Abstract
The result of a complex mineralogical study of the first discovery of the rare hydrated magnesium carbonate minerals of Nesquehonite and Dypingite in the Obnazhennaya kimberlite pipe (of the Yakutian kimberlite province) is presented. The methods of X-ray phase analysis, electronic microscopy, and Raman spectroscopy have established that the main minerals of the samples found in the form of white crust on a small area of rock outcrop of kimberlite breccia are hydrated carbonates: Nesquehonite is MgCO3•3H2O, Dypingite is Mg5(CO3)4(OH)2•5H2O. The formation of Dypingite over Nesquehonite was shown using Raman imaging for the first time. Nesquehonite is represented as aggregates consisting of chaotically oriented prismatic crystals or kidney-shaped formations. Dypingite in the examined samples appears less frequently as rose-shaped aggregates formed from lamellar crystals. It is assumed that the formation of rare carbonates of the Obnazhennaya kimberlite pipe is mainly the result of the weathering of silicates, formation of mineralized solutions, and their subsequent crystallization, including the capture of CO2 from the air.
1. Introduction
Carbonates are one of the common endogenous minerals in kimberlites. They are formed at all stages of mineral formation associated with kimberlite rocks; from formation in diamond, mantle xenoliths, and inclusion in magmatic minerals of kimberlites to crystallization in mesostasis and in hydrothermal formations [1,2,3,4,5]. Enrichment of endogenous carbonates is a prerequisite for the appearance of exogenous carbonate minerals under certain conditions. For example, hydrated carbonate—Colingite Mg10Fe2(CO3) (OH)24∙2H2O—was found in the Manchary kimberlite pipe of the Khompu–Mayskoe field (Central Yakutia). It was formed in milonitized tectonically disturbed kimberlite rocks as a result of their interaction with groundwater [6].
Basalts, peridotites, and serpentinites absorb a significant amount of atmospheric CO2 with the formation of low-temperature secondary carbonates [7,8,9]. This feature of rocks draws attention to the problem of the carbonization of kimberlite rocks that are reworked during diamond recovery [10,11,12]. Studies of kimberlite rocks show that olivine, serpentine, brusite, and smectite have the highest reactivity to interact with carbon dioxide. For a sample, from the Udachnaya pipe, the experimental value of absorbed carbon dioxide over the 10-year life cycle of processed kimberlite ore, measured with elemental analysis, is 8.2% [12]. Kimberlite from the Obnazhennaya pipe has a similar mineral composition. It seems to be a natural object with which the formation of carbonates “in situ” as a result of the carbonization process is associated.
This study presents the data of the complex mineralogical study of the first discovery of Nesquehonite and Dypingite (hydrated magnesium carbonate minerals) in the Obnazhennaya kimberlite pipe, located northeast of the Siberian platform.
Nesquehonite is a hydrated magnesium carbonate MgCO3•3H2O formed in near-surface conditions, and it was first discovered in the Nesquehoning coal mine, Pennsylvania, USA [13]. The mineral is white or colorless, and most often performs pseudomorphosis with lansfordite MgCO3•5H2O, and also forms pure and transparent lamellar crystals [14,15]. Nesquehonite is in paragenesis with the low-temperature minerals lansfordite, Dypingite, hydromagnesite, artinite, calcite, brucite, aragonite, pyroaurite, and monohydrocalcite. In ultramafic rocks, dolomite-bearing schists, and serpentinites, it appears in paragenesis with minerals, which are formed due to magnesium leaching. In Russia, the mineral was first discovered in the Baleisky gold-ore deposit [14]. In addition, it is known to be found in volcanic exhalations in Kamchatka [16], among newly formed minerals in the Chelyabinsk coal dumps [17], magnesian skarns of the Korshunovskoe deposit [18], and the Titov magnesian-skarn borate-ore deposit [19].
Dypingite is a hydrated magnesium carbonate Mg5(CO3)4(OH)2•5H2O which was found in a Dypingdal serpentine-magnesite deposit in Norway as a thin white deposit. The mineral is of secondary origin, formed under surface conditions during weathering of waste materials [20]. Associative minerals of Dypingite include Hydrotalcite, Magnesite, Brusite, Bruniatellite, Pyroaurite, Artinite, Hydromagnesite, and Nesquehonite. It also occurs on the surface of serpentinites [9]. Earlier in Russia, the mineral was found in Kamchatka in the products of the fumarole activity of volcanoes near the crater edge, and in the Chelyabinsk coal basin in the form of granular crusts, grown on altered fossil wood and on spherulites of iosikawaite [16,17].
At present, this discovery of Nesquehonite and Dypingite in kimberlite rocks is the first.
2. Materials and Methods
2.1. Materials
The Obnazhennaya pipe is located on the right bank within the Kuoyka field of the Yakutian kimberlite province (Figure 1).
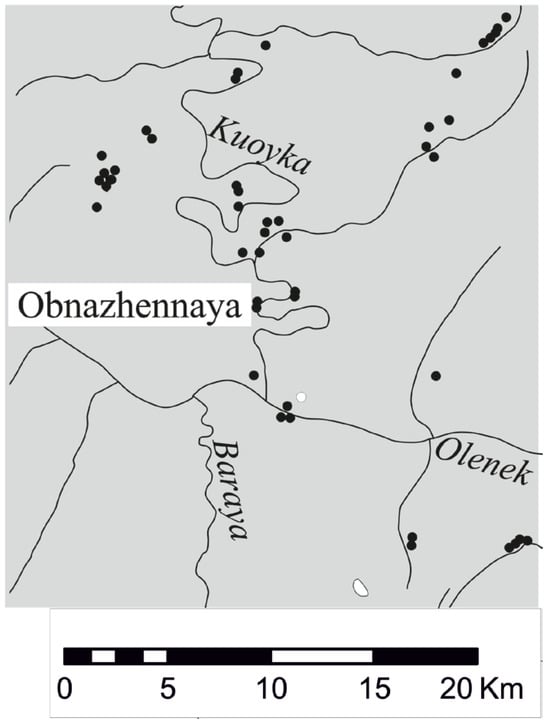
Figure 1.
Kimberlite bodies of the Kuoyka field of the Yakutian kimberlite province, the Obnazhennaya pipe (70°30′58″ N, 120°30′30″ E).
Contacts of the kimberlite body with the host dolomites of the Turgut Formation of the Upper Vendian are hidden from three sides under steeply falling talus. The body is elliptical in shape, with its long axis oriented to the northwest. The size of the pipe is 20 × 30 m. The Obnazhennaya pipe (Figure 2) belongs to the Middle Mesozoic era of kimberlite formation. It is proved by isotopic methods [21,22] and confirmed by the age of the present fragments of belemnite rostra of the genus Pachyteuthis, characteristic of the Upper Jurassic and Lower Cretaceous [23] and attributed to the Jurassic and Late Jurassic Cylindroteuthis species [24,25]. It is also indicated using a xenolith with wood fragments, defined as Protocedroxylon, which is of Jurassic-Lower Cretaceous age [26].

Figure 2.
Photo of the Obnazhennaya kimberlite pipe.
The appearance of the 18 m high rocky outcrop of the Obnazhennaya pipe is connected with the formation of modern relief in the area of the Kuoyka field (Kuoysko-Daldynskoye uplift), which began to form as a result of tectonic activity in the Late Pliocene-Early Pleistocene. During the onset of denudational development of the region in the Eopleistocene and continuing until the middle Middle Neopleistocene, intensive dissection of the plain took place throughout the territory. The neotectonic regime of the postglacial period led to the formation of erosion-denudation relief, and the region continues to experience insignificant local uplifts at the present stage [27].
The Obnazhennaya pipe is composed of kimberlite breccia with a large number of variations in composition xenoliths of practically unaltered peridotites, websterites, eclogites, metamorphic rocks, dolerites, as well as sedimentary rocks in an amount from 10 to 40%. In the kimberlite pipe, there are blocks composed of both kimberlite breccia with massive cement texture, located in the northwestern part of the pipe, and autolithic kimberlite breccia, located in the southeastern part of the body. The names of the breccias are given in accordance with the classification presented in the paper [28]. According to [29], these breccias are volcaniclastic kimberlite and pyroclastic kimberlite, respectively. Magmatic contacts between these two varieties of kimberlite breccias, despite the ideal rock outcrop, is not established. The structures of the kimberlites are medium- and large-porphyritic. The rocks are composed mainly of unaltered phenocrysts of olivine (about 50%), phlogopite (up to 5.5%), calcite (about 20%), and serpentine (20–40%). The size of rounded idiomorphic and angular olivine grains ranges from 0.05 to 5 mm. The mesostasis rocks are micro- or fine-grained calcite-serpentine, with chromospinelide grains and isolated oval apatite. Calcite is present both as microlites and as xenomorphic precipitates [28].
A small section of the rock outcrop of autolithic kimberlite breccia, beneath a projecting ledge, in an area of less than 1 m2 revealed crusts of white up to 5 mm thick (Figure 3). The needle-like crusts separated easily, along with the darker fragments of kimberlite rock. On rock outcrops and in open fractures, kimberlite breccias are also covered with white crusts, which have a microgranular dense structure, but are composed of calcite.

Figure 3.
Rock outcrop of the Obnazhennaya pipe (a) and white crust of the hydrated magnesium carbonate minerals Nesquehonite and Dypingite on the kimberlite breccia (b).
2.2. Methods
The comprehensive study of samples was carried out using the methods of electron microscopy, X-ray phase and thermal analysis, Infrared and Raman spectroscopy. Chemical composition and micromorphology of grains, crystals, and aggregates of hydrated carbonates of the Obnazhennaya kimberlite pipe were studied on the scanning electron microscopes Jeol JSM-7800F and Jeol JSM-6480LV, with the energy attachments INCA Energy 350 Oxford Instruments (20 kV, 1 nA, beam diameter 1 µm), and INCA Wave Oxford Instruments (20 kV, 45 nA, beam diameter 1 μm, standards: MgKα: olivine, SKα: barite, KKα: orthoclase, CaKα: fluorapatite). To study the mineral composition, small fragments of carbonate crusts were filled with epoxy resin, polished, and sprayed with carbon. Initial mineral diagnosis was performed on a D2 PHASER X-ray diffractometer; a PDF 2 database was used on powder samples. Only transparent grains were selected for thermal and spectroscopic studies. Thermal analysis (DTA, TG, DTG) was performed on a STA 449 C Jupiter, with a sample mass 7 mg, heating rate 10°/min, and Ar C Jupiter atmosphere. IR spectra of the preparation were obtained on IR Fourier Protege 40 and FT-801 spectrometers. Samples were prepared according to the standard scheme. The spectra were taken in the range 600–4000 cm−1 with a resolution of 2 cm−1 and 256 scans. Considering that the crusts under study represent an aggregate of at least the two minerals Nesquehonite and Dypingite, the samples were additionally studied with Raman spectroscopy on an automated Raman-mapping system, Apyron (WITec), with a 488 nm laser, 50 mW, with a scan area of 220 × 80 µm (880 × 320 pixels); the interval of spectrum accumulation in each point was 0.5 s.
3. Results
3.1. Micromorphology and Chemical Composition of the Studied Hydrated Magnesium Carbonate Minerals
Nesquehonite occurs in the form of aggregates consisting of unoriented, not tightly adjoining, prismatic crystals (Figure 4a), the maximum size of which reaches 100 µm. Rarely, parallel prismatic Nesquehonite grains, which form aggregates in the form of translucent kidney-shaped crusts up to 0.4 mm thick, are observed (Figure 4b).
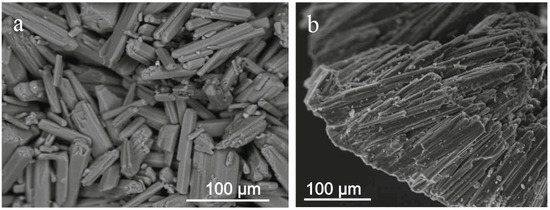
Figure 4.
SEM image of fragments of Nesquehonite aggregates: unoriented crystals (a), kidney-shaped formations (b).
Nesquehonite and Dypingite are epigenetic low-temperature minerals. According to the phase transitions in the Mg-CO2-H2O system, Dypingite is formed during the transformation of Nesquehonite into Hydromagnesite Mg5(CO3)4(OH)2•4H2O, which subsequently transforms into the more stable Magnesite MgCO3 [30]. In the investigated samples, Dypingite occurs less frequently than Nesquehonite. It forms rose-shaped aggregates of lamellar crystals up to 30 µm in size (Figure 5a,b), or patches on prismatic Nesquehonite crystals.
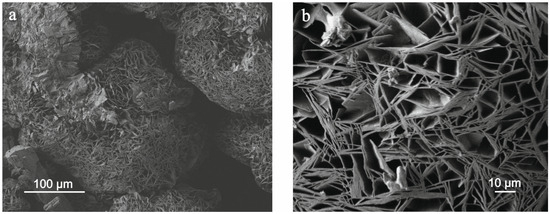
Figure 5.
SEM images of rose-shaped aggregates of Dypingite (a) formed by lamellar crystals (b).
Due to the presence of a large number of voids in the aggregates of crystals and the presence of numerous cracks in them, the burnout of minerals under the probe beam means it is difficult to obtain quantitative compositions of minerals. In EDS spectra of nesquegonite (transparent grains), other components, except MgO, were not found. In the composition of dipingite, there is an admixture of SO2 of up to 2%, and also in unstable and minimal amounts—K2O and CaO (Table 1).

Table 1.
Chemical composition of studied hydrated magnesium carbonate minerals.
3.2. X-ray Phase Analysis
The data of the X-ray phase analysis of the bulk samples of carbonate crusts with fragments of the main mass of the rock showed that the studied material is a mixture of the usual minerals of kimberlites: serpentine, mica, calcite, olivine with a significant amount of Nesquehonite and traces (quite low concentration) of Dypingite (Figure 6). According to the results of the study of preparations selected from the crusts, it was found that the transparent grains were represented by almost-pure Nesquehonite, and white aggregates were a mixture of Dypingite and Nesquehonite (Figure 7).
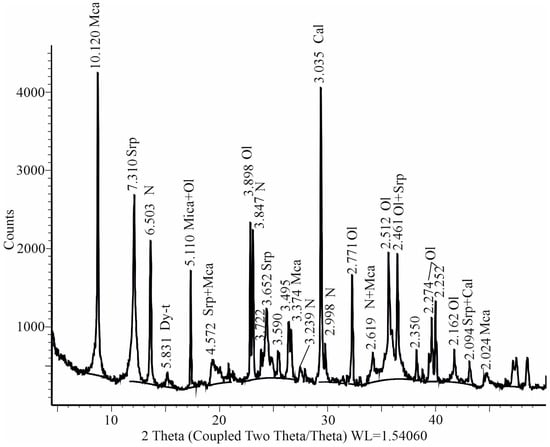
Figure 6.
The fragment of an X-ray diffractogram of samples of carbonate crusts with fragments of the main mass of kimberlite rock: Cal is Calcite, Dy-t is Dypingite, Mca is Mica, N is Nesquehonite, Ol is Olivine, Srp is Serpentine.
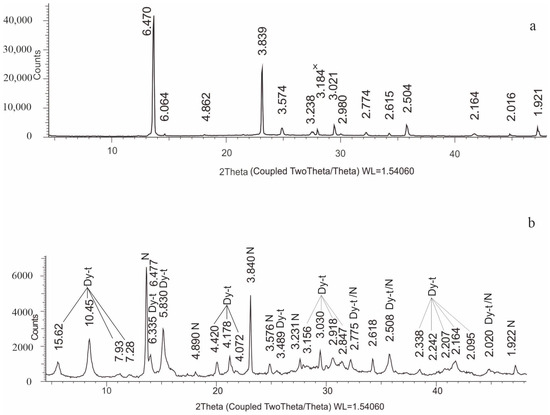
Figure 7.
The fragments of X-ray diffractograms of transparent crystals (a) and white aggregate (b): Dy-t is Dypingite, N is Nesquehonite, x is the impurity line.
3.3. Thermogravimetric Characteristics and IR Spectra
The thermogram of transparent grains is characterized by five endothermic effects at 125, 167, 219, 440 and 493 °C (Figure 8). The first three correspond to the removal of three water molecules; the mass loss of approximately 37% is close to the amount of water in the mineral. Decomposition of carbonate occurs at 440 °C; mass loss of ~30% is close to the theoretical, then there is the crystallization of a new phase of MgO. Our results are very close to the data of synthetic Nesquehonite [31].
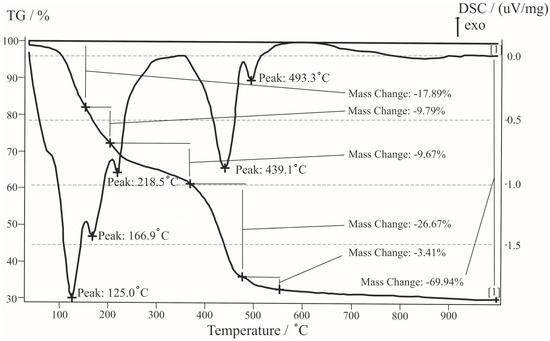
Figure 8.
Thermogram of Nesquehonite.
The sample prepared from transparent grains was studied on two IR Fourier Protege 40 and “FT-801” spectrometers. The obtained IR spectra are similar to those described in the paper [32], characterized by the absorption bands typical for Nesquehonite such as the symmetric modes of stretching (v1) and bending (v2) (CO3)2 at 1099 cm−1 and 854 cm−1, respectively. The three bands at 1520, 1469, and 1421 cm−1 are attributed to asymmetric stretching with splitting mode (v3) [33,34,35]. Stretching of OH groups and H2O molecules gives broad bands in the region between 2500 and 4000 cm−1 [33,36]. The 3125 and 3261 cm−1 bands correspond to the stretching vibrations of water. The 3455 and 3559 cm−1 bands can be attributed to the OH stretching modes of the valence bond of water in the crystal structure [37,38]. The two peaks observed at 699 cm−1 and 607 cm−1 correspond to the bending mode in the (v4) HCO3 plane [39,40]. (Figure 9 and Figure 10 (S1).
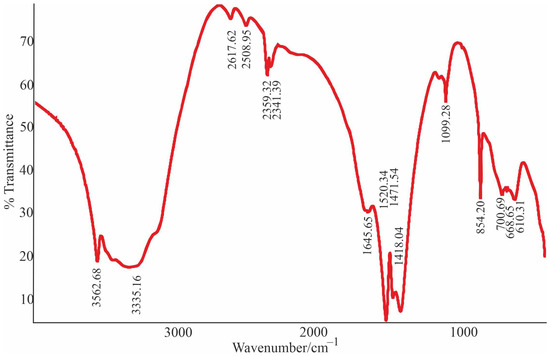
Figure 9.
IR spectrum of Nesquehonite.
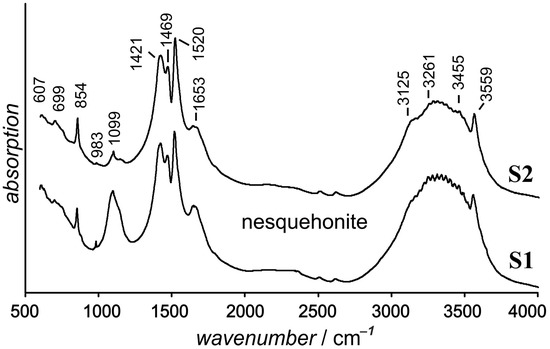
Figure 10.
IR spectra of preparations prepared from outer water-transparent (S1) and inner white (S2) aggregate crystals.
The spectra of the preparation made of white microgranular aggregates (Figure 10, S2) were obtained on the spectrometer “FT-801”. The spectra of the white material (Figure 10, S2) differ from the spectra of the elongated transparent crystals (Figure 10, S1) only in the intensity of the bands of ~1099 cm−1 and 983 cm−1. The bands close to 1099 cm−1 refer to the symmetric stretching mode of (CO3)2. The occurrence of several bands in this region may indicate the presence of several non-equivalent carbonate links in the Nesquehonite structure. The broadening of the 1653 cm−1 band of bending water oscillations [37,38,41] indicates a greater variety of hydrogen bonds.
3.4. Raman Spectroscopy
The samples consisting of transparent prismatic crystals and the micrograin aggregates replacing them were selected for study using Raman spectroscopy. In the spectra of prismatic crystals, only the Dypingite bands appear (950–1098, 3141–3649 cm−1) (Figure 11a). The spectrum of microgranular aggregates is characterized by bands 705, 986, 1117–1124 and 3141–3649 cm−1, corresponding to Nesquehonite and Dypingite (Figure 11b). The study of samples using Raman mapping, applied for the first time in the study of hydrated magnesia carbonate minerals, confirms the obtained data and shows the formation of Dypingite by Nesquehonite (Figure 12).
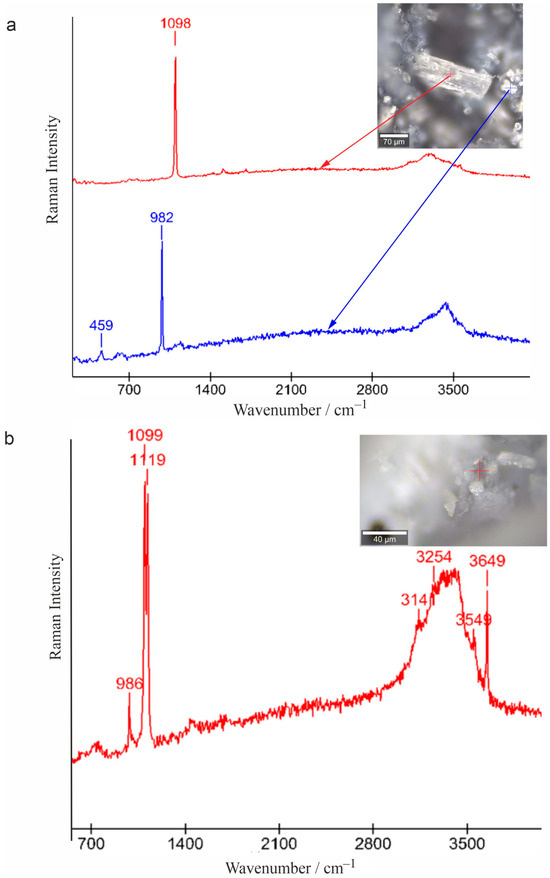
Figure 11.
Raman spectra of prismatic Dypingite crystal (a) and microgranular aggregates (Dypingite and Nesquehonite) (b); the points where they were obtained are indicated in the inset photos.
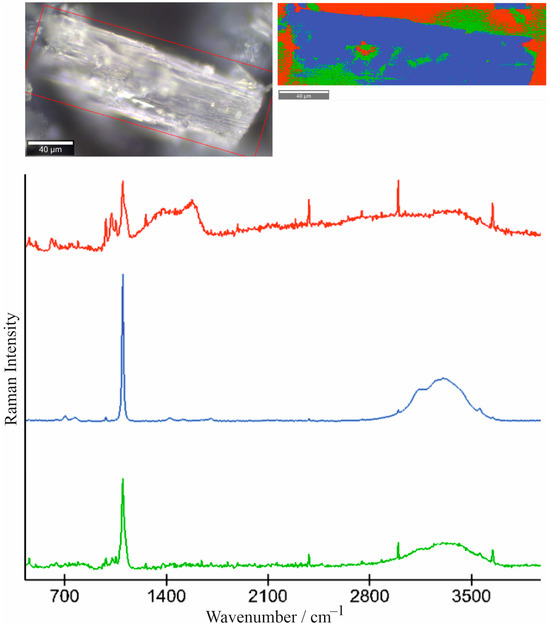
Figure 12.
The data of Raman mapping. The color of the spectra and the image corresponding: green indicates Nesquehonite, blue is Dypingite, red is air and what has fallen into the focus of the lens.
4. Discussion and Conclusions
For the first time in kimberlite rocks (on rock outcrops of kimberlite breccia of the Obnazhennaya pipe), the hydrated magnesium carbonates Nesquehonite and Dypingite have been established. They are also associated with the process of the weathering of endogenous minerals, as well as magnesium carbonate minerals from the dumps of the Clynton Creek chrysotile deposit, Yukon Territory [31]. The scheme of formation of the studied hydrated carbonate minerals, apparently, is close to the proposed in the paper [31], when CO2 from air is used for formation of magnesium carbonates. In our case, it is most likely that rainfall or water, formed as a result of snow melting, are filtered through the talus located in the upper part of the outcrop of the Obnazhennaya kimberlite pipe. At the same time, the solutions are enriched with Mg and CO2 contained in the minerals, as evidenced by the chemical composition of the autolithic kimberlite breccia (Chemical composition, % SiO2 28.66; TiO2 0.50; Al2O3 1.00; Cr2O3 0.19; Fe2O3 2.01; FeO 4.35%; MnO 0.22; MgO 28.67; CaO 14.45; Na2O 0.73; K2O 1.25; P2O5 1.13; SO3 0.16; P p.p. 5.03; CO2 11.2; Sum 99.72) [29], on which aggregates of the minerals under study are formed after seepage solutions get on a vertical rock wall of kimberlite breccia where, in modern conditions as a result of evaporation, there is a deposition of hydrated carbonate minerals found by us. Protruding over the rock wall, the canopy plays an important role in creating conditions for the appearance of Nesquehonite and Dypingite. In rock outcrops that do not have canopies in the upper part, there are no crusts formed by Nesquehonite and Dypingite.
In summary, despite the fact that the introduction of the kimberlite pipe took place in the Middle Mesozoic epoch of kimberlite formation [22], the mineral formation of Nesquehonite and Dypingite, associated with kimberlite rocks, occurs in modern times due to the coincidence of several natural factors, and, primarily, the presence of a unique natural outcrop of kimberlite rocks.
Author Contributions
Conceptualization, S.S.U., O.B.O. and N.V.Z.; methodology, S.S.U., O.B.O. and N.V.Z.; investigation, S.S.U., O.B.O. and N.V.Z.; writing—original draft preparation, S.S.U., O.B.O. and N.V.Z.; writing—review and editing S.S.U. and N.V.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Diamond and Precious Metals Geology Institute FUEM-2019-0003.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to N.N. Emelyanova, I.N. Zueva and A.E. Molotkov for help and advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zinchuk, N.N. Postmagmatic Minerals of Kimberlites; Nedra: Moscow, Russia, 2000; p. 538. [Google Scholar]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatskiy, V.S.; Arauho, D.; Griffin, V.L. Carbonate and silicate media of crystallization of fibrous diamonds from placers in the northeast of the Siberian platform. Geol. Geophys. 2011, 52, 1649–1664. [Google Scholar]
- Logvinova, A.M.; Virt, R.; Tomilenko, A.A.; Afanasyev, V.P.; Sobolev, N.V. Features of the phase composition of nanosized inclusions in alluvial diamonds of the Northeastern Siberian platform. Geol. Geophys. 2011, 52, 1634–1648. [Google Scholar]
- Ionov, D.A.; Doucet, L.S.; Xu, Y.; Golovin, A.V.; Oleinikov, O.B. Reworking of Archean mantle in the NE Siberian craton by carbonatite and silicate melt metasomatism: Evidence from a carbonate-bearing, dunite-to websterite xenolith suite from the Obnazhennaya kimberlite. Geochim. Cosmochim. Acta 2018, 224, 132–153. [Google Scholar] [CrossRef]
- Golovin, A.V.; Kamenetsky, V.S. Compositions of kimberlite melts: A review of studies of melt inclusions in kimberlite minerals. Petrology 2023, 31, 115–152. [Google Scholar] [CrossRef]
- Zayakina, N.V.; Oleinikov, O.B.; Vasileva, T.I.; Oparin, N.A. Coalingite from kimberlite breccia of the Manchary pipe, Central Yakutia. Geol. Ore Depos. 2015, 57, 732–736. [Google Scholar] [CrossRef]
- Hansen, L.D.; Dipple, G.M.; Gordon, T.M.; Kellet, D.A. Carbonated serpentinite (listwanite) at Atlin, British Columbia: A geological analogue to carbon dioxide sequestration. Can. Mineral. 2005, 43, 225–239. [Google Scholar] [CrossRef]
- Wilson, S.A.; Raudsepp, M.; Dipple, G.M. Verifying and quantifying carbon fixation in minerals from serpentine-rich mine tailings using the Rietveld method with X-ray powder diffraction data. Am. Mineral. 2006, 91, 1331–1341. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabites, J.E.; Southam, G. Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: Examples from the Clinton Creek and Cassiar chrysotile deposits. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Garanin, V.K.; Posukhova, T.V.; Garanin, K.V. Mineralogy of Diamond Deposits; Max-Press: Moscow, Russia, 2012; p. 329. [Google Scholar]
- Mervine, E.M.; Wilson, S.A.; Power, I.M.; Dipple, G.M.; Turvey, C.C.; Hamilton, J.L.; Vanderzee, S.; Raudsepp, M.; Southam, C.; Matter, J.M.; et al. Potential for offsetting diamond mine carbon emissions through mineral carbonation of processed kimberlite: An assessment of De Beers mine sites in South Africa and Canada. Mineral. Petrol. 2018, 112 (Suppl. S2), 755–765. [Google Scholar] [CrossRef]
- Masanov, A.Y.; Dubovichev, M.A.; Tolstov, A.V.; Anisimova, P.S.; Garanin, K.V.; Dorokhov, A.V.; Baranovskaya, V.B. Offseting potential of GHG emissions of ALROSA Group enterprises due to carbonation of waste kimberliteю Ratsional’noye osvoyeniye nedr. Miner. Min. Conserv. (MMC) 2021, 4, 64–73. [Google Scholar]
- Genth, F.A.; Penfield, S.L. On lansfordite, nesquehonite, a new mineral, and pseudomorphs of nesquehonite after lansfordite. Am. J. Sci. 1890, 139, 121–137. [Google Scholar] [CrossRef][Green Version]
- Pisarsky, B.I.; Konev, A.A. About the find of nesvegonite in Transbaikalia. DAN SSSR 1971, 200, 1423–1425. [Google Scholar]
- Giester, G.; Lengauer, C.; Rieck, B. The crystal structure of nesquehonite, MgCO3·3H2O, from Lavrion, Greece. Mineral. Petrol. 2000, 70, 153–163. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Filatov, S.K.; Serafimova, U.K.; Sergeeva, S.V. Chlorartinite Mg2(CO3)ClOH 3H2O—A new mineral of volcanic exhalations. ZVMO 1998, 2, 55–59. [Google Scholar]
- Cesnokov, B.; Kotrly, M.; Nisanbajev, T. Brennende Abraumhalden und Aufschlüsse im Tscheljabinsker Kohlenbecken-eine reiche Mineralienküche. Mineralien-Welt 1998, 9, 54–63. [Google Scholar]
- Mazurov, M.P.; Grishina, S.N.; Istomin, V.E.; Titov, A.T. Metasomatism and Ore Formation at Contacts of Dolerite with Saliferous Rocks in the Sedimentary Cover of the Southern Siberian Platform. Geol. Ore Depos. 2007, 49, 271–284. [Google Scholar] [CrossRef]
- Pavlovsky, A.B.; Pechenkin, I.G.; Lugovskaya, I.G. Geological and Industrial Types of Mineral Deposits; Tin. Study Guide; VIMS: Moscow, Russia, 2015. [Google Scholar]
- Raade, G. Dypingite, a new hydrous basic carbonate of magnesium from Norvay. Am. Mineral. 1970, 55, 1457–1465. [Google Scholar]
- Brakhfogel, F.F. Geological Aspects of Kimberlite Magmatism in the North-Eastern Siberian Platform; Yakutian Branch of SO AN USSR Press: Yakutsk, Russia, 1984; p. 128. [Google Scholar]
- Zaitsev, A.I.; Smelov, A.P. Isotope Geochronology of Kimberlite Rock Formation of the Yakutsk Province; Ofset: Yakutsk, Russia, 2010; p. 108. [Google Scholar]
- Milashev, V.A.; Shulgina, N.I. New data on the age of kimberlites of the Siberian platform. DAN USSR 1959, 126, 1320–1322. [Google Scholar]
- Malkov, B.A.; Gustomesov, V.A. Jurassic fauna in the kimberlites of the Olenek uplift and the age of kimberlite volcanism in the northeast of the Siberian platform. DAN USSR 1976, 229, 435–438. [Google Scholar]
- Malkov, B.A.; Silin, Y.I.; Tsovbun, Y.M. Radiological evidence of xenogenicity of “porphyry inclusions” of olivine, pyrope, chromdiopside in kimberlites. Rep. USSR Acad. Sci. 1979, 245, 927–929. [Google Scholar]
- Kostrovitsky, S.I.; Admakin, L.A. The discovery of xenolith wood in a kimberlite pipe Exposed. Geol. Geophys. 1991, 11, 82–84. [Google Scholar]
- Grinenko, V.S.; Yuganova, L.A.; Truschelev, A.N.; Malanin, Y.A.; Smetannikova, L.I.; Knyazev, V.G.; Prokopiev, A.V.; Kazakova, G.G.; Protopopov, R.I. State Geological Map of the Russian Federation. Scale 1:1,000,000 (Third Generation); Sheet R-51–Jarjan. Explanatory Note; Publishing House of St. Petersburg Kartfabriki VSEGEI: St. Petersburg, Russia, 2012; p. 379. [Google Scholar]
- Kornilova, V.P.; Nikolaev, L.I. Petrography and chemical nature of kimberlite and comagmatic rocks of the Kuoyka field. In Kimberlite and Basite Magmatism of the Olenek Uplift Area. Yakutsk; Ofset: Yakutsk, Russia, 1980; pp. 92–106. [Google Scholar]
- Smith, B.S.; Nowicki, T.E.; Russell, J.K.; Webb, K.; Mitchell, R.H.; Hetman, C.; vA Robey, J. A Glossary of Kimberlite and Related Terms (Part 1, Part 2, Part 3); Scott-Smith Petrology Inc.: Vancouver, BC, Canada, 2017. [Google Scholar]
- Ballirano, P.; De Vito, C.; Mignardi, S.; Ferrini, V. Phase transitions in the MgCO2H2O system and the thermal decomposition of dypingite, Mg3 (CO3)4(OH)2·5H2O: Implications for geosequestration of carbon dioxide. Chem. Geol. 2013, 40, 59–67. [Google Scholar] [CrossRef]
- Jauffret, G.; Morrison, J.; Glasser, F.P. On the thermal decomposition of nesquehonite. J. Therm. Anal. Calorim. 2015, 122, 601–609. [Google Scholar] [CrossRef]
- Skliros, V.; Anagnostopoulou, A.; Tsakiridis, P.; Perraki, M. Mineralogical and spectroscopic study of nesquehonite synthesized by reaction of gaseous CO2 with Mg chloride solution. Bull. Geol. Soc. Greece 2016, 50, 2009–2017. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Martens, W.N.; Nothdurft, L.; Duong, L.V.; Webb, G.E. Low temperature synthesis and characterization of nesquehonite. J. Mater. Sci. Lett. 2003, 22, 825–829. [Google Scholar] [CrossRef]
- Coleyshaw, E.; Crump, G.; Griffith, W. Vibrational spectra of the hydrated carbonate minerals ikaite, monohydrocalcite, lansfordite and nesquehonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2231–2239. [Google Scholar] [CrossRef]
- Morgan, B.; Wilson, S.A.; Madsen, I.C.; Gozukara, Y.M.; Habsuda, J. Increased thermal stability of nesquehonite (MgCO3•3H2O) in the presence of humidity and CO2: Implications for lowtemperature O2 storage. Int. J. Greenh. Gas Control 2015, 39, 366–376. [Google Scholar] [CrossRef]
- Ferrini, V.; De Vito, C.; Mignardi, S. Synthesis of nesquehonite by reaction of gaseous CO2 with Mg chloride solution: Its potential role in the sequestration of carbon dioxide. J. Hazard. Mater. 2009, 168, 832–837. [Google Scholar] [CrossRef]
- Hopkinson, L.; Rutt, K.; Cressey, G. The transformation of nesquehonite to hydromagnesite in the system CaO-MgO-H2O-CO2: An experimental spectroscopic study. J. Geol. 2008, 116, 387–400. [Google Scholar] [CrossRef]
- Hopkinson, L.; Kristova, P.; Rutt, K.; Cressey, G. Phase transitions in the systemMgO-CO2-H2O during CO2 degassing of Mg-bearing solutions. Geochim. Cosmochim. Acta 2012, 76, 1–13. [Google Scholar] [CrossRef]
- Hales, M.C.; Frost, R.L.; Martens, W.N. Thermo-Raman spectroscopy of synthetic nesquehonite—implication for the geosequestration of greenhouse gases. J. Raman Spectrosc. 2008, 39, 1141–1149. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Infrared and infrared emission spectroscopy of nesquehonite Mg(OH)(HCO3)•2H2O-implications for the formula of nesquehonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Lanas, J.; Alvarez, J.I. Dolomitic lime: Thermal decomposition of nesquehonite. Thermochim. Acta 2004, 421, 123–132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).