Suitability of Auger Pressing Briquettes for Blast Furnace Use Based on Laboratory Tests
Abstract
1. Introduction
2. Materials and Methods
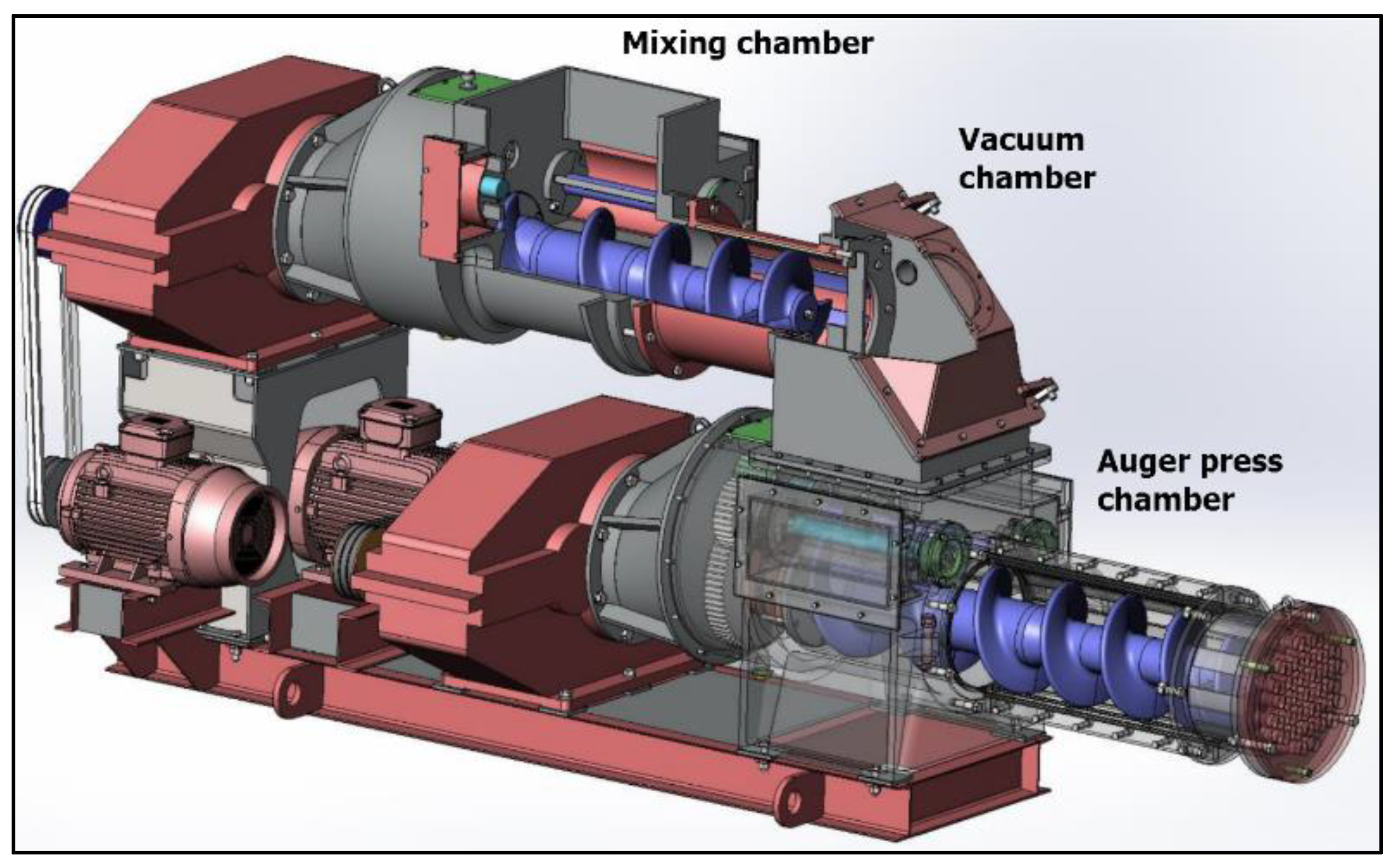
2.1. Auger Pressing Briquettes
- Mixing chamber including double shaft mixer and pre-compaction function;
- Vacuum chamber;
- Auger press chamber.
2.2. Iron Ore Pellets
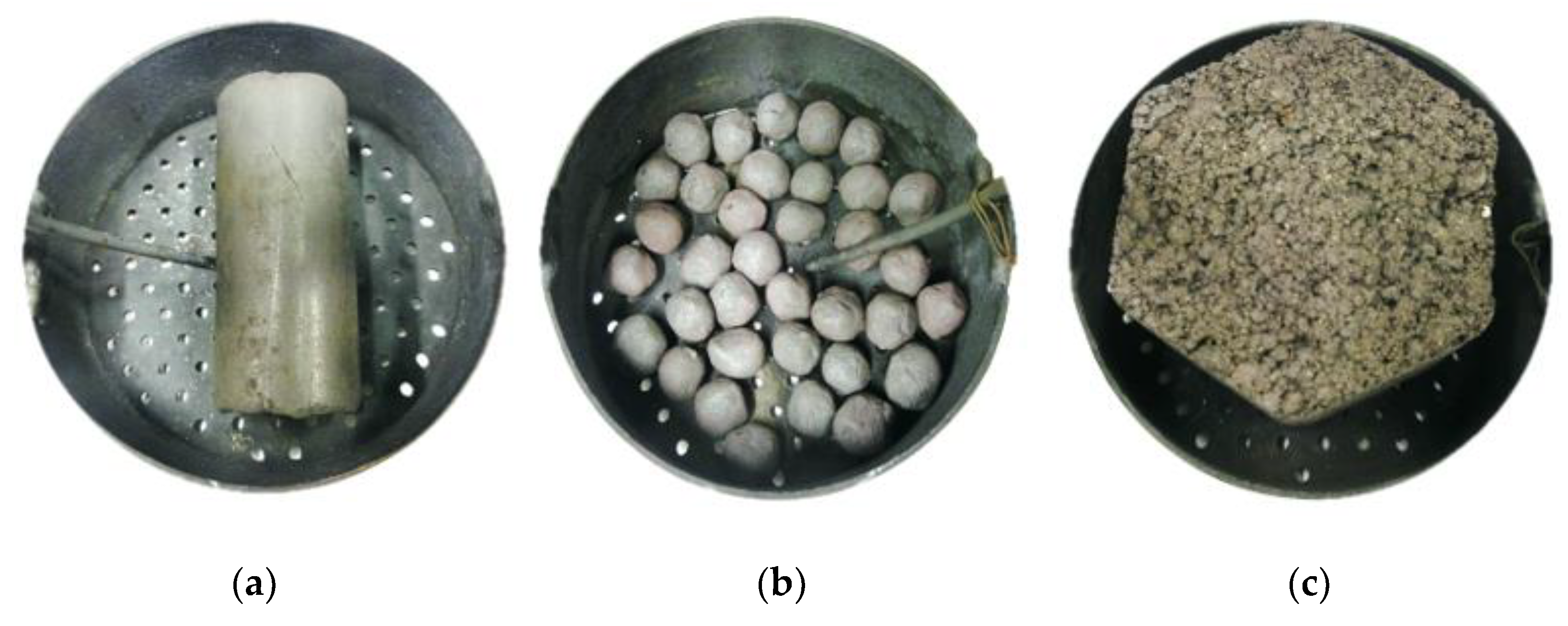
2.3. Industrial Blast Furnace Briquettes
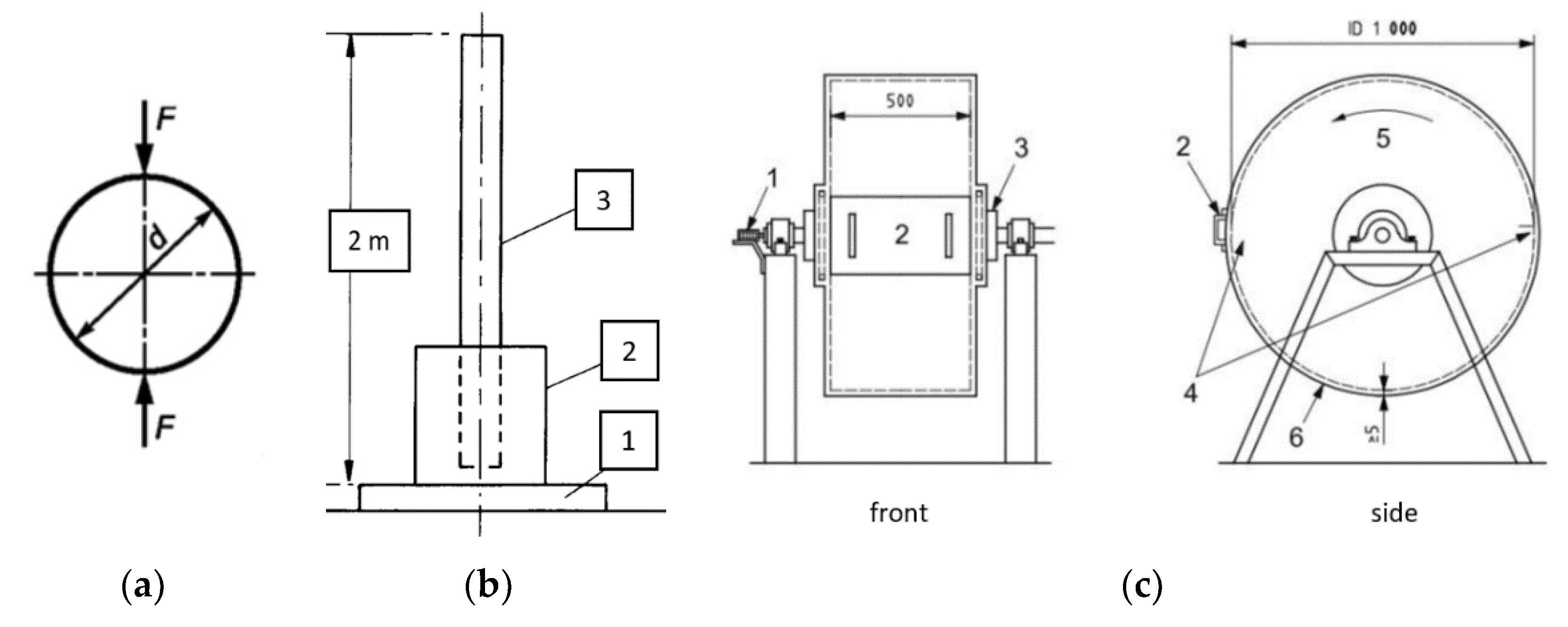
2.4. Strength Tests
2.5. Sample Preparation
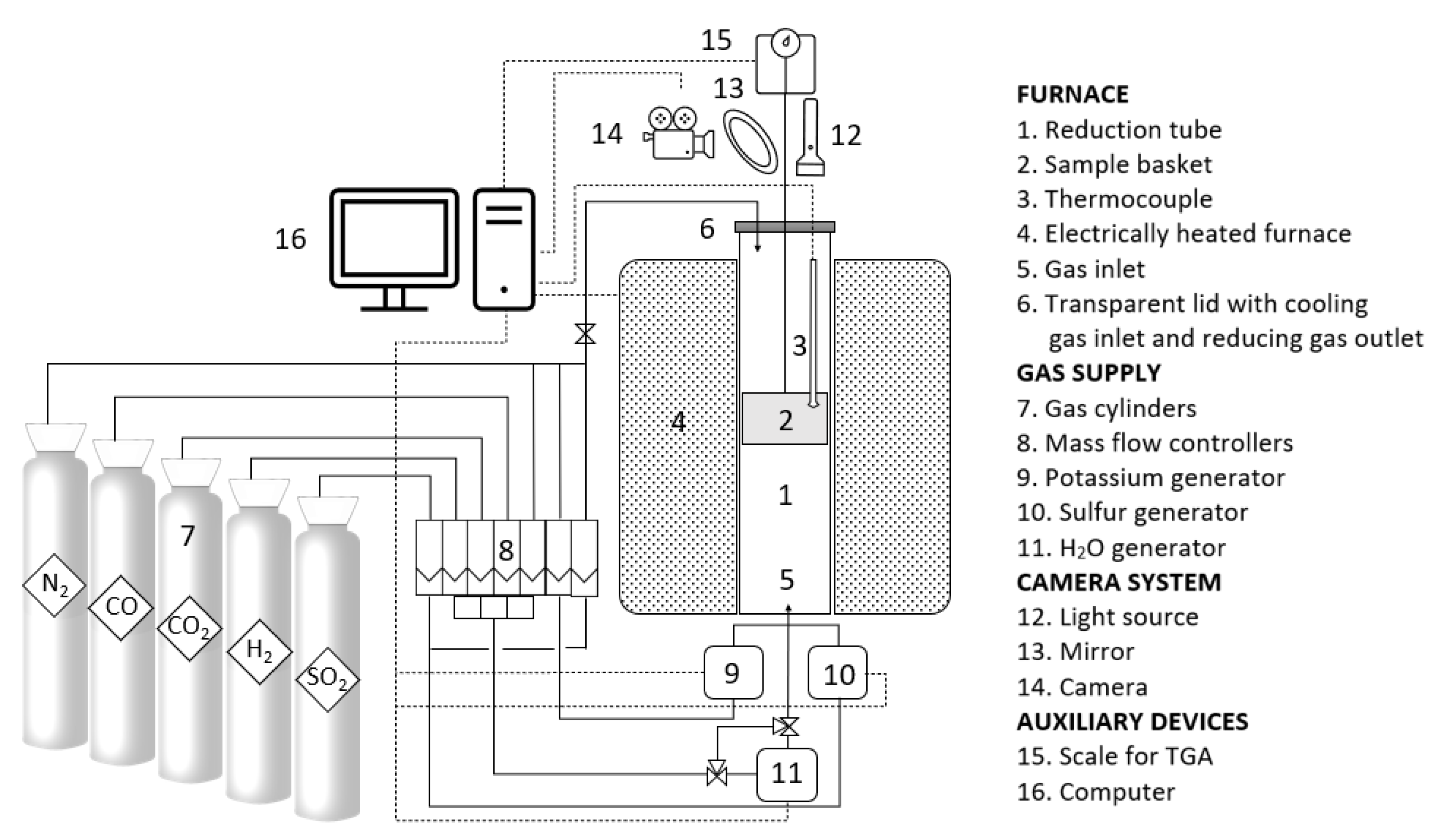
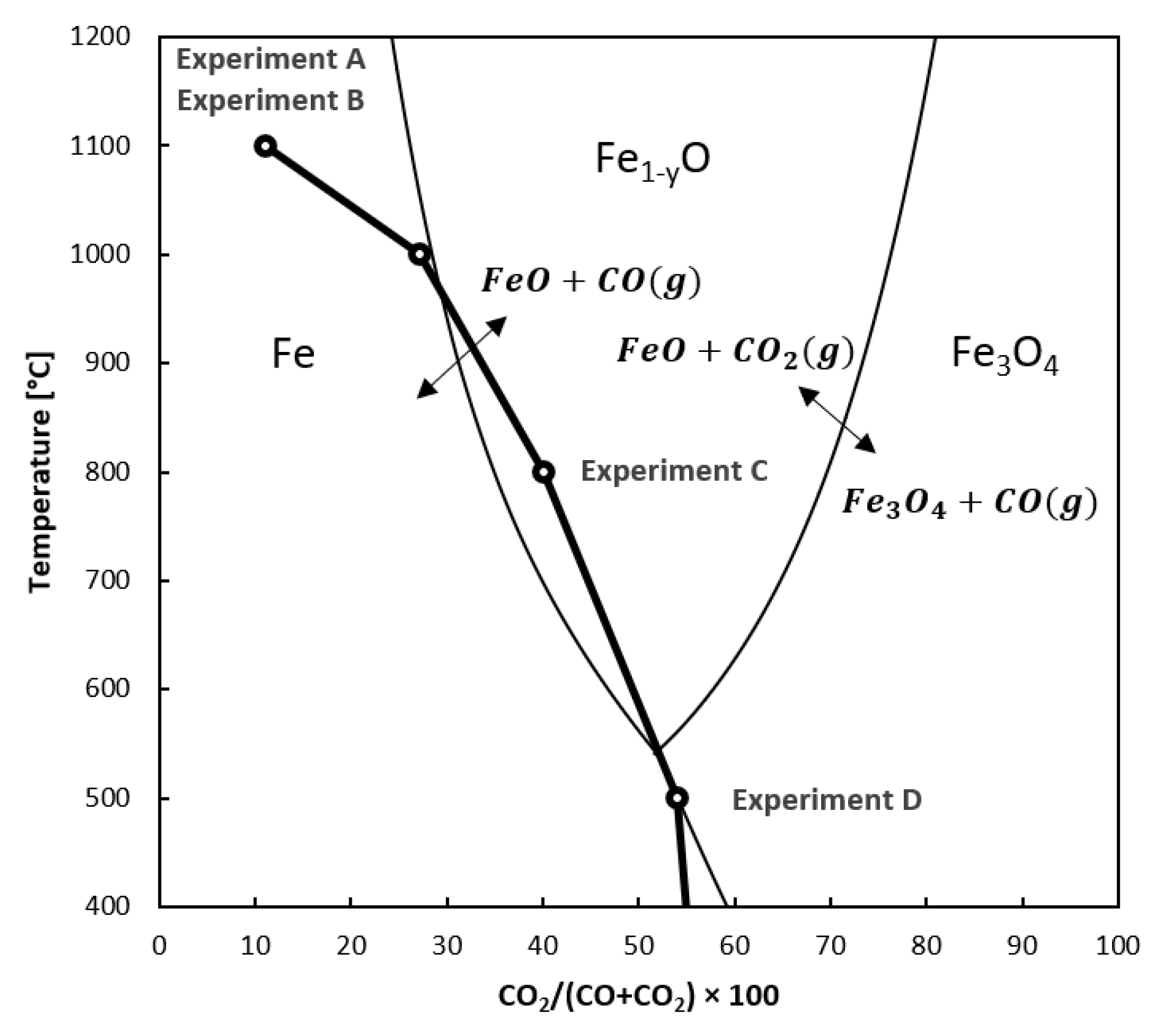
2.6. Blast Furnace Simulation
2.7. Reduction and Swelling Calculation
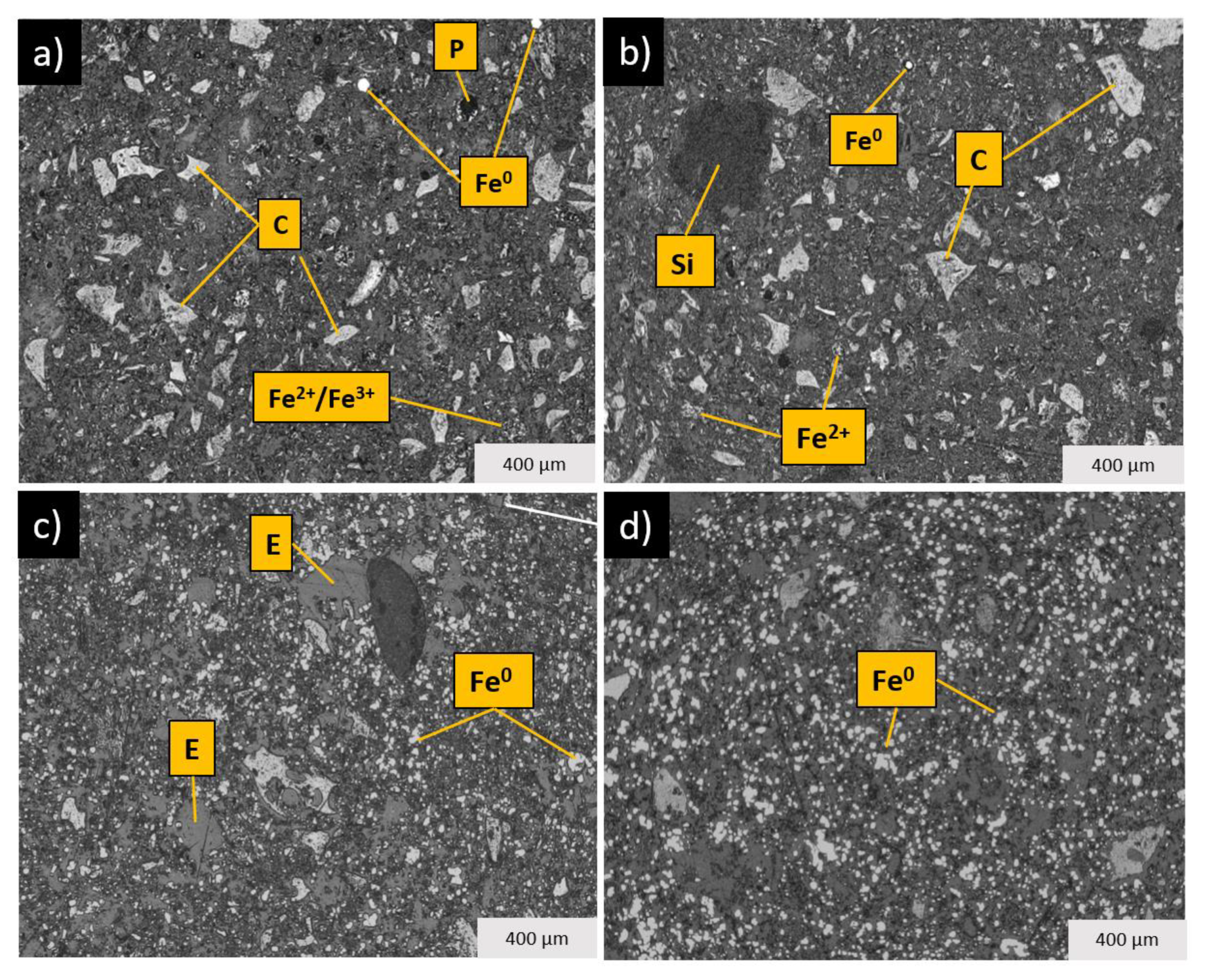
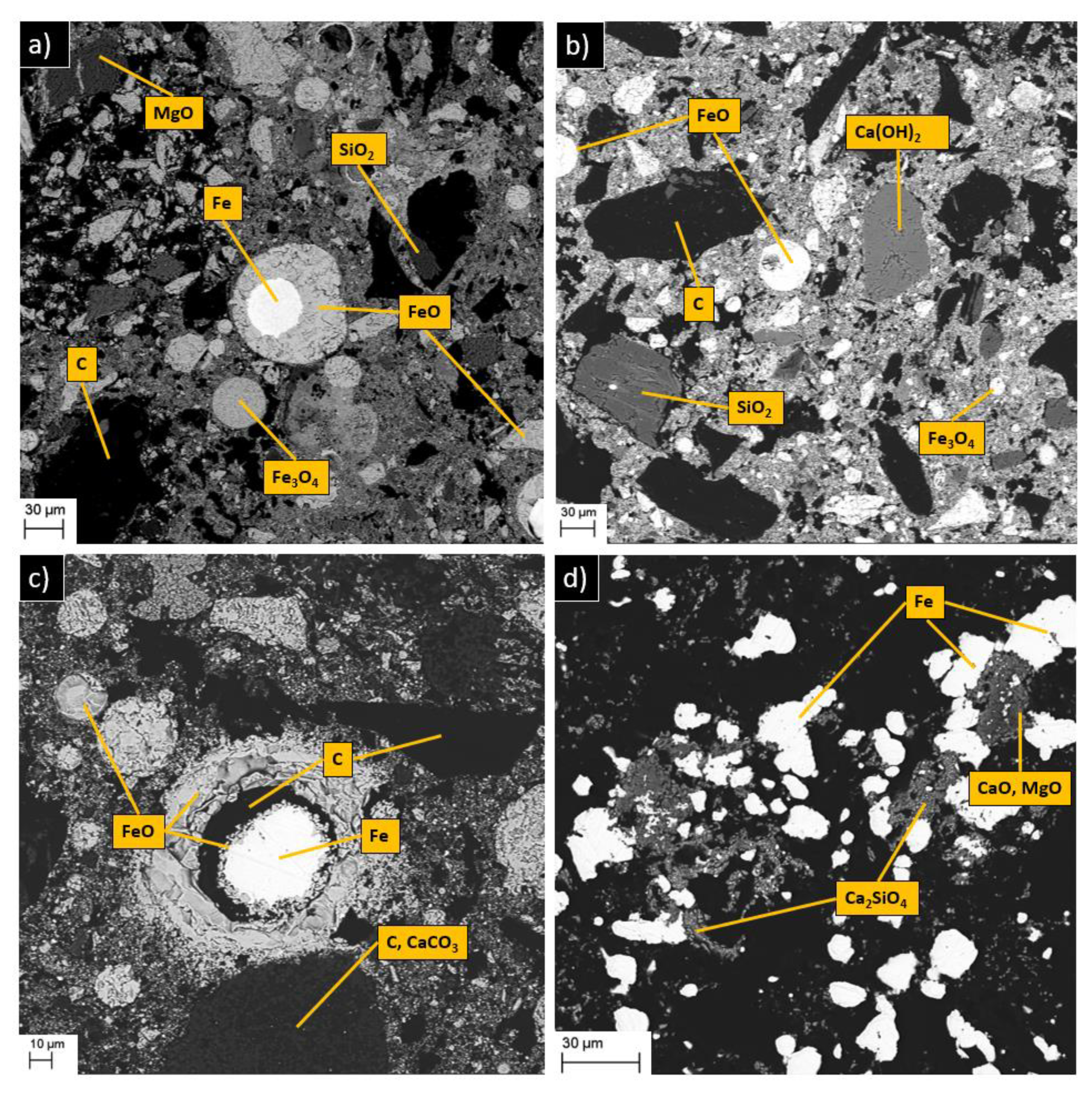
2.8. Mineralogical Characterization
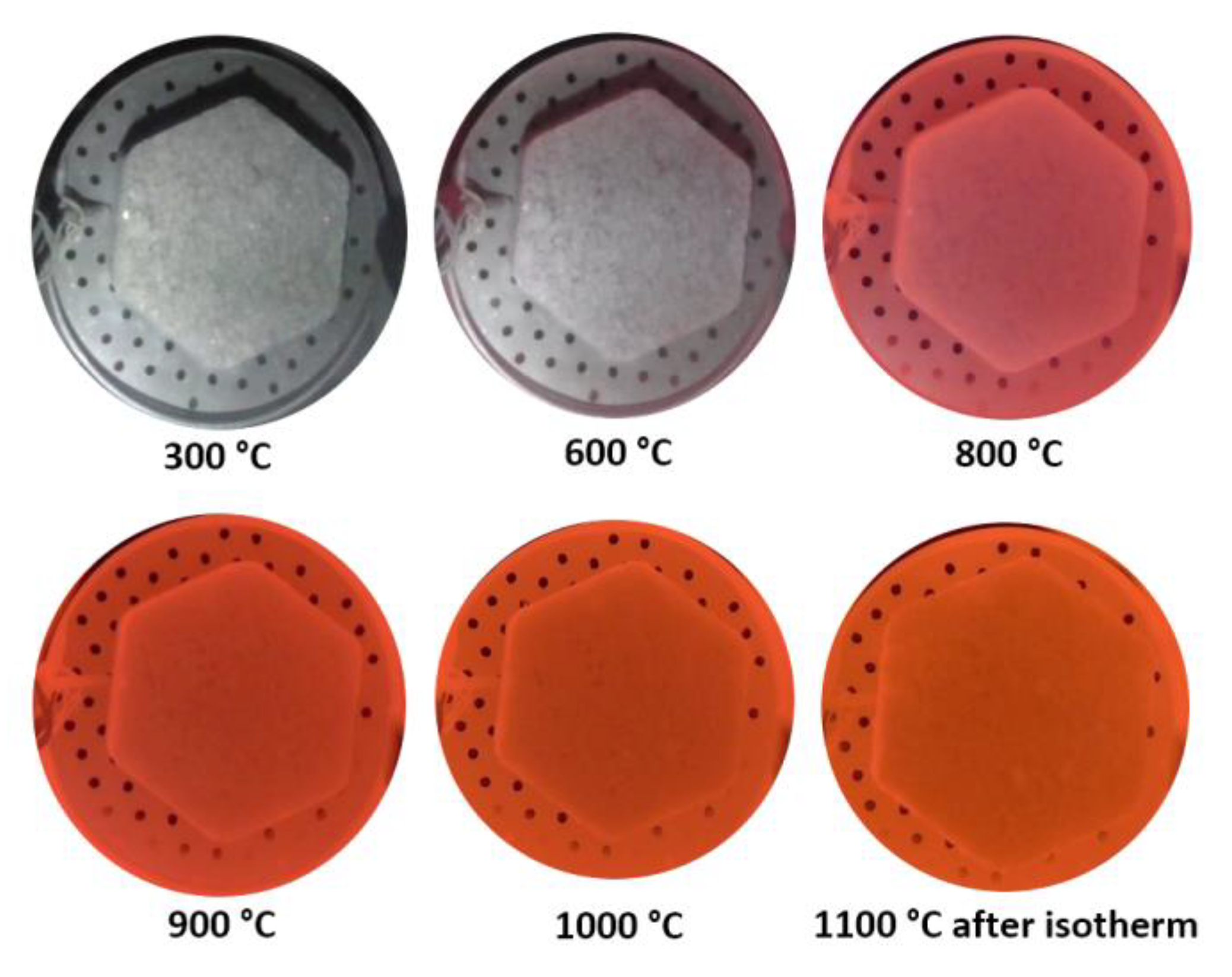
3. Results
3.1. Strength Tests
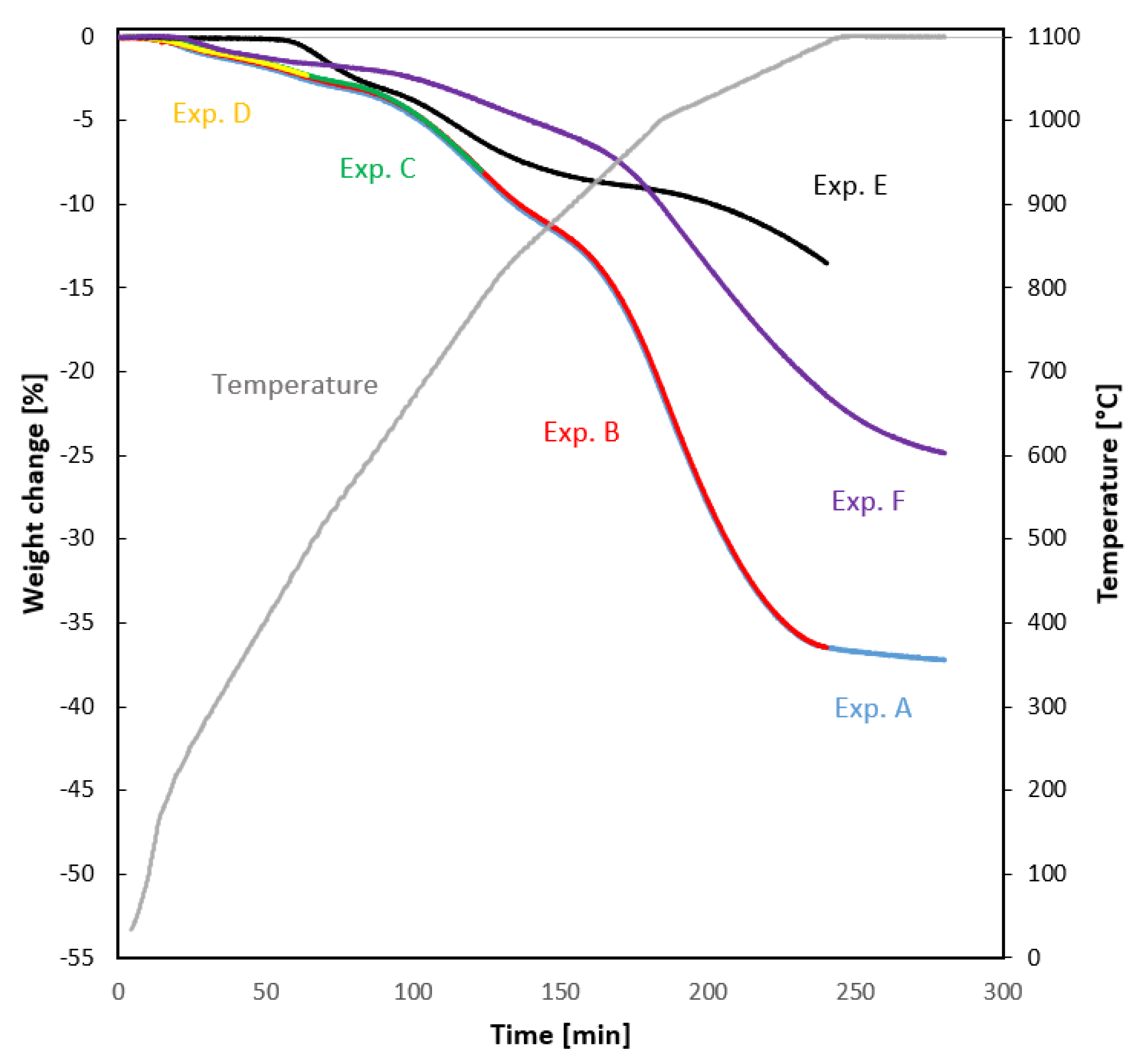
3.2. Reduction Experiments for Auger Pressing Briquettes
3.2.1. Uninterrupted Reduction Experiments
3.2.2. Interrupted Reduction Experiments
3.3. Reduction Experiments for Reference Samples
4. Discussion
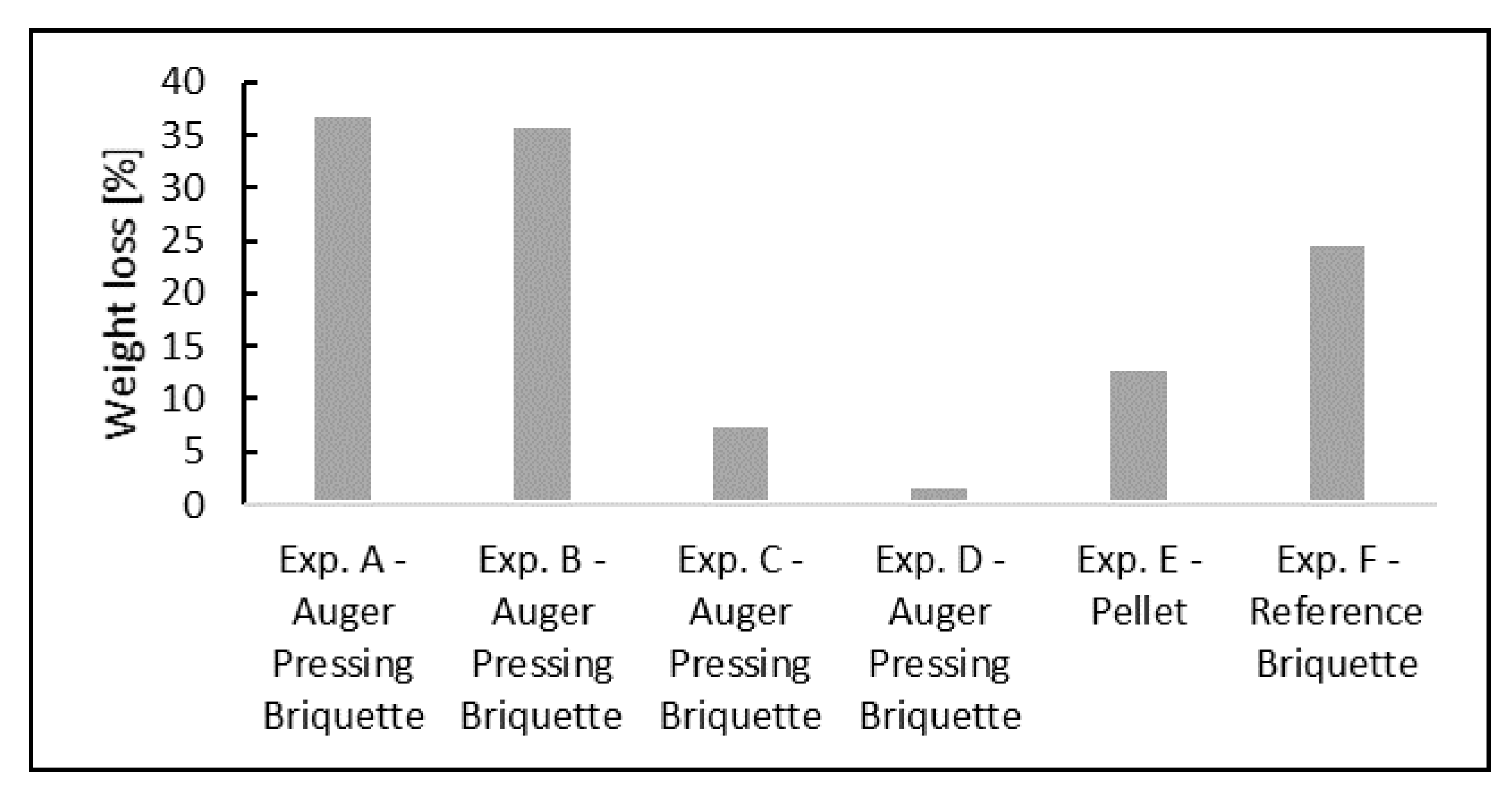
4.1. Weight Loss and Reduction
4.2. Phase Transformations
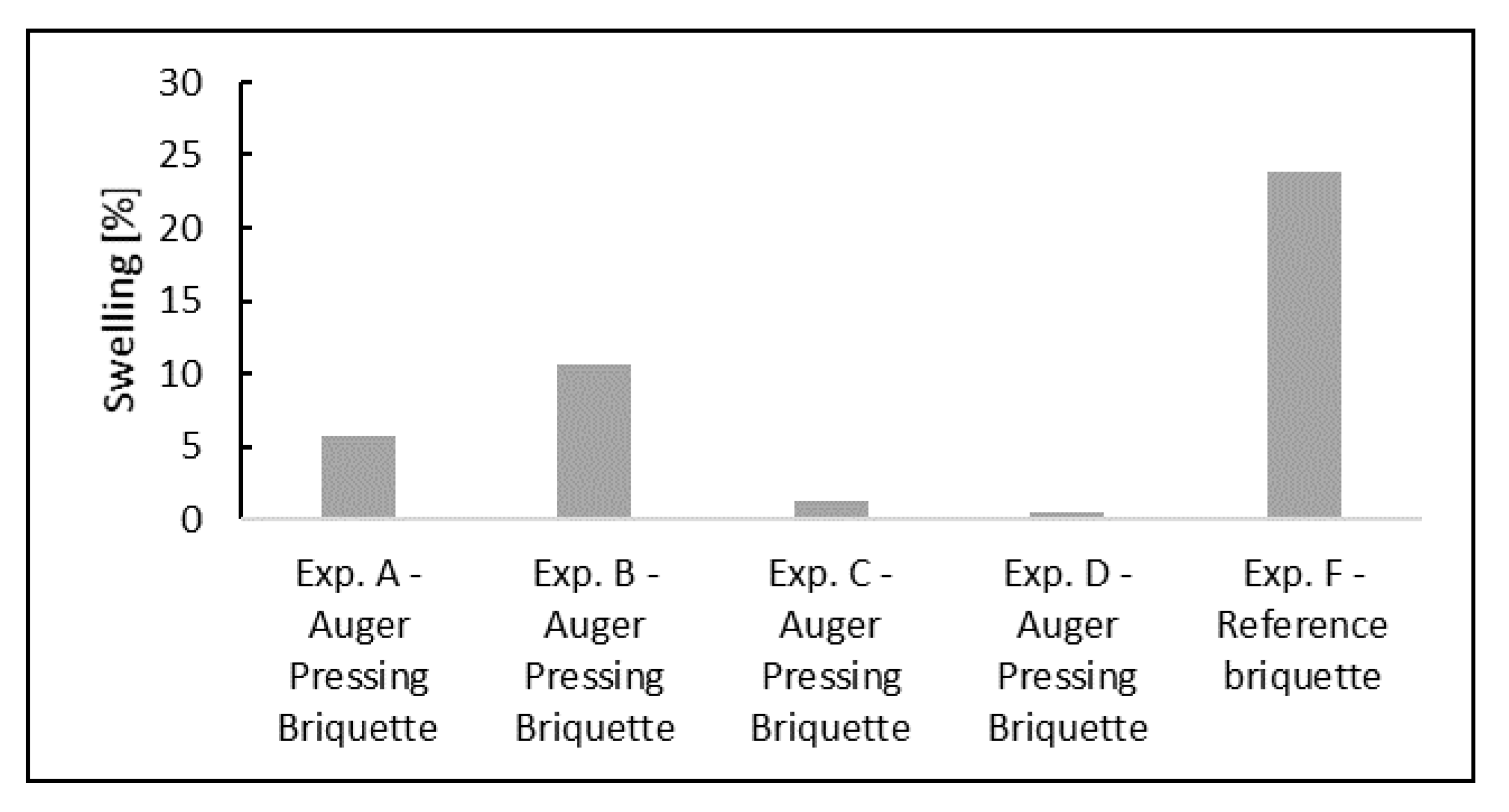
4.3. Swelling and Cracking
4.4. Effect of Chemical Composition
5. Conclusions
- BF sludge-based by-product briquettes made by the vacuum auger pressing technology had a strong self-reducing effect based on the rapid weight losses at temperatures 780–1100 °C compared to the reference samples. The phenomenon is most probably caused by the coal contained in the briquette. This is a particularly good feature in terms of BF productivity.
- Slight swelling behavior was observed, as the volume of the briquettes increased by 5–11% when wüstite was reduced to metallic iron during the uninterrupted reduction experiments. Such a small increase in volume does not cause operational issues in BF.
- The cracking behavior of briquettes at temperatures above 1000 °C was observed, but the briquettes could be handled after the reduction experiments without degradation. Such durability is promising since it is desired to avoid fines ending up in the BF process.
- The chemical composition of the briquettes appears to be a good compromise between reducibility and strength properties, allowing further research into new by-product briquette recipes as well.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Steel Association. World Steel in Figures 2021; World Steel Association: Brussels, Belgium, 2021. [Google Scholar]
- Steel Industry Co-Products; World Steel Association: Brussels, Belgium, 2019.
- Khunte, M. Process Waste Generation and Utilization in Steel Industry. Int. J. Ind. Manuf. Syst. Eng. 2018, 3, 1. [Google Scholar] [CrossRef]
- Mäkelä, M.; Paananen, T.; Heino, J.; Kokkonen, T.; Huttunen, S.; Makkonen, H.; Dahl, O. Influence of Fly Ash and Ground Granulated Blast Furnace Slag on the Mechanical Properties and Reduction Behavior of Cold-Agglomerated Blast Furnace Briquettes. ISIJ Int. 2012, 52, 1101–1108. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Mohassab, Y. Ironmaking and Steelmaking Processes: Greenhouse Emissions, Control, and Reduction; Cavaliere, P., Ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-39527-2. [Google Scholar]
- Omran, M.; Fabritius, T.; Paananen, T. Effect of Blast Furnace Sludge (BFS) Characteristics on Suitable Recycling Process Determining. J. Miner. Mater. Charact. Eng. 2017, 5, 185–197. [Google Scholar] [CrossRef][Green Version]
- Baricová, D.; Pribulová, A.; Buľko, B.; Demeter, P. Recycling of the Steelmaking By-Products into the Oxygen Converter Charge. New Trends Prod. Eng. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Reuter, M.; Xiao, Y.; Boin, U. Recycling and Environmental Issues of Metallurgical Slags and Salt Fluxes. In Proceedings of the VII International Conference on Molten Slags Fluxes & Salts, Cape Town, South Africa, 25–28 January 2004; pp. 349–356. [Google Scholar]
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ricketts, J.A. Modern Blast Furnace Ironmaking: An Introduction, 3rd ed.; IOS Press: Amsterdam, The Netherlands, 2015; ISBN 978-1-61499-498-5. [Google Scholar]
- Lu, L.; Pan, J.; Zhu, D. Quality Requirements of Iron Ore for Iron Production. In Iron Ore; Elsevier: Amsterdam, The Netherlands, 2015; pp. 475–504. ISBN 978-1-78242-156-6. [Google Scholar]
- Andersson, A.; Gullberg, A.; Kullerstedt, A.; Ahmed, H.; Sundqvist-Ökvist, L.; Samuelsson, C. Upgrading of Blast Furnace Sludge and Recycling of the Low-Zinc Fraction via Cold-Bonded Briquettes. J. Sustain. Metall. 2019, 5, 350–361. [Google Scholar] [CrossRef]
- Soria-Aguilar, M.d.J.; Davila-Pulido, G.I.; Carrillo-Pedroza, F.R.; Gonzalez-Ibarra, A.A.; Picazo-Rodriguez, N.; Lopez-Saucedo, F.d.J.; Ramos-Cano, J. Oxidative Leaching of Zinc and Alkalis from Iron Blast Furnace Sludge. Metals 2019, 9, 1015. [Google Scholar] [CrossRef]
- Esezobor, D.E.; Balogun, S.A. Zinc Accumulation during Recycling of Iron Oxide Wastes in the Blast Furnace. Ironmak. Steelmak. 2006, 33, 419–425. [Google Scholar] [CrossRef]
- Mohanty, M.K.; Mishra, S.; Mishra, B.; Sarkar, S.; Samal, S.K. A Novel Technique for Making Cold Briquettes for Charging in Blast Furnace. IOP Conf. Ser. Mater. Sci. Eng. 2016, 115, 012020. [Google Scholar] [CrossRef]
- Pietsch, W. Agglomeration Processes: Phenomena, Technologies, Equipement; Wiley-VCH: Weinheim, Germany, 2002; ISBN 978-3-527-30369-4. [Google Scholar]
- Concrete Block Manufacturing Systems—HESS GROUP. Available online: https://www.topwerk.com/en/hess-group/products/concrete-block-manufacturing-systems (accessed on 14 June 2022).
- Lemos, L.R.; da Rocha, S.H.F.S.; de Castro, L.F.A. Reduction Disintegration Mechanism of Cold Briquettes from Blast Furnace Dust and Sludge. J. Mater. Res. Technol. 2015, 4, 278–282. [Google Scholar] [CrossRef]
- Maschinenfabrik Köppern—Der Spezialist für Walzenpressentechnologien: Home. Available online: https://www.koeppern-international.com/ (accessed on 14 June 2022).
- Iljana, M. Iron Ore Pellet Properties under Simulated Blast Furnace Conditions: Investigation on Reducibility, Swelling and Softening. Ph.D. Dissertation, University of Oulu, Oulu, Finland, 2017. Available online: http://urn.fi/urn:isbn:9789526215709 (accessed on 27 June 2022).
- Park, T.J.; Min, S.K.; Kim, H. Estimation of Reducibility of Sinter with Continuous Measurement of Reduction Degradation Test. Met. Mater. Int. 2020, 26, 532–540. [Google Scholar] [CrossRef]
- Iljana, M.; Mattila, O.; Alatarvas, T.; Visuri, V.-V.; Kurikkala, J.; Paananen, T.; Fabritius, T. Dynamic and Isothermal Reduction Swelling Behaviour of Olivine and Acid Iron Ore Pellets under Simulated Blast Furnace Shaft Conditions. ISIJ Int. 2012, 52, 1257–1265. [Google Scholar] [CrossRef]
- ISO 4695:2021; Iron Ores for Blast Furnace Feedstocks—Determination of the Reducibility by the Rate of Reduction Index. ISO: Geneva, Switzerland, 2015; pp. 1–7.
- ISO 7215:2015; Iron Ores for Blast Furnace Feedstocks—Determination of the Reducibility by the Final Degree of Reduction Index. ISO: Geneva, Switzerland, 2015; pp. 1–6.
- Hooey, P.L.; Sterneland, J.; Hallin, M. Evaluation of high temperature properties of blast furnace burden. In Proceedings of the 1st International Meeting on Ironmaking, Belo Horizonte, Brazil, 24 September 2001; Volume 24. [Google Scholar]
- de Moraes, S.L.; de Lima, J.R.B.; Ribeiro, T.R. Iron Ore Pelletizing Process: An Overview. In Iron Ores and Iron Oxide Materials; Shatokha, V., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-320-9. [Google Scholar]
- Paananen, T. Terästehtaan sivuvirroista masuunibrikettiä. In Proceedings of the Prosessiteollisuuden Sivuvirtojen Hyödyntäminen Seminar, Oulu, Finland, 10 January 2013. [Google Scholar]
- ISO 3271:2015; Iron Ores for Blast Furnace and Direct Reduction Feedstocks—Determination of the Tumble and Abrasion Indices. ISO: Geneva, Switzerland, 2015; pp. 1–5.
- Kemppainen, A.; Iljana, M.; Heikkinen, E.-P.; Paananen, T.; Mattila, O.; Fabritius, T. Reduction Behavior of Cold-Bonded Briquettes under Simulated Blast Furnace Conditions. ISIJ Int. 2014, 54, 1539–1545. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Zymla, V.; Osório, E.; Vilela, A.C.F. Carbon Gasification in Self-Reducing Mixtures. ISIJ Int. 2014, 54, 2687–2696. [Google Scholar] [CrossRef]
- Liu, G.; Strezov, V.; Lucas, J.A.; Wibberley, L.J. Thermal Investigations of Direct Iron Ore Reduction with Coal. Thermochim. Acta 2004, 410, 133–140. [Google Scholar] [CrossRef]
- Menéndez, E.; Andrade, C.; Vega, L. Study of Dehydration and Rehydration Processes of Portlandite in Mature and Young Cement Pastes. J. Therm. Anal. Calorim. 2012, 110, 443–450. [Google Scholar] [CrossRef]
- Singh, M.; Björkman, B. Swelling Behaviour of Cement-Bonded Briquettes-Proposed Model. ISIJ Int. 2004, 44, 482–491. [Google Scholar] [CrossRef]
- Wang, H.; Sohn, H.Y. Effects of Firing and Reduction Conditions on Swelling and Iron Whisker Formation during the Reduction of Iron Oxide Compact. ISIJ Int. 2011, 51, 906–912. [Google Scholar] [CrossRef]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Pisilä, E.; Kondrakov, M.; Fabritius, T. Effect of Adding Limestone on the Metallurgical Properties of Iron Ore Pellets. Int. J. Miner. Process. 2015, 141, 34–43. [Google Scholar] [CrossRef]
- ISO 13930:2015; Iron Ores for Blast Furnace Feedstocks—Determination of Low Temperature Reduction-Disintegration Indices by Dynamic Method. ISO: Geneva, Switzerland, 2015; pp. 1–6.
- Lundkvist, K.; Rosendahl, S.; Nyman, F.; Bölke, K.; Gustavsson, L.; Söderström, D.; Wedholm, A. OXYFINES Technique for Upgrading Zinc Containing Blast Furnace Sludge—Part 1: Pilot Trials. Metals 2020, 10, 1468. [Google Scholar] [CrossRef]
- Lundkvist, K.; Rosendahl, S.; Nyman, F.; Bölke, K.; Gustavsson, L.; Söderström, D.; Wedholm, A. OXYFINES Technique for Upgrading Zinc Containing Blast Furnace Sludge—Part 2: System Analysis. Metals 2020, 10, 1471. [Google Scholar] [CrossRef]


| Constituents | Elements (%) | Weight (g) | Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fetot | P | SiO2 | CaO | MgO | Al2O3 | Zn | H2O | C | |||
| BF sludge (0–3 mm) | 44.4 | 0.17 | 5.12 | 3.12 | 3.9 | 2.2 | 0.43 | 23.0 | 12,000 | 40 | |
| Mill scale (0–3 mm) | 70.0 | 16,500 | 55 | ||||||||
| Slaked lime (0–3 mm) | 80.0 | 20.0 | 900 | 3 | |||||||
| Binder AMCOM VA3500 | 33.3 | 600 | 2 | ||||||||
| Calculated Total Content | 56.3 | 0.07 | 2.05 | 3.65 | 1.56 | 0.88 | 0.17 | 0.60 | 9.87 | 30,000 | 100 |
| Added water | 100.0 | 4500 | |||||||||
| Fetot | FeO | SiO2 | CaO | MgO | Al2O3 | TiO2 | V2O5 |
|---|---|---|---|---|---|---|---|
| 66.7 | 0.6 | 1.85 | 0.43 | 1.3 | 0.32 | 0.35 | 0.26 |
| C | Na2O | MgO | Al2O3 | SiO2 | S | K2O | CaO | Fe | Basicity |
|---|---|---|---|---|---|---|---|---|---|
| 8.28 | 0.32 | 2.1 | 2.4 | 8.1 | 0.41 | 0.21 | 11.1 | 48.4 | 1.37 |
| Length (mm) | Force (kg) | Strength (kg/cm) | Strength (kg/cm2) | |
|---|---|---|---|---|
| 77.0 | 183.0 | 23.8 | 196.3 | |
| 81.0 | 192.0 | 23.7 | 195.4 | |
| 75.0 | 184.0 | 24.5 | 202.1 | |
| 68.0 | 170.0 | 25.0 | 206.2 | |
| 73.0 | 189.0 | 25.9 | 213.6 | |
| 73.0 | 198.0 | 27.1 | 223.5 | |
| 67.0 | 151.0 | 22.5 | 185.6 | |
| AVG | 73.4 | 181.0 | 24.6 | 203.2 |
| SD | 4.9 | 15.9 | 1.5 | 12.6 |
| Number of Revolutions | Content (%) | |
|---|---|---|
| >5 mm | >0.5 mm | |
| 25 | 93 | 95 |
| 50 | 89 | 90 |
| 100 | 80 | 82 |
| 200 | 64 | 69 |
| Sample | Fetot | SiO2 | CaO | MgO | Al2O3 | Zn | H2O | C | Basicity |
|---|---|---|---|---|---|---|---|---|---|
| Auger Pressing Briquette | 56.3 | 2.05 | 3.65 | 3.9 | 0.88 | 0.17 | 0.60 | 9.87 | 1.78 |
| Ref. Briquette | 48.4 | 8.1 | 11.1 | 2.1 | 2.4 | 0.01 | ~7.5 | 8.28 | 1.37 |
| Ref. Pellet | 66.7 | 1.85 | 0.43 | 1.3 | 0.32 | <0.003 | 1.5 | - | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitikka, O.; Iljana, M.; Heikkilä, A.; Tkalenko, I.; Koriuchev, N.; Shehovsov, D.; Malkki, A.; Fabritius, T. Suitability of Auger Pressing Briquettes for Blast Furnace Use Based on Laboratory Tests. Minerals 2022, 12, 868. https://doi.org/10.3390/min12070868
Vitikka O, Iljana M, Heikkilä A, Tkalenko I, Koriuchev N, Shehovsov D, Malkki A, Fabritius T. Suitability of Auger Pressing Briquettes for Blast Furnace Use Based on Laboratory Tests. Minerals. 2022; 12(7):868. https://doi.org/10.3390/min12070868
Chicago/Turabian StyleVitikka, Olli, Mikko Iljana, Anne Heikkilä, Illia Tkalenko, Nikita Koriuchev, Daniel Shehovsov, Andrey Malkki, and Timo Fabritius. 2022. "Suitability of Auger Pressing Briquettes for Blast Furnace Use Based on Laboratory Tests" Minerals 12, no. 7: 868. https://doi.org/10.3390/min12070868
APA StyleVitikka, O., Iljana, M., Heikkilä, A., Tkalenko, I., Koriuchev, N., Shehovsov, D., Malkki, A., & Fabritius, T. (2022). Suitability of Auger Pressing Briquettes for Blast Furnace Use Based on Laboratory Tests. Minerals, 12(7), 868. https://doi.org/10.3390/min12070868







