1. Introduction
The amount of water used for mineral processing varies significantly within the industry. Based on the data provided by mining companies, water used for mining and mineral processing fluctuates between 0.3 and 6.3 m
3 per ton of ore processed [
1]. The make-up water consumption depends on factors such as the type ore being processed and the geographical location, as in some cases water must be transported to site from a long distance [
2,
3]. In some cases, the regulations for tailings management limit the freshwater intake [
4]. However, the freshwater consumption can be significantly decreased by recirculating the process water.
When the water circulation rate at a concentrator plant is increased, impurities accumulate in the process water and begin influencing on the metallurgical performance. For instance, the Kevitsa Ni-Cu-PGE concentrator plant in northern Finland has been experiencing a measurable impact of water quality on the flotation process. At Kevitsa, more than 95% of the process water is recycled and over time the effect of recycled water has become more noticeable. According to the plant experience, the quality of the process water in copper flotation circuit does not impact much on copper recovery, but for the nickel flotation circuit, the increase in impurities in the nickel circuit process water decreases the nickel recoveries. Recycled water from nickel thickener overflow accounts for less than 10% of the water added to the process, but its quality still has a negative impact on the nickel recovery in flotation and it needs to be cleaned before adding it back to the circuit to minimize the nickel recovery losses. [
5,
6]
The successful separation of pentlandite from silicate minerals depends on several factors that are already discussed in literature. Firstly, the serpentine group minerals, generally described by the formula (Mg, Fe)3Si2O5(OH)4, have been found to affect nickel recovery negatively by forming a hydrophilic slime coating on the pentlandite grain surfaces because of opposite surface potentials so that serpentine group minerals are positively charged, and surfaces of pentlandite grains are negatively charged [
7,
8,
9,
10,
11]. Micrographs from scanning electron microscopy study have revealed that fine serpentine fibers coat pentlandite mineral surfaces and interfere with the collector adsorption [
12]. Secondly, Cu, Ni and Fe metal ions form metal hydroxides at alkaline pH in process water that may adsorb on the surfaces of valuable minerals, make them less hydrophobic and decrease the recovery [
9,
13,
14]. Thirdly, thiosulphate ions have been found to affect negatively on collector adsorption on sulphide minerals which may lead to decreased pentlandite recovery [
5,
6]. All these forms of impurities and harmful solids have been identified in Kevitsa nickel concentrate thickener overflow water.
Dissolved air flotation (DAF) has been widely used in industrial scale water treatment since the mid-1990s, with the main applications being at drinking water plants [
15]. The separation of impurities in a DAF unit is based on microbubbles with a diameter less than 100 µm. The bubbles are generated by dissolving air into water under pressure, and the dissolution of air into water follows Henry’s law. The pressurized water is then released into a flotation basin. The impurities in the water attach to the air bubbles that raise to the top of the DAF reactor, while the clarified water is recovered from the bottom of the basin. DAF treatment can remove various type of impurities from water, including colloids (between 1 nm and 0.1 µm), fine (2.5 µm or less) and ultrafine (0.1 µm or less) particles, precipitates, and certain ions. Coagulant and flocculant are needed to collect the particles into flocs that can attach to the air bubbles. In earlier studies, the optimal particle floc size for removal has been on average between 15 and 30 µm [
16,
17]. However, the weight of the floc also has an impact on the recovery rate and the floc weight varies depending on the application and on the impurities being removed. At mineral processing plants, the impurities accumulating in the process water originate mainly from the ore [
18,
19,
20]. Some water treatment trials have shown that DAF can remove more than 90% of suspended solids [
21]. The advantage of DAF compared to other treatment methods, such as filtration, is that it can handle varying loads of solids in the water stream being treated. Therefore, DAF is a viable option for treating the internal streams at mineral processing plants where disturbances in process conditions often take place.
To implement DAF, water treatment test work on site is needed to avoid any changes in the composition of process water samples during transportation. It has previously been shown that the water composition may change rapidly [
22]. In earlier studies, the use of DAF-treated processed water has improved the flotation performance for apatite [
23]. Microflotation together with microfiltration has also been used for the removal of copper from mine effluent water [
24]. The challenges in pentlandite recovery can be overcome to some extent by optimizing the collector and depressant reagent dosages in flotation [
25,
26]. However, treatment of the process water can also help to improve the recovery of valuable minerals. To be cost efficient it is often more practical to selectively treat internal water streams instead of combining all process water streams before treatment. In this study, the effect of purifying the nickel concentrate thickener overflow water with DAF on the metallurgical performance of nickel flotation was investigated. The target was to find out whether investing in DAF treatment would be technically and economically feasible. The economical calculations show that even a slight improvement in the metallurgical performance pays back the investment.
2. Materials and Methods
The flotation and DAF treatment tests were carried out at Boliden’s Kevitsa concentrator plant. Kevitsa is a low-grade Ni-Cu-PGE deposit, where nickel is carried mainly by pentlandite and copper by chalcopyrite. Platinum group metals are mainly associated with nickel minerals, while gold reports to the copper concentrate. Pyroxene group minerals are the main non-sulphide gangue in the ore. The grade of nickel carried by sulphide minerals is on average 0.22%. The average mineral composition of the Kevitsa ore is shown in
Table 1 [
27].
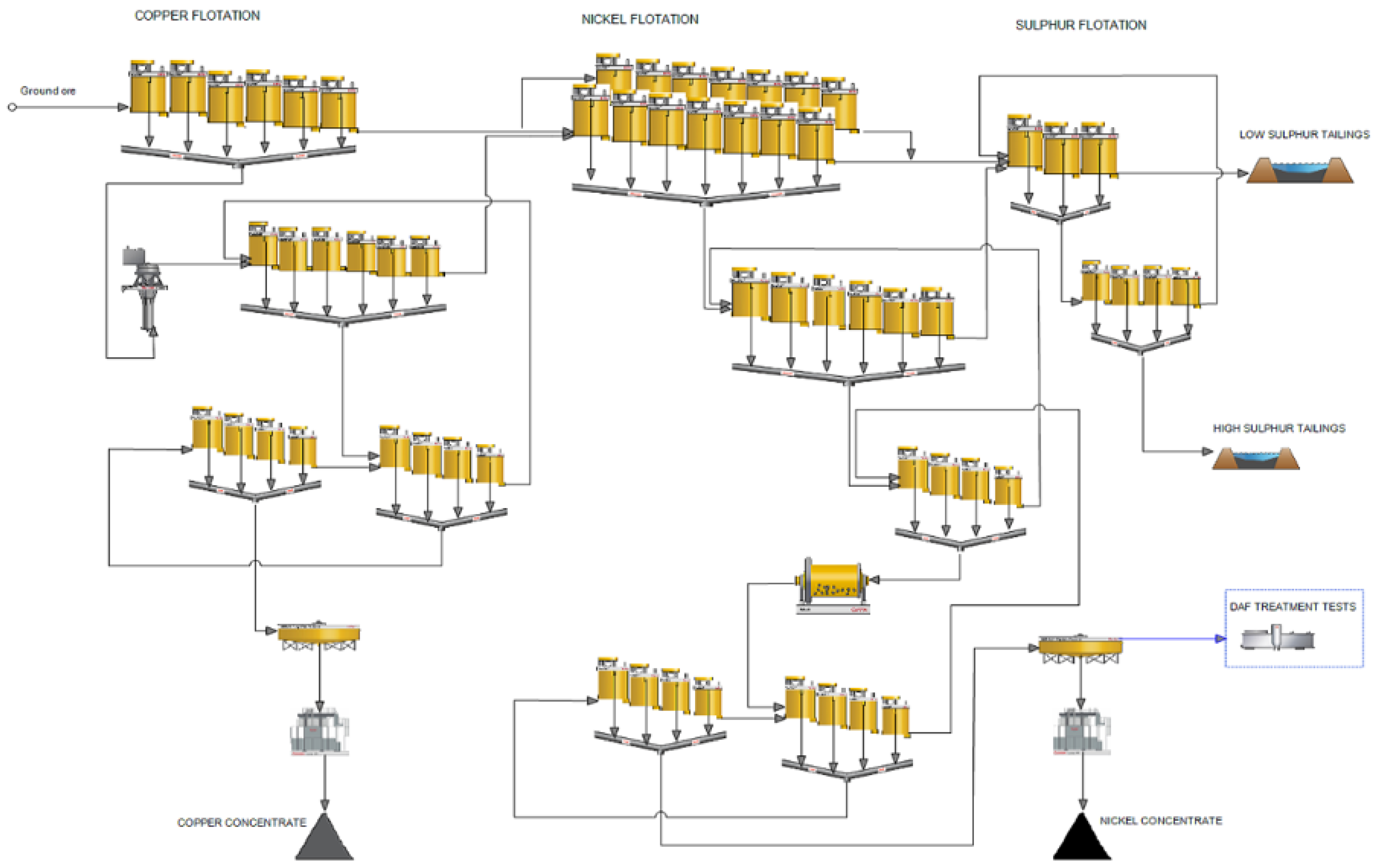
The flowsheet of the Kevitsa flotation circuit is shown in
Figure 1. Copper and nickel minerals are separated by sequential flotation. Copper is floated first, and the copper scavenger tails is the feed to nickel flotation. Finally, pyrrhotite and other remaining sulphur-bearing minerals are floated and sent to the high sulphur tailings pond. The water samples used in the test work were taken from the plant nickel concentrate thickener overflow. Currently the overflow water is circulated in the process as such. [
27]
2.1. Laboratory Flotation Tests
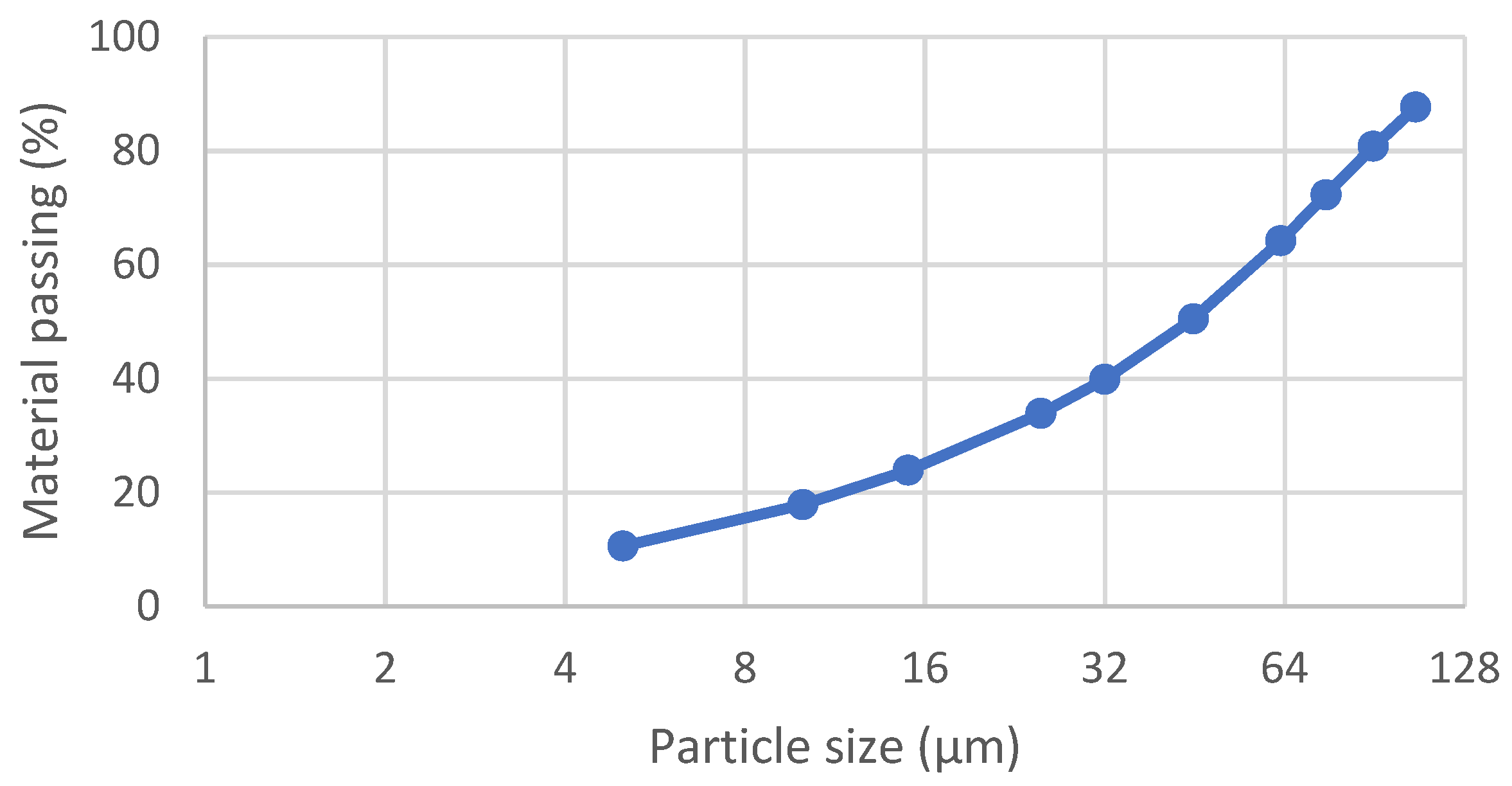
The Kevitsa Cu-Ni ore was used as feed material in the flotation tests carried out at the site laboratory. The sample was collected from the products of secondary and tertiary crushers in the concentrator process. The original sample size was less than 10mm, and it was screened at the site laboratory using a 4 mm screen. The 4 mm material was homogenized and split into 1 kg plastic bags using rotary splitter. Each flotation feed batch was ground in a laboratory grinding mill right before flotation to avoid excess oxidation of mineral surfaces. The target grind size was 75% passing 75 µm. The particle size distribution after grinding is shown in
Table 2 and
Figure 2.
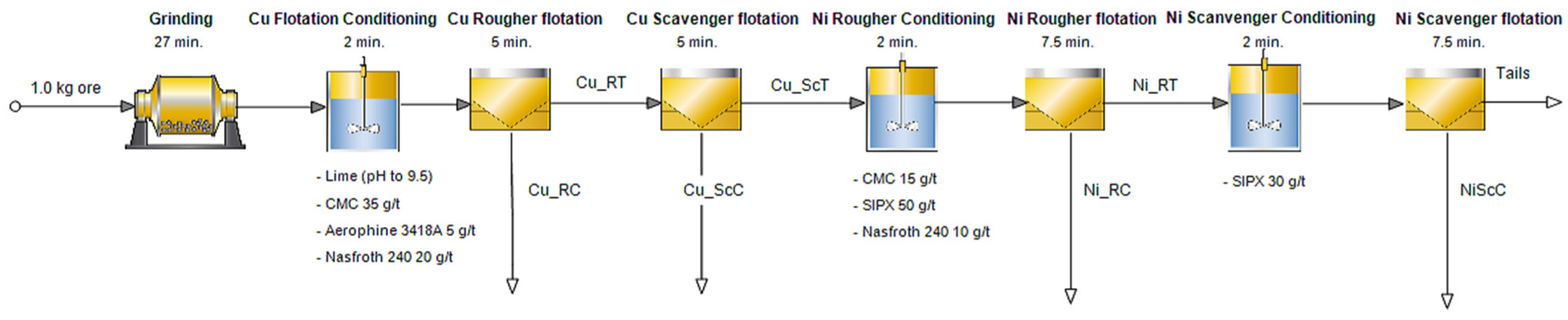
The flotation tests were carried out shortly after taking the water sample. The laboratory flotation test flowsheet is shown in
Figure 3.
Before starting the laboratory flotation test, process water pH was adjusted to 9.5 by lime addition. All tests were carried out at a temperature of 20 °C. Prior to flotation, the test feed batch was ground in a laboratory mill at 70% solids for 27 min. The tests were performed using an Outotec GTK LabCell flotation machine with a 2 L flotation cell. The rotor mixing speed was 1300 rpm and air feed rate 5 NL/min. The slurry was first conditioned for two minutes with 35 g/t CMC, 5 g/t Aerophine 3418A and 20 g/t Nasfroth 240 reagents. After that, copper rougher-scavenger flotation was carried out for 10 min. Copper scavenger tailings were conditioned again with 15 g/t CMC, 50 g/t SIPX and 10 g/t Nasfroth 240 reagents for two minutes before recovering the nickel rougher concentrate during 7.5 min of flotation. After another conditioning of slurry with 30 g/t SIPX for two minutes, nickel scavenger flotation was performed for 7.5 min.
2.2. DAF Treatment
The DAF treatment was conducted in a FlooDaf B5 container test unit. The pilot test system was equipped with a chemical dosing system, a compressor, and a local PLC (programmable logic control) with online monitoring manufactured by Metso Outotec. The air to generate bubbles in the size range of 50–100 μm was fed to the system at 6 bar pressure. The surface area of the rectangular flotation tank was 5 m2. An inclined baffle was fixed 60° to the horizontal between the contact and separation zones to reduce turbulence and to elevate the bubble-floc agglomerates towards the surface of the tank. The capacity of the standard FlooDaf B5 unit is up to 35 m3/h.
After optimizing the test conditions, the chemical dosages used were 90 ppm for cationic coagulant PAX XL-100 and 1.3 ppm for anionic flocculant Superfloc A120 for the nickel concentrate thickener overflow. pH was raised to 9 by using Ca(OH)2 at a dosing rate of 10 L/h as 20% milk of lime. The water feed flow rate to DAF treatment was 6.6 m3/h.
2.3. Analysis Methods
The non-treated and purified water samples were analysed after sampling on site, and for certain components at Metso Outotec Research Center, as specified in
Table 3. ICP-OES (inductively coupled plasma optical emission spectrometer) was applied. The analyses were carried out both after filtration with a 0.45 µm filter and after microwave assisted dissolving. The analysis after filtration gives the amount of concentration of the impurity dissolved in the water sample. The microwave assisted dissolving shows both colloidal and dissolved forms of the analysed element. The concentrations of dissolved Cu, Ni, Fe and SO
42− were determined on-site by UV-Vis (ultraviolet-visible) spectrophotometry utilizing cuvette tests specific for each component and concentration range from the Hach company.
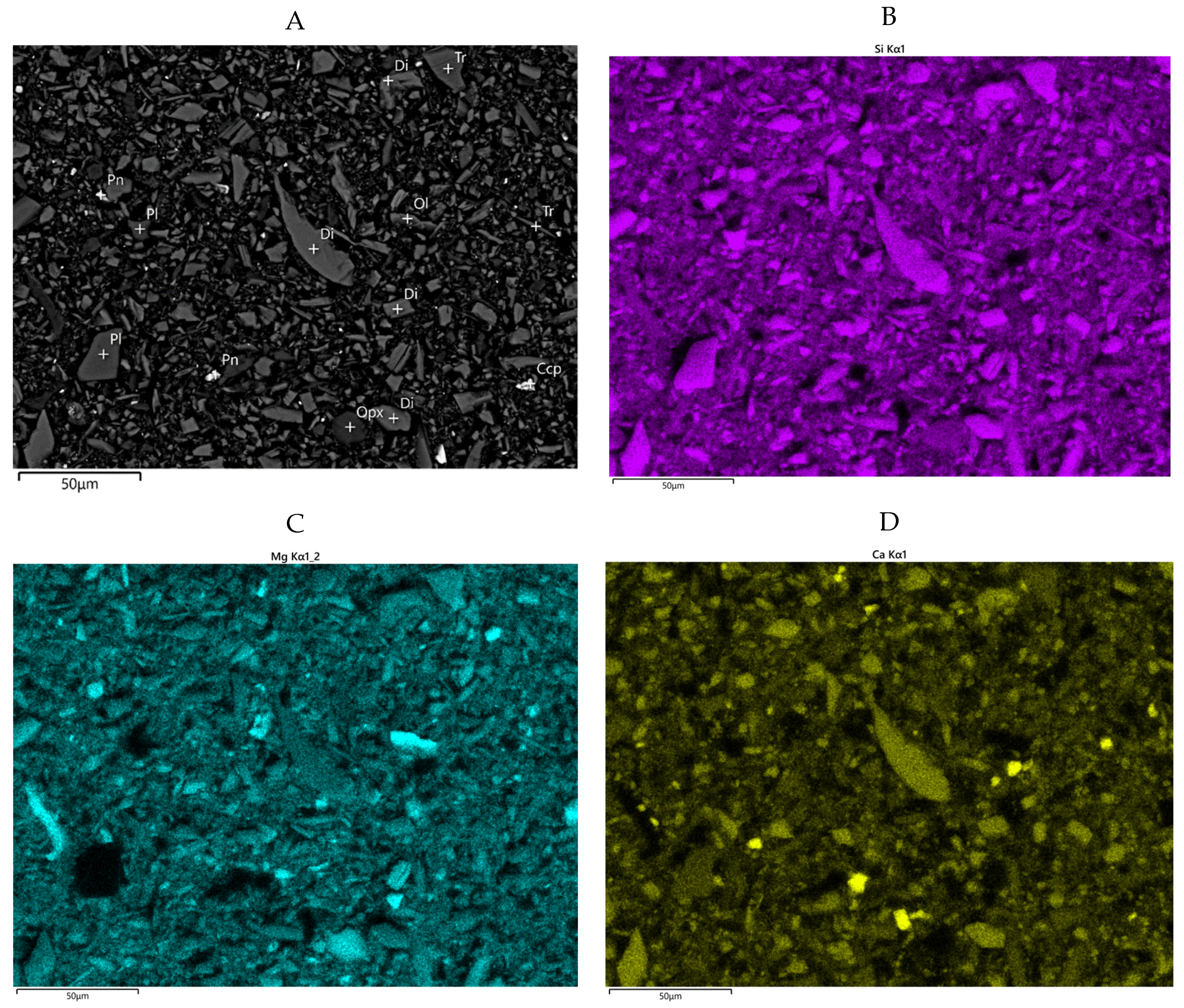
The solids removed by the DAF treatment from the Ni concentrate thickener overflow water were characterized at Metso Outotec Research Center. The main elements of the sample were analysed using ICP-OES after total dissolution. Nickel and iron content after bromine-methanol dissolution were analysed using ICP-OES to differentiate between Ni and Fe content in sulphide and silicate minerals. Sulphur and carbon contents were measured using an Eltra CS-2000 automatic analyser. The quantity of silica was analysed colorimetrically using a Hach DR 5000 UV-Vis spectrophotometer. The ferromagnetic material was analyzed by using Satmagan analyser. Polished resin section was prepared for mineralogical studies using a JEOL JSM-6490LV and 7000F scanning electron microscopes (SEM) equipped with an Oxford Instruments energy dispersive spectrometer (EDS). The imaging and EDS analyses were performed under routine conditions using 20 kV acceleration voltage and 1 nA beam current. Mineral quantification was performed using HSC Chemistry® software.
Student’s t-test was used to compare the mean values of the flotation test results. The analysis was performed with Microsoft Excel.
4. Conclusions
It is known that pentlandite flotation is sensitive to fine solids. The target of the study was to reduce the overall solids load in the circulating thickener overflow process water. The results indicate that DAF treatment efficiently improves Ni concentrate thickener OF quality and thereby the nickel recovery in flotation. DAF removes silicate minerals, metal hydroxides and thiosulphate ions, each of them being individually harmful for nickel recovery based on earlier studies [
7,
8,
9,
10,
11,
12,
13,
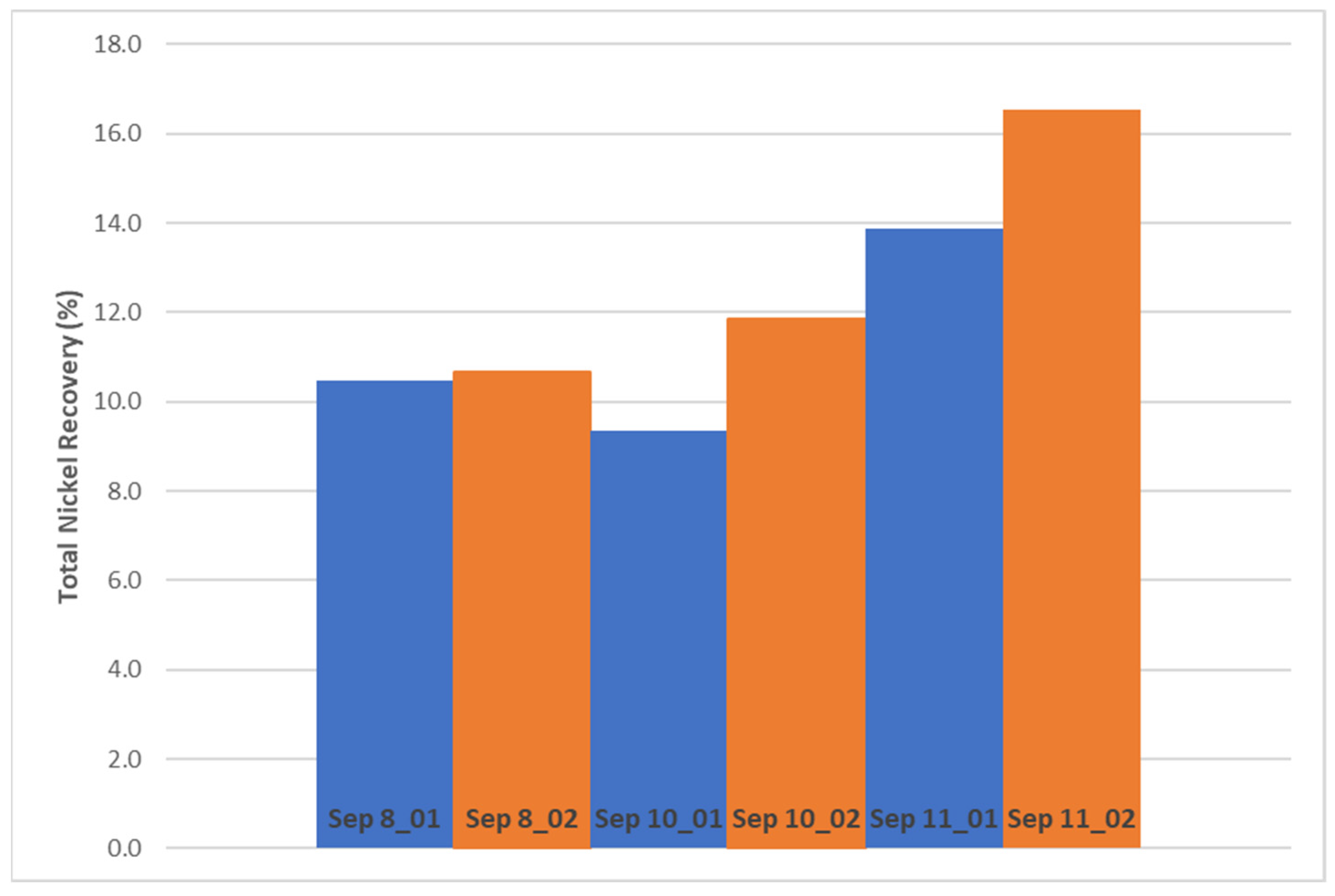
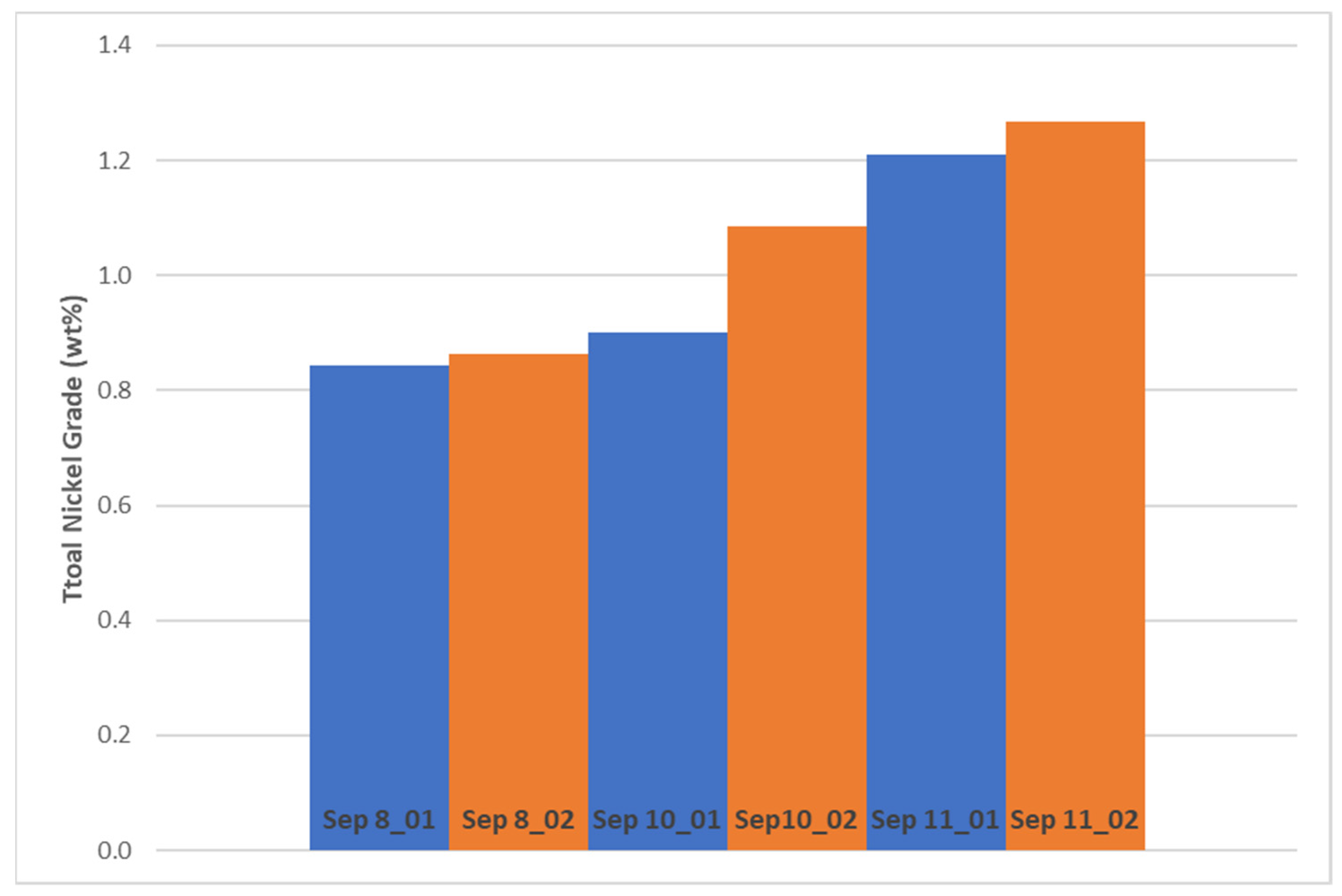
14]. The total nickel recovery to the Kevitsa Ni rougher-scavenger concentrate in laboratory scale increased by 2.6 percentage points at 0.06 percentage points increase in Ni grade when carrying out DAF treatment at optimized conditions compared to non-treated water. This is a significant improvement in the metallurgical performance of the flotation circuit. Improved nickel recovery was seen also with other corresponding test pairs of the same campaign. Statistical testing indicates that there is a 92% probability that the observed increase in Ni recovery is due to DAF treatment.
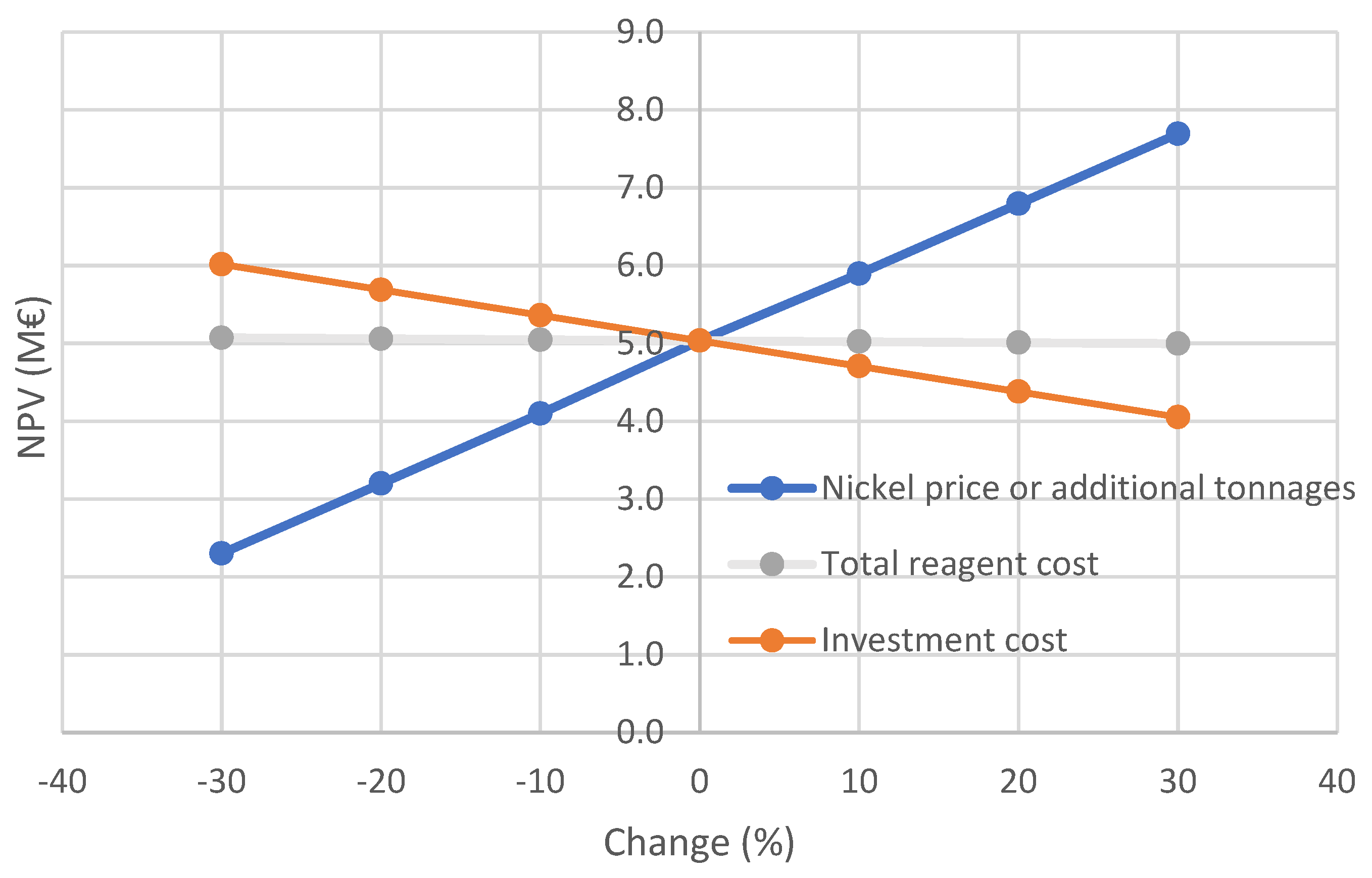
Economic calculations indicate that even with conservative assumptions on the nickel price and additional concentrate tonnages the net present value over ten years is positive. Consequently, DAF would be a feasible and profitable investment for the concentrator plant. It aids the closing of the plant water circuits and the saving of fresh water. As the nickel thickener overflow comprises less than 10% of the water added to the Kevitsa process, it would be worth studying if treating other process water streams could bring additional improvements on the flotation performance. The results can be seen to be encouraging also for other nickel concentrators to test improving flotation performance by treating the circulating water streams using DAF, as the harmful effects of impure process water on pentlandite recovery have been shown widely.
To further improve the reliability of the results, and to investigate the effect of each impurity in the process water on the nickel recovery in flotation, a pilot campaign of several months with automated flocculant and coagulant dosing system would be needed.