Selective Recovery of Copper from the Mixed Metals Leach Liquor of E-Waste Materials by Ion-Exchange: Batch and Column Study
Abstract
1. Introduction
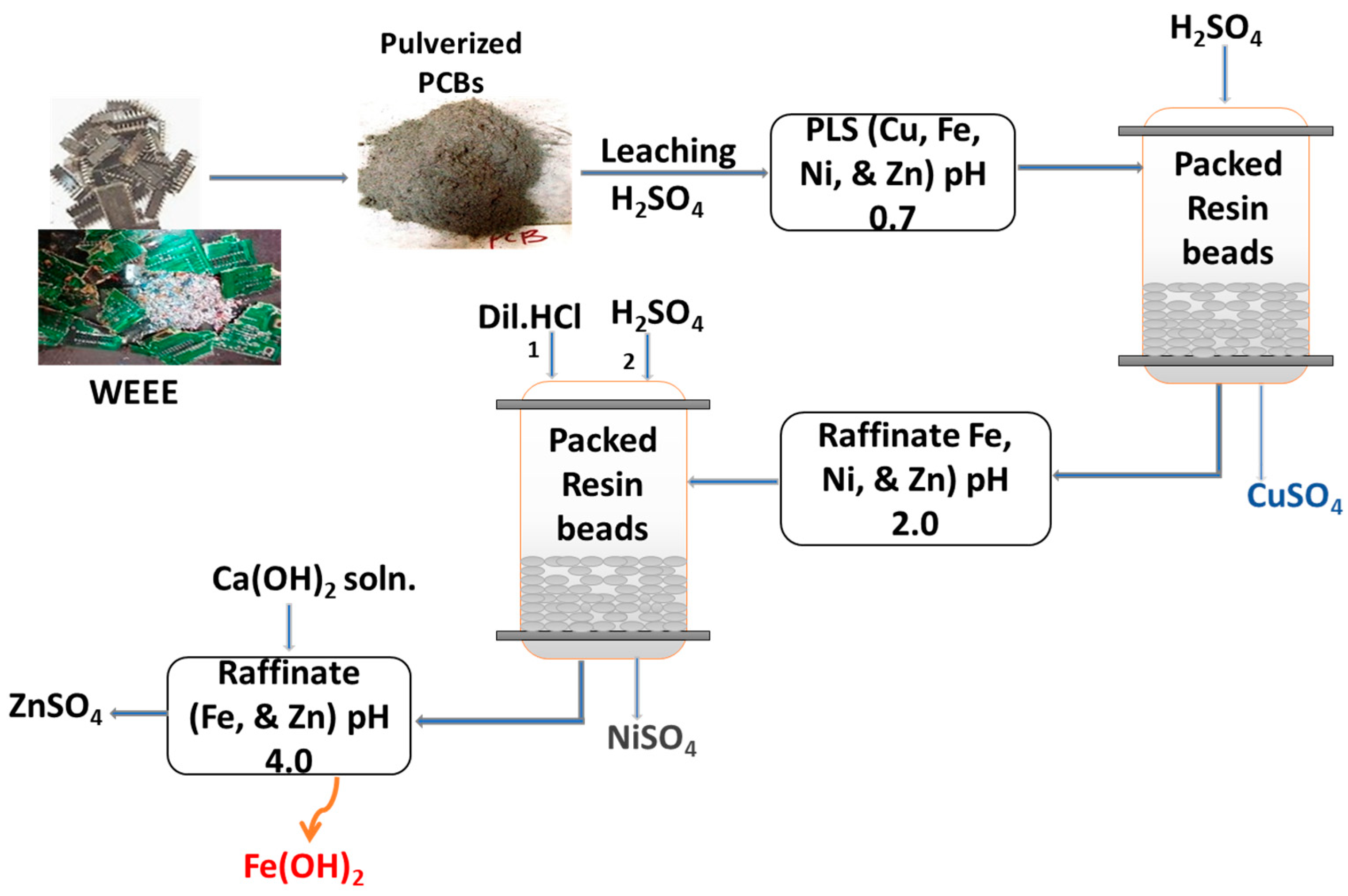
2. Materials and Methods
2.1. Sorbate and Chelating Resin Characteristics
2.2. Experimental Procedure
- Batch experiments for copper adsorption and elution using Dowex M4195 with determination of isotherms.
- Fixed bed adsorption and desorption studies of Cu2+ ions.
- Treatment of raffinate for the removal of Ni2+ using spent Dowex M4195 and removal of Fe3+ from solution.
2.2.1. Batch Experiment
2.2.2. Fixed Bed Sorption Study
2.2.3. Column Sorption Models
Thomas Model
Yoon–Nelson Model
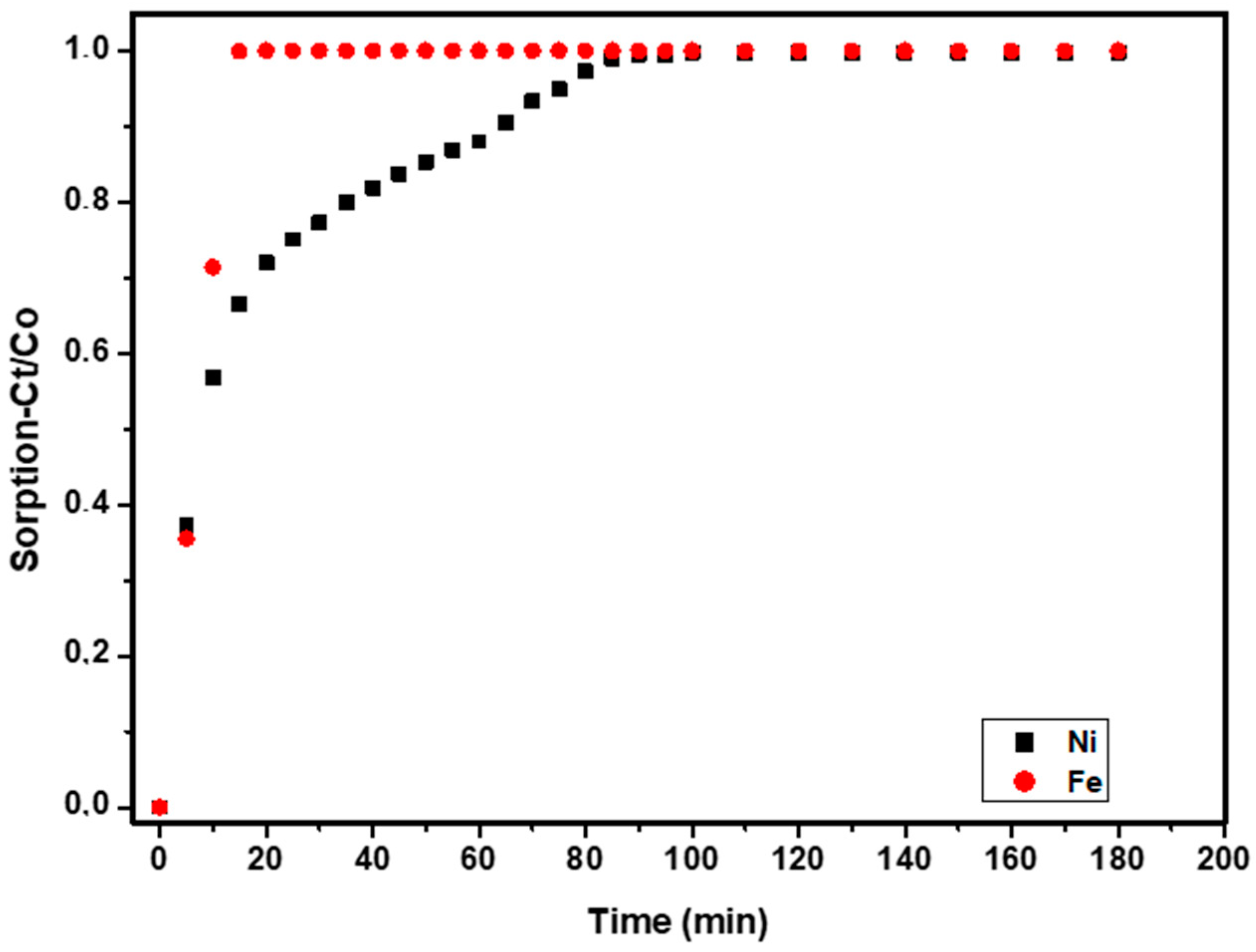
2.2.4. Sorption of Nickel and Removal of Impurities
3. Results and Discussion
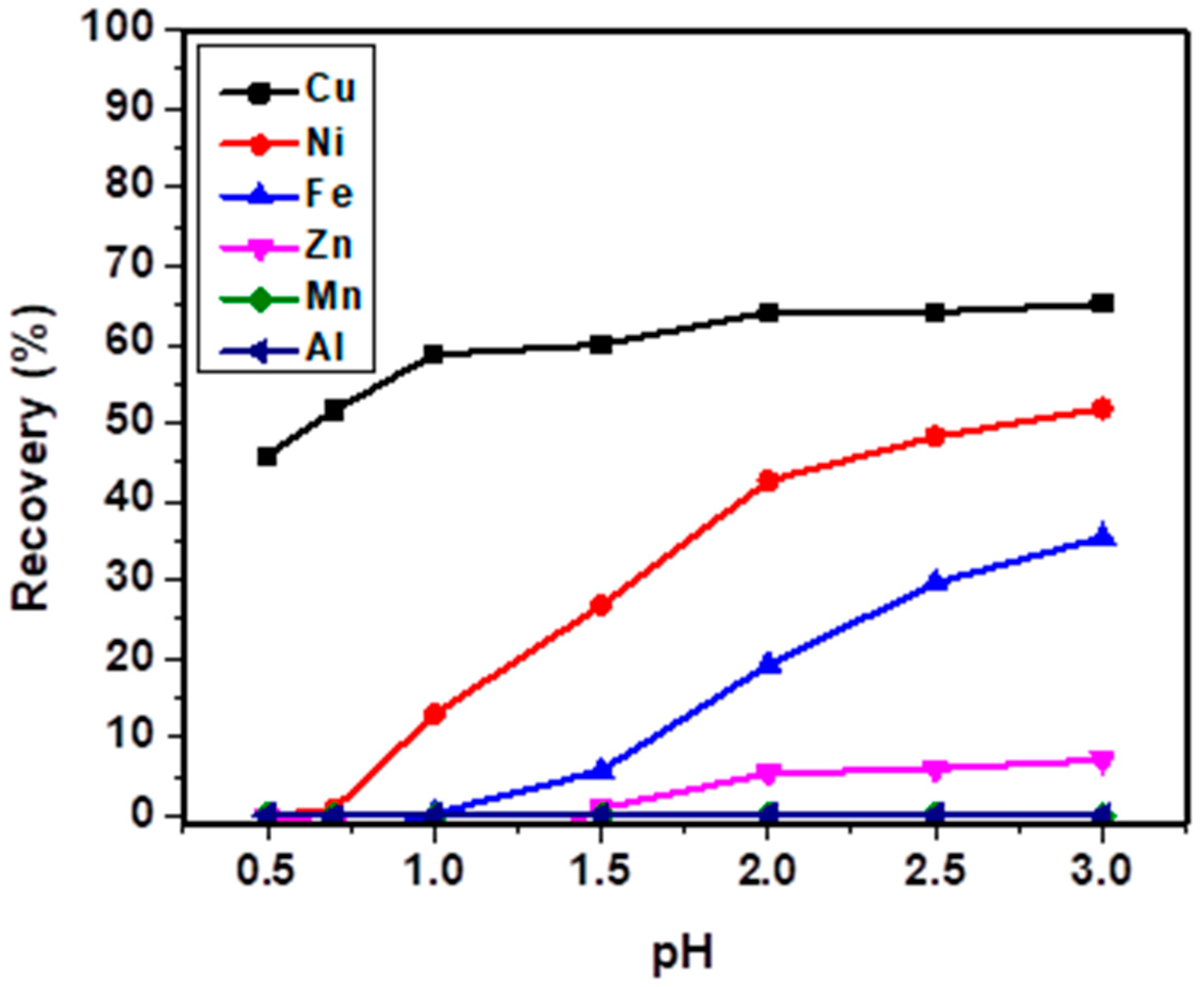
3.1. Batch Sorption Study
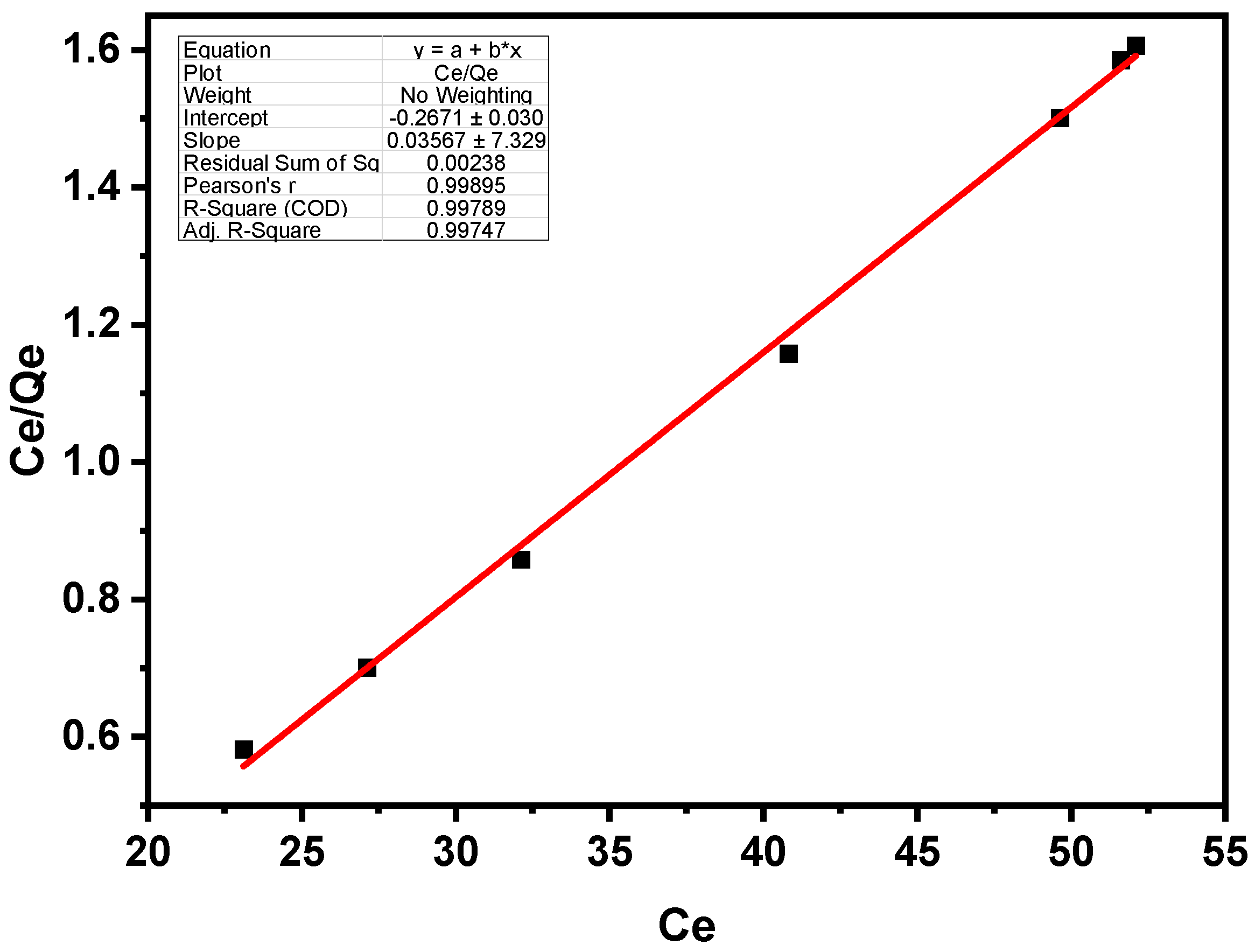
3.2. Batch Sorption Isotherm
3.2.1. Langmuir Isotherm
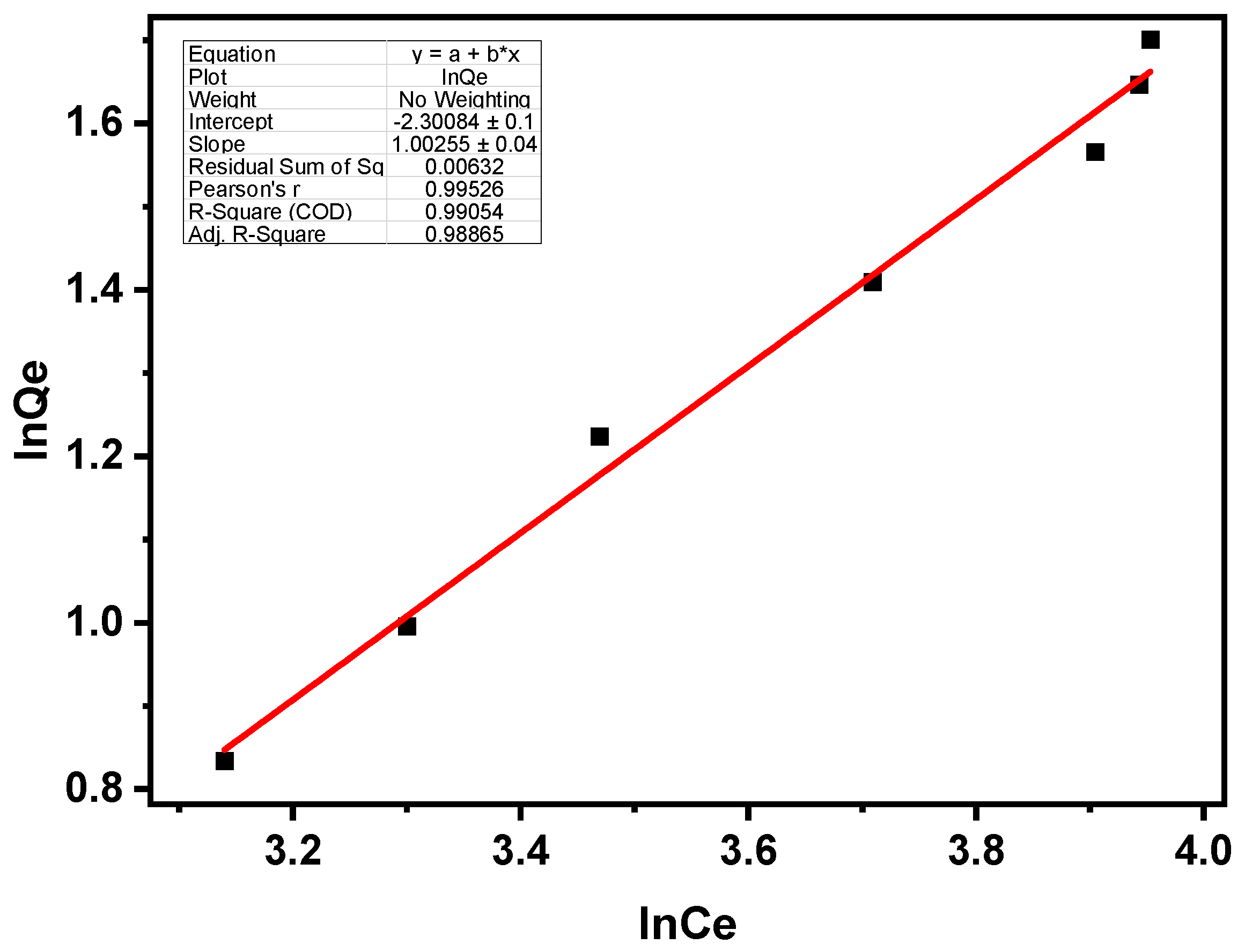
3.2.2. Freundlich Isotherm
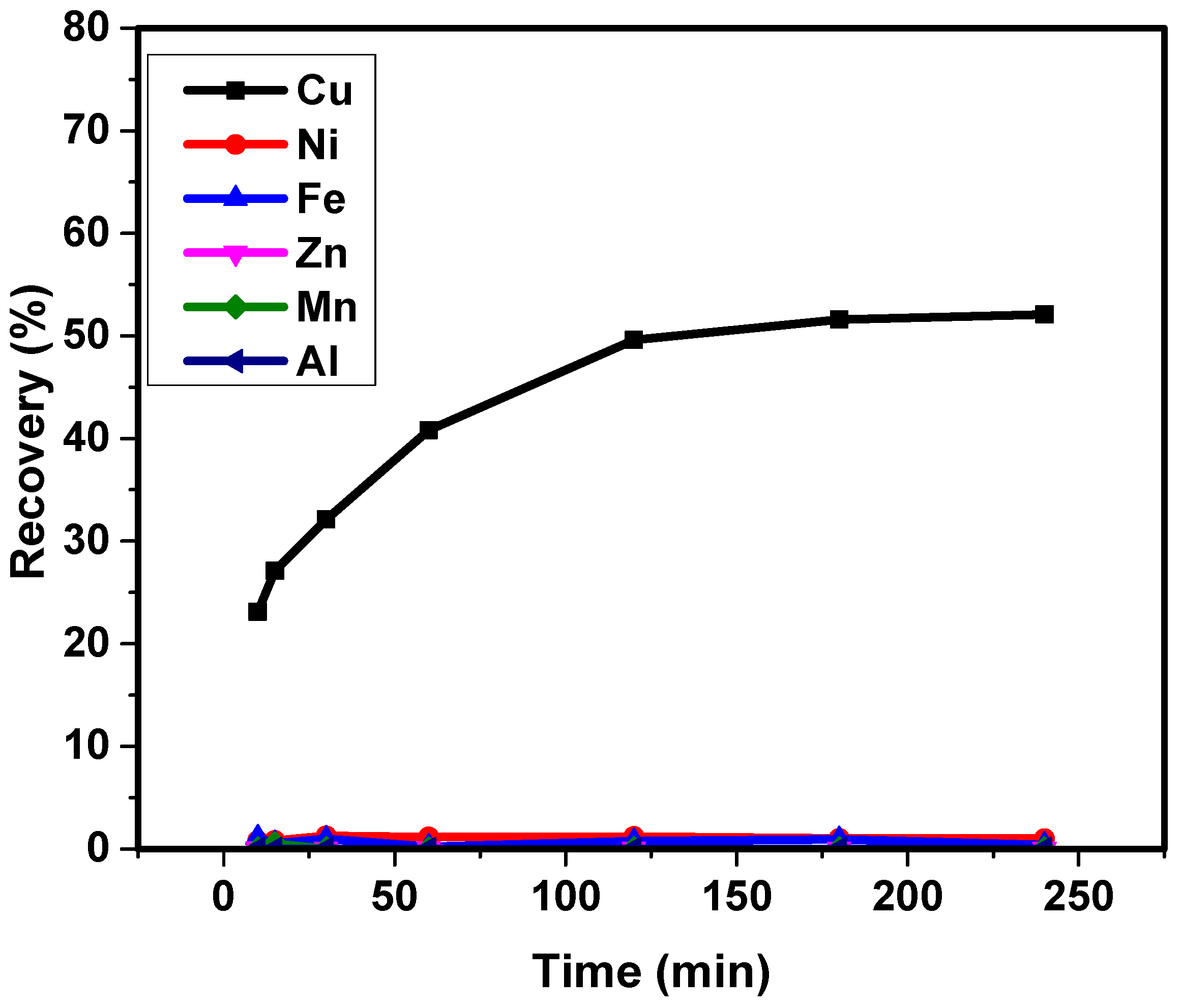
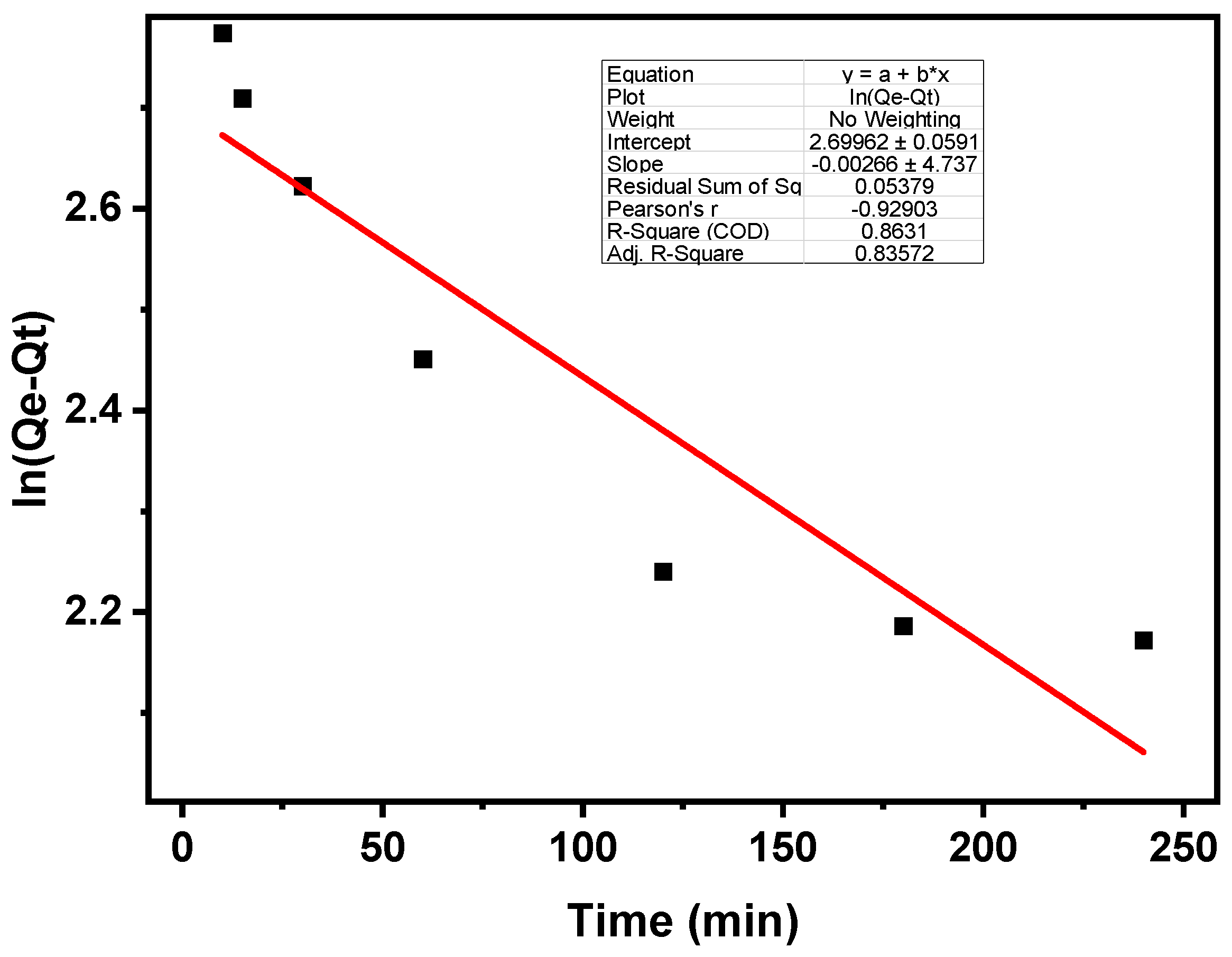
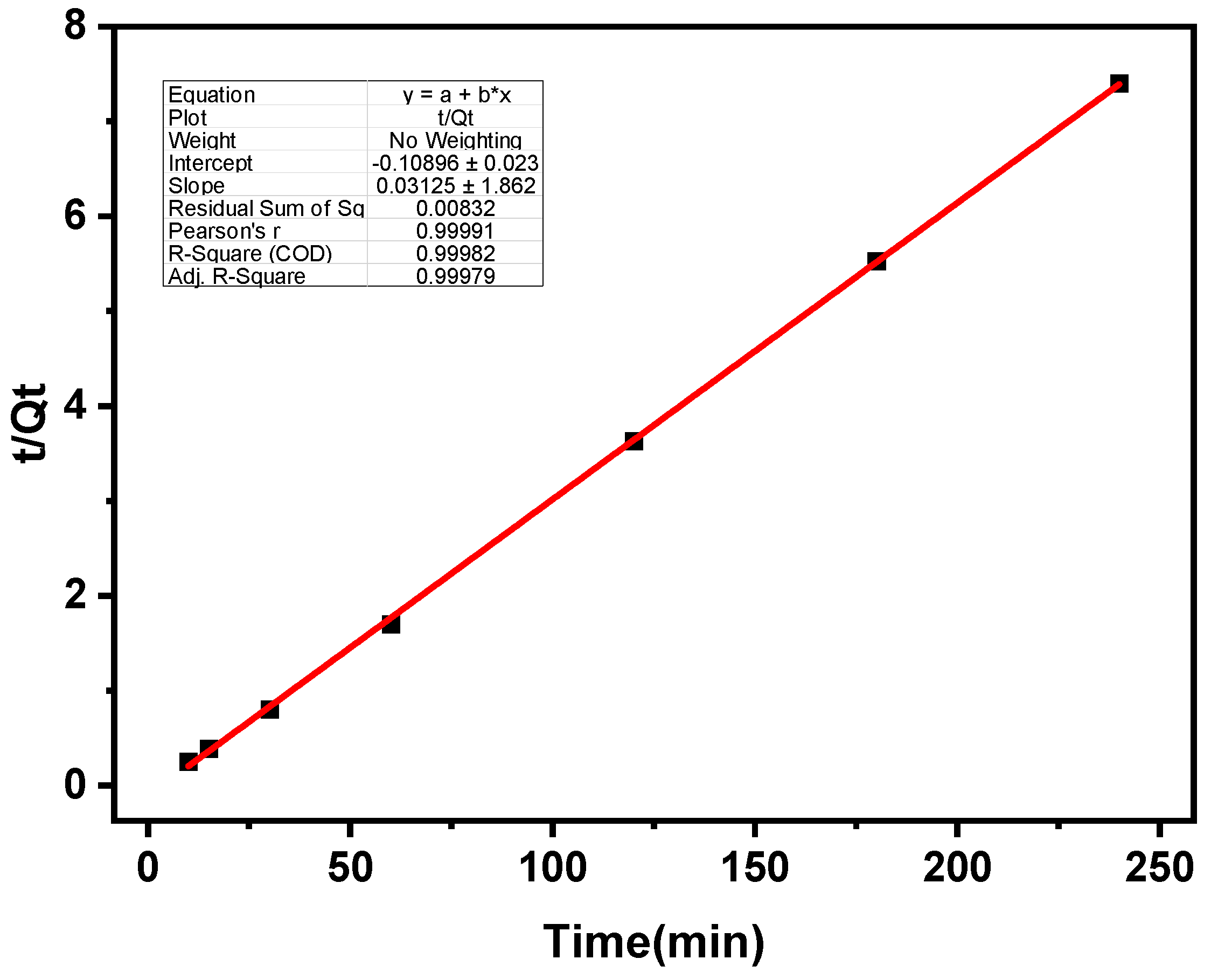
3.3. Sorption Kinetics
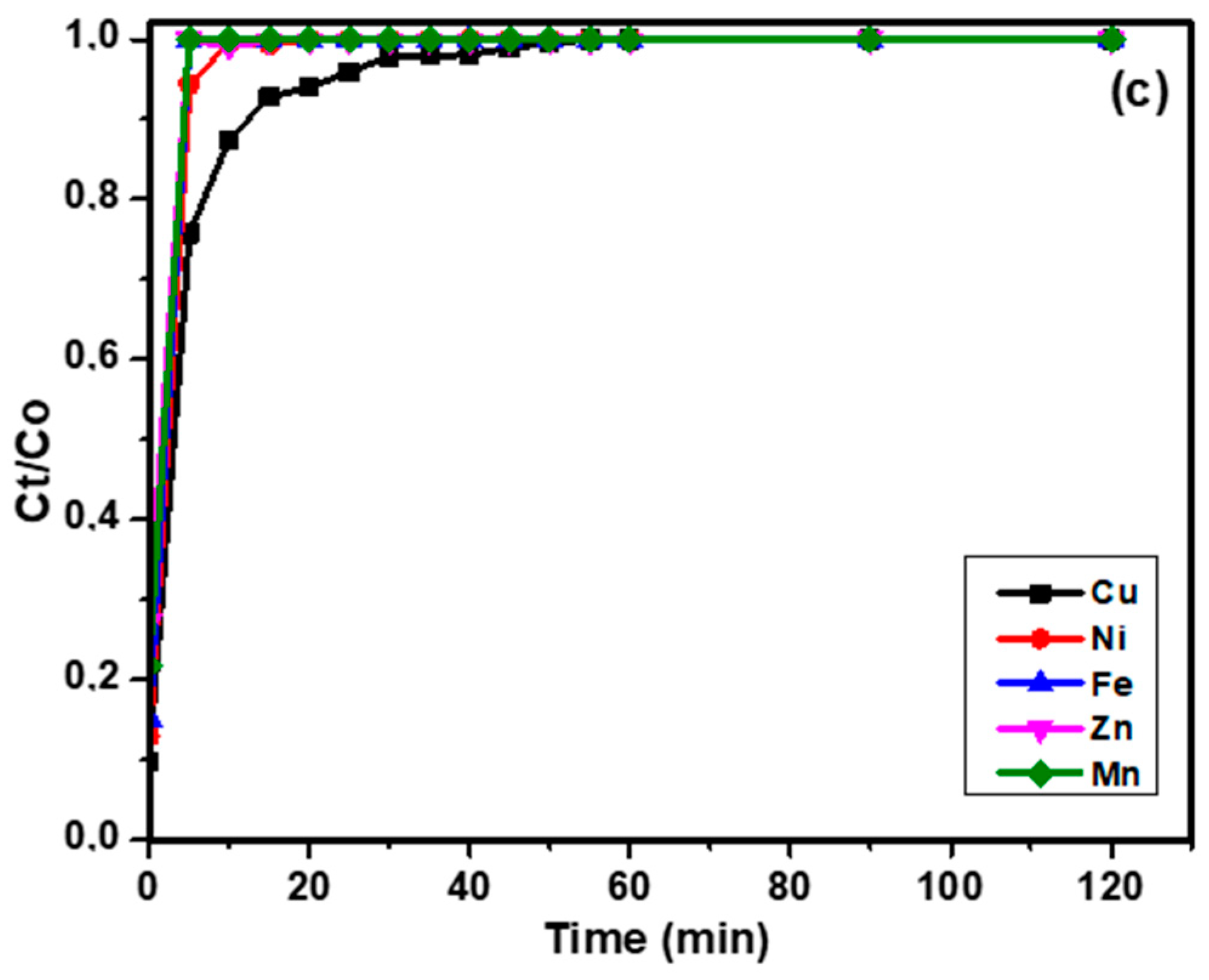
3.4. Column Sorption of Copper
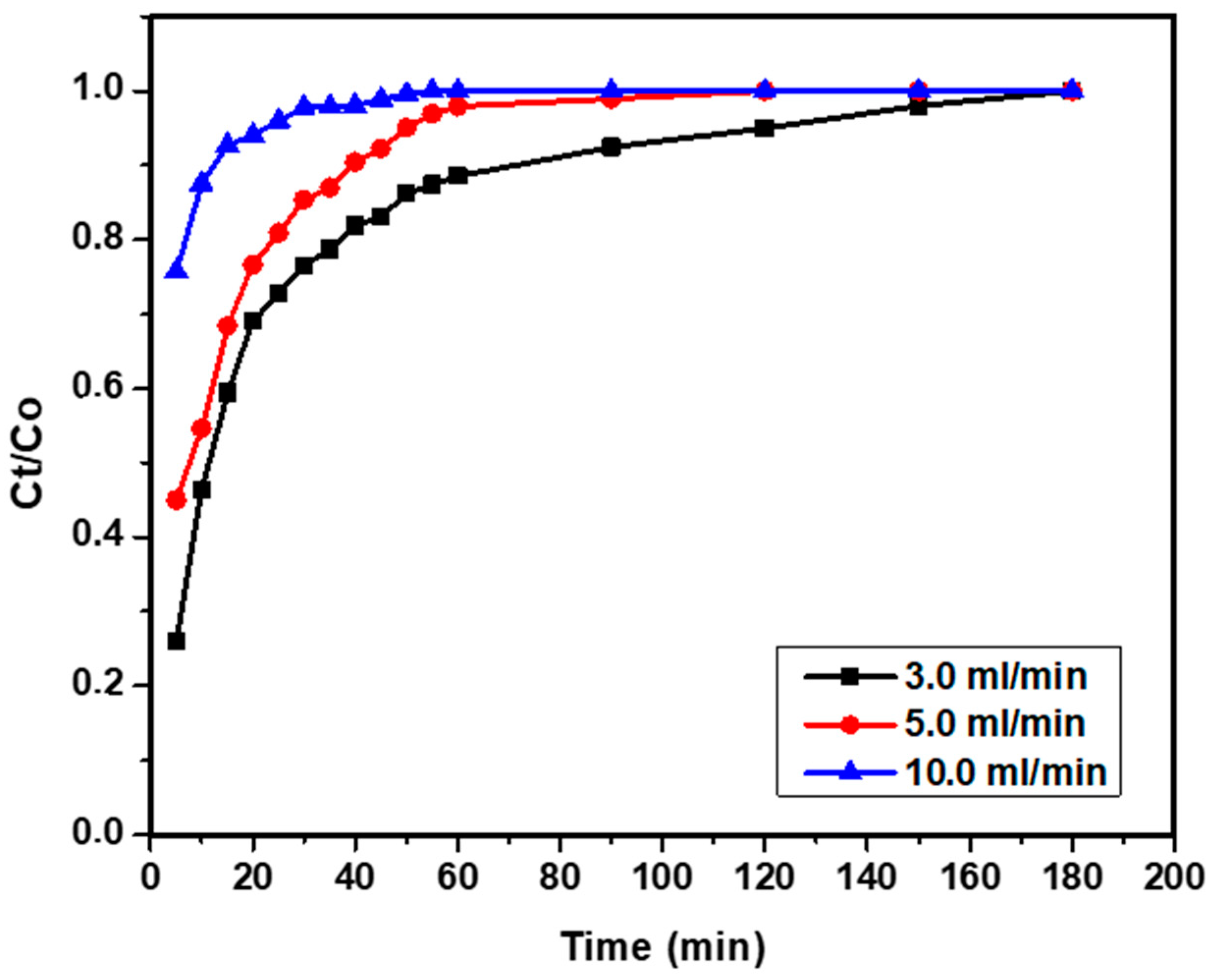
3.4.1. Effect of Flow Rate on Breakthrough Curve
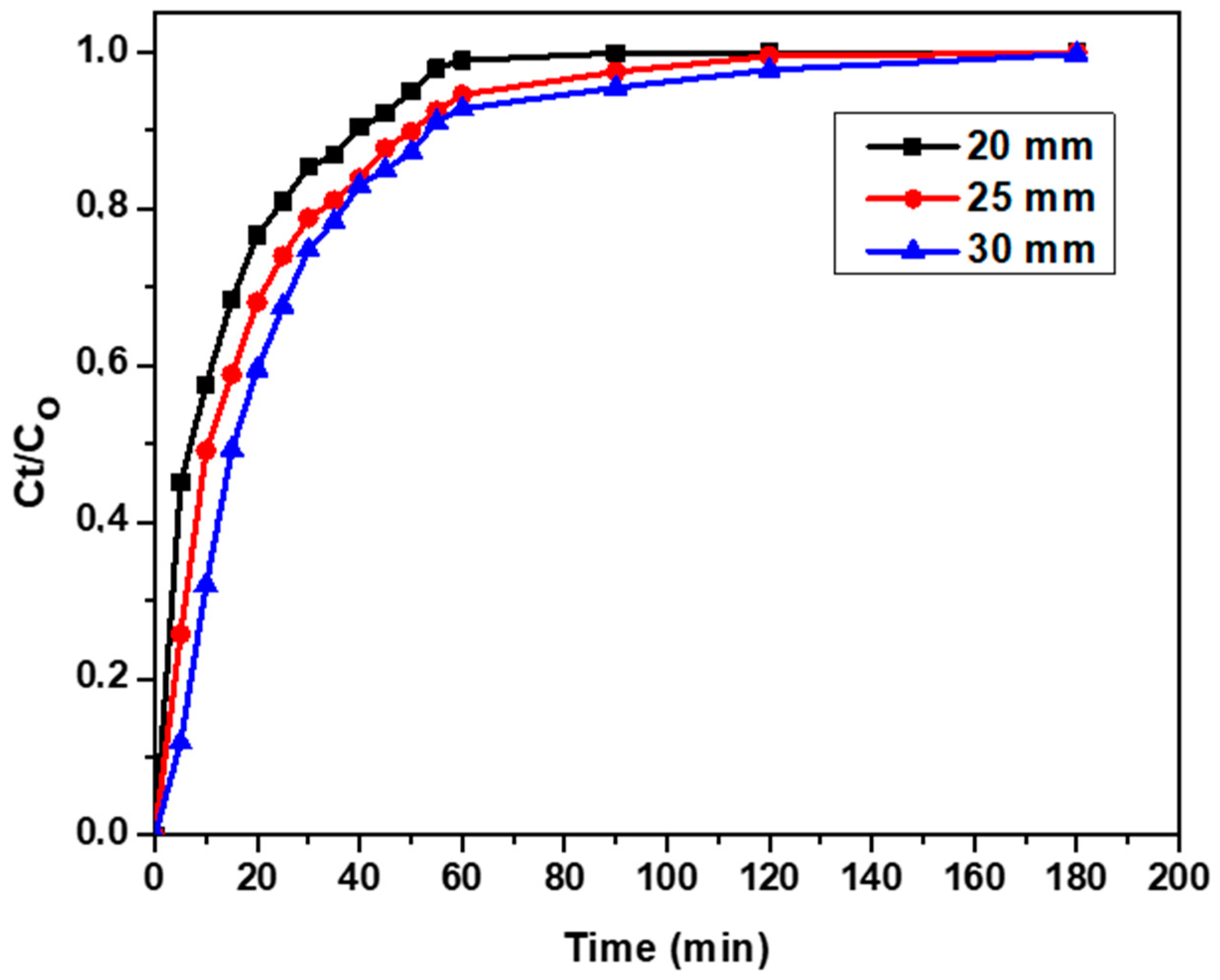
3.4.2. Effect of Bed Height on Breakthrough Curve
3.5. Determination of Column Parameters
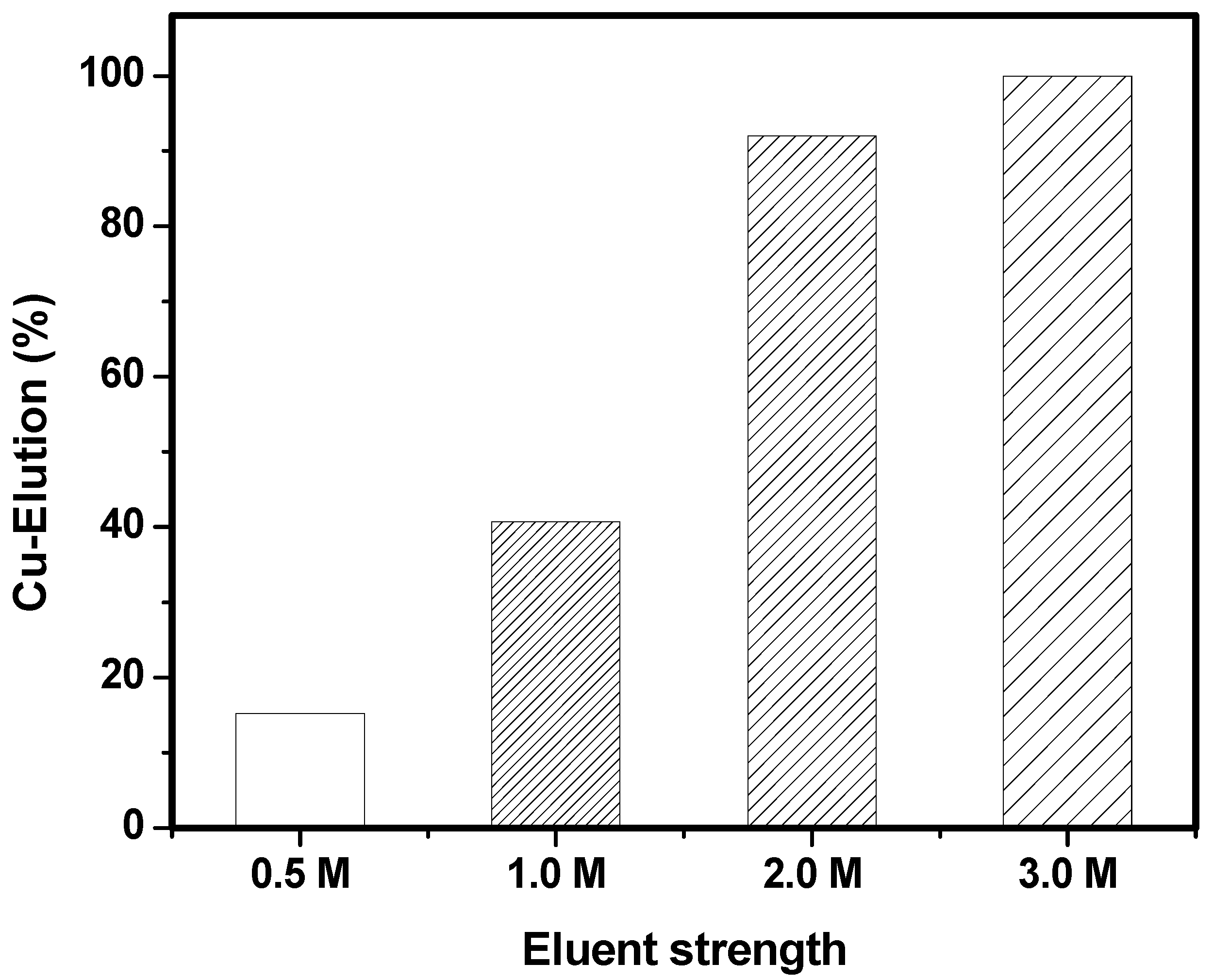
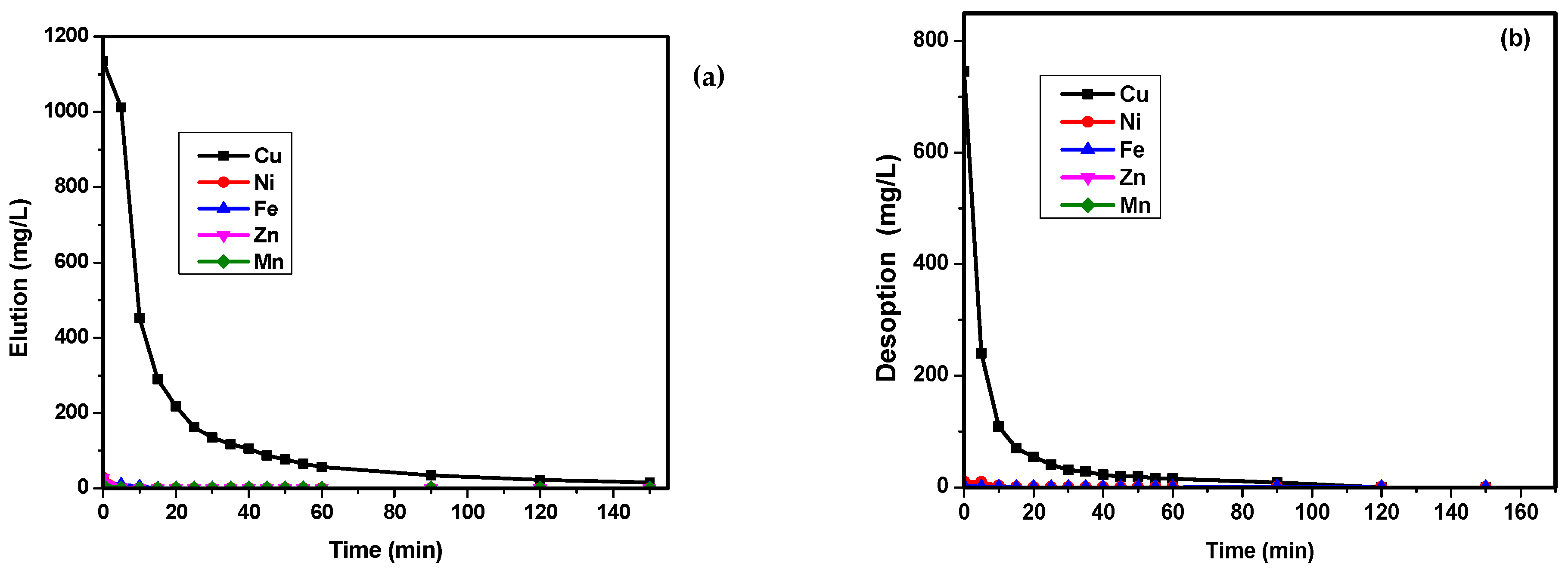
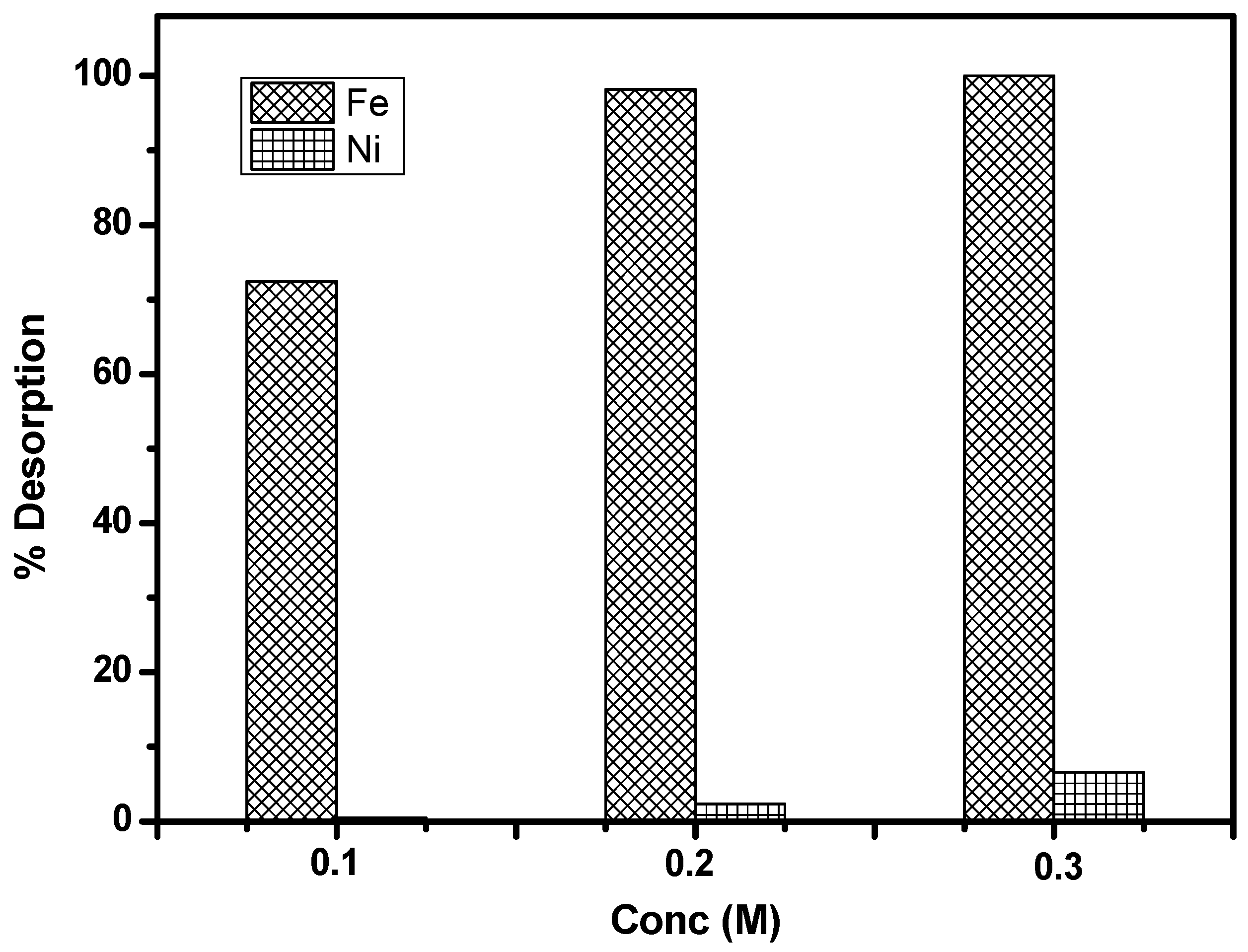
3.6. Batch and Column Desorption
3.7. Modelling of Column Data
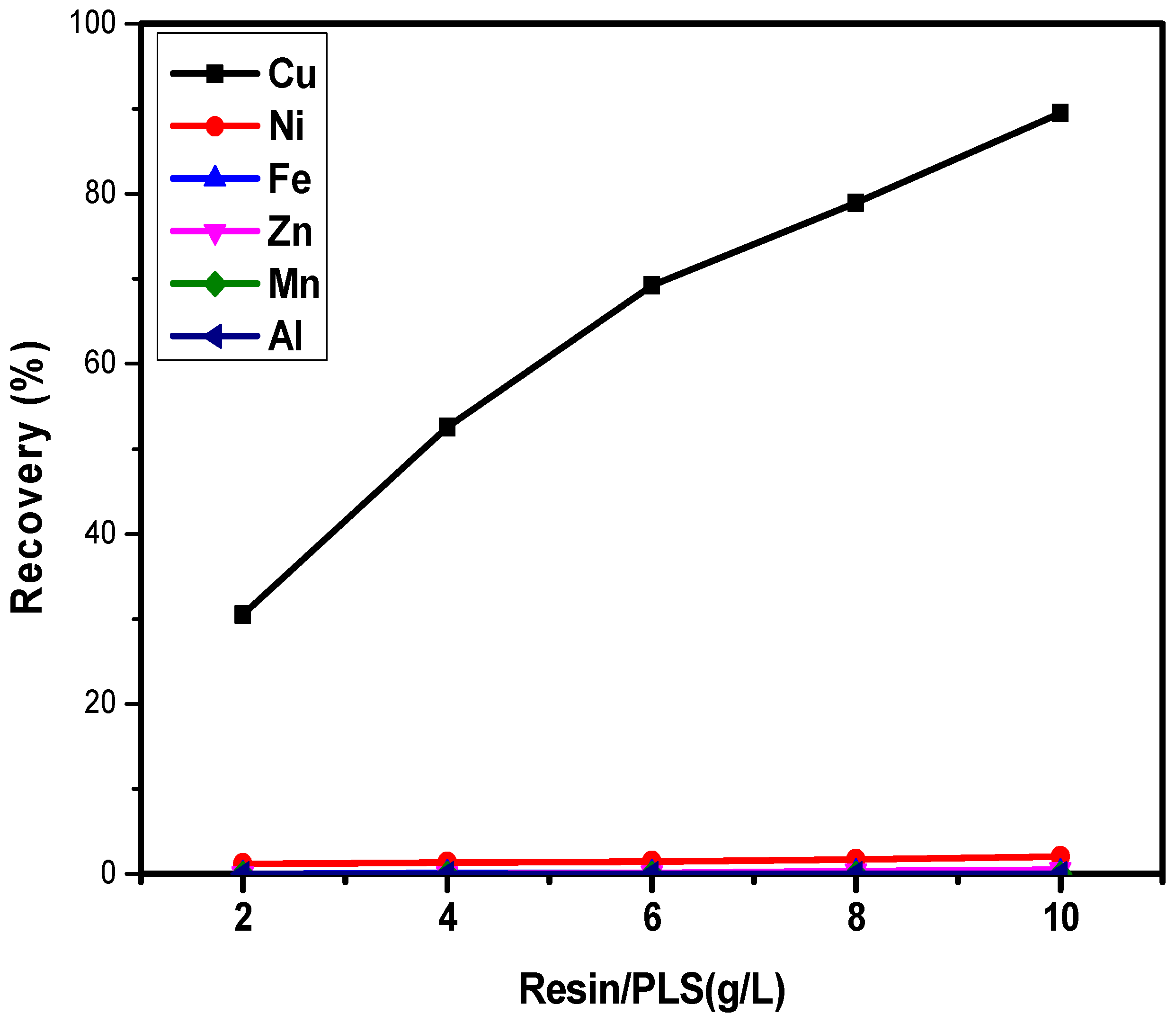
3.8. Treatment of Raffinate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor 2017: Quantities, Flows, and Resources; United Nations University: Bonn, Germany; Geneva, Switzerland; Vienna, Austria, 2017. [Google Scholar]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University (UNU): Bonn, Germany; United Nations Institute for Training and Research (UNITAR)—Co-hosted SCYCLE Programme, International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Rotterdam, The Netherlands, 2020. [Google Scholar]
- Luda, M.P. Recycling of Printed Circuit Boards. In Integrated Waste Management Volume II; Kumar, S., Ed.; InTech Publishers: London, UK, 2011; Available online: http://www.intechopen.com/books/integrated-waste-management-volume-ii/recycling-of-printed-circuit-boards (accessed on 28 September 2023).
- Das, S.C.; Gopala Krishna, P. Effect of Fe(III) during copper electrowinning at higher current density. Int. J. Miner. Process. 1996, 46, 91–105. [Google Scholar] [CrossRef]
- Shaw, D.R.; Dreisinger, D.B.; Lancaster, T.; Richmond, G.D.; Tomlinson, M. The commercialization of the FENIX iron control system for purifying copper electrowinning electrolytes. J. Mater. 2004, 56, 38–41. [Google Scholar] [CrossRef]
- Ehsani, A.; Yazici, E.Y.; Deveci, H. The influence of impurity ions on the electrowinning of copper from waste PCBs leaching solutions. In Proceedings of the XIII International Mineral Processing Symposium (IMPS), Bodrum, Turkey, 10–12 October 2012. [Google Scholar]
- Patterson, J.W.; Minear, R.A. Physical-Chemical Methods of Heavy Metal Removal. In Heavy Metals in the Aquatic Environment; Kenkel, P.A., Ed.; Pergamon Press: Oxford, UK, 1975; pp. 261–276. [Google Scholar]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and Their Application in Hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Arslan, C.; Arslan, F. Thermochemical Review of Jarosite and Goethite Stability Regions at 25 and 95 C. Eng. Environ. Sci. Acad. J. 2003, 27, 45–52. [Google Scholar]
- Yazici, E.Y.; Bas, A.D.; Deveci, H. Removal of Iron as Goethite from Leach Solutions of Waste of Printed Circuit Boards (WPCB). In Proceedings of the 27th International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Diniz, C.; Doyle, F.; Ciminelli, V. Effect of pH on the adsorption of selected heavy metal ions from concentrated chloride solutions by chelating resin Dowex M4195. Sep. Technol. 2002, 37, 3169–3185. [Google Scholar] [CrossRef]
- Habashi, S. Kinetics of Metallurgical Processes, 2nd ed.; Metallurgie Extractive Quebec: Quebec, QC, Canada, 1999. [Google Scholar]
- Davenport, W.G.I.; King, M.; Schlesinger, M.; Biswas, A.K. Extractive Metallurgy of Copper, 5th ed.; Pergamon Press: Oxford, UK, 2011. [Google Scholar]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Long Le, H.; Jeong, J.; Lee, J.C.; Pandey, B.D.; Yoo, J.M.; Huyunh, T.H. Hydrometallurgical process for copper recovery from waste printed circuit boards (PCBs). Miner. Process. Extr. Metall. Rev. 2011, 32, 90–104. [Google Scholar] [CrossRef]
- Provazi, K.; Campos, B.A.; Espinosa, D.C.R.; Tenório, J.A.S. Metal separation from mixed types of batteries using selective precipitation and liquid-liquid extraction techniques. Waste Manag. 2011, 31, 59–64. [Google Scholar] [CrossRef]
- Nayl, A.A.; Hamed, M.M.; Rizk, S.E. Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2015, 55, 119–125. [Google Scholar] [CrossRef]
- Kumari, A.; Jha, M.K.; Singh, R.P. Recovery of metals from pyrolyzed PCBs by hydrometallurgy techniques. Hydrometallurgy 2016, 165, 97–105. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, Y.E.; Panda, S. A novel approach based on solvent displacement crystallisation for iron removal and copper recovery from solutions of semi-pilot scale bioleaching of WPCBs. J. Clean. Prod. 2021, 294, 126346. [Google Scholar] [CrossRef]
- Mendes, F.D.; Martins, A.H. Recovery of nickel and cobalt from acid leach pulp by ion exchange using chelating resin. Miner. Eng. 2005, 18, 945–954. [Google Scholar] [CrossRef]
- Sahni, S.K.; Reedijk, J. Coordination chemistry of chelating resins and ion exchangers. Coord. Chem. Rev. 1984, 59, 1–139. [Google Scholar] [CrossRef]
- Busche, B.; Wiacek, R.; Davidson, J.; Koonsiripaiboon, V.; Yantasee, W.; Addleman, R.S.; Fryxell, G.E. Synthesis of nanoporous iminodiacetic acid sorbents for binding transition metals. Inorg. Chem. Commun. 2009, 12, 312–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zainol, Z.; Nicol, M. Comparative study of chelating ion exchange resins for the recovery of nickel and cobalt from laterite leach tailings. Hydrometallurgy 2009, 96, 283–287. [Google Scholar] [CrossRef]
- Laatikainen, K.; Lahtinen, M.; Laatikainen, M.; Paatero, E. Copper removal by chelating adsorption in solution purification of hydrometallurgical zinc production. Hydrometallurgy 2010, 104, 14–19. [Google Scholar] [CrossRef]
- Hubicki, Z.; Kołodyńska, D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. In Ion Exchange Technologies; InTech Publishers: London, UK, 2012; p. 193. [Google Scholar]
- Kołodyńska, D.; Sofińska-Chmiel, W.; Mendyk, E.; Hubicki, Z. DOWEX M4195 and LEWATIT®MonoPlus TP 220 in heavy metal ions removal from acidic streams. Sep. Sci. Technol. 2014, 49, 2003–2015. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, F.; Xu, C.; Gao, J.; Chen, D.; Li, A. Enhanced removal of Cu(II) and Ni(II) from saline solution by novel dual-primary-amine chelating resin based on anion-synergism. J. Hazard. Mater. 2015, 287, 234–242. [Google Scholar] [CrossRef]
- Suwannahong, K.; Sirilamduan, C.; Deepatana, A.; Kreetachat, T.; Wongcharee, S. Characterization and Optimization of Polymeric Bispicolamine Chelating Resin: Performance Evaluation via RSM Using Copper in Acid Liquors as a Model Substrate through Ion Exchange Method. Molecules 2022, 27, 7210. [Google Scholar] [CrossRef]
- Ajiboye, E.A.; Olasheinde, E.F.; Adebayo, A.O.; Ajayi, O.O.; Ghosh, M.K.; Basu, S. Extraction of copper and zinc from waste printed circuit boards. Recycling 2019, 4, 36. [Google Scholar] [CrossRef]
- Rusen, A.; Sunkar, A.S.; Topkaya, Y. Zinc and lead extraction from Cinkur leach residues by using hydrometallurgy method. Hydrometallurgy 2008, 93, 45–50. [Google Scholar] [CrossRef]
- Diniz, C.V.; Ciminelli, V.S.T.; Doyle, F.M. The use of the chelating resin Dowex M-4195 in the adsorption of selected heavy metals ions from manganese solutions. Hydrometallurgy 2005, 78, 147–155. [Google Scholar] [CrossRef]
- Ajiboye, E.A.; Olasehinde, E.F.; Adebayo, A.O.; Ajayi, O.O. Recovery of Copper and Nickel from Polymetallic Sulphate Leach Solution of Printed Circuit Boards using Dowex M4195. Physicochem. Probl. Miner. Process. 2019, 5, 1156–1164. [Google Scholar]
- Ajiboye, E.A.; Olasehinde, E.F.; Adebayo, A.O.; Ajayi, O.O.; Ghosh, M.K.; Basu, S. Extraction of Cu, Zn, and Ni from waste silica-rich integrated circuits by sulfation roasting and water leaching. Chem. Pap. 2020, 74, 663–671. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Selective adsorption of palladium (II) complexes onto the chelating ion exchange resin Dowex M4195-Kinetic studies. Solvent Extr. Ion Exch. 2010, 28, 124–159. [Google Scholar] [CrossRef]
- Lara, J.; Tejada, C.; Villabona, Á.; Arrieta, A.; Granados Conde, C. Adsorción de plomo y cadmio en sistema continuo de lecho fijo sobre residuos de cacao. Rev. Ion 2017, 29, 113–124. [Google Scholar] [CrossRef]
- Grinstead, R. Selective absorption of copper, nickel, cobalt and other transition metal ions from sulphuric acid solutions with the chelating ion exchange resin Dow XFS 4195. Hydrometallurgy 1984, 12, 387–400. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Ansari, R.; Khodavirdilo, B. Removal of Copper ions from aqueous solutions using polymer derivations of poly (styrene-alt-maleic anhydride). Egypt. J. Pet. 2016, 26, S1110062116300320. [Google Scholar] [CrossRef]
- Futalan, C.M.; Kan, C.-C.; Dalida, M.L.; Pascua, C.; Wan, M.-W. Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydr. Polym. 2011, 83, 697–704. [Google Scholar] [CrossRef]
- Revathi, M.; Saravanan, M.; Chiya, A.B.; Velan, M. Removal of Copper, Nickel, and Zinc Ions from Electroplating Rinse Water. Clean Soil Air Water 2012, 40, 66–79. [Google Scholar] [CrossRef]
- Rudnicki, P.; Hubicki, Z.; Kołodyńska, D. Evaluation of heavy metal ions removal from acidic wastewater streams. Chem. Eng. J. 2014, 252, 362–373. [Google Scholar] [CrossRef]
- Perez, I.D.; Correa, M.M.J.; Cianga Silvas, F.P.; Tenório, J.A.S.; Espinosa, D.C.R. Effect of Flow Rate on Metals Adsorption of Synthetic Solution Using Chelating Resin Dowex XUS43605 in Column Experiments. In Energy Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 483–491. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. Continuous fixed-bed column study and adsorption modeling: Removal of cadmium (II) and lead (II) ions in aqueous solution by dead calcareous skeletons. Biochem. Eng. J. 2014, 87, 50–61. [Google Scholar] [CrossRef]
- Punrattanasin, P.; Sariem, P. Adsorption of Copper, Zinc, and Nickel using Loess as Adsorbent by Column Studies. Pol. J. Environ. Stud. 2015, 24, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.C.; Groot, D.R. Improving cathode morphology at a copper electrowinning plant by optimizing Magnafloc 333 and chloride concentrations. J. S. Afr. Inst. Min. Metall. 2019, 119, 983–987. [Google Scholar] [CrossRef]
- Beukes, N.; Badenhorst, J. Copper electrowinning: Theoretical and practical design. J. S. Afr. Inst. Min. Metall. 2009, 106, 343–356. [Google Scholar]
- Wołowicz, A.; Hubicki, Z. Enhanced removal of copper (II) from acidic streams using functional resins: Batch and column studies. J. Mater. Sci. 2020, 55, 13687–13715. [Google Scholar] [CrossRef]
- Verduzco-Navarro, I.P.; Rios-Donato, N.; Jasso-Gastinel, C.F.; Martinez-Gomez, A.D.J.; Mendizábal, E. Removal of Cu (II) by fixed-bed columns using Alg-Ch and Alg-ChS hydrogel beads: Effect of operating conditions on the mass transfer zone. Polymers 2020, 12, 2345. [Google Scholar] [CrossRef]
- Zimmerman, M.D.; Bennett, P.C.; Sharp, J.M., Jr.; Choi, W.J. Experimental determination of sorption in fractured flow systems. J. Contam. Hydrol. 2002, 58, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Yahorava, V.; Kotze, M.; Auerswald, D. Evaluation of different adsorbents for copper removal from cobalt electrolyte. J. S. Afr. Inst. Min. Metall. 2014, 114, 383–390. [Google Scholar]
- Angel, V.; Candelaria, T.; Rodrigo, O.; Keily, P.; Ciro, B. Impact of temperature, bed height, and particle size on Ni (II) removal in a continuous system: Modelling the break curve. Pol. Acad. Sci. 2022, 52, 257–264. [Google Scholar]

| Metals | Cu | Ni | Mn | Fe | Zn | Al | Sn | Pb |
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 5230 | 312 | 90.9 | 1220 | 477 | 305 | 5.50 | 2.01 |
| Isotherm | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Qm (mg/g) | KL (L/mg) | RL | R2 | Kf | 1/n | R2 |
| Values | 5.89 | 3.74 | 0.015 | 0.99 | 36156.8 | 0.975 | 0.99 |
| Kinetics | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| Parameters | Qe | k1 | R2 | Qe | k2 | R2 |
| Values | 50.0 | 0.006 | 0.86 | 1.3 | 0.072 | 0.998 |
| H | C0 | Q | qt | mt | qeq | Ceq | %R |
|---|---|---|---|---|---|---|---|
| 20 | 1099 | 3 | 19.8 | 494.6 | 19.8 | 1055.5 | 4 |
| 20 | 974 | 5 | 17.4 | 541.4 | 17.4 | 945 | 3.2 |
| 20 | 924.5 | 10 | 3.6 | 554.7 | 3.6 | 918.5 | 0.7 |
| 25 | 894.5 | 5 | 20.1 | 536.7 | 10.1 | 861 | 3.8 |
| 30 | 902 | 5 | 27.4 | 541.5 | 11.0 | 865.5 | 4.1 |
| Column Parameters | Thomas Model | Yoon-Nelson Model | ||||||
|---|---|---|---|---|---|---|---|---|
| H | C0 | Q | kTH × 10−5 | Q0 | R2 | kYN | τ | R2 |
| 2 | 1099 | 3 | 4.187 | 32.47 | 0.93 | 0.0519 | 11.34 | 0.95 |
| 2 | 974 | 5 | 4.663 | 34.1 | 0.91 | 0.0523 | 10 | 0.95 |
| 2 | 924.5 | 10 | 10.22 | 64.77 | 0.90 | 0.097 | 6.62 | 0.94 |
| 2.5 | 894.5 | 5 | 4.8452 | 39.7 | 0.91 | 0.04681 | 8 | 0.95 |
| 3 | 902 | 5 | 6.0798 | 49.01 | 0.92 | 0.05648 | 16.3 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajiboye, E.A.; Aishvarya, V.; Petersen, J. Selective Recovery of Copper from the Mixed Metals Leach Liquor of E-Waste Materials by Ion-Exchange: Batch and Column Study. Minerals 2023, 13, 1285. https://doi.org/10.3390/min13101285
Ajiboye EA, Aishvarya V, Petersen J. Selective Recovery of Copper from the Mixed Metals Leach Liquor of E-Waste Materials by Ion-Exchange: Batch and Column Study. Minerals. 2023; 13(10):1285. https://doi.org/10.3390/min13101285
Chicago/Turabian StyleAjiboye, Emmanuel A., V. Aishvarya, and Jochen Petersen. 2023. "Selective Recovery of Copper from the Mixed Metals Leach Liquor of E-Waste Materials by Ion-Exchange: Batch and Column Study" Minerals 13, no. 10: 1285. https://doi.org/10.3390/min13101285
APA StyleAjiboye, E. A., Aishvarya, V., & Petersen, J. (2023). Selective Recovery of Copper from the Mixed Metals Leach Liquor of E-Waste Materials by Ion-Exchange: Batch and Column Study. Minerals, 13(10), 1285. https://doi.org/10.3390/min13101285







