Preparation of Bimetallic Au-Pd/MWCNTs Electrode for Detection of Dopamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
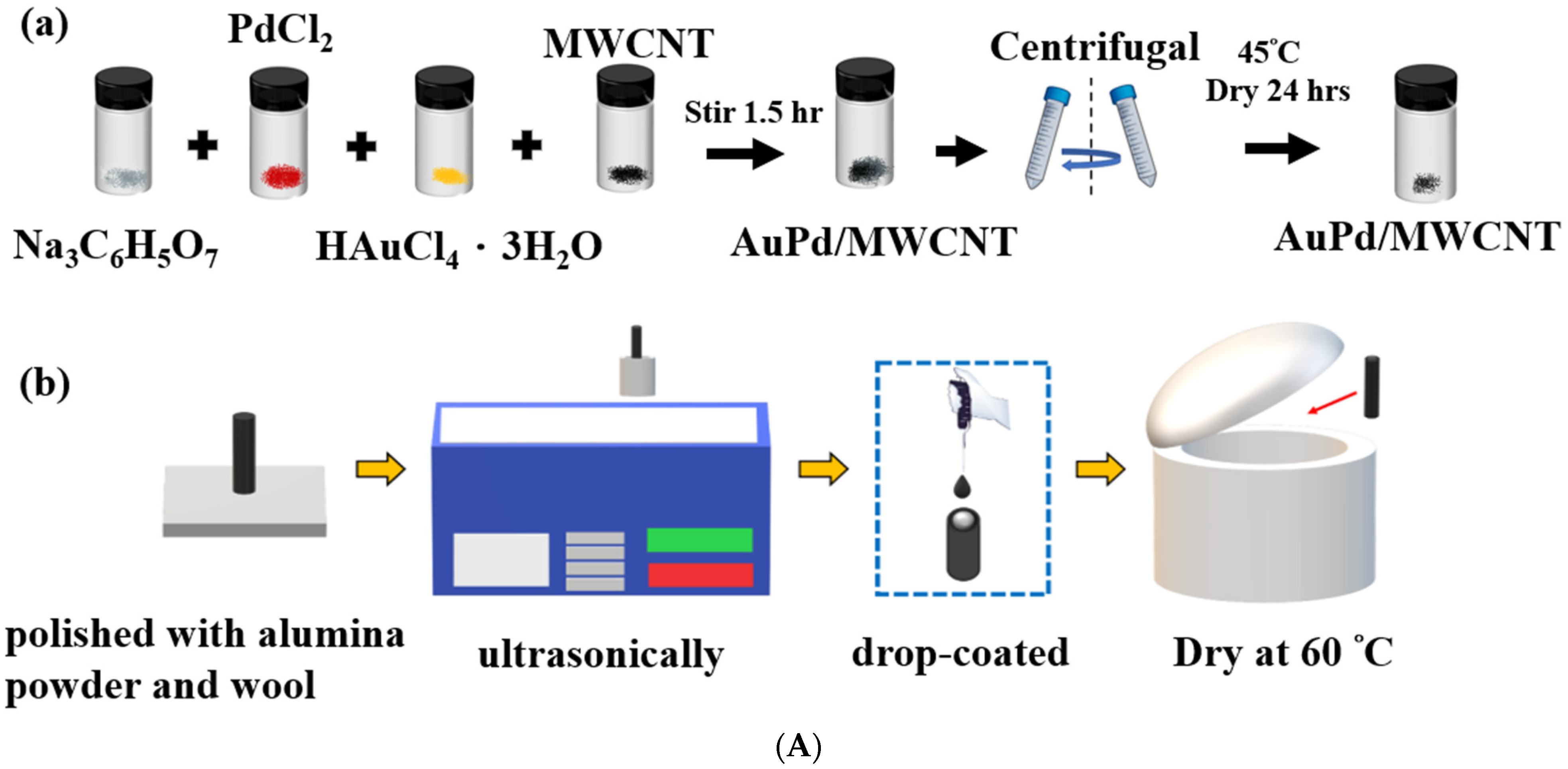
2.2. Electrochemical Sensors Preparation
2.3. Characterization Apparatus
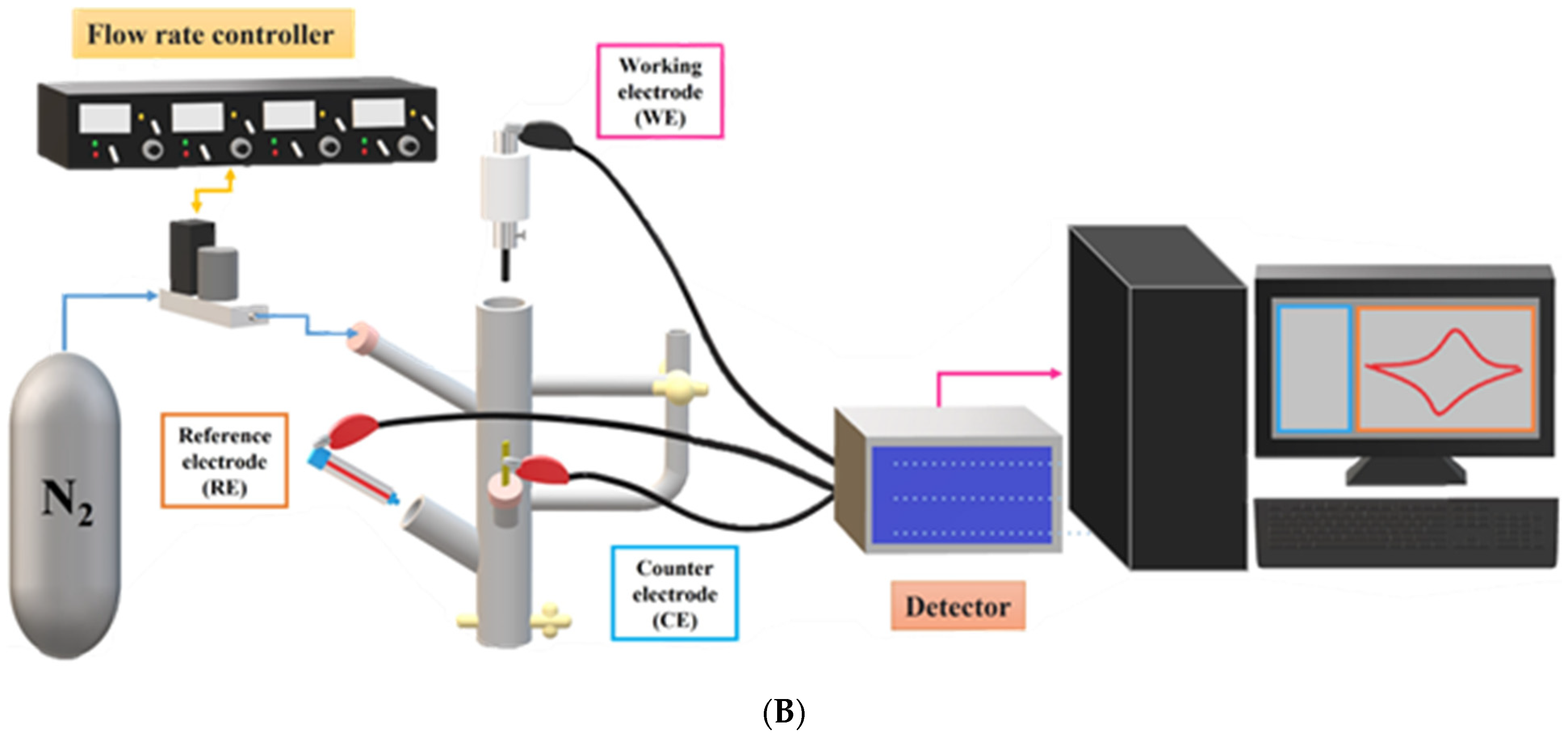
2.4. Electrode Preparation and Electrochemical Measurements
3. Results
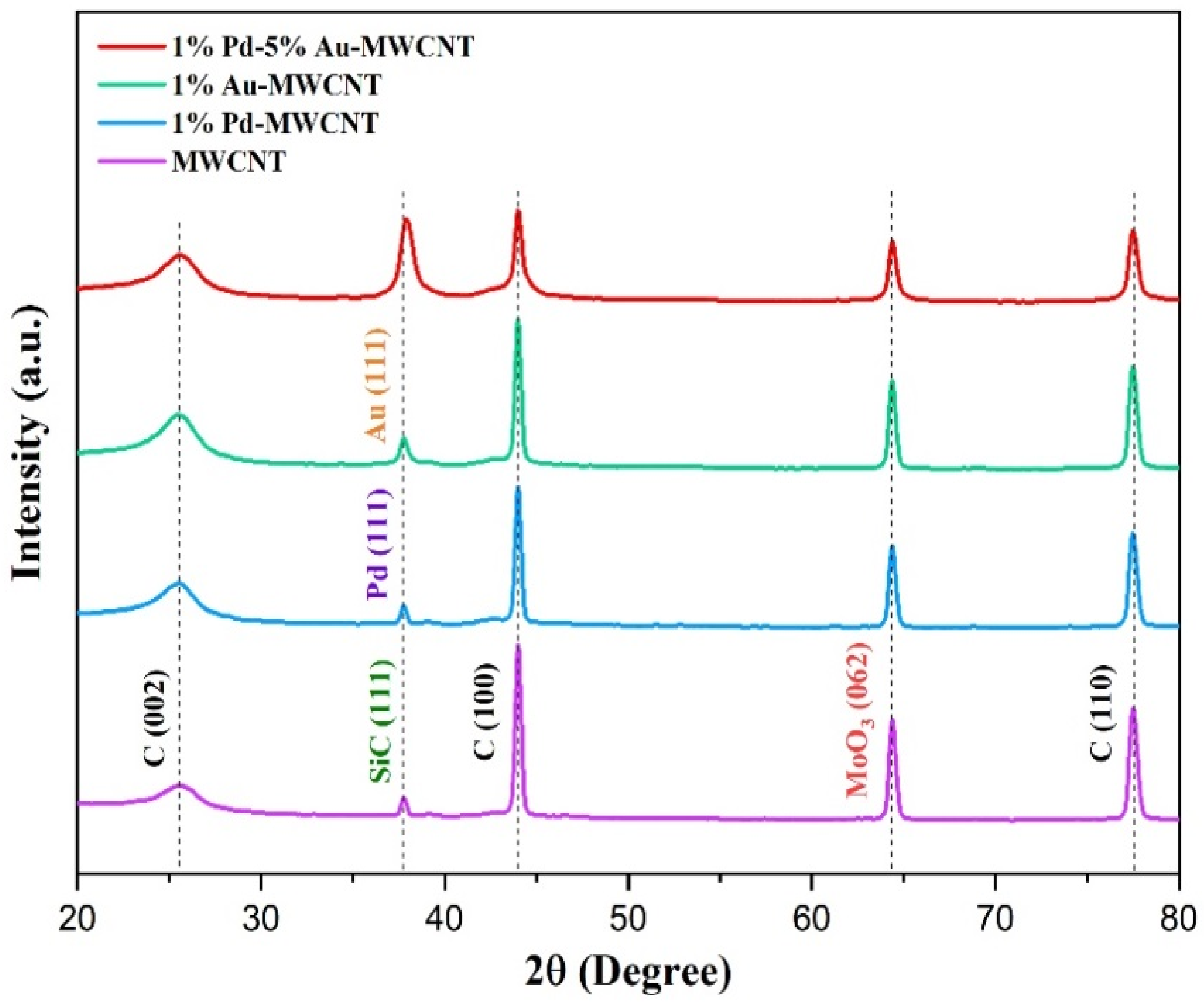
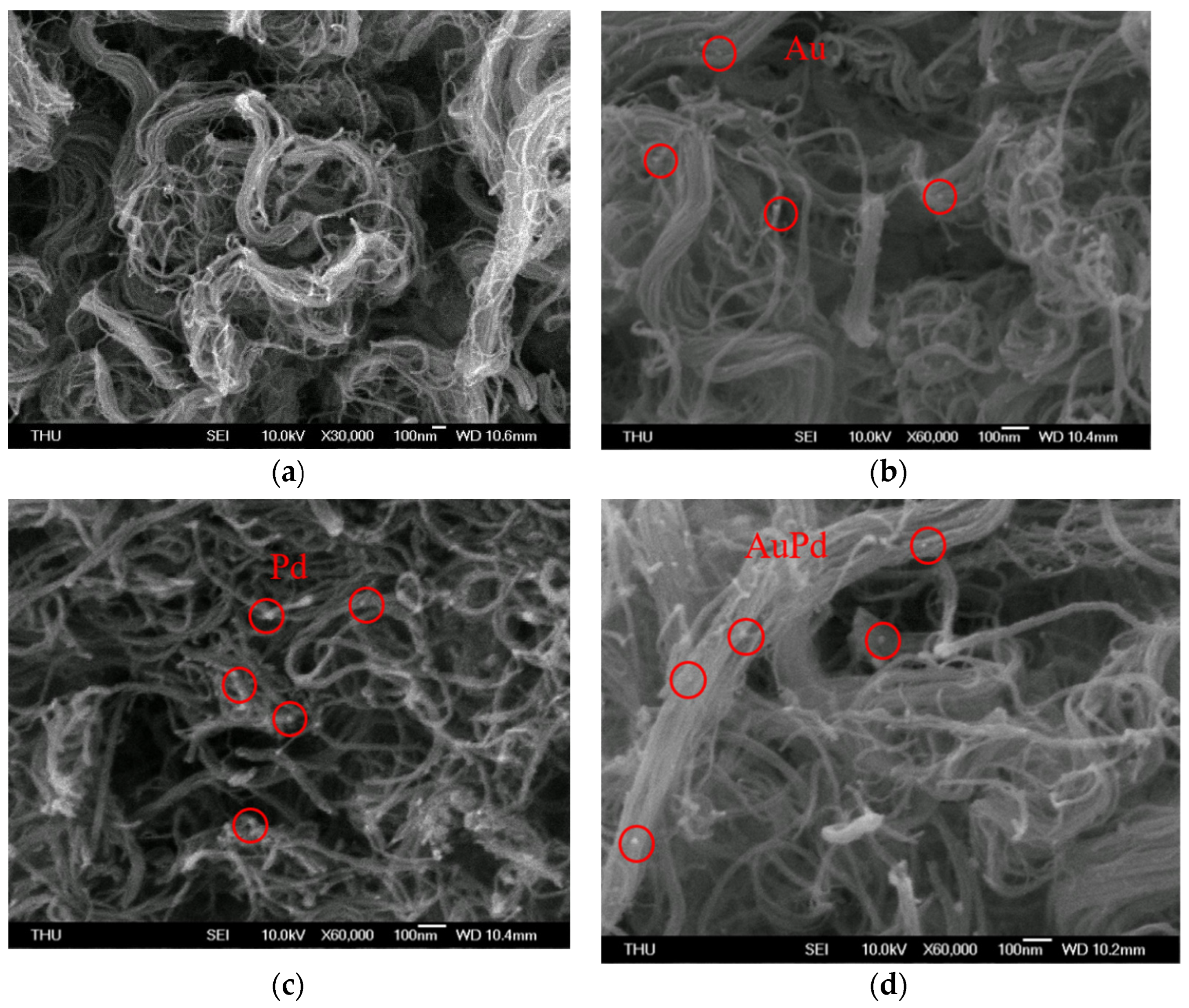
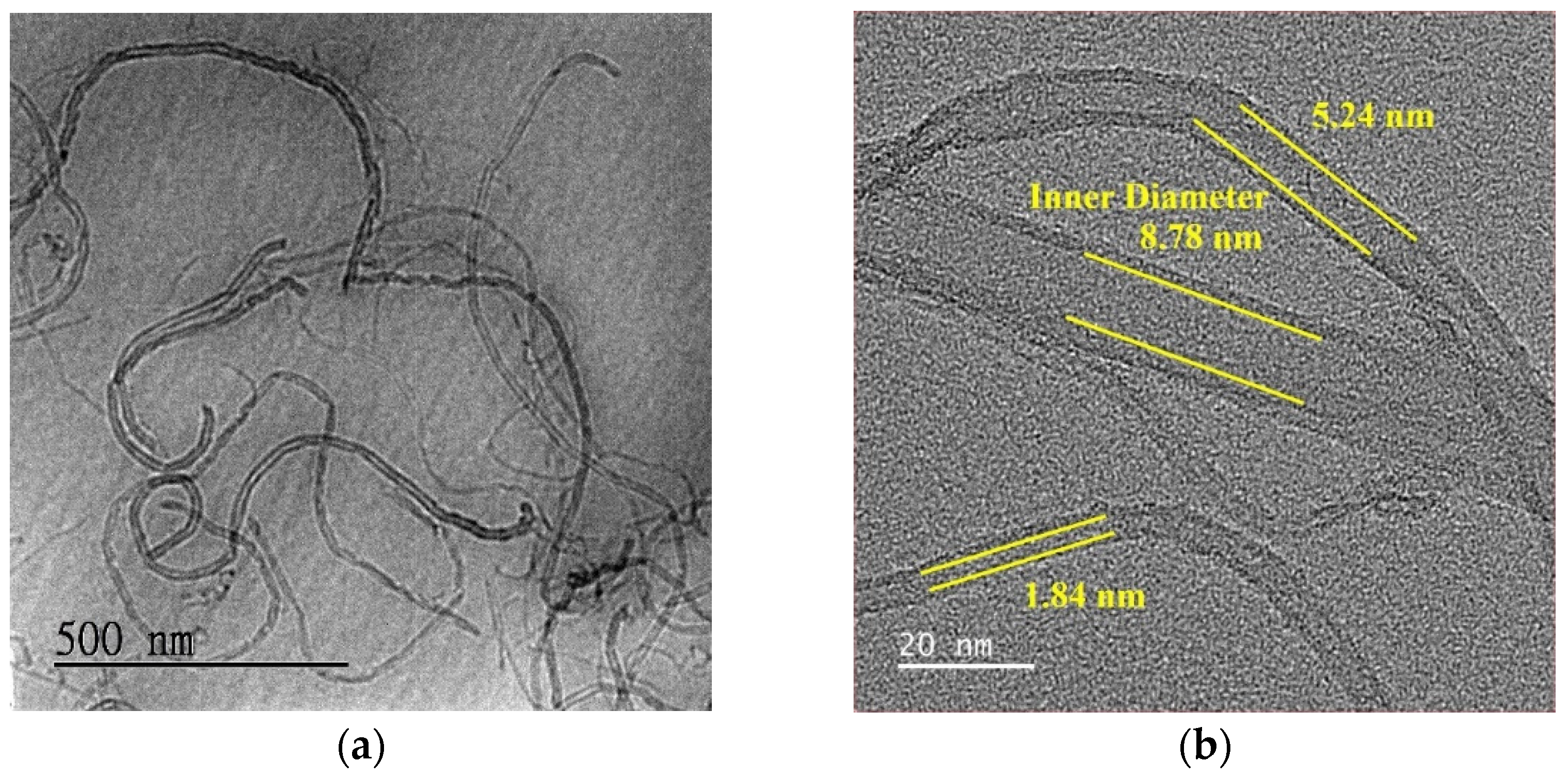
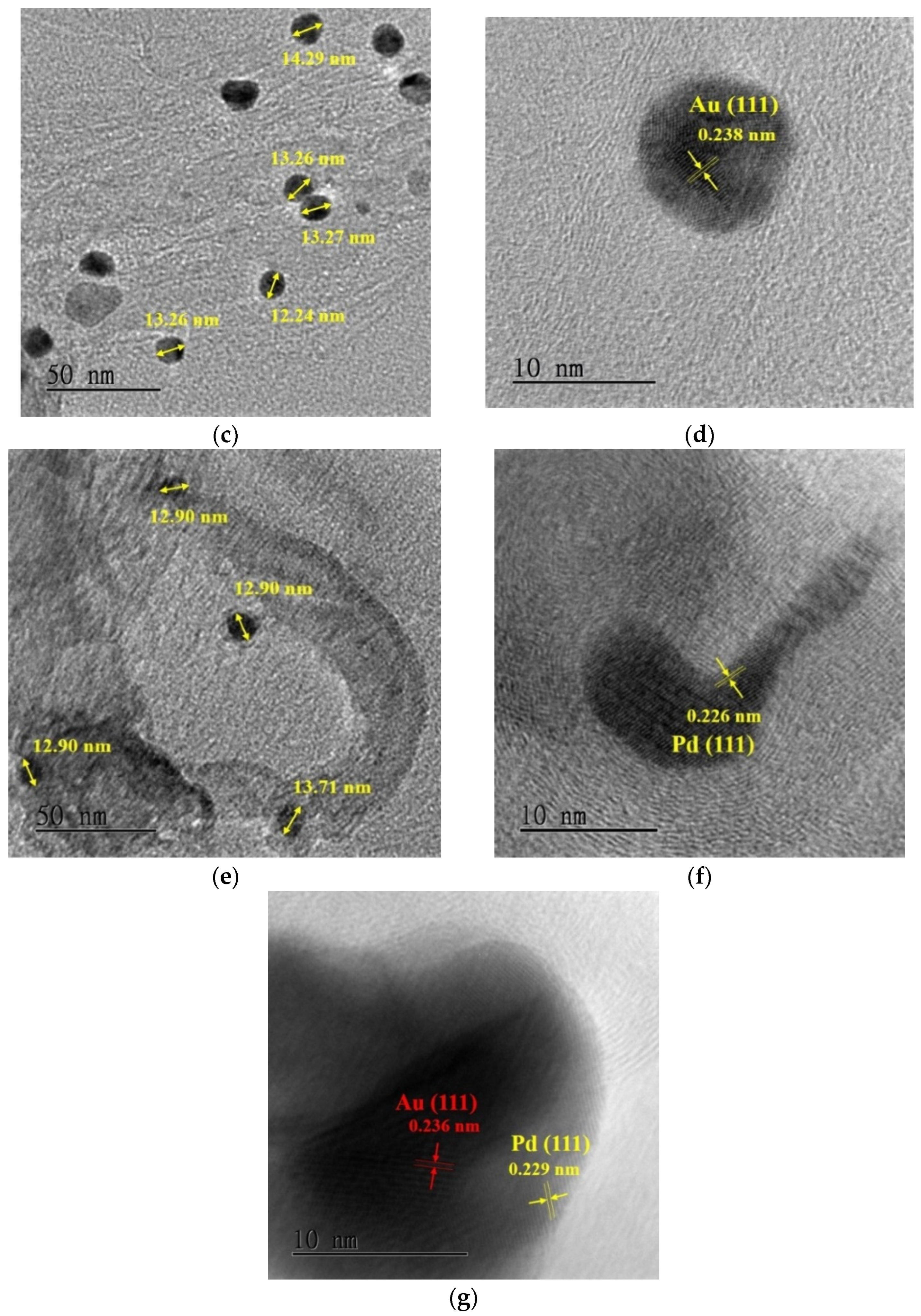
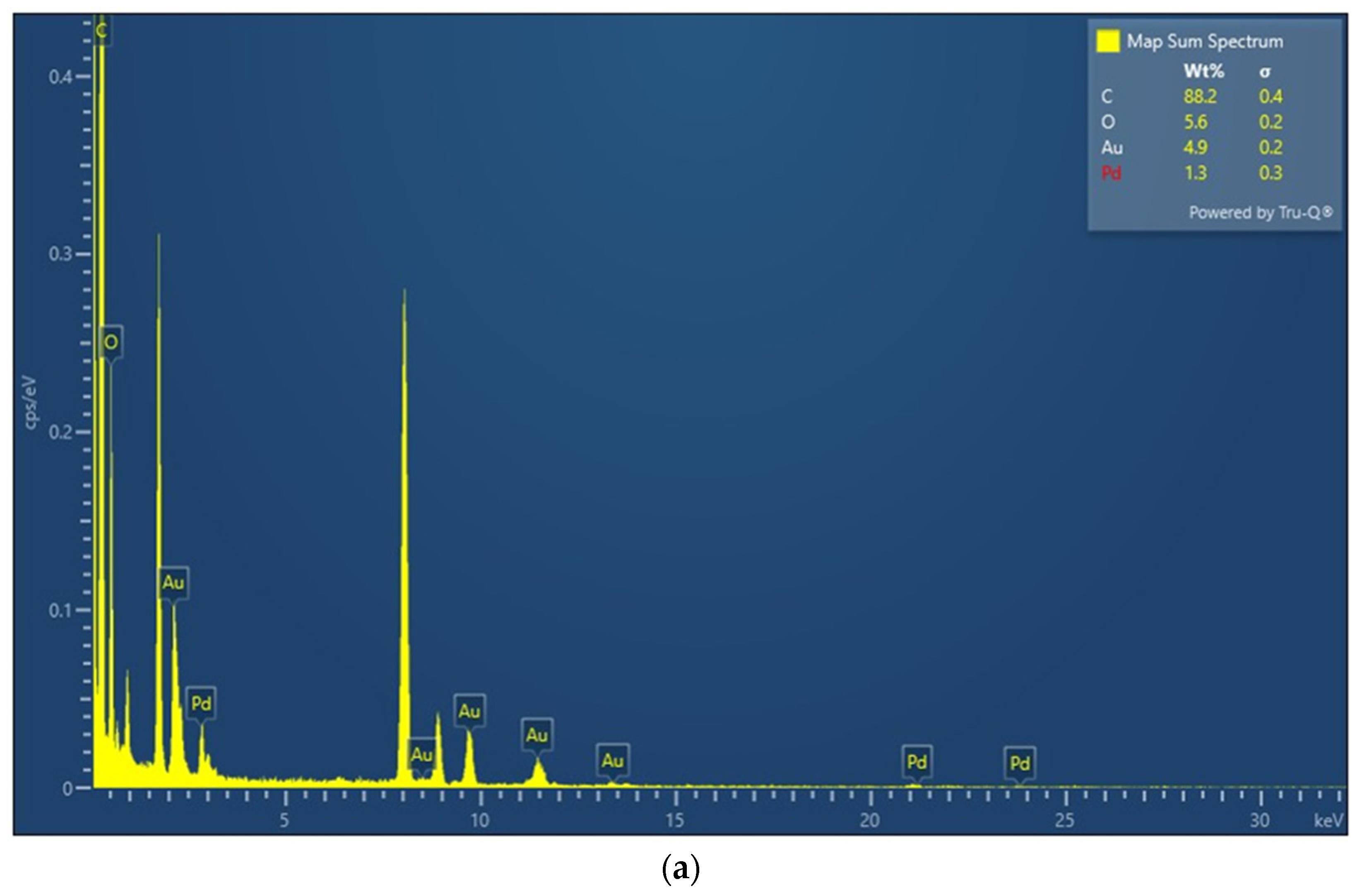
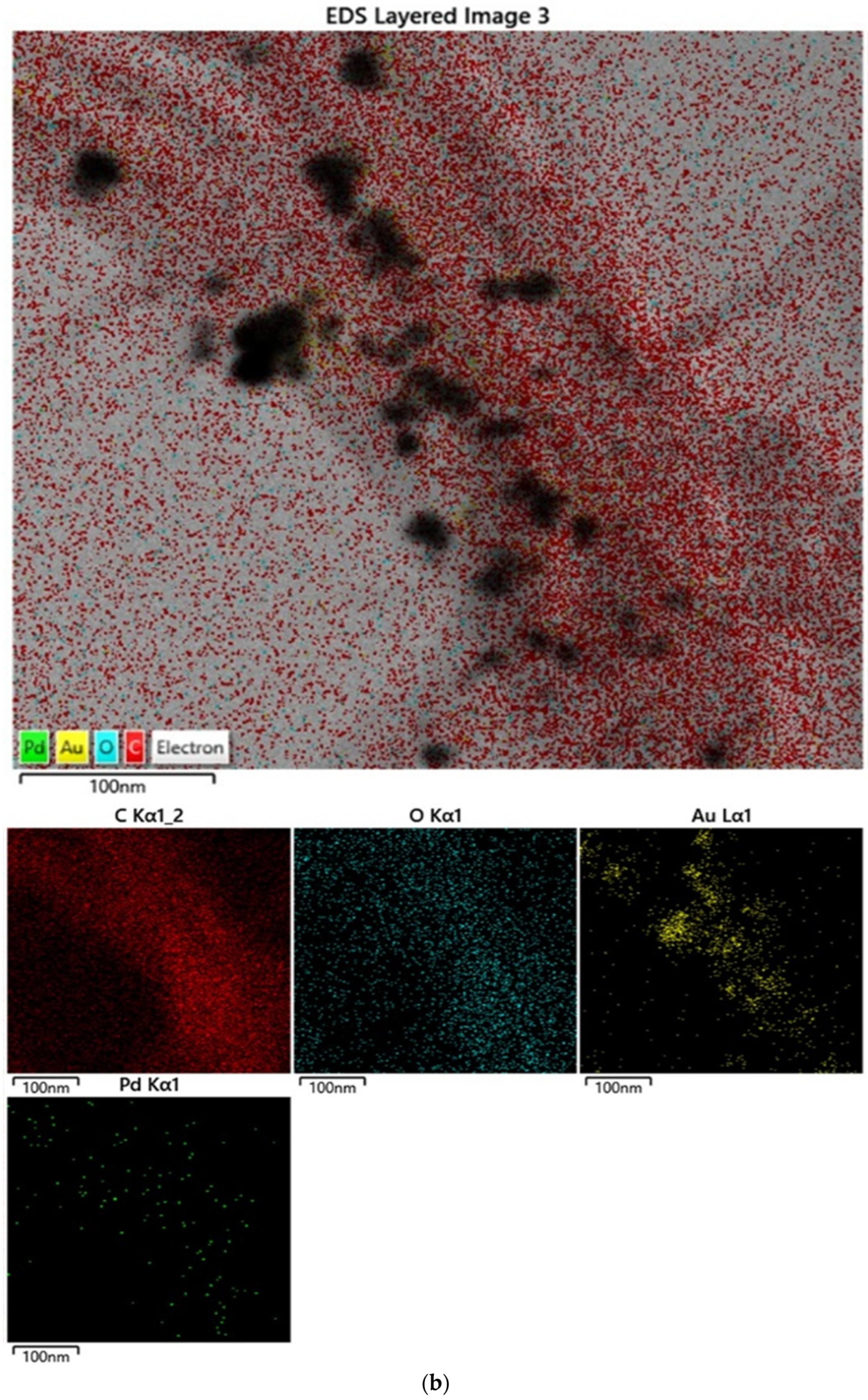
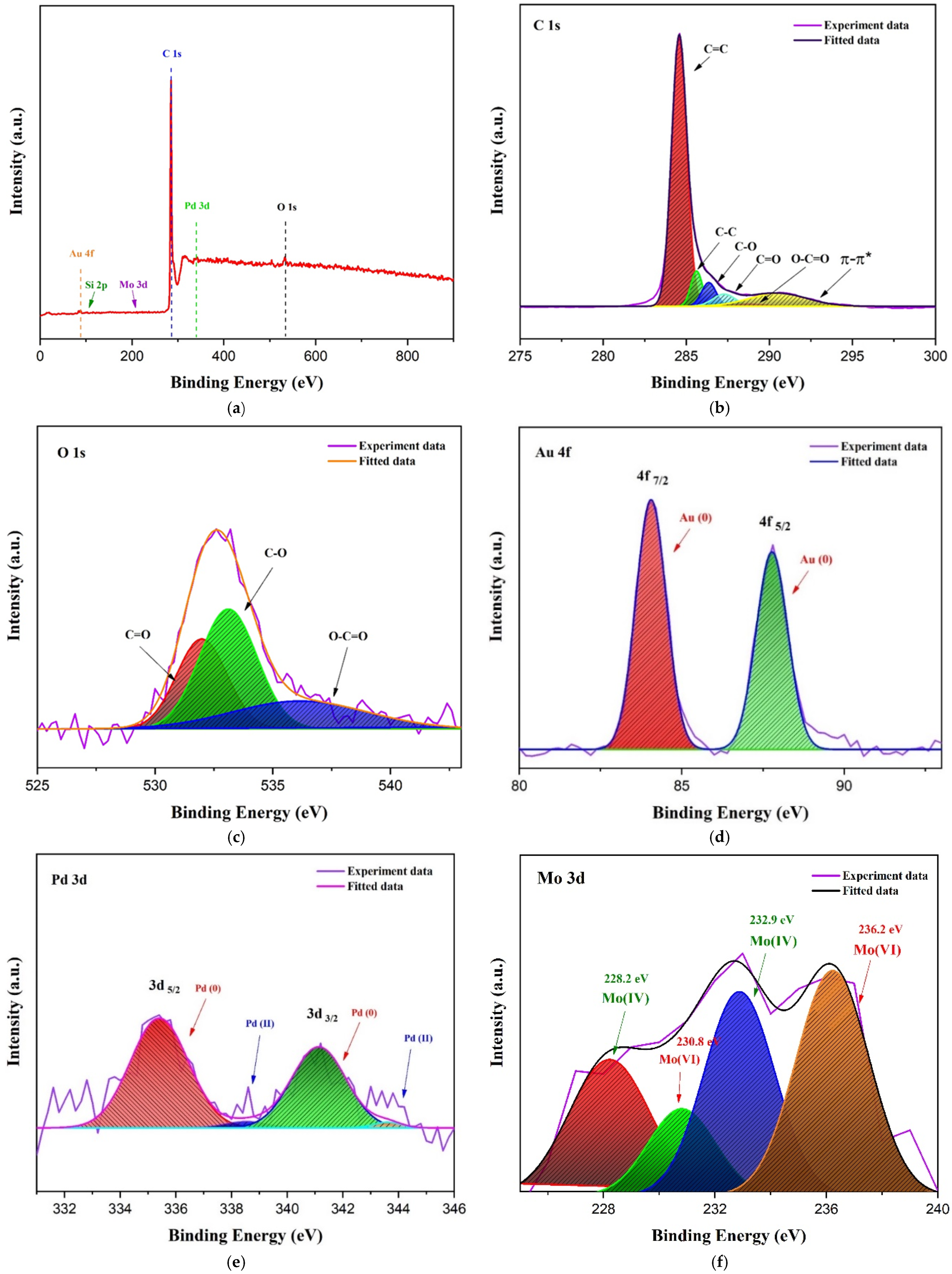
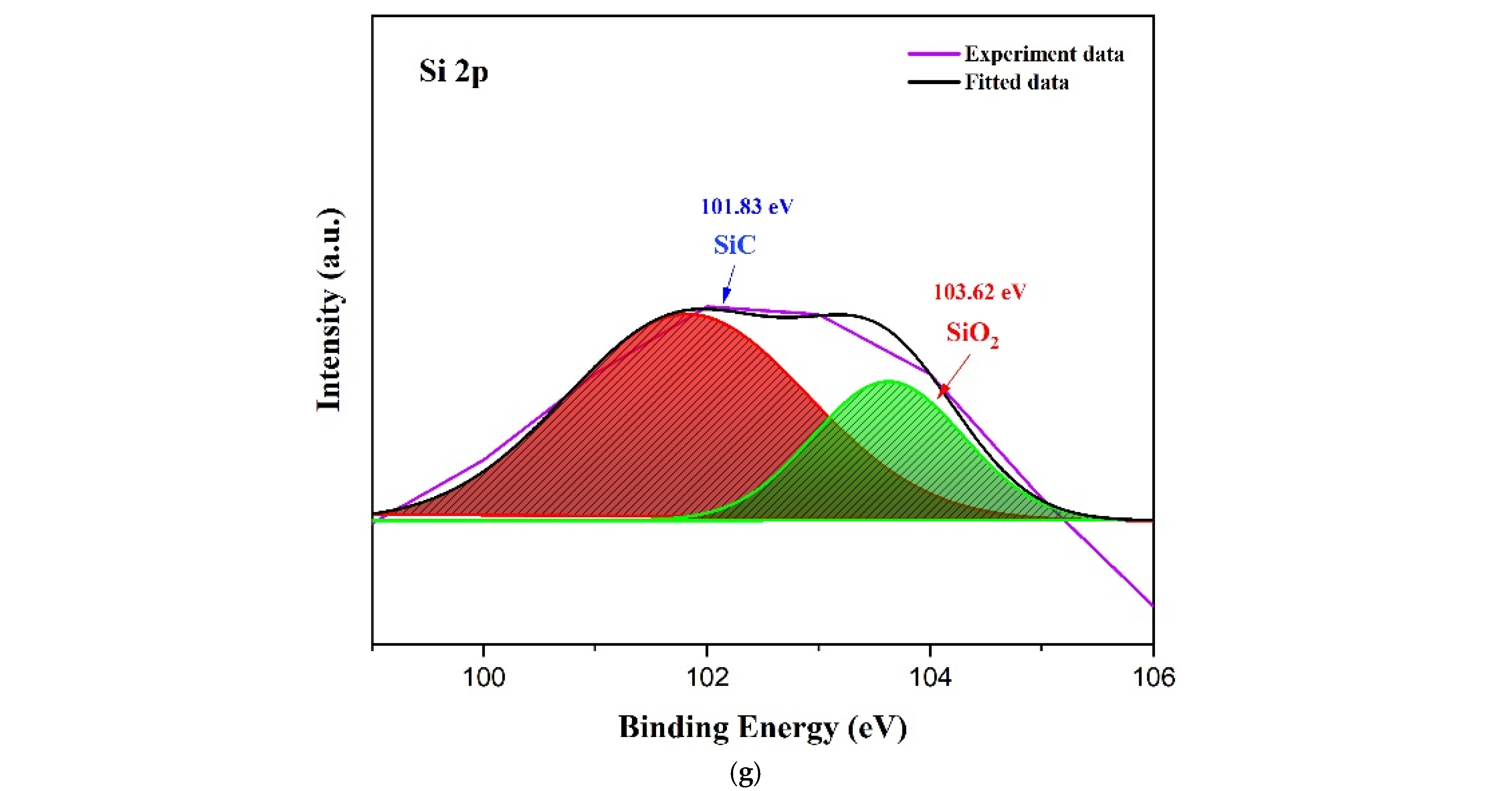
3.1. Sensing Material Characterization by XRD, SEM, TEM and XPS
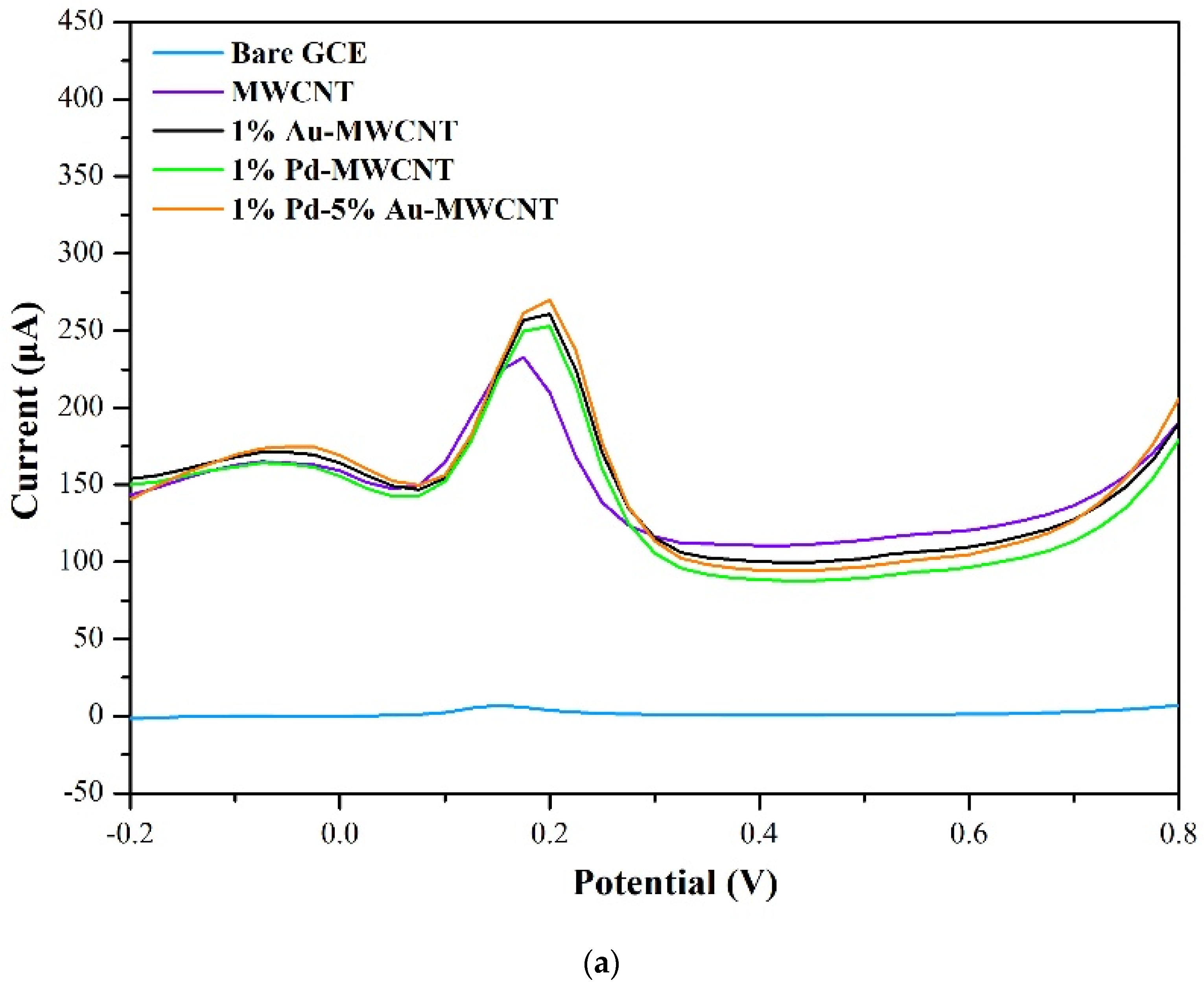
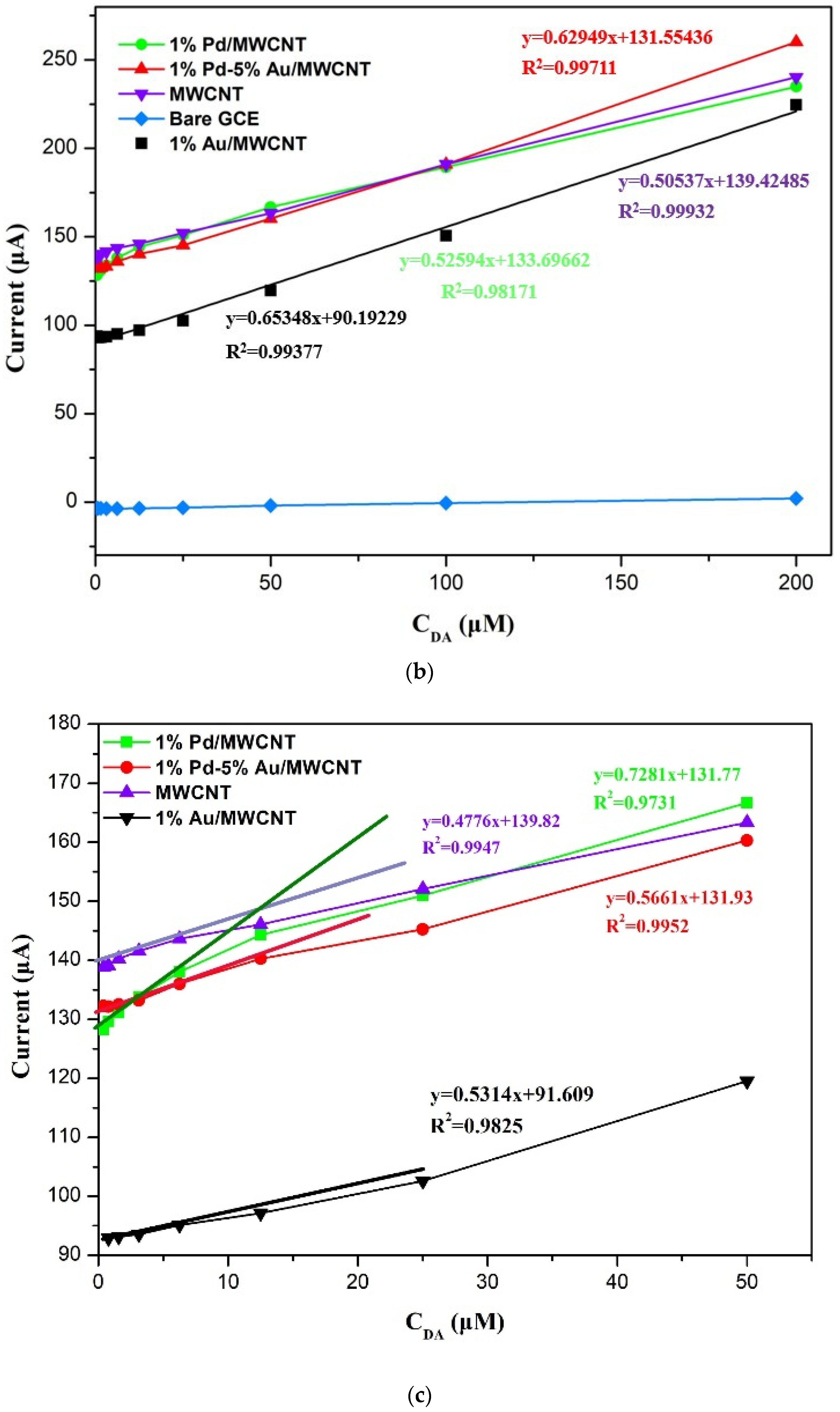
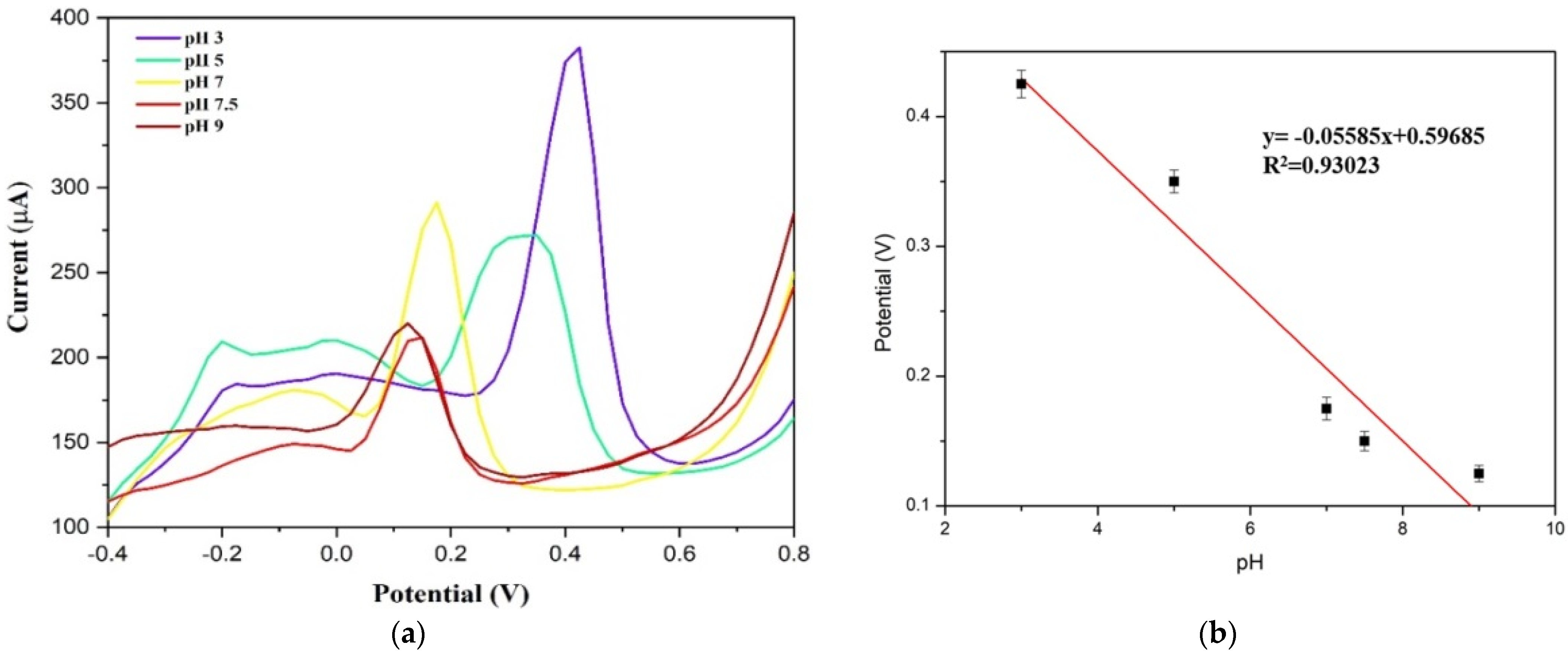
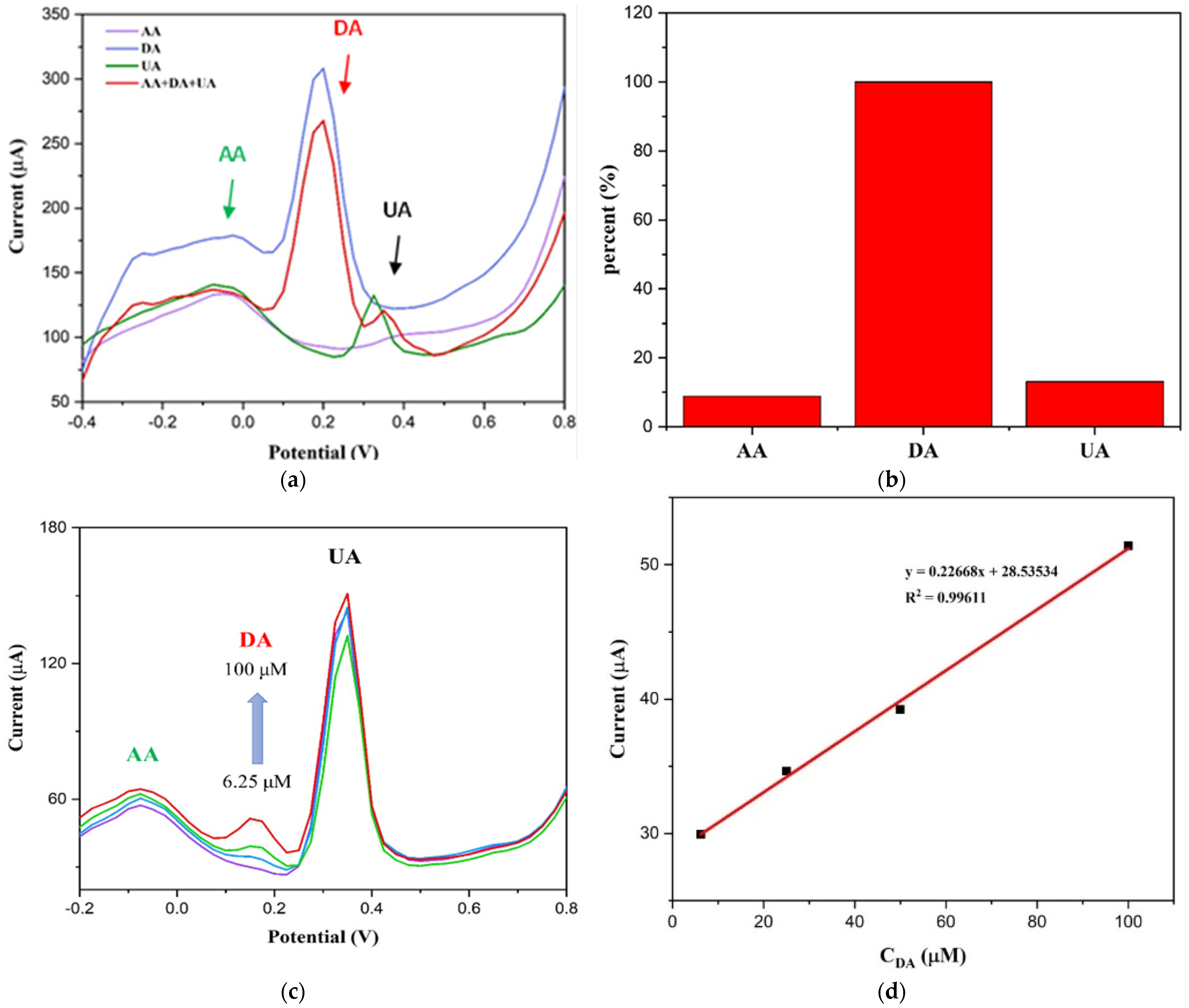
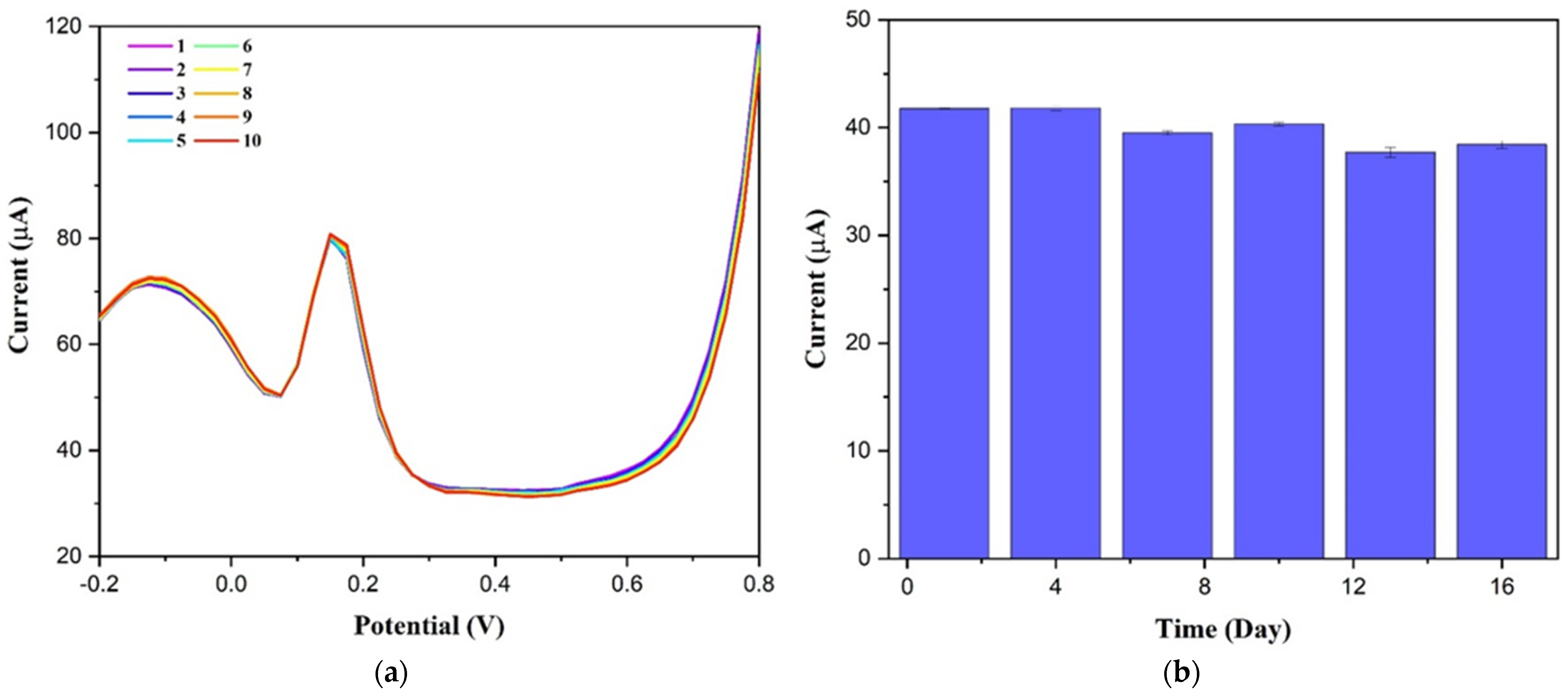
3.2. Sensing Properties
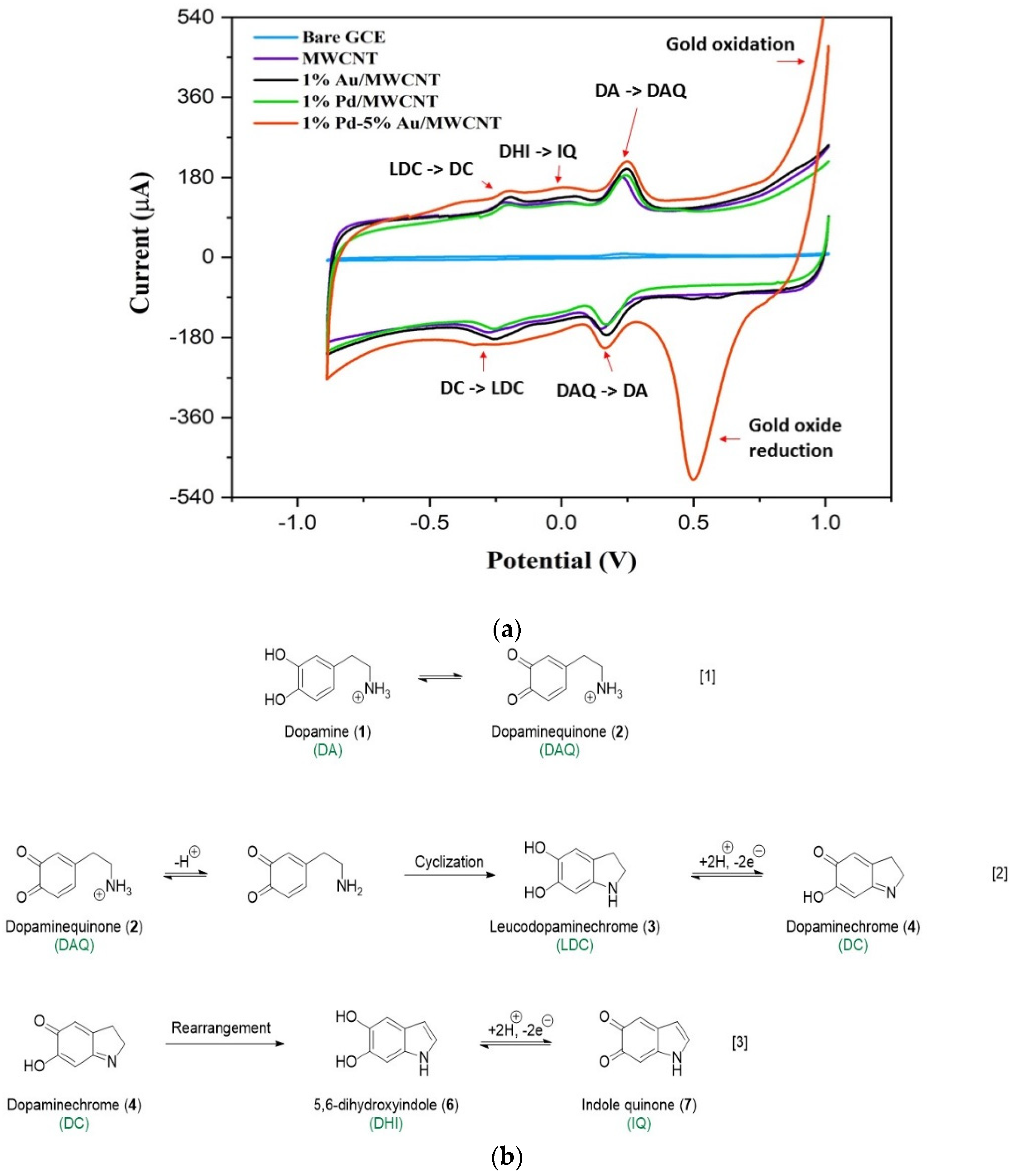
3.3. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coddington, L.T.; Dudman, J.T. Learning from Action: Reconsidering Movement Signaling in Midbrain Dopamine Neuron Activity. Neuron 2019, 104, 63–77. [Google Scholar] [CrossRef]
- Schultz, W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013, 23, 229–238. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Razia, D.M.; Ashiq, M.; Ghaffar, A.; Akram, M.; Allam, A.E.; Bouyahya, A.; Garipova, L.; Shariati, M.A.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta. 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Scott, J.G. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl. Psychiat. 2018, 8, 30. [Google Scholar] [CrossRef]
- de Benedetto, G.E.; Fico, D.; Pennetta, A.; Malitesta, C.; Nicolardi, G.; Lofrumento, D.D.; Nuccio, F.D.; Pesa, V.L. A rapid and simple method for the determination of 3,4-dihydroxyphenylacetic acid, norepinephrine, dopamine, and serotonin in mouse brain homogenate by HPLC with fluorimetric detection. J. Pharm. Biomed. Anal. 2014, 98, 266–270. [Google Scholar] [CrossRef]
- Gao, W.-Y.; Qi, L.-M.; Liu, Z.-Y.; Majeed, S.; Kitte, S.A.; Xu, G.-B. Efficient lucigenin/thiourea dioxide chemiluminescence system and its application for selective and sensitive dopamine detection. Sens. Actuators B Chem. 2017, 238, 468–472. [Google Scholar] [CrossRef]
- Zhang, L.; Qv, S.; Wang, Z.; Cheng, J. Determination of dopamine in single rat pheochromocytoma cell by capillary electrophoresis with amperometric detection. J. Chromatogr. B 2003, 792, 381–385. [Google Scholar] [CrossRef]
- Mahshid, S.; Li, C.; Mahshid, S.S.; Askari, M.; Dolati, A.; Yang, L.; Luo, S.; Cai, Q. Sensitive determination of dopamine in the presence of uric acid and ascorbic acid using TiO2 nanotubes modified with Pd, Pt and Au nanoparticles. Analyst 2011, 136, 2322–2329. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, C.; Wang, C.; Pu, F.; Ren, J.; Qu, X. Silver nanoprobe for sensitive and selective colorimetric detection of dopaminevia robust Ag–catechol interaction. Chem. Commun. 2011, 47, 1181–1183. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, L.; Zhang, X.Y.; Wang, X.H.; Zhou, J.; Sun, Z.; Li, N.B.; Luo, H.Q. Ratiometric fluorescence detection of dopamine based on effect of ligand on the emission of Ag nanoclusters and aggregation-induced emission enhancement. Sens. Actuators B Chem. 2020, 310, 127858. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, P.; Yang, L.; Huang, C.; Li, Y. Facile in Situ Synthesis of Silver Nanoparticles on the Surface of Metal-Organic Framework for Ultrasensitive Surface-Enhanced Raman Scattering Detection of Dopamine. Anal. Chem. 2015, 87, 12177–12182. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.; El-Nour, K.M.A.; Brajter-Toth, A. Detection of transient dopamine antioxidant radicals using electrochemistry in electrospray ionization mass spectrometry. Electrochim. Acta 2017, 249, 145–154. [Google Scholar] [CrossRef]
- Ahmadi, N.; Bagherzadeh, M.; Nemati, A. Comparison between electrochemical and photoelectrochemical detection of dopamine based on titania-ceriagraphene quantum dots nanocomposite. Biosens. Bioelectron. 2020, 151, 111977. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Wei, X. A sensitive and selective sensor for dopamine determination based on a molecularly imprinted electropolymer of o-aminophenol. Sens. Actuators B Chem. 2009, 140, 663–669. [Google Scholar] [CrossRef]
- Palomar, Q.; Gondran, C.; Lellouche, J.-P.; Cosnier, S.; Holzinger, M. Functionalized tungsten disulfide nanotubes for dopamine and catechol detection in a tyrosinasebased amperometric biosensor design. J. Mater. Chem. B 2020, 8, 3566–3573. [Google Scholar] [CrossRef]
- Emadoddin, M.; Mozaffari, S.A.; Ebrahimi, F. An antifouling impedimetric sensor based on zinc oxide embedded polyvinyl alcohol nanoplatelets for wide range dopamine determination in the presence of high concentration ascorbic acid. J. Pharm. Biomed. Anal. 2021, 205, 114278. [Google Scholar] [CrossRef]
- Lv, Q.; Chen, L.-S.; Liu, H.-X.; Zou, L.-L. Peony-like 3D-MoS2/graphene nanostructures with enhanced mimic peroxidase performance for colorimetric determination of dopamine. Talanta 2022, 247, 123553. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajheidari, N. ZnO–CuxO/polypyrrole nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine, and uric acid. Anal. Biochem. 2015, 473, 53–62. [Google Scholar] [CrossRef]
- Lin, J.; Huang, B.; Dai, Y.-F.; Wei, J.; Chen, Y.-W. Chiral ZnO nanoparticles for detection of dopamine. Mater. Sci. Eng. C 2018, 93, 739–745. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Liu, J.; Gan, W.; Ye, B.-C.; Li, Y.-C. Highly sensitive and selective voltammetric determination of dopamine using a gold electrode modified with a molecularly imprinted polymeric film immobilized on flaked hollow nickel nanospheres. Microchim. Acta 2017, 184, 1285–1294. [Google Scholar] [CrossRef]
- Atchudan, R.; Pandurangan, A.; Joo, J. Effects of Nanofillers on the Thermo-Mechanical Properties and Chemical Resistivity of Epoxy Nanocomposites. J. Nanosci. Nanotechnol. 2015, 15, 4255–4267. [Google Scholar] [CrossRef] [PubMed]
- Tülbez, S.; Esen, Z.; Dericioglu, A.F. Effect of CNT impregnation on the mechanical and thermal properties of C/C-SiC composites. Adv. Compos. Hybrid Mater. 2020, 3, 177–186. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Dien, N.D.; Vu, X.H. Facile synthesis of silver/gold alloy nanoparticles for ultra-sensitive rhodamine B detection. RSC Adv. 2021, 11, 21475–21488. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Wang, W.; Liu, C.-J. Synthesis of AuPd alloyed nanoparticles via room-temperature electron reduction with argon glow discharge as electron source. Nanoscale Res. Lett. 2014, 9, 405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mo, F.; Xie, J.; Wu, T.; Liu, M.; Zhang, Y.; Yao, S. A sensitive electrochemical sensor for bisphenol A on the basis of the AuPd incorporated carboxylic multi-walled carbon nanotubes. Food Chem. 2019, 292, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Pakdee, U.; Chiangga, S.; Suwannatus, S.; Limsuwan, P. Growth of MWCNTs on Flexible Stainless Steels without Additional Catalysts. J. Nanomater. 2017, 2017, 5672728. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Jia, Q.-C.; Kong, L.-B. Molybdenum dioxide supported carbon nanotubes@carbon constructs disordered nanocluster particles as anodes for lithium-ion capacitors with long-term cycling stability. J. Mater. Sci. Mater. Electron. 2021, 32, 18912–18930. [Google Scholar] [CrossRef]
- Meškinis, Š.; Vasiliauskas, A.; Andrulevičius, M.; Peckus, D.; Tamulevičius, S.; Viskontas, K. Diamond Like Carbon Films Containing Si: Structure and Nonlinear Optical Properties. Materials 2020, 13, 1003. [Google Scholar] [CrossRef]
- Xie, Y.-L.; Yuan, J.; Ye, H.-L.; Song, P.; Hu, S.-Q. Facile ultrasonic synthesis of graphene/SnO2 nanocomposite and its application to the simultaneous electrochemical determination of dopamine, ascorbic acid, and uric acid. J. Electroanal. Chem. 2015, 749, 26–30. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Q.; Wu, C.; Zhang, Y.; Zeng, L. PtNi bimetallic nanoparticles loaded MoS2 nanosheets: Preparation and electrochemical sensing application for the detection of dopamine and uric acid. Anal. Chim. Acta 2019, 1055, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-N.; Zou, J.; Teng, J.; Liu, Q. A novel electrochemical sensor based on self-assembled platinum nanochains-Multi-walled carbon nanotubes-graphene nanoparticles composite for simultaneous determination of dopamine and ascorbic acid. Ecotoxicol. Environ. Saf. 2019, 172, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fu, D. An Electrochemical Sensor for Dopamine Detection Based on Ternary Pd-Au-P Composites Supported on PDDA/RGO. Int. J. Electrochem. Sci. 2019, 14, 9909–9920. [Google Scholar] [CrossRef]
- Arvinte, A.; Crudu, I.-A.; Doroftei, F.; Timpu, D.; Pinteala, M. Electrochemical codeposition of silver-gold nanoparticles on CNT-based electrode and their performance in electrocatalysis of dopamine. J. Electroanal. Chem. 2018, 829, 184–193. [Google Scholar] [CrossRef]
- Yan, J.; Liu, S.; Zhang, Z.; He, G.; Zhou, P.; Liang, H.; Tian, L.; Zhou, X.; Jiang, H. Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on graphene anchored with Pd-Pt nanoparticles. Colloids Surf. B 2013, 111, 392–397. [Google Scholar] [CrossRef]
- Jiang, J.; Du, X. Sensitive electrochemical sensors for simultaneous determination of ascorbic acid, dopamine, and uric acid based on Au@Pd-reduced graphene oxide nanocomposites. Nanoscale 2014, 6, 11303–11309. [Google Scholar] [CrossRef]
- Wei, X.; Yang, P.; Li, H.; Wang, S.; Xing, Y.; Liu, X.; Zhang, S. Synthesis and properties of mesoporous Zn-doped Li1.2Mn0.54Co0.13Ni0.13O2 as cathode materials by a MOFs-assisted solvothermal method. RSC Adv. 2017, 7, 35055. [Google Scholar] [CrossRef]
| Electrode | Linear Range (μM) | LOD (μM) | References |
|---|---|---|---|
| PtNi@MOS2 | 0.5–150 | 0.1 | [31] |
| PtNCs-MWCNTs-GNPs | 2–50 | 0.5 | [32] |
| Pd-Au-P/PDDA/rGO | 3.5–125 | 0.7 | [33] |
| Ag-Au/CNT | 1–173 | 0.052 | [34] |
| Pd3Pt1/PDDA-RGO | 4–200 | 0.04 | [35] |
| Au@Pd-RGO | 0.01–100 | 0.024 | [36] |
| 1% Pd/MWCNT | 0.098–6.8 | 0.045 | This work |
| 1% Pd-5%Au/MWCNT | 0.098–6.8 | 0.058 | This work |
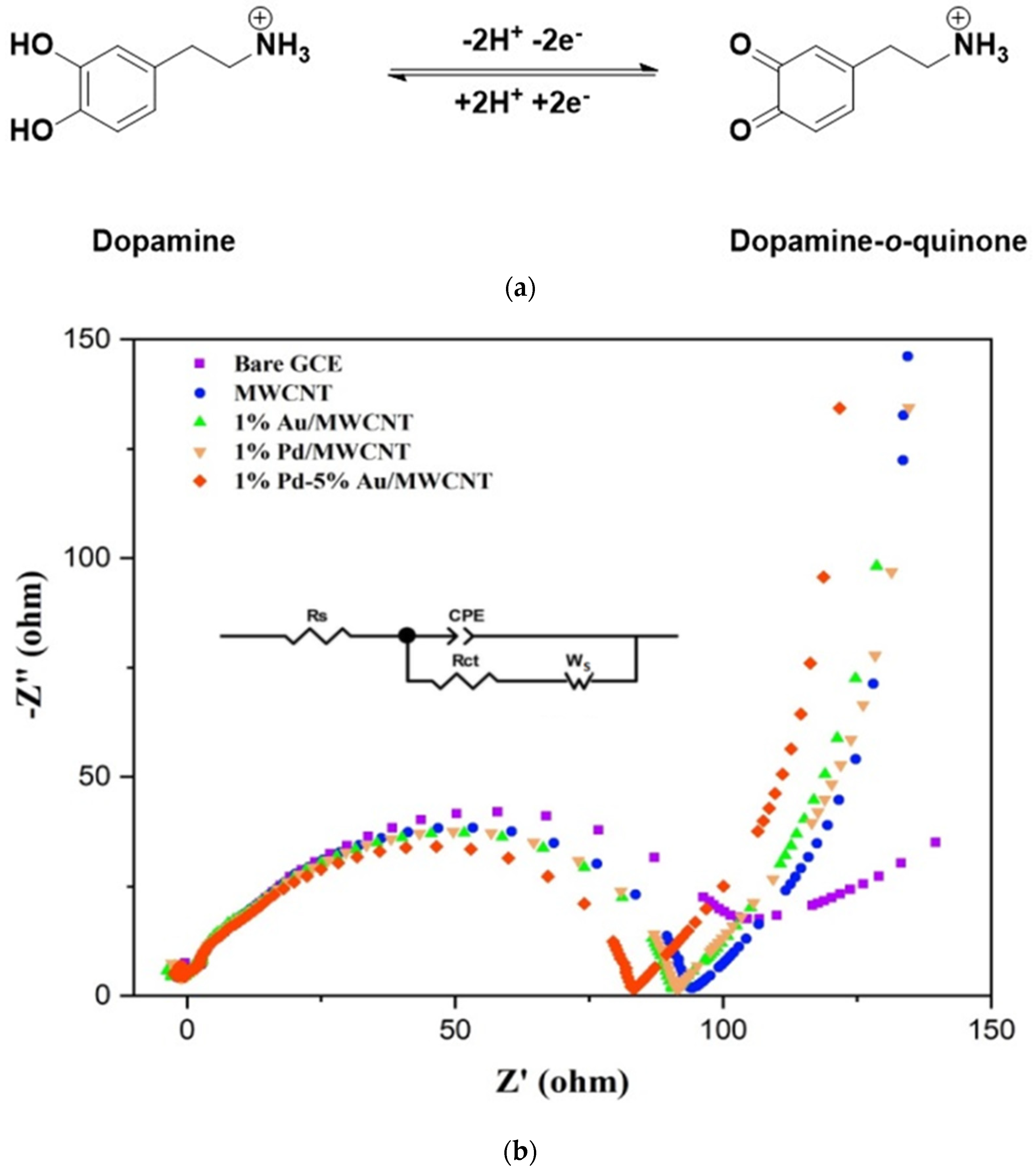
| Electrode | Rs (Ω) | Rct (Ω) | CPE | Ws (Ω) |
|---|---|---|---|---|
| Bare GCE | 20.78 | 106.72 | 8.24 × 10−12 | 69.94 |
| MWCNT | 85.74 | 94.26 | 4.00 × 10−20 | 47.88 |
| 1% Au/MWCNT | 33.05 | 90.98 | 1.20 × 10−3 | 58.67 |
| 1% Pd/MWCNT | 31.13 | 91.42 | 8.70 × 10−4 | 102.3 |
| 1% Pd-5% Au/MWCNT | 18.82 | 83.21 | 5.54 × 10−4 | 41.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Luk, H.-N.; Liu, Y.-S.; Wu, R.-J.; Chung, M.-H.; Chang, X.-J. Preparation of Bimetallic Au-Pd/MWCNTs Electrode for Detection of Dopamine. Minerals 2022, 12, 1145. https://doi.org/10.3390/min12091145
Zhu Z, Luk H-N, Liu Y-S, Wu R-J, Chung M-H, Chang X-J. Preparation of Bimetallic Au-Pd/MWCNTs Electrode for Detection of Dopamine. Minerals. 2022; 12(9):1145. https://doi.org/10.3390/min12091145
Chicago/Turabian StyleZhu, Zhen, Hsiang-Ning Luk, Yu-Shih Liu, Ren-Jang Wu, Ming-Hung Chung, and Xu-Jia Chang. 2022. "Preparation of Bimetallic Au-Pd/MWCNTs Electrode for Detection of Dopamine" Minerals 12, no. 9: 1145. https://doi.org/10.3390/min12091145
APA StyleZhu, Z., Luk, H.-N., Liu, Y.-S., Wu, R.-J., Chung, M.-H., & Chang, X.-J. (2022). Preparation of Bimetallic Au-Pd/MWCNTs Electrode for Detection of Dopamine. Minerals, 12(9), 1145. https://doi.org/10.3390/min12091145








