Texture and Trace Element Geochemistry of Quartz: A Review
Abstract
:1. Introduction
2. Characteristics of Magmatic Quartz
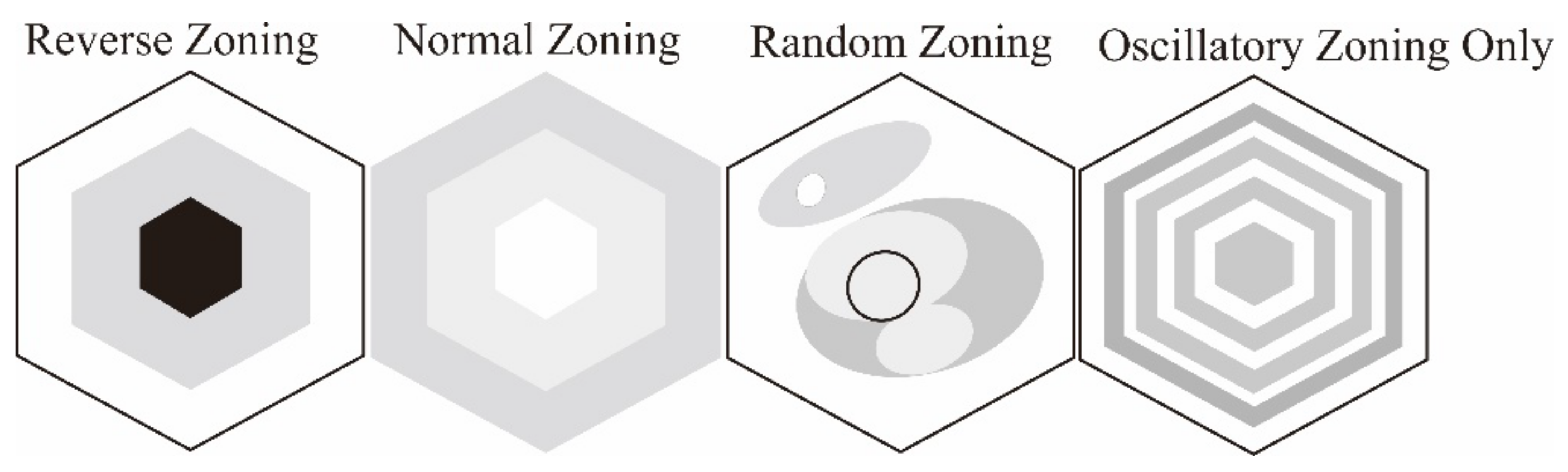
2.1. Internal Texture
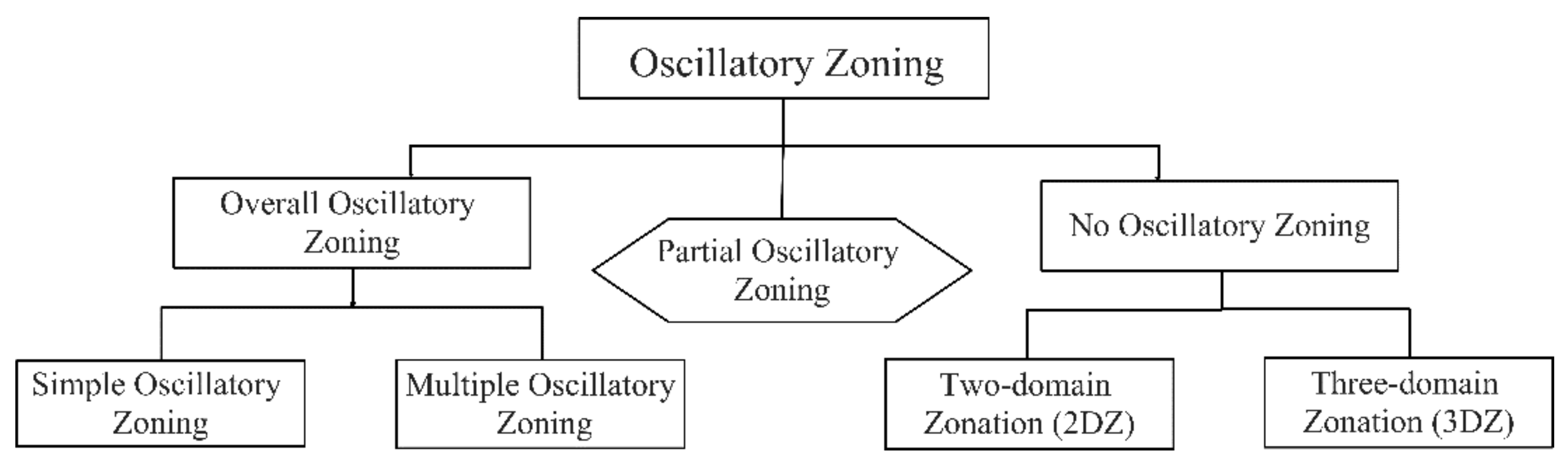
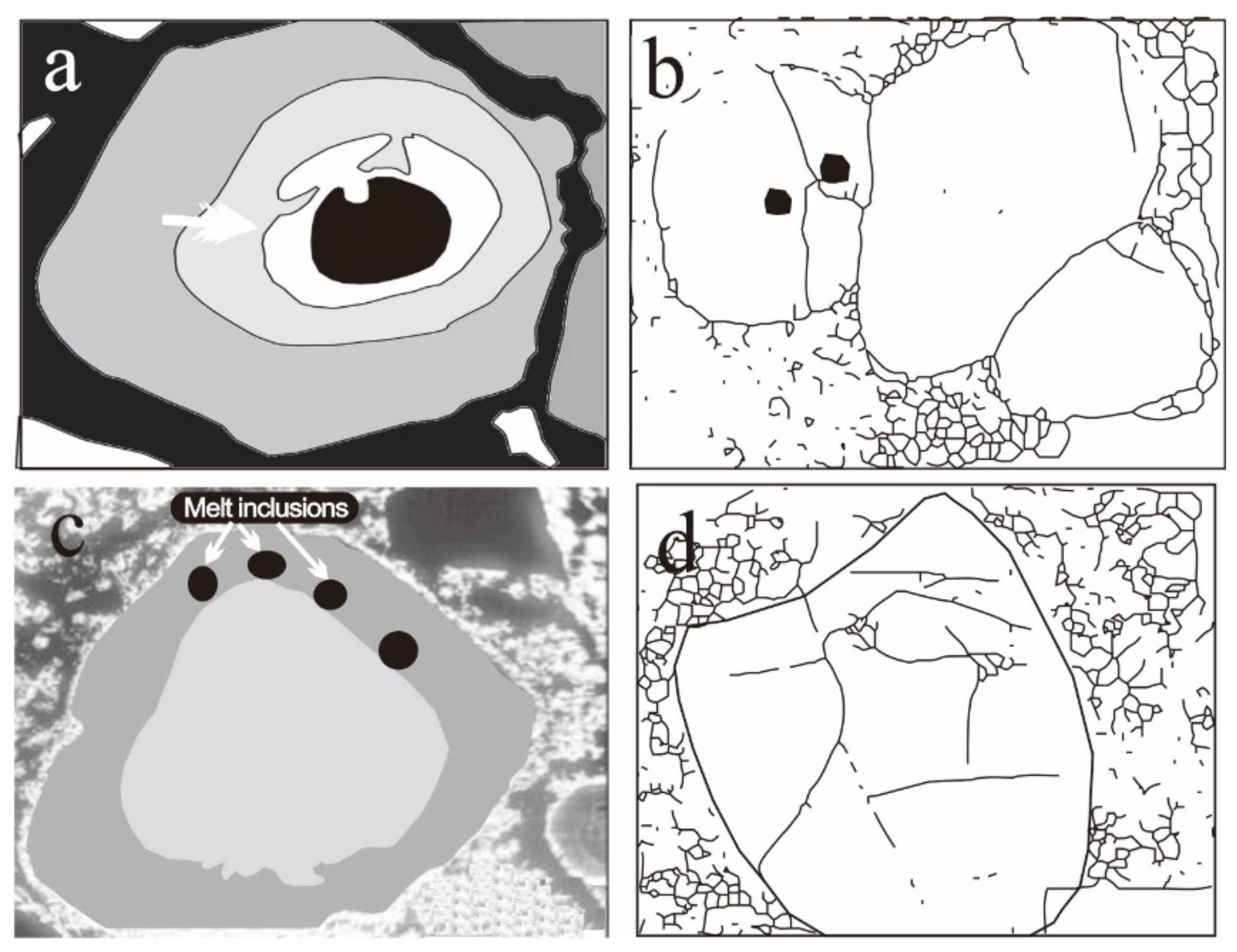
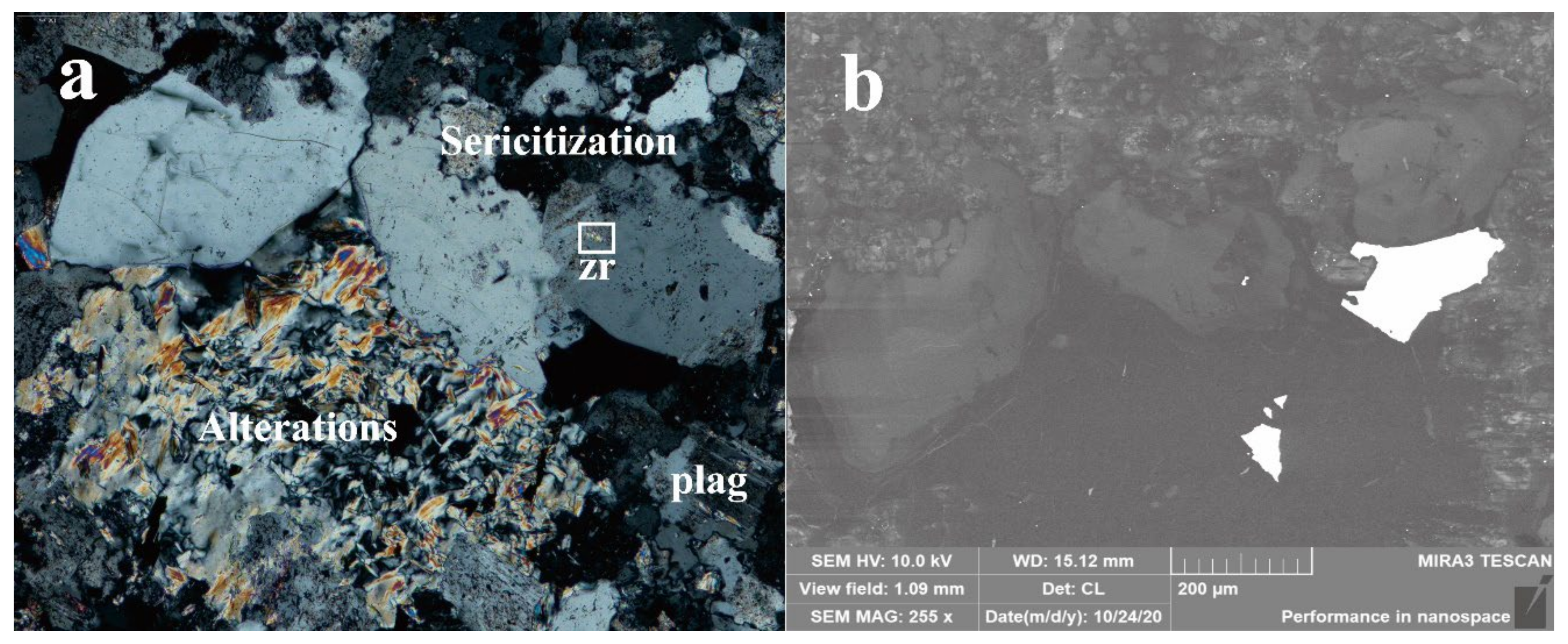
Cathodoluminescence Features
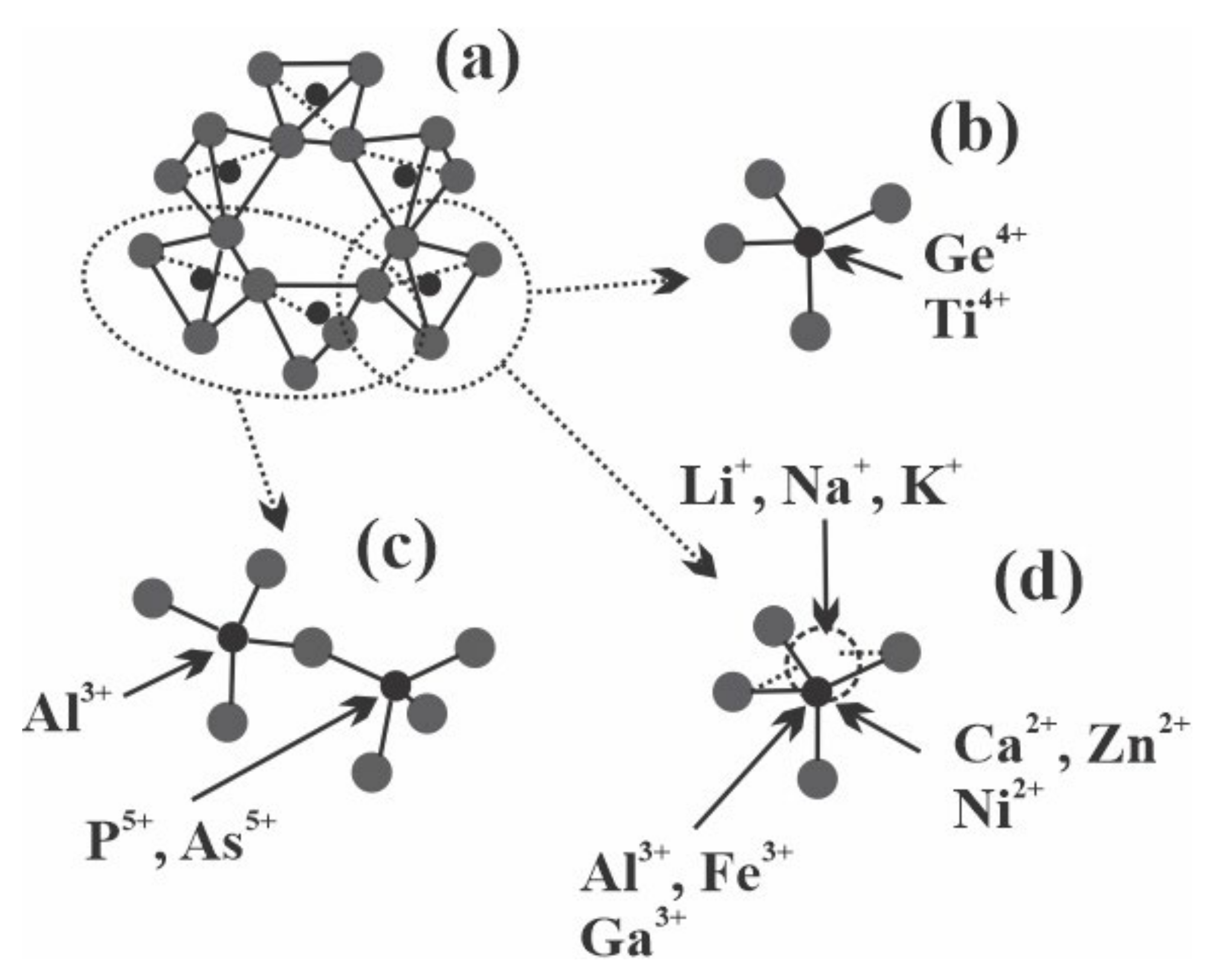
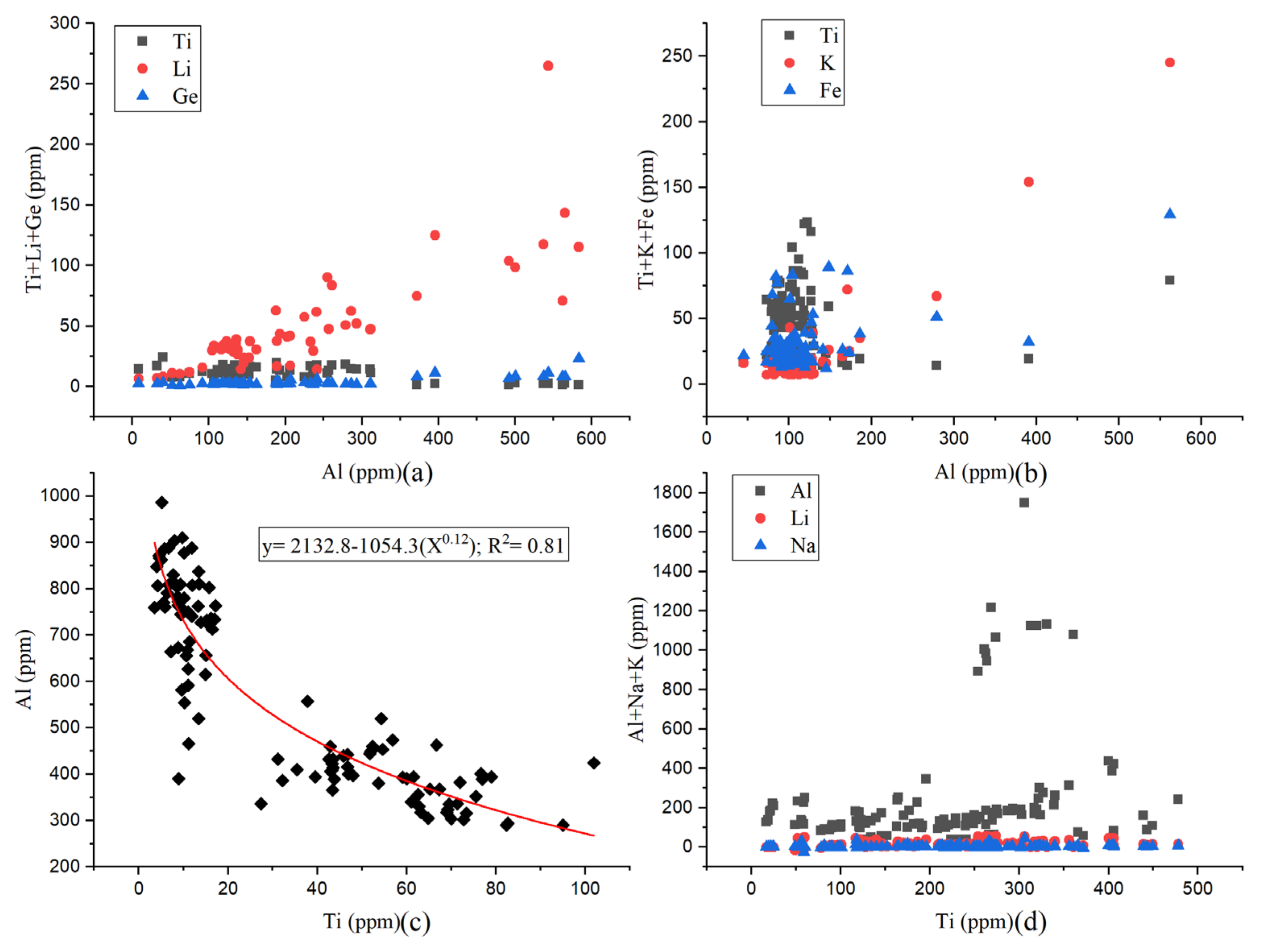
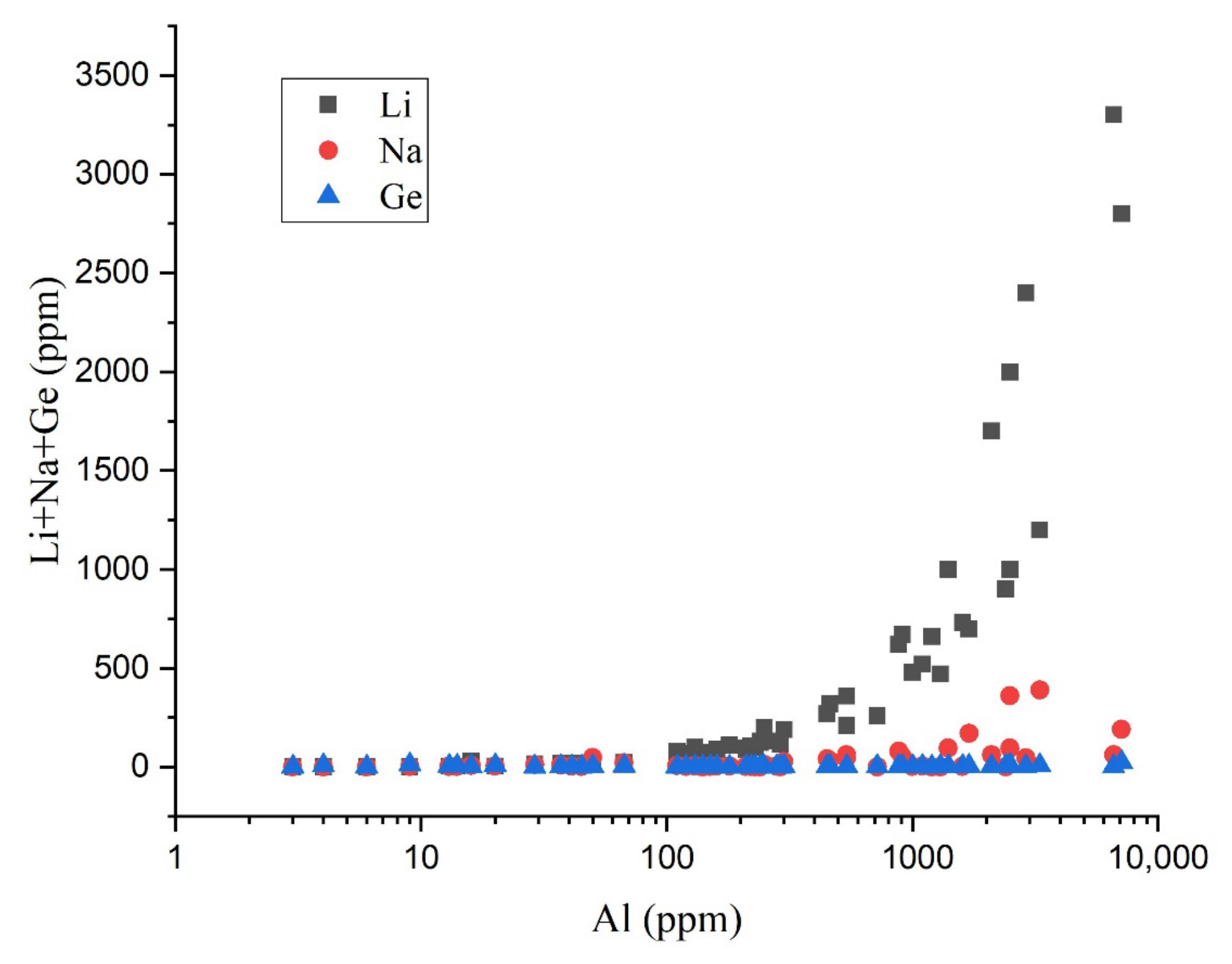
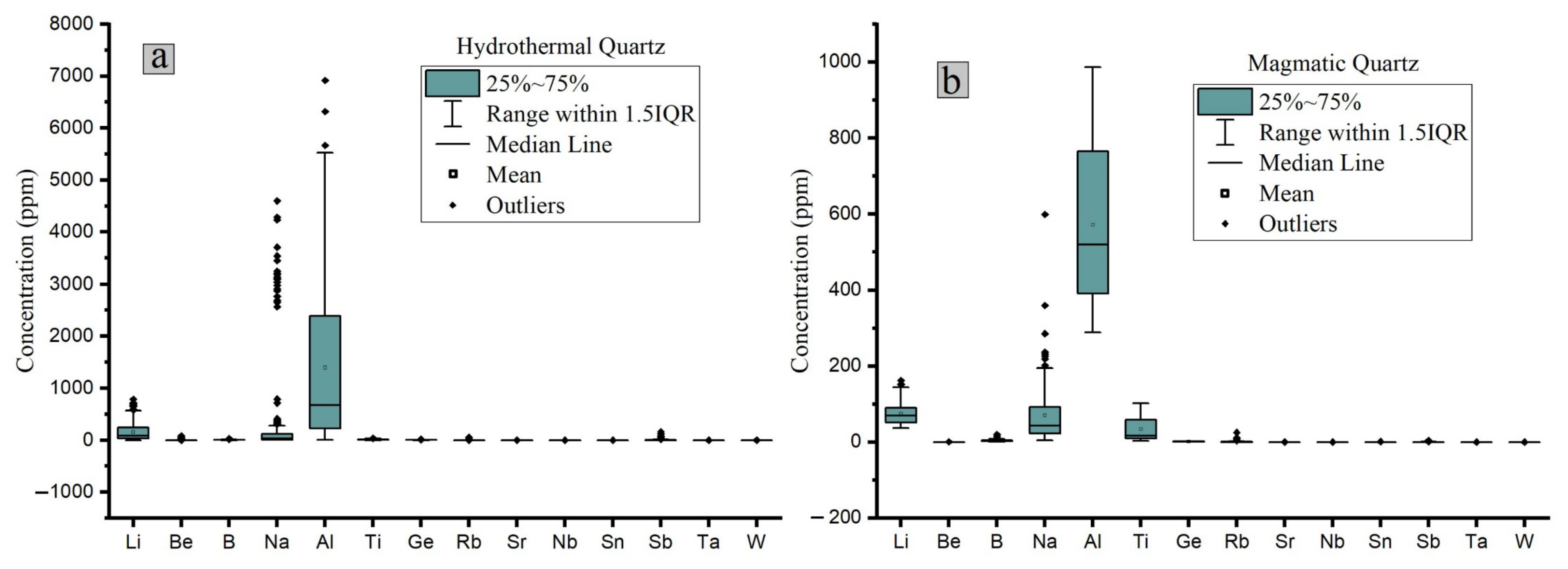
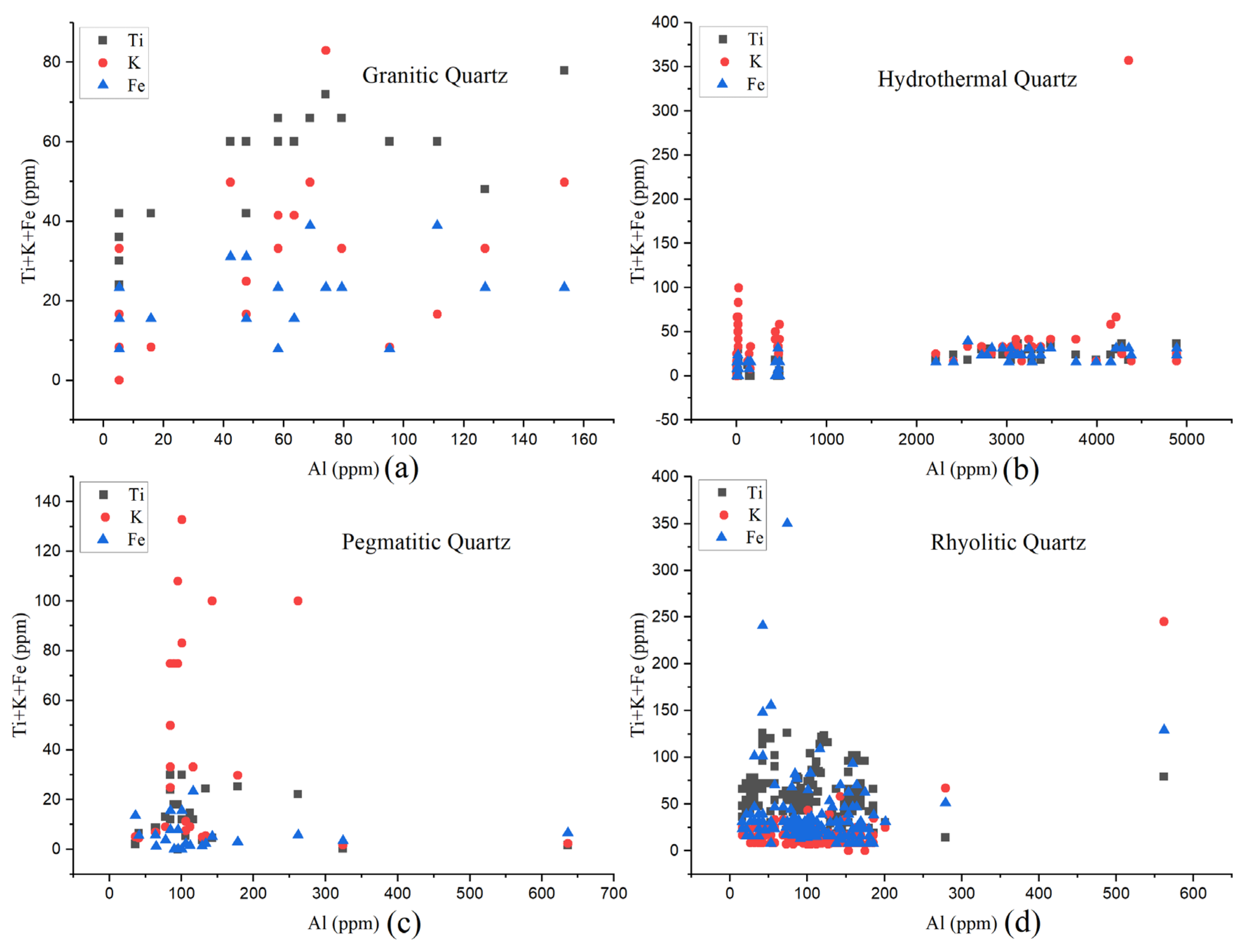
2.2. Chemical Composition
2.3. Relation between Internal Texture and Trace Elements
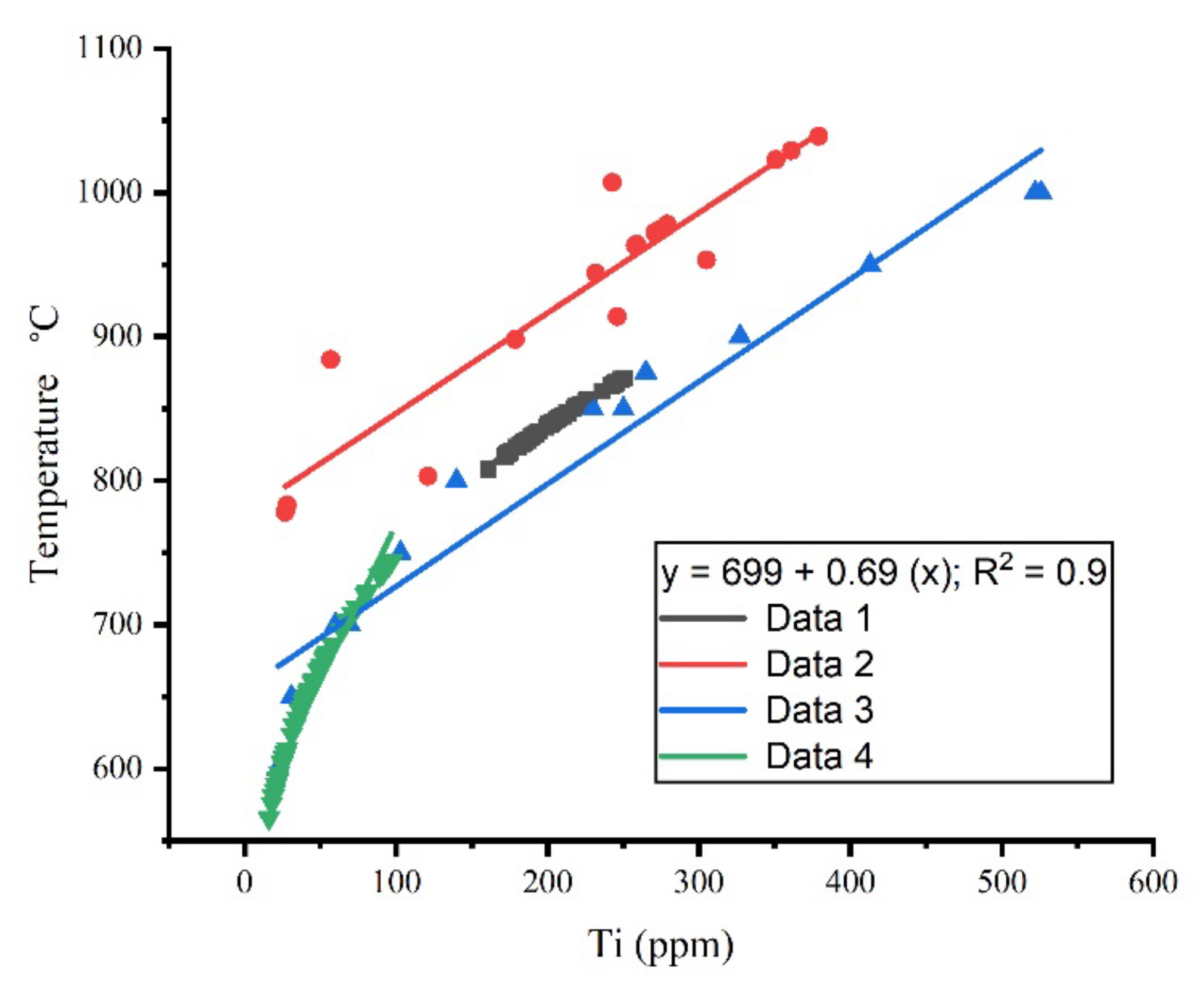
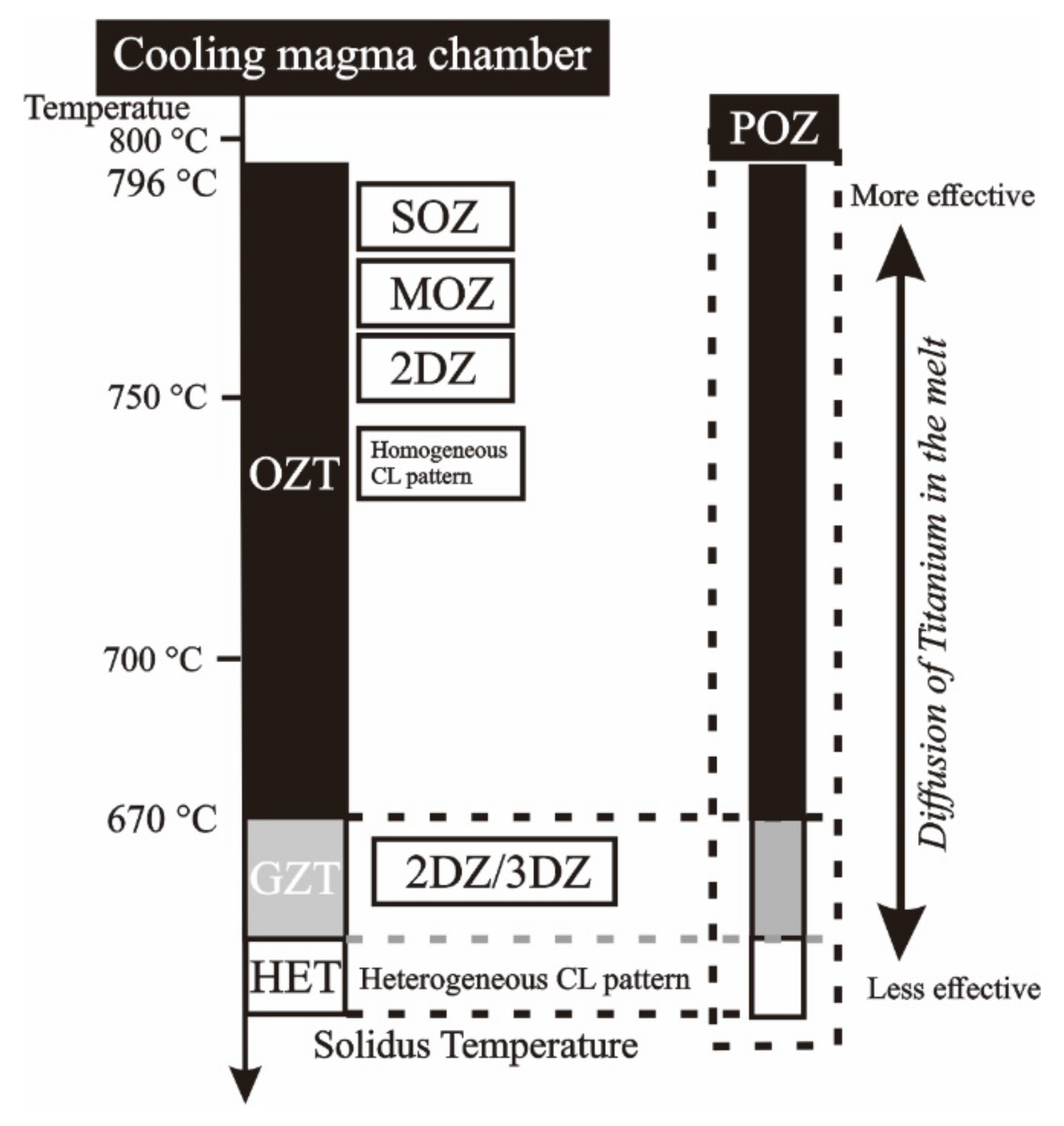
2.4. Titanium Diffusion
2.5. TitaniQ Thermometry and Genetic Programming (GP)
3. Characteristic of Hydrothermal Quartz
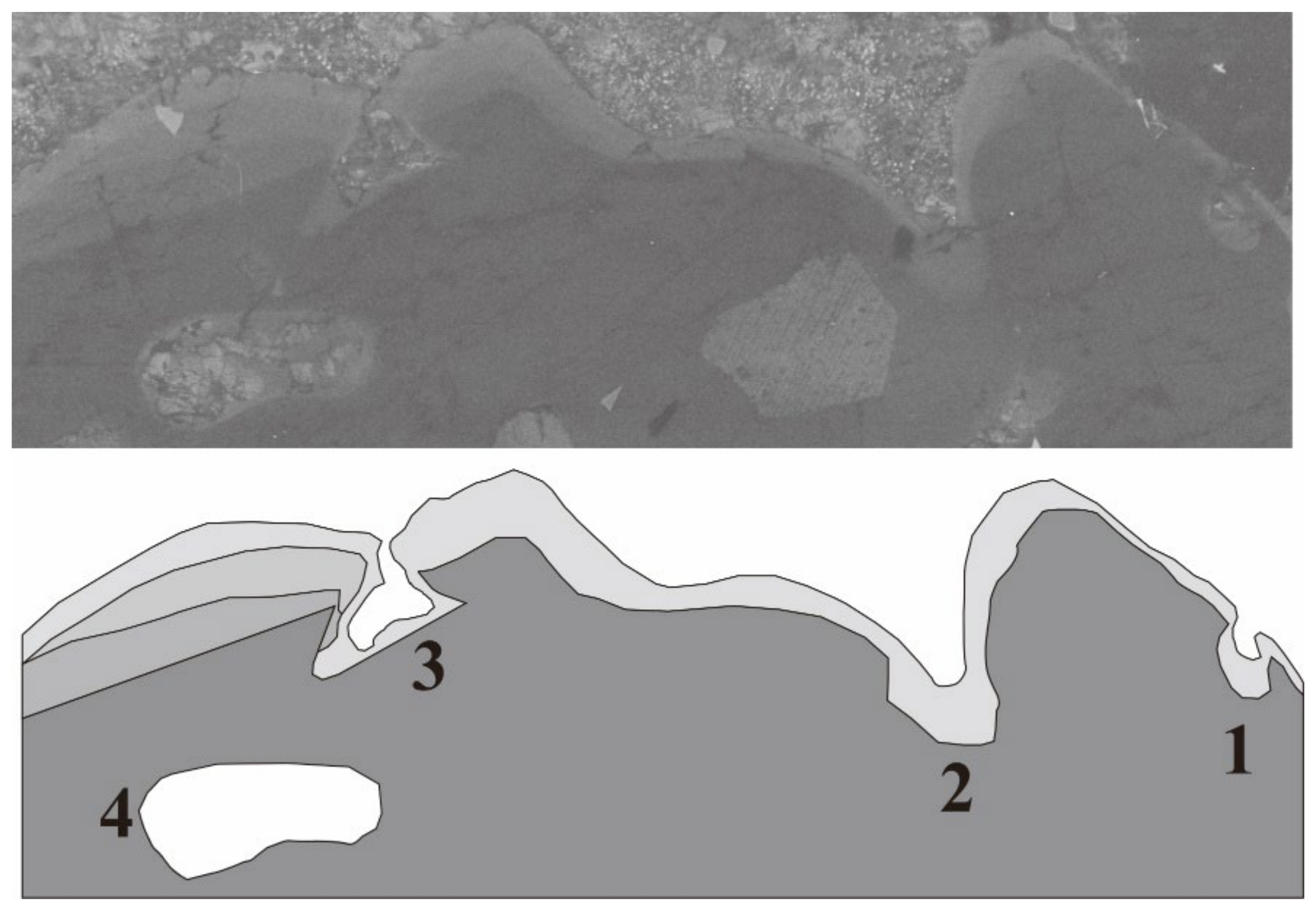
3.1. Internal Texture
| Sketch of Texture | Texture Type | Grain Size | Grain Form | Comments | References |
|---|---|---|---|---|---|
 | Oscillatory Zoned Euhedral Crystal | Variable | Prismatic |
| [35,127] |
 | Spherical texture | Fine | Anhedral |
| [35] |
 | Mosaic texture | Fine | Anhedral |
| [35,132] |
 | Spider and cobweb texture | Fine | Prismatic/mimics host |
| [35] |
 | Sector Zoning | Variable |
| [86,125,131,133,134] | |
 | Rounded concentric zonation | Variable | Variable | Changes in the chemical composition of the fluid or temperature of crystallization | [131,133] |
3.2. Chemical Composition
4. Alpha and Beta Quartz
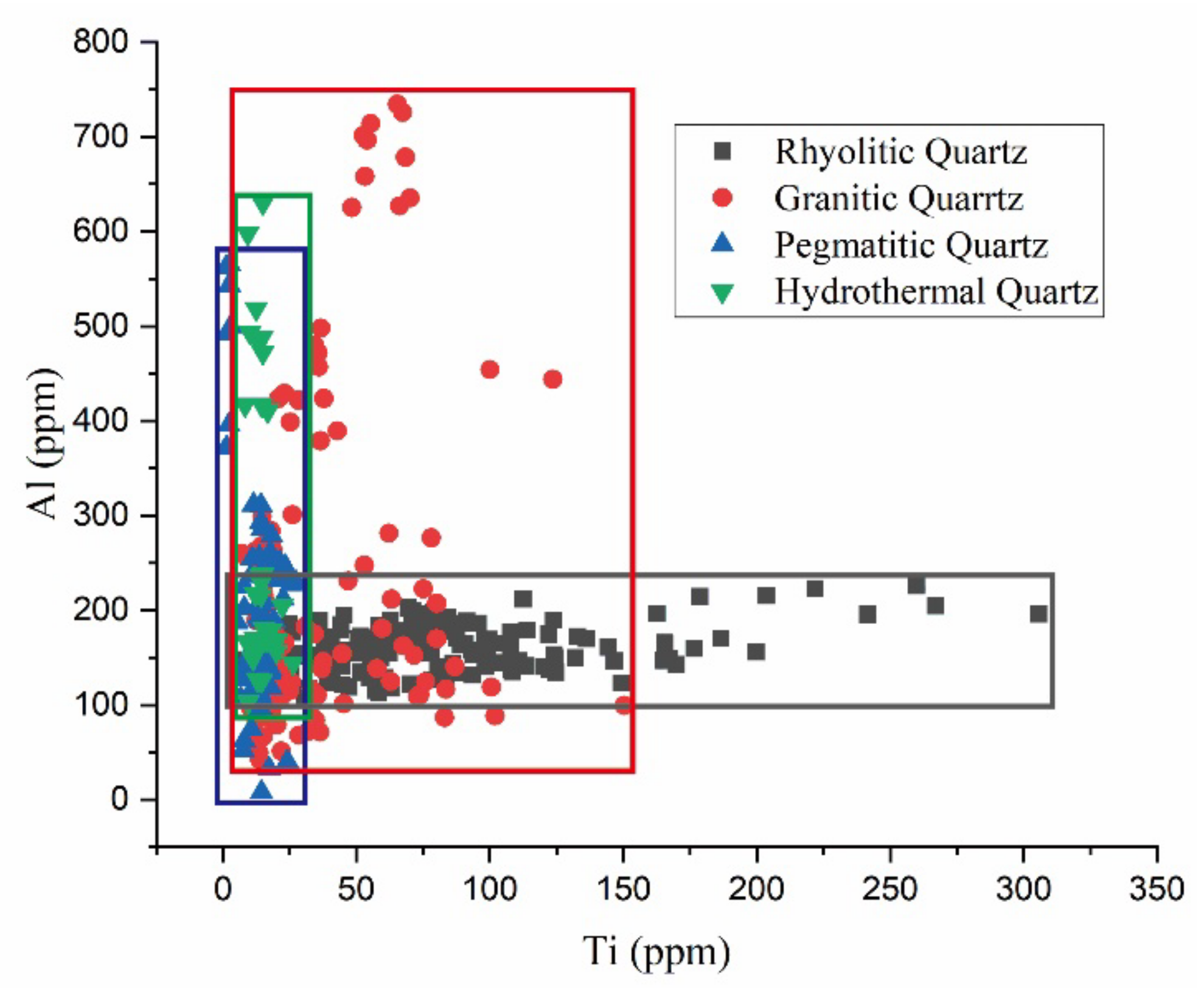
5. Comparison between Hydrothermal and Magmatic Quartz
6. Conditions Necessary for Mineralization
7. Implications for Hydrothermal Systems and Ore Deposits
8. Applications of Quartz and CL in Geoscience
9. Conclusions
- Titanium is the most important element in magmatic quartz, while Al is the most important in hydrothermal quartz;
- Cathodoluminescence intensity is directly proportional to the Ti concentration, but the concentrations of other trace elements also affect the CL intensity;
- By using luminescence properties, we can differentiate between magmatic and hydrothermal quartz;
- Each type of ore deposit has its own unique CL characteristics;
- Barren quartz veins contain high Ti contents compared to fertile ones;
- The Ge concentration is higher in magmatic quartz compared to hydrothermal quartz;
- Li, Na,
- and K act as charge compensators in quartz lattices;
- The Ge/Al ratios of magmatic quartz and hydrothermal quartz are less than 0.008 and greater than 0.008, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Augustsson, C.; Reker, A. Cathodolumenescence Spectra of Quartz As Provenance Indicators Revisited. J. Sediment. Res. 2012, 82, 559–570. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Boggs, S. Provenance interpretation of Tertiary sandstones from the Cheju Basin (NE East China Sea): A comparison of conventional petrographic and scanning cathodoluminescence techniques. Sediment. Geol. 2002, 152, 29–43. [Google Scholar] [CrossRef]
- Palmer, S.E.; Kyser, T.; Hiatt, E.E. Provenance of the Proterozoic Thelon Basin, Nunavut, Canada, from detrital zircon geochronology and detrital quartz oxygen isotopes. Precambrian Res. 2003, 129, 115–140. [Google Scholar] [CrossRef]
- Augustsson, C.; Rusing, T.; Adams, C.J.; Chmiel, H.; Kocabayoglu, M.; Buld, M.; Zimmermann, U.; Berndt, J.; Kooijman, E. Detrital Quartz and Zircon Combined: The Production of Mature Sand with Short Transportation Paths Along the Cambrian West Gondwana Margin, Northwestern Argentina. J. Sediment. Res. 2011, 81, 284–298. [Google Scholar] [CrossRef]
- Müller, A.; Seltmann, R.; Behr, H.J. Application of cathodoluminescence to magmatic quartz in a tin granite—Case study from the Schellerhau Granite Complex, Eastern Erzgebirge, Germany. Miner. Depos. 2000, 35, 169–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, J.; Tian, J.; Pan, J.; Sun, S.; Zhang, L.; Zhang, S.; Chu, G.; Zhao, Y.; Lai, C. Texture and trace element geochemistry of quartz in skarn system: Perspective from Jiguanzui Cu–Au skarn deposit, Eastern China. Ore Geol. Rev. 2019, 109, 535–544. [Google Scholar] [CrossRef]
- Huang, R.; Audétat, A. The titanium-in-quartz (TitaniQ) thermobarometer: A critical examination and re-calibration. Geochim. Cosmochim. Acta 2012, 84, 75–89. [Google Scholar] [CrossRef]
- Yuguchi, T.; Ogita, Y.; Kato, T.; Yokota, R.; Sasao, E.; Nishiyama, T. Crystallization processes of quartz in a granitic magma: Cathodoluminescence zonation pattern controlled by temperature and titanium diffusivity. J. Southeast Asian Earth Sci. 2020, 192, 104289. [Google Scholar] [CrossRef]
- Müller, A.; Herklotz, G.; Giegling, H. Chemistry of quartz related to the Zinnwald/Cínovec Sn-W-Li greisen-type deposit, Eastern Erzgebirge, Germany. J. Geochem. Explor. 2018, 190, 357–373. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Dosbaba, M. Chemical signature of quartz from S- and A-type rare-metal granites—A summary. Ore Geol. Rev. 2020, 125, 103674. [Google Scholar] [CrossRef]
- Mashkovtsev, R.I.; Balitsky, V.S.; Pan, Y. EPR characteristics of radiation-induced defects in Ge-rich α-quartz. Solid State Sci. 2022, 125, 106833. [Google Scholar] [CrossRef]
- Rusk, B.G.; Lowers, H.A.; Reed, M.H. Trace elements in hydrothermal quartz: Relationships to cathodoluminescent textures and insights into vein formation. Geology 2008, 36, 547. [Google Scholar] [CrossRef]
- Monnier, L.; Lach, P.; Salvi, S.; Melleton, J.; Bailly, L.; Béziat, D.; Gouy, S. Quartz trace-element composition by LA-ICP-MS as proxy for granite differentiation, hydrothermal episodes, and related mineralization: The Beauvoir Granite (Echassières district), France. Lithos 2018, 320–321, 355–377. [Google Scholar] [CrossRef]
- Pacák, K.; Zachariáš, J.; Strnad, L. Trace-element chemistry of barren and ore-bearing quartz of selected Au, Au-Ag and Sb-Au deposits from the Bohemian Massif Trace-element chemistry of barren and ore-bearing quartz of selected Au, Au–Ag and Sb–Au deposits from the Bohemian Massif. J. Geosci. 2019, 64, 19–35. [Google Scholar] [CrossRef]
- Breiter, K.; Svojtka, M.; Ackerman, L.; Švecová, K. Trace element composition of quartz from the Variscan Altenberg—Teplice caldera (Krušné hory/Erzgebirge Mts, Czech Republic/Germany): Insights into the volcano-plutonic complex evolution. Chem. Geol. 2012, 326, 36–50. [Google Scholar] [CrossRef]
- Dong, G.; Morrison, G.; Jaireth, S. Quartz textures in epithermal veins, Queensland; classification, origin and implication. Econ. Geol. 1995, 90, 1841–1856. [Google Scholar] [CrossRef]
- Jourdan, A.-L.V.; Mullis, J.; Ramseyer, K.; Spiers, C. Evidence of growth and sector zoning in hydrothermal quartz from Alpine veins. Eur. J. Miner. 2009, 21, 219–231. [Google Scholar] [CrossRef]
- Miyoshi, N.; Yamaguchi, Y.; Makino, K. Successive zoning of Al and H in hydrothermal vein quartz. Am. Miner. 2005, 90, 310–315. [Google Scholar] [CrossRef]
- Larsen, G.; Polvé, B.R.; Juve, M. Granitic pegmatite quartz from Evje-Iveland: Trace element chemistry and implications for high purity quartz formation. Bull. Geol. Surv. Norw. 2000, 436, 57–65. [Google Scholar]
- Müller, A.; Lennox, P.; Trzebski, R. Cathodoluminescence and micro-structural evidence for crystallisation and deformation processes of granites in the Eastern Lachlan Fold Belt (SE Australia). Contrib. Miner. Pet. 2002, 143, 510–524. [Google Scholar] [CrossRef]
- Behr, H.-J.; Kronz, A. Trace elements and cathodoluminescence of igneous quartz in topaz granites from the Hub Stock (Slavkovský Les Mts., Czech Republic). Miner. Pet. 2003, 79, 167–191. [Google Scholar] [CrossRef]
- Götze, J.; Plötze, M.; Graupner, T.; Hallbauer, D.K.; Bray, C.J. Trace element incorporation into quartz: A combined study by ICP-MS, electron spin resonance, cathodoluminescence, capillary ion analysis, and gas chromatography. Geochim. Cosmochim. Acta 2004, 68, 3741–3759. [Google Scholar] [CrossRef]
- Larsen, R.B.; Henderson, I.; Ihlen, P.M.; Jacamon, F. Distribution and petrogenetic behaviour of trace elements in granitic pegmatite quartz from South Norway. Contrib. Miner. Pet. 2004, 147, 615–628. [Google Scholar] [CrossRef]
- Jacamon, F.; Larsen, R.B. Trace element evolution of quartz in the charnockitic Kleivan granite, SW-Norway: The Ge/Ti ratio of quartz as an index of igneous differentiation. Lithos 2009, 107, 281–291. [Google Scholar] [CrossRef]
- Dowling, K.; Morrison, G. Application of quartz textures to the classification of gold deposits using North Queensland examples. Econ. Geol. Monogr. 1989, 6, 342–355. [Google Scholar]
- Breiter, K.; Ďurišová, J.; Dosbaba, M. Quartz chemistry—A step to understanding magmatic-hydrothermal processes in ore-bearing granites: Cínovec/Zinnwald Sn-W-Li deposit, Central Europe. Ore Geol. Rev. 2017, 90, 25–35. [Google Scholar] [CrossRef]
- Beurlen, H.; Müller, A.; Silva, D.; Da Silva, M.R.R. Petrogenetic significance of LA-ICP-MS trace-element data on quartz from the Borborema Pegmatite Province, northeast Brazil. Miner. Mag. 2011, 75, 2703–2719. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R. Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Müller, A.; van den Kerkhof, A.M.; Behr, H.J.; Kronz, A.; Koch-Müller, M. The evolution of late-Hercynian granites and rhyolites documented by quartz—A review. Earth Environ. Sci. Trans. R. Soc. Edinb. 2010, 100, 185–204. [Google Scholar] [CrossRef]
- Götze, D.; Plötze, J.; Habermann, M. Origin, spectral characteristics, and practical applications of the cathodoluminescence of quartz—A review. Mineral. Petrol. 2001, 71, 225–250. [Google Scholar] [CrossRef]
- Flem, B.; Mansfeld, J.; Lahaye, Y.; Larsen, R.B.; Dundas, S. LA-HR-ICP-MS analysis of quartz and principles governing the distribution and speciation of structural impurities in igneous quartz. Rep. Geol. Surv. Norw. 2000, 81, 1–42. [Google Scholar]
- Muller, A.; Welch, M.D. Frontiers in quartz research. Miner. Mag. Themat Issue 2009, 73, 517–518. [Google Scholar]
- Larsen, R.B.; Jacamon, F.; Kronz, A. Trace element chemistry and textures of quartz during the magmatic hydrothermal transition of Oslo Rift granites. Miner. Mag. 2009, 73, 691–707. [Google Scholar] [CrossRef]
- Müller, A.; Herrington, R.; Armstrong, R.; Seltmann, R.; Kirwin, D.J.; Stenina, N.G.; Kronz, A. Trace elements and cathodoluminescence of quartz in stockwork veins of Mongolian porphyry-style deposits. Miner. Depos. 2010, 45, 707–727. [Google Scholar] [CrossRef]
- Rusk, B. Cathodoluminescent textures and trace elements in hydrothermal quartz. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–329. [Google Scholar]
- Müller, A.; Koch-Müller, M. Hydrogen speciation and trace element contents of igneous, hydrothermal and metamorphic quartz from Norway. Miner. Mag. 2009, 73, 569–583. [Google Scholar] [CrossRef]
- Götte, T.; Pettke, T.; Ramseyer, K.; Koch-Müller, M.; Mullis, J. Cathodoluminescence properties and trace element signature of hydrothermal quartz: A fingerprint of growth dynamics. Am. Miner. 2011, 96, 802–813. [Google Scholar] [CrossRef]
- Cherniak, D.; Watson, E.; Wark, D. Ti diffusion in quartz. Chem. Geol. 2007, 236, 65–74. [Google Scholar] [CrossRef]
- Baele, J.; Decrée, S.; Rusk, B. Cathodoluminescence applied to ore geology and exploration. In Ore Deposits: Origin, Exploration, and Exploitation; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 131–161. [Google Scholar]
- Ding, T.; Ma, D.; Lu, J.; Zhang, R. Garnet and scheelite as indicators of multi-stage tungsten mineralization in the Huangshaping deposit, southern Hunan province, China. Ore Geol. Rev. 2018, 94, 193–211. [Google Scholar] [CrossRef]
- Wark, D.A.; Watson, E.B. TitaniQ: A titanium-in-quartz geothermometer. Contrib. Mineral. Petrol. 2006, 152, 743–754. [Google Scholar] [CrossRef]
- Wark, D.; Hildreth, W.; Spear, F.; Cherniak, D.; Watson, E. Pre-eruption recharge of the Bishop magma system. Geology 2007, 35, 235. [Google Scholar] [CrossRef]
- Götze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Götze, J.; Schertl, H.-P.; Neuser, R.D.; Kempe, U.; Hanchar, J.M. Optical microscope-cathodoluminescence (OM–CL) imaging as a powerful tool to reveal internal textures of minerals. Miner. Pet. 2012, 107, 373–392. [Google Scholar] [CrossRef]
- Chang, Z.; Meinert, L.D. The magmatic–hydrothermal transition—Evidence from quartz phenocryst textures and endoskarn abundance in Cu–Zn skarns at the Empire Mine, Idaho, USA. Chem. Geol. 2004, 210, 149–171. [Google Scholar] [CrossRef]
- Wilcock, J.; Goff, F.; Minarik, W.G.; Stix, J. Magmatic recharge during the formation and resurgence of the Valles Caldera, New Mexico, USA: Evidence from quartz compositional zoning and geothermometry. J. Petrol. 2013, 54, 635–664. [Google Scholar] [CrossRef]
- Bégué, F.; Deering, C.D.; Gravley, D.M.; Kennedy, B.M.; Chambefort, I.; Gualda, G.A.R.; Bachmann, O. Extraction, Storage and Eruption of Multiple Isolated Magma Batches in the Paired Mamaku and Ohakuri Eruption, Taupo Volcanic Zone, New Zealand. J. Pet. 2014, 55, 1653–1684. [Google Scholar] [CrossRef]
- Graeter, K.A.; Beane, R.J.; Deering, C.D.; Gravley, D.; Bachmann, O. Formation of rhyolite at the Okataina Volcanic Complex, New Zealand: New insights from analysis of quartz clusters in plutonic lithics. Am. Miner. 2015, 100, 1778–1789. [Google Scholar] [CrossRef]
- Pamukcu, A.S.; Ghiorso, M.S.; Gualda, G.A.R. High-Ti, bright-CL rims in volcanic quartz: A result of very rapid growth. Contrib. Miner. Pet. 2016, 171, 105. [Google Scholar] [CrossRef]
- Befus, K.S.; Manga, M. Supereruption quartz crystals and the hollow reentrants. Geology 2019, 47, 710–714. [Google Scholar] [CrossRef]
- Vasyukova, O.V.; Kamenetsky, V.S.; Goemann, K.; Davidson, P. Diversity of primary CL textures in quartz from porphyry environments: Implication for origin of quartz eyes. Contrib. Miner. Pet. 2013, 166, 1253–1268. [Google Scholar] [CrossRef]
- Wiebe, R.A.; Wark, D.A.; Hawkins, D.P. Insights from quartz cathodoluminescence zoning into crystallization of the Vinalhaven granite, coastal Maine. Contrib. Miner. Pet. 2007, 154, 439–453. [Google Scholar] [CrossRef]
- Rusk, B.; Koenig, A.; Lowers, H. Visualizing trace element distribution in quartz using cathodoluminescence, electron microprobe, and laser ablation-inductively coupled plasma-mass spectrometry. Am. Mineral. 2011, 96, 703–708. [Google Scholar] [CrossRef]
- Landtwing, T.; Pettke, M. Relationships between SEM-cathodoluminescence response and trace element composition of hydrothermal vein quartz. Am. Miner. 2005, 90, 122–131. [Google Scholar] [CrossRef]
- Cruz-Uribe, A.M.; Mertz-Kraus, R.; Zack, T.; Feineman, M.D.; Woods, G.; Jacob, D.E. A new LA-ICP-MS method for Ti in quartz: Implications and application to high pressure rutile–quartz veins from the Czech Erzgebirge. Geostand. Geoanalytical Res. 2016, 41, 29–40. [Google Scholar] [CrossRef]
- Müller, A.; Kronz, A.; Breiter, K. Trace elements and growth patterns in quartz: A fingerprint of the evolution of the subvolcanic Podlesí Granite System (Krušné hory Mts., Czech Republic). Vestn. Ces. Geol. Ust. 2002, 77, 135–145. [Google Scholar] [CrossRef]
- Müller, A. Cathodoluminescence and Characterisation of Defect Structures in Quartz with Applications to the Study of Granitic Rocks. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2000. [Google Scholar]
- Ehrlich, K.; Verš, E.; Kirs, J.; Soesoo, A. Titaan-kvartsis geotermomeetria meetodi rakendamine paleoproterosoilise Suursaare kvartsporfüüri kristalliseerumistemperatuuride määramisel. Est. J. Earth Sci. 2012, 61, 195–204. [Google Scholar] [CrossRef]
- Boggs, S.; Kwon, Y.-I.; Goles, G.G.; Rusk, B.G.; Krinsley, D.; Seyedolali, A. Is Quartz Cathodoluminescence Color a Reliable Provenance Tool? A Quantitative Examination. J. Sediment. Res. 2002, 72, 408–415. [Google Scholar] [CrossRef]
- Thomas, J.B.; Watson, E.B.; Spear, F.S.; Shemella, P.T.; Nayak, S.K.; Lanzirotti, A. TitaniQ under pressure: The effect of pressure and temperature on the solubility of Ti in quartz. Contrib. Miner. Pet. 2010, 160, 743–759. [Google Scholar] [CrossRef]
- Ashley, K.T.; Webb, L.E.; Spear, F.S.; Thomas, J.B. P-T-D histories from quartz: A case study of the application of the TitaniQ thermobarometer to progressive fabric development in metapelites. Geochem. Geophys. Geosystems 2013, 14, 3821–3843. [Google Scholar] [CrossRef]
- Bromiley, G.D.; Hiscock, M. Grain boundary diffusion of titanium in polycrystalline quartz and its implications for titanium in quartz (TitaniQ) geothermobarometry. Geochim. Cosmochim. Acta 2016, 178, 281–290. [Google Scholar] [CrossRef]
- Buthelezi, M.; Ashwal, L.; Horváth, P. Application of titanium-in-quartz geothermometry to magmatic quartz in evolved rocks from the Bushveld Complex, South Africa. South Afr. J. Geol. 2017, 120, 241–250. [Google Scholar] [CrossRef]
- Matthews, N.E.; Pyle, D.; Smith, V.; Wilson, C.; Huber, C.; Van Hinsberg, V. Quartz zoning and the pre-eruptive evolution of the ~340-ka Whakamaru magma systems, New Zealand. Contrib. Miner. Pet. 2011, 163, 87–107. [Google Scholar] [CrossRef]
- Götze, J. Application of Cathodoluminescence Microscopy and Spectroscopy in Geosciences. Microsc. Microanal. 2012, 18, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Bernet, M.; Bassett, K. Provenance Analysis by Single-Quartz-Grain SEM-CL/Optical Microscopy. J. Sediment. Res. 2005, 75, 492–500. [Google Scholar] [CrossRef]
- Tanner, D.; Henley, R.W.; Mavrogenes, J.A.; Holden, P. Combining in situ isotopic, trace element and textural analyses of quartz from four magmatic-hydrothermal ore deposits. Contrib. Miner. Pet. 2013, 166, 1119–1142. [Google Scholar] [CrossRef]
- Muller, A. The genetic significance of snowball quartz in high fractionated tin granites of the Krušne Hory/Erzgebirge Miner. Depos. Miner. Depos. Process. Process. Rotterdam Balkema. 1999, 1, 409–412. [Google Scholar]
- Breiter, K.; Müller, A.; Leichmann, J.; Gabašová, A. Textural and chemical evolution of a fractionated granitic system: The Podlesí stock, Czech Republic. Lithos 2005, 80, 323–345. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals: A common phenomenon. Can. Miner. 1996, 34, 1111–1126. [Google Scholar]
- Fowler, C.; Prokoph, A.; Stern, A.; Dupuis, R. Organization of oscillatory zoning in zircon: Analysis, scaling, geochemistry, and model of a zircon from Kipawa, Quebec, Canada. Geochim. Consmochim. Acta 2002, 66, 311–328. [Google Scholar] [CrossRef]
- Yuguchi, T.; Iwano, H.; Kato, T.; Sakata, S.; Hattori, K.; Hirata, T.; Sueoka, S.; Danhara, T.; Ishibashi, M.; Sasao, E.; et al. Zircon growth in a granitic pluton with specific mechanisms, crystallization temperatures and U–Pb ages: Implication to the ‘spatiotemporal’ formation process of the Toki granite, central Japan. J. Mineral. Petrol. Sci. 2016, 111, 9–34. [Google Scholar] [CrossRef]
- Yuguchi, T.; Sueoka, S.; Iwano, H.; Izumino, Y.; Ishibashi, M.; Danhara, T.; Sasao, E.; Hirata, T.; Nishiyama, T. Position-by-position cooling paths within the Toki granite, central Japan: Constraints and the relation with fracture population in a pluton. J. Southeast Asian Earth Sci. 2018, 169, 47–66. [Google Scholar] [CrossRef]
- Götte, T.; Ramseyer, K. Trace element characteristics, luminescence properties and real structure of quartz. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 265–285. [Google Scholar]
- Yacobi, B.G.; Holt, D.B. Cathodoluminescence Microscopy of Inorganic Solids; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Rusk, B.G.; Reed, M.H.; Dilles, J.H.; Kent, A. Intensity of quartz cathodoluminescence and trace-element content in quartz from the porphyry copper deposit at Butte, Montana. Am. Miner. 2006, 91, 1300–1312. [Google Scholar] [CrossRef]
- Zinkernagel, U. Cathodoluminescence of quartz and its application to sandstone petrology. In Contributions to Sedimentary Geology; Schweizerbart Science Publishers: Stuttgart, Germany, 1978. [Google Scholar]
- Frelinger, S.N.; Ledvina, M.D.; Kyle, J.R.; Zhao, D. Scanning electron microscopy cathodoluminescence of quartz: Principles, techniques and applications in ore geology. Ore Geol. Rev. 2015, 65, 840–852. [Google Scholar] [CrossRef]
- Müller, A.; Breiter, K.; Seltmann, R.; Pécskay, Z. Quartz and feldspar zoning in the eastern Erzgebirge volcano-plutonic complex (Germany, Czech Republic): Evidence of multiple magma mixing. Lithos 2005, 80, 201–227. [Google Scholar] [CrossRef]
- Boggs, S.; Krinsley, D.H.; Goles, G.G.; Seyedolali, A.; Dypvik, H. Identification of shocked quartz by scanning cathodoluminescence imaging. Meteorit. Planet. Sci. 2001, 36, 783–791. [Google Scholar] [CrossRef]
- Müller, A.; Seltmann, R.; Halls, C.; Siebel, W.; Dulski, P.; Jeffries, T.; Spratt, J.; Kronz, A. The magmatic evolution of the Land’s End pluton, Cornwall, and associated pre-enrichment of metals. Ore Geol. Rev. 2006, 28, 329–367. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, Z.; Paquette, J.; White, N.C.; Hou, Z.; Ge, L. Magmatic Au mineralization at the Bilihe Au deposit, China. Econ. Geol. 2015, 110, 1661–1668. [Google Scholar] [CrossRef]
- Takahashi, R.; Müller, A.; Matsueda, H.; Okrugin, V.M.; Ono, S.; van den Kerkhof, A.; Kronz, A.; Elena, D.A. Cathodoluminescence and trace elements in quartz: Clues to metal precipitation mechanisms at the Asachinskoe gold deposit in Kamchatka. In Origin and Evolution of Natural Diversity, Proceedings of the International Symposium, The Origin and Evolution of Natural Diversity, Sapporo, Japan, 1–5 October 2007; COE for Neo-Science of Natural History, Hokkaido University: Sapporo, Japan, 2008; pp. 175–184. [Google Scholar]
- Van den Kerkhof, A.M.; Scherer, T.; Riganti, A. Cathodoluminescence and EPR analysis of Archean quarzites from the Nondweni Greenstone Belt, South Africa. In Proceedings of the International Conference on Cathodoluminescence and Related Techniques in Geosciences and Geomaterials, Nancy, France, 2–4 September 1996; p. 75. [Google Scholar]
- Vasyukova, O.V. Types and origin of quartz and quartz-hosted fluid inclusions in mineralised porphyries. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2011. [Google Scholar]
- Watt, G.R.; Wright, P.; Galloway, S.; McLean, C. Cathodoluminescence and trace element zoning in quartz phenocrysts and xenocrysts. Geochim. Cosmochim. Acta 1997, 61, 4337–4348. [Google Scholar] [CrossRef]
- Vasyukova, O.; Goemann, K.; Kamenetsky, V.; MacRae, C.; Wilson, N. Cathodoluminescence properties of quartz eyes from porphyry-type deposits: Implications for the origin of quartz. Am. Miner. 2012, 98, 98–109. [Google Scholar] [CrossRef]
- Kontak, D.J.; Clark, A.H. The Minastira peraluminous granite, Puno, southeastern Peru: A quenched, hypabyssal intrusion recording magma commingling and mixing. Miner. Mag. 1997, 61, 743–764. [Google Scholar] [CrossRef]
- Burt, R.M.; Cole, J.W.; Vroon, P.Z. Volcanic geology and geochemistry of Motuhora (Whale Island), Bay of Plenty, New Zealand. N. Zeal. J. Geol. Geophys. 1996, 39, 565–580. [Google Scholar] [CrossRef]
- Manning, D. The effect of fluorine on liquidus phase relationships in the system Qz-Ab-Or with excess water at 1 kb. Contrib. Miner. Pet. 1981, 76, 206–215. [Google Scholar] [CrossRef]
- Eklund, O.; Shebanov, A.D. The origin of rapakivi texture by sub-isothermal decompression. Precambrian Res. 1999, 95, 129–146. [Google Scholar] [CrossRef]
- Kuşcu, G.G.; Floyd, P.A. Mineral compositional and textural evidence for magma mingling in the Saraykent volcanics. Lithos 2001, 56, 207–230. [Google Scholar] [CrossRef]
- Frondel, C. Origin of the Segmental Coloration of Amethyst and Smoky Quartz. American Museum Novitates; no. 758; American Museum of Natural History: New York, NY, USA, 1934. [Google Scholar]
- Gurbanov, S.V.; Chernukha, A.G.; Koshchug, D.G.; Kurasova, D.G.; Fedyushchenko, S.P. EPR spectroscopy and geochemistry of rock-forming quartz as an indicator of the superimposed processes in rocks of igneous associations of various ages in the Greater Caucasus. Geochem. Int. 1999, 37, 519–604. [Google Scholar]
- Hiraga, T.; Anderson, I.M.; Kohlstedt, D.L. Chemistry of grain boundaries in mantle rocks. Am. Miner. 2003, 88, 1015–1019. [Google Scholar] [CrossRef]
- Hiraga, T.; Anderson, I.M.; Kohlstedt, D.L. Grain boundaries as reservoirs of incompatible elements in the Earth’s mantle. Nature 2004, 427, 699–703. [Google Scholar] [CrossRef]
- Lan, Q.; Lin, J.; Fu, S.; Luo, J. Cathodoluminescent textures and trace element signatures of hydrothermal quartz from the granite-related No. 302 uranium deposit, South China: A reconnaissance study for their genetic significances. J. Geochem. Explor. 2021, 224, 106740. [Google Scholar] [CrossRef]
- Muller, A.; Wiedenbeck, M.; van den Kerkhof, A.M.; Kronz, A.; Simon, K. Trace elements in quartz-a combined electron microprobe, secondary ion mass spectrometry, laser-ablation ICP-MS, and cathodoluminescence study. Eur. J. Mineral. 2003, 15, 747–763. [Google Scholar] [CrossRef]
- Leeman, W.P.; MacRae, C.M.; Wilson, N.C.; Torpy, A.; Lee, C.A.; Student, J.J.; Thomas, J.B.; Vicenzi, E.P. A study of cathodoluminescence and trace element compositional zoning in natural quartz from volcanic rocks: Mapping titanium content in quartz. Microsc. Microanal. 2012, 18, 1322–1341. [Google Scholar] [CrossRef]
- Breiter, K.; Müller, A. Evolution of rare-metal granitic magmas documented by quartz chemistry. Eur. J. Mineral. 2009, 21, 335–346. [Google Scholar] [CrossRef]
- Shane, P.; Smith, V.C.; Nairn, I. Millennial timescale resolution of rhyolite magma recharge at Tarawera volcano: Insights from quartz chemistry and melt inclusions. Contrib. Miner. Pet. 2008, 156, 397–411. [Google Scholar] [CrossRef]
- Spear, F.S.; Wark, D.A. Cathodoluminescence imaging and titanium thermometry in metamorphic quartz. J. Metamorph. Geol. 2009, 27, 187–205. [Google Scholar] [CrossRef]
- Wehrle, E.A.; McDonald, A.M. Cathodoluminescence and trace-element chemistry of quartz from Sudbury offset dikes: Observations, interpretations, and genetic implications. Can. Mineral. 2019, 57, 947–963. [Google Scholar] [CrossRef]
- Ruffini, R.; Borghi, A.; Cossio, R.; Olmi, F.; Vaggelli, G. Volcanic Quartz Growth Zoning Identified by Cathodoluminescence and EPMA Studies. Mikrochim. Acta 2002, 139, 151–158. [Google Scholar] [CrossRef]
- Barbee, O.; Chesner, C.; Deering, C. Quartz crystals in Toba rhyolites show textures symptomatic of rapid crystallization. Am. Mineral. J. Earth Planet. Mater. 2020, 105, 194–226. [Google Scholar] [CrossRef]
- Donovan, J.J.; Lowers, H.A.; Rusk, B.G. Improved electron probe microanalysis of trace elements in quartz. Am. Mineral. 2011, 96, 274–282. [Google Scholar] [CrossRef]
- Ramseyer, K.; Mullis, J. Factors influencing short-lived blue cathodoluminescence of alpha-quartz. Am. Mineral. 1990, 75, 791–800. [Google Scholar]
- Perny, B.; Eberhardt, P.; Ramseyer, K.; Mullis, J.; Pankrath, R. Microdistribution of Al, Li, and Na in α quartz: Possible causes and correlation with short-lived cathodoluminescence. Am. Mineral. 1992, 77, 534–544. [Google Scholar]
- Chamberlain, K.J.; Morgan, D.; Wilson, C. Timescales of mixing and mobilisation in the Bishop Tuff magma body: Perspectives from diffusion chronometry. Contrib. Miner. Pet. 2014, 168, 1034. [Google Scholar] [CrossRef]
- Gualda, G.A.R.; Pamukcu, A.S.; Ghiorso, M.S.; Sutton, A.S.R., Jr.; Rivers, M.L. Timescales of Quartz Crystallization and the Longevity of the Bishop Giant Magma Body. PLoS ONE 2012, 7, e37492. [Google Scholar] [CrossRef]
- Jollands, M.C.; Bloch, E.; Müntener, O. New Ti-in-quartz diffusivities reconcile natural Ti zoning with time scales and temperatures of upper crustal magma reservoirs. Geology 2020, 48, 654–657. [Google Scholar] [CrossRef]
- Mercer, C.; Reed, M.H. Time Scales of Porphyry Cu Deposit Formation: Insights from Titanium Diffusion in Quartz. Econ. Geol. 2015, 110, 587–602. [Google Scholar] [CrossRef]
- Ackerson, M.R.; Mysen, B.O.; Tailby, N.D.; Watson, E.B. Low-temperature crystallization of granites and the implications for crustal magmatism. Nature 2018, 559, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Spear, F.S.; Ashley, K.T.; Webb, L.E.; Thomas, J.B. Ti diffusion in quartz inclusions: Implications for metamorphic time scales. Contrib. Miner. Pet. 2012, 164, 977–986. [Google Scholar] [CrossRef]
- Cole, J.W.; Deering, C.D.; Burt, R.M.; Sewell, S.; Shane, P.A.R.; Matthews, N.E. Okataina Volcanic Centre, Taupo Volcanic Zone, New Zealand: A review of volcanism and synchronous pluton development in an active, dominantly silicic caldera system. Earth-Sci. Rev. 2014, 128, 1–17. [Google Scholar] [CrossRef]
- Ostapenko, G.T.; Gamarnik, M.Y.; Gorogotskaya, L.I.; Kuznetsov, G.V.; Tarashchan, A.N.; Timoshkova, L.P. Isomorphism of titanium substitution for silicon in quartz: Experimental data. Miner. Zh 1987, 9, 30–40. [Google Scholar]
- Behr, W.M.; Platt, J.P. A naturally constrained stress profile through the middle crust in an extensional terrane. Earth Planet. Sci. Lett. 2011, 303, 181–192. [Google Scholar] [CrossRef]
- Reid, M.R.; Vazquez, J.A.; Schmitt, A.K. Zircon-scale insights into the history of a Supervolcano, Bishop Tuff, Long Valley, California, with implications for the Ti-in-zircon geothermometer. Contrib. Mineral. Petrol. 2011, 161, 293–311. [Google Scholar] [CrossRef]
- Ehrlich, K.; Vers, E.; Kirs, J.; Soesoo, A. Using a titanium-in-quartz geothermometer for crystallization temperature estimation of the Palaeoproterozoic Suursaari quartz porphyry. Est. J. Earth Sci. 2012, 61, 195. [Google Scholar] [CrossRef]
- Girard, G.; Stix, J. Rapid extraction of discrete magma batches from a large differentiating magma chamber: The Central Plateau Member rhyolites, Yellowstone Caldera, Wyoming. Contrib. Mineral. Petrol. 2010, 160, 441–465. [Google Scholar] [CrossRef]
- Ostapenko, G.T.; Tarashchan, A.N.; Mitsyuk, B.M. Rutile-quartz geothermobarometer. Geochem. Int. 2007, 45, 506–508. [Google Scholar] [CrossRef]
- Ferreira, C. Gene Expression Programming: Mathematical Modeling by an Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2006; Volume 21. [Google Scholar]
- Iqbal, M.F.; Liu, Q.-F.; Azim, I.; Zhu, X.; Yang, J.; Javed, M.F.; Rauf, M. Prediction of mechanical properties of green concrete incorporating waste foundry sand based on gene expression programming. J. Hazard. Mater. 2019, 384, 121322. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.F.; Amin, M.N.; Shah, M.I.; Khan, K.; Iftikhar, B.; Farooq, F.; Aslam, F.; Alyousef, R.; Alabduljabbar, H. Applications of Gene Expression Programming and Regression Techniques for Estimating Compressive Strength of Bagasse Ash based Concrete. Crystals 2020, 10, 737. [Google Scholar] [CrossRef]
- Jourdan, A.-L.; Vennemann, T.W.; Mullis, J.; Ramseyer, K. Oxygen isotope sector zoning in natural hydrothermal quartz. Miner. Mag. 2009, 73, 615–632. [Google Scholar] [CrossRef]
- Allan, M.M.; Yardley, B.W. Tracking meteoric water infiltration into a magmatic hydrothermal system: A cathodoluminescence, oxygen isotope, and trace element study of quartz from Mt. Leyshon, Australia. Chem. Geol. 2007, 240, 343–360. [Google Scholar] [CrossRef]
- Lehmann, K.; Berger, A.; Gotte, T.; Ramseyer, K.; Wiedenbeck, M. Growth related zonations in authigenic and hydrothermal quartz characterized by SIMS-, EPMA-, SEM-CL-and SEM-CC-imaging. Mineral. Mag. 2009, 73, 633–643. [Google Scholar] [CrossRef]
- Northrup, P.A.; Reeder, R.J. Evidence for the importance of growth-surface structure to trace element incorporation in topaz. Am. Mineral. 1994, 79, 1167–1175. [Google Scholar]
- Wass, S.Y. The origin and petrogenetic significance of hour-glass zoning in titaniferous clinopyroxenes. Miner. Mag. 1973, 39, 133–144. [Google Scholar] [CrossRef]
- Watson, E.B.; Liang, Y. A simple model for sector zoning in slowly grown crystals: Implications for growth rate and lattice diffusion, with emphasis on accessory minerals in crustal rocks. Am. Mineral. 1995, 80, 1179–1187. [Google Scholar] [CrossRef]
- Onasch, C.M.; Vennemann, T.W. Disequilibrium partitioning of oxygen isotopes associated with sector zoning in quartz. Geology 1995, 23, 1103–1106. [Google Scholar] [CrossRef]
- Rusk, M.; Reed, B. Scanning electron microscope: Cathodoluminescence analysis of quartz reveals complex growth histories in veins from the Butte porphyry copper deposit, Montana. Geology 2002, 30, 727–730. [Google Scholar] [CrossRef]
- Nadoll, P.; Rehm, M.; Duschl, F.; Klemd, R.; Kraemer, D.; Sośnicka, M. REY and Trace Element Chemistry of Fluorite from Post-Variscan Hydrothermal Veins in Paleozoic Units of the North German Basin. Geosciences 2018, 8, 283. [Google Scholar] [CrossRef]
- Ihinger, P.D.; Zink, S.I. Determination of relative growth rates of natural quartz crystals. Nature 2000, 404, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Monecke, T.; Kempe, U.; Götze, J. Genetic significance of the trace element content in metamorphic and hydrothermal quartz: A reconnaissance study. Earth Planet. Sci. Lett. 2002, 202, 709–724. [Google Scholar] [CrossRef]
- Fu, S.; Lan, Q.; Yan, J. Trace element chemistry of hydrothermal quartz and its genetic significance: A case study from the Xikuangshan and Woxi giant Sb deposits in southern China. Ore Geol. Rev. 2020, 126, 103732. [Google Scholar] [CrossRef]
- Cherniak, D. Diffusion in Quartz, Melilite, Silicate Perovskite, and Mullite. Rev. Miner. Geochem. 2010, 72, 735–756. [Google Scholar] [CrossRef]
- Ramseyer, K.; Mullis, J. Geologic application of cathodoluminescence of silicates. In Cathodoluminescence in Geosciences; Springer: Berlin/Heidelberg, Germany, 2000; pp. 177–191. [Google Scholar]
- Rusk, B.G.; Reed, M.H.; Dilles, J.H. Fluid Inclusion Evidence for Magmatic-Hydrothermal Fluid Evolution in the Porphyry Copper-Molybdenum Deposit at Butte, Montana. Econ. Geol. 2008, 103, 307–334. [Google Scholar] [CrossRef]
- Shibue, Y.; Chiba, H.; Kusakabe, M.; Morishita, Y. Temperatures and Oxygen Isotopic Compositions of Hydrothermal Fluids for the Takatori Tungsten-copper Deposit, Japan. Resour. Geol. 2005, 55, 101–110. [Google Scholar] [CrossRef]
- Johnson, S.E.; Song, W.J.; Cook, A.C.; Vel, S.S.; Gerbi, C.C. The quartz α↔β phase transition: Does it drive damage and reaction in continental crust? Earth Planet. Sci. Lett. 2021, 553, 116622. [Google Scholar] [CrossRef]
- Aparicio, A.; Bustillo, M. Cathodoluminescence Spectral Characteristics of Quartz and Feldspars in Unaltered and Hydrothermally Altered Volcanic Rocks (Almeria, Spain). Spectrosc. Lett. 2012, 45, 104–108. [Google Scholar] [CrossRef]
- Schaarschmidt, A.; Haase, K.M.; de Wall, H.; Bestmann, M.; Krumm, S.; Regelous, M. Upper crustal fluids in a large fault system: Microstructural, trace element and oxygen isotope study on multi-phase vein quartz at the Bavarian Pfahl, SE Germany. Geol. Rundsch. 2018, 108, 521–543. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Pirajno, F.; Li, N.; Guo, D.-S.; Lai, Y. Isotope systematics and fluid inclusion studies of the Qiyugou breccia pipe-hosted gold deposit, Qinling Orogen, Henan province, China: Implications for ore genesis. Ore Geol. Rev. 2009, 35, 245–261. [Google Scholar] [CrossRef]
- Yu, D.; Xu, D.; Zhao, Z.; Huang, Q.; Wang, Z.; Deng, T.; Zou, S. Genesis of the Taolin Pb-Zn deposit in northeastern Hunan Province, South China: Constraints from trace elements and oxygen-sulfur-lead isotopes of the hydrothermal minerals. Miner. Deposita 2020, 55, 1467–1488. [Google Scholar] [CrossRef]
- Shu, Q.; Lai, Y. Fluid Inclusion and Oxygen Isotope Constraints on the Origin and Hydrothermal Evolution of the Haisugou Porphyry Mo Deposit in the Northern Xilamulun District, NE China. Geofluids 2017, 2017, 10–12. [Google Scholar] [CrossRef]
- Seward, T.M. Metal transport by hydrothermal ore fluids. Geochem. Hydrothermal Ore Depos. 1997, 3, 435–486. [Google Scholar]
- Hensler, A.-S.; Hagemann, S.G.; Brown, P.E.; Rosière, C. Using oxygen isotope chemistry to track hydrothermal processes and fluid sources in itabirite-hosted iron ore deposits in the Quadrilátero Ferrífero, Minas Gerais, Brazil. Miner. Depos. 2013, 49, 293–311. [Google Scholar] [CrossRef]
- Lubben, J.D. Silicification across the Betze-Post Carlin-Type Gold Deposit: Clues to Ore Fluid Properties and Sources, Northern Carlin Trend, Nevada. Master’s Thesis, University of Nevada, Reno, NV, USA, 2003. [Google Scholar]
- Götze, J.; Plötze, M.; Trautmann, T. Structure and luminescence characteristics of quartz from pegmatites. Am. Mineral. 2005, 90, 13–21. [Google Scholar] [CrossRef]
- Müller, A.; Wanvik, J.E.; Ihlen, P.M. Petrological and chemical characterisation of high-purity quartz deposits with examples from Norway. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 71–118. [Google Scholar]
- Harben, P.W. The Industrial Mineral Handybook—A Guide to Markets, Specifications and Prices, 4th ed.; Industrial Mineral Information: Worcester Park, UK, 2002; p. 412. [Google Scholar]
- Richter, D.K.; Götte, T.; Götze, J.; Neuser, R.D. Progress in application of cathodoluminescence (CL) in sedimentary petrology. Mineral. Petrol. 2003, 79, 127–166. [Google Scholar] [CrossRef]
- Gorobets, B.S.; Rogozine, A.A. Luminescent Spectra of Minerals; RPC VIMS: Moscow, Russia, 2002. [Google Scholar]
- Gaft, M.; Reisfeld, R.; Panczer, G. Luminescence Spectroscopy of Minerals and Materials; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Boggs, S., Jr.; Krinsley, H. Application of Cathodoluminescence Imaging to the Study of Sedimentary Rocks; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Gucsik, A. Cathodoluminescence and Its Application in the Planetary Sciences; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
| Linking Function Assessment | |||||
|---|---|---|---|---|---|
| Model | No. of Chromosome | Head Size | Genes | Linking Function | R2 |
| Gene Expression Programming (GEP+) | 200 | 8 | 3 | Addition | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.A.; Shao, Y.; Zhang, Y.; Zhao, H.; Zhao, L. Texture and Trace Element Geochemistry of Quartz: A Review. Minerals 2022, 12, 1042. https://doi.org/10.3390/min12081042
Shah SA, Shao Y, Zhang Y, Zhao H, Zhao L. Texture and Trace Element Geochemistry of Quartz: A Review. Minerals. 2022; 12(8):1042. https://doi.org/10.3390/min12081042
Chicago/Turabian StyleShah, Sajjad Ahmad, Yongjun Shao, Yu Zhang, Hongtao Zhao, and Lianjie Zhao. 2022. "Texture and Trace Element Geochemistry of Quartz: A Review" Minerals 12, no. 8: 1042. https://doi.org/10.3390/min12081042
APA StyleShah, S. A., Shao, Y., Zhang, Y., Zhao, H., & Zhao, L. (2022). Texture and Trace Element Geochemistry of Quartz: A Review. Minerals, 12(8), 1042. https://doi.org/10.3390/min12081042






