1. Introduction
Coal is a complex geological material transformed from plant residue through a series of complex biochemical, physical chemical and geochemical processes [
1,
2,
3,
4], and it is mainly composed of organic components (macerals) and mineral matter. The terms mineral matter and mineral in coal are prone to be used interchangeably, without discretion in some cases. The mineral matter in coal generally contains all inorganic components, including discrete crystalline minerals (minerals in the traditional sense), non-crystalline mineraloids, inorganic elements combined with organic matter (mainly Ca, Fe, Mg, Al and Na) and elements dissolved in coal pore water [
4,
5,
6,
7,
8].
Minerals play a most significant role in the assessment of coal resources utilization. On the one hand, they directly reduce the economic value of coal by diluting the energy; on the other hand, these minerals being the main components of fly ash particles, residual bottom ash and slag generated by coal combustion will contribute to the slagging, agglomeration, corrosion and erosion of the boiler [
9]. In addition, the minerals in coal have an impact on human health and the environment in the process of coal mining operations and utilization activities. For example, inhalation of high-content nano-quartz released by burning coal indoor in Xuan Wei County, Yunnan Province, China, is the main cause of the high local rate of lung cancer in women [
10,
11,
12]. In addition, fine-grained quartz and pyrite inhaled by coal miners are also important contributors to Coal Workers Pneumoconiosis (Black Lung Disease) [
3,
13]. Some sulfides are major hosts of toxic elements (such as As and Hg), which have adverse effects on human health (arsenonsis) and the environment [
4,
14,
15]. Although minerals reduce the economic value of coal and release harmful substances, some critical elements are closely related to minerals in some way (e.g., rare earth elements and Y and Ga are mostly associated with clays, phosphate and carbonate minerals), which have attracted much attention from researchers [
3,
8,
16,
17,
18,
19,
20,
21]. In addition, coal ash is attracting increasing attention as a potential source of critical elements. For instance, the fly ash derived from Inner Mongolia Haerwusu coal deposits with an Al
2O
3 concentration up to 51 wt% has been used as a source for Al extraction [
22].
From the genetic point of view, the mineral matter in coal, like the organic matter, is a product of various geological processes associated with peat accumulation and rank advance, as well as changes in subsurface fluids and other aspects of sediment diagenesis [
5,
6,
23,
24]. Minerals can provide information for the genesis of coal and geological background of coal-accumulating basins and can also help to clarify coalification and explain basic theoretical issues, such as regional geological historical evolution [
3,
6,
21,
25,
26,
27,
28,
29].
At present, at least 200 minerals have been observed in coal [
3,
4]. The contents of minerals are generally between a few percent and dozens of percent. The modes of occurrence and origins of most of these minerals have been comprehensively reviewed by Ward [
5,
6].
Despite the fact that XRD analyses of raw coals of varied ranks have been used by many researchers [
30,
31,
32], the presence of a “hump” due to the high content of organic matter makes accurate quantification of mineralogical compositions of coals difficult, particularly in low-rank coals. During the high-temperature (815 °C) ashing process, the phases of minerals would be transformed: for example, kaolinite may release structural water, and carbonate minerals would release CO
2 by mineral decomposition [
33]. Several researchers chose to reduce the temperature for heating coal for a protracted period at around 370 °C to destroy the organic matter. This is still a sufficiently high temperature for many minerals in coal, such as pyrite, siderite, gypsum and some clay minerals, as they may undergo irreversible changes of mass and/or crystal structure [
5,
34,
35,
36]. For instance, the dehydration of gypsum and/or the reaction between liberated CaO from organic matter and SOx from the breakdown of pyrite could result in the formation of bassanite or anhydrite during the ashing process or when treating samples before XRD analysis [
4,
5,
30]. However, at low temperature (120~150 °C), in vacuum and under high pressure, the oxygen plasma excited by electrons reacts with the organic matter in coal to produce water and carbon dioxide [
37], during which the minerals can be isolated from higher-rank coals without major alteration [
8,
17,
38,
39,
40,
41]. X-ray diffractograms combined with Siroquant analysis is one of the effective means to identify and quantify the minerals in coal [
42].
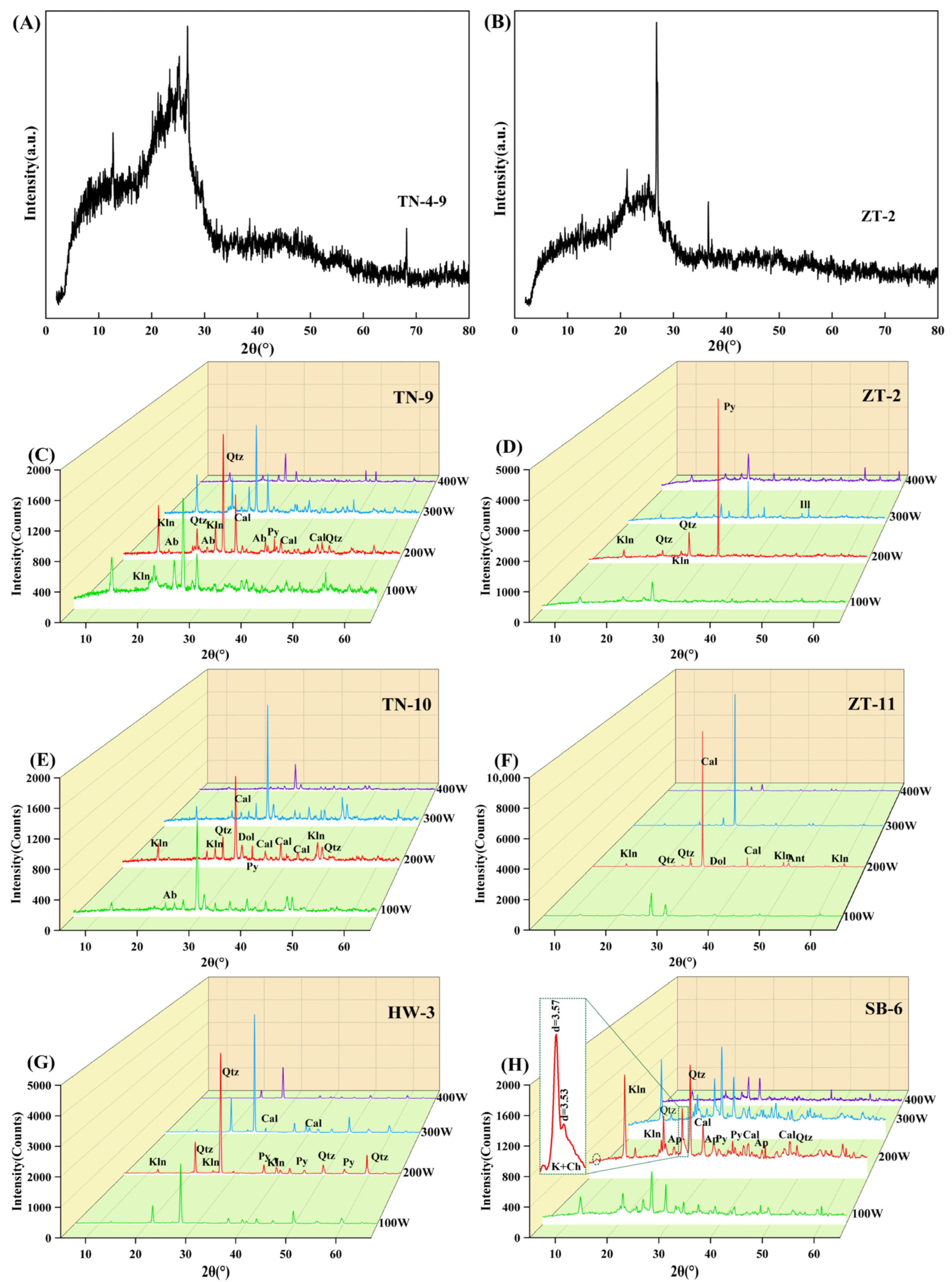
Due to the high crystallinity of minerals in anthracite and bituminous coal, LTAs-XRD analyses can effectively determine the properties of different minerals in coal [
42,
43,
44]. However, lower-rank coals contain a large number of NMIE (non-mineral inorganic elements) with active chemical properties, and minerals of low crystallinity are lower, resulting in higher background values in XRD spectra in the form of a bulge. In the process of low-temperature ashing, the NMIEs (such as Ca, Al and Mg) react with organic sulfur to form sulfate phases in the residue, affecting the qualitative and quantitative analyses of minerals in low-temperature ashes [
6,
36,
45,
46].
The Quorum K1050X Plasma Asher is widely used in low-temperature coal ashing because of its simple operation, low consumable requirements and economic benefits [
8,
17,
41]. When there are a few of samples to be ashed, it can meet the requirements of researchers. However, it is often necessary to ash a large number of coal samples in scientific research. This instrument can only ash a single sample at a time, which may require spending a lot of time on the preliminary treatment. Therefore, the efficiency of low-temperature ashing has been an urgent problem to be solved. As the demand for ashing and the maturity of low-temperature oxygen plasma ashing technology increase, low-temperature plasma ashers have made progress. The IoN 40 low-temperature plasma asher is produced by the American PVA TePla, which is a batch-mode plasma system for etching, stripping, cleaning and surface treatments. The IoN Series combines field-proven features with the process flexibility of computer control to offer ultimate performance: high throughput, low particulate generation, process versatility, precise parameter control and fault isolation.
At present, the IoN 40 plasma system is widely used in the field of materials, especially membrane materials, such as membrane preparation by CF4 plasma treatment conducted on an IoN 40 with 150 W or 200 W [
47,
48,
49,
50]. In addition, the IoN 40 at a 100 W operating power as used when az5214e was spin coated on the ZnO Nanorod array at 90 °C [
51]. LTA was carried out using the IoN 40 at a constant pressure of approximately 40 psi, prior to the study of the effect of acid baking on the minerals present in the tailings using X-ray diffraction [
52]. However, previous publications have not provided any details of the exact low-temperature degrees and corresponding working conditions of the ashers, and this could lead to two adverse effects on coal ashing without proper operating guidance: (1) a relatively high temperature (e.g., >150 °C) may cause the alteration of minerals (particularly clay minerals), and (2) a relatively low temperature (e.g., <80 °C) may cause a long ashing time and incomplete ashing of organic matter. In this study, the authors introduced the most frequently used low-temperature plasma ashers (PVA TePla IoN 40 and Quorum K1050X) to reveal optimal operating parameters for low-temperature ashing and made a comparison of the ashing effects of the two plasma ashers.
2. Samples and Analytical Programs
2.1. Instruments
Low-temperature ashing was conducted using the IoN 40 plasma system (PVA TePla, Corona, CA, USA), the chamber of which is pumped by a rotary vacuum pump. In the near-vacuum condition, the oxygen plasma, which is activated by gas mixed with atoms and ions generated by a high-frequency electric field, reacts with coal samples loaded into the working chamber. The oxygen plasma oxidizes and decomposes the organic matter in the coal at a low temperature, without changing the structure and properties of the minerals. A Quorum K1050X (Quorum, London, UK), with the same operating principle as the IoN 40, was used as a comparative device in this study.
Table 1 lists the working parameters for the two instruments’ settings.
The XRD analysis for the low-temperature ashes (LTAs) was performed on the Thermo Fisher Scientific ARL EQUINOX 3000 (Thermo Fisher, Paris, France) X-ray powder diffractometer with PSD real-time detector. The XRD pattern was recorded with a 2.6–120° 2θ interval with 1 s minimum collection time and 0.05 resolution.
2.2. Samples and Analytical Methods
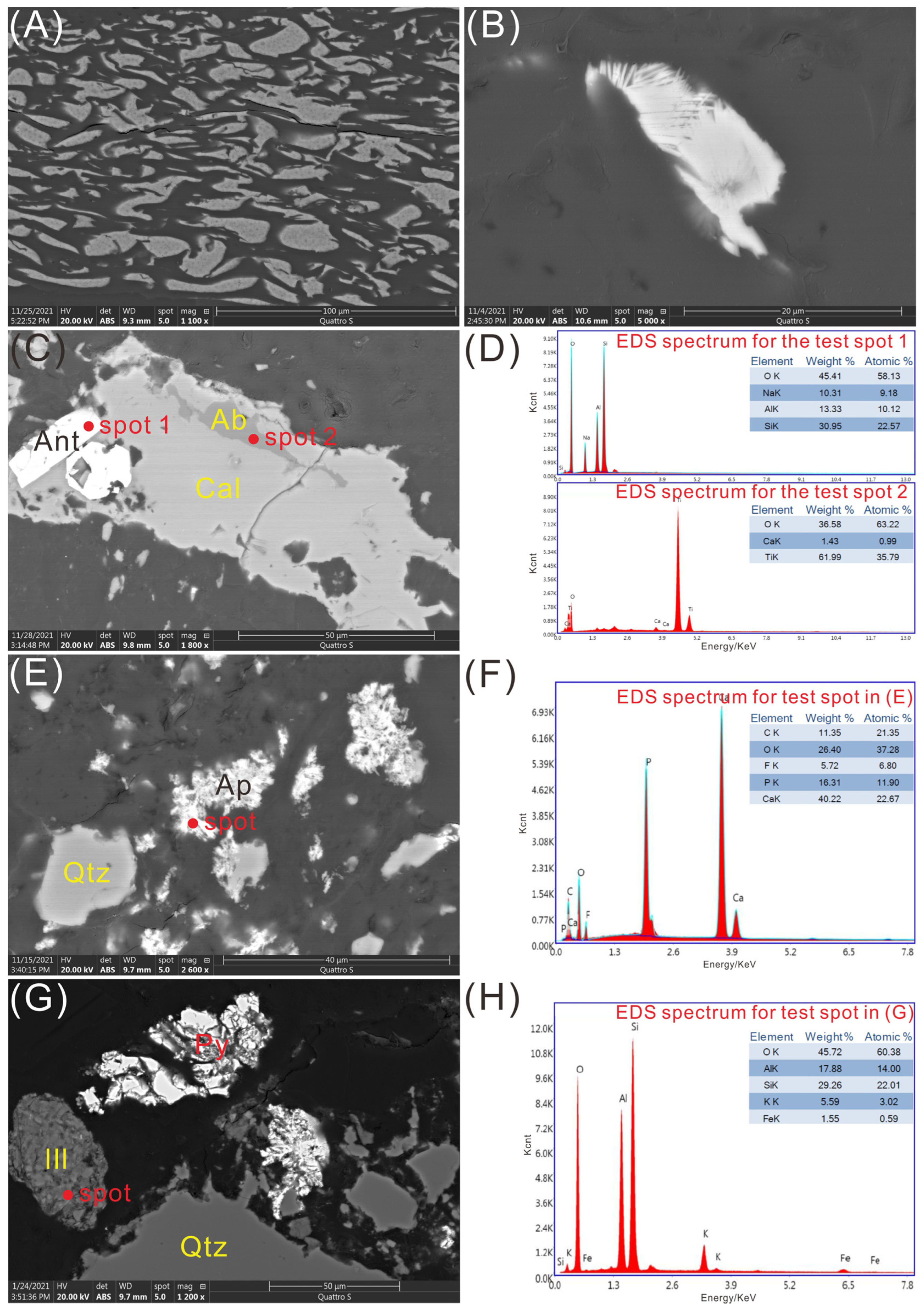
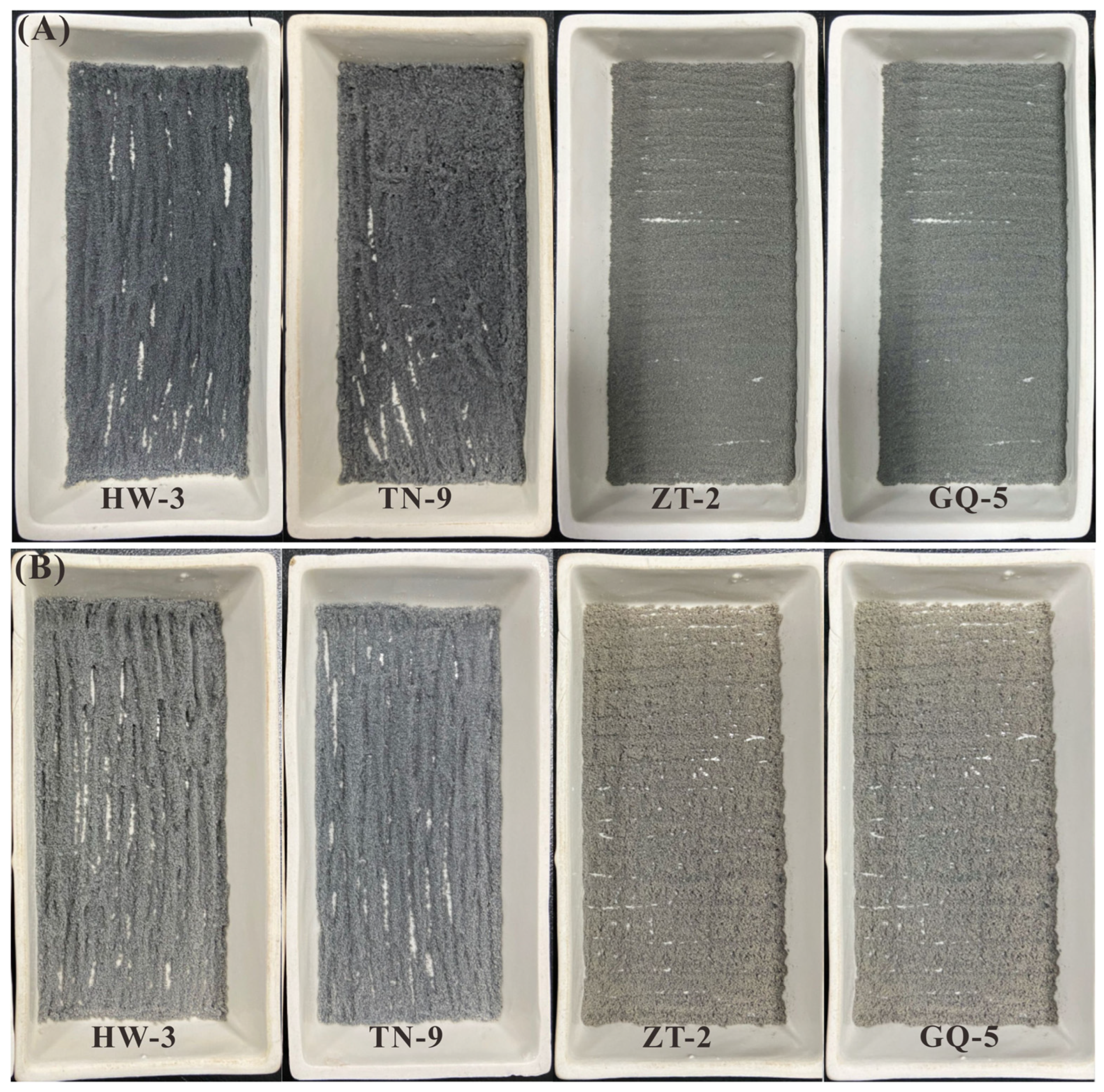
A total of 8 coal samples from coal mines of Shaanxi and Hebei Province, China, were chosen for the ashing process, including samples TN-9 and TN-10 (Tingnan Mine, Shaanxi Province), ZT-2 and ZT-11 (Gaojiapu Mine, Shaanxi Province), SB-6 (Shengbo Mine, Shaanxi Province), HW-3 (Haiwan Mine, Shaanxi Province) of Middle Jurassic; GQ-4 and GQ-5 (Gequan Mine, Hebei Province) of Late Carboniferous. All samples were immediately stored in plastic bags to minimize contamination and oxidation after collection. Each sample was crushed and ground by using a tungsten carbide mill before analysis.
Proximate analysis was conducted using the methods described in standards of ASTM D3173/D3173M-17a, D3174-12 and D3175-18 [
53,
54,
55]. An elemental analyzer (vario MACRO) was used to determine the percentages of C, H and N in the coals, following ASTM D5373-16 [
56]. The total sulfur and forms of sulfur were determined following ASTM Standards D4239-18e1 [
57] and D2492-02 [
58], respectively.
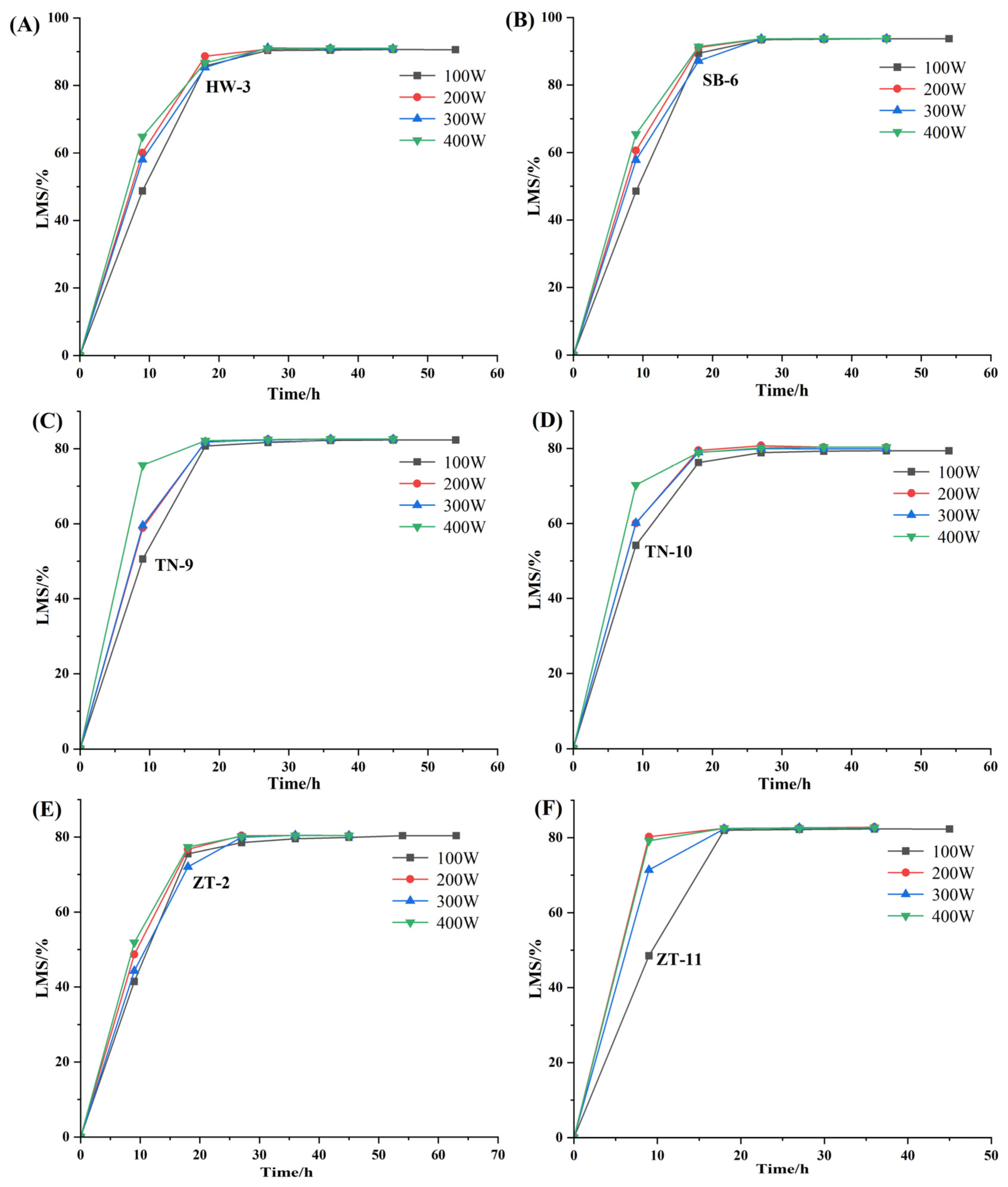
Approximately 2 g (±0.005 g) of each coal (less than 200 mesh) sub-sample was tiled in the reaction boat (60 × 90 mm) and then placed on the electrodes in the IoN 40 asher. Ghe running power was set as 100 (Program A), 200 (Program B), 300 (Program C) and 400 (Program D) watts to compare the ashing effects on the same subsplit coal sample after the ashing completed. Each ashing interval was set to 9 h.
The gas intake affects the glow emission degree of oxygen plasma. When setting the oxygen flow rate, the following factors have to be fulfilled: (1) reaching the degree of plasma glow radiation; (2) the powder sample could not be blown away. Based on the above conditions, the maximum oxygen flow rate was set to 150 mL/min (
Table 1).
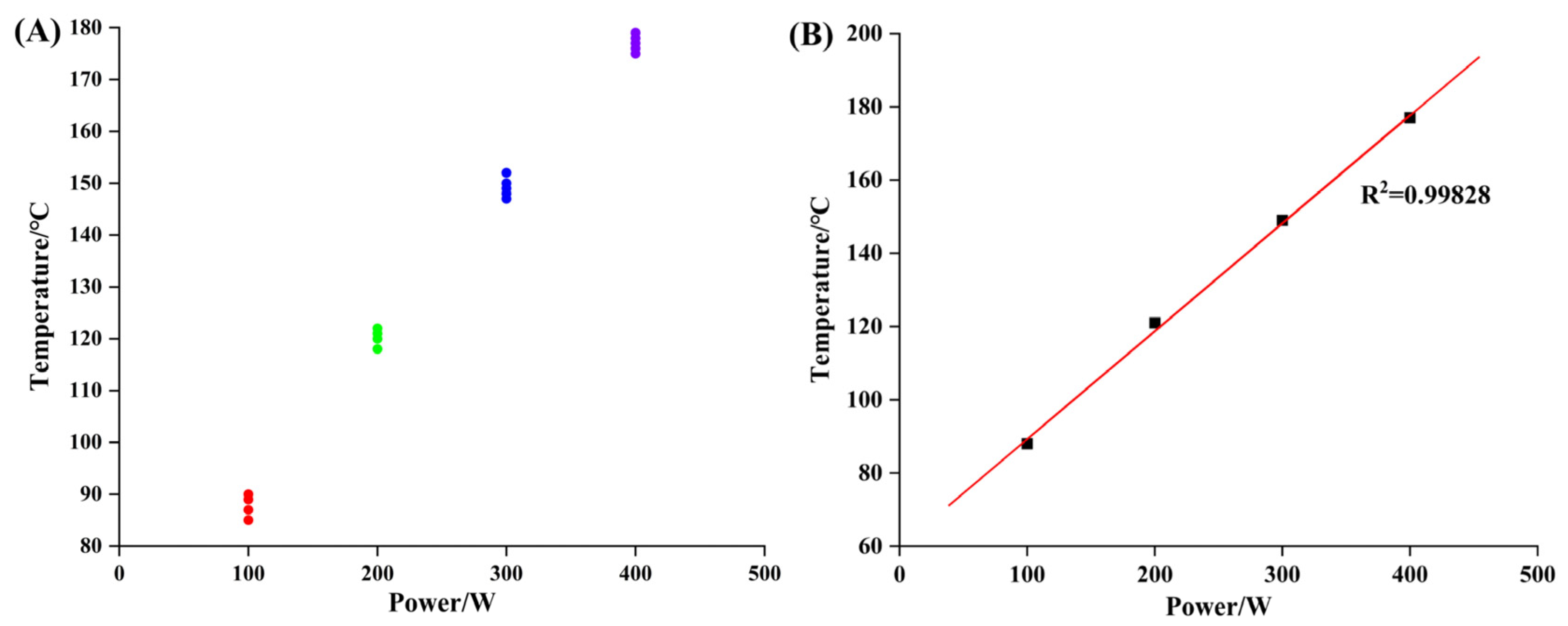
In the experiment, the most important parameter was the operating temperature in the working chamber of the plasma asher. Some researchers obtained control temperatures of 120~150 °C from the research of coal ashing to separate minerals from coal [
5,
18,
36,
45,
59,
60,
61,
62]. The temperature of the working chamber under varied powers was monitored to obtain the corresponding specific ashing temperature, as shown in
Table 2.
A reaction boat with a coal sample was placed into the working chamber of the K1050X. The running power was set to 75 watts (123 °C), and a 20 mL/min oxygen flow rate was used. The ashing interval was also set to 9 h. This program was denoted by E.
After each ashing interval, the samples were weighed and stirred and then continued to be ashed until the mass changed within 4 mg, which was regarded as constant mass.
Approximately 5 g (±0.005 g) of each sub-sample of coal (less than 200 mesh) was spread in each reaction boat and then placed into a muffle furnace, following the temperature and time described in ASTM International Standard [
54], to obtain high-temperature ashes (at 815 °C). The obtained ashes were pressed into flakes for XRF analysis.
Samples were crushed to <1 mm and then prepared as epoxy-bound particulate pellets to polish surface treatment, following International Organization for Standardization (ISO) 7404-2, 2009 [
63], and examined with 50× oil-immersion, reflected-light optics on microscopes. The random reflectance of vitrinite in each polished coal samples was determined on a Leica DM4P microscope equipped with a Craic UCC-300 spectrophotometer, according to the procedure described in ISO 7404-5 (2009) [
64].
A field emission-scanning electron microscope (FE-SEM, FEI Quattro S), in conjunction with an energy-dispersive X-ray spectrometer (EDS; EDAX Team), was used to study the mineral morphology of coal samples and the distribution of elements in the minerals. Prior to SEM-EDS analysis, the sample briquettes were gold-coated using a Quorum Q150T ES sputtering coater. SEM images were captured via a retractable solid-state backscatter electron detector. The working distance of the FE-SEM-EDS was ~10 mm, beam voltage 20.0 kV and micron spot size 5.
2.3. Qualitative and Quantitative Analysis of Minerals
The LTAs were loaded into the sample cup attached to the XRD, and the powder surface was gently flattened by a cover glass for scanning. The working voltage and current of the XRD is 40 kV and 30 mA, respectively, with a dwell time of 1200 s.
Jade 5.0 software was used to identify the types of minerals in LTAs based on the XRD patterns.
X-ray diffractograms of the LTAs were subjected to quantitative mineralogical analysis using Rietveld-based Siroquant™, a commercial software system initially described by Taylor [
65]. This graphic-oriented system allows the different crystallographic parameters for each mineral in the sample to be adjusted interactively to accommodate variations due to atomic substitution, layer disordering, preferred orientation and other factors in the individual phases [
39,
65,
66] and has the characteristics of fast calculation speed, small error and high accuracy. At present, Siroquant™ has been used by many researchers for quantitative analysis of minerals in LTA and whole coal samples [
5,
17,
36,
60,
61,
62,
67,
68,
69,
70,
71].
2.4. XRF Analysis
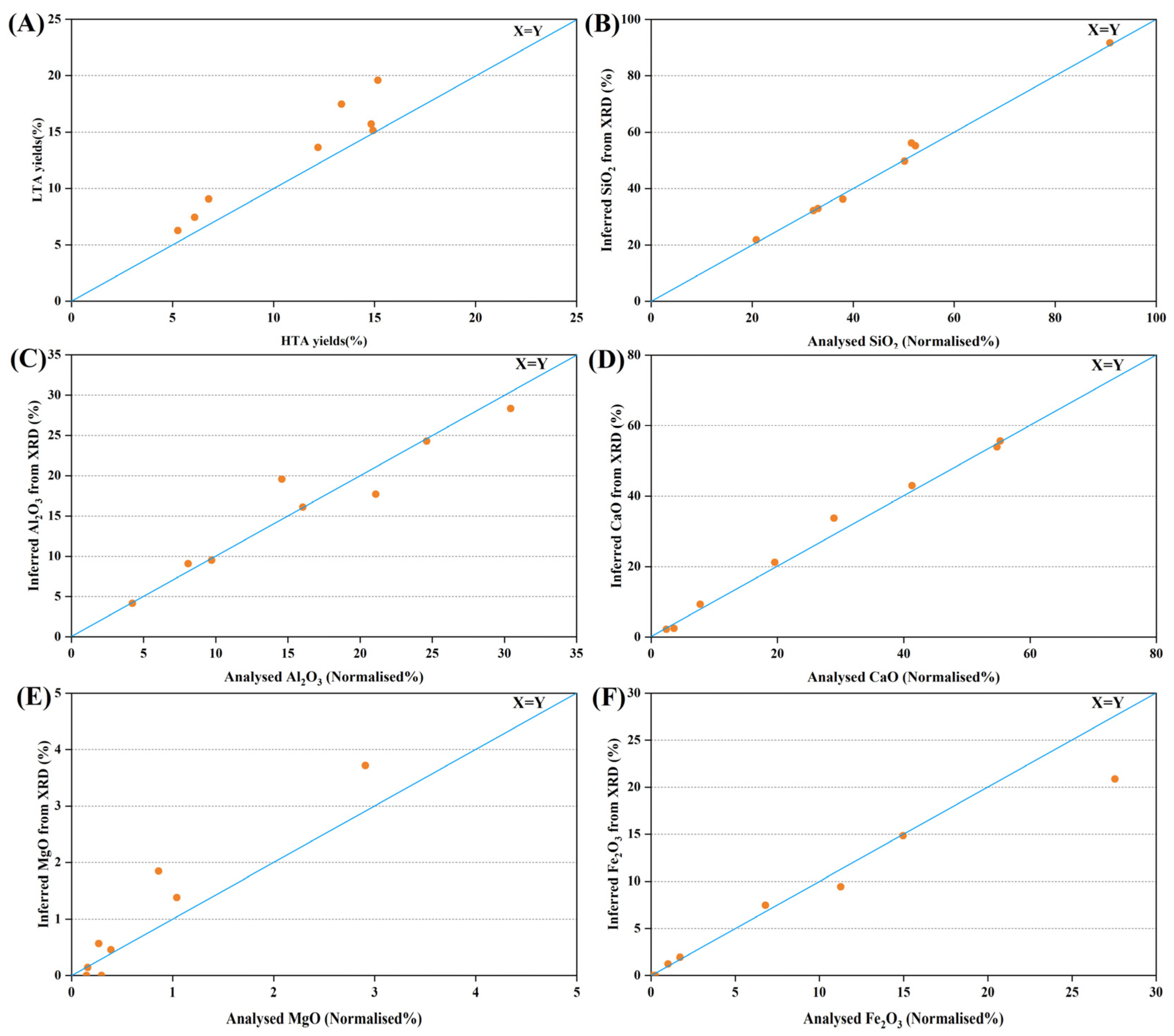
X-ray fluorescence (XRF) spectrometry (Thermo Scientific ARL PERFORM’ X 4200) was used to determine the contents of major oxides, including SiO2, Al2O3, CaO, K2O, Na2O, Fe2O3, MnO, MgO, TiO2 and P2O5, in the coal ash samples (at 815 °C).
5. Conclusions
(1) Selection of the Plasma Ashers
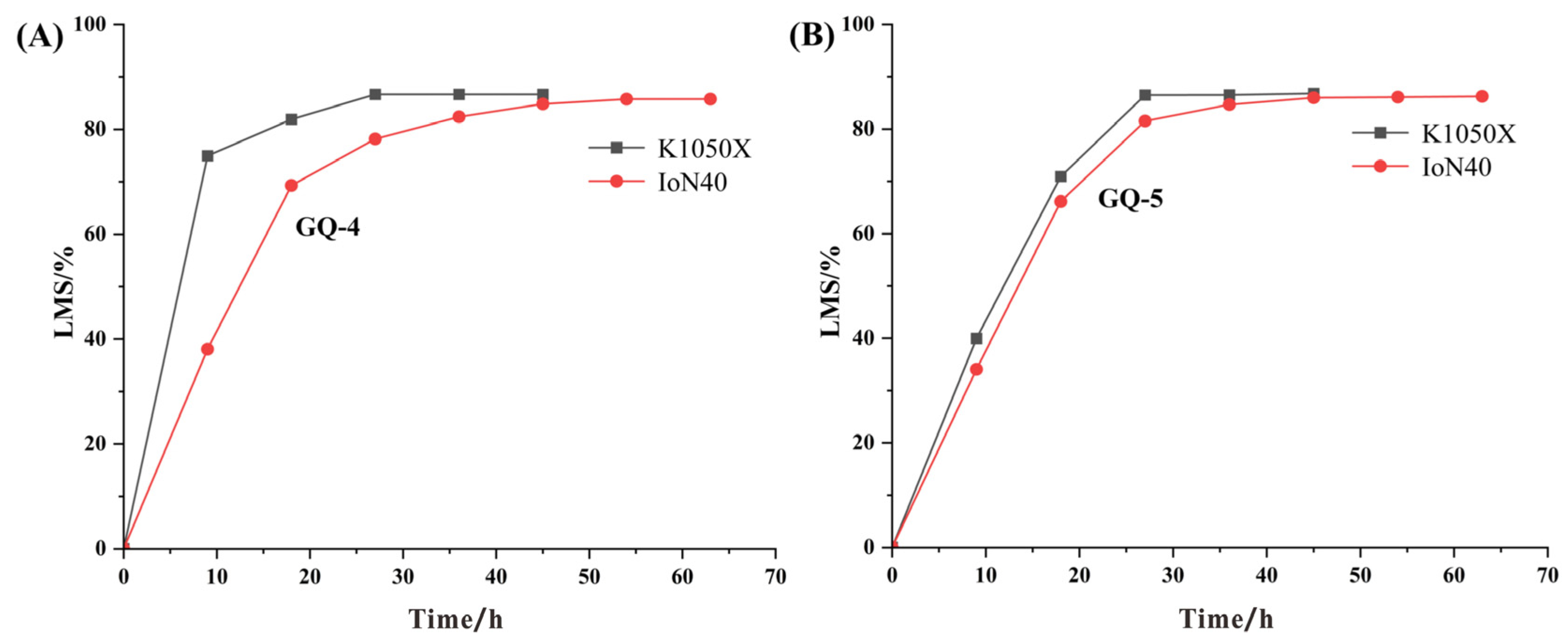
The IoN 40 plasma system with a big chamber can be used to ash the coal samples, facilitating the isolation of the minerals from bituminous coals, which meets the needs of low-temperature ashing of a large number of samples (at least 48 samples) in a short time. The K1050X with a small chamber (one sample at a time) is able to ash coal samples faster and more efficiently.
(2) Optimal work program of IoN 40 plasma system
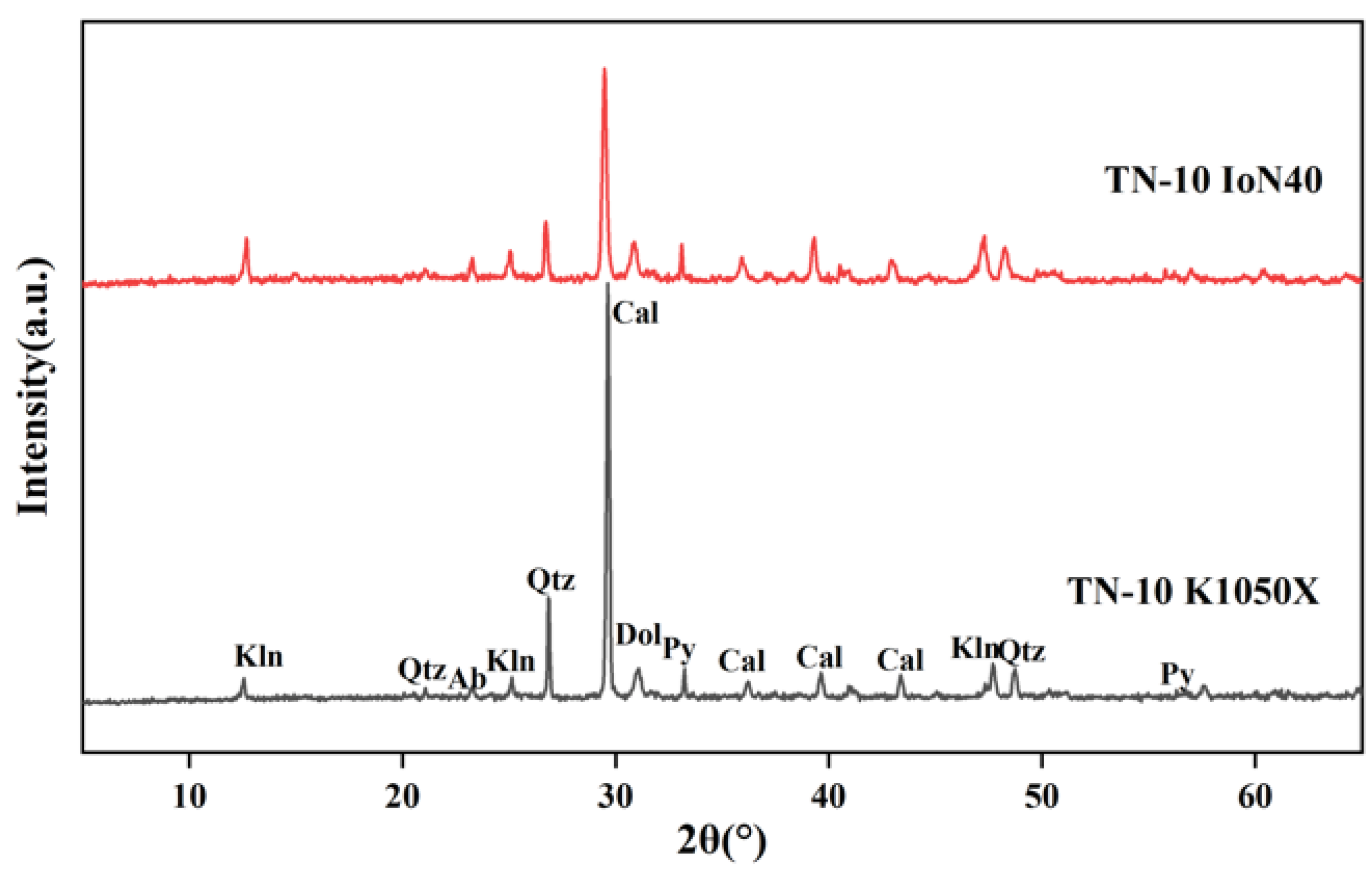
The optimal operation parameters of the IoN 40 plasma system are put forward as follows: the gas intake is 150 mL/min, the pressure is 500 mTorr and the power is 200 watts, especially for clay minerals, whose crystal structure is easy to change or undergo pyrolysis reaction. At XRD analysis of LTAs obtained at running power of 200 watts, it is found that the diffraction peaks of minerals are intact, by which kaolinite d(002) and chlorite d(004) can be clearly distinguished. In addition, the qualitative and quantitative analyses of minerals by XRD + Siroquant are accurate and reliable at the optimal working program.
(3) The effects of different low temperatures on crystalline mineral matter
When the ashing temperature is lower than 80 °C (about 100 W), it is not high enough to remove the organic matter completely. When the temperature increases to 150 °C (about 300 W), the symmetry and integrity of the mineral crystal structures begin to become worse. At the same time, the intensity of diffraction peak decreases with increase in ashing temperature, leading to the disappearance of some trace mineral peaks. In summary, the mineral peak intensity and symmetry would not be changed under the ashing temperature of 121 °C and is thus fit for accurate quantitative analysis.