Abstract
Phosphate ore is an important strategic mineral resource. The efficient utilization of phosphate resources faces challenges such as low grade of raw ore and difficulty in discharging gangue minerals. One of the key problems to be solved urgently in the reverse flotation of phosphate ore is the effective depression of apatite. However, research on the influence mechanism of acid depressants on the surface properties and adsorption characteristics of apatite is still insufficient. In this study, the influence of different depressants (such as sulfuric acid, phosphoric acid or mixed acid of sulfur acid and phosphorus acid) on the flotation separation performance of an artificial mixture of apatite and dolomite (gangue mineral) was investigated through laboratory flotation tests. On this basis, with the addition of different depressants, the contact angle, zeta potential, XPS and TOC were used to investigate the surface wettability, surface charge, surface species and the adsorption characteristics of the collector (sodium oleate) on the apatite surface, and, accordingly, the inhibiting mechanism was discussed. The results show that, when mixed acid of sulfur acid and phosphorus acid is used as a depressant, a concentrate with a P2O5 grade of 33.53% and a recovery of 88.92% can be obtained, and the parameters are better than when using phosphoric acid with a P2O5 grade of 30.15% and a recovery of 80.12% or sulfuric acid with a P2O5 grade of 30.12% and a recovery of 80.58%. Our analysis shows that the mixed acid has the best inhibiting effect on apatite, which is mainly due to the following: (a) after adding the mixed acid, chemicals such as CaSO4, CaHPO4/Ca(H2PO4)2 are generated on the surface of apatite, resulting in a significant reduction in the contact angle and stronger surface hydrophilicity; (b) the mixed acid reduces the zeta potential of apatite, produces new species and weakens the non-selective adsorption of negatively charged oleate on the surface of apatite, thus preventing the apatite from floating.
1. Introduction
Phosphorus is an essential element for plant growth, and phosphate fertilizer is produced from phosphate rock, which contains the necessary nutrient phosphorus for crops; phosphate rock is thus an irreplaceable and important mineral resource to ensure food security for human society [1,2]. In recent years, with the rapid development of the new energy industry, the mass production of Lithium-ion Ferrous Phosphate batteries (LFP batteries) has further increased the demand for phosphorus ores, and thus many countries have included phosphorus ores in the list of strategic minerals. According to statistics, by 2021, the world’s phosphate ore reserves were approximately 71 billion tons, mainly distributed in 60 countries and regions in Africa, North America, Asia, the Middle East and South America. Nevertheless, if the annual consumption of phosphate ore is predicted at a growth rate of 3% per year, the world’s phosphate ore resources will be depleted by 2065 [3]. This puts forward very high requirements for improving the utilization efficiency of phosphorus resources. China is a country with abundant reserves of phosphate rock resources, accounting for 4.6% of the globe’s total reserves and ranking second in the world [4]. However, China’s phosphate rock is mainly sedimentary phosphate rock, with a low to medium grade, and has characteristics such as high impurity content and fine mineral embedding particle size [5]. Therefore, the efficient utilization of phosphate resources faces severe challenges.
The development of efficient beneficiation technology for medium to low grade phosphate ore is crucial for improving the grade of concentrate and reducing impurity content [6]. Flotation is the main beneficiation method for medium to low grade phosphorus ores, which achieves separation between minerals based on differences in the physical and chemical properties of the solid–water interface of mineral particles (mainly manifested as differences in wettability) [7,8,9]. The valuable mineral in the sedimentary calcium magnesium phosphate ore is apatite, while the gangue minerals are mainly dolomite, calcite, etc. The reverse flotation process is often used to enrich apatite in practice because, under acidic conditions, apatite can be depressed while dolomite presents a good floatability [10,11]. However, apatite and dolomite or calcite are all salt minerals, with similar crystal structure and surface physical and chemical properties, and little difference in floatability, which leads to difficult flotation separation and a complex separation mechanism [12,13]. Therefore, effective depressants must be added to regulate the flotation behavior of apatite and gangue minerals, with the purpose of depressing apatite from floating [14].
Sulfuric acid or phosphoric acid is usually added to the pulp to depress apatite and serve as the pH regulator of the pulp, and then the carbonate gangue minerals (dolomite and calcite) are floated with anionic collectors (such as fatty acids) [15,16,17]. Much research has been carried out in view of the depression of sulfuric acid or phosphoric acid on apatite in a reverse flotation system. For example, Abouzeid [18] reviewed the depression mechanism of apatite in a carbonate–phosphate reverse flotation system, and believed that it is various soluble phosphate radicals that depressed apatite. Elgillani [19] analyzed the thermodynamics of the “phosphate–carbonate–water” system, and believed that the water-soluble CaHPO4 or CaH2PO4+ produced by apatite dissolution under acidic conditions is the component that produced depression. Zou [20] studied the depression mechanism of sulfuric acid on apatite during the reverse flotation of apatite and dolomite, and found that sulfate can react with calcium on the surface of apatite to generate calcium sulfate, which hinders the adsorption of sodium oleate (collector) and calcium sites on the surface of apatite, making the surface hydrophilic, while sulfate cannot shield magnesium sites on the surface of dolomite, so dolomite still has good floatability. Chen [21] investigated the depression effect of both phosphoric acid and hydrochloric acid on collophane during phosphate ore flotation, and the result showed that the depression effect of phosphoric acid on collophane is better than that of hydrochloric acid because phosphoric acid could ionize H2PO4− and adsorb on the collophane surface to make it hydrophilic. Liu [22] studied the selective depression mechanism of phosphoric acid in the flotation system of apatite and dolomite, and the results show that H2PO4− is the key ion to depress apatite under weak acidic conditions (pH 5–6). Ye [23] also showed that, when phosphoric acid is used as the depressant of apatite, its ionized H2PO4− has the ability to depress apatite and buffer the pH of pulp, and it could obtain flotation indicators similar to sulfuric acid at low dosage, which is suitable for calcite-containing phosphate ore. Zhang [24] studied the flotation performance of a collophanite ore by employing sulfur–phosphorus mixed acid as a depressant, and found that the sulfur–phosphorus mixed acid could depress the apatite effectively; a concentrate with P2O5 content of 28.4% and a corresponding recovery of 85% was obtained. It should be noted that the waste acid water, composed of phosphoric acid and sulfuric acid in the wet process of phosphoric acid, has also been proven to be an effective and inexpensive depressant [25].
In the reverse flotation process for phosphate ore, the effective depression of apatite is one of the key problems that needs to be solved urgently. Although many scholars have carried out relevant research, the mechanism of the influence of acid depressants, especially mixed acids (i.e., sulfuric acid and phosphoric acid), on the surface properties and adsorption characteristics of apatite is still insufficient. Based on previous research, this study takes pure apatite and dolomite from a phosphate ore from China’s Guizhou Province as the research object to investigate the effect of sulfuric acid, phosphoric acid and mixed acid (sulfur acid and phosphorus acid) on the flotation separation. The mechanism of mixed acid depressing the flotation of apatite is discussed in light of the surface species and non-selective adsorption of collectors to apatite. The research results provide a certain scientific reference for the efficient utilization of medium-low grade calcium–magnesium phosphate ores by reverse flotation.
2. Methods and Materials
2.1. Materials
The phosphate ore sample used in this study was taken from the Wengfu phosphate mine in Guizhou, SW China. The sample was crushed to −2 mm by a laboratory crusher, and then apatite and dolomite in the ore were hand-picked and ground, respectively. Then, the samples were screened into the fraction of 35–75 μm due to its good floatability, which were used for subsequent flotation experiments and mechanism research. X-ray diffraction (XRD) and X-ray fluorescence spectrum (XRF) were used to analyze the property of apatite and dolomite samples, and the results are shown in Figure 1 and Table 1. Due to the presence of small amounts of impurities in the sample, such as SiO2, Fe2O3, Al2O3, etc., the purity of apatite and dolomite samples are 90% and 98%, respectively, which meets the requirements of the pure mineral flotation test. In addition, an artificial ore sample was prepared by mixing apatite and dolomite in the mass ratio of 1:1, with the P2O5 content of 20.21%. It is notable that, in Table 1, the sum of different elements of apatite and dolomite is not close to 100%; this is because carbon and some minor elements in these two minerals are not included.
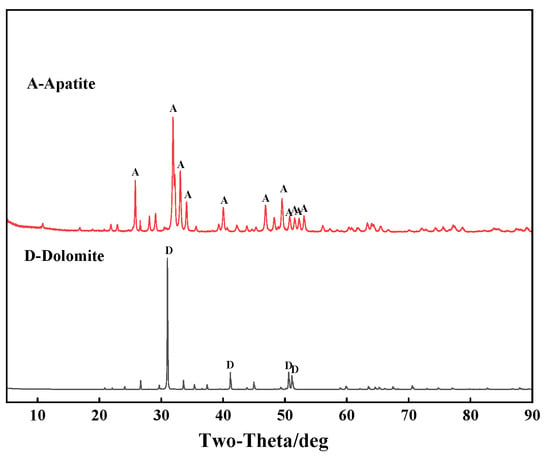
Figure 1.
X-ray diffraction pattern of the purified apatite and purified dolomite.

Table 1.
The chemical composition of purified apatite, dolomite and artificial ore, %.
2.2. Methods
Microflotation tests: Deionized water (40 mL) and apatite or artificial ore sample (2 g) were put into the flotation cell (50 mL) (Jilin Exploration Machinery Plant, China), stirred for 1 min and then a certain amount of sulfuric acid (0–1.6 mmol/L), phosphoric acid (0–5 mmol/L), or a mixture of sulfur acid and phosphorus acid as depressants and pH regulator were added to the slurry (stirred for 1 min), then collector (sodium oleate, 0.2–1.6 mmol/L) was added and stirred for 2 min and, finally, gas was charged at a rate of 0.6 cm3/h and flotation was conducted for 5 min. The floats and sinks were filtered, respectively, and then dried to constant weight at 45℃. The phosphoric acid and sulfuric acid used in the flotation test were of superior purity, while sodium oleate was of analytical purity. It was found that the data errors of grade and recovery do not exceed 2%, thus one flotation test was conducted for each condition.
Contact angle: Contact angle can directly characterize the wettability of mineral surfaces. A block sample of apatite (a cube with a side length of about 2 cm) was prepared, and a smooth surface of which was made by grinding and polishing. The contact angle of the polished surface was determined using a contact angle tester (HARKE-SPCAX3, Beijing Hake test instrument factory, China). The measurement was repeated for 5 times and an average value was calculated. In order to investigate the influence of reagents on the wettability and surface morphology of the apatite, the smooth surface was immersed in depressant or collector solution for a period of time, and then dried before testing.
Zeta potential: After being ground to −35 μm with an agate mortar, the apatite sample was put in a beaker containing a certain amount of depressant or collector solution for 10 min, then filtered and dried. An amount of 20 mg of the sample was then put into another beaker; 20 mL NaCl solution (mmol/L) was added, then vibrated in an ultrasonic cleaner for 10 min. After that, 3 mL of the suspension was sampled and the zeta potential was measured at 25 °C using a Zetasizer Delsa™ Nano C system (Beckman Coulter, Brea, CA, USA); the test was repeated for 3 times for an average value.
Total Organic Carbon Analysis (TOC): TOC (TOC-LCPH, Shimadzu, Kyoto, Japan) was used to measure the content of organic carbon in the pulp. An amount of 2 g of the sample was put in a beaker which contains 40 mL deionized water and a certain concentration of sodium oleate (collector), stirred in a laboratory water bath (25 °C) for 5 min and then treated in a centrifugal machine at a rotate speed of 4000 r/min for 10 min. The concentration of total organic carbon in the supernatant was determined, thus the adsorption amount of sodium oleate on the mineral surfaces was calculated based on the difference between the initial and the residual concentration of sodium oleate in the pulp. The test was repeated 3 times for an average value.
X-ray photoelectron spectroscopy (XPS): Al Kα ray is used as a radiation source (hv = 1486.6 eV), with an anode voltage and current of 9 kV and 18.5 mA, respectively. The test voltages for full spectrum and narrow spectrum are 100 eV and 30 eV, respectively, with an energy scanning step of 0.05 eV, a residence time of 40 ms~50 ms and an analysis chamber vacuum of 10−8 Pa. An amount of 2 g of apatite was added to 40 mL depressant solution, which was stirred, centrifuged and vacuum-dried before being tested using X-ray photoelectron spectroscopy (ESCALAB 250Xi, Thermo Fisher, Waltham, MA, USA).
Bubble–particle wrap angle test: In the flotation process, the hydrophobicity of the mineral surface directly affects the interaction between mineral particles and bubbles, which is the key to mineral flotation. Bubble–particle wrap angle is a comprehensive reflection of bubble–particle attachment, which has been used to simulate the flotation process and indicate the situation of particles who attached onto bubble surfaces [26]. The test was conducted by using a self-constructed bubble–particle attachment testing device, which includes of a computer, a camera, a needle capillary (internal diameter 2 mm), a micro syringe pump and a rectangular quartz cuvette. First, 0.5 g mineral sample and 40 mL deionized water were added into the quartz cuvette (3.5 × 3.5 × 5 cm), stirred for 1 min, then depressants (acids) and collector (sodium oleate) were added and conditioned for 2 min. After that, an air bubble with a diameter of about 3 mm was generated from the capillary and a rotor speed of 250 rpm was maintained during the experiment and the wrap angles of the particles on the bubble surface were determined at 10, 20, 30, 60 and 180 s, respectively. The picture of bubble–particles wrap angle was taken by the camera when the suspension was clear.
3. Results and Discussion
3.1. Effect of Mixture of Sulfur Acid and Phosphorus Acid on Flotation Separation of Apatite and Dolomite
A reverse flotation test was carried out for the artificial ore of apatite and dolomite, and the influence of the sodium oleate dosage on the flotation separation performance of apatite dolomite was discussed. As shown in Figure 2, with the increase in dosage of sodium oleate, the P2O5 grade of phosphorus concentrate increases from 25.77% to 27.90%. When the dosage of sodium oleate is 1.6 mmol/L, the P2O5 grade of the phosphorus concentrate reaches its maximum value of 27.90%, but the corresponding recovery is only 52.87%. Excessive sodium oleate led to the decrease in P2O5 recovery, which may be attributed to the enhanced non-selective adsorption of sodium oleate on apatite, which causes apatite to float up into the tailings. Overall, when the dosage of sodium oleate is 0.98 mmol/L, optimal flotation performance is obtained with a P2O5 grade of 27.64% and a recovery of 78.03%.
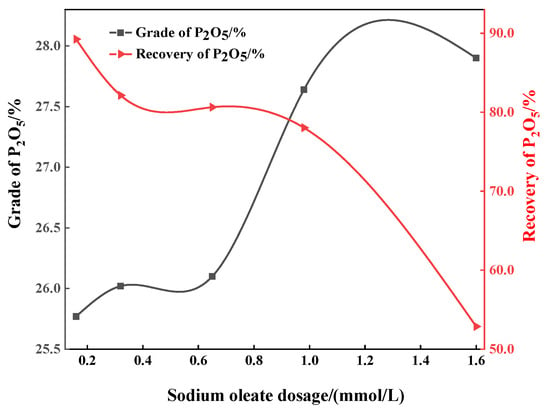
Figure 2.
Effect of sodium oleate dosage on P2O5 grade and recovery of the concentrate.
When the collector dosage is maintained at 0.98 mmol/L, the effect of sulfuric acid as a depressant on apatite flotation has also been studied. When the amount of sulfuric acid increases from 0.2 mmol/L to 0.8 mmol/L, the P2O5 grade of the concentrate increases with the increase in sulfuric acid dosage, but when the sulfuric acid dosage is greater than 0.8 mmol/L, the concentrate grade shows a downward trend. When the consumption of sulfuric acid is 0.8 mmol/L, the P2O5 grade and recovery are 30.12% and 80.58%, respectively (Figure 3). Flotation tests were also conducted using phosphoric acid as a depressant under the same collector conditions. With the increase in phosphoric acid dosage, the P2O5 grade of the phosphate concentrate increases gradually. At a phosphoric acid dosage of 3.27 mmol/L, the P2O5 grade of the concentrate is 30.15%, and the recovery is 80.12% (Figure 4), which is better than the separation performance when sulfuric acid is used as a depressant. The results above show that a good flotation separation effect of apatite dolomite can be achieved with sulfuric acid or phosphoric acid as the depressant (the concentrate P2O5 grade > 30%), but the problem is that the recovery is relatively low.
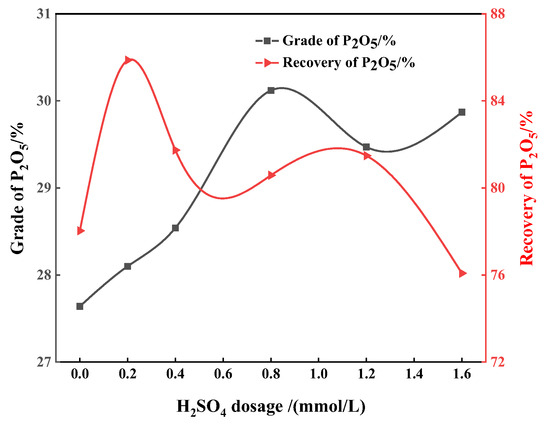
Figure 3.
Effect of the dosage of sulfuric acid on P2O5 grade and recovery.
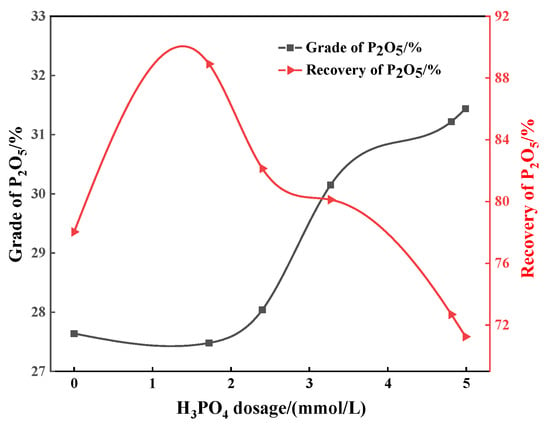
Figure 4.
Effect of phosphoric acid dosage on P2O5 grade and recovery.
In order to further enhance the flotation separation performance, the influence of the mixture of sulfuric acid and phosphoric acid (hereinafter referred to as mixed acid) on the flotation of apatite and dolomite was also explored. When the dosage of sodium oleate and phosphoric acid are maintained as 0.98 mmol/L and 3.27 mmol/L, respectively, a certain amount of sulfuric acid is added to the pulp to study the effect of different proportions of mixed acid on the flotation. As shown in Figure 5, with the increase in sulfuric acid dosage in the mixed acid, the P2O5 grade of the concentrate shows a trend of first increasing and then decreasing. When a mixed acid composed of 3.27 mmol/L phosphoric acid and 0.8 mmol/L sulfuric acid is added, the P2O5 grade of the phosphorus concentrate is 33.53% and the recovery is 88.92%. Therefore, compared with the use of sulfuric acid or phosphoric acid, respectively, the use of mixed acid can significantly enhance the flotation separation of apatite and dolomite.
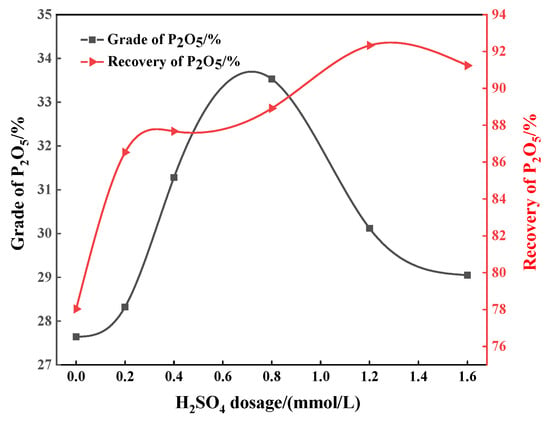
Figure 5.
Effect of sulfuric acid content in sulfur–phosphorus mixed acid on grade and recovery of phosphorus concentrate P2O5.
3.2. Mechanism of Depression of Apatite by Mixed Acid
3.2.1. Effect of the Mixed Acid on the Surface Wettability of Apatite
In order to explore the flotation depression mechanism of acid depressants on apatite, flotation tests were carried out on apatite to study the relationship between the surface wettability of apatite (quantified by contact angle) under the action of different depressants and its depression effect (quantified by the yield of the floats, the lower the yield, the better the depression effect). It can be seen from Figure 6, Figure 7 and Figure 8 that regardless of whether phosphoric acid, sulfuric acid or mixed acid are used as depressants, the contact angle and floats yields of apatite gradually decrease with the increase in depressant dosage. This shows that increasing the amount of acid can enhance the depression effect on apatite, and also shows that there is a good positive correlation between the surface contact angle of apatite and its floats yield. For example, when the amount of sulfuric acid is 0.8 mmol/L, the contact angle of apatite reaches the minimum value of 46.20° and the corresponding floats yield is also the smallest at only 26.03% (Figure 6). Similar situations can also be observed in Figure 7 and Figure 8. It can be inferred that the acid depressants act with the surface of apatite, reducing its surface contact angle and enhancing its hydrophilicity, thus preventing it from floating up. In addition, it can be found that, when the mixed acid is used as the depressant, the contact angle and floats yield of apatite are significantly lower than those using sulfuric acid or phosphoric acid, respectively (Figure 6, Figure 7 and Figure 8). This is consistent with the flotation test results of artificial ore.
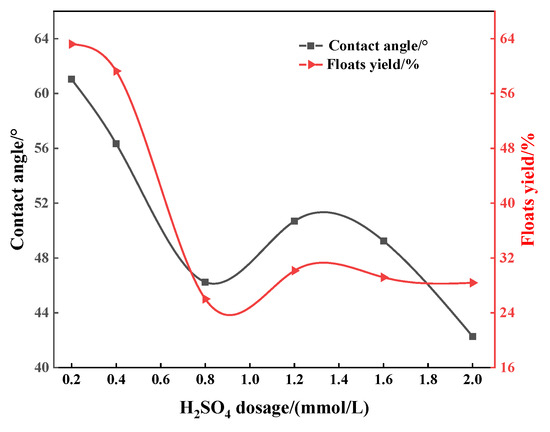
Figure 6.
Influence of amount of sulfuric acid on contact angle between surface and apatite.
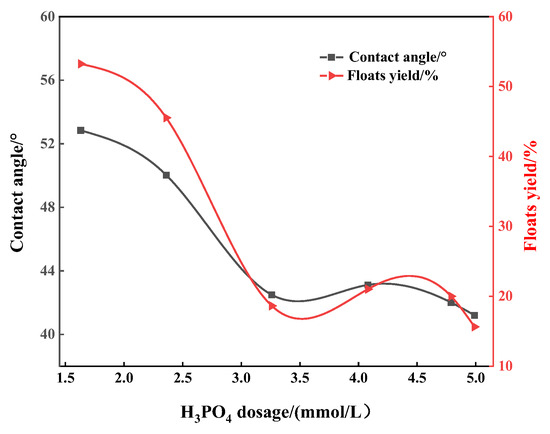
Figure 7.
Effect of phosphoric acid dosage on contact angle of apatite floating on surface.
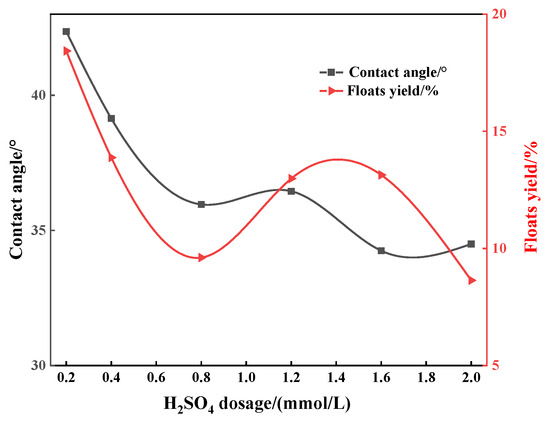
Figure 8.
Influence of sulfuric acid dosage on the contact angle between apatite floating and surface under the condition of sulfur–phosphorus mixed acid.
3.2.2. Effect of Mixed Acid on Surface Charge of Apatite
The zeta potential of the slurry was tested under the action of different depressants. Specifically, when the consumption of sulfuric acid ranges from 0.2 to 2.0 mmol/L, the pulp pH varies from 3.19 to 2.26. When the consumption of phosphoric acid ranges from 1.63 to 4.99 mmol/L, the pulp pH varies from 2.62 to 2.33 while, in the case of mixed acid, the pH ranges from 2.45 to 2.25. The results show that, as the dosage of depressants increases, the absolute value of zeta potential decreases gradually (Figure 9 and Figure 10). Specifically, under the action of mixed acid, the absolute zeta potential of the slurry is the highest, followed by sulfuric acid and phosphoric acid. When mixed acid is used, the absolute value of slurry zeta potential is relatively large and the flotation rate of apatite is also the lowest under corresponding conditions (Figure 8). This may be because the zeta potential value on the apatite surface is small (negative value), which is the same as the charge symbol of oleate (collector, with negative charge), and the electrostatic repulsion is large, which reduces the non-selective adsorption of the collector on the apatite surface, thus preventing apatite from floating up.
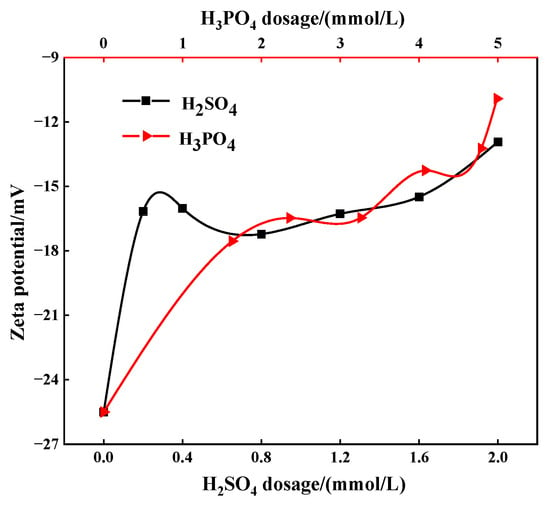
Figure 9.
Zeta potential of apatite surface under different concentrate and depressant conditions.
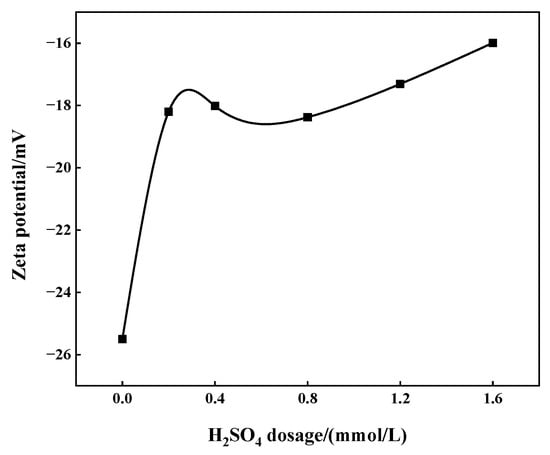
Figure 10.
Effect of sulfuric acid dosage on zeta potential of apatite surface under the condition of parathion mixed acid (phosphoric acid dosage 3.27 mmol/L).
3.2.3. Effect of Mixed Acid on Surface Composition of Apatite
Apatite is first mixed with sulfuric acid (0.8 mmol/L), phosphoric acid (3.27 mmol/L), or mixed sulfur phosphorus acid (H2SO4 = 0.8 mmol/L, H3PO4 = 3.27 mmol/L), and then XPS is used to determine the composition of the substances generated on the surface of apatite. The XPS full spectrum, element binding energy and content are shown in Figure 11 and Table 2, respectively. When apatite is treated with sulfuric acid or mixed acid, S element is detected on its surface, but S element is not detected on the surface of raw ore and sample treated with phosphoric acid, which indicates that CaSO4 may be generated on the surface of apatite after being treated with sulfuric acid or mixed acid.
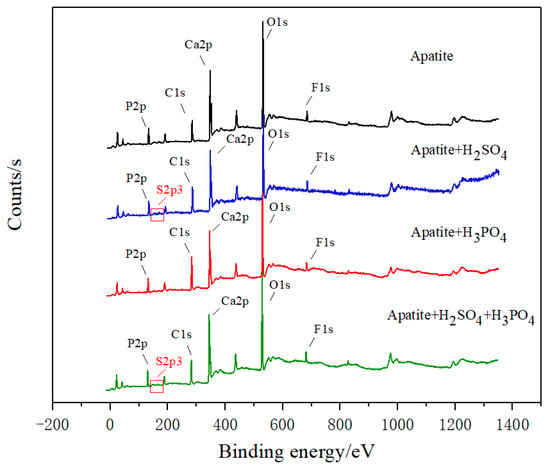
Figure 11.
X-ray photoelectron spectroscopy of apatite surface with depressant.

Table 2.
Results of element concentration and binding energy on apatite surface.
The narrow spectrum of Ca on the surface of apatite under different depressant conditions is shown in Figure 12. Without the addition of any depressant, the 2p double peaks of Ca in apatite appear at 351.03 eV and 347.35 eV, representing Ca2p1/2 and Ca2p3/2, respectively. When adding sulfuric acid, these two peaks become 351.00 eV and 347.45 eV; when adding phosphoric acid, it becomes 351.00 eV and 347.46 eV; and after adding the mixed acid, it is 351.23 eV and 347.45 eV, respectively. The difference of binding energy of Ca2p is more than 0.20eV only under the condition of mixed acid, while that of Ca2p is less than 0.05 eV under the condition of using sulfuric acid or phosphoric acid. This indicates that, under the action of mixed acid, strong chemical reactions occur and the new substances generated at the Ca site.
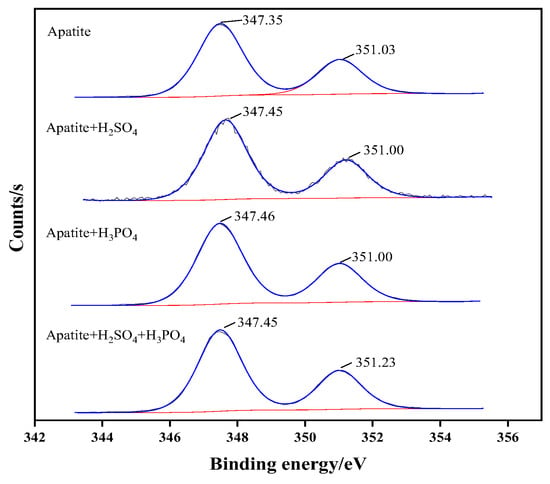
Figure 12.
High-resolution narrow map of Ca2p on apatite surface with different inhibitor.
A further analysis of the energy spectrum of P2p shows that apatite has a characteristic peak at 133.61 eV (Figure 13), representing Ca5(PO4)3F, and no other peaks are observed. The binding energy of Ca5(PO4)3F moves from 133.61 eV to 133.54 eV, 133.62 eV and 133.64 eV, respectively, after being treated with sulfuric acid, phosphoric acid and mixed acid, all with small changes. In addition, a new CaHPO4 is generated on the surface of apatite after the action of any one of the three depressants, and Ca(H2PO4)2 is also found under the action of phosphoric acid or mixed acid.
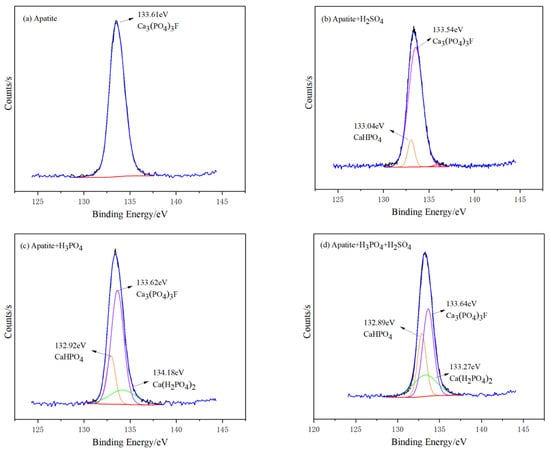
Figure 13.
X-ray photoelectron spectroscopy of high resolution for P2p: (a) Apatite; (b) Apatite + H2SO4; (c) Apatite + H3PO4; and (d) Apatite + H3PO4 + H2SO4.
A high-resolution spectrum analysis of P2p shows that under, sulfuric acid conditions, the photoelectron intensity and peak area of CaHPO4 are relatively small, at 635.28 Counts·S−1 and 598.23 CPS·eV, respectively; while, under phosphoric acid conditions, this value increases to 3932.38 Counts·S−1 and 4883.36 CPS·eV; and, when under the condition of mixed acid, the value rises to 6885.75 Counts·S−1 and 9034.77 CPS·eV (Figure 14). The values of photoelectron intensity and peak area represent the amount of product. Therefore, under the condition of mixed acid, the content of CaHPO4 or Ca (H2PO4)2 newly formed on the surface of apatite is the highest, followed by phosphoric acid and sulfuric acid.
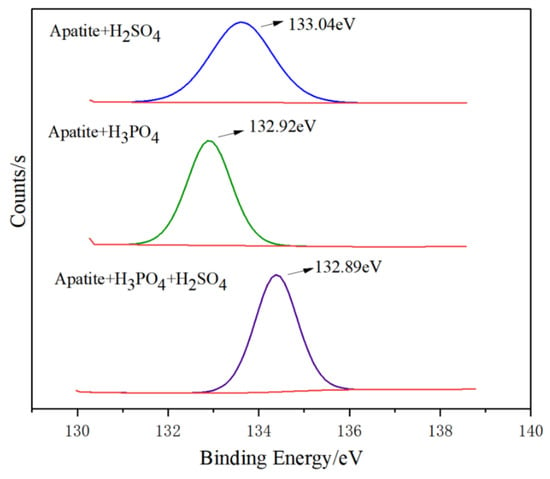
Figure 14.
X-ray photoelectron spectroscopy of calcium hydrogen phosphate of P2p under different conditions.
3.2.4. Adsorption of Sodium Oleate on Apatite after Depressant Treatment
In the flotation separation process of apatite and dolomite, the purpose of adding the collector is to make it adsorbed on the surface of dolomite, enhance its surface hydrophobicity and float it. However, the collector may also adsorb on the surface of apatite to enhance its hydrophobicity, so that apatite and dolomite will float together, resulting in a decrease in flotation recovery, which is undesirable in the flotation separation process. In this study, a certain amount of sulfuric acid, phosphoric acid or mixed acid are added, respectively, to the slurry containing apatite, and, after a period of action, a certain amount of sodium oleate is added and is stirred for 10 min. At last, the adsorption of sodium oleate on apatite was tested using TOC. The amount of sodium oleate adsorbed by apatite without acid depressant treatment is taken as a blank reference sample. Therefore, the amount of sodium oleate in the slurry before and after the addition of different depressants were measured, respectively, and the non-selective adsorption of sodium oleate on apatite is thus calculated.
As shown in Figure 15, in general, with the increase in the initial concentration of sodium oleate in the slurry (from 0.16 to 1.60 mmol/L), the adsorption capacity of sodium oleate on the apatite surface also increases. This is because the fatty acid could form stable chemisorption on the apatite surface by the O of fatty acids bonding with the Ca1 site [27]. However, no matter how much the initial addition amount of sodium oleate changes (0.16–1.6 mmol/L), when the mixed acid is used as a depressant, the adsorption amount of sodium oleate on apatite surface is the lowest; this is because the hydrophilic substances CaSO4, CaHPO4/Ca(H2PO4)2 formed in the presence of the mixed acid and adsorbed on the surface of apatite hinder the adsorption of sodium oleate [28]. Under the conditions of phosphoric acid, the adsorption capacity of sodium oleate is slightly smaller than that of sulfuric acid, but both of which are significantly higher than that of the mixed acid. Moreover, as the initial addition amount of sodium oleate increases, this rule becomes more obvious.
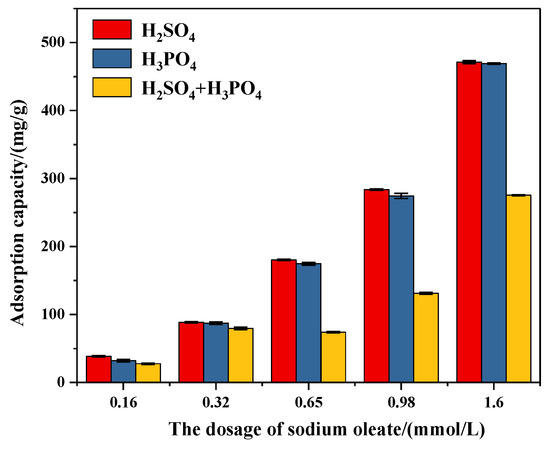
Figure 15.
Adsorption of sodium oleate on apatite after being treated by different acidic depressants.
For example, under the condition of an initial addition of 1.6 mmol/L sodium oleate, if the mixed acid is used (H2SO4 = 0.8 mmol/L, H3PO4 = 3.27 mmol/L) as a depressant, the adsorption capacity of sodium oleate on the apatite is 237.25 mg/L, while if phosphoric acid (3.27 mmol/L) or sulfuric acid (0.8 mmol/L) is used as the depressant, the adsorption capacity of sodium oleate on apatite is 425.02 mg/L and 440.11 mg/L, respectively, which are significantly higher than that of the mixed acid. The above test results show that, first, there is non-selective adsorption of sodium oleate on the surface of apatite pretreated by acid depressants; and second, compared with the use of phosphoric acid or sulfuric acid, the mixed acid can significantly weaken the adsorption of the collector on the surface of apatite, thereby reducing the flotation of apatite and optimizing the flotation index.
On the basis of the above tests, the adsorption kinetics characteristics of the collector on the apatite treated with depressants were further investigated. The consumption of sodium oleate is maintained at 0.98 mmol/L. As shown in Figure 16, after mixed acid treatment, the adsorption kinetics of the collector on apatite conform to the pseudo first order kinetics model [29], while after sulfuric acid or phosphoric acid treatment, the adsorption kinetics of the collector on apatite present different characteristics, with a high degree of fitting with the second order kinetics model. In addition, compared with sulfuric acid or phosphoric acid, the equilibrium adsorption capacity of the collector on apatite is smaller (consistent with that shown in Figure 15) and the adsorption rate is also lower when the mixed acid is used as the depressant. This shows that, as a depressant, the mixed acid can effectively weaken, although not completely eliminate, the adsorption of collector on the surface of apatite, and its performance is better than that of phosphoric acid or sulfuric acid.
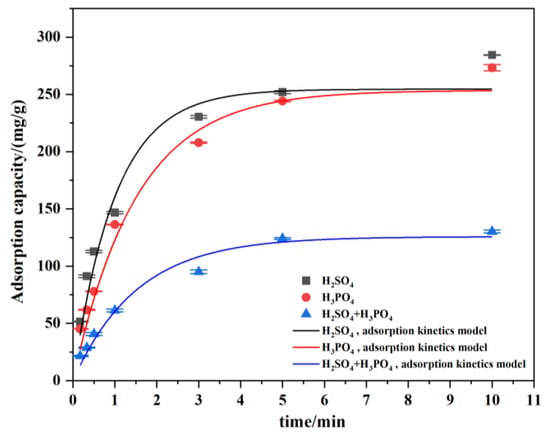
Figure 16.
Kinetic model fitting for sodium oleate adsorption under the condition of different depressants.
3.2.5. Effect of Mixed Acid on Bubble–Particle Attachment
The collision and adhesion between particles and bubbles are key influencing factors in the flotation process. In the reverse flotation system of phosphate ore, the main purpose of the depressant is to reduce the probability of collision and adhesion between apatite and bubbles. The collector adsorbed on the surface of apatite will also increase the adsorption probability of apatite and bubbles to some extent, which is also an unfavorable factor for flotation separation.
A bubble–particle wrap angle testing system was used in this study to evaluate the effect of various depressants on the aggregation of bubbles and apatite particles. It can be seen from Figure 17 and Figure 18 that, with the increase in reaction time (from 10 s to 180 s), the adsorption amount of apatite on the bubble surface increases gradually after the treatment of the three depressants, and the corresponding wrapping angles also increase. However, after the treatment of the mixed acid, the adsorption amount of apatite on the bubble surface is relatively small, while that for phosphoric acid and sulfuric acid is relatively large. This shows that the mixed acid can better depress apatite, weaken the collision and adhesion between apatite and bubbles and avoid the loss of apatite.
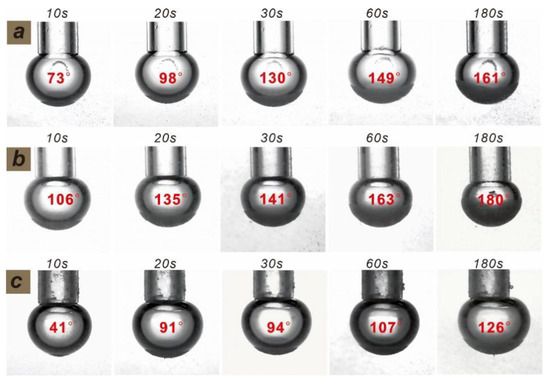
Figure 17.
Variation of adsorption characteristics (wrapping angle) of apatite and bubbles with time under the action of different depressants: (a) sulfur acid; (b) phosphoric acid; and (c) sulfur acid + phosphoric acid.
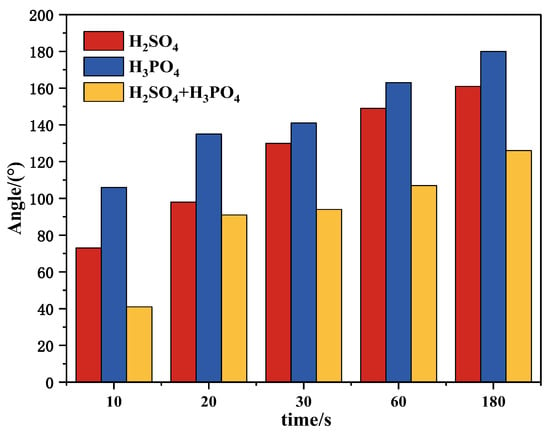
Figure 18.
Wrapping angles of apatite after being treated with different depressants.
After being treated with a depressant (acids) and collector (sodium oleate) in turn, apatite particles’ adsorption characteristics on the bubble surface are shown in Figure 19 and Figure 20. Compared with that shown in Figure 17 and Figure 18, more apatite was adsorbed on the bubble surface after the action of the depressant and collectors. This shows that apatite can still adsorb the collector (sodium oleate) to a certain extent after acting with depressants, thus enhancing its surface hydrophobicity and its collision and adhesion capacity with bubbles. In addition, by comparing the effect of different depressants, it can be seen that, after the action of “sulfuric acid + phosphoric acid + sodium oleate”, the amount of apatite adsorbed on the bubble surface is less than that after the action of “phosphoric acid + sodium oleate” or “sulfuric acid + sodium oleate”. This also shows that the effect of the mixed acid can more effectively weaken the adsorption of collector on the surface of apatite, thus strengthening the depression effect.

Figure 19.
Variation of adsorption characteristics (wrapping angle) of apatite and bubbles under the action of different depressants and sodium oleate: (a) sulfur acid and sodium oleate; (b) phosphoric acid and sodium oleate; and (c) sulfur acid and phosphoric acid and sodium oleate.
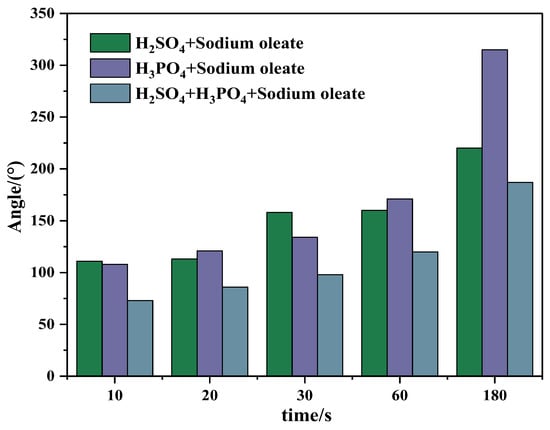
Figure 20.
Wrapping angles of apatite after being treated with different depressants and sodium oleate.
4. Conclusions
- Reverse flotation tests of artificial mixed ore of apatite and dolomite show that the depression effect of the mixture of sulfur acid and phosphorus acid is better than that of sulfuric acid or phosphoric acid. This is because the contact angle of apatite is the smallest under the action of mixed acid, resulting in the depression of apatite. When the amounts of sulfuric acid and phosphoric acid in the mixed acid are 3.27 mmol/L and 0.8 mmol/L, a phosphorus concentrate with a P2O5 grade of 32.53% and a recovery of 88.92% can be obtained.
- Under different depressant conditions, the zeta potential of apatite is all negative, but the absolute value of zeta potential of apatite is the largest under the action of mixed acid. The enhancement of surface hydrophilicity is not conducive to the adsorption of apatite particles and bubbles, while the increase in the absolute value of zeta potential is not conducive to the adsorption of anionic collectors on the mineral surface, both resulting in the effective depression of the flotation of apatite.
- After adding sulfuric acid, phosphoric acid or mixed acid, the apatite surface generates reactants like CaHPO4. Under the action of sulfuric acid or mixed acid, a small amount of CaSO4 is also detected on the mineral surface. Compared with the addition of sulfuric acid or phosphoric acid, after the addition of mixed acid, the surface reaction of apatite is stronger, and the content of CaHPO4 and Ca(H2PO4)2 with stronger hydrophilicity is the highest. These new products may be an important factor to enhance the hydrophilicity of the apatite surface.
- After adding different acid depressants, the hydrophilic substances CaSO4, CaHPO4/Ca(H2PO4)2 adsorbed on the surface of apatite, hindering the adsorption of sodium oleate and resulting in the adsorption capacity of sodium oleate on apatite surface decreasing significantly. Under the action of mixed acid, the adsorption amount of sodium oleate on the surface of apatite is the lowest and the adsorption rate is relatively low, which indicates that mixed acid greatly weakens the non-selective adsorption of the collector and strengthens the depression of apatite.
Author Contributions
W.C. conceived and designed the experiments; X.L. and X.P. performed the experiments; X.L. and W.H. analyzed the data; W.C. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R & D project (2018YFE0110300).
Data Availability Statement
The raw data required to reproduce the above findings cannot be shared at this time as the data also form part of an ongoing study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santana, R.C.; Duarte, C.R.; Ataíde, C.H.; Barrozo, M.A.S. Flotation selectivity of phosphate ore: Effects of particle size and reagent concentration. Sep. Sci. Technol. 2011, 46, 1511–1518. [Google Scholar] [CrossRef]
- Wang, L.; Tian, M.; Khoso, S.A.; Hu, Y.; Sun, W.; Gao, Z. Improved flotation separation of apatite from calcite with benzohydroxamic acid collector. Miner. Process. Extr. Metall. Rev. 2019, 40, 427–436. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries; National Minerals Information Center: Reston, VA, USA, 2022.
- Wu, F.; Wang, J.; Liu, J.; Zeng, G.; Xiang, P.; Hu, P.; Xiang, W. Distribution, geology and development status of phosphate resources. Geol. China 2021, 48, 82–101, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y.; He, J.; Liu, C. Thermodynamics versus kinetics in nanosynthesis. Angew. Chem. Int. Ed. 2015, 54, 2022–2051. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nguyen, A.V.; Miller, J.D. Selective attachment and spreading of hydroxamic acid–alcohol collector mixtures in phosphate flotation. Int. J. Miner. Process. 2006, 78, 122–130. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Sun, H.; Yin, W.; Sun, Q.; Sun, H.; Han, H.; Sheng, Q.; Yao, J. Selective adsorption of an eco-friendly and efficient depressant PBTCA onto dolomite for effective flotation of fluorapatite from dolomite. Chem. Eng. J. 2020, 400, 125780. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Sun, H.; Yin, W.; Hong, J.; Cao, S.; Tang, Y.; Zhao, C.; Yao, Y. Improving flotation separation of apatite from dolomite using PAMS as a novel eco-friendly depressant. Miner. Eng. 2020, 156, 106492. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; Hakkou, R.; Benzaazoua, M. Review of the Main Factors Affecting the Flotation of Phosphate Ores. Minerals 2020, 10, 1109. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Y.; Guo, X.; Guo, W. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Luo, H.; Cheng, R.; Liu, F. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Chander, S.; Fuerstenau, D.W. Interfacial properties and equilibria in the apatite-aqueous solution system. J. Colloid Interface Sci. 1979, 70, 506–516. [Google Scholar] [CrossRef]
- Horta, D.; Mello Monte, M.B.; Salles Leal Filho, L. The effect of dissolution kinetics on flotation response of apatite with sodium oleate. Int. J. Miner. Process. 2016, 146, 97–104. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Adsorption and contact angle of single and binary mixtures of surfactants on apatite. Miner. Eng. 2003, 16, 839–848. [Google Scholar] [CrossRef]
- Ruan, Y.; He, D.; Chi, R. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef]
- Dong, L.; Wei, Q.; Qin, W.; Jiao, F. Selective adsorption of sodium polyacrylate on calcite surface: Implications for flotation separation of apatite from calcite. Sep. Purif. Technol. 2020, 241, 116415. [Google Scholar] [CrossRef]
- Abouzeid, A.-Z.M.; Negm, A.T.; Elgillani, D.A. Upgrading of calcareous phosphate ores by flotation: Effect of ore characteristics. Int. J. Miner. Process. 2009, 90, 81–89. [Google Scholar] [CrossRef]
- Elgillani, D.A.; Abouzeid, A.-Z.M. Flotation of carbonates from phosphate ores in acidic media. Int. J. Miner. Process. 1993, 38, 235–256. [Google Scholar] [CrossRef]
- Zou, H.; Cao, Q.; Liu, D.; Yu, X.; Lai, H. Surface features of fluorapatite and dolomite in the reverse flotation process using sulfuric acid as a depressor. Minerals 2019, 9, 33. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Hart, B.; Ye, J. Study on the effect of collector and inhibitor acid on the floatability of collophane and dolomite in acidic media by TOF-SIMS and XPS. Surf. Interface Anal. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, Y.; Li, C.; Cheng, R. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. Int. J. Miner. Process. 2017, 167, 95–102. [Google Scholar] [CrossRef]
- Ye, J. Study on Interfacial Regulation of Fine Apatite Flotation. Ph.D. Thesis, Guizhou University, Guiyang, China, 2019. [Google Scholar]
- Zhang, H.; Zhou, F.; Liu, M.; Jin, Y.; Xiao, L.; Yu, H. Employing sulfur–phosphorus mixed acid as a depressant: A novel investigation in flotation of collophanite. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Liu, R.; Liu, C.; Liu, L.; Liu, R.; Zhang, H.; Pang, J.; Liu, D. Application of waste acid from phosphogysum dam as an eco-friendly depressant in collophane flotation. J. Clean. Prod. 2020, 267, 122184. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q. Interaction behavior between coarse and fine particles in the reverse flotation of fluorapatite and dolomite. Langmuir 2023, 39, 12931–12943. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Li, X.; Zhang, Q. DFT study of coadsorption of fatty acid and kerosene on fluorapatite (001) surface. Physicochem. Probl. Miner. Process. 2023, 59, 161890. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Q.; Li, X. Interaction of Ca2+, Mg2+ with dolomite (104) surface and its effect on caproic acid adsorption: DFT calculation. Appl. Surf. Sci. 2023, 614, 156244. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q. Effect of Molecular Structure of Organic Acids on the Crystal Habit of α-CaSO4·0.5H2O from Phosphogypsum. Crystals 2020, 10, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).