Evaluation of Heavy Metal Presence in Agricultural Samples of Lactuca sativa and Trifolium alexandrinum Using Picosecond Laser-Induced Breakdown Spectroscopy and Flame Atomic Absorption Spectroscopy in Banha and Giza Governorates, Egypt
Abstract
1. Introduction
2. Material and Methods
2.1. Samples Collection
2.2. Samples Preparation
2.3. Theoretical Background
2.3.1. Pollution Index (PI)
2.3.2. Bioaccumulation Factor (BF) and Translocation Factor (TF)
2.4. Statistical Analysis
2.5. Instrumentations and Sample Analysis
2.5.1. FAAS System
2.5.2. LIBS System
3. Results
3.1. Major and Trace Heavy Metals Content in Lactuca sativa (Lettuce) and Trifollium alexandrinum (Clover) parts (Roots, Shoots) and Their Associated Soil
3.2. Pollution Index (PI) of the Soils
3.3. Bioaccumulation and Translocation Factors of Metals by Plants
3.4. Ps-LIBS Spectrum Studies
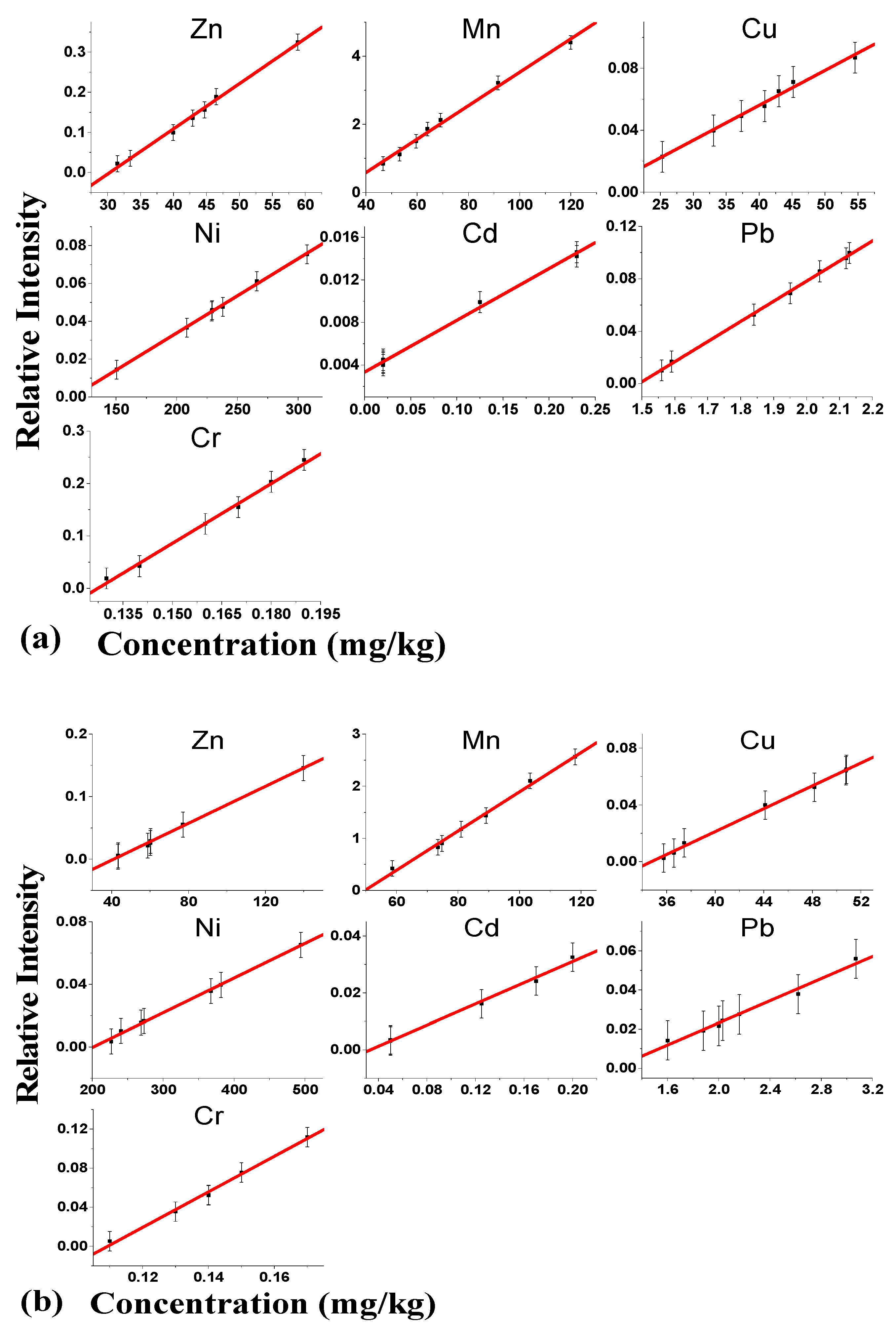
3.5. Content Calibration Studies
4. Discussion
4.1. Heavy Metals Content in Lactuca sativa (Lettuce) and Trifollium alexandrinum (Clover) Parts (Roots and Shoots) and Their Associated Soil
4.2. Pollution Index (PI) of the Soils
4.3. Bioaccumulation and Translocation Factors of Metals by Plants
4.4. Ps-LIBS Spectrum Studies
4.5. Content Calibration Studies
4.6. Factors Influencing Heavy Metal Content
4.6.1. Contaminated Water Irrigation
4.6.2. Environmental Conditions
4.6.3. Soil Characteristics
4.6.4. Plant Species Attributes
4.6.5. Climatic Influences
4.6.6. Human Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, B.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Liu, Z.; Zhang, Y.; Zhang, C.; Cheng, M.; Liu, Y.; et al. Evaluation Methods for Assessing Effectiveness of in Situ Remediation of Soil and Sediment Contaminated with Organic Pollutants and Heavy Metals. Environ. Int. 2017, 105, 43–55. [Google Scholar]
- Yohannes, H.; Elias, E. Contamination of Rivers and Water Reservoirs in and Around Addis Ababa City and Actions to Combat It. Environ. Pollut. Clim. Chang. 2017, 1, 116. [Google Scholar] [CrossRef]
- Proshad, R.; Kormoker, T.; Mursheed, N.; Monirul Islam, M.; Bhuyan, M.I.; Sazedul Islam, M.; Mithu, T.N. Heavy Metal Toxicity in Agricultural Soil Due to Rapid Industrialization in Bangladesh: A Review. Int. J. Adv. Geosci. 2018, 6, 83. [Google Scholar] [CrossRef]
- Ahmed, A.; Sara Taha, A.; Sundas, R.Q.; Man-Qun, W. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar]
- Ding, Y.; Xia, G.; Ji, H.; Xiong, X. Accurate Quantitative Determination of Heavy Metals in Oily Soil by Laser Induced Breakdown Spectroscopy (LIBS) Combined with Interval Partial Least Squares (IPLS). Anal. Methods 2019, 11, 3657–3664. [Google Scholar] [CrossRef]
- Tadesse, M.; Tsegaye, D.; Girma, G. Assessment of the Level of Some Physico-Chemical Parameters and Heavy Metals of Rebu River in Oromia Region, Ethiopia. MOJ Biol. Med. 2018, 3, 99–118. [Google Scholar] [CrossRef]
- Shobha, N.; Kalshetty, B.M. Assessment of Heavy Metals in Green Vegetables and Cereals Collected from Jamkhandi Local Market, Bagalkot, India. Rasayan J. Chem. 2017, 10, 124–135. [Google Scholar] [CrossRef]
- Kilicel, F.; Karapinar, H.S.; Uğuz, A. Determination of Some Heavy Metal Concentrations of Sage Tea with FAAS. Int J Second Metab 2017, 4, 391–399. [Google Scholar] [CrossRef][Green Version]
- Tsanuo, K.M. Determination of lead, cadmium and nickel in henna leaves and cosmetic henna products within lamu county, kenya. Ph.D. Thesis, Kenyatta University, Nairobi, Kenya, 2018. [Google Scholar]
- Fayek, N.; Tawfik, W.; Khalafallah, A.; Hamed, S.; Mousa, W. Spectroscopic Study of Cu, Mn, Cd as Heavy Metals in Agricultural Samples. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1171, 012001. [Google Scholar] [CrossRef]
- Mankoula, A.F.; Tawfik, W.; Gagnon, J.E.; Fryer, B.J.; El-Mekawy, F.; Shaheen, M.E. Assessment of Heavy Metals Content in the Agricultural Soils of Kafr El-Zayat Egypt Using Laser Ablation Inductively Coupled Plasma Mass Spectrometry and Inductively Coupled Plasma Optical Emission Spectroscopy. Egypt. J. Chem. 2021, 64, 1167–1177. [Google Scholar] [CrossRef]
- Seddon, E.A.; Poole, M.W. The Origins and Development of Free-Electron Lasers in the UK. Notes Rec. R. Soc. J. Hist. Sci. 2023, 1–49. [Google Scholar] [CrossRef]
- Khelif, L.; Bellout, Y.; Djebbar, R.; Abrous-Belbachir, O. Efficacy of Avena sativa L. and Trifolium alexandrinum L. Test System for Detecting the Genotoxic and Phytotoxic Potential of Water Pollution. Appl. Ecol. Environ. Res. 2016, 14, 325–342. [Google Scholar] [CrossRef]
- Tsioubri, M.; Gasparatos, D.; Economou-Eliopoulos, M. Selenium Uptake by Lettuce (Lactuca sativa L.) and Berseem (Trifolium alexandrinum L.) as Affected by the Application of Sodium Selenate, Soil Acidity and Organic Matter Content. Plants 2020, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Harmon, R.S.; Senesi, G.S. Laser-Induced Breakdown Spectroscopy—A Geochemical Tool for the 21st Century. Appl. Geochem. 2021, 128, 104929. [Google Scholar] [CrossRef]
- Zettner, A. Principles and applications of atomic absorption spectroscopy. Adv. Clin. Chem. 1964, 7, 1–62. [Google Scholar] [PubMed]
- Anabitarte, F.; Cobo, A.; Lopez-Higuera, J.M. Laser-Induced Breakdown Spectroscopy: Fundamentals, Applications, and Challenges. ISRN Spectrosc. 2012, 2012, 285240. [Google Scholar] [CrossRef]
- Tawfik, W. Recent Advances in the Investigation of Textiles Using Laser-Induced Breakdown Spectroscopy (LIBS). Preserv. Restor. Tech. Anc. Egypt. Text. 2022, 143–163. [Google Scholar] [CrossRef]
- Wang, Z.; Afgan, M.S.; Gu, W.; Song, Y.; Wang, Y.; Hou, Z.; Song, W.; Li, Z. Recent Advances in Laser-Induced Breakdown Spectroscopy Quantification: From Fundamental Understanding to Data Processing. Trends Anal. Chem. 2021, 143, 116385. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Al-Zahrani, A.A. Preservation and Restoration Techniques for Ancient Egyptian Textiles; IGI Global: London, UK, 2023; p. 272. [Google Scholar]
- Naeem, M.A.; Iqbal, M.; Amin, N.; Musadiq, M.; Jamil, Y.; Cecil, F. Measurement of Electron Density and Temperature of Laser-Induced Copper Plasma. Asian J. Chem. 2013, 25, 2192–2198. [Google Scholar] [CrossRef]
- Farooq, W.A.; Rasool, K.G.; Tawfik, W.; Aldawood, A.S. Application of Laser Induced Breakdown Spectroscopy in Early Detection of Red Palm Weevil: (Rhynchophorus ferrugineus) Infestation in Date Palm. Plasma Sci. Technol. 2015, 17, 948–952. [Google Scholar] [CrossRef]
- Soliman, M.; Tawfik, W.; Harith, M. Quantitative Elemental Analysis of Agricultural Drainage Water Using Laser Induced Breakdown Spectroscopy. In Proceedings of the First Cairo Conference on Plasma Physics and Applications: CCPPA 2003, Cairo, Egypt, 11–15 October 2003; Volume 34, pp. 240–243. [Google Scholar]
- Sawaf, S.; Tawfik, W. Analysis of Heavy Elements in Water with High Sensitivity Using Laser Induced Breakdown Spectroscopy. Optoelectron. Adv. Mater. Rapid Commun. 2014, 8, 414–417. [Google Scholar]
- Tawfik, W.; Sawaf, S. Approaching the Ppb Detection Limits for Copper in Water Using Laser Induced Breakdown Spectroscopy. In Proceedings of the SPIE Sensing Technology + Applications, Baltimore, MD, USA, 5–9 May 2014; p. 9101. [Google Scholar]
- Tawfik, W.; Farooq, W.; Al-Mutairi, F.; Alahmed, Z. Monitoring of Inorganic Elements in Desert Soil Using Laser-Induced Breakdown Spectroscopy. Lasers Eng. 2015, 32, 129–140. [Google Scholar]
- Farooq, W.A.; Tawfik, W.; Al-Mutairi, F.N.; Alahmed, Z.A. Qualitative Analysis and Plasma Characteristics of Soil from a Desert Area Using LIBS Technique. J. Opt. Soc. Korea 2013, 17, 548–558. [Google Scholar] [CrossRef]
- Tawfik, W.; Bousiakou, L.G.; Qindeel, R.; Farooq, W.A.; Alonizan, N.H.; Fatani, A.J. Trace Analysis of Heavy Metals in Groundwater Samples Using Laser Induced Breakdown Spectroscopy (LIBS). Optoelectron. Adv. Mater. Rapid Commun. 2015, 9, 185–192. [Google Scholar]
- Xu, Y.; Yamaji, N.; Shen, R.; Ma, J.F. Sorghum Roots Are Inefficient in Uptake of EDTA-Chelated Lead. Ann. Bot. 2007, 99, 869–875. [Google Scholar] [CrossRef]
- Allan, J.; Mitchell, T.; Harborne, N.; Bohm, L.; Crane-Robinson, C. Roles of H1 Domains in Determining Higher Order Chromatin Structure and H1 Location. J. Mol. Biol. 1986, 187, 591–601. [Google Scholar] [CrossRef]
- Scientific, T.F. Thermo Solaar S4; Thermo Fisher Scientific: Waltham, MA. USA, 2023. [Google Scholar]
- Liu, W.H.; Zhao, J.Z.; Ouyang, Z.Y.; Söderlund, L.; Liu, G.H. Impacts of Sewage Irrigation on Heavy Metal Distribution and Contamination in Beijing, China. Environ. Int. 2005, 31, 805–812. [Google Scholar] [CrossRef]
- Schnoor, J.L. Phytoremediation of Soil and Groundwater; Technol. Eval. Rep. TE-02-01; Ground-Water Remediation Technologies Analysis Center (GWRTAC): Pittsburgh, PA, USA, 2002. [Google Scholar]
- Baker, A.J.; Walker, P.L. Ecophysiology of Metal Uptake by Tolerant Plants. In Heavy Metal Tolerance in Plants: Evolutionary Aspects; CRC Press: Boca Raton, USA, 1990; pp. 155–177. [Google Scholar]
- Fitz, W.J.; Wenzel, W.W. Arsenic Transformations in the Soil-Rhizosphere-Plant System: Fundamentals and Potential Application to Phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Baker, A.J.; Brooks, R. Terrestrial Higher Plants Which Hyperaccumulate Metallic Elements. A Review of Their Distribution, Ecology and Phytochemistry. Bio. Recover. 1989, 1, 81–126. [Google Scholar]
- Al-Salman, I.M.; Abdul-Aziz, M. Lead Bio-Accumulation in Baghdad City-Iraq. Jordan J. Appl. Sci. 2002, 4, 6–11. [Google Scholar]
- Kaba, P.; Shushi, S.; Gyimah, E.; Husein, M.; Abomohra, A. Multivariate Analysis of Heavy Metals and Human Health Risk Implications Associated with Fish Consumption from the Yangtze River in Zhenjiang City, China. Water 2023, 15, 1999. [Google Scholar] [CrossRef]
- Walker, S.; Stevenson, B.; Peterson, J.; Donati, G.; Jones, B. Determining Micro- and Macro-Elements by Flame Atomic Absorption Determining Micro- and Macro Metals by Flame Atomic Absorption Spectrometry. Chem. Educator 2016, 21, 264–272. [Google Scholar]
- Fikry, M.; Tawfik, W.; Omar, M.M. Spectroscopic Determination of the Stark Broadening Parameters of Al (I) 305.007 Nm in Atmospheric Air Using Picosecond Time-Resolved LIPS. J. Mol. Struct. 2023, 1276, 134796. [Google Scholar] [CrossRef]
- Elliethy, M.; Ragab, A.A.; Bedair, R.; Khafagi, O.M. Assessment of Nutrients and Heavy Metals Content in Soil and Some Vegetables Cultivated in Agricultural Land around El-Khashab Canal (Helwan-El Saff Area). Int. J. Theor. Appl. Res. 2022, 1, 27–37. [Google Scholar] [CrossRef]
- California EPA. California Environmental Protection Agency, State Water Resources Control Board. Total Maximum Daily Load Program: Background and Information; California Environmental Protection Agency: Sacramento, CA, USA, 2010. [Google Scholar]
- Food Agriculture Organization; World Health Organization. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007; Available online: https://iris.who.int/bitstream/handle/10665/43411/WHO?sequence=1 (accessed on 8 March 2023).
- Fikry, M.; Tawfik, W.; Omar, M. Controlling the Plasma Electron Number Density of Copper Metal Using NIR Picosecond Laser-Induced Plasma Spectroscopy. Opt. Appl. 2021, 51, 365–374. [Google Scholar] [CrossRef]
- Kramida, A.; Yu, R.; Reader, J. Team 2013 NIST Atomic Spectra Database; Version 5-4; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization. Safety Evaluation of Certain Mycotoxins in Food; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Hussein, M.; Ali, M.; Abbas, M.; Bassouny, M. Effects of Industrialization Processes in Giza Factories (Egypt) on Soil and Water Quality in Adjacent Territories. Egypt J. Soil Sci. 2022, 62, 253–2661. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Goni, M.A. Heavy Metal Contamination in Water, Soil, and Vegetables of the Industrial Areas in Dhaka, Bangladesh. Environ. Monit. Assess. 2010, 166, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Heiberger, R.M.; Holland, B.; Heiberger, R.M.; Holland, B. One-Way Analysis of Variance. In Statistical Analysis and Data Display; Springer: New York, NY, USA, 2015; pp. 167–197. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ghoneim, M.A. Effect of Polluted Water on Soil and Plant Contamination by Heavy Metals in El-Mahla El-Kobra, Egypt. Solid Earth 2016, 7, 703–711. [Google Scholar] [CrossRef]
- El-Bady, M.S.M.; Metwally, H.I. A Study of Heavy Metals Contamination in the Agricultural Soils along the Highway between Damietta and Cairo in the Nile Delta, Egypt. Middle East. J. Appl. Sci. 2019, 9, 125–133. [Google Scholar]
- El-Bady, M. Road Dust Pollution by Heavy Metals along the Sides of Expressway between Benha and Cairo, Southern of Nile Delta, Egypt. Geochem. J. 2014, 4, 177–191. [Google Scholar]
- Osman, R.; Melegy, A.; Dawood, Y.; Gad, A. Distribution of Some Potentially Toxic Heavy Metals in the Soil of Shoubra El Kheima, Egypt. Egypt. J. Chem. 2021, 64, 1965–1980. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Su, R.; Xie, T.; Yao, H.; Chen, Y.; Wang, H.; Dai, X.; Wang, Y.; Shi, L.; Luo, Y. Lead Responses and Tolerance Mechanisms of Koelreuteria Paniculata: A Newly Potential Plant for Sustainable Phytoremediation of Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 14968. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Ou, Q.; Wang, H.; Dai, X.; Chen, Y.; Luo, Y.; Yao, H.; Ouyang, D.; Li, Z.; Wang, Z. Organic–Inorganic Composite Modifiers Enhance Restoration Potential of Nerium oleander L. to Lead–Zinc Tailing: Application of Phytoremediation. Environ. Sci. Pollut. Res. 2023, 30, 56569–56579. [Google Scholar] [CrossRef]
- Łukasiewicz, S. The Physical Structure of the Land, Organic Substances Content, and the Chemical Composition of Soil Comprising the Subsoil of 21 Urban Greenery Locations in the Territory of Poznań. Part IV. Content of Microelements: Cl, Fe, Mn, Zn, Cu, B and Na. Physiogr. Res. 2012, 63, 49–75. [Google Scholar]
- Foti, L.; Dubs, F.; Gignoux, J.; Lata, J.C.; Lerch, T.Z.; Mathieu, J.; Nold, F.; Nunan, N.; Raynaud, X.; Abbadie, L.; et al. Trace Element Concentrations along a Gradient of Urban Pressure in Forest and Lawn Soils of the Paris Region (France). Sci. Total Environ. 2017, 598, 938–948. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Arbuckle-Keil, G.; Naderi Beni, N.; Bartelt-Hunt, S.L.; De Villiers, S.; Prata, J.C.; da Costa, J.P.J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T.; et al. Study of Marine Debris around a Tourist City in East China: Implication for Waste Management. Sci. Total Environ. 2019, 161, 278–289. [Google Scholar]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Removal of Nickel and Phenanthrene from Kaolin Soil Using Different Extractants. Environ. Eng. Sci. 2004, 21, 691–704. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar]
- Ahmad, T.; Ahmad, K.; Khan, Z.I.; Munir, Z.; Khalofah, A.; Al-Qthanin, R.N.; Alsubeie, M.S.; Alamri, S.; Hashem, M.; Farooq, S.; et al. Chromium Accumulation in Soil, Water and Forage Samples in Automobile Emission Area. Saudi J. Biol. Sci. 2021, 28, 3517–3522. [Google Scholar] [CrossRef]
- Alfaro, M.R.; Ugarte, O.M.; Lima, L.H.V.; Silva, J.R.; da Silva, F.B.V.; da Silva Lins, S.A.; do Nascimento, C.W.A. Risk Assessment of Heavy Metals in Soils and Edible Parts of Vegetables Grown on Sites Contaminated by an Abandoned Steel Plant in Havana. Environ. Geochem. Health 2022, 44, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A Review on Bioremediation Approach for Heavy Metal Detoxification and Accumulation in Plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, F.; Ma, J.; Khan, Z.I.; Hussain, M.I.; Javaid, I.; Ahmad, K.; Nazar, S.; Akhtar, S.; Ejaz, A.; et al. Comparative Evaluation of Groundwater, Wastewater and Canal Water for Irrigation on Toxic Metal Accumulation in Soil and Vegetable: Pollution Load and Health Risk Assessment. Agric. Water Manag. 2022, 264, 107515. [Google Scholar] [CrossRef]
- Eltaher, G.T.; Ahmed, D.A.; El-Beheiry, M.; Sharaf El-Din, A. Biomass Estimation and Heavy Metal Accumulation by Pluchea Dioscoridis (L.) DC. in the Middle Nile Delta, (Egypt): Perspectives for Phytoremediation. S. Afr. J. Bot. 2019, 127, 153–166. [Google Scholar] [CrossRef]
- Kumar, P.; Dipti; Kumar, S.; Singh, R.P. Severe Contamination of Carcinogenic Heavy Metals and Metalloid in Agroecosystems and Their Associated Health Risk Assessment. Environ. Pollut. 2022, 301, 118953. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Li, Z.; Yu, A.; Guan, W.; Wang, Z.; Yu, H.; Zou, L. Phytoremediation of Cadmium-Contaminated Soils by Solanum nigrum L. Enhanced with Biodegradable Chelating Agents. Environ. Sci. Pollut. Res. 2022, 29, 56750–56759. [Google Scholar] [CrossRef]
- Zoufan, P.; Jalali, R.; Karimiafshar, A.; Motamedi, H. Assessment of Heavy Metal Accumulation and Antibacterial Activity of Some Medicinal Herbs Grown in an Industrial Area of Steel Production, Ahvaz. Iran. J. Pharm. Sci. 2017, 13, 73–86. [Google Scholar]
- Hu, W.; Huang, B.; Tian, K.; Holm, P.E.; Zhang, Y. Heavy Metals in Intensive Greenhouse Vegetable Production Systems along Yellow Sea of China: Levels, Transfer and Health Risk. Chemosphere 2017, 167, 82–90. [Google Scholar] [CrossRef]
- Chandra, R.; Kumar, V. Phytoextraction of Heavy Metals by Potential Native Plants and Their Microscopic Observation of Root Growing on Stabilised Distillery Sludge as a Prospective Tool for in Situ Phytoremediation of Industrial Waste. Environ. Sci. Pollut. Res. 2017, 24, 2605–2619. [Google Scholar] [CrossRef]
- Ali, J.; Khan, S.; Khan, A.; Waqas, M.; Nasir, M.J. Contamination of Soil with Potentially Toxic Metals and Their Bioaccumulation in Wheat and Associated Health Risk. Environ. Monit. Assess. 2020, 192, 3746. [Google Scholar] [CrossRef]
- Sabir, M.; Baltrėnaitė-Gedienė, E.; Ditta, A.; Ullah, H.; Kanwal, A.; Ullah, S.; Faraj, T.K. Bioaccumulation of Heavy Metals in a Soil–Plant System from an Open Dumpsite and the Associated Health Risks through Multiple Routes. Sustainability 2022, 14, 13223. [Google Scholar] [CrossRef]
- Fikry, M.; Alhijry, I.A.; Aboulfotouh, A.M.; El Sherbini, A.M. Feasibility of Using Boltzmann Plots to Evaluate the Stark Broadening Parameters of Cu(I) Lines. Appl. Spectrosc. 2021, 75, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, K.; Tawfik, W.; Khater, E.M.A.; Kayed, S.T.; Fikry, M. Fast Determination of Phosphorus Concentration in Phosphogypsum Waste Using Calibration-Free LIBS in Air and Helium. Opt. Quantum Electron. 2022, 54, 96. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-Induced Breakdown Spectroscopy (LIBS), Part II: Review of Instrumental and Methodological Approaches to Material Analysis and Applications to Different Fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef]
- Aragón, C.; Aguilera, J.A. Characterization of Laser Induced Plasmas by Optical Emission Spectroscopy: A Review of Experiments and Methods. Spectrochim. Acta Part. B At. Spectrosc. 2008, 63, 893–916. [Google Scholar] [CrossRef]
- Ziku, M. Digital Cultural Heritage and Linked Data: Semantically-Informed Conceptualisations and Practices with a Focus on Intangible Cultural Heritage. Lib. Q. J. Assoc. Eur. Res. Libr. 2020, 30, 1–16. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-Induced Breakdown Spectroscopy (LIBS), Part I: Review of Basic Diagnostics and Plasmaparticle Interactions: Still-Challenging Issues within the Analytical Plasma Community. Appl. Spectrosc. 2010, 64, 335–366. [Google Scholar] [CrossRef]
- Musazzi, S.; Perini, U. Laser-Induced Breakdown Spectroscopy Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642450846. [Google Scholar]
- Galbács, G. A Critical Review of Recent Progress in Analytical Laser-Induced Breakdown Spectroscopy. Anal. Bioanal. Chem. 2015, 407, 7537–7562. [Google Scholar]
- Fikry, M.; Tawfik, W.; Omar, M.M. Investigation on the Effects of Laser Parameters on the Plasma Profile of Copper Using Picosecond Laser Induced Plasma Spectroscopy. Opt. Quantum Electron. 2020, 52, 249. [Google Scholar] [CrossRef]
- Fikry, M.; Tawfik, W.; Omar, M. Measurement of the Electron Temperature in a Metallic Copper Using Ultrafast Laser-Induced Breakdown Spectroscopy. J. Russ. Laser Res. 2020, 41, 484–490. [Google Scholar] [CrossRef]
- Qi, H.; Lai, H. Micromachining of Metals and Thermal Barrier Coatings Using a 532 Nm Nanosecond Fiber Laser. Phys. Procedia 2012, 39, 603–612. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Anwar, M. Chemosphere Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Bradl, H.B. Adsorption of Heavy Metal Ions on Soils and Soils Constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of Metals and Metalloids from Contaminated Soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]







| Element | Wavelength (nm) | Lamp Current (mA) | Fuel Flow (L/min) | Band Pass (nm) | Flame Type | RSD % |
|---|---|---|---|---|---|---|
| Mg | 285.2 | 4 | 1.1 | 0.5 | Air/acetylene | 0.2 |
| K | 766.5 | 8 | 1.2 | 0.5 | Air/acetylene | 2.41 |
| Fe | 248.3 | 15 | 0.9 | 0.2 | Air/acetylene | 1.2 |
| Zn | 213.9 | 10 | 1.1 | 0.5 | Air/acetylene | 0.2 |
| Mn | 279.5 | 12 | 1.1 | 0.2 | Air/acetylene | 0.4 |
| Cu | 324.8 | 5 | 1.0 | 0.5 | Air/acetylene | 3.1 |
| Ni | 232.0 | 15 | 0.9 | 0.2 | Air/acetylene | 2.3 |
| Cd | 228.8 | 8 | 1.2 | 0.5 | Air/acetylene | 3.58 |
| Pb | 217.0 | 10 | 1.1 | 0.5 | Air/acetylene | 2.94 |
| Cr | 357.9 | 12 | 4.2 | 0.5 | Nitrous oxide/acetylene | 1.94 |
| Major and Trace Heavy Metals | Lettuce (mg/kg) | Clover (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S1 | S2 | S3 | S4 | S5 | ||
| Major elements | Mg | 182.3 ± 5.91 a | 183.6 ± 2.83 a | 204 ± 30.9 a | 199.3 ± 13.9 a | 180.2 ± 6.77 a | 197.1 ± 2.22 a | 200.4 ± 4.8 a | 205 ± 0.00 a | 196.4 ± 20.1 a | 192 ± 10.9 a |
| K | 12,050 ± 831 a | 6441 ± 208 cd | 9976 ± 121 b | 5713 ± 311 d | 7396 ± 82 c | 12,819 ± 477 a | 6025 ± 623 c | 9349 ± 623 b | 4778 ± 207 c | 5402 ± 415 c | |
| Fe | 829 ± 13.4 d | 981 ± 44.1 c | 2373 ± 37.5 a | 2031 ± 110.5 b | 640 ± 0.75 e | 37.80 ± 1.65 c | 24.04 ± 0.34 c | 26.05 ± 3.54 c | 3832 ± 96 a | 879 ± 30.5 b | |
| Trace heavy metals | Zn | 33.58 ± 1.21 b | 40.42 ± 3.49 a | 35.18 ± 1.31 b | 20.46 ± 0.79 c | 21.69 ± 1.32 c | 17.15 ± 3.45 c | 22.15 ± 0.16 c | 21.49 ± 1.71 c | 244 ± 15.3 a | 49.69 ± 1.68 b |
| Mn | 47.09 ± 2.03 a | 45.58 ± 1.10 a | 22.41 ± 1.81 b | 23.05 ± 0.02 b | 21.45 ± 0.63 b | 9.07 ± 0.10 d | 23.21 ± 0.04 c | 22.66 ± 0.13 c | 758 ± 9.23 a | 37.77 ± 1.75 b | |
| Cu | 19.75 ± 1.05 c | 24.58 ± 0.30 a | 22.78 ± 2.15 ab | 19.82 ± 0.49 bc | 25.41 ± 0.57 a | 18.49 ± 0.92 c | 23.55 ± 0.73 b | 27.90 ± 1.92 a | 23.11 ± 1.17 b | 22.55 ± 2.16 b | |
| Ni | 12.92 ± 0.86 c | 226 ± 12.92 a | 33.04 ± 0.57 b | 13.84 ± 0.43 c | 24.21 ± 1.69 bc | 27.25 ± 3.45 bc | 26.45 ± 5.29 c | 36.35 ± 2.77 ab | 42.07 ± 3.44 a | 25.83 ± 2.09 c | |
| Cd | 11.56 ± 1.23 a | 8.98 ± 0.86 b | 8.85 ± 0.38 b | 11.44 ± 0.37 a | 10.09 ± 0.25 ab | 10.64 ± 1.05 a | 7.87 ± 0.00 c | 9.96 ± 0.37 ab | 11.13 ± 0.68 a | 8.38 ± 0.23 bc | |
| Pb | 4.44 ± 0.24 c | 6.77 ± 0.12 b | 6.31 ± 0.16 b | 6.64 ± 0.25 b | 8.00 ± 0.86 a | 5.90 ± 0.49 bc | 7.63 ± 0.00 a | 6.77 ± 0.37 ab | 5.66 ± 0.00 c | 6.27 ± 0.37 bc | |
| Cr | 5.12 ± 0.73 ab | 6.30 ± 0.30 a | 6.46 ± 0.20 a | 4.92 ± 0.65 b | 6.34 ± 0.43 a | 4.29 ± 0.32 c | 4.34 ± 0.00 c | 7.07 ± 0.27 a | 6.97 ± 0.36 a | 5.74 ± 0.24 b | |
| Major and Trace Heavy Metals | Lettuce (mg/kg) | Clover (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S1 | S2 | S3 | S4 | S5 | ||
| Major elements | Mg | 298 ± 9.6 b | 291 ± 1.1 b | 286 ± 4.2 b | 336 ± 19.2 a | 327 ± 3.5 a | 351 ± 18 a | 336 ± 4.8 a | 337 ± 5.1 a | 353 ± 8.5 a | 324 ± 12.2 a |
| K | 2239 ± 0.45 a | 2251 ± 0.40 a | 2248 ± 1.00 a | 2064 ± 83.1 a | 2174 ± 430 a | 2246 ± 1.7 a | 2244 ± 7.1 a | 2247 ± 3.2 a | 2292 ± 187 a | 1898 ± 83.2 b | |
| Fe | 480 ± 7.2 a | 421 ± 41.9 b | 485 ± 10.5 a | 419 ± 19.7 b | 349 ± 5.9 c | 1122 ± 22.2 b | 551 ± 21.6 c | 517 ± 20.9 c | 1872 ± 4.9 a | 429 ± 12.6 d | |
| Trace heavy metals | Zn | 31.49 ± 0.79 c | 42.92 ± 0.67 b | 46.47 ± 0.39 b | 58.85 ± 3.95 a | 33.45 ± 1.56 c | 43.27 ± 0.39 d | 58.72 ± 0.20 c | 77.08 ± 2.03 b | 139.8 ± 7.90 a | 43.39 ± 2.65 d |
| Mn | 64.00 ± 0.96 c | 59.55 ± 2.94 c | 64.68 ± 1.40 c | 119.9 ± 4.74 a | 91.57 ± 0.72 b | 74.79 ± 1.51 c | 73.48 ± 2.88 c | 103.4 ± 4.18 b | 118.1 ± 1.77 a | 58.64 ± 3.65 d | |
| Cu | 37.27 ± 2.01 c | 25.28 ± 0.30 d | 40.82 ± 0.20 bc | 54.53 ± 1.31 a | 45.16 ± 2.75 b | 35.75 ± 0.99 b | 48.17 ± 1.85 a | 37.44 ± 1.26 b | 50.76 ± 0.99 a | 50.80 ± 1.15 a | |
| Ni | 238 ± 10 b | 266 ± 22 ab | 150.5 ± 24.5 c | 229 ± 1.6 b | 307 ± 31.7 a | 269 ± 49 b | 273 ± 29 b | 494 ± 14 a | 226 ± 14.3 b | 240 ± 16.6 b | |
| Cd | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.23 ± 0.02 a | 0.23 ± 0.03 a | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 0.17 ± 0.02 a | 0.20 ± 0.00 a | |
| Pb | 1.59 ± 0.03 bc | 1.56 ± 0.12 c | 2.12 ± 0.08 a | 2.13 ± 0.14 a | 1.95 ± 0.25 ab | 3.07 ± 0.17 a | 2.00 ± 0.13 bc | 2.16 ± 0.29 b | 2.03 ± 0.04 bc | 1.60 ± 0.17 c | |
| Cr | 0.17 ± 0.01 b | 0.19 ± 0.01 a | 0.18 ± 0.00 ab | 0.13 ± 0.00 c | 0.14 ± 0.01 c | 0.11 ± 0.00 c | 0.17 ± 0.01 a | 0.17 ± 0.00 a | 0.14 ± 0.00 b | 0.13 ± 0.01 b | |
| Major and Trace Heavy Metals | Lettuce (mg/kg) | Clover (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S1 | S2 | S3 | S4 | S5 | ||
| Major elements | Mg | 300 ± 15.9 c | 310 ± 3 c | 313 ± 1.5 c | 383 ± 6.2 a | 343 ± 0.00 b | 339 ± 5.4 b | 324 ± 27 b | 312 ± 0.00 b | 410 ± 0.00 a | 309 ± 4.4 b |
| K | 2247 ± 5.9 a | 2244 ± 6.7 a | 2246 ± 4.1 a | 1731 ± 166.3 b | 2209 ± 145.5 a | 2247 ± 5.5 a | 2241 ± 1.9 a | 2242 ± 0.15 a | 2002 ± 62.3 b | 2345 ± 84.4 a | |
| Fe | 548 ± 13.7 c | 545 ± 17.1 c | 1430 ± 16.1 a | 300 ± 7.5 d | 816 ± 14.2 b | 529 ± 26.6 c | 559 ± 0.15 c | 2106 ± 3.8 b | 453 ± 2.5 c | 2259 ± 88.6 a | |
| Trace heavy metals | Zn | 57.88 ± 1.64 bc | 60.54 ± 3.78 b | 74.06 ± 0.46 a | 53.34 ± 1.47 c | 35.07 ± 0.68 d | 31.49 ± 1.67 d | 49.75 ± 11.6 bc | 56.29 ± 0.41 b | 112.8 ± 6.5 a | 36.12 ± 0.13 cd |
| Mn | 135.3 ± 0.29 b | 114.4 ± 1.87 c | 212 ± 2.6 a | 93.18 ± 2.85 d | 99.03 ± 6.93 d | 72.22 ± 1.87 b | 74.54 ± 0.02 b | 148.2 ± 8.1 a | 36.01 ± 0.90 c | 81.35 ± 5.34 b | |
| Cu | 37.57 ± 1.91 b | 36.64 ± 2.30 b | 40.91 ± 1.53 b | 47.36 ± 1.20 a | 36.84 ± 1.71 b | 41.39 ± 0.85 b | 36.45 ± 1.97 c | 38.33 ± 2.11 bc | 53.79 ± 1.84 a | 57.91 ± 0.94 a | |
| Ni | 342 ± 28 ab | 342 ± 10 ab | 299 ± 17 b | 370 ± 16 a | 348 ± 9.4 a | 246 ± 18 c | 500 ± 52 a | 534 ± 30 a | 329 ± 16.4 bc | 335 ± 35.7 b | |
| Cd | 0.07 ± 0.00 b | 0.06 ± 0.00 b | 0.06 ± 0.00 b | 0.23 ± 0.01 a | 0.22 ± 0.03 a | 0.06 ± 0.00 b | 0.05 ± 0.01 b | 0.02 ± 0.00 c | 0.20 ± 0.01 a | 0.21 ± 0.01 a | |
| Pb | 0.92 ± 0.00 b | 1.22 ± 0.04 b | 1.16 ± 0.02 b | 1.92 ± 0.34 a | 1.76 ± 0.21 a | 1.66 ± 0.10 b | 2.11 ± 0.19 a | 1.97 ± 0.09 ab | 2.03 ± 0.11 a | 2.21 ± 0.15 a | |
| Cr | 0.18 ± 0.01 ab | 0.18 ± 0.01 a | 0.17 ± 0.01 ab | 0.15 ± 0.01 bc | 0.14 ± 0.01 c | 0.16 ± 0.01 ab | 0.15 ± 0.00 abc | 0.16 ± 0.01 a | 0.14 ± 0.01 bc | 0.14 ± 0.01 c | |
| Heavy Metals | Permissible Levels in Soil (mg/kg) | Permissible Levels in a Plant (mg/kg) |
|---|---|---|
| Zn | 23 [43] | 60 [44] |
| Mn | 437 [43] | 100 [44] |
| Cu | 3 [43] | 2 [44] |
| Ni | 1.6 [43] | 0.5 [44] |
| Cd | 1.7 [43] | 0.5 [44] |
| Pb | 80 [43] | 2 [44] |
| Cr | 100 [43] | 1 [44] |
| Heavy Metals | Selected Wavelengths | |
|---|---|---|
| λ (nm) | Transition Configuration | |
| Zn | 213.85 | 3d104s2 → 3d104s4p(1P°) |
| Mn | 279.48 | 3d54s2 → 3d5(6S)4s4p(1P°) |
| 280.11 | 3d54s2 → 3d5(6S)4s4p(1P°) | |
| Cu | 324.75 | 3d104s → 3d104p |
| 327.39 | 3d104s → 3d104p | |
| Ni | 234.55 | 3d8(3F)4s2 → 3d8(1D)4s4p(3P°) |
| Cd | 228.8 | 4d105s2 → 4d105s5p |
| Pb | 283.31 | 6s26p2 → 6s26p7s |
| Cr | 236.47 | 3d5(6S)4s → 3d5(6S)5p |
| Metals | Standard error | Fitting Equation | ||
|---|---|---|---|---|
| Lettuce | Clover | Lettuce | Clover | |
| Zn | ±0.01% | ±0.05% | ||
| Mn | ±0.02% | ±0.80% | ||
| Cu | ±0.03% | ±0.10% | ||
| Ni | ±0.03% | ±0.02% | ||
| Cd | ±0.001% | ±0.006% | ||
| Pb | ±0.05% | ±0.02% | ||
| Cr | ±0.03% | ±0.05% | ||
| Metals | Standard error | Fitting Equation | ||
|---|---|---|---|---|
| Lettuce | Clover | Lettuce | Clover | |
| Zn | ±0.02% | ±0.02% | ||
| Mn | ±0.20% | ±0.15% | ||
| Cu | ±0.01% | ±0.01% | ||
| Ni | ±0.005% | ±0.008% | ||
| Cd | ±0.001% | ±0.005% | ||
| Pb | ±0.008% | ±0.01% | ||
| Cr | ±0.02% | ±0.01% | ||
| Metals | Standard Error | Fitting Equation | ||
|---|---|---|---|---|
| Lettuce | Clover | Lettuce | Clover | |
| Zn | ±0.005% | ±0.01% | ||
| Mn | ±0.08% | ±0.15% | ||
| Cu | ±0.002% | ±0.005% | ||
| Ni | ±0.001% | ±0.005% | ||
| Cd | ±0.001% | ±0.002% | ||
| Pb | ±0.007% | ±0.005% | ||
| Cr | ±0.005% | ±0.005% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayek, N.; Tawfik, W.; Khalafallah, A.; Hamed, S.; Mousa, W.; Fikry, M. Evaluation of Heavy Metal Presence in Agricultural Samples of Lactuca sativa and Trifolium alexandrinum Using Picosecond Laser-Induced Breakdown Spectroscopy and Flame Atomic Absorption Spectroscopy in Banha and Giza Governorates, Egypt. Minerals 2023, 13, 1300. https://doi.org/10.3390/min13101300
Fayek N, Tawfik W, Khalafallah A, Hamed S, Mousa W, Fikry M. Evaluation of Heavy Metal Presence in Agricultural Samples of Lactuca sativa and Trifolium alexandrinum Using Picosecond Laser-Induced Breakdown Spectroscopy and Flame Atomic Absorption Spectroscopy in Banha and Giza Governorates, Egypt. Minerals. 2023; 13(10):1300. https://doi.org/10.3390/min13101300
Chicago/Turabian StyleFayek, Noura, Walid Tawfik, Ahmed Khalafallah, Sawsan Hamed, Wafaa Mousa, and Mohamed Fikry. 2023. "Evaluation of Heavy Metal Presence in Agricultural Samples of Lactuca sativa and Trifolium alexandrinum Using Picosecond Laser-Induced Breakdown Spectroscopy and Flame Atomic Absorption Spectroscopy in Banha and Giza Governorates, Egypt" Minerals 13, no. 10: 1300. https://doi.org/10.3390/min13101300
APA StyleFayek, N., Tawfik, W., Khalafallah, A., Hamed, S., Mousa, W., & Fikry, M. (2023). Evaluation of Heavy Metal Presence in Agricultural Samples of Lactuca sativa and Trifolium alexandrinum Using Picosecond Laser-Induced Breakdown Spectroscopy and Flame Atomic Absorption Spectroscopy in Banha and Giza Governorates, Egypt. Minerals, 13(10), 1300. https://doi.org/10.3390/min13101300







