Fluid Components in Cordierite from the Rocks of Epidote-Amphibole Facies of the Muzkol Metamorphic Complex, Tajikistan: Pyrolysis-Free GC-MS Data
Abstract
1. Introduction
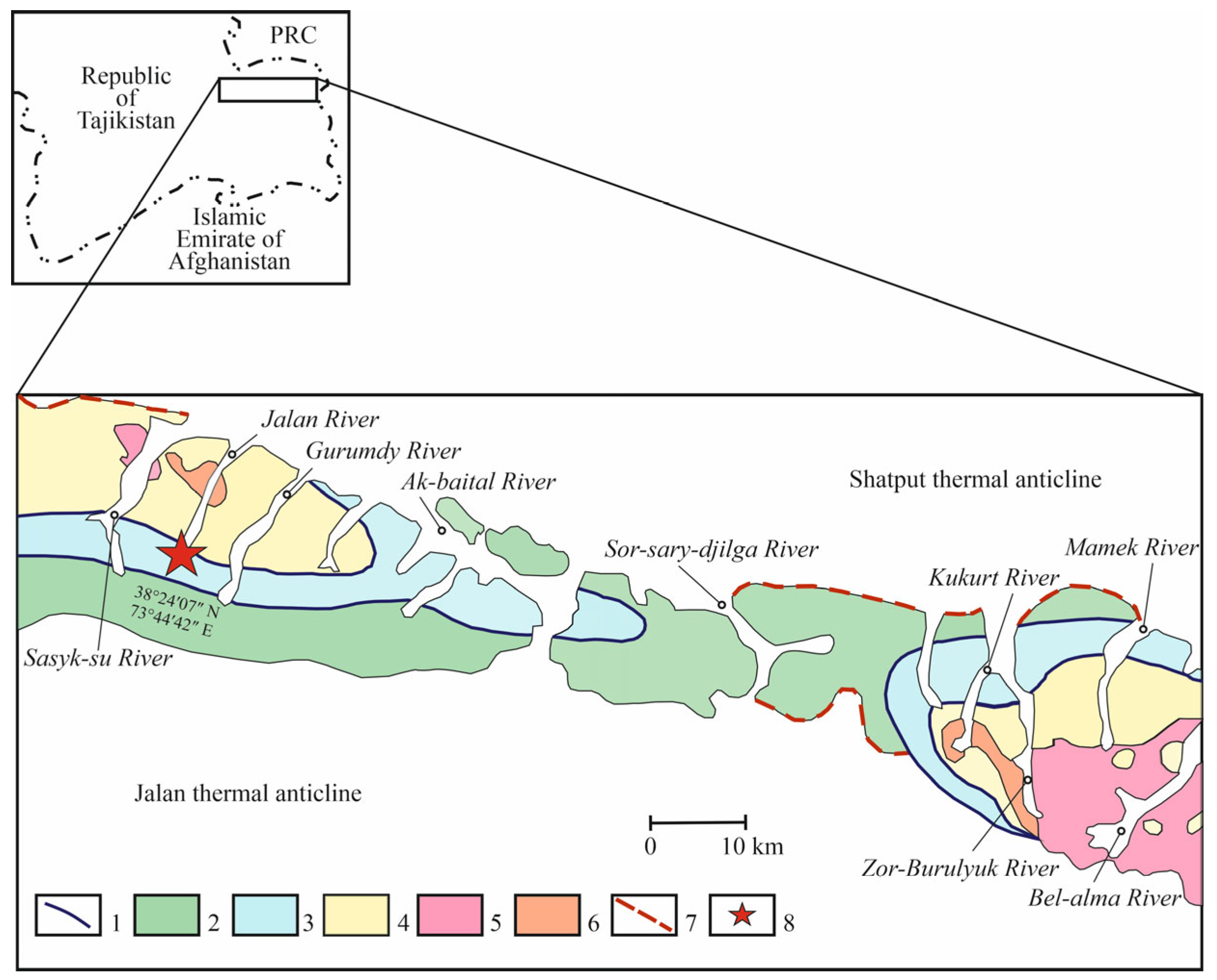
2. Geology
3. Materials and Methods
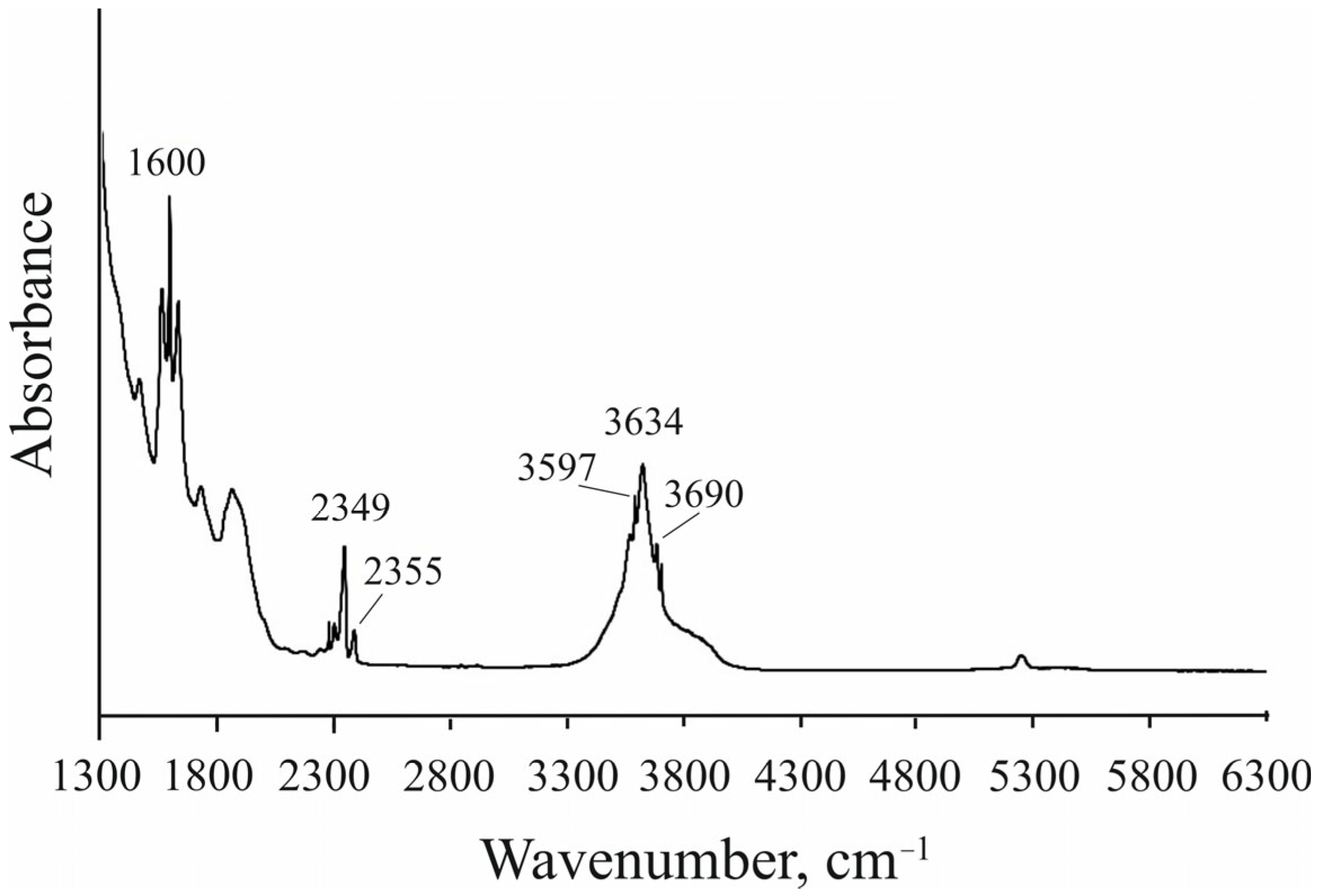
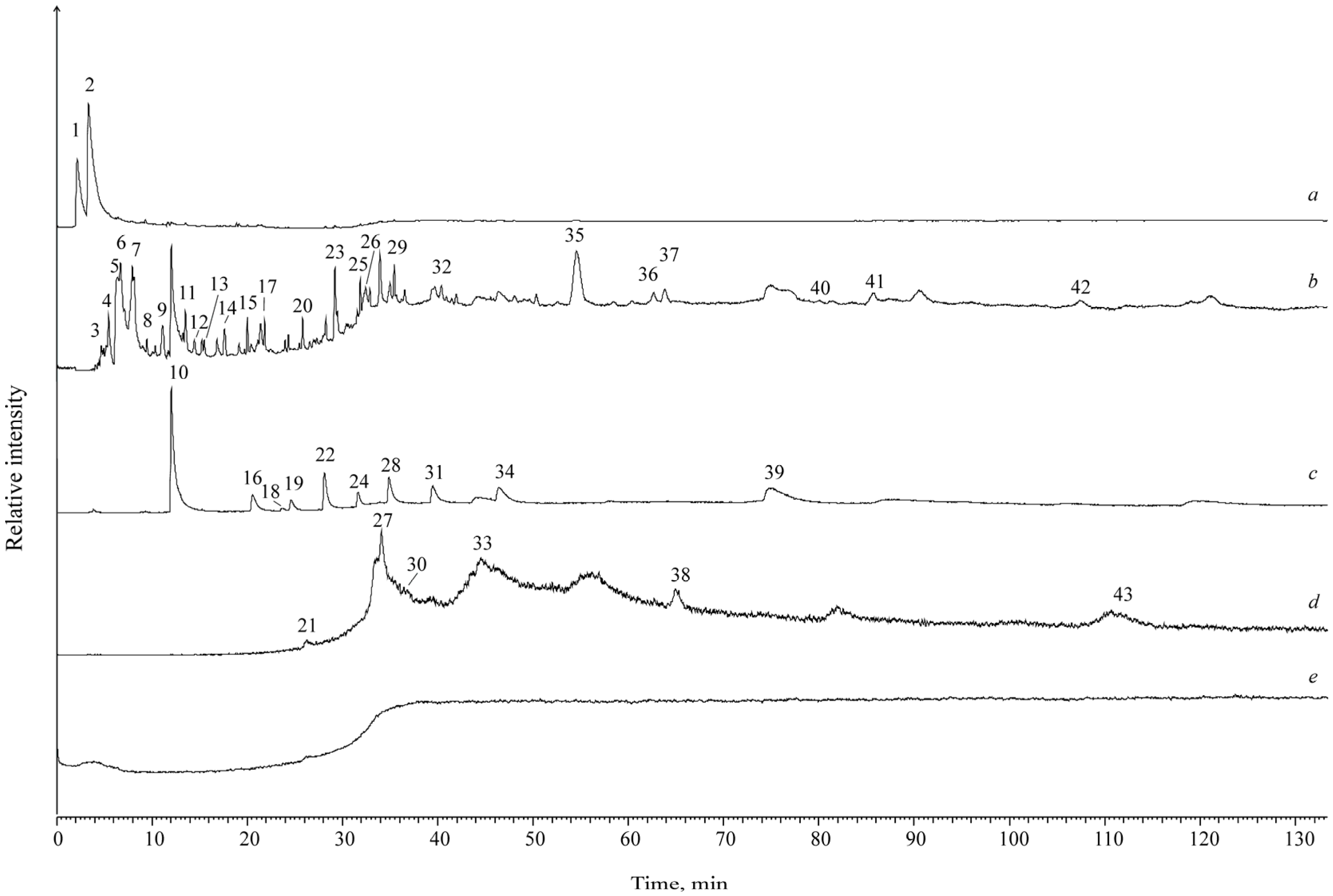
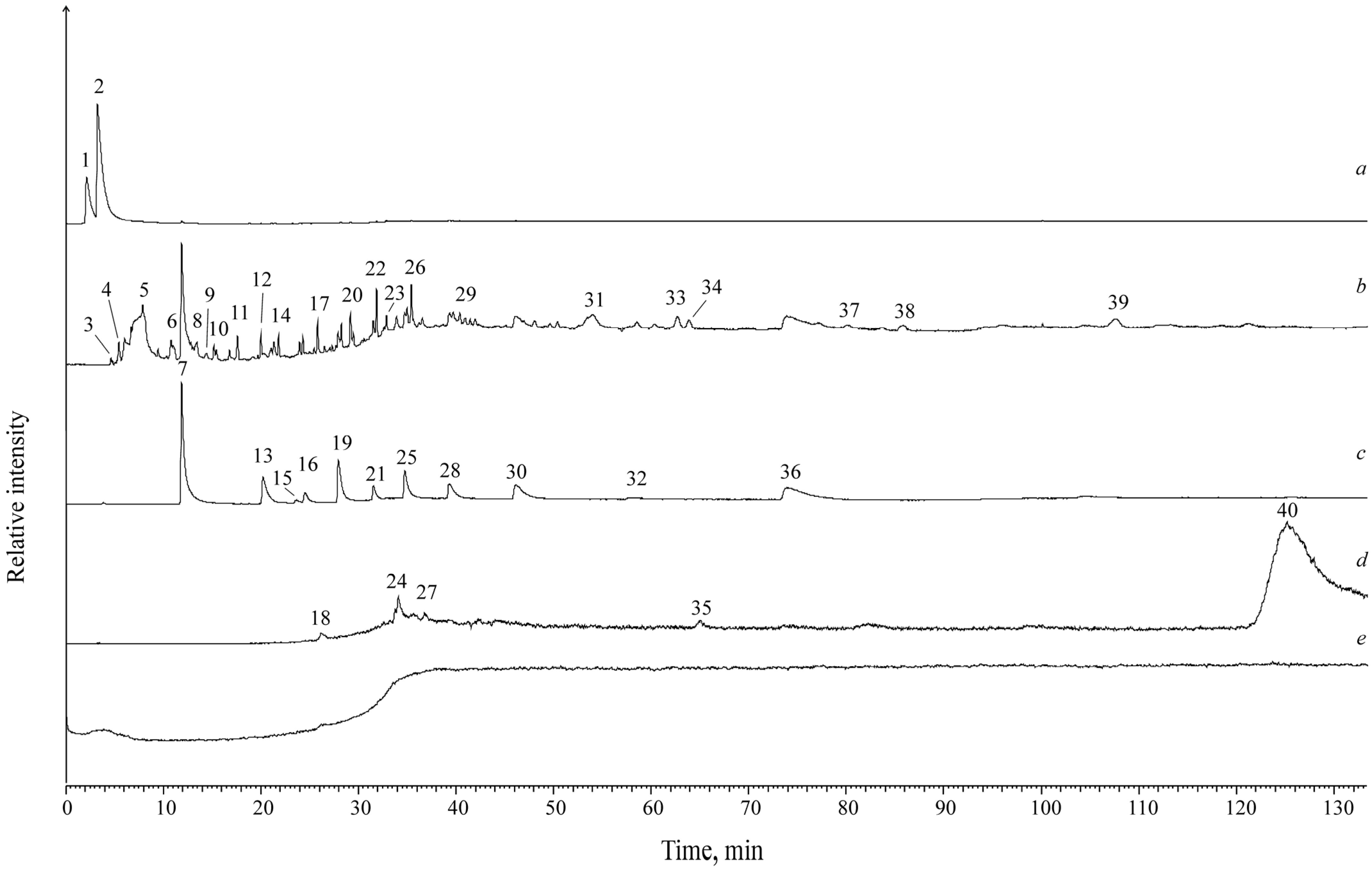
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreyer, W.; Yoder, H.S. Cordierite—H2O system. In Annual Report of the Director of the Geophysical Laboratory; Carnegie Institution: Washington, DC, USA, 1958; p. 197. [Google Scholar]
- Schreyer, W.; Schairer, I.F. Compositions and structural states of anhydrous Mg-cordierites: A reinvestigation of the central part of the system MgO-Al2O3-SiO2. J. Petrol. 1961, 2, 324–406. [Google Scholar] [CrossRef]
- Schreyer, W.; Seifert, F. Compatibility relations of the aluminium silicates in the systems MgO-Al2O3-SiO2-H2O and K2O-MgO-Al2O3-SiO2-H2O at high pressures. Am. J. Sci. 1969, 267, 371–388. [Google Scholar] [CrossRef]
- Seifert, F.; Schreyer, W. Lower temperature stability limit of Mg cordierite in the range 1–7 kb water pressure: A redetermination. Contr. Mineral. Petrol. 1970, 27, 225–238. [Google Scholar] [CrossRef]
- Seifert, F. Low-temperature compatibility relations of cordierite in haplopelites of the system K2O-MgO-Al2O3-SiO2-H2O. J. Petrol. 1970, 11, 73–99. [Google Scholar] [CrossRef]
- Newton, R.C. An experimental determination of the high-pressure stability limits of magnesian cordierite under wet and dry conditions. J. Geol. 1972, 80, 398–420. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Shvedenkova, S.V. Experimental study of the equilibrium of cordierite with potassium feldspar in water-carbon-dioxide fluid. Geol. Geophys. 1998, 39, 851–855. [Google Scholar]
- Bul’bak, T.A.; Shvedenkov, G.Y.; Ripinen, O.I. Kinetiks of exchange reaction water-CO2 in the structural channels of (Mg, Fe2+)-cordierite. Geokhimiya 2005, 4, 429–437. [Google Scholar]
- Bul’bak, T.A.; Shvedenkov, G.Y. Experimental study on incorporation of C-H-O-N fluid components in Mg-cordierite. Eur. J. Mineral. 2005, 17, 829–838. [Google Scholar] [CrossRef]
- Likhacheva, A.Y.; Goryainov, S.V.; Krylov, A.S.; Bul’bak, T.A.; Prasad, P.S.R. Raman spectroscopy of natural cordierite at high water pressure up to 5 GPa. J. Raman Spectrosc. 2012, 43, 559–563. [Google Scholar] [CrossRef]
- Likhacheva, A.Y.; Goryainov, S.V.; Bul’bak, T.A. An X-ray diffraction study of the pressure-induced hydration in cordierite at 4–5 GPa. Am. Mineral. 2013, 98, 181–186. [Google Scholar] [CrossRef]
- Miletich, R.; Gatta, G.D.; Willi, T.; Mirwald, P.W.; Lotti, P.; Merlini, M. Cordierite under hydrostatic compression: Anomalous elastic behavior as a precursor for a pressure-induced phase transition. Am. Mineral. 2014, 99, 479–493. [Google Scholar] [CrossRef]
- Kalt, A.; Altherr, R.; Ludwig, T. Contact metamorphism in pelitic rocks on the island of Kos (Greece, Eastern Aegean Sea): A test for the Na-in-cordierite thermometer. J. Petrol. 1998, 39, 663–688. [Google Scholar] [CrossRef]
- Gerdes, A.; Montero, P.; Bea, F.; Fershater, G.; Borodina, N.; Osipova, T.; Shardakova, G. Peraluminous granites frequently with mantle-like isotope compositions: The continental-type Murzinka and Dzhabyk batholiths of the eastern Urals. Int. J. Earth Sci. 2002, 91, 3–19. [Google Scholar] [CrossRef]
- Sokol, E.V.; Maksimova, N.V.; Nigmatulina, E.N.; Sharygin, V.V.; Kalugin, V.M. Combustion Metamorphism; SB RAN Publisher: Novosibirsk, Russia, 2005; 284p. [Google Scholar] [CrossRef]
- Grapes, R.; Korzhova, S.; Sokol, E.; Seryotkin, Y. Paragenesis of unusual Fe-cordierite (sekaninaite)-containing paralava and clinker from the Kuznetsk coal basin, Siberia, Russia. Contrib. Mineral. Petrol. 2011, 162, 253–273. [Google Scholar] [CrossRef]
- Heinrich, E.W. Cordierite in pegmatite near Micanite, Colorado. Am. Mineral. 1950, 35, 173–184. [Google Scholar]
- Newton, R.C. BeO in pegmatitic cordierite. Mineral. Mag. 1966, 35, 920–927. [Google Scholar] [CrossRef]
- Černý, P.; Povondra, P. Beryllian cordierite from Vĕžná: (Na,K)+Be→Al. Neues Jahrb. Miner. Monatsh. 1966, 2, 36–44. [Google Scholar]
- Černý, P.; Povondra, P. Cordierite in West-Moravian desilicated pegmatites. Acta Univ. Carol. Geol. 1967, 3, 203–221. [Google Scholar]
- Černý, P.; Chapman, R.; Schreyer, W.; Ottolini, L.; Bottazzi, P.; McCammon, C.A. Lithium in sekaninaite from the type locality, Dolní Bory, Czech Republic. Can. Mineral. 1997, 35, 167–173. [Google Scholar]
- Jobin-Bevans, S.; Černý, P. The beryllian cordierite + beryl + spessartine assemblage, and secondary beryl in altered cordierite, Greer Lake granitic pegmatites, southeastern Manitoba. Can. Mineral. 1998, 36, 447–462. [Google Scholar]
- Grew, E.S.; Yates, M.G.; Barbier, J.; Shearer, C.K.; Sheraton, J.W.; Shiraishi, K.; Motoyoshi, Y. Granulite-facies beryllium pegmatites in the Napier Complex in Khmara and Amundsen Bays, western Enderby Land, East Antarctica. Polar Geosci. 2000, 13, 1–40. [Google Scholar]
- Sokol, E.V.; Seryotkin, Y.V.; Bul’bak, T.A. Na-Li-Be-rich cordierite from the Murzinka pegmatite field, Middle Urals, Russia. Eur. J. Mineral. 2010, 22, 565–575. [Google Scholar] [CrossRef]
- Dymek, R.F.; Albee, A.L.; Chodos, A.A. Petrology and origin of Boulders #2 and #3, Apollo 17 Station 2. In Proceedings of the Seventh Lunar Science Conference, Houston, TX, USA, 15–19 March 1976; pp. 2335–2378. [Google Scholar]
- Herzberg, C.T.; Baker, M.B. The cordierite-to-spinel-cataclasite transition: Structure of the lunar crust. Proc. Conf. Lunar Highlands Crust. Geochim. Cosmochim. Acta 1980, 1 (Suppl. 12), 113–132. [Google Scholar]
- Marvin, U.B.; Carey, J.W.; Lindstrom, M.M. Cordierite-Spinel Troctolite, a New Magnesium-Rich Lithology from the Lunar Highlands. Science 1989, 243, 925–928. [Google Scholar] [CrossRef]
- Fuchs, L.H. Occurrence of cordierite and aluminous orthoenstatite in the Allende meteorite. Am. Mineral. 1969, 54, 1645–1653. [Google Scholar]
- Sheng, Y.J.; Hutcheon, I.D.; Wasserburg, G.J. Origin of plagioclase-olivine inclusions in carbonaceous chondrites. Geochim. Cosmochim. Acta 1991, 55, 581–599. [Google Scholar] [CrossRef]
- Petaev, M.I.; Zaslavskaya, N.I.; Clarke, R.S., Jr.; Olsen, E.J.; Jarosewich, E.; Kononkova, N.N.; Holmberg, B.B.; Davis, A.M.; Ustinov, V.I.; Wood, J.A. The Chaunskij meteorite: Mineralogical, chemical and isotope data, classification and proposed origin (abstract). Meteoritics 1992, 27, 276–277. [Google Scholar]
- Petaev, M.I.; Clarke, R.S.; Olsen, E.J.; Jarosewich, E.; Davis, A.M.; Steele, I.M.; Lipschutz, M.E.; Wang, M.S.; Clayton, R.N.; Mayeda, T.K.; et al. Chaunskij: The most highly metamorphosed, shock-modified and metal-rich mesosiderite (abstract). Lunar Planet. Sci. 1993, 24, 1131–1132. [Google Scholar]
- Schreyer, W. Experimental studies on cation substitutions and fluid incorporation in cordierite. Bull. Mineral. 1985, 108, 273–291. [Google Scholar] [CrossRef]
- Zimmermann, J.L. Application petrogenetique de l’etude de la liberation de l’eau et du gaz carbonique des cordierites. CR Acad. Sci. Paris D 1972, 275, 519–522. [Google Scholar]
- Zimmermann, J.L. Etude par spectrometric de masse de la composition des fluides dans quelques cordierites du sud de la Norvege. Soc. Geol. Fr. 1973, 418. [Google Scholar]
- Zimmermann, J.L. La liberation de l’eau, du gaz casrbonique et des hydrocarbures des cordierites. Cinetiques des mecanismes. Determination des sides. Interet petrogenetique. Bull. Mineral. 1981, 104, 325–338. [Google Scholar]
- Beltrame, R.J.; Norman, D.I.; Alexander, E.C.; Savkins, F.J. Volatiles released by step-heating a cordierite to 1200 °C. Trans. Am. Geophys. Union 1976, 57, 352. [Google Scholar]
- Goldman, D.S.; Rossman, G.R.; Dollase, W.A. Channel constituents in cordierite. Am. Mineral. 1977, 62, 1144–1157. [Google Scholar]
- Johannes, B.; Schreyer, W. Experimental introduction of CO2 and H2O into Mg- cordierite. Am. J. Sci. 1981, 281, 299–317. [Google Scholar] [CrossRef]
- Armbruster, T.; Bloss, F.D. Orientation and effects of channel H2O and CO2 in cordierites. Am. Mineral. 1982, 67, 284–291. [Google Scholar]
- Mottana, A.; Fusi, A.; Potenza, B.B.; Crespi, R.; Liborio, G. Hydrocarbon-containing cordierite from Dervio-Colico road tunnel (Como, Italy). Neues Jahrb. Miner. Abh. 1983, 148, 181–199. [Google Scholar]
- Armbruster, T. Ar, N2, and CO2 in the Structural Cavites of Cordierite, an Optical and X-ray Single-Crystal Study. Phys. Chem. Miner. 1985, 12, 233–245. [Google Scholar] [CrossRef]
- Shvedenkov, G.Y.; Lepezin, G.G.; Bul’bak, T.A.; Osorgin, N.Y. Experimental study of saturation of magnesian cordierite with the components of C-O-H fluid. Geokhimiya 1995, 2, 251–262. [Google Scholar]
- Lepezin, G.G.; Bul’bak, T.A.; Sokol, E.V.; Shvedenkov, G.Y. Fluid components in cordierite and their importance for metamorphic petrology. Geol. Geophys. 1999, 1, 97–112. [Google Scholar]
- Khomenko, V.M.; Langer, K. Aliphatic hydrocarbons in structural channels of cordierite: A first evidence from polarized single-crystal IR absorption spectroscopy. Am. Mineral. 1999, 84, 1181–1185. [Google Scholar] [CrossRef]
- Kolesov, E.A.; Geiger, C.A. Cordierite II: The role of CO2 and H2O. Am. Mineral. 2000, 85, 1265–1274. [Google Scholar] [CrossRef]
- Khomenko, V.; Langer, K.; Geiger, C.A. Structural locations of the iron ions in cordierite: A spectroscopic study. Contrib. Mineral. Petrol. 2001, 141, 381–396. [Google Scholar] [CrossRef]
- Kolesov, B.A. Raman spectra of single H2O molecules isolated in crystal cavities. Zhurnal Strukturnoi Khimii 2006, 47, 27–40. [Google Scholar] [CrossRef]
- Smith, J.V.; Schreyer, W. Location of argon and water in cordierite. Miner. Mag. 1962, 33, 226–236. [Google Scholar] [CrossRef]
- Farrel, E.E.; Newnham, R.E. Electronic and vibrational absorption spectra. Am. Mineral. 1967, 52, 380–389. [Google Scholar]
- Armbruster, T.; Bloss, F.D. Channel CO2 in cordierites. Nature 1980, 286, 140–141. [Google Scholar] [CrossRef]
- Carson, D.G.; Rossman, G.R.; Vaughan, R.V. Orientation and motion of water molecules in cordierite: A proton nuclear magnetic resonance study. Phys. Chem. Miner. 1982, 8, 14–19. [Google Scholar] [CrossRef]
- Mirwald, P. Crystal chemical effects of sodium on the incorporation of H2O and CO2 in Mg-cordierite. Terra Cogn. 1983, 3, 163. [Google Scholar]
- Aines, R.D.; Rossman, G.R. The high temperature behavior of water and carbon dioxide in cordierite and beryl. Am. Mineral. 1984, 69, 319–327. [Google Scholar]
- Armbruster, T. The role of Na in the structure of low cordierite. A syngle crystal X-ray study. Am. Mineral. 1986, 71, 746–757. [Google Scholar]
- Giampaolo, C.; Putnis, A. The kinetics of dehydration and order-disorder of molecular H2O in Mg-cordierite. Eur. J. Miner. 1989, 1, 193–202. [Google Scholar] [CrossRef]
- Vry, J.K.; Brown, P.E.; Valley, J.W. Cordierite volatile content and the role of CO2 in high grade metamorphism. Am. Mineral. 1990, 75, 71–88. [Google Scholar]
- Bertoldi, C.; Proyer, A.; Garbe-Schönberg, D.; Behrens, H.; Dachs, E. Comprehensive chemical analyses of natural cordierites: Implications for exchange mechanisms. Lithos 2004, 78, 389–409. [Google Scholar] [CrossRef]
- Majumdar, A.S.; Mathew, G. Raman-Infrared (IR) Spectroscopy Study of Natural Cordierites from Kalahandi, Odisha. J. Geol. Soc. India 2015, 86, 80–92. [Google Scholar] [CrossRef]
- Tropper, P.; Wyhlidal, S.; Haefeker, U.A.; Mirwald, P.W. An experimental investigation of Na incorporation in cordierite in low P/high T metapelites. Miner. Petrol. 2018, 112, 199–217. [Google Scholar] [CrossRef]
- Sugiura, K. The water problem of cordierite. Bull. Tokyo Inst. Technol. Ser. B 1959, 1, 1–26. [Google Scholar]
- Lepezin, G.G.; Melenevskii, V.N. Problem of H2O and CO2 in conrdierites. Dokl. AN SSSR 1983, 269, 920–924. [Google Scholar]
- Winkler, B.; Goddens, G.; Hennion, B. Movement of channel H2O in cordierite observed with quasi-elastic neutron scattering. Am. Mineral. 1994, 79, 801–808. [Google Scholar]
- Winkler, B.; Milman, V.; Payne, M.C. Orientation, location, and total energy of hydration of channel H2O in cordierite investigated by ab-initio total energy calculations. Am. Mineral. 1994, 79, 200–204. [Google Scholar]
- Wood, D.L.; Nassau, K. Infrared spectra of foreign molecules in beryl. J. Chem. Phys. 1967, 47, 2200–2228. [Google Scholar] [CrossRef]
- Sokol, E.V.; Stolpovskaya, V.N.; Lepezin, G.G. New data on water in cordierites (based on IR spectroscopy). Dokl. RAN 1998, 359, 671–675. [Google Scholar]
- Knop, E.; Mirwald, P.W. Cordierite as a monitor of fluid and melt sodium activity in metapelites, migmatites and granites: Constraints from incorporation experiments. J. Conf. Abstr. 2000, 5, 58. [Google Scholar]
- Suknev, V.S.; Kitsul, V.I.; Lazebnik, Y.D.; Brovkin, A.A. On the presence and qualitative estimation of CO2 in cordierites based on data of IR spectroscopy and chemical analysis. Dokl. AN SSSR 1971, 200, 950–952. [Google Scholar]
- Kitsul, V.I.; Lazebnik, Y.D.; Brovkin, A.A.; Suknev, V.O. Diagram for determination of the iron content of cordierites. Dokl. AN SSSR 1971, 200, 1419–1422. [Google Scholar]
- Schreyer, W.; Gordillo, C.E.; Werding, G. A new sodian-berillian cordierite from Soto, Argentina, and the relationship between distortion index, Be content, and state of hydration. Contrib. Miner. Petrol. 1979, 70, 421–428. [Google Scholar] [CrossRef]
- Hofmann, J.; Kaiser, G.; Klemm, W.; Paech, H.-J. K/Na Alter von Dolerite und Metamorphiten der Shackleton Range und der Whichaway Nunataks, Ost- und Südostumrandung des Filchner-Eisschelfs (Antarktis). Z. Geol. Wiss. Berl. 1980, 8, 1227–1232. [Google Scholar]
- Armbruster, T.; Schreyer, W.; Hoefs, J. Very high CO2 cordierite from Norwegian Lapland; mineralogy, petrology, and carbon isotopes. Contrib. Mineral. Petrol. 1982, 81, 262–267. [Google Scholar] [CrossRef]
- Lai, K.K.; Ackermand, D.; Raith, P.; Seifert, F. Sapphirine-containing assemblages from Kiranur, Southern India: A study of chemographic relationships in the Na2O-FeO-MgO-Al2O3-SiO2-H2O system. Neues Jahrb. Miner. Abh. 1984, 150, 121–152. [Google Scholar]
- Kurepin, V.A.; Malyuk, G.A.; Kalinichenko, A.M.; Utochkin, D.V. Volatiles in cordierite from Berdichev granites (the Ukrainian Shield). Mineral. Zhurnal 1986, 8, 70–82. [Google Scholar]
- Perreault, S.; Martignole, J. CO2-rich cordierites in high-temperature migmatites, northeastern Grenville province, Quebec (abs). Geol. Assoc. Can. Program Abstr. 1986, 11, 114. [Google Scholar]
- Perreault, S.; Martignole, J. High-temperature cordierite migmatites in the north-eastern Grenville Province. J. Metamorph. Geol. 1988, 6, 673–696. [Google Scholar] [CrossRef]
- Geverk’yan, S.V.; Kuripin, V.A. CO2 in cordierite structure (IR spectroscopic data). Mineral. Zhurnal 1987, 9, 49–53. [Google Scholar]
- Le Breton, N. Infrared investigation of CO2-containing cordierites. Some implications for the study of metapelitic granulites. Contrib. Mineral. Petrol. 1989, 103, 387–396. [Google Scholar] [CrossRef]
- Osorgin, N.Y. The Kinetics of Degassing of (H2O and CO2) Cordierites and Their Significance for Metamorphic Petrology: Manuscript; OIGGM SB RAN: Novosibirsk, Russia, 1991; 16p. [Google Scholar]
- Peck, H.W.; Valley, J.W. Genesis of cordierite–getrite gneisses, central metasedimentary Belt, boundary thrust zone, Grenville province, Ontario, Canada. Can. Mineral. 2000, 38, 511–524. [Google Scholar] [CrossRef]
- Harley, S.L.; Carrington, D.P. The distribution of H2O between cordierite and granitic melt: H2O incorporation in cordierite and its application to high-grade metamorphism and crustal anatexis. J. Petrol. 2001, 42, 1595–1620. [Google Scholar] [CrossRef]
- Harley, S.L.; Thompson, P.; Hensen, B.J.; Buick, I.S. Cordierite as a sensor of fluid conditions in high-grade metamorphism and crustal anatexis. J. Metamor. Geol. 2002, 20, 71–86. [Google Scholar] [CrossRef]
- Kaindl, R.; Tropper, P.; Deibla, I. A semi-quantitative technique for determination of CO2 in cordierite by Raman spectroscopy in thin sections. Eur. J. Mineral. 2006, 18, 331–335. [Google Scholar] [CrossRef]
- Kaindl, R.; Többens, D.; Haefeker, U. Quantum-mechanical calculations of the Raman spectra of Mg- and Fe-cordierite. Am. Mineral. 2011, 96, 1568–1574. [Google Scholar] [CrossRef]
- Aranovich, L.Y.; Podlessky, K.K.; Shchenochkina, N.I. Experimental determination of CO2 solubility in cordierite. Dokl. AN SSSR 1981, 261, 728–730. [Google Scholar]
- Le Breton, N.; Schreyer, W. Experimental CO2 incorporation in to Mg-cordierite: Nonlinear behaviour of the system. Eur. J. Mineral. 1993, 7, 427–438. [Google Scholar] [CrossRef]
- Farmer, V.C. Infrared Spectra of Minerals; The Mineralogical Society: London, UK, 1974; 539p. [Google Scholar]
- Damon, P.E.; Kulp, J.L. Excess helium and argon in beryl and other minerals. Am. Mineral. 1958, 43, 433–459. [Google Scholar]
- Saito, K.; Alexander, E.C., Jr.; Dragon, J.C.; Zashu, S. Rare gases in cyclosilicates and cogenetic minerals. J. Geophys. Res. 1984, 89, 7891–7901. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Shvedenkov, G.Y.; Lepezin, G.G. On saturation of magnesian cordierite with alkanes at high temperatures and pressures. Phys. Chem. Mineral. 2002, 29, 140–154. [Google Scholar] [CrossRef]
- Boberski, C.; Schreyer, W. Synthesis and water contents of Fe2+—Containing cordierites. Eur. J. Mineral. 1990, 2, 565–584. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Shvedenkov, G.Y.; Lepezin, G.G. Replacement of H2O molecules by D2O and CO2 in the structural channels of cordierite. Geokhimiya 1999, 1, 75–81. [Google Scholar]
- Shvedenkov, G.Y.; Reverdatto, V.V.; Bul’bak, T.A.; Bryksina, N.A. Bimetasomatic diffusion zonality in the CaO–MgO–SiO2–H2O–CO2 system: Experiments using natural samples. Petrology 2006, 14, 515–527. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Shvedenkova, S.V. Dependence of the water content in channels of the cordierite structure on the composition of its Fe-Mg solid solution. Dokl. Akad. Nauk. 2008, 419, 661–664. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Shvedenkova, S.V. Solid solutions of (Mg, Fe2+)-cordierite: Synthesis, water content, magnetic properties. Geokhimiya 2011, 49, 411–426. [Google Scholar] [CrossRef]
- Kurepin, V.A. Cordierite as an indicator of thermodynamic conditions of petrogenesis. Contrib. Mineral. Petrol. 2010, 160, 391–406. [Google Scholar] [CrossRef]
- Zatolokina, K.I.; Tomilenko, A.A.; Bul’bak, T.A.; Lepezin, G.G. Volatile components in cordierite and coexisting tourmaline and quartz from pegmatites of the Kuhilal deposit (Pamirs, Tajikistan). Geol. Geophys. 2021, 62, 1411–1431. [Google Scholar] [CrossRef]
- Dufour, M.S.; Popova, V.A.; Krivets, T.N. Alpine Metamorphic Complex of the Eastern Part of Central Pamirs; Leningrad University: Saint Petersburg, Russia, 1970; 126p. [Google Scholar]
- Gorokhov, I.M.; Dufour, M.S.; Neimark, D.A.; Amelin, Y.V.; Ovchinnikova, G.V.; Gorokhovsky, B.M. Early Paleozoic fragments of Gondwana in the Nappes of the Central Pamirs and Lower Himalayas: Geochemical and Isotopic Characteristics. Stratigr. Geol. Korrelyatsiya 1993, 8, 20–34. [Google Scholar]
- Dufour, M.S.; Popova, V.A. Processes of regional metamorphism in the Muzkol metamorphic complex in the Central Pamirs. Vestn. Leningr. Univ. 1975, 12, 14–20. [Google Scholar]
- Gilev, A.V. Jewelry-quality cordierite of Central Pamir. Mineral. Tadjikistana 1989, 8, 33–47. [Google Scholar]
- Dufour, M.S.; Kotov, N.V. Thermodynamic conditions for the manifestation of metamorphism and metasomatism in the rocks of the eastern part of the Central Pamirs. Izv. USSR Acad. Sci. Ser. Geol. 1972, 10, 24–36. [Google Scholar]
- Dufour, M.S.; Sedova, I.S. On the evolution of P-T parameters during the formation of metamorphic zoning of the Muzkol complex (Central Pamir) according to the study of inclusions in mineral-forming media. Izv. USSR Acad. Sci. Ser. Geol. 1975, 10, 49–58. [Google Scholar]
- Dufour, M.S.; Kol’tsov, A.B.; Zolotarev, A.A.; Kuznetsov, A.B. Corundum-bearing metasomatic rocks in the Central Pamirs. Petrologiya 2007, 15, 160–177. [Google Scholar] [CrossRef]
- Dubessy, J.; Poty, B.; Ramboz, C. Advances in C–O–H–N–S fluid geochemistry based on micro-Raman spectrometric analysis of fluid inclusions. Eur. J. Mineral. 1989, 1, 517–534. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Kuz’min, D.V.; Bul’bak, T.A.; Timina, T.Y.; Sobolev, N.V. Composition of primary fluids and melt inclusions in regenerated olivines from hypabbysal kimberlites of theMalokuonapskaya pipe (Yakutia). Dokl. Akad. Nauk. 2015, 465, 213–217. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Sokol, I.A.; Palyanov, Y.N. Carbon and nitrogen speciation in Npoor C-O-H fluids at 6.3 GPa and 1100–1400 degrees. C. Sci. Rep. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Sokol, A.G.; Palyanov, Y.N.; Tomilenko, A.A.; Bul’bak, T.A.; Palyanova, G.A. Carbon and nitrogen speciation in nitrogen-rich C-O–H–N fluids at 5.5–7.8 GPa. Earth Planet. Sci. Lett. 2017, 460, 234–243. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Tomilenko, A.A.; Wirth, R.; Bul’bak, T.A.; Luk’yanova, L.I.; Fedorova, E.N.; Reutsky, V.N.; Efimova, E.S. Mineral and fluid inclusions in diamonds from the Urals placers, Russia: Evidence for solid molecular N2 and hydrocarbons in fluid inclusions. Geochim. Cosmochim. Acta 2019, 266, 197–219. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Tomilenko, A.A.; Bul`bak, T.A.; Logvinova, A.M. Composition of Hydrocarbons in Diamonds, Garnet, and Olivine from Diamondiferous Peridotites from the Udachnaya Pipe in Yakutia, Russia. Engineering 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Tomilenko, A.A.; Gibsher, N.A.; Sazonov, A.M.; Shaparenko, E.O.; Ryabukha, M.A.; Khomenko, M.O.; Sil’yanov, S.A.; Nekrasova, N.A. Hydrocarbons in fluid inclusions from native gold, pyrite and quartz of the Sovetskoe deposit (Yenisei Ridge, Russia) according to pyrolysis-free gas chromatography-mass spectrometry data. Russ. Geol. Geophys. 2020, 61, 1535–1560. [Google Scholar] [CrossRef]
- Cesare, B.; Maineri, C.; Toaldo, A.B.; Pedron, D.; Vigil, A.A. Immiscibility between carbonic fluids and granitic melts during crustal anatexis: A fluid and melt inclusion study in the enclaves of the Neogene Volcanic Province of SE Spain. Chem. Geol. 2007, 237, 433–449. [Google Scholar] [CrossRef]
- Santosh, M.; Jackson, D.H.; Harris, N.B.W. The Significance of Channel and Fluid-Inclusion CO2 in Cordierite: Evidence from Carbon Isotopes. J. Petrol. 1993, 34, 233–258. [Google Scholar] [CrossRef]
- Herms, P.; Schenk, V. Fluid inclusions in granulite-facies metapelites of the Hercynian ancient lower crust of the Serre, Calabria, Southern Italy. Contrib. Mineral. Petrol. 1992, 112, 393–404. [Google Scholar] [CrossRef]
- Lepezin, G.G.; Kuznetsova, I.K.; Lavrent’ev, Y.G.; Chmel’nikova, O.S. Optical methods of determination of the water contents in cordierites. Contrib. Mineral. Petrol. 1976, 58, 319–329. [Google Scholar] [CrossRef]
- Lepezin, G.G.; Melenevsky, V.N. On the problem of water diffusion in the cordierites. Lithos 1977, 10, 49–57. [Google Scholar] [CrossRef]
- Mirwald, P.W.; Tropper, P. Structural disequilibrium in cordierites from long-duration experiments using crystal chemical constraints. Mitt. Osterr. Mineral. Gesellschaft. 2015, 161, 88. [Google Scholar]
- Zhdanova, N.V.; Khalif, A.L. Drying of Hydrocarbon Gases; Khimiya Publishing House: Moscow, Russia, 1984; 200p. (In Russian) [Google Scholar]
- Penkala, T. Zapys Krystalochemii; Panstwowe Wydawnictwo Naukowe: Warszawa, Poland, 1972; pp. 401–402. [Google Scholar]
- Andrawes, F.; Holzer, G.; Roedder, E.; Gibson, E.K.; Oro, J. Gas chromatographic analysis of volatiles in fluid and gas inclusions. J. Chromatogr. 1984, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; William-Jones, A.E. Bulk analysis of volatiles in fluid inclusions. In Fluid Inclusions: Analysis and Interpretation; Samson, I., Anderson, A., Marshall, D., Eds.; Mineralogical Association of Canada: Quebec, QC, Canada, 2003; Volume 32, Chapter 10; pp. 10-1–10-30. [Google Scholar]
- Tomilenko, A.A.; Bul’bak, T.A.; Logvinova, A.M.; Sonin, V.M.; Sobolev, N.V. The Composition Features of Volatile Components in Diamonds from the Placers in the Northeastern Part of the Siberian Platform by Gas Chromatography–Mass Spectrometry. Dokl. Akad. Nauk. 2018, 481, 310–314. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Sokol, I.A.; Zaikin, P.A.; Sobolev, N.V. Composition of reduced mantle fluids: Evidence from modelling experiments and fluid inclusions in natural diamond. Russ. Geol. Geophys. 2020, 61, 810–825. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Bul’bak, T.A.; Khomenko, M.O.; Kuz’min, D.V.; Sobolev, N.V. The composition of volatile components in olivines from Yakutian kimberlites of various ages: Evidence from gas chromatography–mass spectrometry. Dokl. Akad. Nauk. 2016, 468, 684–689. [Google Scholar] [CrossRef]
- Schreiber, U.; Mayer, C.; Schmitz, O.J.; Rosendahl, P.; Bronja, A.; Greule, M.; Keppler, F.; Mulder, I.; Sattler, T.; Schöler, H.F. Organic compounds in fluid inclusions of Archean quartz—Analogues of prebiotic chemistry on early Earth. PLoS ONE 2017, 12, e0177570. [Google Scholar] [CrossRef]
- Grozeva, N.G.; Klein, F.; Seewald, J.S.; Sylva, S.P. Chemical and isotopic analyses of hydrocarbon-containing fluid inclusions in olivine-rich rocks. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20180431. [Google Scholar] [CrossRef]
- Ryabukha, M.A.; Gibsher, N.A.; Tomilenko, A.A.; Bul’bak, T.A.; Khomenko, M.O.; Sazonov, A.M. P-T-X parameters of metamorphogene and hydrothermal fluids, isotopy and age of the Bogunai gold deposit, southern Yenisei Ridge (Russia). Geol. Geophys. 2015, 56, 1153–1172. [Google Scholar] [CrossRef]
- Shaparenko, E.; Gibsher, N.; Tomilenko, A.; Sazonov, A.; Bul’bak, T.; Ryabukha, M.; Khomenko, M.; Silyanov, S.; Nekrasova, N.; Petrova, M. Ore–Bearing Fluids of the Blagodatnoye Gold Deposit (Yenisei Ridge, Russia): Results of Fluid Inclusion and Isotopic Analyses. Minerals 2021, 11, 1090. [Google Scholar] [CrossRef]
- Karlsen, D.A.; Nedkvitne, T.; Larter, S.R.; Bjørlykke, K. Hydrocarbon composition of authigenic inclusions: Application to elucidation of petroleum reservoir filling history. Geochim. Cosmochim. Acta 1993, 57, 3641–3659. [Google Scholar] [CrossRef]
- George, S.C.; Volk, H.; Dutkiewicz, A. Mass Spectrometry Techniques for Analysis of Oil and Gas Trapped in Fluid Inclusions. In Mass Spectrometry Handbook; Lee, M.S., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 645–673. [Google Scholar] [CrossRef]
- Lepezin, G.G.; Osorgin, N.Y. Estimation of fluid composition of metamorphic complexes of moderate pressure. Dokl. RAN 1992, 324, 648–653. [Google Scholar]


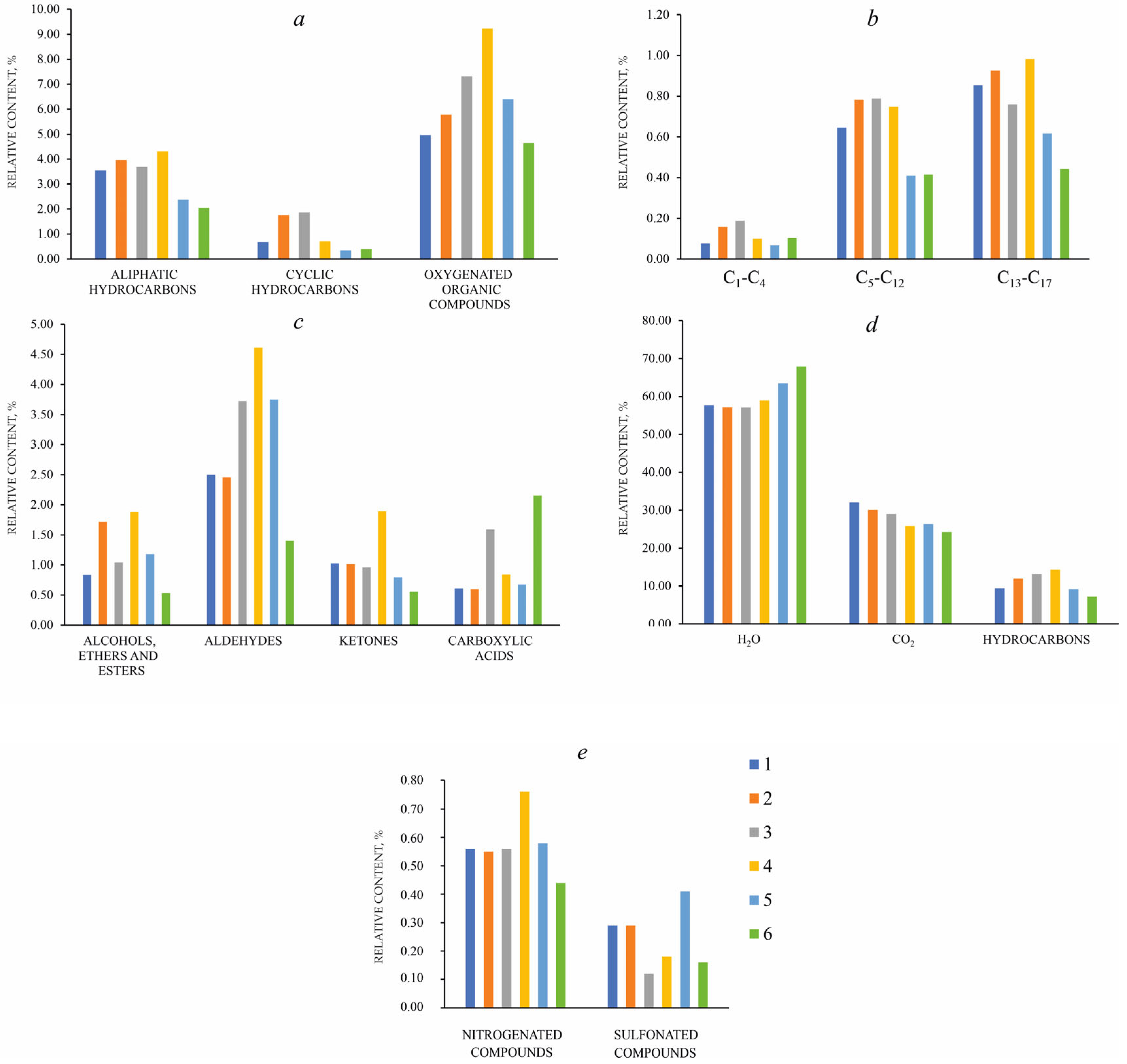
| Component | Cordierite from the Muzkol Complex | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| SiO2 | 49.44 | 49.53 | 49.40 | 49.34 | 49.37 | 49.56 | 49.46 | 49.49 | 49.54 | 49.44 | 49.55 | 49.44 |
| TiO2 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 |
| Al2O3 | 32.68 | 32.61 | 32.70 | 32.71 | 32.75 | 32.73 | 32.86 | 32.80 | 32.69 | 32.79 | 32.93 | 32.79 |
| FeO | 2.95 | 2.87 | 2.87 | 2.94 | 2.91 | 2.89 | 2.91 | 2.93 | 2.89 | 2.92 | 2.95 | 2.90 |
| MnO | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 |
| MgO | 12.21 | 12.38 | 12.39 | 12.38 | 12.49 | 12.56 | 12.52 | 12.55 | 12.53 | 12.46 | 12.38 | 12.40 |
| CaO | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 |
| Na2O | 0.40 | 0.35 | 0.43 | 0.39 | 0.43 | 0.40 | 0.44 | 0.42 | 0.39 | 0.38 | 0.38 | 0.38 |
| K2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Ʃ | 97.72 | 97.78 | 97.83 | 97.80 | 97.99 | 98.18 | 98.23 | 98.23 | 98.08 | 98.05 | 98.22 | 97.97 |
| Formula for 18 O atoms | ||||||||||||
| Si | 5.00 | 5.01 | 4.99 | 4.99 | 4.99 | 5.00 | 4.99 | 4.99 | 5.00 | 4.99 | 4.99 | 4.99 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 3.90 | 3.89 | 3.89 | 3.90 | 3.90 | 3.890 | 3.90 | 3.90 | 3.89 | 3.90 | 3.91 | 3.90 |
| Fe | 0.25 | 0.24 | 0.24 | 0.25 | 0.25 | 0.240 | 0.25 | 0.25 | 0.24 | 0.25 | 0.250 | 0.25 |
| Mn | 0.002 | 0.003 | 0.002 | 0.002 | 0.002 | 0.003 | 0.002 | 0.002 | 0.003 | 0.003 | 0.002 | 0.003 |
| Mg | 1.84 | 1.87 | 1.870 | 1.87 | 1.88 | 1.890 | 1.88 | 1.89 | 1.88 | 1.88 | 1.86 | 1.87 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.001 | 0.001 | 0.001 | 0.001 | 0.00 | 0.002 |
| Na | 0.078 | 0.069 | 0.084 | 0.077 | 0.084 | 0.078 | 0.085 | 0.082 | 0.077 | 0.074 | 0.074 | 0.075 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ʃ | 11.070 | 11.082 | 11.076 | 11.089 | 11.106 | 11.097 | 11.108 | 11.114 | 11.09 | 11.101 | 11.089 | 11.094 |
| XMg | 0.880 | 0.885 | 0.885 | 0.881 | 0.882 | 0.886 | 0.882 | 0.882 | 0.886 | 0.881 | 0.881 | 0.881 |
| Temperature | 672 | 690 | 660 | 674 | 660 | 672 | 657 | 664 | 674 | 680 | 680 | 678 |
| Name | MW | Sample Number (Numbering Corresponds to Figure 2) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Aliphatic hydrocarbons: | 3.54 | 3.96 | 3.69 | 4.31 | 2.37 | 2.05 | |
| Paraffins (CH4-C17H36) | 32–240 | 1.57 | 1.86 | 1.74 | 1.83 | 1.10 | 0.96 |
| Olefins (C2H2-C17H34) | 28–238 | 1.97 | 2.10 | 1.95 | 2.48 | 1.28 | 1.09 |
| Cyclic hydrocarbons: | 0.68 | 1.76 | 1.86 | 0.71 | 0.34 | 0.38 | |
| Naphthenes (C6H10-C8H14) | 82–110 | 0.06 | 0.17 | 0.11 | 0.07 | 0.03 | 0.03 |
| Arenes (C6H6-C18H28) | 78–204 | 0.62 | 1.58 | 1.73 | 0.62 | 0.29 | 0.35 |
| PAH (C10H8-C11H10) | 128–142 | <0.01 | 0.01 | 0.03 | 0.01 | 0.01 | <0.01 |
| Oxygenated organic compounds: | 4.97 | 5.78 | 7.32 | 9.23 | 6.40 | 4.65 | |
| Alcohols, Esters and ethers (CH4O-C14H18O4) | 32–250 | 0.84 | 1.72 | 1.04 | 1.88 | 1.18 | 0.53 |
| Aldehydes (CH2O-C15H30O) | 44–226 | 2.50 | 2.46 | 3.72 | 4.61 | 3.75 | 1.40 |
| Ketones (C3H6O- C15H30O) | 58–226 | 1.03 | 1.01 | 0.97 | 1.89 | 0.79 | 0.56 |
| Carboxylic acids (C2H4O2-C13H26O2) | 60–214 | 0.61 | 0.60 | 1.59 | 0.84 | 0.67 | 2.15 |
| Heterocyclic organic compounds: | 0.24 | 0.45 | 0.35 | 0.31 | 0.12 | 0.15 | |
| Dioxanes (C4H8O2) | 88 | 0.05 | 0.11 | 0.04 | 0.07 | 0.01 | 0.03 |
| Furans (C5H6O-C15H26O) | 82–222 | 0.19 | 0.34 | 0.31 | 0.24 | 0.11 | 0.12 |
| Nitrogenated compounds: (N2-C14H29NO) | 28–124 | 0.56 | 0.55 | 0.56 | 0.76 | 0.58 | 0.44 |
| Sulfonated compounds: (H2S-C10H16S) | 34–168 | 0.29 | 0.29 | 0.12 | 0.18 | 0.41 | 0.16 |
| Chlorinated compounds: (C5H8Cl2O2) | 170 | <0.01 | 0.13 | <0.01 | 0.02 | <0.01 | <0.01 |
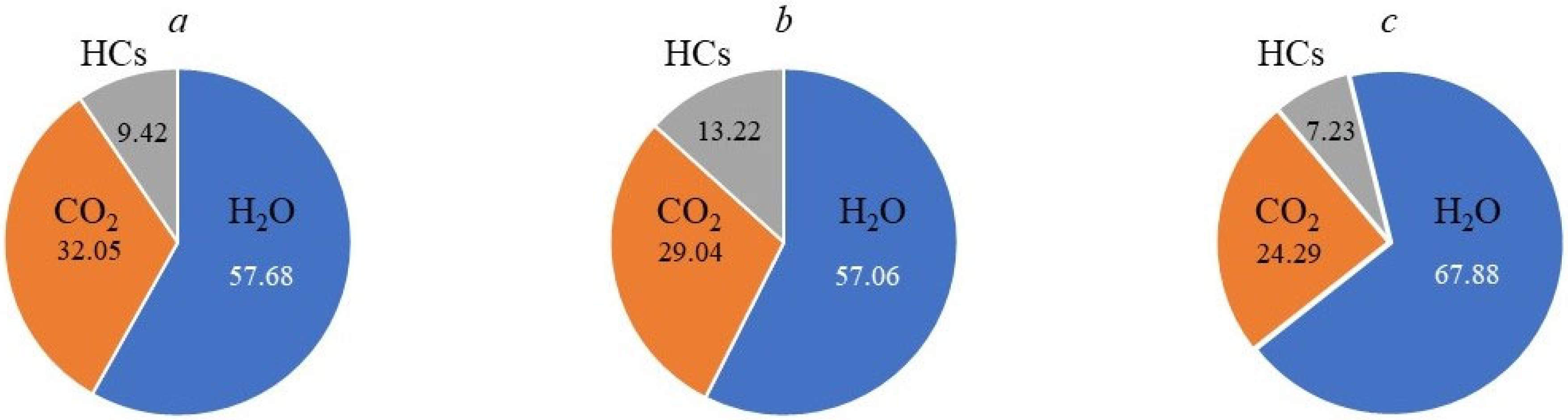
| CO2 | 44 | 32.05 | 30.12 | 29.04 | 25.80 | 26.31 | 24.29 |
| H2O | 18 | 57.68 | 57.09 | 57.06 | 58.91 | 63.49 | 67.88 |
| Total number of components | 165 | 169 | 166 | 170 | 172 | 165 | |
| CO2/(H2O + CO2) | 0.36 | 0.35 | 0.34 | 0.30 | 0.29 | 0.26 | |
| Alkanes/Alkenes | 0.80 | 0.89 | 0.89 | 0.74 | 0.86 | 0.88 | |
| Name | MW | Muz-1c | Muz-1k | Kukh-1c | Kukh-1k |
|---|---|---|---|---|---|
| Aliphatic hydrocarbons: | 3.54 | 2.05 | 1.27 | 3.44 | |
| Paraffins (CH4-C17H36) | 32–240 | 1.57 | 0.96 | 0.52 | 1.34 |
| Olefins (C2H2-C17H34) | 28–238 | 1.97 | 1.09 | 0.75 | 2.10 |
| Cyclic hydrocarbons: | 0.68 | 0.38 | 0.17 | 0.29 | |
| Naphthenes (C6H10-C8H14) | 82–110 | 0.06 | 0.03 | 0.02 | 0.04 |
| Arenes (C6H6-C18H28) | 78–204 | 0.62 | 0.35 | 0.14 | 0.24 |
| PAH (C10H8-C11H10) | 128–142 | <0.01 | <0.01 | 0.01 | 0.01 |
| Oxygenated organic compounds: | 4.97 | 4.65 | 3.70 | 6.68 | |
| Alcohols, Esters and ethers (CH4O-C14H18O4) | 32–250 | 0.84 | 0.53 | 0.60 | 1.04 |
| Aldehydes (CH2O-C15H30O) | 44–226 | 2.50 | 1.40 | 2.22 | 3.75 |
| Ketones (C3H6O-C15H30O) | 58–226 | 1.03 | 0.56 | 0.32 | 0.89 |
| Carboxylic acids (C2H4O2-C13H26O2) | 60–214 | 0.61 | 2.15 | 0.56 | 1.00 |
| Heterocyclic organic compounds: | 0.24 | 0.15 | 0.03 | 0.05 | |
| Dioxanes (C4H8O2) | 88 | 0.05 | 0.03 | 0.01 | 0.01 |
| Furans (ethers) (C5H6O-C15H26O) | 82–222 | 0.19 | 0.12 | 0.02 | 0.04 |
| Nitrogenated compounds: (N2-C14H29NO) | 28–124 | 0.56 | 0.44 | 0.11 | 0.40 |
| Sulfonated compounds: (H2S-C10H16S) | 34–168 | 0.29 | 0.16 | 0.02 | 0.04 |
| Chlorinated compounds: (C5H8Cl2O2, C16H33Cl) | 170–240 | <0.01 | <0.01 | 0.03 | 0.06 |
| CO2 | 44 | 32.05 | 24.29 | 5.5 | 23.50 |
| H2O | 18 | 57.68 | 67.88 | 89.2 | 65.60 |
| Total number of components | 165 | 165 | 166 | 170 | |
| CO2/(H2O + CO2) | 0.36 | 0.26 | 0.06 | 0.26 | |
| Alkanes/Alkenes | 0.80 | 0.88 | 0.69 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zatolokina, K.I.; Tomilenko, A.A.; Bul’bak, T.A. Fluid Components in Cordierite from the Rocks of Epidote-Amphibole Facies of the Muzkol Metamorphic Complex, Tajikistan: Pyrolysis-Free GC-MS Data. Minerals 2023, 13, 323. https://doi.org/10.3390/min13030323
Zatolokina KI, Tomilenko AA, Bul’bak TA. Fluid Components in Cordierite from the Rocks of Epidote-Amphibole Facies of the Muzkol Metamorphic Complex, Tajikistan: Pyrolysis-Free GC-MS Data. Minerals. 2023; 13(3):323. https://doi.org/10.3390/min13030323
Chicago/Turabian StyleZatolokina, Ksenia Igorevna, Anatoly Alexeyevich Tomilenko, and Taras Alexandrovich Bul’bak. 2023. "Fluid Components in Cordierite from the Rocks of Epidote-Amphibole Facies of the Muzkol Metamorphic Complex, Tajikistan: Pyrolysis-Free GC-MS Data" Minerals 13, no. 3: 323. https://doi.org/10.3390/min13030323
APA StyleZatolokina, K. I., Tomilenko, A. A., & Bul’bak, T. A. (2023). Fluid Components in Cordierite from the Rocks of Epidote-Amphibole Facies of the Muzkol Metamorphic Complex, Tajikistan: Pyrolysis-Free GC-MS Data. Minerals, 13(3), 323. https://doi.org/10.3390/min13030323





