Abstract
In recent years, most of the studies have been adapted to determine the optimum conditions for the flotation of very fine minerals. In this context, besides parameters such as particle size, morphology, and pH, the effects of frother type and its concentration present a very significant role in optimizing the flotation conditions. Therefore, the effects of froth stability during flotation can be considered one of the most important issues. Considering that knowledge in mind, in this study, the foamability and froth decay rate of six frothers (PPG200, PPG400, PPG600, BTEG, BTPG, and BDPG) having different molecular weights but similar polyglycol structures were investigated. In addition, methyl isobutyl carbinol (MIBC) which is a well-known frother type in the industry was also used as a reference. Additionally, a series of tests were also performed in the presence of collectors (Dodecylamine hydrochloride, DAH, and sodium oleate, NaOL) + frother mixtures to mimic the flotation conditions. The results of these tests indicated that the bubble size became finer at even low concentrations of PPG600 and PPG400 frothers. Following that, a significant decrease in bubble size was also found for the collector + frother mixtures system regardless of the concentration of the frothers.
1. Introduction
Flotation of fine-size minerals has become particularly important in recent years, as advances in milling have allowed the economical use of low-grade ore deposits [1]. Also, mineral wastes, mostly composed of fine particles, are becoming a critical problem for the mining industry due to environmental concerns (for instance, acid mine drainage) [2,3]. Accordingly, the flotation of fine particles depends on the recovery of valuable hydrophobic mineral particles from the pulp by adhering to fine-sized air bubbles. Thus, low recovery values of fine particles by flotation can be attributed to the low probability of bubble-particle collision, which decreases with decreasing particle size [4]. However, bubble-particle interactions such as electrostatic and hydrophobic forces are important in determining the selectivity of the flotation separation [5]. Frothers and surface–active collectors are often used for the flotation of different minerals [6,7]. Both of them often exhibit synergy in particle recovery but the possible synergetic effects on the froth stability have not been studied.
Derjaguin et al. (1984) [8] formally proposed that the flotation general rate constant is equal to the product of three possibilities or efficiencies: Bubble-particle collision, adhesion, and stability. The flotation rate constant, and hence the flotation recovery, is a function of the probability of these subprocesses [9]. Forty years after Derjaguin and Dukhin’s proposal, Miettinen et al. (2010) [10] published a review on the flotation limits of fine particles, showing the importance of key parameters such as bubble size, particle aggregation, and flow conditions in fine particle flotation. Moreover, in a recent paper of ours [5] we proved theoretically that the collision rate between the fine particles and the bubbles is sufficient [11,12] to sustain high recovery if all of the collisions are effective. In addition, there exists a thermodynamic low size limit for the flotation of fine hydrophobized particles (θ = 80°). This limit is in the range of 0.15–0.4 µm) and below it the capture of the fine particles is impossible. Particles with sizes in the range of 0.4–2 µm can be floated but with a small flotation rate due to the very small level of exceeding the total push force over the resistance force for the formation of a three-phase contact line (TPCL). Such particles with sizes above 2 µm are floated due to the cavitation of dissolved gases. So, there exist two ways to float fine particles with high recovery for short time—(i) to initiate electrostatic attraction between bubbles and particles; (ii) to hydrophobize them significantly (θ > 90°). The second way is more powerful.
Nevertheless, improving the recovery of fine-sized particles (−20 μm) has been a long-standing goal in the mineral processing industry. The relationship between particle size and buoyancy is presented in detail by research conducted by Gaudin et al. (1931) [13], which shows that fine particles are more difficult to recover than medium-size particles. As the particle size decreases, the effectiveness of the bubble–particle collisions reduces. Introducing fine bubbles increases the fine particle recovery due to (i) increasing the number of bubbles in the flotation reactor, thus increasing the collision rate; (ii) larger capillary pressure during the bubble–particle collision due to the increased pressure inside of the fine bubbles. The latter factor is the powerful driving force for the capture of the particles by the bubbles.
Such methods for the production of small bubbles have been the focus of many studies including dissolved air flotation [14] and pico bubbles [15]. Because the problem of dealing with fine particles has preoccupied mineral flotation engineers for more than a century. This issue is even more important today, driven by the energy and water minimization requirements as well as the mineralogy and liberation requirements of many ore deposits. Considering that knowledge in mind, it is the objective of this study to systematically determine the froth stability characteristics of seven commercial frothers by the “Dynamic Froth Analysis” method as a function of frother and collector concentration.
2. Materials and Methods
2.1. Materials
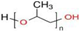
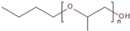
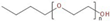
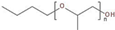
In this study, the foamability and the rate of froth decay for the selected frothers obtained from BASF, Ludwigshafen, Germany, namely, polypropylene glycols (PPG200, 400, and 600), tri propylene glycol (BTPG), triethylene glycol (BTEG), dipropylene glycol (BDPG), and isobutyl carbinol (MIBC) was determined using a dynamic foam analyzer. In addition to frothers, the effects of collectors, namely, dodecylamine hydrochloride (DAH, >98% purity, Acros Organics) and sodium oleate (NaOL, >99% purity, Sigma-Aldrich) were also investigated as collector + frother mixtures in certain ratios (see Table 1). Some chemical and physical properties of frothers and collectors are presented in Table 1 and Table 2, respectively.

Table 1.
Some chemical and physical properties of frothers.

Table 2.
Physical and chemical properties of collectors.
2.2. Methods
2.2.1. Dynamic Foam Stability Experiments
The foamability experiments were carried out using the KRUSS dynamic foam analyzer DFA100 (KRUSS GmbH, Hamburg Germany) shown in Figure 1. This device measures the kinetics of foam formation, and froth decay. An optical sensor is attached to the foam column and measures the discharge of liquid (foam drainage) from the foam lamellas and the amount of foam produced. The foam generator assembly is connected to a computer serving as a data collection and monitoring unit. All measurements, result evaluation, and analysis are controlled by the installed foam analysis software. Statistical accuracy was ensured by measuring the results at least three times.

Figure 1.
Set up for dynamic foam stability experiments using the Dynamic foam analyzer (DFA100).
Geldenhuys et al. (2018) [16] reported that pore opening influences dynamic foam stability, but the results should be comparable to each other as the pore opening is constant for all suspensions tested. The foam is then created by bubbling air through a ceramic frit at an airflow rate for a constant time (standard conditions). Its height is recorded to evaluate the dynamic parameters of foaming ability, the rate of foam decay, and time-dependent bubble size distribution.
For the dynamic foam stability experiments, 10,000 ppm (1 g/100 mL) stock solutions were first prepared for each frother. Then, standard solutions at CCC values for each frother were prepared. All these solutions were prepared by mixing distilled water (23° ± 1) at room temperature and 500 rpm mixing speed for 5 min. As a collector, 7 solutions were prepared in the concentration range of 1.10−5–1.10−3 mol/L DAH. Similar to DAH, 7 concentrations of NaOL between 1.10−5 M–1.10−3 mol/L were prepared with distilled water (23° ± 1) as 100 mL for NaOL. In addition to CCC of frothers with the collectors 5.10−5 mol/L mix system. Concentrations of 1.10−5–1.10−3 mol/L of collectors were used in the presence of frother PPG600 3 ppm. The experiments were carried out depending on the mixed solution concentrations of the collectors and frothers given in Table A1, Table A2, Table A3, Table A4, Table A5, Table A6 and Table A7.
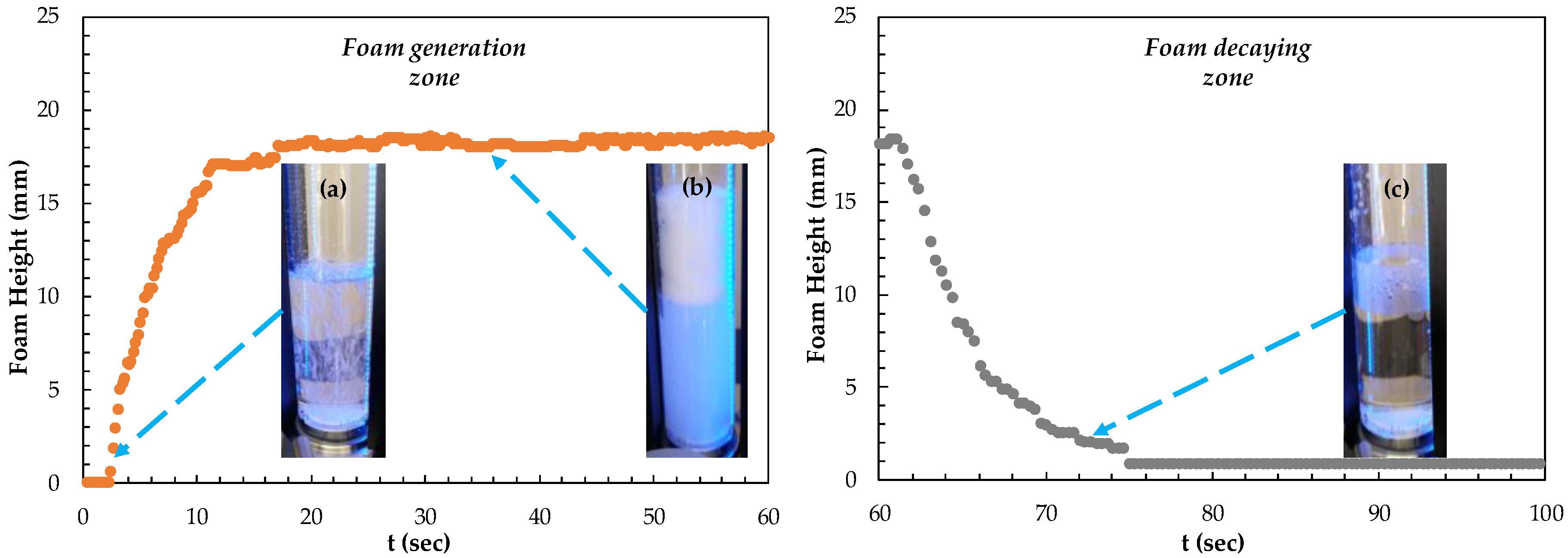
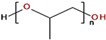
In the experiments, 100 mL of solutions is first transferred into a tempered glass column with a diameter of 40 mm and a height of 250 mm. Then, when the device is started, the air is distributed through a porous filter plate (12–25 μm) in the system (from electronic gas flow control) at an airflow rate of 0.2 L/min (Figure 2a), resulting in the desired foam formation. The foam formation time is set at 60 s and the foam height in the system is measured during this time (Figure 2b). Then, the gas flow is stopped automatically at 60 s, and the foam degradation is measured for 40 s (Figure 2c).
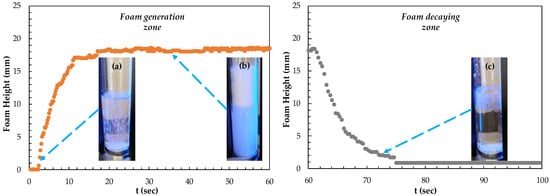
Figure 2.
Dynamic foam stability experiments (a) foam formation (b) stable foam (c) foam decaying.
Our previous study indicated that there was not much difference between the air and N2 gases in the experiments performed with a 10 ppm concentration of PPG600 frother depending on the time in terms of foamability [17]. For this reason, the air was used in the measurements.
2.2.2. Modeling Studies
The foaminess and foam decay curves were processed, thus calculating the Bickerman unit of foaminess [18] and foam production [19]. The Bickerman unit of foaminess is the ratio between the height of the stationary froth column and the superficial gas flow given through the porous bottom:
where is the stationary height of the foam, while is the gas flow rate (cm/s).
The foam production is the ratio between the Bickerman unit of foaminess and the initial speed of froth decay after stopping the gas flow:
where is the initial linear foam decay rate.
Each experimental test on foamability and foam decay was processed, thus obtaining the Bickerman unit of foaminess, the initial speed of froth decay, and the foam production. It will be shown here what these three parameters look like for one of the cases and after that, it will be operated only with foam production as a basic result of this study.
3. Results and Discussion
Frothers are used to facilitate the dispersion of air into fine-sized bubbles and to stabilize the foam. And, as reported in our previous study that the strength of frothers can be determined by either the dynamic foamability index (DFI) or their critical coalescence concentration values [17,20].
For this aim, a dynamic foam analyzer (DFA) is used to evaluate the foamability and the rate of foam decay. The foam height and lifetime of a bubble are quantitative parameters that represent dynamic stability. In the DFA technique, the foam rises in a column, and the height is measured as a function of time. The maximum height can be well correlated with foamability [21,22]. The flotation process used in ore preparation depends on the formation of a carefully controlled and stable foam. It also controls the amount of water recovered and thus the amount of mechanical transport. Measuring the foam height for a given frother dosage at a given airflow rate in a metering cylinder is a simple, semi-quantitative method of estimating the frothing power of a frother. Frothers adsorb at the gas-liquid interface and change the interface properties. Surfactants play an important role in foam stability. The effect of surfactants on foam behavior is not clear. In flotation, the foam must be dynamically stable as the foam is formed, that is the foam must be stable to bubble coalescence. However, Surfactants play an important role in foam stability. Unstability is undesirable in processes such as pumping or filtration. But it should be noted that both foamability and foam decay are mainly controlled by the particles rather than the surfactant molecules [23,24].
3.1. Dynamic Foam Stability Experiments
3.1.1. Foam Stability and Foam Decaying of Frothers
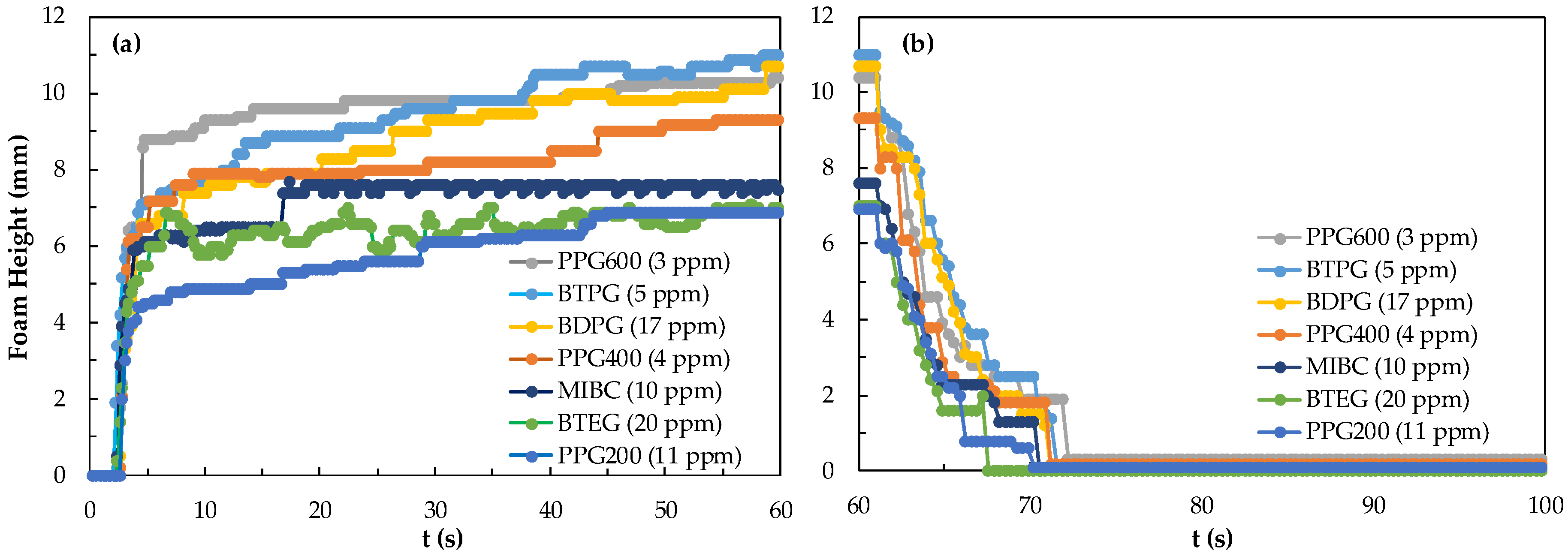
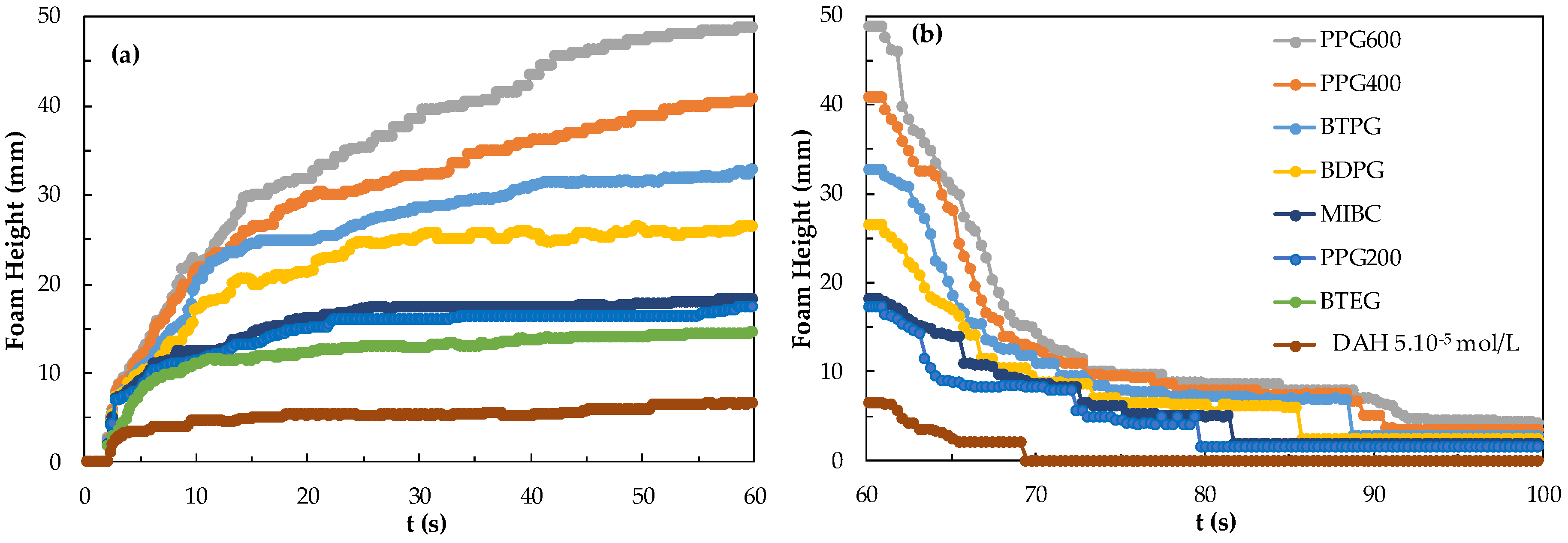
First, the differences in foamability and foam decay at different concentrations of frothers were investigated. In Figure 3, the results of foamability and foam decaying are shown separately in the critical coalescence concentrations (CCC) values for each frother. The values for these frothers were both experimentally determined and theoretically calculated in our previous studies [25,26].
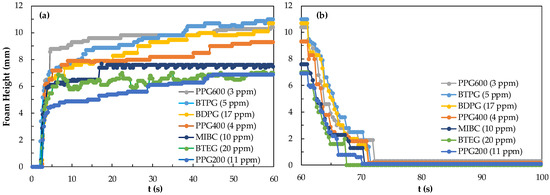
Figure 3.
(a) Foamability and (b) foam decay of frothers at their CCC values.
A unique relationship was found between foam decay (FD), Mw (Molecular weight), and foamability of the homologous foaming series. However, when the functional groups of the molecule are changed, different results were obtained. Therefore, seven foams with specific values of CCC were analyzed. According to the CCC values of seven foams obtained from this study, foaming agents should be considered as PPG, BTPG, BDPG, MIBC, and BTEG depending on the homologous series with increasing carbon atoms, taking into account the type and location of functional groups. As seen in Figure 3, the highest foamability measured as 11 mm and lowest foam decay measured as 6.8 mm were obtained with PPG600 at CCC values, while the lowest foamability and foam decaying values measured as 0 mm were obtained with PPG200.
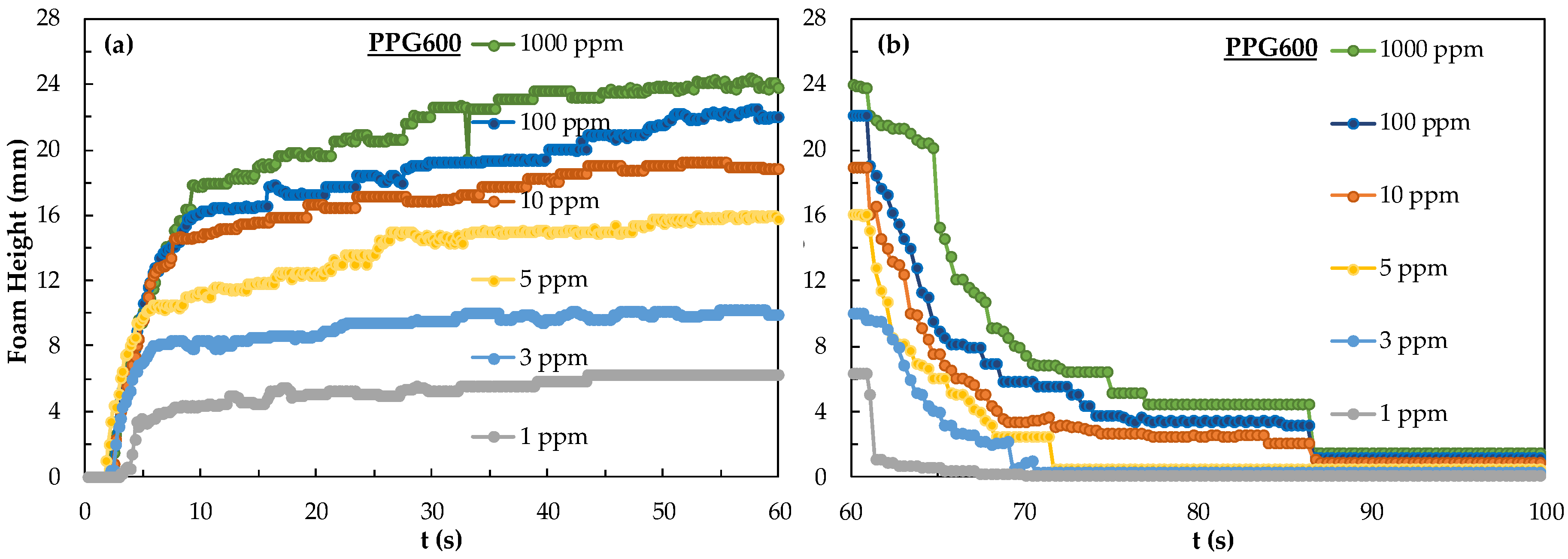
In Figure 4, the foamability and foam decay of PPG600 frother from 1 ppm to 1000 ppm were examined. The results of this series of tests showed that no significant difference could be obtained for concentrations over 10 ppm. Although foam height at the CCC value of 3 ppm at 60 s is 10 mm, the foam decay at and below 3 ppm is rather sharp indicating the need for higher frother concentrations to maintain relatively acceptable foam stabilities.
Figure 4.
(a) Foamability and (b) foam decay of PPG600 frother at different concentrations.
3.1.2. Foam Stability and Foam Decaying of Collectors
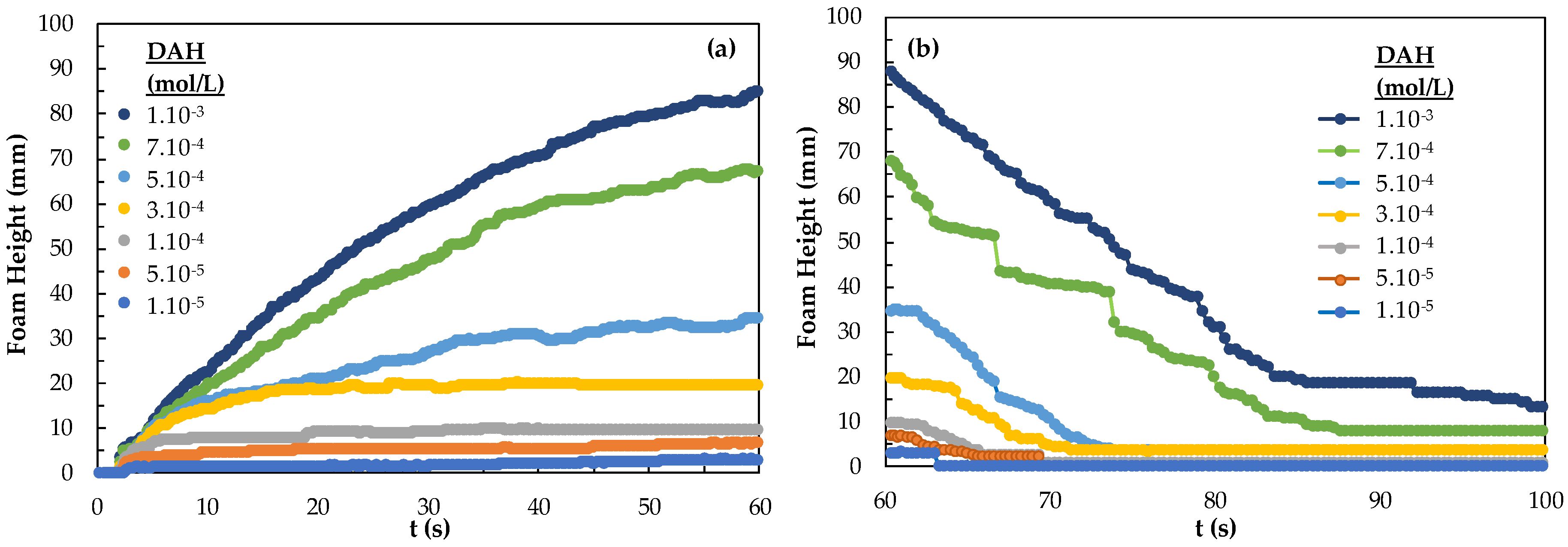
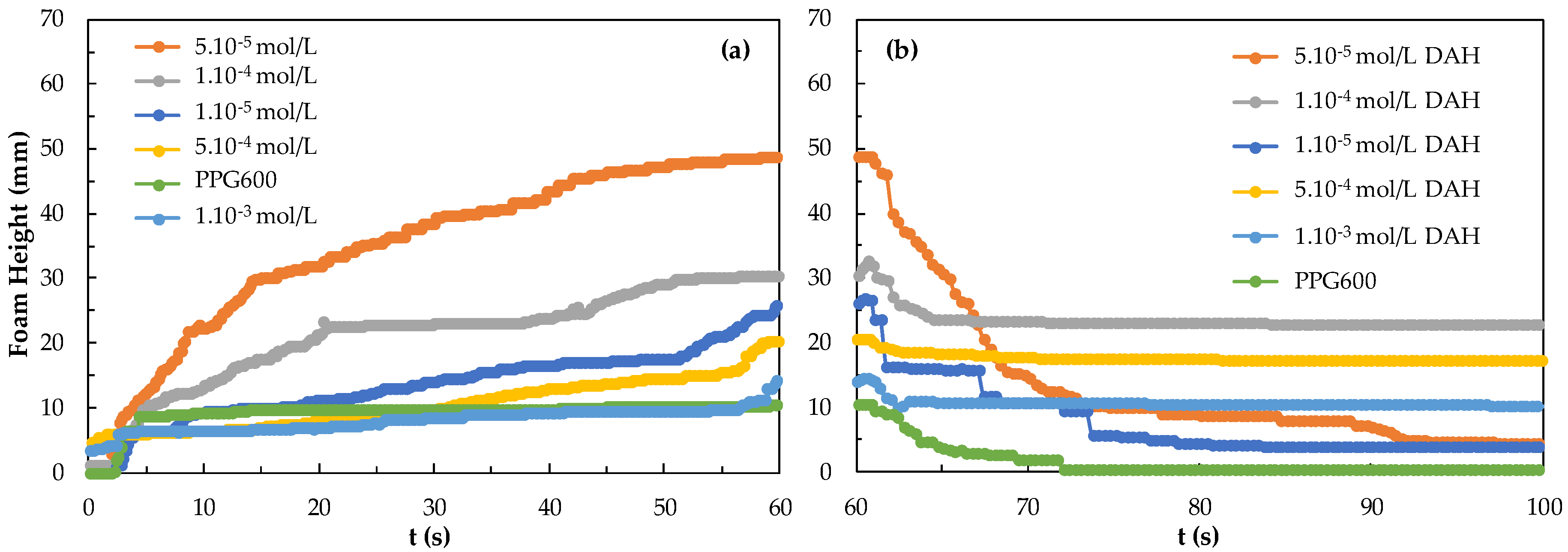
As it is known, foamability and foam decay effect are two of the most important classification parameters for determining foam properties. Interestingly excluding sulfide minerals and in some cases coal flotation systems, all other minerals require some kind of frother-collector combinations. Therefore, the effect of collector concentration should be considered to comment on foamability and foam decay properties, and, accordingly, the flotation of fine-sized particles. In addition to these parameters, the bubble sizes in terms of Sauter Mean Diameter (SMD) values and the bubble size distribution (BSD) under the control of different parameters such as gas flow rate, the pore size of the porous frit may also be effective on tuning the flotation conditions of fine particles [27]. Thus, in this study, the characteristics of different frothers and frother + collector mixtures were studied in order to provide a different perspective on the aforementioned issue of the flotation of very fine particles. Here, both the difference between the collectors and the foamability and foam decay measurements were carried out at different concentrations of the collectors. As seen in Figure 5, the foamability was found to be more durable and foam decay time increased as the concentration of the DAH collector increased. As seen in Figure 5, foaming begins at a DAH concentration of 5.10−5 mol/L and increases, more above 1.10−4 mol/L. It seems that the foam decaying continues for 80 s at the concentration of 7.10−4 mol/L DAH and 1.10−3 mol/L DAH.
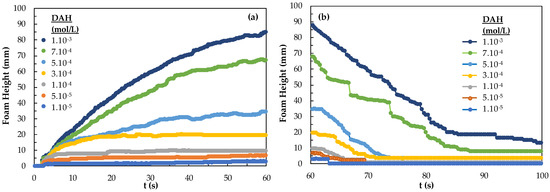
Figure 5.
(a) Foamability and (b) foam decay of DAH collector as a function concentration.
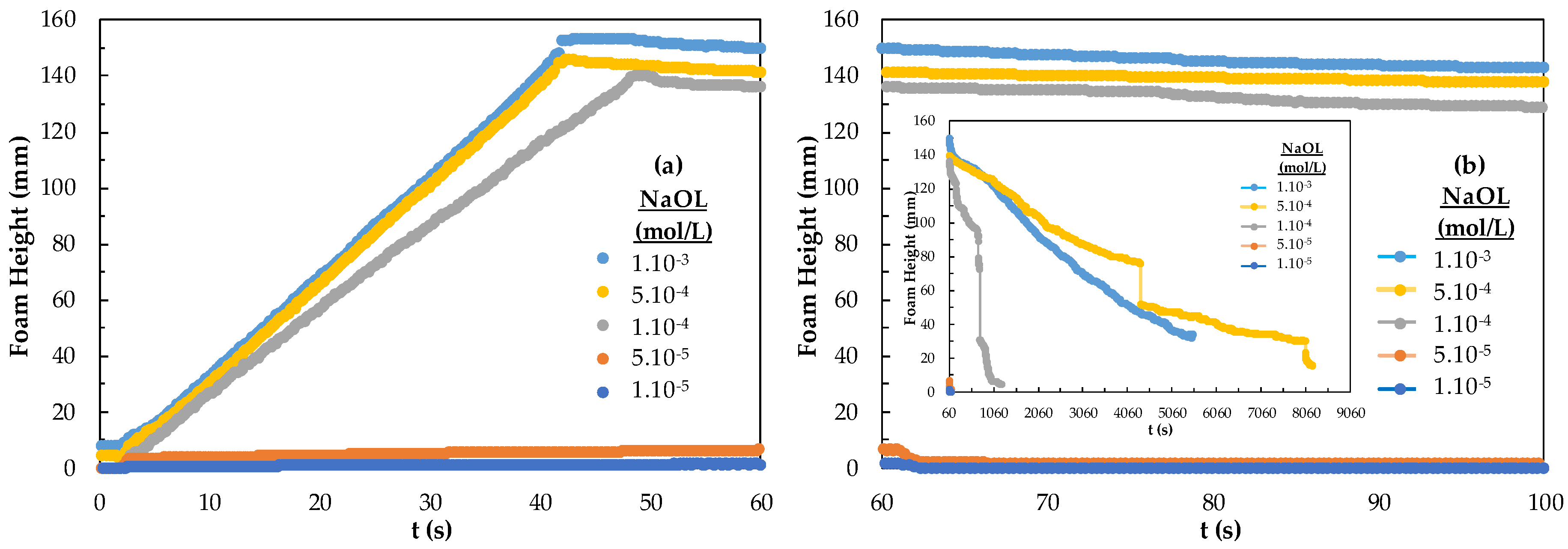
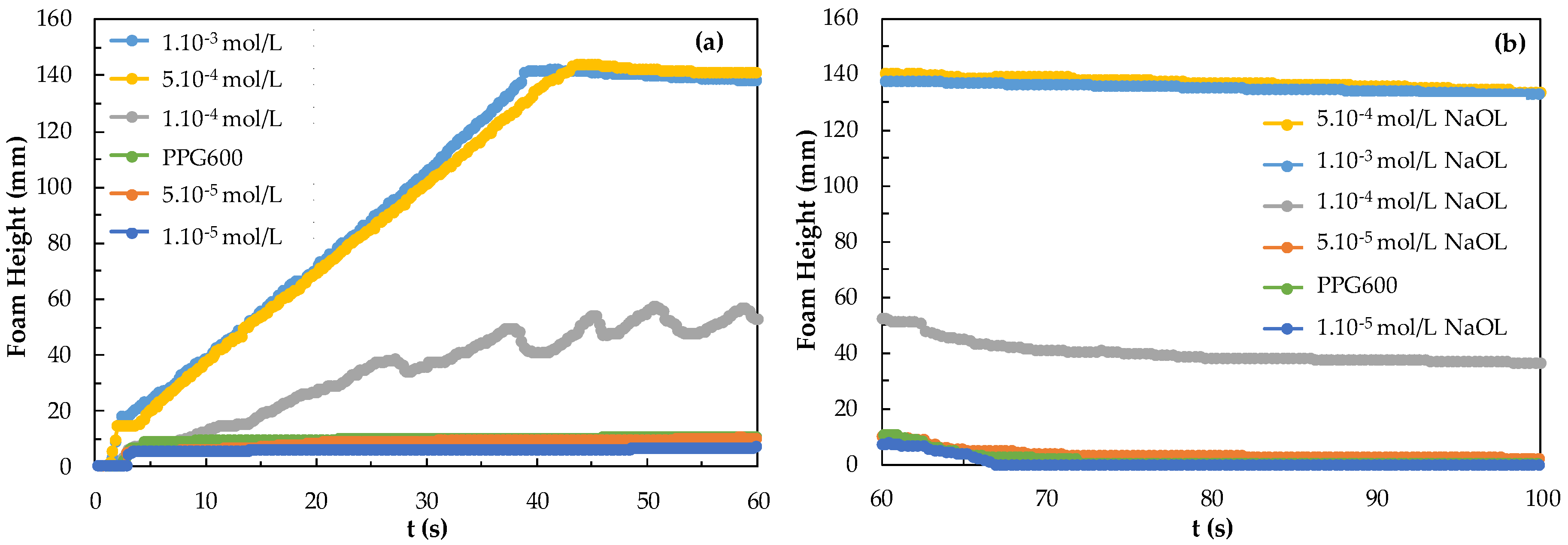
The results of foamability and foam decay of NaOL are given in Figure 6. In Figure 6, foam starts to rise at the 5.10−5 mol/L concentration of NaOL and shows the highest foaming at 5.10−4 mol/L. Foam decay appears to be 18 mm after 2 h 25 min for 5.10−4 mol/L (inset figure). NaOL 1.10−3 mol/L has the highest stability and the foam decaying was the lowest. Also, too-high foam stability is not suitable for flotation and was considered too high a concentration. The effective concentration range of NaOL on foam behavior is much different from that of the DAH collector. That is NaOL has 18 carbon atoms, and 33 hydrogen atoms, the molecular mass is 304.4 g/mol, and the functional group is carboxyl, while DAH has 12 carbon atoms, 28 hydrogen atoms, the molecular mass is 221.8, and one amino functional group. The low number of hydrogens in the chain greatly affects the surface and the number and location of functional groups. Because the chemistry adsorption of NaOL is slower, NaOL increases the potential for hydrogen formation. Infrared spectrophotometry studies showed that the RCOO− ion was chemically adsorbed on the magnesite surface and physically adsorbed on the serpentine surface, while the NH3+ ion was physically adsorbed on the surface of both minerals [28]. The higher the concentration of the DAH collector, the higher the foam whereas the foam rise between 5.10−5–5.10−4 mol/L is sufficient for NaOL. This difference is related to the molecular weight, content, and molecular structure of the collectors.
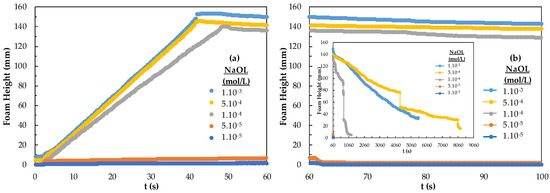
Figure 6.
(a) Foamability and (b) foam decay according to the concentration of the NaOL collector.
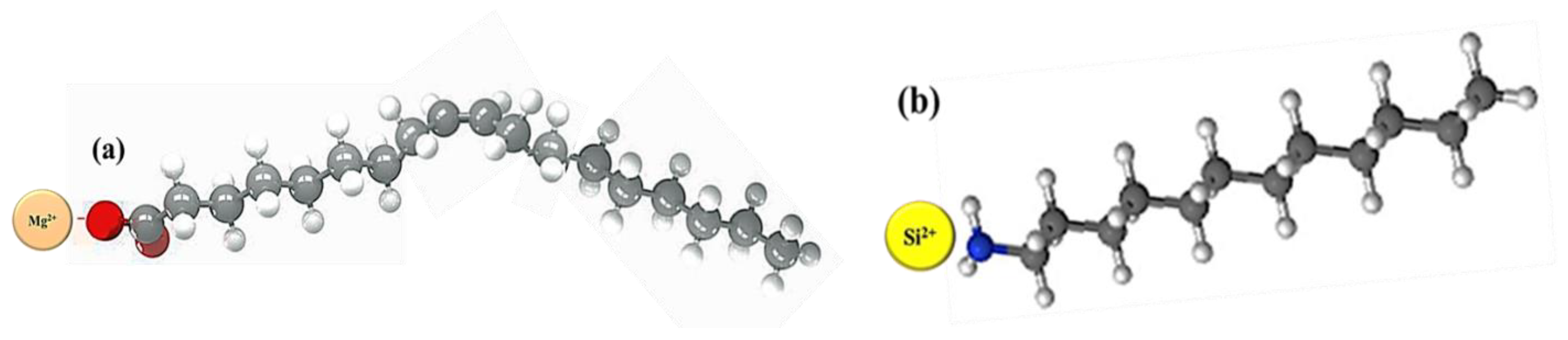
It was also found that physical adsorption had a medium impact on foamability and foam decay while chemical adsorption maintained better foamability and foam decay. Thus, the chemical adsorption of NaOl with magnesite particles and the physical adsorption characteristics of DAH with quartz are shown in Figure 7.
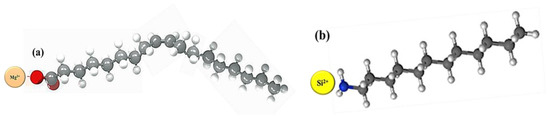
Figure 7.
(a) chemistry adsorption of magnesite with NaOL and (b) physical adsorption of quark with DAH.
3.1.3. Frothers in the Presence of Collectors
Continuous drainage for enrichment depends on the stability of the foam produced in the flotation cell. Therefore, foam structure and stability are very important in the flotation process. To achieve adequate separation in flotation, additional reagents that act as frothers, collectors, or both must be used [29,30]. The type and amount of reagent added are important parameters for flotation. On the other hand, the type of the collector in particular also significantly affects the characteristics of the frother in the mixture. In a recent study [31], it was found that the use of DAH as a component of a frother + collector mixture resulted in lowering the CCC values of frothers such as PPG200, 400, and 600 due to its additional frother property during flotation of particles. This in turn will result in finer bubble sizes which will affect the flotation recoveries of very fine particles. Taking into account that knowledge in mind, in this study, the effects of mixtures of collectors and frothers on foamability and foam decay were investigated. In the first experiment, the effects of pure reagents on foamability and foam decay performance were determined. The effects of reagent type and dosage on foamability and foam decay were investigated in foam flotation. This study aims to explain the relationship between foamability, foam decay, and mixing system depending on the effect of the reagent. The results of foam stability and foam degradation measurements for frothers (CCC values) as a function of DAH appear in Figure 8.
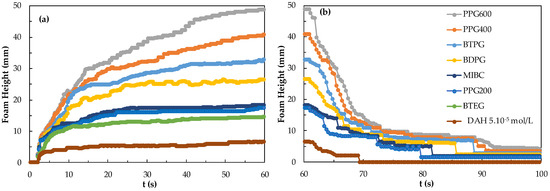
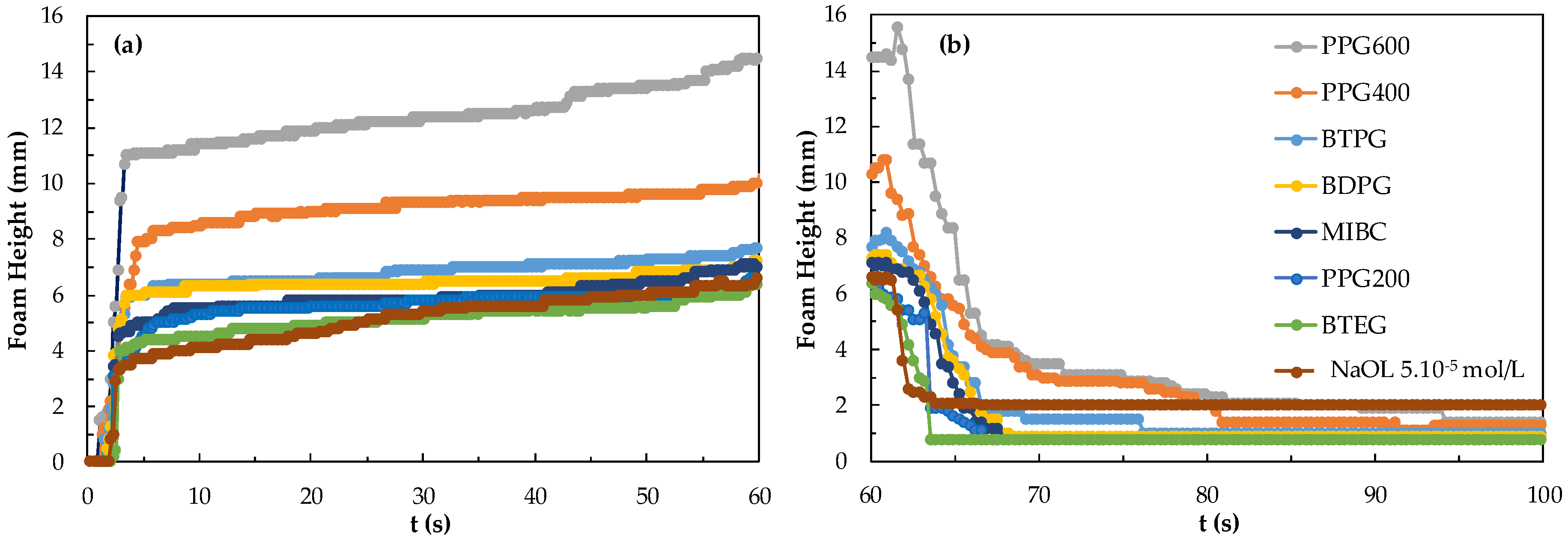
Figure 8.
(a) Foamability and (b) foam decay for the frothers at their CCC values in the presence of 5.10−5 mol/L DAH.
As seen in Figure 8, larger foamability is achieved with PPG600 + DAH compared to other frothers whereas the least foamability was observed in the presence of BTEG + DAH. When 5.10−5 mol/L DAH was mixed with a frother, the foam formed with 3 ppm PPG600 mixture increased up to 48.8 mm within 60 s and the foam degradation decreased to 4.3 mm after 70 s.
The results of foamability and foam degradation measurements by mixing 5.10−5 mol/L NaOL with the frothers at CCC concentrations are shown in Figure 9. As can be seen from Figure 9, the CCC values of the frothers in the presence of 5.10−5 mol/L NaOL show the highest foamability and lowest foam decay in the PPG600 mix system, while BTEG shows the lowest foam stability and highest foam decay. Thus, from this, it is determined that NaOL and PPG600 are more suitable for using a mixing system.
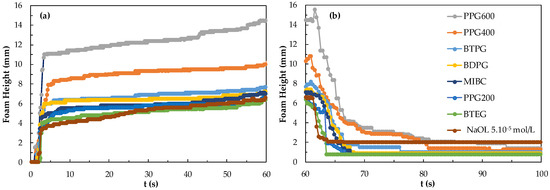
Figure 9.
(a) Foamability and (b) foam decay for the frothers at their CCC values in the presence of 5.10−5 mol/L NaOL.
Also, the mixed system with 5.10−5 mol/L DAH and frothers showed better foamability than the mixed system with 5.10−5 mol/L NaOL and frothers; this probably depends on the length of the hydrocarbon and the adsorption kinetics. This is because longer hydrocarbon chains not only adsorb more slowly but also produce more stable adsorption. The molecular mass and chain length of DAH are lower than that of NaOL, which means better interaction with foaming agents and better foamability. Concentration is shown to be important when mixing a strong collector with a strong foamer. Therefore, the foamability and foam decay of frother and collector mixture with 3 ppm of PPG600 at different concentrations of collectors were investigated.
Figure 10 shows the foamability and foam degradation results for the DAH header in the presence of 3 ppm PPG600 frother. Here, the highest foamability and lowest foam degradation of 5.10−5 mol/L DAH are given, while the lowest foamability of 1.10−3 mol/L M DAH is shown, according to the mixing system of PPG600 3 ppm and DAH collector concentrations. In the presence of 3 ppm PPG600, the foamability of the DAH mix system decreased to 5.10−5 mol/L > 1.10−4 mol/L > 1.10−5 mol/L > 5.10−4 mol/L >1.10−3 mol/L, and the foam decay was 1.10−4 mol/L > 5.10−4 mol/L > 1.10−3 mol/L > 5.10−5 mol/L > 1.10−5 mol/L. In the presence of a strong frother PPG600 and strong collector DAH mix system, low concentration seems to be more suitable. It also means that it is more suitable to use a mixing system with DAH collector + PPG600 (3 ppm) frother.
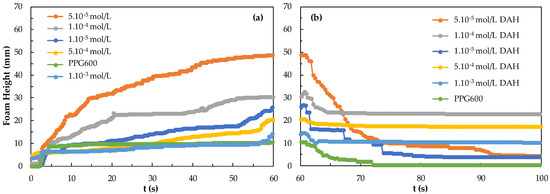
Figure 10.
(a) Foamability and (b) foam decay results for the DAH collector in the presence of 3 ppm PPG600 frother.
Figure 11 shows the foamability and foam decay of the mixed system with the concentrations of NaOL in the presence of 3 ppm PPG600. The highest foamability and foam decay in the mixed system of 3 ppm PPG600 and 5.10−4 mol/L and 1.10−3 mol/L NaOL, the least foamability and foam decay were observed in the 1.10−5 M and 5.10−5 mol/L NaOL with 3 ppm PPG600 system. Therefore, it shoractically should be understood that pure NaOL showed to act both as a collector and frother.
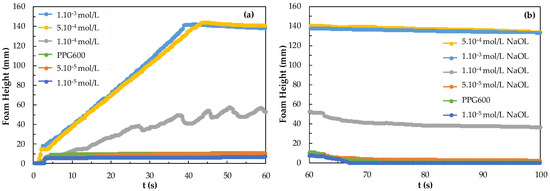
Figure 11.
(a) Foamability and (b) foam decay results for NaOL collector in the presence of 3 ppm PPG600.
3.2. Modeling Studies
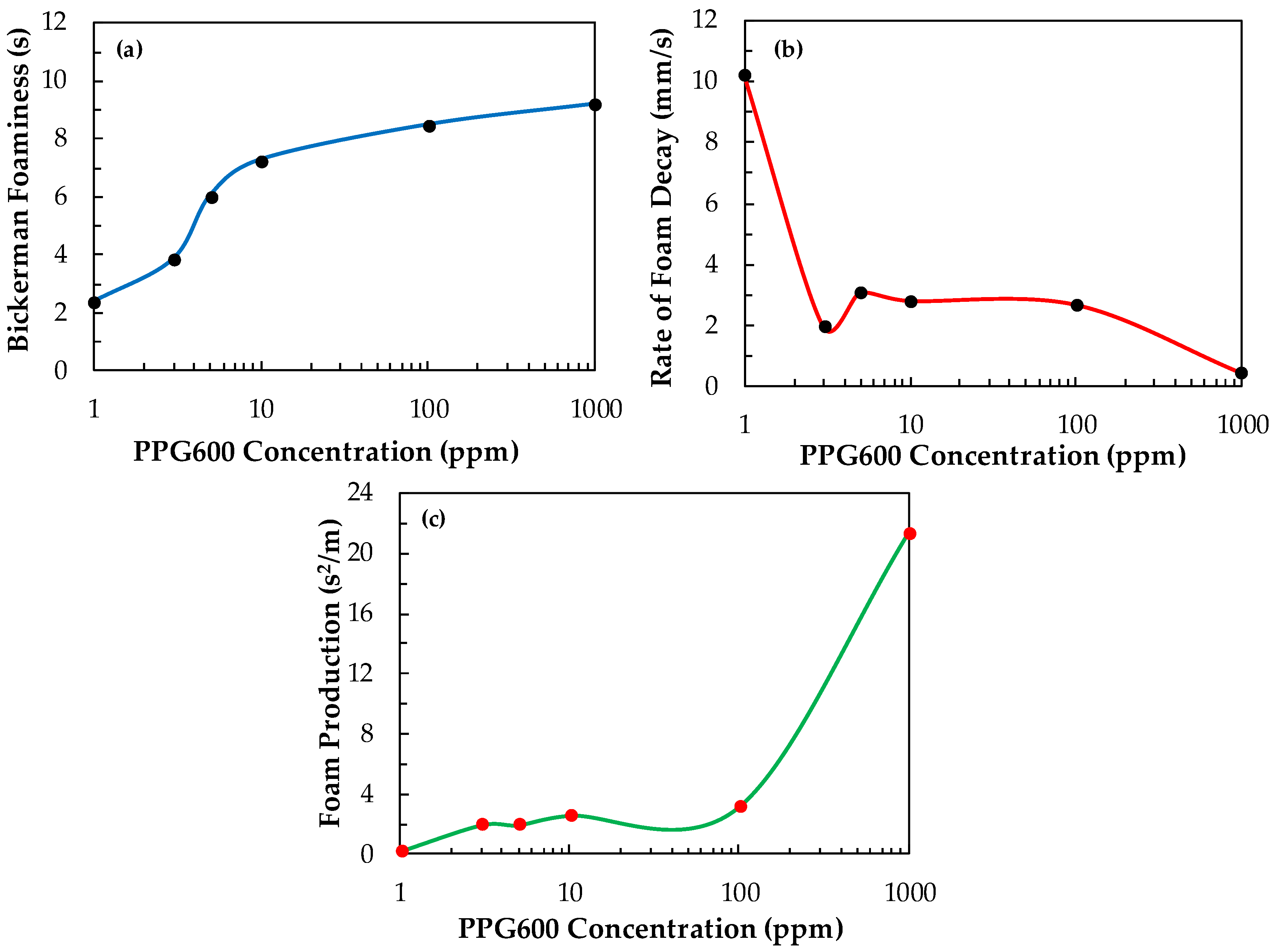
Figure 12 shows the foaminess, the initial speed of froth decay, and the foam production of PPG600 versus its concentration. One can be seen in Figure 12 that as the foaminess increases, the rate of foam decay generally decreases, and the foam production increases with the increase of the concentration of PPG600. Yet, it is interesting the way, in which these variations of the parameters occur because foam production is the line between foamability and foam decay. These two parameters are usually competing, thus confusing the appropriate reading of the experimental data.
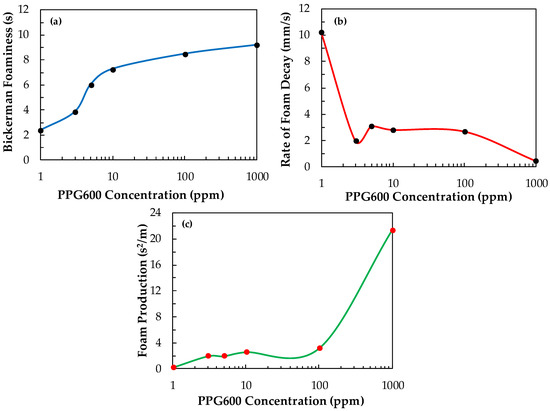
Figure 12.
(a) Bickerman foaminess, (b) rate of foam decay, and (c) foam production vs. the concentration of PPG600.
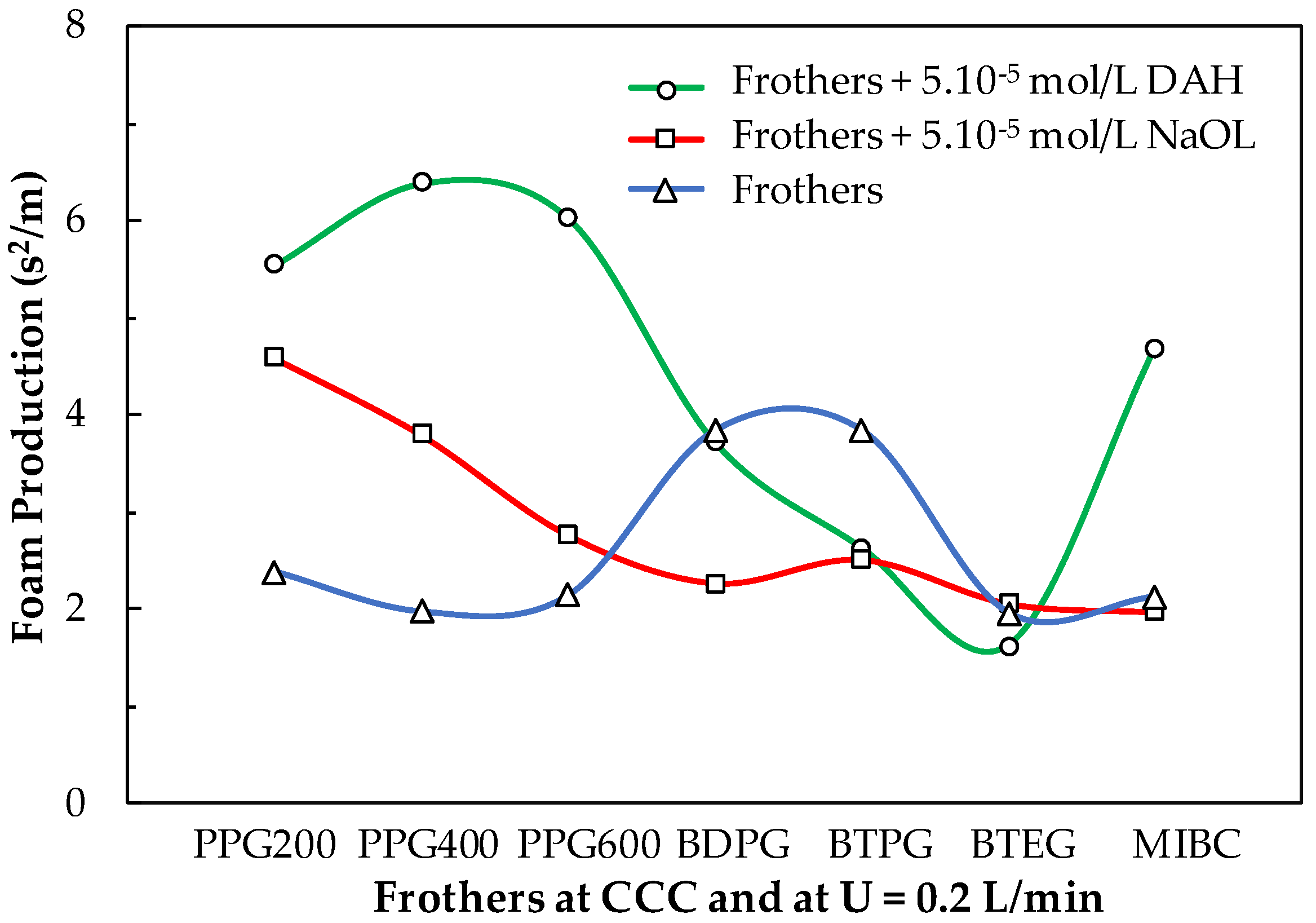
Figure 13 shows the foam production (FP) [19] of the basic frothers, studied in this work, at their CCC values in the absence and presence of collectors (DAH and NaOL). Also, in the absence of collectors PPG200, PPG400, and PPG600, BTEG, and MIBC have almost the same FP values, while BDPG and BTPG produce larger FP values. It is interesting to know how the FP values of the frothers change upon adding 5.10−5 mol/L collectors. The latter concentration of the collectors is operational in the flotation process. For example, the addition of DAH to PPG200, PPG400, and PPG600 increase significantly their FP values, The FP value of BDPG is not affected while the FP values of BTPG and BTEG are decreased, while this one of MIBC is increased. Upon the addition of NaOL, the increase of the FP values of PPG200, PPG400, and PPG600 is weaker. The FP values of BDPG and BTPG are decreased. This corresponds to lower stationary foam height and/or faster foam decay and indicates the quality of froth according to analysis. The frothers of BTEG and MIBC are not practically affected by the presence of NaOL. This information can be exploited in the real frother/collectors formulations to decrease the level of entrainment of particles. Low foam production corresponds to faster-decaying foam, thus releasing the water on top of the foam containing entrained particles. The collectors (NaOL and DAH) have certain surface-active properties. Therefore, their froth performance was studied separately.
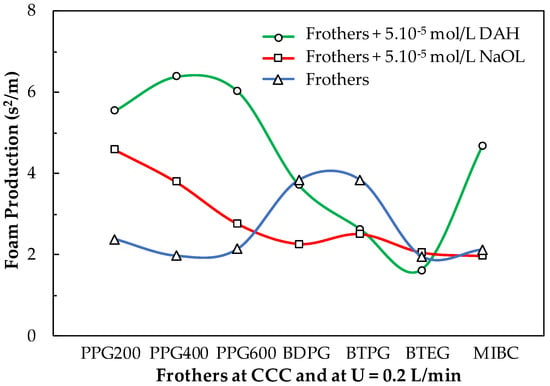
Figure 13.
Foam production of the basic frothers, studied in this work, with concentrations equal to their CCC values in the absence and presence of 5.10−5 mol/L collectors (DAH and NaOL).
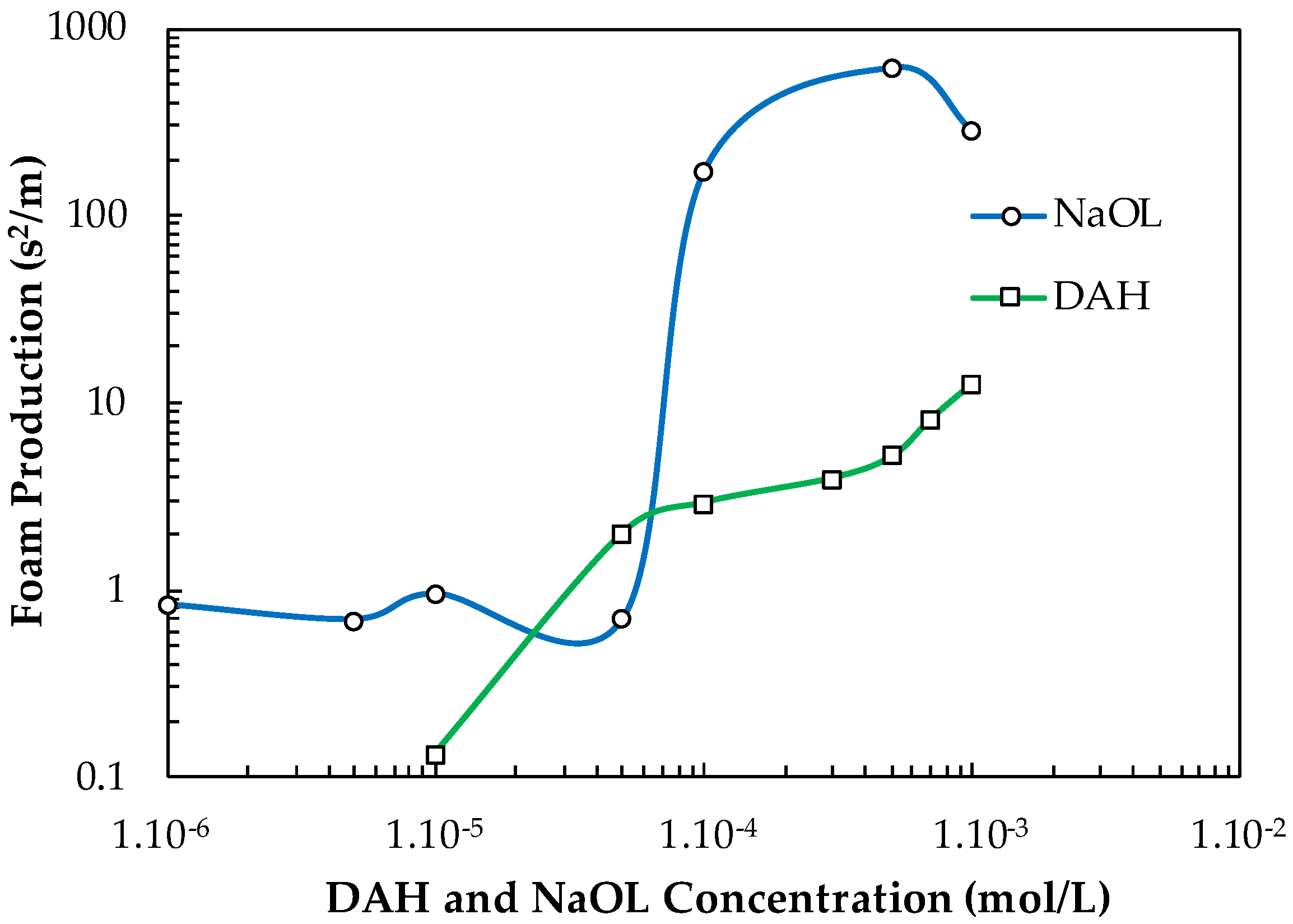
Figure 14 shows how the FP values of NaOL and DAH generally increase with increasing their concentration. For example, NaOL has small FP values until C = 5.10−5 mol/L, above which, its FP value increases substantially, i.e., it converts into a strong frother. The FP values of DAH increase with its concentration in the way given in Figure 13. As far as PPG 600 is the best-recognized frother, producing fine bubbles in the pulp zone, it was focused on its performance in presence of these two frothers. The interaction between frother and collector, which is difficult to predict, will result in their mutual froth performance.
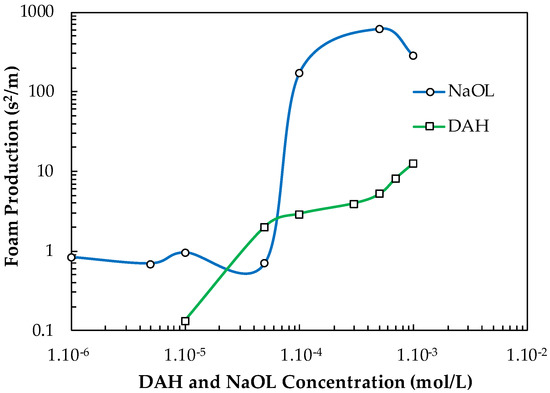
Figure 14.
Foam production of NaOL and DAH versus their concentration.
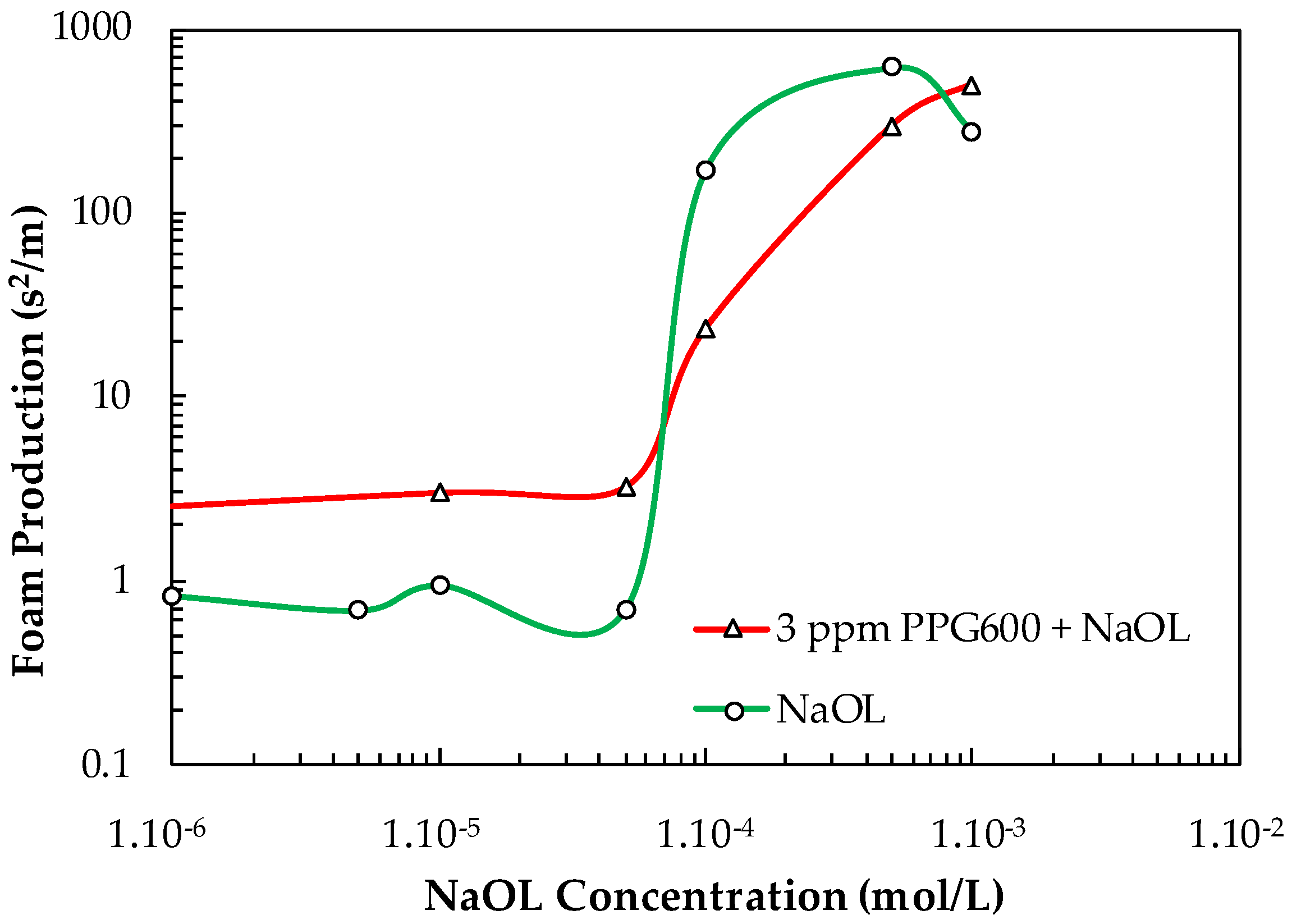
Figure 15 shows the FP values of NaOL and NaOL + 3 ppm PPG600 vs. NaOL concentration. It is seen from Figure 15 that the addition of 3 ppm PPG600 has an additive effect on the total FP value until the critical concentration C = 5.10−5 mol/L, above which interaction between them occurs, thus resulting in a smaller total FP value compared with that of the single collector.
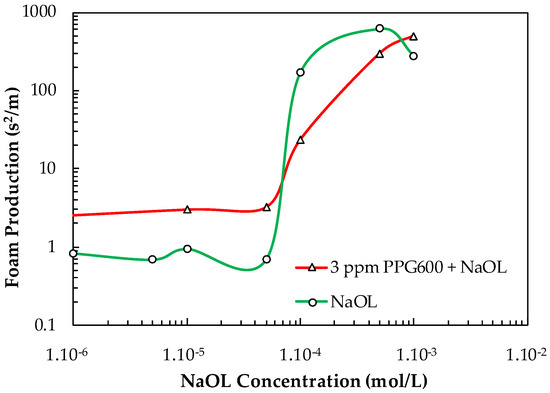
Figure 15.
Foam production value of NaOL and NaOL + 3 ppm PPG600 vs. NaOL concentration.
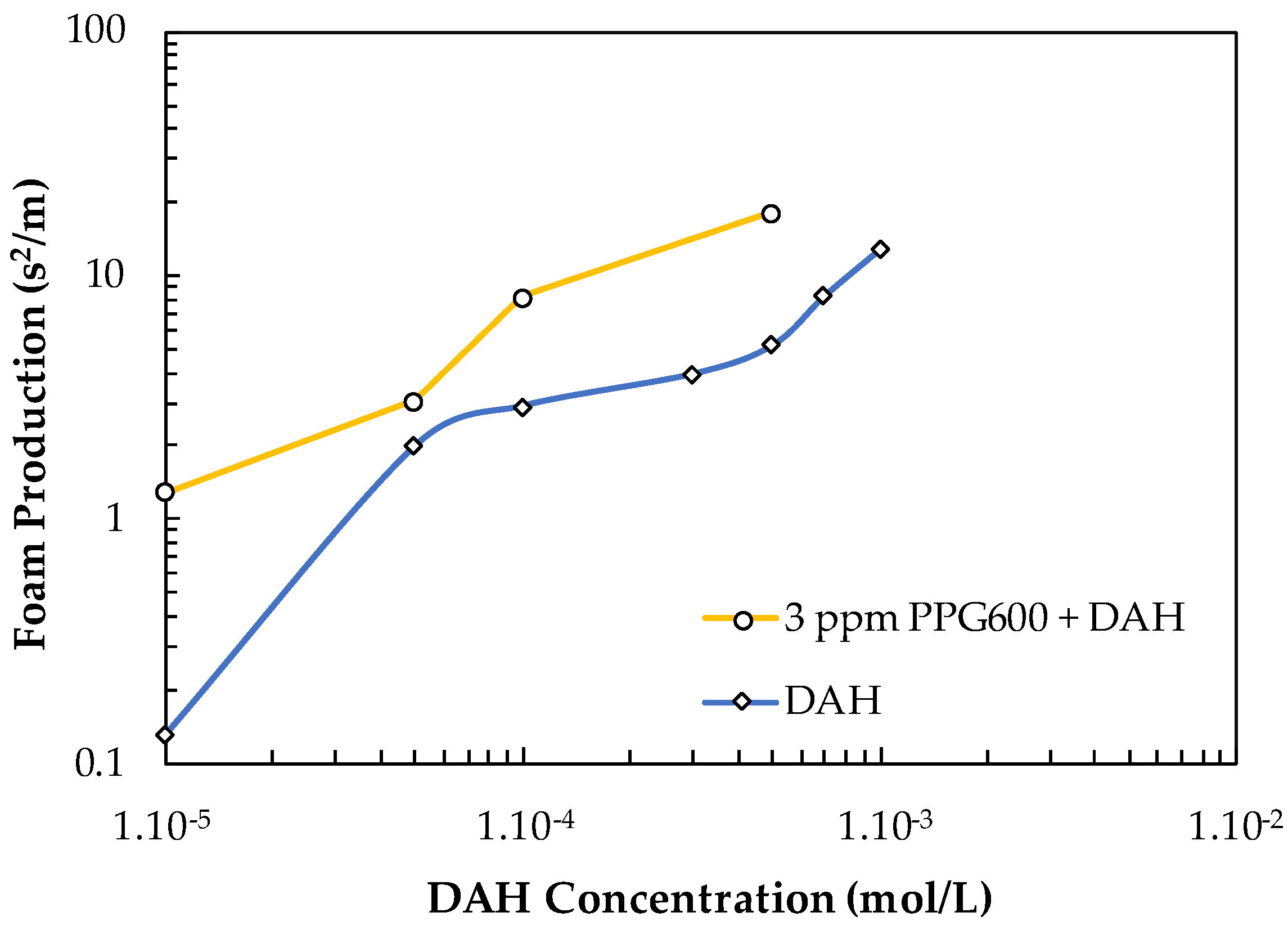
The FP values of DAH and DAH + 3 ppm PPG600 vs. the concentration of DAH are shown in Figure 16. One can see that the contribution of PPG600 to this system is practically additive.
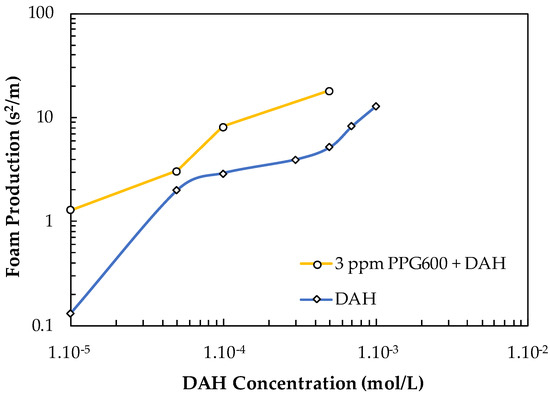
Figure 16.
Foam production value of DAH and DAH + 3 ppm PPG600 vs. DAH concentration.
The frothing performance of a mixture of frother and collector in terms of foam production could be used for the proper selection of reagent formulation at industrial conditions. Of course, it depends on the operational conditions of each plant, but this approach can be used for every specific industrial case. For example, the presence of coarse bubbles in the froth zone minimizes the entrainment of particles. This corresponds to small foam production (FP) value. Therefore, it was interesting to choose such a combination of frother and collector, which results in as small as possible FP value. For example, the combination of 5.10−5 mol/L DAH and 3 ppm PPG600 have a small FP value and can be used for obtaining a froth zone with coarse bubbles and a minimal level of entrainment. According to Figure 15, 5.10−5 mol/L NaOL + 3 ppm PPG600 is the most suitable combination having a small FP value. One should not use a concentration of NaOL larger than 5.10−5 mol/L because its FP values significantly increased.
4. Conclusions
The results of this study revealed that foamability and foam decay significantly vary with the kind of reagent. In the presence of frother, while the highest foamability and low foam decaying were obtained with PPG600 at its CCC values, the lowest foamability, and high foam decay were found in the presence of BTEG. Among all the frothers, foam stability, and foam degradation were exhibited in the order BTEG < PPG200 ≈ MIBC < BDPG < BTPG < PPG400 < PPG600.
In the presence of sodium oleate (NaOL) and DAH collectors, foam decay began at a concentration of 5.10−5 mol/L. It was observed that as the concentration of the collectors increased, the foamability was larger and the foam decay time increased. The highest foam formation and the highest foam height were found to be ~98 mm at 1.10−3 mol/L for DAH and 152 mm at 5.10−4 mol/L for NaOL. The foam heights obtained with NaOL reached decayed in 40 s at the shortest time. NaOL and DAH collectors are indicated to support frothers in foam formation. It was determined that when the concentration of NaOL increased in the presence of 3 ppm PPG600, the foam height increased further, and the foamability was larger. This was found to be supported by NaOL and DAH collectors in the presence of 3 ppm PPG600 frother. It is shown that it is more appropriate to use a combination of 3 ppm PPG600 frother with DAH or NaOL collectors.
Overall, it can be concluded from this study that the foam production concept can be used for the proper selection of frothers and collectors producing coarse bubbles with small entrainment in the froth zone. And, the mixture of frother and collector results in either: (i) additive effect or (ii) interaction between them, which can either decrease or increase the FP value of the mixture.
The preliminary theoretical prediction of the frothing performance of frother + collector is a quite complex subject, but its experimental study, shown in this work, could help us to better understand the absence or presence of synergy between them.
Author Contributions
Experimental methodology, O.G., K.B., O.O., F.B.; validation, S.I.K., N.A.G.; investigation, O.G., K.B., O.O.; resources, O.G., O.O., F.B., M.S.Ç.; original draft preparation, O.G. and O.O.; writing—review and editing, O.G., O.O., S.I.K., M.S.Ç.; supervision, O.O., M.S.Ç.; project administration, M.S.Ç. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is supported by European Union’s Horizon 2020 research and innovation program under grant agreement No. 821265, project FineFuture (Innovative technologies and concepts for fine particle flotation: unlocking future fine-grained deposits and Critical Raw Materials resources for the EU).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The following data was obtained from the results of modeling studies:

Table A1.
CCC values, Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value of selected frothers.
Table A1.
CCC values, Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value of selected frothers.
| Frothers | CCC (ppm) | Foaminess (s) | dh/dt (mm/s) | Foam Production (s2/mm) | St. Dev. (s2/mm) |
|---|---|---|---|---|---|
| PPG200 | 11 | 2.60 | 1.09 | 2.38 | 0.18 |
| PPG400 | 4 | 3.51 | 1.78 | 1.97 | 0.11 |
| PPG600 | 3 | 3.92 | 1.84 | 2.13 | 0.11 |
| BDPG | 17 | 4.03 | 1.05 | 3.84 | 0.19 |
| BTPG | 5 | 4.15 | 1.08 | 3.85 | 0.19 |
| BTEG | 20 | 2.68 | 1.37 | 1.96 | 0.15 |
| MIBC | 10 | 2.90 | 1.37 | 2.12 | 0.15 |

Table A2.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of PPG600.
Table A2.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of PPG600.
| C (ppm) | Foaminess (s) | dh/dt (mm/s) | Foam Production (s2/mm) | St. Dev. (s2/mm) | |
|---|---|---|---|---|---|
| PPG600 | 1 | 2.38 | 10.21 | 0.23 | 0.02 |
| 3 | 3.85 | 1.95 | 1.97 | 0.10 | |
| 5 | 6.03 | 3.05 | 1.98 | 0.07 | |
| 10 | 7.28 | 2.79 | 2.61 | 0.07 | |
| 100 | 8.48 | 2.67 | 3.18 | 0.04 | |
| 1000 | 9.20 | 0.43 | 21.38 | 0.23 |

Table A3.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of DAH.
Table A3.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of DAH.
| C (mol/L) | Foaminess (s) | dh/dt (mm/s) | Foam Production (s2/mm) | St. Dev. (s2/mm) | |
|---|---|---|---|---|---|
| DAH | 1.10−5 | 1.17 | 8.82 | 0.13 | 0.01 |
| 5.10−5 | 2.49 | 1.23 | 2.02 | 0.08 | |
| 1.10−4 | 3.69 | 1.26 | 2.93 | 0.08 | |
| 3.10−4 | 7.65 | 1.93 | 3.96 | 0.05 | |
| 5.10−4 | 13.12 | 2.53 | 5.19 | 0.04 | |
| 7.10−4 | 27.37 | 3.31 | 8.26 | 0.03 | |
| 1.10−3 | 32.35 | 2.52 | 12.82 | 0.04 |

Table A4.
CCC values, Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value of the frothers at 5.10−5 mol/L DAH.
Table A4.
CCC values, Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value of the frothers at 5.10−5 mol/L DAH.
| CCC (ppm) | Foaminess (s) | dh/dt (mm/s) | Foam Production (s2/mm) | St. Dev. (s2/mm) | |
|---|---|---|---|---|---|
| PPG200 | 11 | 6.56 | 1.18 | 5.54 | 0.08 |
| PPG400 | 4 | 15.42 | 2.41 | 6.39 | 0.04 |
| PPG600 | 3 | 18.40 | 3.05 | 6.03 | 0.03 |
| BDPG | 17 | 10.03 | 2.70 | 3.72 | 0.04 |
| BTPG | 5 | 12.37 | 4.70 | 2.63 | 0.02 |
| BTEG | 20 | 5.50 | 3.40 | 1.62 | 0.03 |
| MIBC | 10 | 6.90 | 1.47 | 4.69 | 0.07 |

Table A5.
CCC values, Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value of the frothers at 5.10−5 mol/L NaOL.
Table A5.
CCC values, Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value of the frothers at 5.10−5 mol/L NaOL.
| CCC (ppm) | Foaminess (s) | dh/dt (mm/s) | Foam Production (s2/mm) | St. Dev. (s2/mm) | |
|---|---|---|---|---|---|
| PPG200 | 11 | 2.49 | 0.54 | 4.59 | 0.18 |
| PPG400 | 4 | 4.07 | 1.07 | 3.79 | 0.09 |
| PPG600 | 3 | 5.88 | 2.13 | 2.76 | 0.05 |
| BDPG | 17 | 2.79 | 1.23 | 2.27 | 0.08 |
| BTPG | 5 | 3.09 | 1.23 | 2.51 | 0.08 |
| BTEG | 20 | 2.41 | 1.17 | 2.06 | 0.09 |
| MIBC | 10 | 2.68 | 1.36 | 1.97 | 0.07 |

Table A6.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of DAH in presence of 3 ppm PPG600.
Table A6.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of DAH in presence of 3 ppm PPG600.
| C (mol/L) | Foaminess (s) | dh/dt (mm/s) | Foam Production (s2/mm) | St. Dev. (s2/mm) | |
|---|---|---|---|---|---|
| DAH | 0 | 3.92 | 1.84 | 2.13 | 0.11 |
| 1.10−5 | 11.65 | 8.94 | 1.30 | 0.01 | |
| 5.10−5 | 20.06 | 6.53 | 3.07 | 0.02 | |
| 1.10−4 | 20.85 | 2.53 | 8.22 | 0.04 | |
| 5.10−4 | 14.14 | 0.78 | 18.12 | 0.13 |

Table A7.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of NaOL in presence of 3 ppm PPG600.
Table A7.
Bikerman unit of foaminess, the initial rate of foam decay, foam production, and the standard deviation of the foam production value for different concentrations of NaOL in presence of 3 ppm PPG600.
| C (mol/L) | Foaminess (s) | dh/dt (mm/s) | Foam Production (s2/mm) | St. Dev. (s2/mm) | |
|---|---|---|---|---|---|
| NaOL | 0 | 3.92 | 1.84 | 2.13 | 0.11 |
| 1.10−5 | 2.83 | 0.94 | 3.00 | 0.11 | |
| 5.10−5 | 4.60 | 1.43 | 3.22 | 0.07 | |
| 1.10−4 | 35.32 | 1.50 | 23.51 | 0.07 | |
| 5.10−4 | 104.54 | 0.35 | 297.24 | 0.28 | |
| 1.10−3 | 103.52 | 0.21 | 498.42 | 0.48 |
References
- George, P.; Nguyen, A.V.; Jameson, G.J. Assessment of true flotation and entrainment in the flotation of submicron particles by fine bubbles. Miner. Eng. 2004, 17, 847–853. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussiere, B.; Kongolo, M.; McLaughlin, J.; Marion, P. Environmental desulphurization of four Canadian mine tailings using froth flotation. Int. J. Miner. Process. 2000, 5, 57–74. [Google Scholar] [CrossRef]
- Mbamba, C.K.; Harrison, S.T.L.; Franzidis, J.P.; Broadhurst, J.L. Mitigating acid rock drainage risks while recovering low-sulfur coal from ultrafine colliery wastes using froth flotation. Miner. Eng. 2012, 29, 13–21. [Google Scholar] [CrossRef]
- Yoon, R.H. The role of hydrodynamic and surface forces in bubble–particle interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozev, N.A.; Ozdemir, O.; Guven, O.; Ata, S.; Bournival, G.; Batjargal, K.; Boylu, F.; Hristova, S.; Çelik, M.S. Physical restrictions of the flotation of fine particles and ways to overcome them. Physicochem. Probl. Miner. Process. 2022, 58, 153944. [Google Scholar] [CrossRef]
- Severov, V.V.; Filippova, I.V.; Filippov, L.O. Use of fatty acids with an ethoxylated alcohol for apatite flotation from old fine-grained tailings. Miner. Eng. 2022, 188, 107832. [Google Scholar] [CrossRef]
- Foucaud, Y.; Collet, A.; Filippova, I.V.; Badawi, M.; Filippov, L.O. Synergistic effects between fatty acids and non-ionic reagents for the selective flotation of scheelite from a complex tungsten skarn ore. Miner. Eng. 2022, 182, 107566. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Dukhin, S.S.; Rulyov, N.N. Kinetic Theory of Flotation of Small Particles. In Surface and Colloid Science; Matijević, E., Good, R.J., Eds.; Springer: Boston, MA, USA, 1984. [Google Scholar]
- Pyke, B.; Fornasiero, D.; Ralston, J. Bubble particle heterocoagulation under turbulent conditions. J. Colloid Interface Sci. 2003, 265, 141–151. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Abrahamson, J. Collision rates of small particles in a vigorously turbulent fluid. Chem. Eng. Sci. 1975, 30, 1371–1379. [Google Scholar] [CrossRef]
- Schubert, H. On the turbulence-controlled microprocesses in flotation machines. Int. J. Miner. Process. 1999, 56, 257–276. [Google Scholar] [CrossRef]
- Gaudin, A.M.; Groh, J.O.; Henderson, H.B. Effect of Particle Size on Flotation; AIME Technical Publications: Englewood, NJ, USA, 1931; Volume 414, pp. 3–23. [Google Scholar]
- Yalcin, T.; Byers, A. Dissolved gas flotation in mineral processing. Miner. Process. Extr. Metall. Rev. 2006, 27, 87–97. [Google Scholar] [CrossRef]
- Tao, Y.J.; Liu, J.T.; Yu, S.; Tao, D. Picobubble enhanced fine coal flotation. Sep. Sci. Technol. 2006, 41, 3597–3607. [Google Scholar] [CrossRef]
- Geldenhuys, S.; McFadzean, B. Column diameter effects on dynamic froth stability measurement. In Proceedings of the XXIX International Mineral Processing Congress (IMPC 2018), Moscow, Russia, 17–21 September 2018. [Google Scholar]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Boylu, F.; Çelik, M.S. Effect of frother and collector mixture on froth stability in ultra-fine size quartz/DAH flotation system. In Proceedings of the 17th International Mineral Processing Symposium (IMPS 2022), Istanbul, Turkiye, 15–17 December 2022. [Google Scholar]
- Bikerman, J.J. The unit of foaminess. Trans. Faraday Soc. 1938, 34, 0634–0638. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Georgiev, P.; Balashev, K. Foam production—Ratio between foaminess and rate of foam decay. J. Colloid Interface Sci. 2012, 379, 144–147. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozev, N.A.; Ozdemir, O.; Batjargal, K.; Guven, O.; Ata, S.; Bournival, G.; Boylu, F.; Çelik, M.S. On the frother’s strength and its performance. Miner. Eng. 2021, 171, 107093. [Google Scholar] [CrossRef]
- Pugh, R.J. Bubble and Foam Chemistry; CUP, Cambridge Press: Cambridge, UK, 2016; Volume 12, pp. 405–419. [Google Scholar]
- Schwarz, S. The Relationship between Froth Recovery and Froth Structure. Ph.D. Thesis, Ian Wark Research Institute, University of South Australia, Adelaide, Australia, 2004. [Google Scholar]
- Neethling, S.J.; Brito-Parada, P.R. Predicting flotation behaviour the interaction between froth stability and performance. Miner. Eng. 2018, 120, 60–65. [Google Scholar] [CrossRef]
- Holmberg, K. Surfactant and Polymers in Aqueous Solution, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Guven, O.; Batjargal, K.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Experimental procedure for the determination of the critical coalescence concentration (CCC) of simple frothers. Minerals 2020, 10, 617. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozev, N.A.; Batjargal, K.; Guven, O.; Özdemir, O.; Boylu, F.; Çelik, M.S. Correlations for easy calculation of the critical coalescence concentration (CCC) of simple frothers. Coatings 2020, 10, 612. [Google Scholar] [CrossRef]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions. Minerals 2021, 11, 304. [Google Scholar] [CrossRef]
- Gence, N.; Ozdag, H. Surface properties of magnesite and surfactant adsorption mechanism. Int. J. Miner. Process. 1995, 43, 37–47. [Google Scholar] [CrossRef]
- Woodburn, E.T.; Flynn, S.A.; Cressey, B.A.; Cressey, G. The effect of froth stability on the beneficiation of low-rank coal by flotation. Powder Technol. 1984, 40, 167–177. [Google Scholar] [CrossRef]
- Subrahmanyam, T.V.; Forssberg, E. Froth Stability Particle Entrainment and Drainage in Flotation. Int. J. Miner. Process. 1988, 23, 33–53. [Google Scholar] [CrossRef]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, S.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Bubbling properties of frothers and collectors mix system. Physicochem. Probl. Miner. Process. 2022, 58, 152890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).