Reducing Negative Effects of Oxidation on Flotation of Complex Cu–Zn Sulfide Ores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore Samples
2.2. EDTA Tests
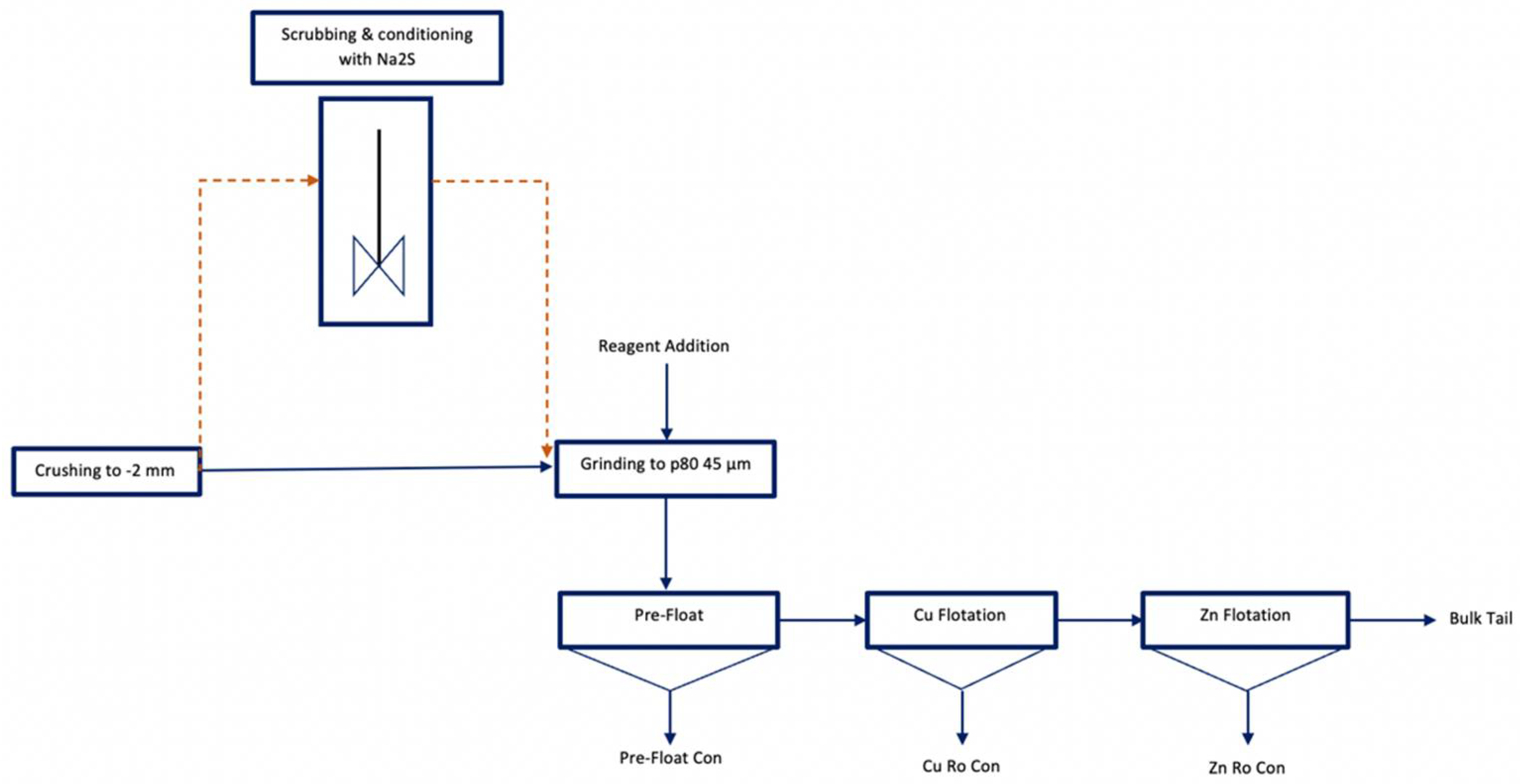
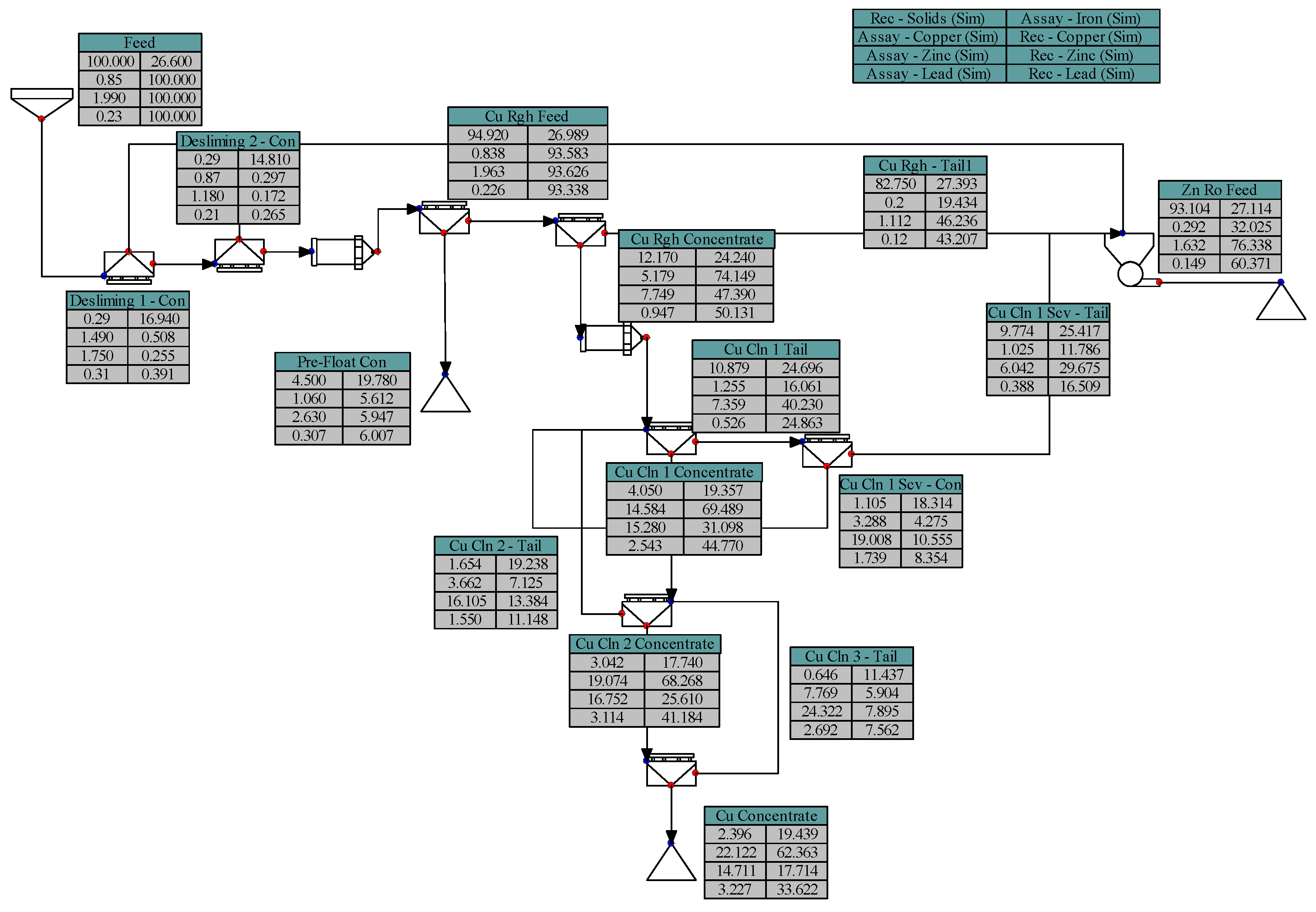
2.3. Batch Flotation Tests
- Pre-Floatation: 25 µL MIBC.
- Copper Flotation:3 kg/t MBS, 1 kg/t ZnSO4, 500 g/t Na2S added in the millpH 6–790 g/t Sodium Aerofloat (NaAF, a dithiophosphate-type collector) + 30 g/t Aero8761 (modified thionocarbamate).
- Zinc Flotation:pH 11.5600 g/t CuSO440 g/t SIPX (Sodium Isopropyl Xanthate).
3. Results and Discussion
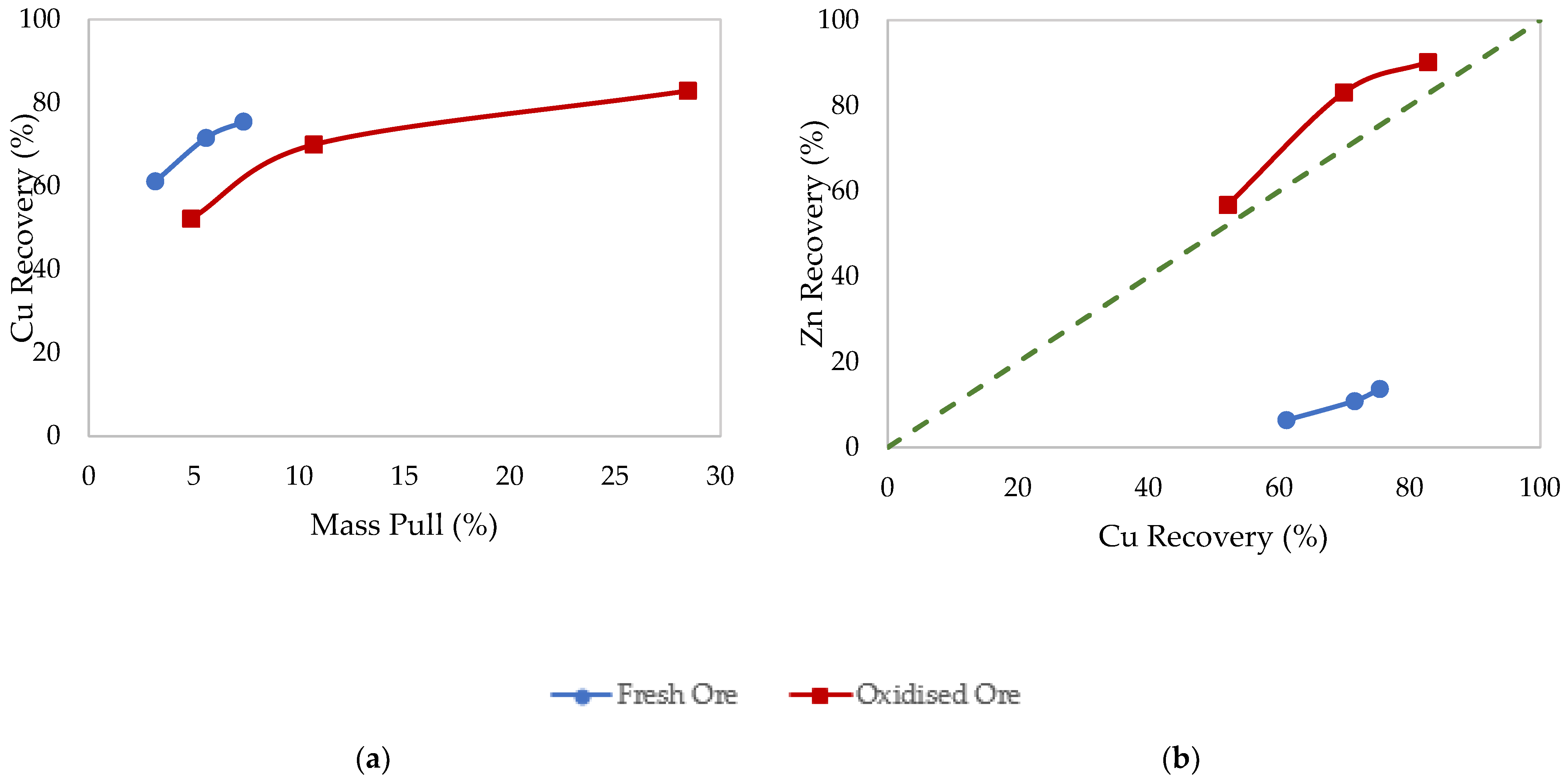
3.1. Effects of Surface Oxidation
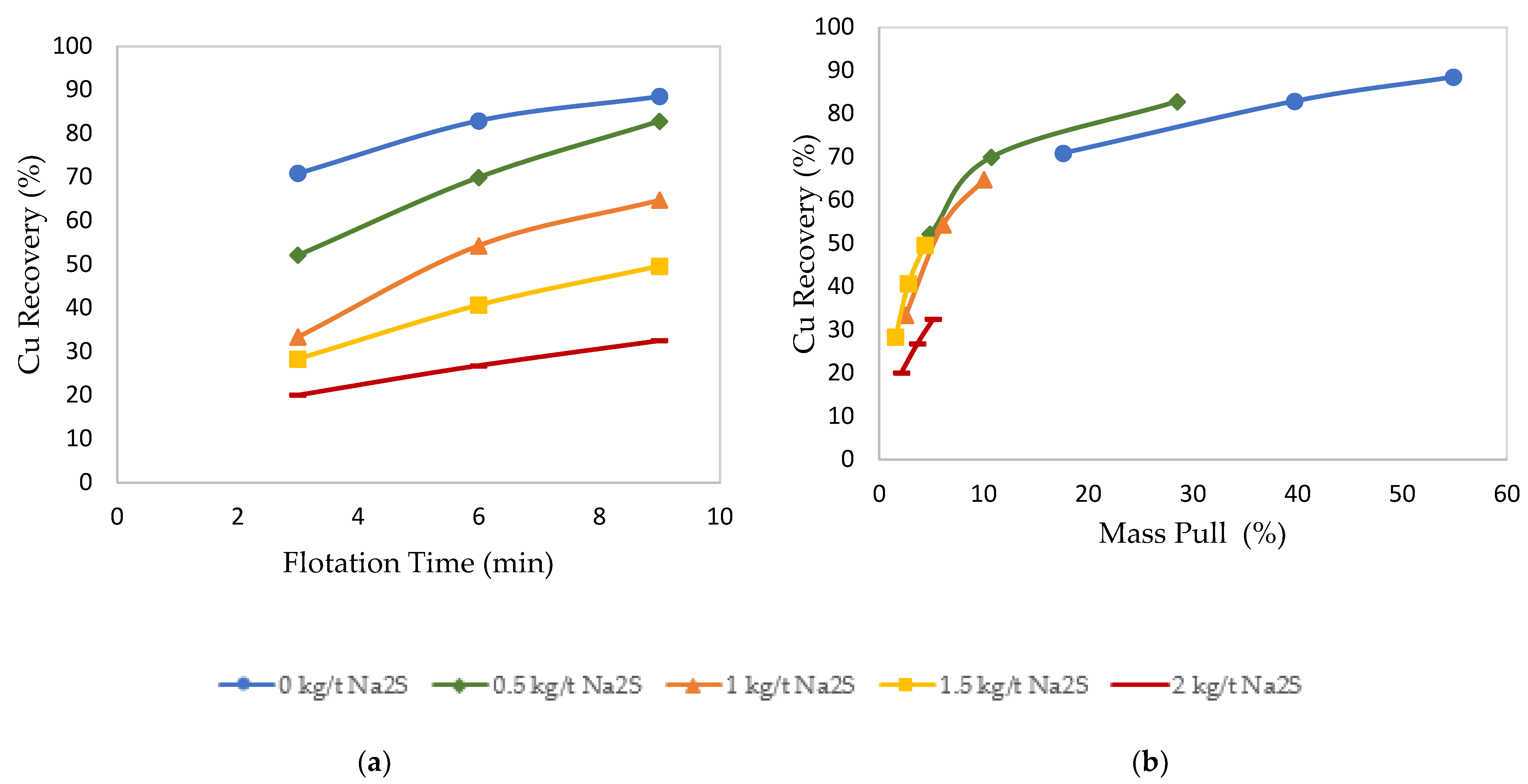
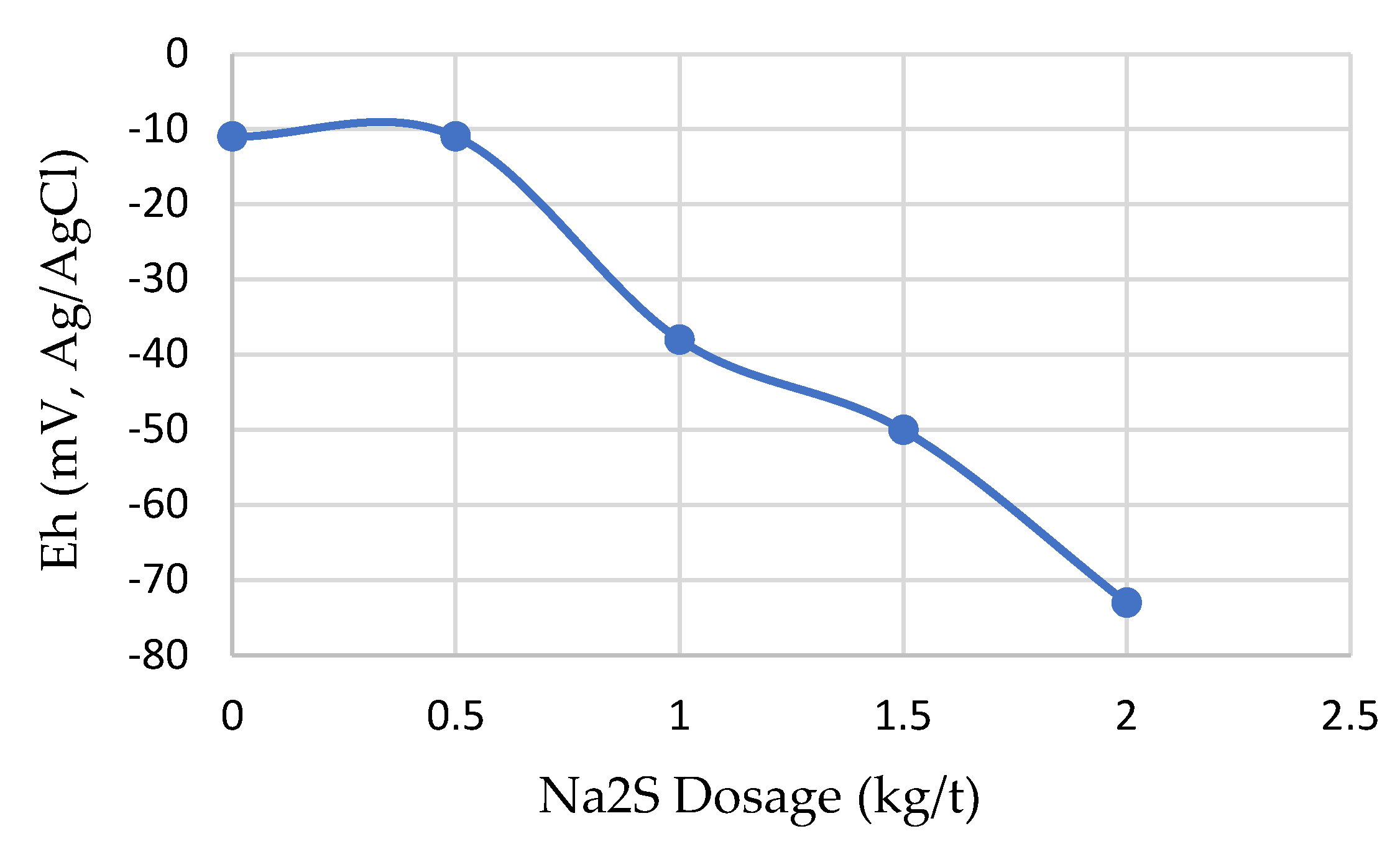
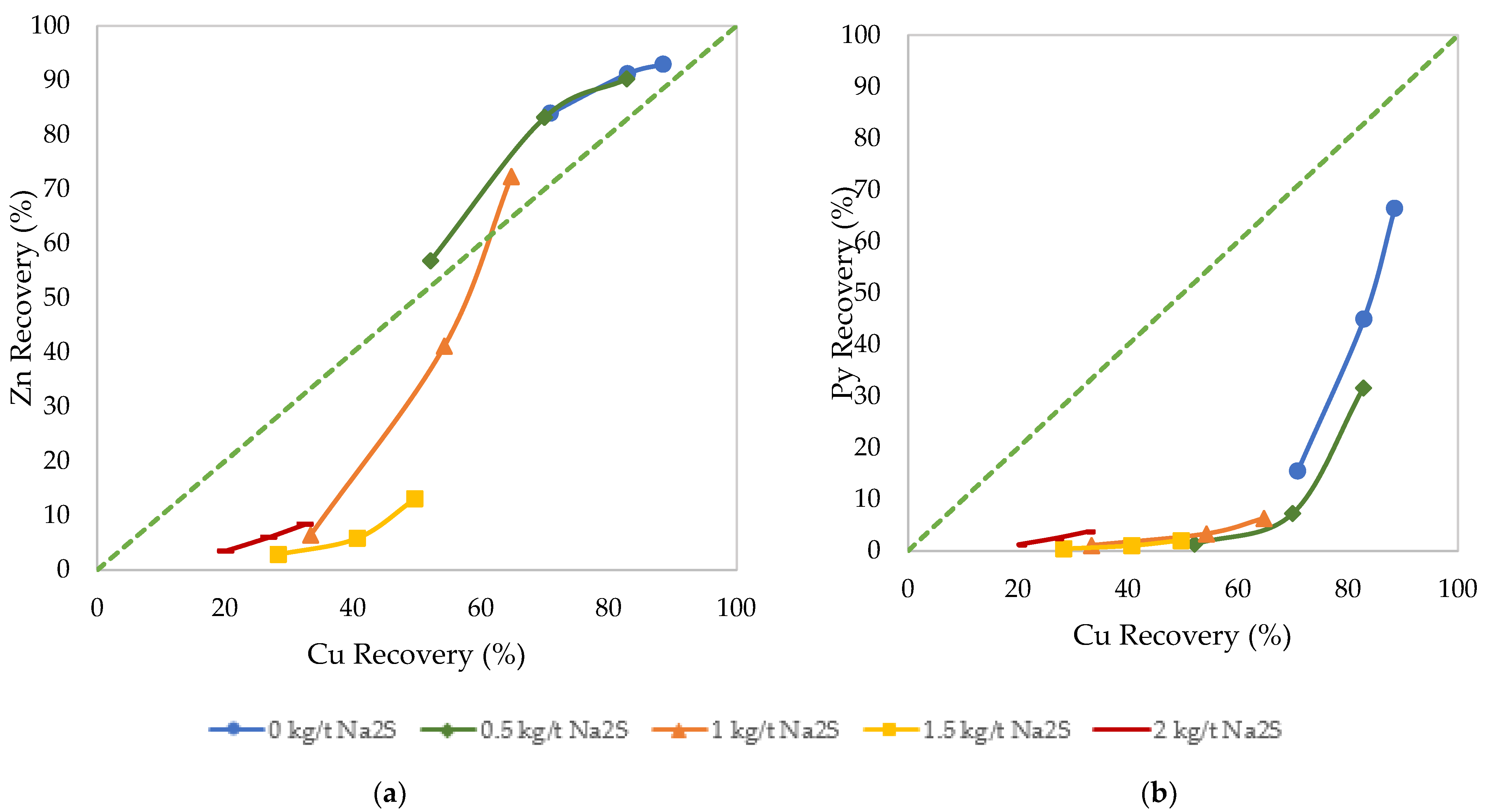
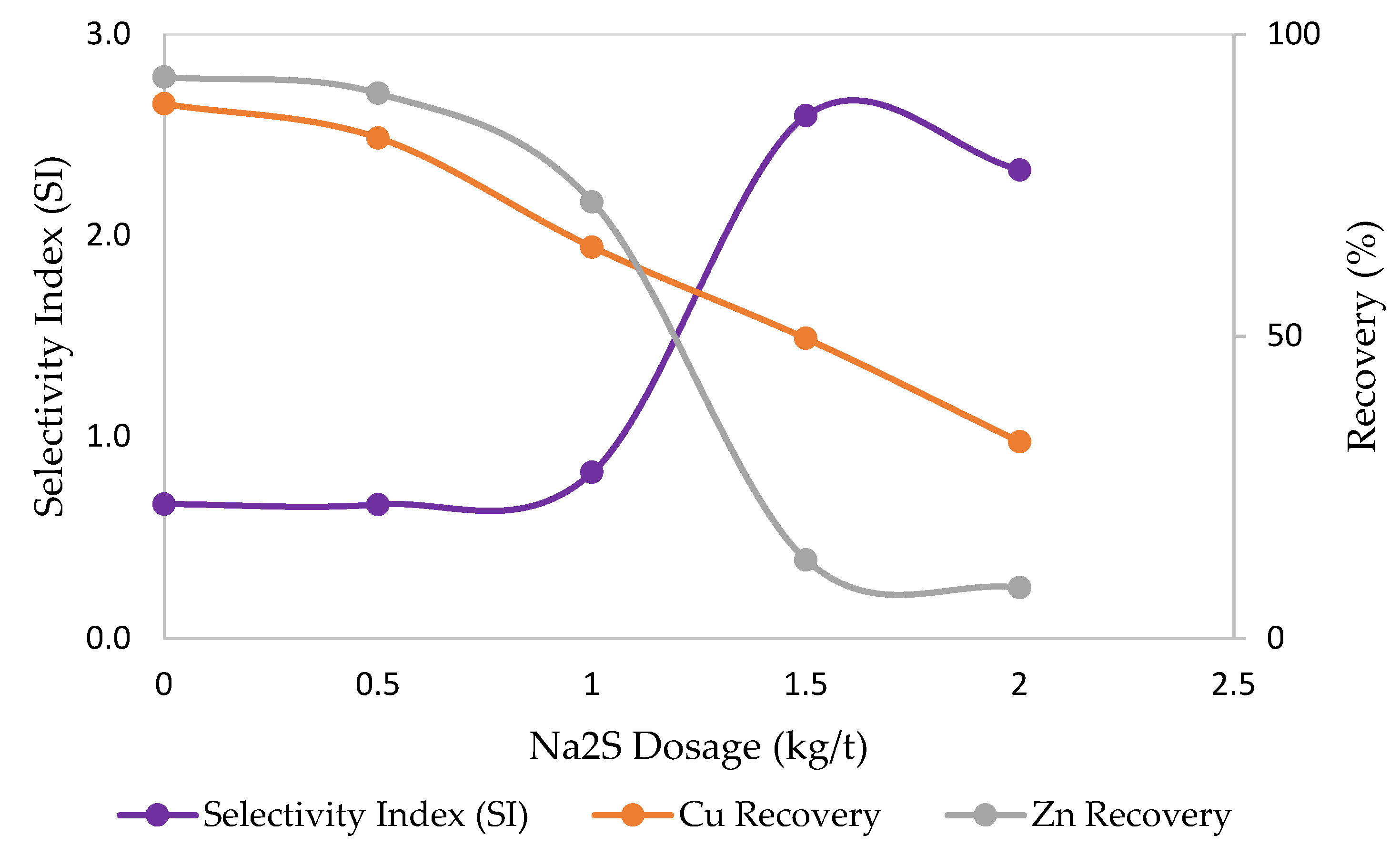
3.2. Effects of Sulfidization
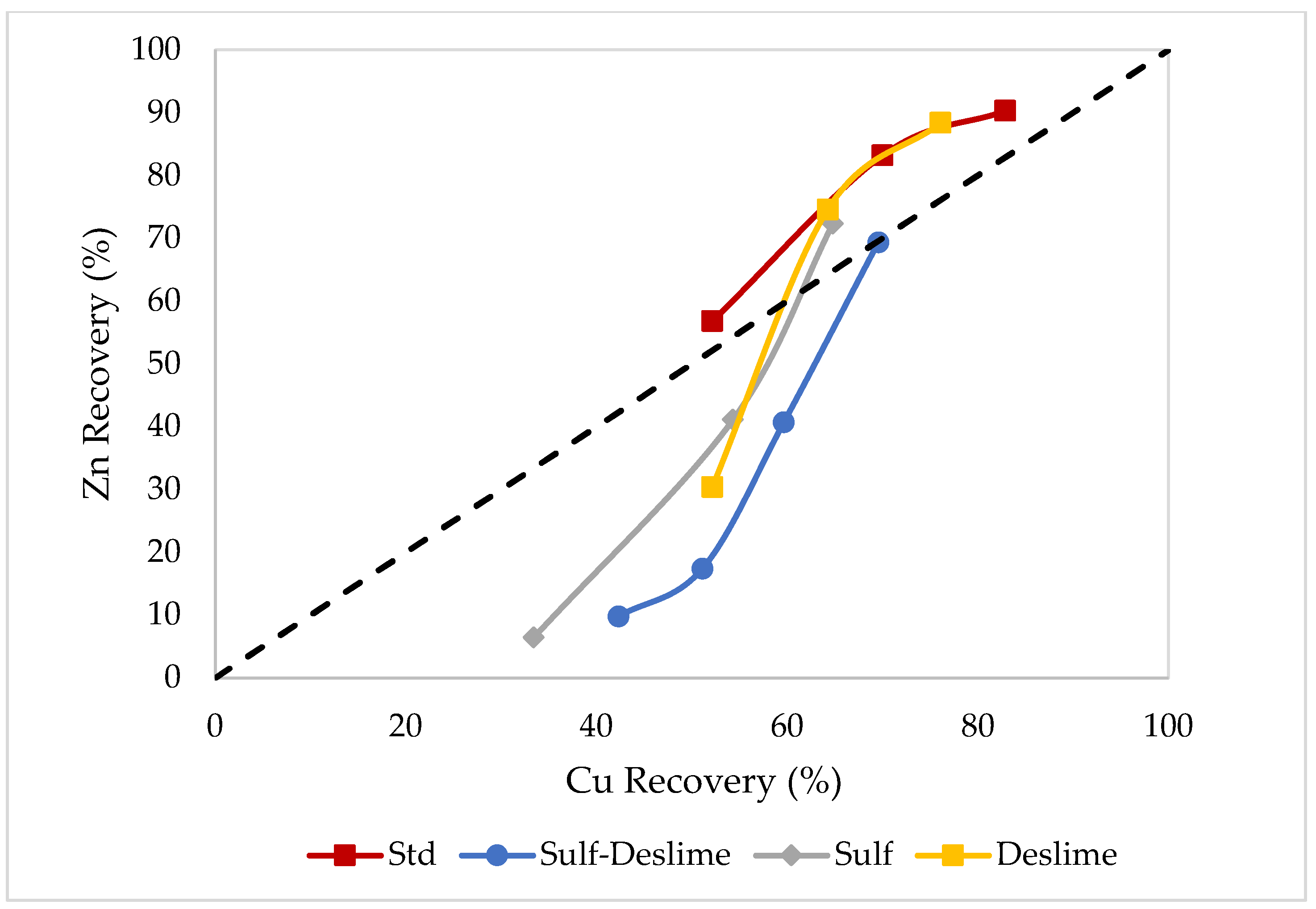
3.3. Effects of Desliming
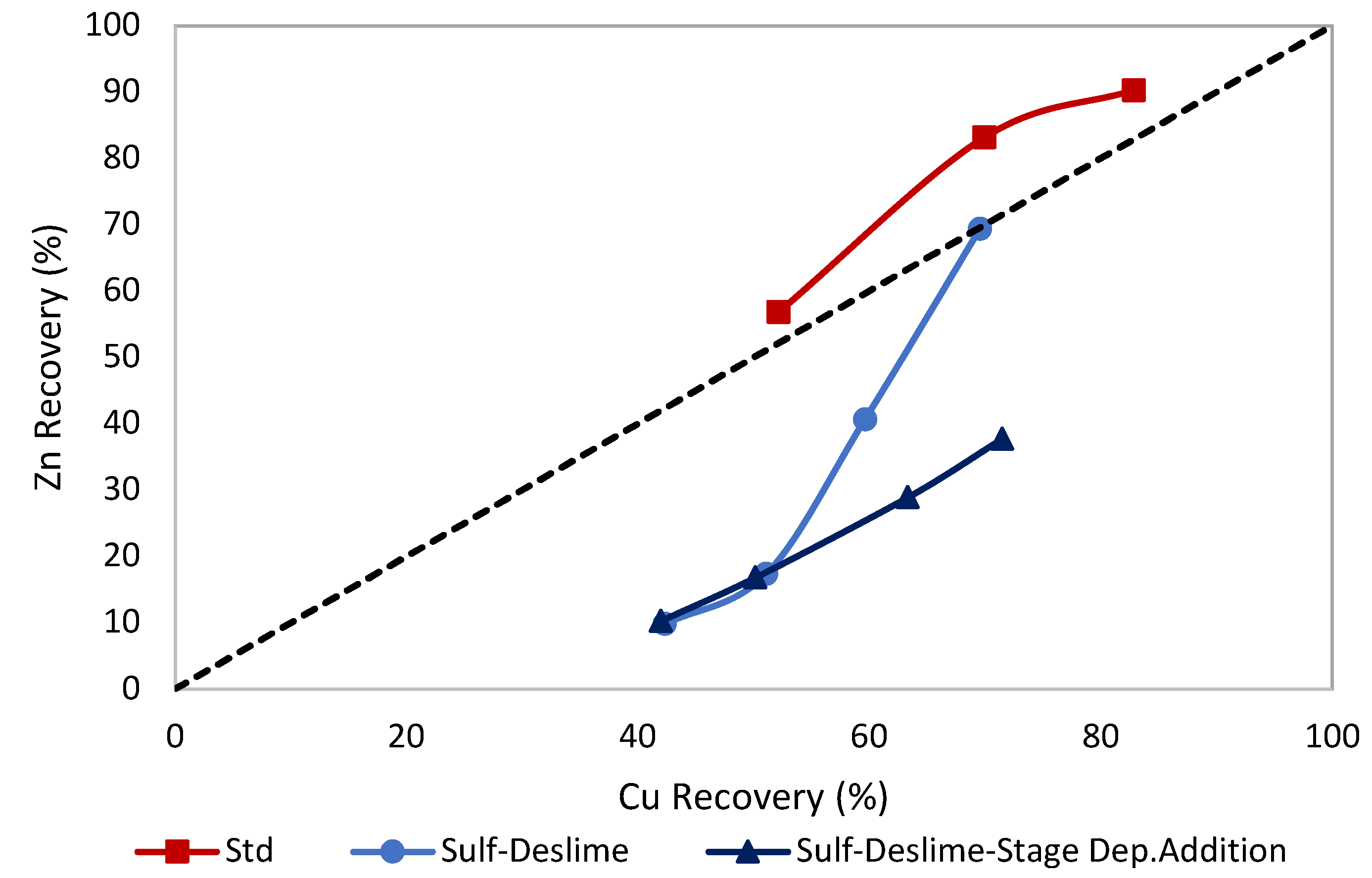
3.4. Effects of Stage of Depressants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, S.R.; Labonte, G.; Finch, J.A. Electrochemistry in the plant. In Innovations in Flotation Technology; Springer: Dordrecht, The Netherlands, 1992; pp. 57–100. [Google Scholar]
- Ralston, J.; Fornasiero, D.; Grano, S. Pulp and solution chemistry. In Froth Flotation: A Century of Innovation; Fuerstenau, M.C., Jameson, G.J., Yoon, R.H., Eds.; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2005. [Google Scholar]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Smart, R.S.C. Surface layers in base metal sulfide flotation. Miner. Eng. 1991, 4, 891–909. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Amarantidis, J.; Skinner, W.; Prestidge, C.A.; La Vanier, L.; Grano, S. Surface analytical studies of oxidation and collector adsorption in sulphide mineral flotation. Scan. Microsc. 1998, 12, 553–583. [Google Scholar]
- Vaughan, D.J.; England, K.E.R.; Kelsall, G.H.; Yin, Q. Electrochemical oxidation of chalcopyrite (CuFeS2) and the related metal-enriched derivatives Cu4Fe5S8, Cu9Fe9S16 and Cu9Fe8S16. Am. Mineral. 1995, 80, 725–731. [Google Scholar] [CrossRef]
- Senior, G.D.; Trahar, W.J. The influence of metal hydroxides and collector on the flotation of chalcopyrite. Int. J. Miner. Processing. 1991, 33, 321–341. [Google Scholar] [CrossRef]
- Rumball, J.A.; Richmond, G.D. Measurement of oxidation in a base metal flotation circuit by selective leaching with EDTA. Int. J. Miner. Process. 1996, 48, 1–20. [Google Scholar] [CrossRef]
- Ekmekçi, Z.; Buswell, M.A.; Bradshaw, D.J.; Harris, P.J. The value and limitations of electrochemical measurements in flotation of precious metal ores. Miner. Eng. 2005, 18, 825–831. [Google Scholar] [CrossRef]
- Greet, C.; Smart, R.S.C. Diagnostic leaching of galena and its oxidation products with EDTA. Min. Eng. 2002, 15, 515–522. [Google Scholar] [CrossRef]
- Clark, D.W.; Newell, A.J.H.; Chilman, G.F.; Capps, P.G. Improving flotation recovery of copper sulphides by nitrogen gas and sulphidisation conditioning. Miner. Eng. 2000, 13, 1197–1206. [Google Scholar] [CrossRef]
- Baker, M.; Pietrobon, M.; Ralston, J.; Smart, R.S.C. Iron sulphide interactions and their influence on sulphide mineral flotation. In AMIRA Project P260 Final Report; Australian Mineral Industries Research Association Limited: Parkville, Austria, 1991; Volume 4. [Google Scholar]
- Houot, R.; Duhamet, D. Importance of oxygenation of pulps in the flotation of sulfide ores. Int. J. Miner. Process. 1990, 29, 77–87. [Google Scholar] [CrossRef]
- Kant, C.; Rao, S.R.; Finch, J.A. Distribution of surface metal ions among the products of chalcopyrite flotation. Miner. Eng. 1994, 7, 905–916. [Google Scholar] [CrossRef]
- Smart, R.St. Surface Analysis. In AMIRA P260 Report No: 8; Australian Mineral Industries Research Association Limited: Parkville, Austria, 1990. [Google Scholar]
- James, R.O.; Healy, T.W. Adsorption of hydrolyzable metal ions at the oxide-water interface. J. Colloid Interf. Sci. 1972, 3, 299–313. [Google Scholar]
- Aldrich, C.; Feng, D. Effect of ultrasonic preconditioning of pulp on the flotation of sulphide ores. Miner. Eng. 1999, 12, 701–707. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Feng, Q.; Wen, S.; Luo, B. Surface cleaning and oxidative effects of ultrasonication on the flotation of oxidized pyrite. Powder Technol. 2017, 311, 390–397. [Google Scholar] [CrossRef]
- Clarke, P.; Fornasiero, D.; Ralston, J.; Smart, R.S.C. A study of the removal of oxidation products from sulfide mineral surfaces. Miner. Eng. 1995, 8, 1347–1357. [Google Scholar] [CrossRef]
- Yoon, R. Collectorless flotation of chalcopyrite and sphalerite ores by using sodium sulfide. Int. J. Miner. Process. 1981, 8, 31–48. [Google Scholar] [CrossRef]
- Newell, A.J.H.; Bradshaw, D.J. The development of a sulfidisation technique to restore the flotation of oxidised pentlandite. Miner. Eng. 2007, 20, 1039–1046. [Google Scholar] [CrossRef]
- Engel, M.D.; Middlebrook, P.D.; Jameson, G.J. Advances in the study of high intensity conditioning as a means of improving mineral flotation performance. Miner. Eng. 1997, 10, 55–68. [Google Scholar] [CrossRef]
- Chen, G.; Grano, S.; Sobieraj, S.; Ralston, J. The effect of high intensity conditioning on the flotation of a nickel ore. Part 1: Size-by-size analysis. Miner. Eng. 1999, 12, 1185–1200. [Google Scholar] [CrossRef]
- Yu, Y.X.; Ma, L.Q.; Wu, L.; Ye, G.C.; Sun, X.F. The role of surface cleaning in high intensity conditioning. Powder Technol. 2017, 319, 26–33. [Google Scholar] [CrossRef]
- Ekmekçi, Z.; Bıçak, Ö. Metallurgical Study on a Cu-Zn Sulfide Deposit DFS Phase-Part II; Hacettepe Mineral Technologies: Ankara, Turkey, 2019; (Unpublished Report). [Google Scholar]
- Shannon, L.K.; Trahar, W.J. The role of collector in sulphide ore flotation. In Advances in Mineral Processing; Somasundaran, P., Ed.; Proc. Symp: Littleton, CO, USA, 1986; pp. 408–425. [Google Scholar]
- Grano, S.R.; Ralston, J.; Johnson, N.W. Characterization and treatment ofheavy medium slimes in the Mt. Isa mines lead-zinc concentrator. Miner. Eng. 1988, 1, 137–150. [Google Scholar] [CrossRef]
- Bicak, O. Prediction of Closed Circuit Performance by Simulation for a Complex Sulphide Ore. In Proceedings of the IMPC Eurasia Conference, Sayfa, Antalya, Türkiye, 31 October–2 November 2019; pp. 924–930. [Google Scholar]
- Tayebi-Khorami, M.; Manlapig, E.; Forbes, E.; Bradshaw, D. The effect of non-sulphide gangue minerals in enargite separation from other copper sulphides in complex ore systems. In Proceedings of the IMPC 2018-29th International Mineral Processing Congress, Moscow, Russia, 17–21 September 2018. [Google Scholar]
- Owusu, C.; E Abreu, S.B.; Skinner, W.; Addai-Mensah, J.; Zanin, M. The influence of pyrite content on the flotation of chalcopyrite/pyrite mixtures. Miner. Eng. 2014, 55, 87–95. [Google Scholar] [CrossRef]
- Peng, Y.J.; Zhao, S.L. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation. Miner. Eng. 2011, 24, 1687–1693. [Google Scholar] [CrossRef]
- Von Oertzen, G.U.; Skinner, W.M.; Nesbitt, H.W.; Pratt, A.R.; Buckley, A.N. Cu adsorption on pyrite (1 0 0): Ab initio and spectroscopic studies. Surf. Sci. 2007, 601, 5794–5799. [Google Scholar] [CrossRef]
- Todd, E.C.; Sherman, D.M.; Purton, J.A. Surface oxidation of chalcopyrite (CuFeS2) under ambient atmospheric and aqueous (pH 2-10) conditions: Cu, Fe L-and O K-edge X-ray spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 2137–2146. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, D.; Finch, J.A. Quantifying accidental activation. Part I. Cu ion production. Miner. Eng. 2002, 15, 567–571. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Taheri, B.; Abdollahy, M.; ShafaeiTonkaboni, S.Z.; Javadian, S.; Yarahmadi, M. Dual effects of sodium sulfide on the flotation behavior of chalcopyrite: I. Effect of pulp potential. Int. J. Miner. Metall. Mater. 2014, 21, 415–422. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery: Butterworth-Heinemann; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
| Cu (%) | Fe (%) | Zn (%) | Pb (%) | S (%) | |
|---|---|---|---|---|---|
| Fresh Ore | 0.70 | 35.60 | 1.78 | 0.16 | 40.11 |
| Oxidized Ore | 0.78 | 29.7 | 1.95 | 0.27 | 32.7 |
| % Mass in the Sample | |
|---|---|
| Pyrite | 76.6 |
| Sphalerite | 2.70 |
| Galena | 0.56 |
| Arsenopyrite | 0.02 |
| Chalcopyrite and Cu-sulfide intergrowths | 2.06 |
| Covellite/chalcocite | 0.50 |
| Tennantite-tetrahedrite | 0.03 |
| Talc and similar | 1.02 |
| All other silicates | 7.48 |
| Fe-(Ti)-oxides/oxyhydroxides/carbonates | 4.26 |
| Ankerite-dolomite/calcite | 2.12 |
| Barite | 2.55 |
| All other minerals/steel | 0.26 |
| Total | 100 |
| EDTA Extractable Metal/Total Metal (%) | ||||
|---|---|---|---|---|
| Cu | Fe | Pb | Zn | |
| Fresh Ore Sample | 3.48 | 0.07 | 31.81 | 0.24 |
| Oxidized Ore | 15.87 | 0.21 | 40.52 | 2.67 |
| Grade (%) | Recovery (%) | |||||
|---|---|---|---|---|---|---|
| Cu | Pb | Zn | Cu | Pb | Zn | |
| Standard Condition | 11.74 | 1.47 | 38.1 | 41.13 | 20.61 | 61.07 |
| Stage Add. of Dep. | 22.13 | 3.22 | 14.71 | 62.36 | 33.61 | 17.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özçelik, S.; Ekmekçi, Z. Reducing Negative Effects of Oxidation on Flotation of Complex Cu–Zn Sulfide Ores. Minerals 2022, 12, 1016. https://doi.org/10.3390/min12081016
Özçelik S, Ekmekçi Z. Reducing Negative Effects of Oxidation on Flotation of Complex Cu–Zn Sulfide Ores. Minerals. 2022; 12(8):1016. https://doi.org/10.3390/min12081016
Chicago/Turabian StyleÖzçelik, Seda, and Zafir Ekmekçi. 2022. "Reducing Negative Effects of Oxidation on Flotation of Complex Cu–Zn Sulfide Ores" Minerals 12, no. 8: 1016. https://doi.org/10.3390/min12081016
APA StyleÖzçelik, S., & Ekmekçi, Z. (2022). Reducing Negative Effects of Oxidation on Flotation of Complex Cu–Zn Sulfide Ores. Minerals, 12(8), 1016. https://doi.org/10.3390/min12081016






