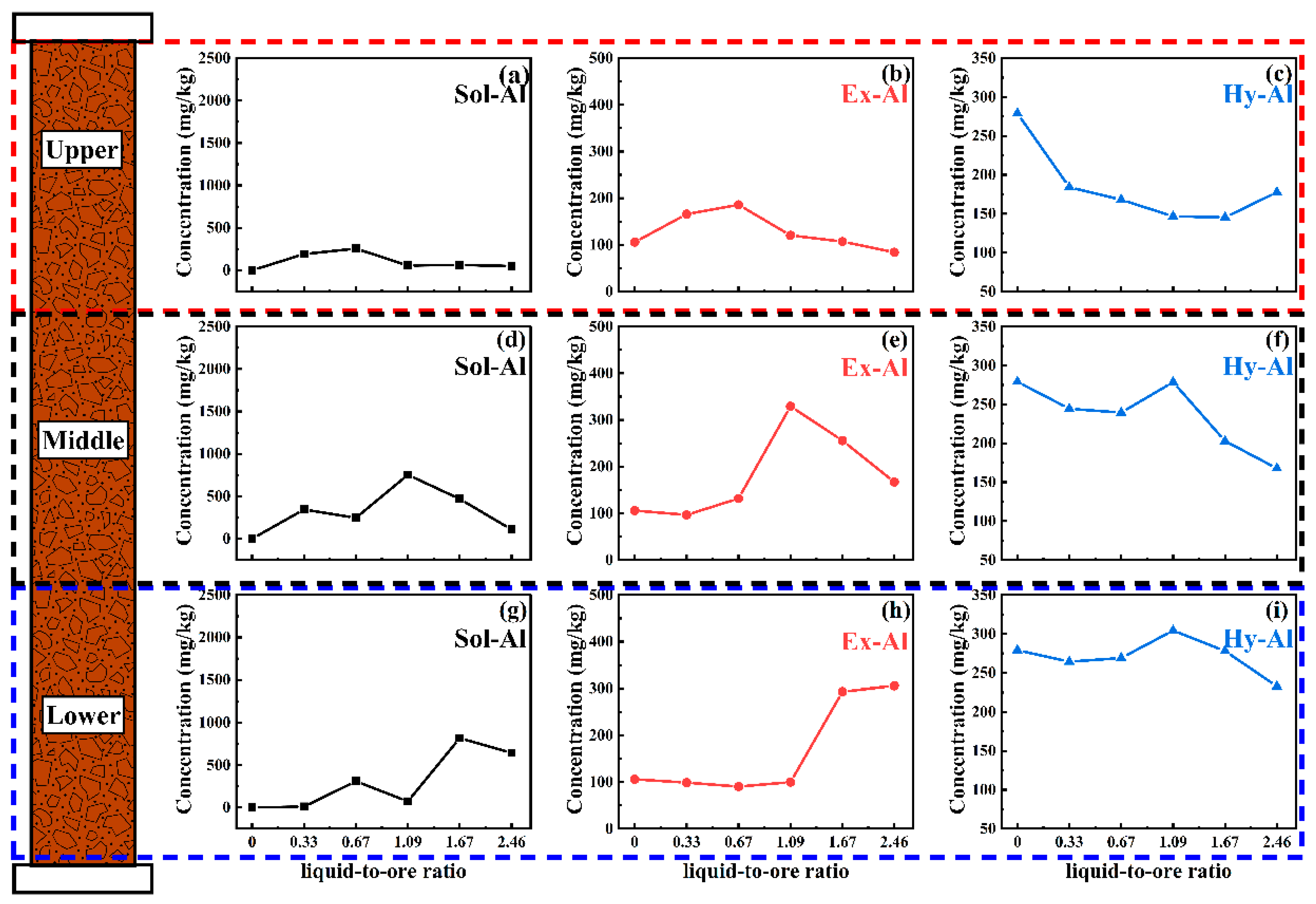
The concentration of magnesium sulfate was controlled at 0.5 wt.%, and the pH of the leaching agent was at 5. The occurrences and content changes of aluminum in the leaching tailings at different parts of the ore column were investigated at different liquid-to-ore ratios, with the results shown in
Figure 4.
According to
Figure 4, when the liquid-to-ore ratio was 0.33, the Sol-Al content in the upper part remained basically unchanged (
Figure 4a), the Ex-Al content dropped from 106 mg/kg to 63 mg/kg (
Figure 4b), and the Hy-Al content was generally unchanged (approximately 280 mg/kg) (
Figure 4c). In the middle part, the Sol-Al (
Figure 4d) and Hy-Al (
Figure 4f) contents increased from 1 mg/kg and 279 mg /kg to 190 mg/kg and 330 mg/kg, respectively, and the Ex-Al content (
Figure 4e) decreased to 89 mg/kg. In the lower part, the Sol-Al content was basically unchanged, the Ex-Al content (
Figure 4h) decreased to 90 mg/kg, and the Hy-Al content increased to 324 mg/kg. This indicates that the ion exchange reaction of aluminum ions occurred in the entire ore column at this stage (Equation (1) [
19]). The transition of Ex-Al to Sol-Al caused the Ex-Al content of the whole ore column to be reduced. In addition, a large amount of Sol-Al migrated to the middle part with the leach solution, leading to an increase in the Sol-Al content in the middle part. The increase in the Hy-Al content in the middle (
Figure 4f) and lower (
Figure 4i) parts was caused by the transition of Sol-Al to Hy-Al (Equation (2) [
18,
27] and Equation (3) [
20]).
When the liquid-to-ore ratio increased to 0.62, the Sol-Al (
Figure 4d) and Hy-Al (
Figure 4f) contents in the middle part decreased to 43 mg/kg and 304 mg/kg, respectively, while in the lower part, the Sol-Al content increased to 330 mg/kg, and the Hy-Al content decreased to 283 mg/kg. This is because the Sol-Al accumulated in the middle part migrates to the lower part during the leaching process so that the Sol-Al content in the lower part rose while the Hy-Al content in the middle and lower parts decreased, which proves the transition of Hy-Al and Ex-Al to Sol-Al at this stage.
As the liquid-to-ore ratio increased to 1.90, the Hy-Al content of the entire ore column dropped, and the Sol-Al content in the lower part decreased to 18 mg/kg, which suggests that the transition of Hy-Al and Ex-Al to Sol-Al continued at this stage until all of the Sol-Al flowed out from the ore column. In addition, the Hy-Al at this stage finally dropped to the level of the original rare earth ore, meaning that at this pH, Hy-Al in the rare earth ore did not undergo occurrence transition during the column leaching; therefore, the aluminum in the leach solution is transformed mainly from Ex-Al in the rare earth ore to Sol-Al.
According to
Figure 5, when the pH of the leaching agent was controlled at 5 and the concentration of magnesium sulfate increased to 4 wt.%, the peaks of the Sol-Al, Ex-Al, and Hy-Al contents of the entire ore column moved to lower liquid-to-ore ratios, indicating that when the pH of leaching agent was 5, the increase of magnesium sulfate concentration could promote the transition of Ex-Al to Sol-Al and the transition of Sol-Al to Hy-Al in the column leaching as well as accelerate the elution of Sol-Al.
3.2.2. Magnesium Sulfate Leaching Agent with pH of 2
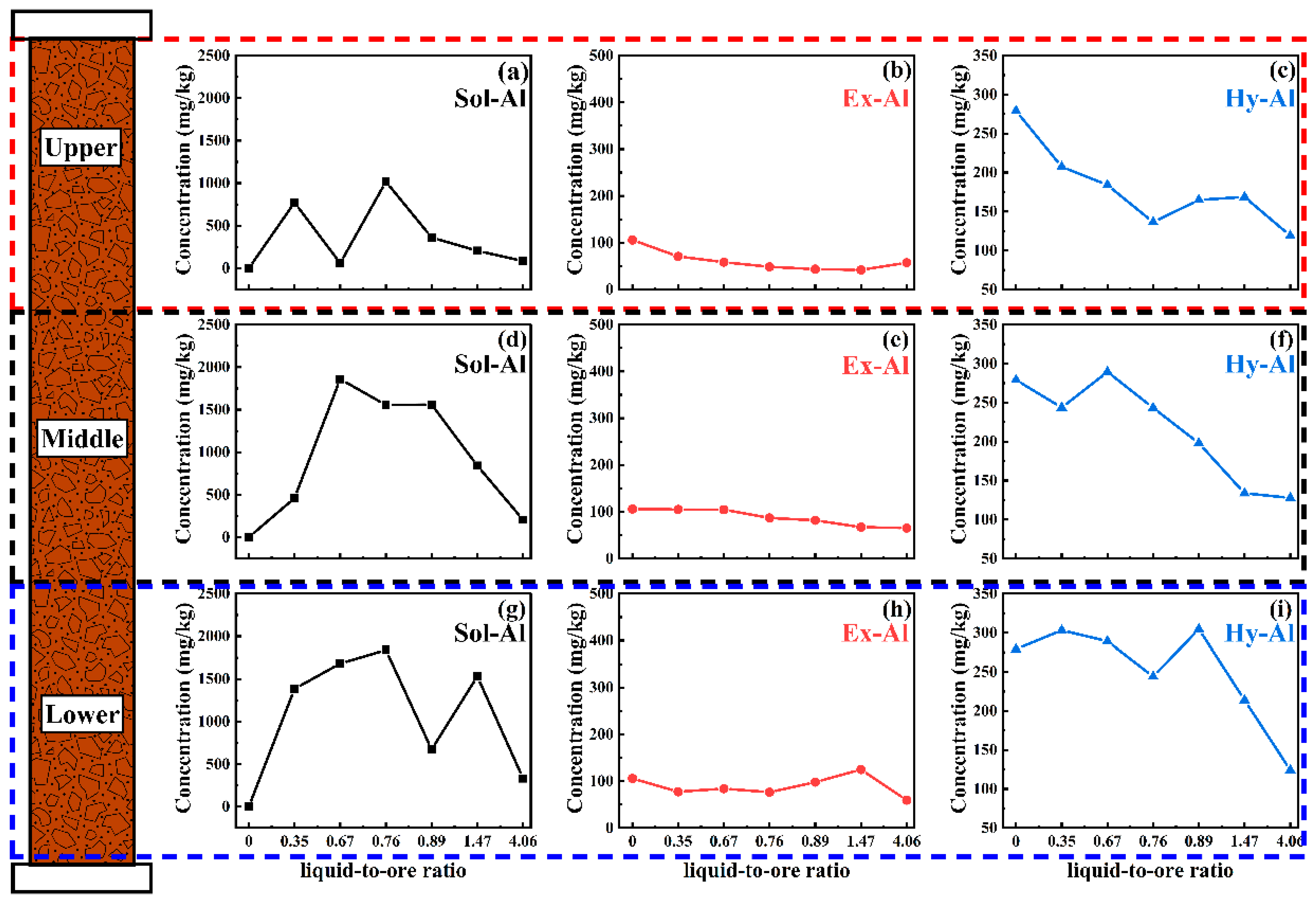
Magnesium sulfate was used as the leaching agent and the pH of the leaching agent was controlled at 2. The occurrences of aluminum and its content changes in the leaching tailings from different parts of the ore column under different magnesium sulfate concentrations (0.5 wt.% and 4 wt.%) and liquid-to-ore ratios during the column leaching were investigated, and the results are shown in
Figure 6 and
Figure 7.
It can be observed from
Figure 6 that the pH decrease in the leaching agent led to the transition of Hy-Al to Sol-Al in the initial stage of leaching (liquid-to-ore ratio < 0.67), and there was a peak (256 mg/kg) of Sol-Al content in the upper part. Furthermore, due to the low concentration of magnesium sulfate in the leaching agent, the Sol-Al in the upper part was back-absorbed by the rare earth ore and transited into Ex-Al, resulting in an increase in the Ex-Al content to 186 mg/kg.
With the increase in the liquid-to-ore ratio (0.67–1.67), Sol-Al continued to migrate downward, and the back-adsorption area of aluminum gradually moved from the upper part to the middle and lower parts. In addition, there were peaks of Sol-Al, Ex-Al, and Hy-Al content in the middle and lower parts, respectively. The peaks of Sol-Al, Ex-Al, and Hy-Al content in the middle part were 753 mg/kg, 330 mg/kg, and 279 mg/kg, respectively, and the liquid-to-ore ratio corresponding to the peaks was 1.09. The peaks of the Sol-Al, Ex-Al, and Hy-Al content in the lower part were 817 mg/kg, 293 mg/kg, and 269 mg/kg, respectively, and the liquid-to-ore ratio corresponding to the peaks was 1.67. This means that the occurrence transition of Sol-Al to Ex-Al and Hy-Al occurred in the middle and lower parts at this stage. The leaching curve in
Figure 3a shows that the concentration of Al
2O
3 decreased from 183 mg/L to 83 mg/L (liquid-to-ore ratios of 0.72–1.25).
When the liquid-to-ore ratio increased to 2.46, the Sol-Al, Ex-Al and Hy-Al content in the middle and lower parts all decreased, indicating the occurrence transition of Hy-Al and Ex-Al to Sol-Al and continuous elution of Sol-Al at this stage.
According to comparison with the condition of magnesium sulfate concentration at 0.5 wt.% and pH of leaching agent at 5 (
Figure 4), it can be concluded that although lowering the pH of the leaching agent can induce the transition of the Hy-Al that naturally occurs in the rare earth ore into Sol-Al, it can then be leached out. However, due to the low magnesium sulfate concentration in the leaching agent, the downward migration of Sol-Al can be back-adsorbed onto the rare earth ore and converted to Ex-Al, leading to an increase in the Ex-Al content compared with the original rare earth ore. In addition, the soil’s inherent acid buffering capacity can also convert a portion of the Sol-Al that migrates downward into Hy-Al during the leaching process. Therefore, when the magnesium sulfate concentration is 0.5 wt.%, lowering the pH of the leaching agent does not significantly increase the content of aluminum in the leach solution.
As shown in
Figure 7, when the pH of the leaching agent was controlled at 2 and the concentration of magnesium sulfate increased to 4 wt.%, the peaks of Sol-Al, Ex-Al, and Hy-Al content all moved to lower liquid-to-ore ratios, while there was a decrease in the Ex-Al content in leaching process, indicating that the increase of the magnesium sulfate concentration helped the transition of Ex-Al to Sol-Al and the transition of Hy-Al to Sol-Al and accelerated the elution of Sol-Al. Moreover, the leaching of aluminum was attributed mostly to the transition of Hy-Al in the rare earth ore to Sol-Al.
3.3. Leaching Mechanism of Aluminum
Based on the leaching curves and the occurrence transition of aluminum in column leaching, occurrence transition occurred between different occurrences of aluminum in the rare earth ore (
Figure 8). Ex-Al in the rare earth ore underwent an ion exchange reaction with Mg
2+ in the leaching agent and transited into Sol-Al (Equation (1)), while the exchangeable rare earths were also leached by ion exchange reaction with Mg
2+ (Equation (4) [
23]); thus, the Mg
2+ content in the leaching agent has a great influence on the mutual transition of Ex-Al and Sol-Al and the leaching of exchangeable rare earths. The transition between Hy-Al and Sol-Al was the formation of aluminum hydroxide on the surface of clay minerals and the hydrolysis of aluminum (Equations (2) and (3)), and it was greatly affected by the pH of the leaching agent.
Based on the analysis of the occurrence transformation of aluminum in the ion-adsorption rare earth ore, the conversion process of aluminum in the rare earth ore can be regulated by adjusting the magnesium sulfate concentration and pH of the leaching agent. The following are the specific methods for regulation:
When the concentration of magnesium sulfate was low (such as 0.5 wt.%), the low pH (such as 2) of the leaching agent led to the transition of Hy-Al to Sol-Al (
Figure 6), but Sol-Al would be back-adsorbed by the rare earth ore and transit into Ex-Al due to the low concentration of Mg
2+ in the leaching agent. Finally, the aluminum content in the leach solution will not increase significantly. Therefore, the application of a lower concentration of magnesium sulfate for the leaching of the rare earth ore can properly decrease the pH of the leaching agent, which accelerates the leaching of rare earths and shortens the leaching time.
In the case of a high concentration (such as 4 wt.%) of magnesium sulfate, the pH of the leaching agent (such as 2–5) had little effect on the transition of Ex-Al to Sol-Al and the desorption of exchangeable rare earths, but the increase in the pH of leaching agent could significantly inhibit the transition of Hy-Al to Sol-Al (
Figure 5), thereby greatly reducing the content of aluminum in the leach solution. Therefore, the use of a high concentration of magnesium sulfate for the leaching of the rare earth ore can properly increase the pH of the leaching agent to inhibit the occurrence transition of Hy-Al present in the rare earth ore and reduce the content of aluminum in the leach solution.
In conclusion, in the actual leaching of ion-adsorption rare earth ore with magnesium sulfate, the pH of the leaching agent should be appropriately increased in the stage of solution injection using high-concentration magnesium sulfate to reduce the leaching of Hy-Al in the rare earth ore. It should then be appropriately lowered in the stage of solution injection using low-concentration magnesium sulfate so as to accelerate the desorption of rare earths and shorten the leaching time. Thusly, the purpose of reducing the leaching of impurity aluminum, accelerating the leaching of rare earths, and reducing the consumption of leaching agent will be finally achieved.