Abstract
This study contributes to our understanding of the evolution of Halılar Cu-Pb (±Zn) mineralization (NW Turkey) based on mineralogical and geochemical results and sulfur isotope data. The study area represents local Cu-Pb with some Zn brecciated-stockwork vein type mineralization along the NE–SW fault gouge zone at the lower boundary of the Sakarkaya and Düztarla granitoid rocks. Two main zones, consisting of sericite–quartz–chlorite ± kaolinite ± pyrite (i.e., zone-1) and calcite–epidote–albite ± chlorite ± sericite (i.e., zone-2), were observed within the central ore mineral zone at the mining site. Different mineralization assemblages were recorded; the main ore mineral contains chalcopyrite, galena, pyrite, and sphalerite within alteration zone-1, and the oxidation/supergene mineralization includes covellite and goethite. The mass balance calculations show that the samples of zone-1 show an increase in SiO2, Fe2O3, K2O, and LOI along with Ag, As, Cu, Mo, Pb, S, Sb, and Zn, reflecting high pyritization with sericitization and silicification. On the other hand, the samples from zone-2 are rich in CaO; Na2O; P2O5; TiO2; LOI; and carbon-reflecting calcite, epidote, and albite alterations. A uniform magmatic sulfur source of Halılar sulfides is suggested by their mean δ34S value of −1.62‰. Furthermore, the primary metal source is metasediments and intrusive Düztarla granitoid magmatism. These observations suggest that the Halılar metasediment-hosted Cu-Pb (±Zn) mineralization was formed by epigenetic hydrothermal processes after sedimentation/diagenesis and metamorphism.
1. Introduction
Studies of hydrothermal alteration are important in the exploration of copper deposits in order to determine the processes of ore formation, as well as to identify potential ore zones [1]. Spectroscopic methods, geophysics, or multispectral remote sensing techniques are used in mapping alteration zones, as well as in identifying their mineral assemblages [1,2,3,4,5,6,7,8,9]. In addition, the geochemical changes from host rock to alteration zones provide alteration type and its degree, as well as the genesis and evolution of the hydrothermal system [5,10,11,12,13,14,15,16,17,18,19]. Hydrothermal alteration processes are responsible for mineralogical and chemical changes in the rock-forming minerals as a result of interactions between the hydrothermal fluids and host rocks along fracture zones and grain boundaries [1,2,20,21]. Schwartz [22] stated that the alteration generally depends on: (1) temperature, pressure, and chemical composition of the fluid; (2) the chemical and physical nature of the wall rocks; and (3) the water–rock ratio. The mechanism and types of mineral deposits are assigned by the nature of the alteration assemblages and the different hydrothermal systems. In addition, the mineral assemblages of the altered rocks are important to help identify the alteration types (e.g., phyllic alteration refers to assemblages of quartz + sericite + pyrite minerals; potassic alteration: orthoclase + biotite + sericite; propylitic alteration: epidote + chlorite + albite) [23]. Gifkins et al. [24] defined different types of mineral deposits by their alteration type and mineralogy, such as porphyry Cu deposits having potassic, phyllic, argillic, and propylitic alterations, while the low-sulfidation, epithermal, geothermal, VHMS, and sediment-hosted massive sulphide deposits having sericitic (or phyllic) and propylitic (or saussuritization) alterations.
In Turkey, mineralization in the structural zone of the Anatolian tectonic belt represents part of the Tethyan–Eurasian metallogenic belt (TEMB), which formed during the Mesozoic and Early Cenozoic [25]. This mineralization was controlled by extensional events that formed after the Neo-Tethys closure. It is associated with calc–alkaline magmatic activity during the Oligocene–Miocene/Pliocene within the post-collision continent-continent environments and led to the formation of Pb-Zn, Sb, As, and Au-Cu deposits [25].
The study area (Halılar area) is located about 25–30 km northeast of Edremit in Balıkesir Province (Biga Peninsula, Turkey) (Figure 1). Halılar Cu-Pb (±Zn) mineralization occurs in a vein-type deposit that formed in the volcanogenic metasediments of the Sakarkaya Formation. It is associated with the NE–SW fault gouge zone along with the lower boundary of the Bağcağız Formation and the Düztarla granitoid intrusion.
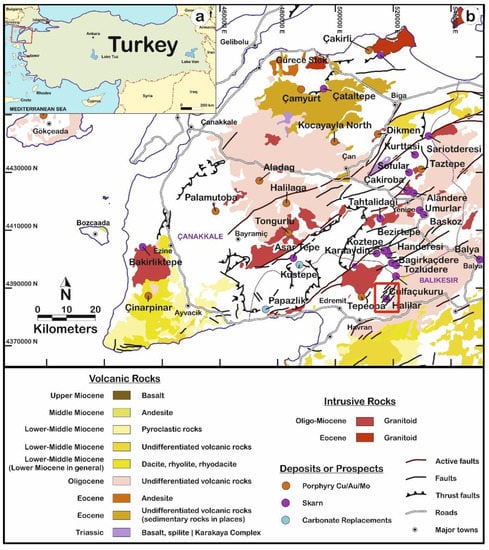
Figure 1.
(a) Geological map of Turkey; (b) study area around Halılar in the Northeast of Edremit in Balıkesir after Yigit [27].
Although geological and geochemical studies of the Halılar area have been published [26], the genesis of base-metal Cu-Pb (-Zn) mineralization in this area remains ambiguous, as it has not been studied in detail. Therefore, this study focuses on mineralization in the Halılar area by reporting new data obtained from mineralogical, petrographical, and geochemical investigations of the mineralization and altered host rock. Using mass balance calculations, enrichment and/or depletion in the chemical components of the different alteration zones associated with this mineralization were calculated on the basis of their mass/volume changes (gain and loss). Sulfur isotope data from the sulfide minerals, including pyrite, chalcopyrite, and galena, were collected to understand the sulfur source(s), as well as to determine the δ34SH2S values of the hydrothermal fluid that caused the Halılar Cu-Pb-(±Zn) mineralization.
2. Geological Setting
The Halılar area contains two groups: the clastic Halılar Group, which is slightly metamorphosed and overlain by the pre-Late Triassic age or Permian limestone [28], and the Bilecik group. These two groups are in contact with the intrusive rocks to the N and NW of Halılar village (Figure 2). The Halılar Group consists of two formations: the Bağcağız and Sakarkaya Formations; the Bilecik Group is represented by two formations: the Taşçıbayırı Formation and Günören Limestone (Figure 2). The granitoid rocks intruded the Sakarkaya and Bağcağız Formations of the Halılar Group in the study area (Figure 2).
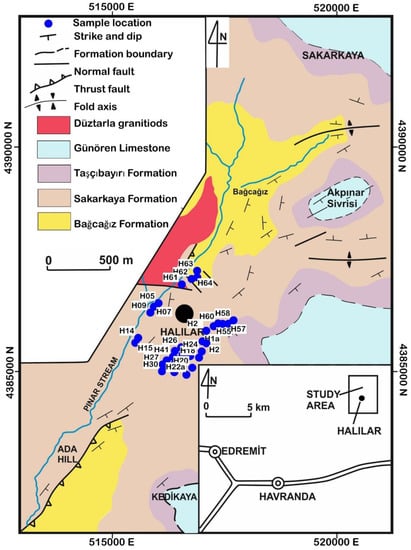
Figure 2.
Geologic map of the Halılar area after Altiner et al. [26].
The Halılar Group was classified by Krushensky, Akcay, and Karaege [28] into the Bağcağız Formation (sandstone and shale) and the Sakarkaya Formation (sandstone and conglomerate). The Bağcağız Formation (sample IDs: H63 and H64) was intruded by the Düztarla granitoid at its lower boundary (Figure 2). It has dark siltstone at its upper boundary, which is overlain by the sandstone of the Sakarkaya Formation. This formation also has sandstone and siltstone alternations from bottom to top, consisting of dark-grayish-colored siltstones and silty shales with yellowish-colored, medium-bedded sandstones from the Lower Triassic to Middle Jurassic. The Bağcağız Formation is represented by carbonaceous dark metasiltstone and rhyolitic metatuffs (Figure 3a). The rhyolitic metatuffs are fine-grained light gray to yellowish rocks (Figure 3a) microscopically consisting of microperthite and quartz crystals embedded in a finer-grained tuffaceous matrix of kaolinitized and carbonatized feldspar, quartz, and Fe-oxide (Figure 3b).

Figure 3.
(a) Rhyolitic metatuffs of the Bağcağız Formation; (b) XPL photomicrograph of the mineral composition of rhyolitic metatuffs; (c) yellowish-colored metasandstone of the Sakarkaya Formation; (d,e) XPL photomicrograph of the poorly sorted quartz, feldspar, and mica grains bounded by iron oxide in the subarkosic-to-quartz arenitic of the metasandstone; (f) general view of the Bilecik Limestone; (g) XPL photomicrograph of the calcite with feldspar, mica, and volcanic rock fragments in the sandy limestone of the Taşçıbayırı Formation; (h) XPL photomicrograph of the calcite and dolomite with Fe-oxide minerals in Günören dolomitic limestone; (i) granodiorite of Düztarla intrusive rocks; (j) XPL photomicrograph of the oligoclase, quartz, and microperthite with subordinate amount of biotite and Fe-oxide minerals in granodiorite; (k) granite from the Düztarla intrusion invaded into the Bağcağız Formation; (l) XPL photomicrograph of the mineral composition of the Düztarla granite intrusion. Abbreviations: biotite (bt), calcite (cal), dolomite (dol), K-feldspar (kfs), kaolinite (kln), muscovite (ms), opaque (opq), plagioclase (pl), quartz (qz), and sericite (ser).
The Sakarkaya Formation (sample IDs: H05, H07, H09, H14, H15, H18, H20, H22a, H55, and H60) outcrops approximately 500 m south of Sakarkaya Hill and 1.5–2 km north and northeast of Halılar village (Figure 2). It is represented by fine-grained, yellowish-colored metasandstone (Figure 3c). It has a sharp contact with the dark metasiltstones of the Bağcağız Formation. The unit rests with a distinct contact on the Bositra-bearing dark silty shale of the Bağcağız Formation [26]. The metasandstone ranges from subarkosic to wackes in composition and consists of poorly sorted quartz, sericitized and kaolinitized feldspar, and mica grains cemented by iron oxide (Figure 3d,e). These components are embedded in altered feldspar and silicified fine-grained matrix (Figure 3d,e). The upper portion of the formation is represented by cross-stratified beds.
The Bilecik Group is part of the Callovian–Hauterivian (Middle Jurassic–Lower Cretaceous) stratigraphy in NW Anatolia known as the Bilecik Limestone (Figure 3f–h). It has been divided into the two formations; Taşçıbayırı and Günören Limestone formations. The Taşçıbayırı Formation (sample IDs: H56, H57, and H58) underlies the Günören Limestone (sample ID: H59); they contain sandy limestone and dolomitic limestone, respectively. The sandy limestone of the Taşçıbayırı Formation is composed of calcite with feldspar, mica, and volcanic rock fragments (Figure 3g). The volcanic rock fragments are composed of broken and/or eroded volcanic rocks consisting of quartz and feldspar (Figure 3g), while the Günören dolomitic limestone consists of calcite and dolomite with Fe-oxide minerals (Figure 3h).
The Düztarla granitoid rocks (sample IDs: H61 and H62) reflect Upper Oligocene–Lower Miocene post-collisional magmatic activity in the study area (Figure 2), differentiated into granodiorite and granitic rocks (Figure 3i–l). The granodiorite consists of plagioclase (oligoclase in composition; 35–50 vol.%), quartz (20–35 vol.%), and microperthite (8–17 vol.%) with subordinate amounts of biotite and Fe-oxide minerals (Figure 3j). The plagioclase is slightly affected by sericite and kaolinite alteration (Figure 3j). The granite is composed of microperthite (30–45 vol.%), quartz (20–35 vol.%), and plagioclase (albite in composition; 20–30 vol.%) with muscovite and Fe-oxide minerals (Figure 3l).
The Halılar area has a well-described Upper Triassic–Liassic continuous succession (Figure 1 and Figure 2). The tectonic sedimentary rocks formed at the Sakarya divergent margin, which evolved in the Late Triassic–Aptian interval [29,30]. As a result of the diachronic closure of the Tethys basin in western Anatolian, the Upper Triassic black shales were deposited in the Lias in the Karakaya euxinic basin throughout the Edremit region. This shale and the Hettangian arkosic sandstones were later intruded by the Düztarla granodioritic–granitic body due to the southward subduction of the Paleo-Tethys [29].
3. Sampling and Analytical Methods
A total of 45 host rocks, altered rocks, and mineralized samples were collected from the study area. Thin sections and a subset of polished sections were examined optically using transmitted and reflected light microscopes. Whole-rock major, trace, and rare earth element analyses were conducted at the Geochemistry Research Laboratories of Istanbul Technical University (ITU/JAL). The samples were grounded using a tungsten carbide milling device. Major elements were analyzed using a BRUKER S8 TIGER model X-ray fluorescence spectrometer (XRF) (Östliche Rheinbrückenstraße 49, 76187 Karlsruhe, Germany) with a wavelength range from 0.01–12 nm. Trace elements were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) using an ELAN DRC-e Perkin Elmer model (PerkinElmer, Waltham, MA, US). Approximately 100 mg of powdered sample was digested in two steps. The first step was completed with 6 mL of 37% HCl, 2 mL of 65% HNO3, and 1 mL of 38%–40% HF in a pressure- and temperature-controlled Teflon beaker using a Berghoff Microwave™ at an average temperature of 180 °C. The second step was completed with the addition of 6 mL of 5% boric acid solution. The remaining solution sample was analyzed by ICP-MS. The altered rocks were also analyzed for mineralogy using a BRUKER X-ray diffractometer (XRD) (Östliche Rheinbrückenstraße 49, 76187 Karlsruhe, Germany). Calculation of the normative mineral abundances from the major element analyses and rare earth element diagrams were created using Igpet 2.3 [31]. The GEOISO-Windows of Coelho [32] were used to determine the absolute mobility of the elements using equations from Gresens [33] and isocon diagrams from Grant [34,35].
Sulfide minerals for sulfur isotope analysis were separated from slightly crushed (200 mesh) lode samples (>95 % pure pyrite, chalcopyrite, and galena). They were washed and handpicked under a binocular microscope. These analyses were carried out at the Geochron Laboratory (USA) using EA-IRMS (Elemental Analysis-Isotope Ratio Mass Spectrometry) techniques. All stable isotope data are reported in the delta (δ) notation, relative to Vienna-Canyon Diablo Troilite (V-CDT) for sulfur isotopes with 0.5‰ (1 σ) analytical uncertainty.
4. Halılar Cu-Pb (±Zn) Mineralization
The Halılar base metal mineralization represents Cu-Pb with some Zn brecciated-stockwork-veining-type mineralization. The mineralization is restricted to a fault gouge zone directed NE–SW, as well as along the lower boundary of the Sakarkaya and Düztarla granitoid rocks (Figure 2). It is also closely associated with intense hydrothermal alteration within the breccia and quartz stockwork veining (Figure 4a–d). Based on the field investigation and petrographic and mineralogical (XRD) data, the mineralized quartz veins and brecciated ore bodies are accompanied by two types of hydrothermal alteration zone with gradational boundaries: zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite) and zone-2 (calcite–epidote–albite ± chlorite ± sericite).
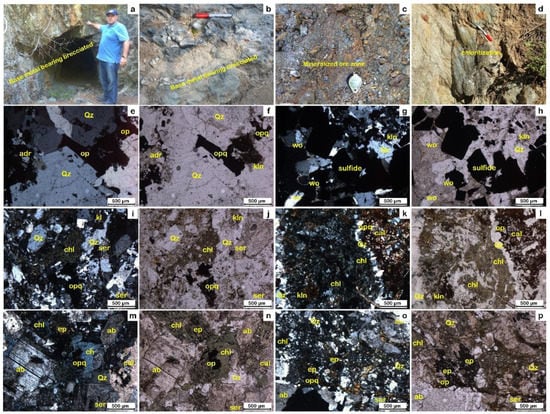
Figure 4.
(a,b) Brecciation and quartz stockwork veining along with intense hydrothermal alteration; (c) base -metal-bearing brecciated zone; (d) pervasive chlorite and epidote alteration in the host rock; (e–h) XPL and PPL photomicrographs of alteration zone-1 containing quartz, wollastonite, kaolinite, and andradite with calcite in a mineralized and brecciated quartz vein; (i–l) XPL and PPL photomicrographs of alteration zone-2 with a high amount of sericite, quartz, chlorite, kaolinite, and opaque minerals; (m–p) XPL and PPL photomicrographs of the calcite, epidote, albite, and quartz with chlorite in alteration zone-2. Abbreviations: calcite (cal), chlorite (chl), epidote (ep), kaolinite (kln), opaque (opq), quartz (qz), sericite (ser), and wollastonite (wo).
The ore zone is represented by mineralized and brecciated quartz stockwork veining (Figure 4a–c). It has high amounts of Cu (9.9 %), Pb (11.3 %), and Zn (0.29 %) mineralization, with high amounts of chalcopyrite and galena with sphalerite and pyrite (Figure 4a–c). It contains quartz with a subordinate amount of wollastonite, kaolinite, andradite, and calcite (Figure 4e–h and Figure 5 and Appendix A). These calc–silicate assemblages refer to the skarn that resulted from the metasomatism of sandy limestone in the Taşçıbayırı Formation in association with andradite (Figure 4e–h and Figure 5 and Appendix A). The XRD data show quartz (low), wollastonite (1A, manganoan), kaolinite (1A), microcline, calcite, chalcopyrite, andradite, anglesite, and cubanite (high) with smaller amounts of pyrite, sphalerite (ferrous), galena, and quartz (high) (Figure 5 and Appendix A).
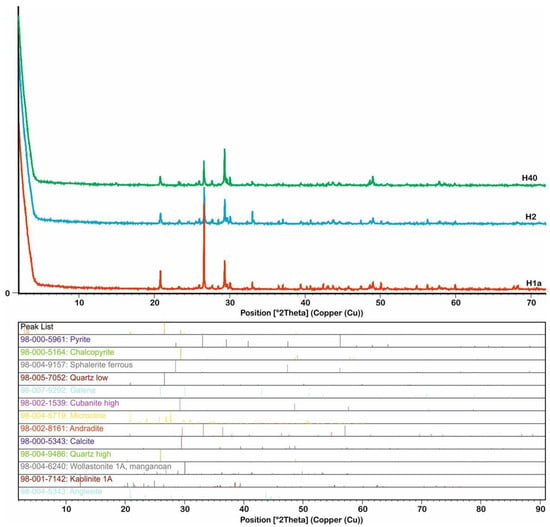
Figure 5.
XRD patterns of the representative samples from the ore zone, having quartz; wollastonite; kaolinite; microcline; calcite; chalcopyrite; andradite; anglesite; and cubanite with pyrite, sphalerite, galena.
4.1. Hydrothermal Alteration Types
The hydrothermal alterations associated with Cu-Pb-Zn mineralization include extensive silicification (Figure 4e–h), sulfidation (Figure 4e–h), and carbonatization (Figure 4k–n), with some chloritization (Figure 4i–p), sericitization (Figure 4i–p), and calc–silicate alteration (Figure 4e–p) distributed in two alteration zones around the mineralized orebodies.
Alteration zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite) forms the main alteration zone developed outwards from the ore zone and has high amounts of sericite and quartz, with lesser amounts of chlorite, kaolinite, and pyrite (Figure 4i–l and Figure 6 and Appendix B). It is characterized by the preferential replacement of the original K-feldspar and/or plagioclase–biotite by sericite/muscovite–kaolinite. XRD studies reveal a paragenesis of quartz (low), kaolinite (1A), clinochlore (1MIa), and sericite (2M1) with a subordinate amount of chamosite (1MIIb), pyrite, and chalcopyrite (Figure 6, Appendix B).
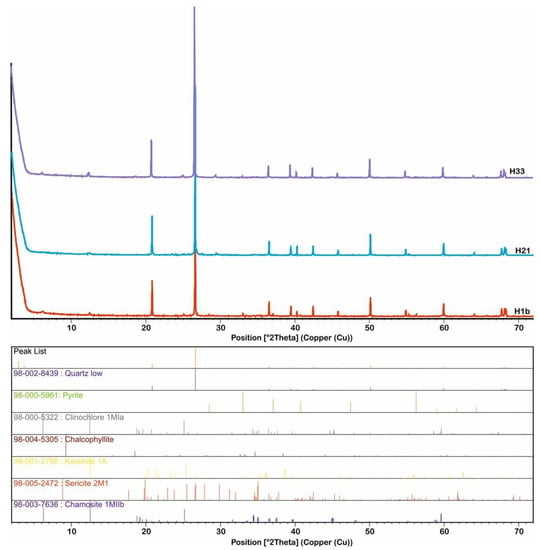
Figure 6.
XRD patterns of representative samples from alteration zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite).
Alteration zone-2 (calcite–epidote–albite ± chlorite ± sericite) represents the distal zone and has higher amounts of calcite, epidote, and albite, with a subordinate amount of chlorite (Figure 4m–p and Figure 7 and Appendix C). The sulfide minerals are less abundant in this zone (Figure 4d). The XRD data reveal that this alteration zone consists of quartz (low), albite (low), muscovite (2M1), clinochlore (IIb-4), microcline, sericite (2M1), and calcite, with lesser amounts of orthoclase, chamosite (1MIIb), and epidote (Figure 7 and Appendix C).
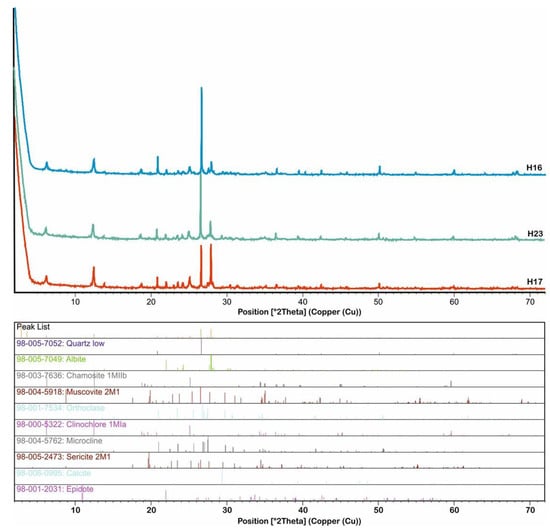
Figure 7.
XRD patterns of representative samples from alteration zone-2 (calcite–epidote–albite ± chlorite ± sericite).
4.2. Ore Mineralogy
The ore mineral assemblage includes chalcopyrite, galena, pyrite, and sphalerite with covellite and goethite in abundant gangue minerals such as quartz, sericite, chlorite, and calcite forming along the quartz stockwork veins as well as in the brecciated ore zones (Figure 4, Figure 5, Figure 6 and Figure 7 and Appendix A, Appendix B and Appendix C). Chalcopyrite and galena are the most common sulfide minerals in the ore bodies, occurring as yellow and whitish gray in color and with a subhedral granular texture (up to 2 mm), respectively (Figure 8a,b). Pyrite is either associated with or occurs as inclusions in chalcopyrite (Figure 8c,d). Sphalerite is characterized by dark gray coloring associated with chalcopyrite and pyrite, forming exsolution textures produced by chalcopyrite (Figure 8b,c,e). These minerals were developed in the main ore mineralization phase (Figure 9). On the other hand, the oxidation and supergene mineralization events represent the second phase of mineralization, including covellite and goethite formed after chalcopyrite and pyrite, respectively (Figure 8 and Figure 9).
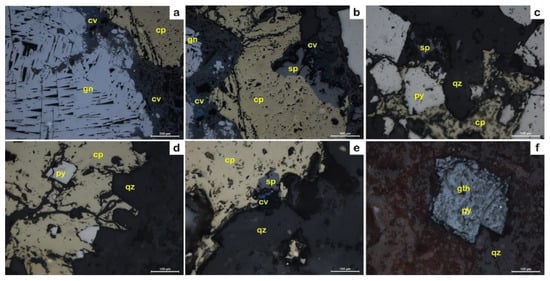
Figure 8.
PPL photomicrographs of reflected light microscopy. (a) Galena with triangular cleavage pits associated with chalcopyrite replaced by covellite. (b) Chalcopyrite with sphalerite, galena, pyrite, and covellite. (c–e) Pyrite associated with chalcopyrite and sphalerite in a mineralized quartz vein. (f) Goethite, the main alteration product after pyrite. Abbreviations: chalcopyrite (cp), covellite (cv), galena (gn), goethite (gth), pyrite (py), quartz (qz), and sphalerite (sp).
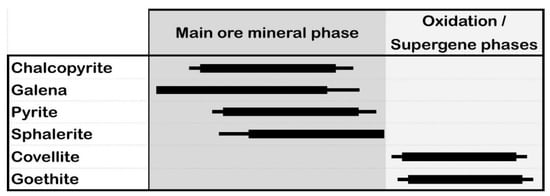
Figure 9.
Paragenetic sequence of mineralization phases in the Halılar area.
5. Geochemical Characteristics
5.1. Geochemistry of the Least-Altered Metasediments
Ten representative samples collected from the least-altered metasediments of the Sakarkaya Formation were analyzed for major, trace, and rare-earth element contents (Table 1). Samples from the metasandstones are classified as mainly wackes and, rarely, Fe-sand and Fe-shale based on the geochemical classification of the terrigenous sandstones and shales by Herron [36] (Figure 10a). The samples have SiO2/Al2O3 ratios ranging from 2.7 to 5.5 with an average of 4.3, which are similar to upper continental crust (UCC) [37] (~4.3 SiO2/Al2O3 ratio), suggesting that they were sourced from the crustal felsic rocks. It also appears in Figure 10b,c that the Sakarkaya metasediments have acidic/intermediate characteristics, which lie mostly in the field of the metavolcanic tuffs, metagreywackes, and arkosic sands [38] according to their low K/Rb ratios (mean = 312.8). In the F1-F2 classification diagram (Figure 10d), the metasediments are mostly comparable with the compositional characteristics of the P4-quartoze sedimentary provenance that form within the passive and active continental margins (Figure 10e) due to recycling from old sedimentary rocks derived from highly weathered felsic terrains. The metasandstones have low total rare earth element contents (∑REE) (up to 145.14 ppm with an average of 88.96 ppm), ∑REE/∑HREE = 6.59–10.43 ppm, (La/Yb)N = 5.38–14.29 ppm, and positive Eu anomaly (Eu/Eu* = 0.68–1.27 ppm) that are similar to the upper continental crust (UCC) of Taylor and McLennan [37] (Figure 10f).

Table 1.
The major oxides and trace and rare-earth elements (REE) of metasediments in the Sakarkaya Formation.
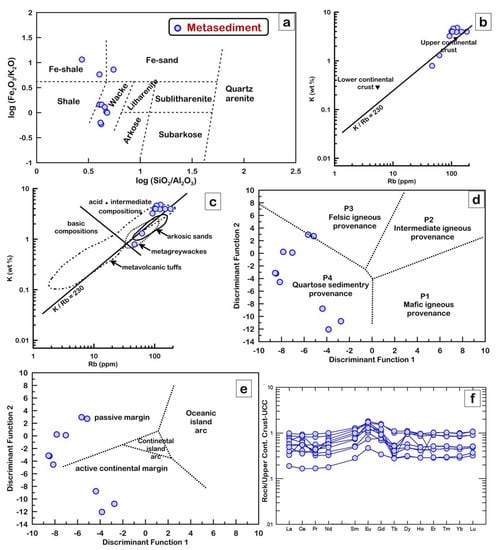
Figure 10.
Geochemical classification diagrams of metasediments: (a) log(Fe2O3/K2O)−log(SiO2/Al2O3) diagram after Herron [36]. (b,c) K − Rb diagrams after Floyd and Leveridge [38]. Fields of unmetamorphosed arkosic sands after van de Kamp et al. [39], low−grade metagreywackes after Condie et al. [40] and Caby et al. [41], and higher-grade metavolcanic tuffs after van de Kamp [42]; (d) plot of samples in discriminant functions F1 vs. F2 (provenance fields are after Roser and Korsch [43]; (e) plot of discriminant scores along Function 1 vs. 2 after Bhatia [44]; (f) upper continental crust (UCC)−normalized REE patterns [37].
5.2. Alteration Geochemistry
Two main alteration zones surround the Cu-Pb±Zn-bearing ore mineralization in the Halılar area. These are represented by zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite) and zone-2 (calcite–epidote–albite ± chlorite ± sericite), and they were analyzed for major, trace, and REEs (Table 2). Based on the alteration index (AI) [45] and advanced argillic alteration index (AAAI) of Williams and Davidson [46], samples from each zone show opposite alteration trends (Figure 11a). The ore zone and alteration zone-1 fall along the trend of silicification/potassic alteration, while alteration zone-2 falls along the carbonation/chloritization alteration trend (Figure 11a). Based on the alteration boxplot relationship between the chlorite–carbonate–pyrite index (CCPI) of Large et al. [47] and the AI of Ishikawa et al. [45], the samples of the ore zone and zone-1 are clustered in the field of strongly altered rock, having chlorite–sericite–pyrite alteration while the ore zone is affected by extensive pyritization (Figure 11b). On the other hand, zone-2, within the carbonate-altered host rock field, shows Mn–carbonate–sericite–chlorite alteration (Figure 11b).

Table 2.
Major oxides and trace and rare-earth elements (REE) of the ore zone and alteration zones 1 and 2 in the Halılar area.
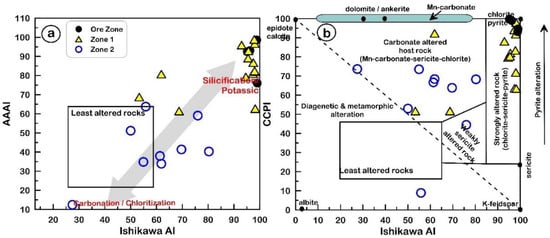
Figure 11.
(a) AI [45] vs. AAAI [46]; (b) AI [45] vs. CCPI [47] of the studied alteration samples from the Halılar area.
5.3. Mass Balance Calculations
The behavior of different elements, excluding immobile ones, is changeable during hydrothermal alteration processes depending on their volume changes and their mass transfer [48,49]. Gresens [33] and Grant [34,35] used mass-balance calculations to quantify hydrothermal alteration effects on the host rock within the mineralized regions and to determine the relative gain and loss of the various major and trace elements during hydrothermal alteration.
Based on the trace element geochemical analyses, the ore zone and alteration zone-1 have high amounts of Cu and Pb, with an average of 9.9% and 11.3%, respectively, for the ore zone, and 0.32% and 0.12%, respectively, for zone-1 (Table 2). They are classified as a Cu-Pb type (Figure 12), which refers to the high concentrations of chalcopyrite and galena. Alteration zone-2 represents the Cu-Pb-Zn type (Figure 12), having low Cu, Pb, and Zn contents, with averages of 28.18ppm, 47.95ppm, and 98.07ppm, respectively.
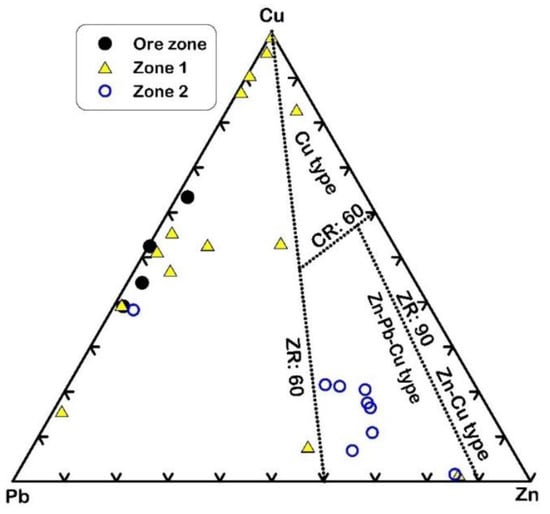
Figure 12.
Metal content classification diagram after Large [50]. The ratios ZR = (100 Zn/(Zn + Pb) and CR = 100 Cu/(Cu + Zn) are based on the average ore grades in mass percent after Large [50].
Al2O3 and TiO2 are immobile in all alteration zones during hydrothermal alteration; therefore, they were selected to assess the chemical changes due to the process of hydrothermal alteration by using the GEOISO-Windows software developed by Coelho [32]. The results of these calculations are illustrated through the isocon diagrams of Grant [34] and show the different patterns of major and trace element gains and losses (Figure 13 and Figure 14 and Table 3). The samples from zone-1 are rich in SiO2, Fe2O3, K2O, and LOI, with lesser increases in the amount of CaO, P2O5, and MnO (Figure 14a). Gains in Ag, As, Cu, Mo, Pb, S, Sb, and Zn are also recognized within this alteration zone (Figure 14b). This zone is characterized by higher amounts of sulfur and iron, with variable copper, lead, and zinc contents reflecting high pyritization, with the main base metals providing higher mass (MC = 170.42) and volume change (VC = 182.1) (Table 3). SiO2 and K2O increases reflect high silicification and sericitization, which are comparable with the petrographic and mineralogical (XRD) data. In zone-2, CaO, Na2O, P2O5, TiO2, LOI, and carbon are enriched, reflecting calcite, epidote, and albite alterations (Figure 14c). The loss of Cu, Pb, and Zn is observed in this zone, providing lower MC (−3.18) and VC (−1.80) values (Figure 14d and Table 3).
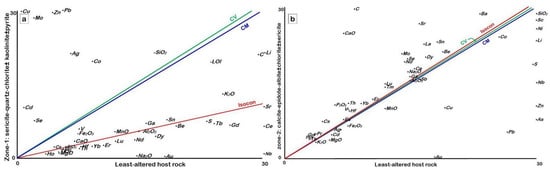
Figure 13.
Isocon diagram comparing the mean composition of least-altered samples and altered samples from (a) alteration zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite) and (b) alteration zone-2 (calcite–epidote–albite ± chlorite ± sericite).
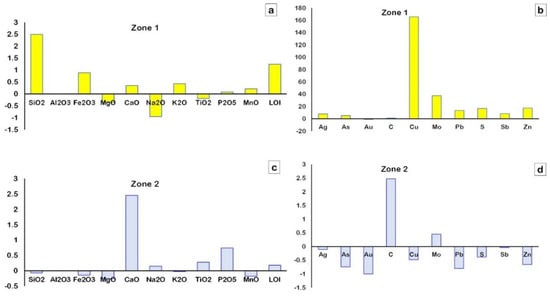
Figure 14.
Gain/loss of major oxides (wt. %) (a,c) and trace elements (ppm) (b,d) in the alteration zones during hydrothermal alteration based on the mean data of the representative least−altered samples as a reference for calculations.

Table 3.
Element/oxide mass changes in relation to the original whole-rock mass ((Mfi-Moi)/Mo) and in relation to the original element/oxide mass in the original rock ((Mfi-Moi)/Moi).
6. Sulfur Isotope (δ34S)
δ34S isotopic data from the sulfide-bearing ore deposits were obtained to determine the source of the sulfur and the origin of the sulfur-bearing fluids [51]. The δ34S isotope values of ten pyrite, chalcopyrite, and galena samples collected from the highly altered and mineralized altered metasediments host rocks are in the range of −1.1 to −0.1‰VCDT (n = 3), −2.7 to −0.5 ‰VCDT (n = 3), and −3.5 to −2.1‰VCDT (n = 4), respectively (Table 4). Pyrites from a quartz vein have an average δ34S of 0.4‰VCDT (Table 4 and Figure 15a). By assuming the H2S as the sulfur species in solution, and based on the fractionation equations of Czamanske and Rye [52] and Ohmoto and Rye [51], the δ34SH2S values of the fluid have a narrow range of −2.54 to −0.08 ‰ VCDT (Table 4 and Figure 15b).

Table 4.
Sulfur isotope values of sulfides from the Halılar area.
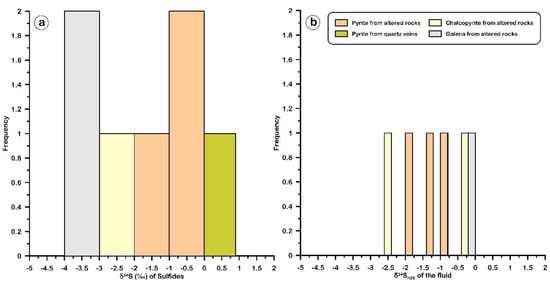
Figure 15.
Histograms of (a) the δ34S isotopic compositions for sulfide minerals (pyrite, chalcopyrite, and galena) and (b) the δ34SH2S of the fluid that formed the sulfides in the Halılar area.
7. Discussion
7.1. Sources of Sulfur
There are many sulfur sources with distinct δ34S values: (1) the mantle source has a 0 ± 3‰ δ34S value [53]; (2) the magmatic source, in which the sulfur resulted from desulfidation and/or dissolution or from magmatic sulfides, has 0 to +9‰ δ34S [54]; (3) the seawater sources have a mean value of +20 ‰ δ34S; and (4) the strongly reduced sulfur source in the sedimentary rocks has very negative δ34S values [55].
In the Halılar area, the mean δ34S value of the sulfides is close to −1.62‰, suggesting a uniform magmatic sulfur source in which the sulfur originates either from the leaching and remobilization of the old magmatic sulfide or from the mantle source (Figure 16). Furthermore, the δ34S values of the studied sulfide minerals decrease from pyrite (−1.1 to 0.4 ‰VCDT) and chalcopyrite (−2.7 to −0.5 ‰VCDT) to galena (−3.5 to −2.1‰VCDT) (Figure 17), which is compatible with the suggested trend of differentiation of Ohmoto and Rye [51]. Thus, the ore-bearing fluid appears to have a magmatic (mantle) source [51] with magmatic–hydrothermal signatures [56] (Figure 18).
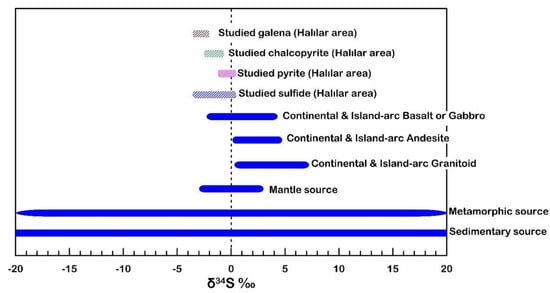
Figure 16.
δ34S values of sulfides from the mantle, the continental igneous setting, and metamorphic and sedimentary sources. Mantle source [53], island arc basalts and gabbros [57,58], andesites [59,60], granitoids [61,62,63], and metamorphic and sedimentary sources [64].
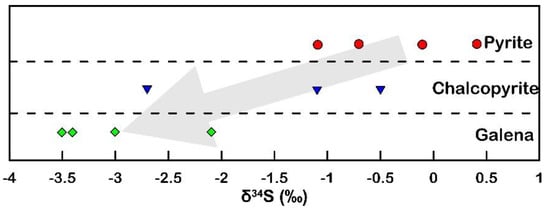
Figure 17.
Distribution of the δ34S values in the studied sulfide minerals from the Halılar area.
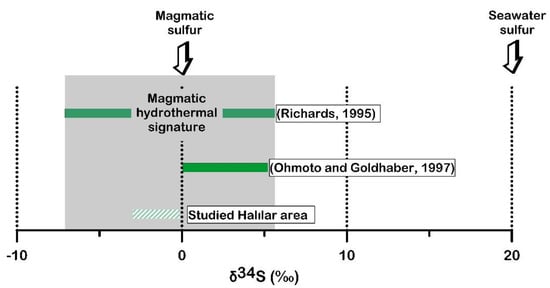
Figure 18.
δ34S values from the Halılar area compared with the magmatic−hydrothermal deposits [51,56].
7.2. Metal Source
The metasediments of the Sakarkaya Formation that host the Halılar Cu-Pb (±Zn) mineralization are slightly enriched in metallic elements (average of Ag = 6.7 ppm, As = 101.9 ppm, Au = 0.04 ppm, Cu = 53.8 ppm, Mo = 1.8 ppm, Pb = 274.7 ppm, S = 389.0 ppm, Sb = 2.0 ppm, and Zn = 371.3 ppm) relative to the average UCC (Table 5). Moreover, the contents of the metallic elements in the Düztarla granitoid rocks also show higher values than typical UCC (mean values of Ag = 1.14 ppm, As = 84.05 ppm, Au = 0.36 ppm, Cu = 368.91 ppm, Mo = 324.68 ppm, Pb = 49.52 ppm, S = 1396.7 ppm, Sb = 2.34 ppm, and Zn = 414.69 ppm).

Table 5.
Upper continental crust (UCC)–normalized elements of metasediments in Sakarkaya Formation and Düztarla granitoid rocks.
When the sulfur is normalized to the UCC of Rudnick et al. [65], it is highly rich in granitoid rocks, but not in metasediments (Figure 19a,b). Thus, the primary metal suppliers appear to be the metasediments and intrusive Düztarla granitoid magmatism; together, they account for the metals in the Halılar brecciated-stockwork-type mineralization. Based on the geologic features and mode of occurrences, the Halılar metasediment-hosted Cu-Pb (±Zn) mineralization appears to be formed by epigenetic hydrothermal processes after sedimentation/diagenesis and metamorphism.
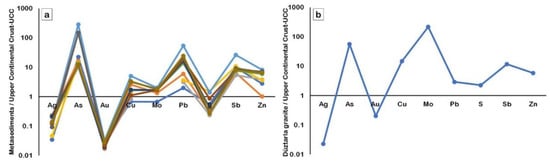
Figure 19.
Upper continental crust (UCC)–normalized elements diagram of the metasediments (a) and Düztarla granitoid (b). After Taylor and McLennan [37] and Rudnick et al. [65].
8. Conclusions
The Halılar area contains two groups: the clastic Halılar Group that overlies the metamorphics of the pre-Late Triassic age or Permian limestones and the Bilecik Group. The Halılar Group consists of the Bağcağız and Sakarkaya Formations, and the Bilecik Group is represented by two formations, including the Taşçıbayırı Formation and the Günören Limestone. The Sakarkaya and Bağcağız Formations were later intruded by Oligo–Miocene Düztarla granitoid rocks.
The Halılar base metal mineralization consists mainly of Cu-Pb sulfide with some Zn sulfide in the brecciated stockworks and veins. This type of vein mineralization is restricted to a fault gouge zone directed NE–SW and along the lower contact of the Sakarkaya and Düztarla granitic rocks. Two types of hydrothermal alteration zones with gradual boundaries can be observed in the main ore zone. These include zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite) and zone-2 (calcite–epidote–albite ± chlorite ± sericite). The main ore mineral assemblage consists of chalcopyrite, galena, pyrite, and sphalerite in an abundant amount of gangue minerals such as quartz, sericite, chlorite, and calcite forming along the quartz stockwork veins, as well as in the brecciated ore zones. The other oxidation and supergene mineralization includes covellite and goethite formed after chalcopyrite and pyrite, respectively.
The least-altered Sakarkaya metasediments are classified mainly as wackes and, rarely, Fe-sand and Fe-shale, which are relatively similar in chemical composition to the upper continental crust (UCC). They are sourced from the crustal felsic rocks and a quartzose sedimentary provenance formed within the passive and active continental margins. Mass-balance calculations reveal that the samples of zone-1 are enriched in SiO2, Fe2O3, K2O, and LOI, with Ag, As, Cu, Mo, Pb, S, Sb, and Zn reflecting a high degree of pyritization with sericitization and silicification. On the other hand, the samples of zone-2 show an increase in CaO; Na2O; P2O5; TiO2; LOI; and carbon-reflecting calcite, epidote, and albite alterations.
The mean δ34S value of the sulfides in the Halılar area is close to −1.62‰, suggesting a uniform magmatic sulfur source in which the sulfur originates either from leaching and remobilization from the old magmatic sulfide or from the mantle source. There is also a sulfur isotope having a differentiation trend from pyrite to galena. The ore-bearing fluid has δ34S values of H2S, ranging from −2.54 to −0.08 ‰, typical of a magmatic–hydrothermal signature [47].
Based on the normalization of the metallic elements in the Sakarkaya metasediments and Düztarla granitoid rocks to the UCC [38] and [65], these metasediments and granitoid rocks represent the primary source of metals forming the Halılar brecciated-stockwork-veining-type mineralization. Overall, the geologic features and the mode of occurrences of the Halılar metasediment-hosted Cu-Pb (±Zn) mineralization suggest that they were formed by epigenetic hydrothermal processes after sedimentation/diagenesis and metamorphism.
Funding
This work was supported by the BAP Project (No. 37872) of Istanbul Technical University (ITU, Turkey).
Data Availability Statement
The data presented in this study are available in this article and its Appendix A, Appendix B and Appendix C.
Acknowledgments
The author would like to acknowledge the assistance of members of the Geochemistry Research Laboratories at Istanbul Technical University (Turkey). Great appreciation goes to M. Kumral (ITU, Turkey) and A. Abdelnasser (Benha University) for their support during all stages of work on the article. Z. Doner (ITU, Turkey) and A. Unal (ITU, Turkey) are also thanked for their help during fieldwork. The help of F. Yavuz (ITU, Turkey) and G. Ustunisik (South Dakota School of Mines, USA) were highly appreciated during the review of the article. The editor and two anonymous reviewers are thanked for carefully reading the manuscript and for their constructive comments.
Conflicts of Interest
The author declares no conflict of interest.
Appendix A

Table A1.
XRD analyses of representative samples from the ore zone.
Table A1.
XRD analyses of representative samples from the ore zone.
| No. | Ref. Code | Compound Name | Chemical Formula | SemiQuant [%] | Average | |||
|---|---|---|---|---|---|---|---|---|
| H1a | H2 | H40 | H25 | |||||
| 1 | 98-005-7052 | Quartz low | O2 Si1 | 35 | 16 | 13 | 51 | 28.75 |
| 2 | 98-004-6240 | Wollastonite 1A, manganoan | Ca2.88 Mn0.12 O9 Si3 | 8 | 11 | 25 | 19 | 15.75 |
| 3 | 98-001-7142 | Kaolinite 1A | H4 Al2 O9 Si2 | 10 | 13 | 16 | 9.75 | |
| 4 | 98-004-5719 | Microcline | Al1 K1 O8 Si3 | 18 | 9 | 1 | 7.00 | |
| 5 | 98-000-5164 | Chalcopyrite | Cu1 Fe1 S2 | 6 | 6 | 13 | 1 | 6.50 |
| 6 | 98-000-5343 | Calcite | C1 Ca1 O3 | 3 | 17 | 5 | 6.25 | |
| 7 | 98-002-8161 | Andradite | Ca3 Fe2 O12 Si3 | 5 | 5 | 7 | 4.25 | |
| 8 | 98-004-5343 | Anglesite | O4 Pb1 S1 | 4 | 4 | 5 | 3 | 4.00 |
| 9 | 98-000-5961 | Pyrite | Fe1 S2 | 4 | 4 | 2 | 6 | 4.00 |
| 10 | 98-002-1539 | Cubanite high | Cu0.3333 Fe0.6667 S1 | 1 | 4 | 7 | 3.00 | |
| 11 | 98-004-9157 | Sphalerite ferrous | Fe0.215 S1 Zn0.785 | 1 | 2 | 1 | 4 | 2.00 |
| 12 | 98-008-5367 | Albite | Al1 Na1 O8 Si3 | 1 | 5 | 1 | 1.75 | |
| 13 | 98-007-8263 | Biotite 1M | H1.47 Al1.92 F1.98 Fe2.59 K2 Mg3.15 Mn0.09 O21.47 Si5.98 Ti0.27 | 6 | 1.50 | |||
| 14 | 98-007-9292 | Galena | Pb1 S1 | 1 | 1 | 2 | 1 | 1.25 |
| 15 | 98-004-9486 | Quartz high | O2 Si1 | 2 | 1 | 1 | 1.00 | |
| 16 | 98-002-2578 | Halite | Br0.8947 Cl0.1053 Na1 | 1 | 2 | 1 | 1.00 | |
| 17 | 98-001-7351 | Goethite | H1 Fe1 O2 | 4 | 1.00 | |||
| 18 | 98-006-2562 | Chalcocite high | Cu2 S1 | 3 | 0.75 | |||
| 19 | 98-005-2707 | Clinochlore IIb-2 | H2 Al2 Mg5 O15 Si3 | 2 | 0.50 | |||
| 20 | 98-002-1503 | Barite high | Ba1 O4 S1 | 1 | 0.25 | |||
Appendix B

Table A2.
XRD analyses of representative samples from alteration zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite).
Table A2.
XRD analyses of representative samples from alteration zone-1 (sericite–quartz–chlorite ± kaolinite ± pyrite).
| No. | Ref. Code | Compound Name | Chemical Formula | SemiQuant [%] | Average | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1b | H3.1 | H3.2 | H5 | H6 | H7 | H8 | H9 | H11 | H12 | H13 | H21 | H26 | H28 | H31 | H33 | |||||
| 1 | 98-002-8439 | Quartz low | O2 Si1 | 48 | 66 | 83 | 51 | 49 | 56 | 53 | 84 | 64 | 65 | 15 | 58 | 38 | 84 | 63 | 46 | 57.69 |
| 2 | 98-001-1941 | Muscovite 2M1 | H2 Al3 K1 O12 Si3 | 40 | 25 | 32 | 12 | 4 | 2 | 10 | 31 | 25 | 11.31 | |||||||
| 3 | 98-001-2798 | Kaolinite 1A | H4 Al2 O9 Si2 | 9 | 18 | 4 | 15 | 15 | 4 | 4 | 4 | 14 | 4 | 7 | 6.13 | |||||
| 4 | 98-001-7363 | Microcline | Al1 K1 O8 Si3 | 6 | 5 | 2 | 6 | 6 | 5 | 4 | 17 | 7 | 10 | 6 | 5 | 4.94 | ||||
| 5 | 98-000-5322 | Clinochlore 1MIa | H8 Al3.3 Fe1.65 Mg2.5 O18 Si2.2 | 4 | 4 | 2 | 2 | 5 | 4 | 4 | 4 | 4 | 5 | 3 | 14 | 14 | 4.31 | |||
| 6 | 98-005-2472 | Sericite 2M1 | H2 Al2.75 Ca0.011 Fe0.032 K0.727 Mg0.022 Na0.17 O12 Si3.128 Ti0.02 | 36 | 22 | 10 | 1 | 4.31 | ||||||||||||
| 7 | 98-001-7679 | Orthoclase | Al1 K0.94 Na0.06 O8 Si3 | 4 | 14 | 3 | 16 | 1 | 2.38 | |||||||||||
| 8 | 98-003-7636 | Chamosite 1MIIb | H16 Al5.024 Fe4.964 Mg5.036 O36 Si5.7 | 4 | 4 | 7 | 5 | 4 | 7 | 1.94 | ||||||||||
| 9 | 98-003-4846 | Albite | Al1 Ge2 Na1 O8 Si1 | 1 | 3 | 1 | 8 | 3 | 1 | 1.06 | ||||||||||
| 10 | 98-002-3975 | Calcite | C1 Ca1 O3 | 2 | 1 | 2 | 6 | 1 | 2 | 1 | 1 | 1.00 | ||||||||
| 11 | 98-002-3340 | Pyrite | Fe1 S2 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0.94 | |||
| 12 | 98-007-8271 | Biotite 1M | H1.47 Al1.92 F1.98 Fe2.59 K2 Mg3.15 Mn0.09 O21.47 Si5.98 Ti0.27 | 7 | 3 | 1 | 4 | 0.94 | ||||||||||||
| 13 | 98-000-5164 | Chalcopyrite | Cu1 Fe1 S2 | 1 | 2 | 1 | 1 | 1 | 1 | 0.44 | ||||||||||
| 14 | 98-006-1164 | Andradite | Ca3 Fe2 O12 Si3 | 6 | 0.38 | |||||||||||||||
| 15 | 98-008-5911 | Dolomite | C2 Ca1 Mg1 O6 | 2 | 1 | 1 | 2 | 0.38 | ||||||||||||
| 16 | 98-004-5305 | Chalcophyllite | H48 Al1 As2 Cu9 O44 S1.5 | 2 | 1 | 1 | 1 | 0.31 | ||||||||||||
| 17 | 98-004-1521 | Magnetite | Fe3 O4 | 1 | 1 | 1 | 1 | 1 | 0.31 | |||||||||||
| 18 | 98-005-1007 | Sphalerite | Fe0.372 S1 Zn0.628 | 1 | 1 | 1 | 1 | 0.25 | ||||||||||||
| 19 | 98-001-7247 | Melanterite | H14 Fe1 O11 S1 | 2 | 1 | 0.19 | ||||||||||||||
| 20 | 98-002-1503 | Barite high | Ba1 O4 S1 | 1 | 1 | 0.13 | ||||||||||||||
| 21 | 98-001-7371 | Galena | Pb1 S1 | 1 | 0.06 | |||||||||||||||
| 22 | 98-005-6956 | Phengite 3T | H1.2 Al1.4 F0.8 Fe0.04 K1 Mg0.75 O11.2 Si3.81 | 1 | 0.06 | |||||||||||||||
| 23 | 98-002-8127 | Ankerite | C2 Ca0.997 Fe0.676 Mg0.273 Mn0.054 O6 | 1 | 0.06 | |||||||||||||||
| 24 | 98-002-2570 | Halite, bromian | Br0.1018 Cl0.8982 Na1 | 1 | 0.06 | |||||||||||||||
Appendix C

Table A3.
XRD analyses of representative samples from alteration zone-2 (calcite–epidote–albite ± chlorite ± sericite).
Table A3.
XRD analyses of representative samples from alteration zone-2 (calcite–epidote–albite ± chlorite ± sericite).
| No. | Ref. Code | Compound Name | Chemical Formula | SemiQuant [%] | Average | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H4 | H10 | H16 | H17 | H19 | H23 | H27 | H29 | H30 | H32 | H41 | |||||
| 1 | 98-004-6436 | Albite low | Al1.005 Na0.986 O8 Si2.995 | 29 | 26 | 14 | 31 | 16 | 19 | 11 | 16 | 16 | 29 | 16 | 20.3 |
| 2 | 98-005-4829 | Quartz low | O2 Si1 | 26 | 7 | 21 | 10 | 12 | 18 | 17 | 8 | 8 | 16 | 38 | 16.5 |
| 3 | 98-004-5914 | Muscovite 2M1 | H2 Al2.9 K1 O12 Si3.1 | 31 | 13 | 2 | 9 | 13 | 17 | 4 | 12 | 1 | 2 | 5 | 9.9 |
| 4 | 98-003-4690 | Clinochlore IIb-4 | H8 Al1.7 Fe0.33 Mg4.95 O18 Si3.02 | 12 | 10 | 14 | 12 | 11 | 7 | 5 | 17 | 14 | 5 | 9.7 | |
| 5 | 98-001-7363 | Microcline | Al1 K1 O8 Si3 | 10 | 9 | 8 | 11 | 9 | 7 | 7 | 7 | 16 | 12 | 8.7 | |
| 6 | 98-005-2473 | Sericite 2M1 | Al2.724 Ca0.011 Fe0.032 K0.776 Mg0.022 Na0.181 O11 Si3.148 Ti0.02 | 8 | 6 | 9 | 28 | 8 | 22 | 7.4 | |||||
| 7 | 98-001-8053 | Anorthite | Al2 Ca1 O8 Si2 | 15 | 10 | 20 | 20 | 12 | 7.0 | ||||||
| 8 | 98-001-2962 | Kaolinite 1A | H4 Al2 O9 Si2 | 6 | 15 | 5 | 12 | 14 | 8 | 3 | 5.7 | ||||
| 9 | 98-005-7051 | Illite 2M1 | H3 Al4 K1 O12 Si2 | 20 | 18 | 3.5 | |||||||||
| 10 | 98-004-5295 | Chlorite IIb+4, chromian | H8 Al1.75 Cr0.25 Mg5 O18 Si3 | 14 | 18 | 2.9 | |||||||||
| 11 | 98-006-0995 | Calcite | C1 Ca1 O3 | 1 | 1 | 2 | 6 | 13 | 2 | 2.3 | |||||
| 12 | 98-001-1876 | Lepidolite 6M | H1 Al1 F1 K1 Mg3 O11 Si3 | 13 | 9 | 2.0 | |||||||||
| 13 | 98-001-7534 | Orthoclase | Al1 K1 O8 Si3 | 9 | 7 | 1.5 | |||||||||
| 14 | 98-003-7636 | Chamosite 1MIIb | H16 Al5.024 Fe4.964 Mg5.036 O36 Si5.7 | 9 | 0.8 | ||||||||||
| 15 | 98-002-2747 | Titanite | Ca1 O5 Si1 Ti1 | 5 | 0.5 | ||||||||||
| 16 | 98-001-2031 | Epidote | H1 Al2 Ca2 Fe1 O13 Si3 | 6 | 0.5 | ||||||||||
| 17 | 98-005-0411 | Dolomite | C2 Ca1 Mg1 O6 | 1 | 1 | 0.2 | |||||||||
| 18 | 98-001-2313 | Phlogopite 3T | H1 Al1 F1 K1 Mg3 O11 Si3 | 2 | 0.2 | ||||||||||
| 19 | 98-000-6129 | Bornite | Cu4.98 Fe1.02 S4 | 1 | 0.1 | ||||||||||
| 20 | 98-007-0040 | Hematite | Fe2 O3 | 1 | 0.1 | ||||||||||
| 21 | 98-004-1521 | Magnetite | Fe3 O4 | 1 | 0.1 | ||||||||||
| 22 | 98-003-8836 | Phengite 3T | H2 Al1.848 K1 Mg0.58 O12 Si3.572 | 1 | 0.1 | ||||||||||
| 23 | 98-001-7325 | Gypsum | H4 Ca1 O6 S1 | 1 | 0.1 | ||||||||||
| 24 | 98-002-8130 | Ankerite | C2 Ca0.997 Fe0.676 Mg0.273 Mn0.054 O6 | 1 | 0.1 | ||||||||||
References
- Rose, A.W.; Burt, D.M. Hydrothermal alteration. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1979; pp. 173–227. [Google Scholar]
- Meyer, C.; Hemley, J.J. Wall rock alteration. Geochem. Hydrothermal Ore Depos. 1967, 1, 166–235. [Google Scholar]
- Creasey, S.C. Some phase relations in the hydrothermally altered rocks of porphyry copper deposits. Econ. Geol. 1959, 54, 351–373. [Google Scholar] [CrossRef]
- Lowell, J.D.; Guilbert, J.M. Lateral and Vertical Alteration-Mineralization Zoning in Porphyry Ore Deposits. Econ. Geol. 1970, 65, 373–408. [Google Scholar] [CrossRef]
- Beane, R.; Bodnar, R. Hydrothermal fluids and hydrothermal alteration in porphyry copper deposits: Arizona Geological Society Digest. Porphyry Copp. Depos. Am. Cordill. 1995, 20, 83–93. [Google Scholar]
- Sabins, F.F. Remote sensing for mineral exploration. Ore Geol. Rev. 1999, 14, 157–183. [Google Scholar] [CrossRef]
- Watanabe, Y.; Hedenquist, J.W. Mineralogic and stable isotope zonation at the surface over the El Salvador porphyry copper deposit, Chile. Econ. Geol. 2001, 96, 1775–1797. [Google Scholar] [CrossRef]
- Seedorff, E.; Dilles, J.H.; Proffett, J.M.; Einaudi, M.T.; Zurcher, L.; Stavast, W.J.; Johnson, D.A.; Barton, M.D. Porphyry deposits: Characteristics and origin of hypogene features. Econ. Geol. 2005. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Tayor, H. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposit. Econ. Geol. 1974, 69, 843–883. [Google Scholar]
- Gustafson, L.B.; Hunt, J.P. The porphyry copper deposit at El Salvador, Chile. Econ. Geol. 1975, 70, 857–912. [Google Scholar] [CrossRef]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. Rev. Mineral. Geochem. 1986, 16, 491–559. [Google Scholar]
- Bowman, J.; Parry, W.; Kropp, W.; Kruer, S. Chemical and isotopic evolution of hydrothermal solutions at Bingham, Utah. Econ. Geol. 1987, 82, 395–428. [Google Scholar] [CrossRef]
- Norman, D.; Parry, W.; Bowman, J.R. Petrology and geochemistry of propylitic alteration at Southwest Tintic, Utah. Econ. Geol. 1991, 86, 13–28. [Google Scholar] [CrossRef]
- Dilles, J.H.; Solomon, G.C.; Taylor, H.P.; Einaudi, M.T. Oxygen and hydrogen isotope characteristics of hydrothermal alteration at the Ann-Mason porphyry copper deposit, Yerington, Nevada. Econ. Geol. 1992, 87, 44–63. [Google Scholar] [CrossRef]
- Clark, A. Are outsize porphyry copper deposits either anatomically or environmentally distinctive? In Giant Ore Deposits; Society of Economic Geologists: Littleton, CO, USA, 1993. [Google Scholar]
- Zaluski, G.; Nesbitt, B.; Muehlenbachs, K. Hydrothermal alteration and stable isotope systematics of the Babine porphyry Cu deposits, British Columbia; implications for fluid evolution of porphyry systems. Econ. Geol. 1994, 89, 1518–1541. [Google Scholar] [CrossRef]
- Wilson, A.J.; Cooke, D.R.; Harper, B.J.; Deyell, C.L. Sulfur isotopic zonation in the Cadia district, southeastern Australia: Exploration significance and implications for the genesis of alkalic porphyry gold-Copper deposits. Miner. Depos. 2007, 42, 465–487. [Google Scholar] [CrossRef]
- Urqueta, E.; Kyser, T.K.; Clark, A.H.; Stanley, C.R.; Oates, C.J. Lithogeochemistry of the Collahuasi porphyry Cu–Mo and epithermal Cu–Ag (–Au) cluster, northern Chile: Pearce element ratio vectors to ore. Geochem. Explor. Environ. Anal. 2009, 9, 9–17. [Google Scholar] [CrossRef]
- Lentz, D.R. Exchange reactions in hydrothermally altered rocks: Examples from biotite-bearing assemblages. Alteration and alteration processes associated with ore-forming systems. Geol. Assoc. Can. Short Course Notes 1994, 11, 69–99. [Google Scholar]
- Reed, M.H. Hydrothermal alteration and its relationship to ore fluid composition. Geochem. Hydrothermal Ore Depos. 1997, 3, 303–365. [Google Scholar]
- Schwartz, G.M. Hydrothermal alteration. Econ. Geol. 1959, 54, 161–183. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems, 1st ed.; Springer: Heidelberg, Germany, 2009; p. 1250. [Google Scholar]
- Gifkins, C.; Herrmann, W.; Large, R.R. Altered Volcanic Rocks: A Guide to Description and Interpretation; Centre for Ore Deposit Research, University of Tasmania: Hobart, Australia, 2005. [Google Scholar]
- Janković, S. The copper deposits and geotectonic setting of the Thethyan Eurasian metallogenic belt. Miner. Depos. 1977, 12, 37–47. [Google Scholar] [CrossRef]
- Altiner, D.; Kocyigit, A.; Farinacci, A.; Nicosia, U.; Conti, M. Jurassic-Lower Cretaceous stratigraphy and paleogeographic evolution of the southern part of north-western Anatolia (Turkey). Geol. Romana 1991, 27, 13–80. [Google Scholar]
- Yigit, O. Mineral deposits of Turkey in relation to Tethyan metallogeny: Implications for future mineral exploration. Econ. Geol. 2009, 104, 19–51. [Google Scholar] [CrossRef]
- Krushensky, R.; Akcay, Y.; Karaege, E. Geology of the Karalar-Yesiler Area, Northwest Anatolia, Turkey; US Geological Survey: Reston, VA, USA, 1974; pp. 1258–2331. [Google Scholar]
- Şengör, A.C.; Yilmaz, Y. Tethyan evolution of Turkey: A plate tectonic approach. Tectonophysics 1981, 75, 181–241. [Google Scholar] [CrossRef]
- Şengör, A.; Yılmaz, Y.; Sungurlu, O. Tectonics of the Mediterranean Cimmerides: Nature and evolution of the western termination of Palaeo-Tethys. Geol. Soc. Lond. Spec. Publ. 1984, 17, 77–112. [Google Scholar] [CrossRef]
- Carr, M. Igpet 2007 for Windows XP or Vista, Terra Softa Inc.: Newark, NJ, USA, 2007.
- Coelho, J. GEOISO—A Windows™ program to calculate and plot mass balances and volume changes occurring in a wide variety of geologic processes. Comput. Geosci. 2006, 32, 1523–1528. [Google Scholar] [CrossRef]
- Gresens, R.L. Composition-Volume relationships of metasomatism. Chem. Geol. 1967, 2, 47–65. [Google Scholar] [CrossRef]
- Grant, J.A. The isocon diagram; a simple solution to Gresens’ equation for metasomatic alteration. Econ. Geol. 1986, 81, 1976–1982. [Google Scholar] [CrossRef]
- Grant, J.A. Isocon analysis: A brief review of the method and applications. Phys. Chem. Earth Parts A/B/C 2005, 30, 997–1004. [Google Scholar] [CrossRef]
- Herron, M.M. Geochemical classification of terrigenous sands and shales from core or log data. J. Sediment. Res. 1988, 58, 820–829. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Hoboken, NJ, USA, 1985. [Google Scholar]
- Floyd, P.; Leveridge, B. Tectonic environment of the Devonian Gramscatho basin, south Cornwall: Framework mode and geochemical evidence from turbiditic sandstones. J. Geol. Soc. 1987, 144, 531–542. [Google Scholar] [CrossRef]
- van de Kamp, P.C.; Leake, B.E.; Senior, A. The petrography and geochemistry of some Californian arkoses with application to identifying gneisses of metasedimentary origin. J. Geol. 1976, 84, 195–212. [Google Scholar] [CrossRef]
- Condie, K.C.; Macke, J.E.; Reimer, T.O. Petrology and geochemistry of early Precambrian graywackes from the Fig Tree Group, South Africa. Geol. Soc. Am. Bull. 1970, 81, 2759–2776. [Google Scholar] [CrossRef]
- Caby, R.; Dostal, J.; Dupuy, C. Upper Proterozoic volcanic graywackes from northwestern Hoggar (Algeria)—Geology and geochemistry. Precambrian Res. 1977, 5, 283–297. [Google Scholar] [CrossRef]
- van de Kamp, P.C. Geochemistry and origin of metasediments in the Haliburton-Madoc area, southeastern Ontario. Can. J. Earth Sci. 1968, 5, 1337–1372. [Google Scholar] [CrossRef]
- Roser, B.; Korsch, R. Determination of tectonic setting of sandstone-Mudstone suites using content and ratio. J. Geol. 1986, 94, 635–650. [Google Scholar] [CrossRef]
- Bhatia, M.R. Plate tectonics and geochemical composition of sandstones. J. Geol. 1983, 91, 611–627. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sawaguchi, T.; Iwaya, S.; Horiuchi, M. Delineation of prospecting targets for Kuroko deposits based on modes of volcanism of underlying dacite and alteration halos. Min. Geol. 1976, 26, 105–117. [Google Scholar]
- Williams, N.C.; Davidson, G.J. Possible submarine advanced argillic alteration at the Basin Lake prospect, Western Tasmania, Australia. Econ. Geol. 2004, 99, 987–1002. [Google Scholar] [CrossRef]
- Large, R.R.; Gemmell, J.B.; Paulick, H.; Huston, D.L. The alteration box plot: A simple approach to understanding the relationship between alteration mineralogy and lithogeochemistry associated with volcanic-hosted massive sulfide deposits. Econ. Geol. 2001, 96, 957–971. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Velasco, F.; Yusta, I. Hydrothermal alteration of felsic volcanic rocks associated with massive sulphide deposition in the northern Iberian Pyrite Belt (SW Spain). Appl. Geochem. 2000, 15, 1265–1290. [Google Scholar] [CrossRef]
- Kumral, M.; Abdelnasser, A.; Budakoglu, M. Geochemistry of Hydrothermal Alteration Associated with Cenozoic Intrusion-Hosted Cu-Pb-Zn Mineralization at Tavşanlı Area, Kütahya, NW Turkey. Minerals 2016, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Large, R.R. Australian volcanic-Hosted massive sulfide deposits; features, styles, and genetic models. Econ. Geol. 1992, 87, 471–510. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R. Isotopes of sulfur and carbon. Geochem. Hydrothermal Ore Depos. 1979, 509–567. [Google Scholar]
- Czamanske, G.K.; Rye, R.O. Experimentally determined sulfur isotope fractionations between sphalerite and galena in the temperature range 600 degrees to 275 degrees C. Econ. Geol. 1974, 69, 17–25. [Google Scholar] [CrossRef]
- Chaussidon, M.; Lorand, J.-P. Sulphur isotope composition of orogenic spinel lherzolite massifs from Ariege (North-Eastern Pyrenees, France): An ion microprobe study. Geochim. Et Cosmochim. Acta 1990, 54, 2835–2846. [Google Scholar] [CrossRef]
- McCuaig, T.C.; Kerrich, R. P—T—t—deformation—fluid characteristics of lode gold deposits: Evidence from alteration systematics. Ore Geol. Rev. 1998, 12, 381–453. [Google Scholar] [CrossRef]
- Rollinson, H. Using Geochemical Data; Cambridge University Press: Cambridge, UK, 1993; p. 352. [Google Scholar]
- Richards, J.P. Alkalic-Type epithermal gold deposits—A review. In Magmas, Fluids, and Ore Deposits, Mineralogical Association of Canada Short Course; Mineralogical Association of Canada: Quebec, QC, Canada, 1995; Volume 23, pp. 367–400. [Google Scholar]
- Ueda, A.; Sakai, H. Sulfur isotope study of Quaternary volcanic rocks from the Japanese Islands Arc. Geochim. Et Cosmochim. Acta 1984, 48, 1837–1848. [Google Scholar] [CrossRef]
- Chaussidon, M.; Albarede, F.; Sheppard, M. Sulphur isotope heterogeneity in the mantle from ion microprobe measurements of sulphide inclusions in diamonds. Nature 1987, 330, 242–244. [Google Scholar] [CrossRef]
- Rye, R.; Luhr, J.; Wasserman, M. Sulfur and oxygen isotopic systematics of the 1982 eruptions of El Chichón Volcano, Chiapas, Mexico. J. Volcanol. Geotherm. Res. 1984, 23, 109–123. [Google Scholar] [CrossRef]
- Luhr, J.F.; Logan, M.A.V. Sulfur isotope systematics of the 1982 El Chichón trachyandesite: An ion microprobe study. Geochim. Et Cosmochim. Acta 2002, 66, 3303–3316. [Google Scholar] [CrossRef]
- Sasaki, A.; Ishihara, S. Sulfur isotopic composition of the magnetite-series and ilmenite-series granitoids in Japan. Contrib. Mineral. Petrol. 1979, 68, 107–115. [Google Scholar] [CrossRef]
- Ishihara, S.; Sasaki, A. Sulfur isotopic ratios of the magnetite-series and ilmenite-series granitoids of the Sierra Nevada batholith—a reconnaissance study. Geology 1989, 17, 788–791. [Google Scholar] [CrossRef]
- Santosh, M.; Masuda, H. Reconnaissance oxygen and sulfur isotopic mapping of Pan-African alkali granites and syenites in the southern Indian Shield. Geochem. J. 1991, 25, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Hoefs, J. Isotope fractionation processes of selected elements. In Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 47–190. [Google Scholar]
- Rudnick, R.; Gao, S.; Holland, H.; Turekian, K. Composition of the continental crust. Crust 2003, 3, 1–64. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).