Unlocking the Saponite Potential in Aided Phytostabilisation of Multi-Metal-Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Saponite Characteristics
2.3. Experiment Design
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
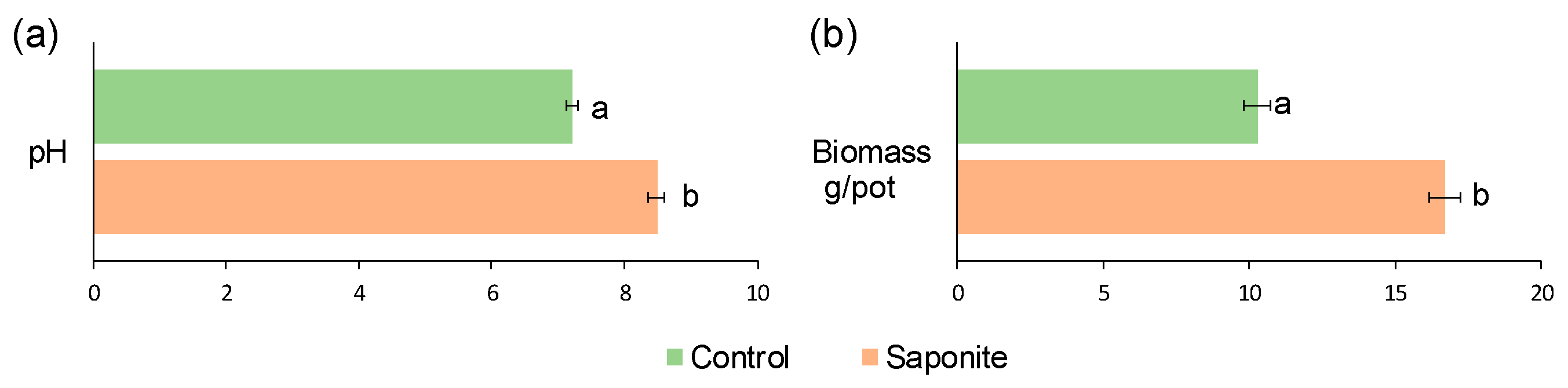
3.1. Soil pH and Plant Biomass
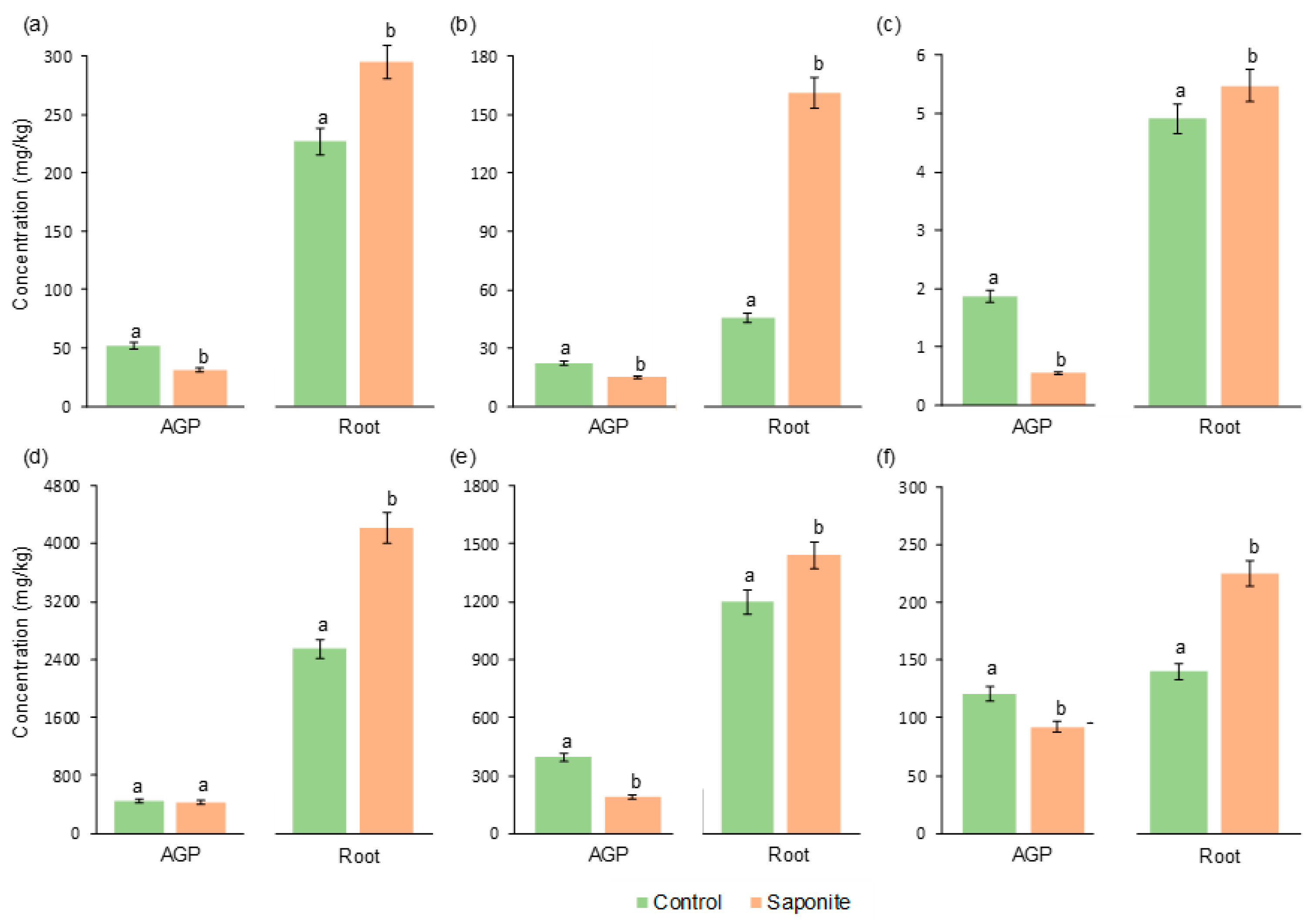
3.2. Metals in Plants following Phytostabilisation
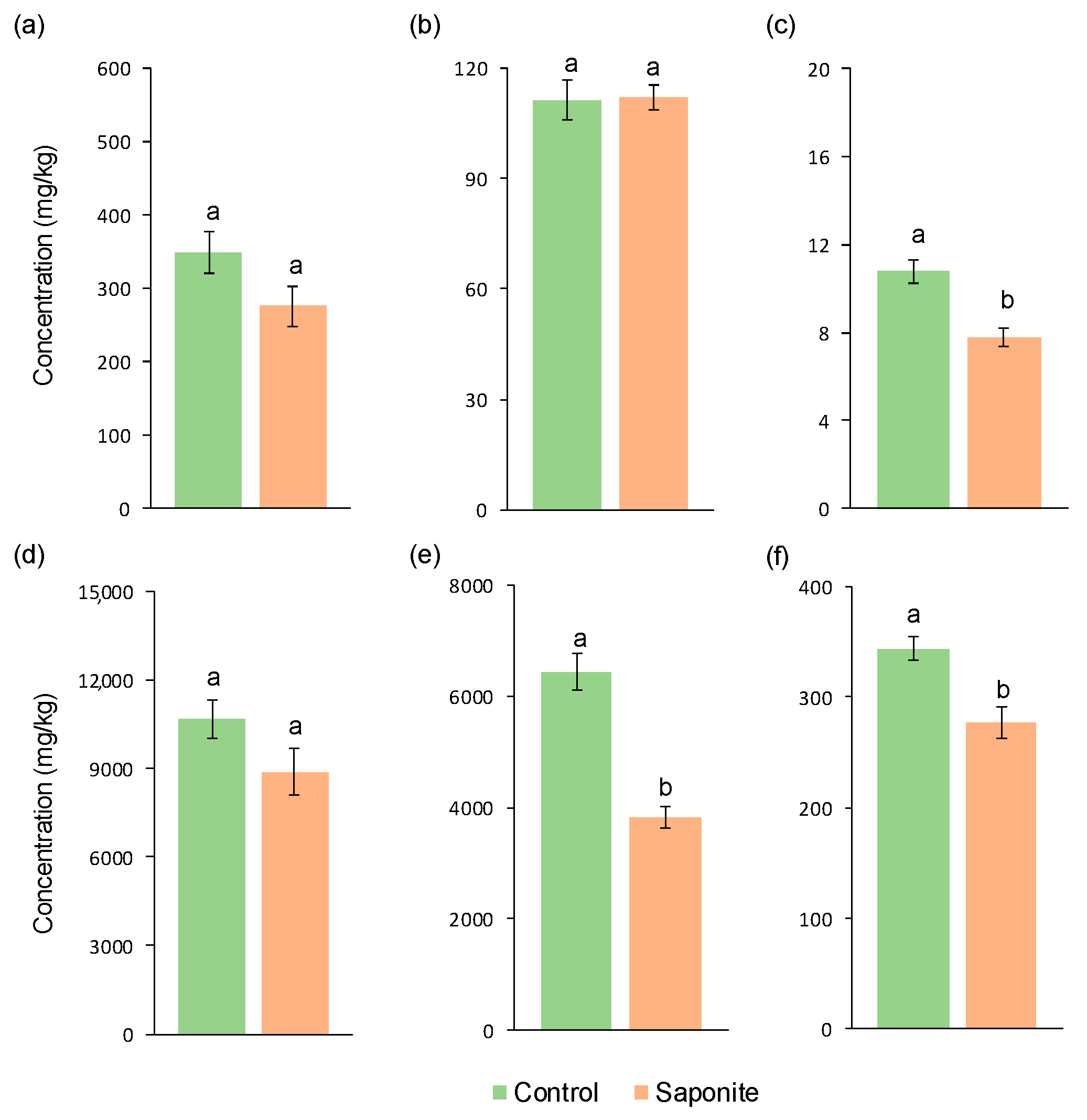
3.3. Metals in the Soil and Exchangeable Fraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gavrilescu, M. Water, Soil, and Plants Interactions in a Threatened Environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- González-Martínez, A.; de Simón-Martín, M.; López, R.; Táboas-Fernández, R.; Bernardo-Sánchez, A. Remediation of Potential Toxic Elements from Wastes and Soils: Analysis and Energy Prospects. Sustainability 2019, 11, 3307. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Yang, J.; Chen, T. Three-Year Field Experiment on the Risk Reduction, Environmental Merit, and Cost Assessment of Four in Situ Remediation Technologies for Metal(Loid)-Contaminated Agricultural Soil. Environ. Pollut. 2020, 266, 115193. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, D.S.; Parthiban, D. Effect of Solid Waste Based Stabilizing Material for Strengthening of Expansive Soil- A Review. Environ. Technol. Innov. 2020, 20, 101108. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A State-of-the-Art Review of Polymers Used in Soil Stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Strawn, D.G. Sorption Mechanisms of Chemicals in Soils. Soil Syst. 2021, 5, 13. [Google Scholar] [CrossRef]
- Firoozi, A.A.; Guney Olgun, C.; Firoozi, A.A.; Baghini, M.S. Fundamentals of Soil Stabilization. Int. J. Geo-Eng. 2017, 8, 26. [Google Scholar] [CrossRef]
- Lan, M.M.; Liu, C.; Liu, S.J.; Qiu, R.L.; Tang, Y.T. Phytostabilization of Cd and Pb in highly polluted farmland soils using ramie and amendments. Int. J. Environ. Res. Public Health 2020, 17, 1661. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Radziemska, M. Influence of chromium (III) and (VI) on the concentration of mineral elements in oat (Avena sativa L.). Fresenius Environ. Bull. 2013, 22, 979–986. [Google Scholar]
- Siebielec, S.; Siebielec, G.; Marzec-Grządziel, A.; Pecio, M.; Stuczyński, T. Testing combined effect of amendments and inoculation with bacteria for improving phytostabilisation of smelter waste extremely contaminated with trace elements. Agronomy 2021, 11, 2064. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.; Yao, Z.; Lin, Q.; Yan, B.; Cui, X.; He, Z.; Yang, X.; Wang, C.H.; Chen, G. Phytoremediation of Cd-Contaminated Farmland Soil via Various Sedum Alfredii-Oilseed Rape Cropping Systems: Efficiency Comparison and Cost-Benefit Analysis. J. Hazard. Mater. 2021, 419, 126489. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Lounès-Hadj Sahraoui, A.; Fontaine, J. Recent Advances in Microbial-Aided Phytostabilization of Trace Element Contaminated Soils. In Advances in Microbe-Assisted Phytoremediation of Polluted Sites, 1st ed.; Kuldeep, B., Ying, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 8, pp. 165–206. [Google Scholar] [CrossRef]
- Klik, B.; Holatko, J.; Jaskulska, I.; Gusiatin, M.Z.; Hammerschmiedt, T.; Brtnicky, M.; Liniauskienė, E.; Baltazar, T.; Jaskulski, D.; Kintl, A.; et al. Bentonite as a Functional Material Enhancing Phytostabilization of Post-Industrial Contaminated Soils with Heavy Metals. Materials 2022, 15, 8331. [Google Scholar] [CrossRef] [PubMed]
- Visconti, D.; Ventorino, V.; Fagnano, M.; Woo, S.L.; Pepe, O.; Adamo, P.; Caporale, A.G.; Carrino, L.; Fiorentino, N. Compost and Microbial Biostimulant Applications Improve Plant Growth and Soil Biological Fertility of a Grass-Based Phytostabilization System. Environ. Geochem. Health 2022, 45, 787–807. [Google Scholar] [CrossRef] [PubMed]
- Radziemska, M.; Gusiatin, Z.M.; Bęś, A.; Czajkowska, J.; Mazur, Z.; Hammerschmiedt, T.; Sikorski, Ł.; Kobzova, E.; Klik, B.K.; Sas, W.; et al. Can the Application of Municipal Sewage Sludge Compost in the Aided Phytostabilization Technique Provide an Effective Waste Management Method? Energies 2021, 14, 1984. [Google Scholar] [CrossRef]
- Sarathchandra, S.S.; Rengel, Z.; Solaiman, Z.M. Remediation of Heavy Metal-Contaminated Iron Ore Tailings by Applying Compost and Growing Perennial Ryegrass (Lolium Perenne L.). Chemosphere 2022, 288, 132573. [Google Scholar] [CrossRef]
- Nagy, A.; Magyar, T.; Kiss, N.É.; Tamás, J. Composted Sewage Sludge Utilization in Phytostabilization of Heavy Metals Contaminated Soils. Int. J. Phytoremediation 2023, 25, 1510–1523. [Google Scholar] [CrossRef]
- Thouin, H.; Norini, M.P.; Battaglia-Brunet, F.; Gautret, P.; Crampon, M.; Le Forestier, L. Temporal evolution of surface and sub-surface geochemistry and microbial communities of Pb-rich mine tailings during phytostabilization: A one-year pilot-scale study. J. Environ. Manag. 2022, 318, 115538. [Google Scholar] [CrossRef]
- Garau, M.; Castaldi, P.; Diquattro, S.; Pinna, M.V.; Senette, C.; Roggero, P.P.; Garau, G. Combining grass and legume species with compost for assisted phytostabilization of contaminated soils. Environ. Technol. Innov. 2021, 22, 101387. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Zhang, S.; Yi, Q.; Zhou, J.; Fang, G.; Zhou, J. Bioavailability and mobility of copper and cadmium in polluted soil after phytostabilization using different plants aided by limestone. Chemosphere 2020, 242, 125252. [Google Scholar] [CrossRef]
- Fronczyk, J.; Radziemska, M.; Mazur, Z. Copper Removal from Contaminated Groundwater Using Natural and Engineered Limestone Sand in Permeable Reactive Barriers. Fresenius Environ. Bull. 2015, 24, 228–234. [Google Scholar]
- Yanushevska, O.I.; Dontsova, T.A.; Aleksyk, A.I.; Vlasenko, N.V.; Didenko, O.Z.; Nypadymka, A.S. Surface and Structural Properties of Clay Materials Based on Natural Saponite. Clays Clay Miner. 2020, 68, 465–475. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy. Available online: https://handbookofmineralogy.org/ (accessed on 31 July 2023).
- Theo Kloprogge, J.; Ponce, C.P. Spectroscopic Studies of Synthetic and Natural Saponites: A Review. Minerals 2021, 11, 112. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhou, Q.; Wu, Q.Q.; Petit, S.; Jiang, X.C.; Xia, S.T.; Li, C.S.; Yu, W.H. Modification, Hybridization and Applications of Saponite: An Overview. Appl. Clay Sci. 2019, 168, 136–154. [Google Scholar] [CrossRef]
- ISO 18400-100:2017; Soil Quality—Sampling—Part 100: Guidance on the Selection of Sampling Standards. International Organization for Standardization: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/67788.html (accessed on 31 July 2023).
- OME. Ordinance of the Minister of Environment on Soil and Ground Quality Standards. J. Law. 2016, 395, 1–86. [Google Scholar]
- Gusiatin, Z.M.; Klik, B.; Kulikowska, D. Tannic Acid for Remediation of Historically Arsenic-Contaminated Soils. Environ. Technol. 2017, 40, 1050–1061. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D.; Klik, B.K.; Hajdukiewicz, K. Ecological Risk Assessment of Sewage Sludge from Municipal Wastewater Treatment Plants: A Case Study. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 1167–1176. [Google Scholar] [CrossRef]
- Pueyo, M.; López-Sánchez, J.F.; Rauret, G. Assessment of CaCl2, NaNO3 and NH4NO3 Extraction Procedures for the Study of Cd, Cu, Pb and Zn Extractability in Contaminated Soils. Anal. Chim. Acta 2004, 504, 217–226. [Google Scholar] [CrossRef]
- R Development Core Team. The R Manuals. Available online: https://cran.r-project.org/manuals.html (accessed on 22 February 2023).
- Nikitina, M.V.; Popova, L.F.; Nakvasina, E.N.; Romanov, E.M.; Zhuravleva, E.A. Possibility Determination of Using Saponite in Agriculture. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 022016. [Google Scholar] [CrossRef]
- Borchardt, G. Smectites. Miner. Soil Environ. 2018, 1, 675–727. [Google Scholar] [CrossRef]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of Heavy Metals in a Contaminated Soil: Effects of Redox Potential and PH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Bosak, V.; Sachyuka, T.; Akulich, M. Application of Saponite-Containing Basaltic Tuffs to Improve the Cultivation of Vegetable Crops. In Proceedings of the Jahrestagung der DBG/BGS Erd-Reich und Boden-Landschaften, Bern, Switzerland, 24–27 August 2019. [Google Scholar]
- Laboski, C. Basic Concepts of Soil Fertility [in] Soil Fertility Basics. Available online: https://extension.soils.wisc.edu/management-topics/soil-fertility-topics/soil-fertility-basics/ (accessed on 31 July 2023).
- O’Geen, A.T. Soil Water Dynamics. Available online: https://www.nature.com/scitable/knowledge/library/soil-water-dynamics-103089121/ (accessed on 31 July 2023).
- Sokol, H.; Sprynskyy, M.; Ganzyuk, A.; Raks, V.; Buszewski, B. Structural, Mineral and Elemental Composition Features of Iron-Rich Saponite Clay from Tashkiv Deposit (Ukraine). Colloids Interfaces 2019, 3, 10. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Tőzsér, D.; Horváth, R.; Simon, E.; Magura, T. Heavy Metal Uptake by Plant Parts of Populus Species: A Meta-Analysis. Environ. Sci. Pollut. Res. 2023, 30, 69416–69430. [Google Scholar] [CrossRef]
- Shojaei, S.; Jafarpour, A.; Shojaei, S.; Gyasi-Agyei, Y.; Rodrigo-Comino, J. Heavy Metal Uptake by Plants from Wastewater of Different Pulp Concentrations and Contaminated Soils. J. Clean. Prod. 2021, 296, 126345. [Google Scholar] [CrossRef]
- Afzal, M.R.; Naz, M.; Wan, J.; Dai, Z.; Ullah, R.; Rehman, S.U.; Du, D. Insights into the Mechanisms Involved in Lead (Pb) Tolerance in Invasive Plants—The Current Status of Understanding. Plants 2023, 12, 2084. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Cetin, S.; Iqbal Bhanger, M. Lead Sorption by Waste Biomass of Hazelnut and Almond Shell. J. Hazard. Mater. 2009, 167, 1203–1208. [Google Scholar] [CrossRef]
- Wu, J.; Hsu, F.C.; Cunningham, S.D. Chelate-Assisted Pb Phytoextraction: Pb Availability, Uptake, and Translocation Constraints. Environ. Sci. Technol. 1999, 33, 1898–1904. [Google Scholar] [CrossRef]
- Luo, C.L.; Shen, Z.G.; Li, X.D. Root Exudates Increase Metal Accumulation in Mixed Cultures: Implications for Naturally Enhanced Phytoextraction. Water Air Soil Pollut. 2008, 193, 147–154. [Google Scholar] [CrossRef]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Competitive Sorption and Desorption of Heavy Metals by Individual Soil Components. J. Hazard. Mater. 2007, 140, 308–315. [Google Scholar] [CrossRef]
- Ouhadi, V.R.; Yong, R.N.; Deiranlou, M. Enhancement of Cement-Based Solidification/Stabilization of a Lead-Contaminated Smectite Clay. J. Hazard. Mater. 2021, 403, 123969. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ou, J.; Wang, X.; Yan, Z.; Zhou, Y. Immobilization of Soil Cadmium Using Combined Amendments of Illite/Smectite Clay with Bone Chars. Environ. Sci. Pollut. Res. 2018, 25, 20723–20731. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, S.; Gu, P.; Liu, S.; Yang, G. Adsorption and Removal of Metal Ions by Smectites Nanoparticles: Mechanistic Aspects, and Impacts of Charge Location and Edge Structure. Appl. Clay Sci. 2021, 201, 105957. [Google Scholar] [CrossRef]
- Müller, H.-J.; Dobler, D.; Schmidts, T.; Rusch, V. Smectite for Medical Use and Their Toxin Binding Capacity. J. Food Nutr. Popul. Health 2019, 3, 16. [Google Scholar] [CrossRef]
- Lucherini, A.; Gonzalez-Ollauri, A.; Mickovski, S.B. The effect of vegetation on soil polluted with galligu: Phytostabilisation and novel approaches to evaluate soil galligu concentration. Environ. Geotech. 2020, 9, 399–411. [Google Scholar] [CrossRef]
| Measurement | Cu | Ni | Cd | Pb | Zn | Cr |
|---|---|---|---|---|---|---|
| (mg/kg) | ||||||
| Concentration | 671.0 | 113.3 | 22.4 | 15,290.0 | 8433.4 | 727.4 |
| Standard deviation | 20.4 | 6.8 | 2.5 | 138.8 | 244.7 | 12.6 |
| Acceptable values | 600 | 500 | 15 | 600 | 2000 | 1000 |
| Characteristic | Unit | Value |
|---|---|---|
| Colour | - | White–grey |
| Hardness | Mohs scale | 1.0 |
| Density | (g/cm3) | 2.1 |
| Specific surface | (m2/g) | 600 |
| Cation exchange capacity | (cmol(+)/kg) | 60 |
| Soil Sample | Cu | Ni | Cd | Pb | Zn | Cr |
|---|---|---|---|---|---|---|
| (mg/kg) | ||||||
| Control | 0.13 ± 0.010 | 0.03 ± 0.001 | 0.05 ± 0.001 | 0.08 ± 0.001 | 0.24 ± 0.006 | 0.74 ± 0.020 |
| Saponite | 0.08 ± 0.001 | 0.02 ± 0.001 | 0.03 ± 0.001 | 0.05 ± 0.001 | 0.17 ± 0.003 | 0.61 ± 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klik, B.; Brtnicky, M.; Jaskulska, I.; Gusiatin, M.Z.; Jaskulski, D.; Holatko, J.; Baltazar, T.; Liniauskiene, E.; Radziemska, M. Unlocking the Saponite Potential in Aided Phytostabilisation of Multi-Metal-Contaminated Soils. Minerals 2023, 13, 1354. https://doi.org/10.3390/min13111354
Klik B, Brtnicky M, Jaskulska I, Gusiatin MZ, Jaskulski D, Holatko J, Baltazar T, Liniauskiene E, Radziemska M. Unlocking the Saponite Potential in Aided Phytostabilisation of Multi-Metal-Contaminated Soils. Minerals. 2023; 13(11):1354. https://doi.org/10.3390/min13111354
Chicago/Turabian StyleKlik, Barbara, Martin Brtnicky, Iwona Jaskulska, Mariusz Zygmunt Gusiatin, Dariusz Jaskulski, Jiri Holatko, Tivadar Baltazar, Ernesta Liniauskiene, and Maja Radziemska. 2023. "Unlocking the Saponite Potential in Aided Phytostabilisation of Multi-Metal-Contaminated Soils" Minerals 13, no. 11: 1354. https://doi.org/10.3390/min13111354
APA StyleKlik, B., Brtnicky, M., Jaskulska, I., Gusiatin, M. Z., Jaskulski, D., Holatko, J., Baltazar, T., Liniauskiene, E., & Radziemska, M. (2023). Unlocking the Saponite Potential in Aided Phytostabilisation of Multi-Metal-Contaminated Soils. Minerals, 13(11), 1354. https://doi.org/10.3390/min13111354










