The Use of Hydrogen as a Potential Reductant in the Chromite Smelting Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sizing of Chromite
2.3. Reduction Procedure
2.4. Analytical Techniques
2.5. Expressing the Extent of Metalization
3. Results and Discussion
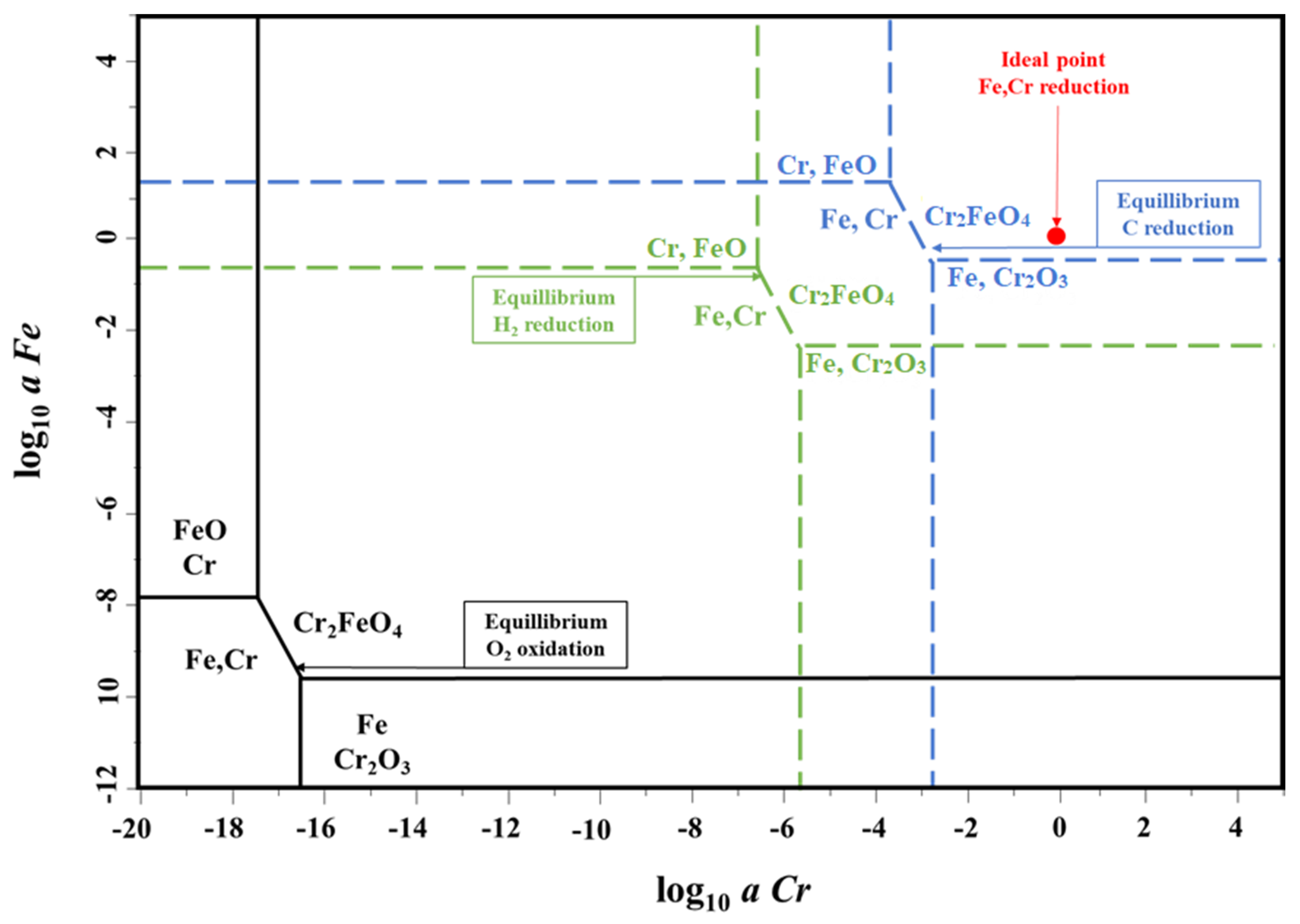
3.1. Thermodynamic Considerations
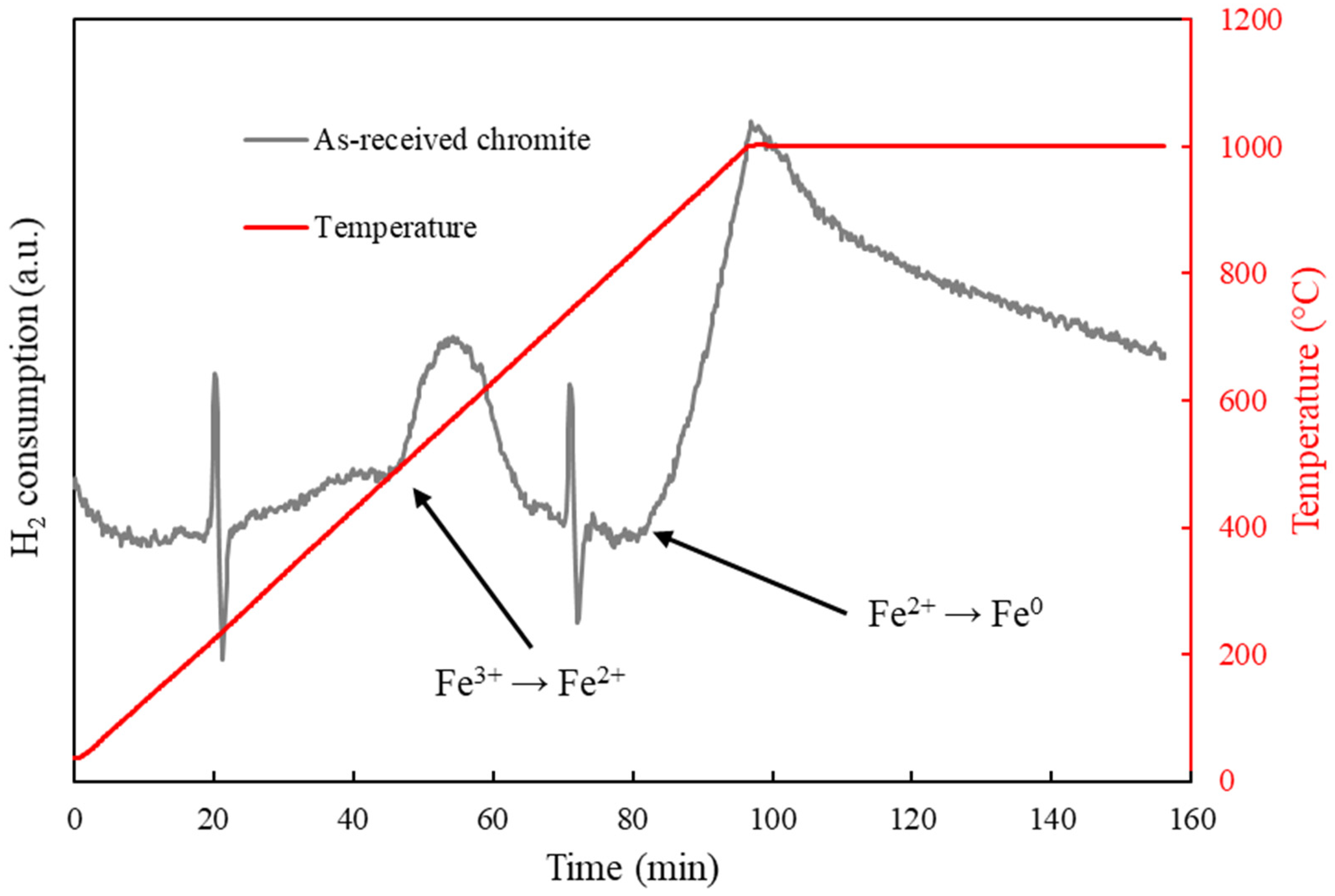
3.2. Reactivity of Chromite with Hydrogen
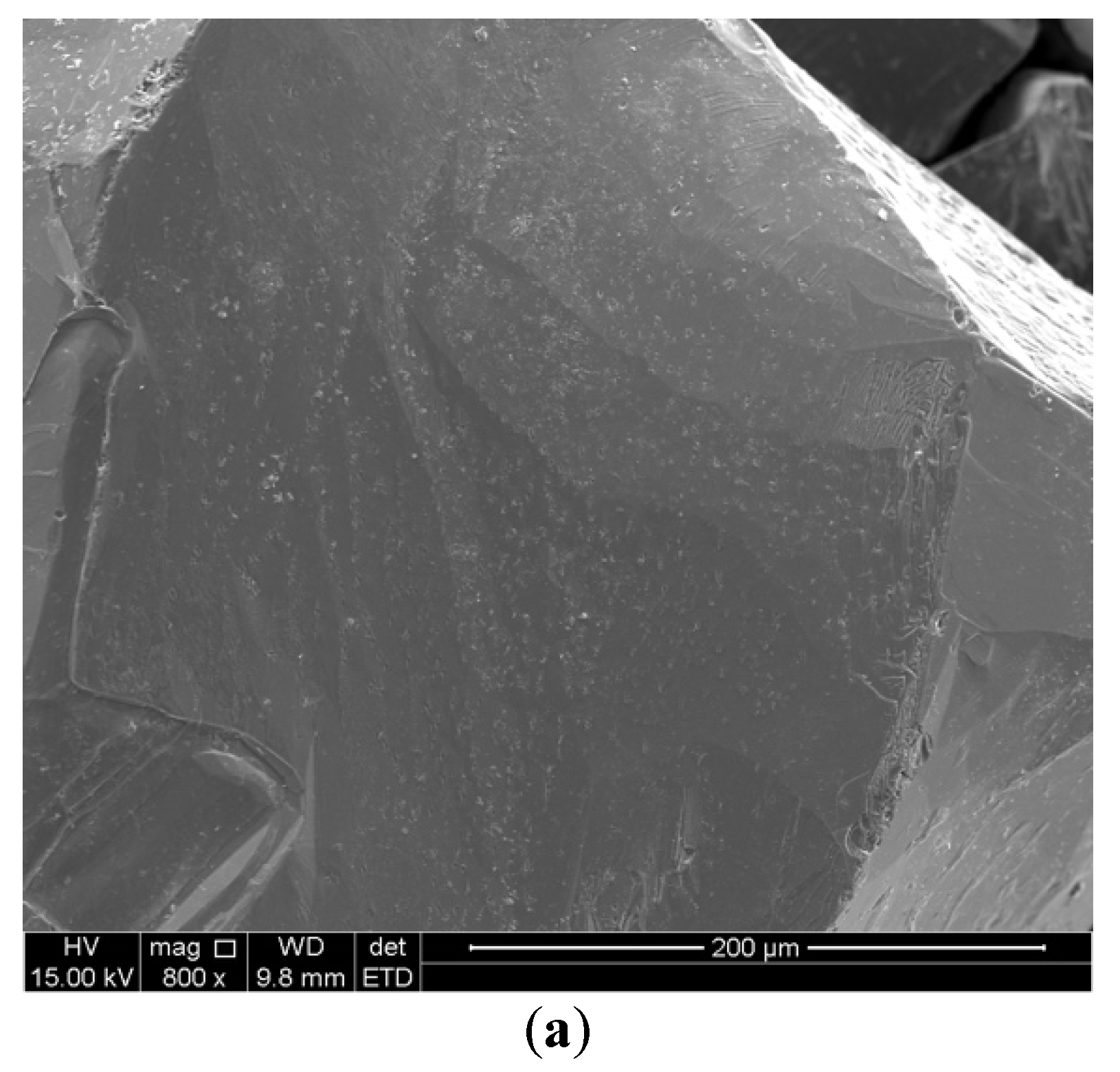
3.3. XRD Characterization of Pre-Reduced Chromite Fines
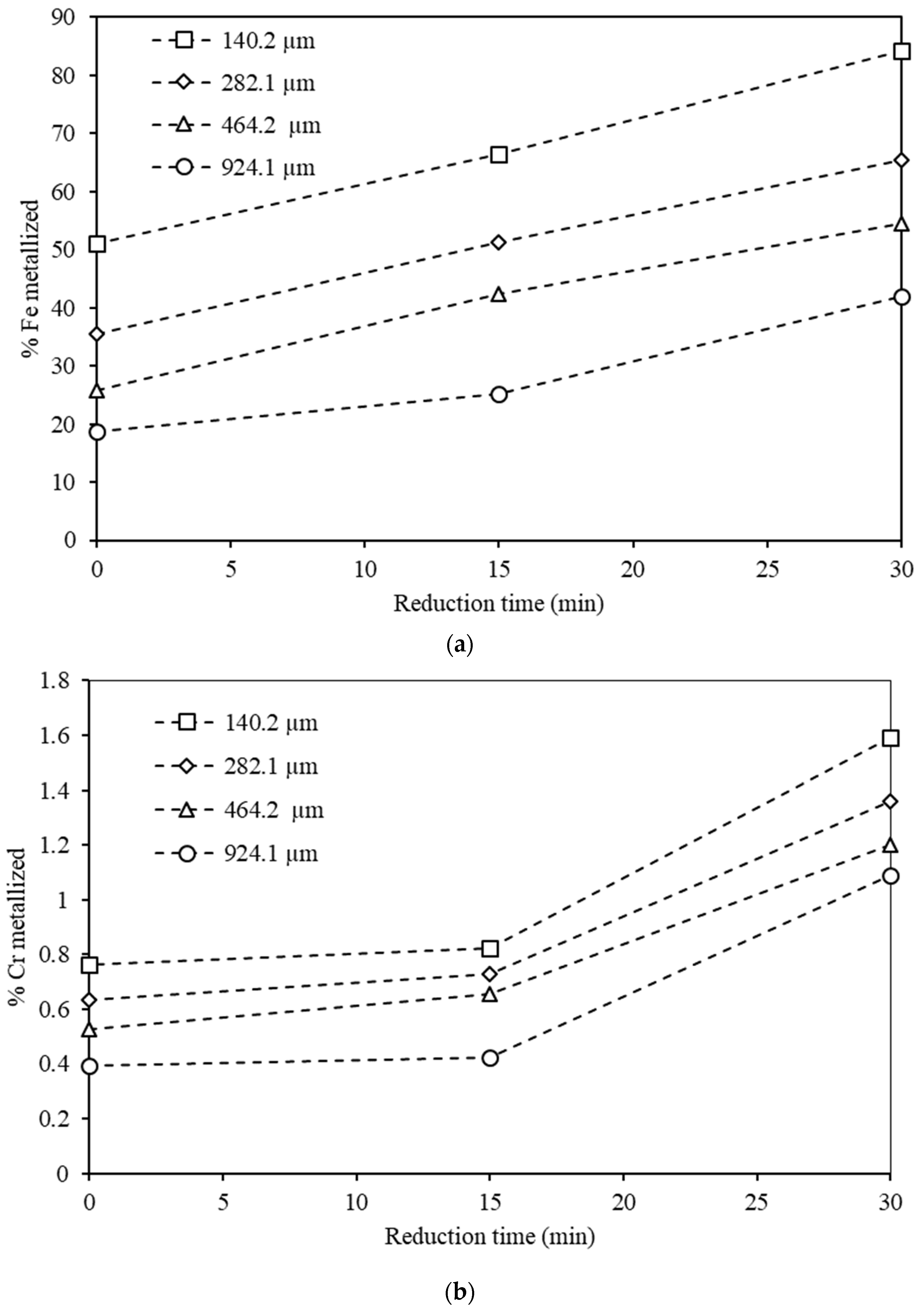
3.4. Effects of Reduction Time and Particle Size on Metalization
3.5. Practical Implementation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tathavadkar, V.D.; Antony, M.; Jha, A. The physical chemistry of thermal decomposition of South African chromite minerals. Metall. Mater. Trans. B 2005, 36, 75–84. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Beukes, J.P.; Paktunc, D.; Van Zyl, P.G.; Jordaan, A. Recycling pre-oxidized chromite fines in the oxidative sintered pellet production process. J. S. Afr. Inst. Min. Metall. 2019, 119, 207–215. [Google Scholar] [CrossRef]
- Riekkola-Vanhanen, M. Finnish expert report on best available techniques in ferrochromium production. Environ. Sci. 1999, 314, 1–49. [Google Scholar]
- Du Preez, S.; Beukes, J.; Van Dalen, W.; Van Zyl, P.; Paktunc, D.; Loock-Hattingh, M. Aqueous solubility of Cr (VI) compounds in ferrochrome bag filter dust and the implications thereof. Water SA 2017, 43, 298–309. [Google Scholar] [CrossRef][Green Version]
- Du Preez, S.; Beukes, J.; Van Zyl, P.; Tangstad, M.; Tiedt, L. Silicon carbide formation enhanced by in-situ-formed silicon nitride: An approach to capture thermal energy of co-rich off-gas combustion. Metall. Mater. Trans. B 2018, 49, 3151–3163. [Google Scholar] [CrossRef]
- Basson, J.; Daavittila, J. High Carbon Ferrochrome Technology. In Handbook of Ferroalloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 317–363. [Google Scholar]
- Murthy, Y.R.; Tripathy, S.K.; Kumar, C.R. Chrome ore beneficiation challenges & opportunities–A review. Miner. Eng. 2011, 24, 375–380. [Google Scholar]
- Beukes, J.P.; Dawson, N.F.; Van Zyl, P.G. Theoretical and practical aspects of Cr (VI) in the South African ferrochrome industry. J. S. Afr. Inst. Min. Metall. 2010, 110, 743–750. [Google Scholar]
- Neizel, B.W.; Beukes, J.P.; van Zyl, P.G.; Dawson, N.F. Why is CaCO3 not used as an additive in the pelletised chromite pre-reduction process? Miner. Eng. 2013, 45, 115–120. [Google Scholar] [CrossRef]
- Ringdalen, E.; Rocha, M.; Figueiredo, P. Energy consumption during HCFeCr production at Ferbasa. INFACON 2015, 2, 668–675. Available online: http://repositoriosenaiba.fieb.org.br/handle/fieb/702 (accessed on 12 January 2022).
- Ringdalen, E.; Rocha, M.; Figueiredo, P.; Tangstad, M. Pretreatment of Jaccurici Ore from Ferbasa-Brazil: Effect on Energy Consumption. In 2015-Sustainable Industrial Processing Summit; Flogen Star OUTREACH: Quebec, QC, Canada, 2015; pp. 83–92. [Google Scholar]
- Barnes, A.; Finn, C.; Algie, S. The prereduction and smelting of chromite concentrate of low chromium-to-iron ratio. J. S. Afr. Inst. Min. Metall. 1983, 83, 49–54. [Google Scholar]
- Niemelä, P.; Krogerus, H.; Oikarinen, P. Formation, Characteristics and Utilisation of CO-Gas Formed in Ferrochromium Smelting. In Tenth International Ferro Alloys Congress; INFACON XI: Cape Town, South Africa, 2004; pp. 68–77. [Google Scholar]
- Du Preez, S.P. Cr (VI) Generation during Flaring of Off-Gas from Closed Ferrochromium Submerged Arc Furnaces; North-West University, Potchefstroom Campus: Potchefstroom, South Africa, 2014. [Google Scholar]
- Du Preez, S.; Beukes, J.; Van Zyl, P. Cr (VI) generation during flaring of CO-rich off-gas from closed ferrochromium submerged arc furnaces. Metall. Mater. Trans. B 2015, 46, 1002–1010. [Google Scholar] [CrossRef]
- Beukes, J.; Du Preez, S.; Van Zyl, P.; Paktunc, D.; Fabritius, T.; Päätalo, M.; Cramer, M. Review of Cr (VI) environmental practices in the chromite mining and smelting industry–Relevance to development of the Ring of Fire, Canada. J. Clean. Prod. 2017, 165, 874–889. [Google Scholar] [CrossRef]
- Winter, F. Production of Chromium Iron Alloys Directly from Chromite Ore. WO2015060951A1, 30 April 2016. [Google Scholar]
- Pheiffer, D.M.; Cookson, K. Ferrochrome Alloy Production. WO2015159268A1, 22 October 2015. [Google Scholar]
- Yu, D.; Paktunc, D. Calcium chloride-assisted segregation reduction of chromite: Influence of reductant type and the mechanism. Minerals 2018, 8, 45. [Google Scholar] [CrossRef]
- Yu, D.; Paktunc, D. Carbothermic Direct Reduction of Chromite Using a Catalyst for the Production of Ferrochrome Alloy. WO2018201218A1, 8 November 2021. [Google Scholar]
- Yu, D.; Paktunc, D. Direct production of ferrochrome by segregation reduction of chromite in the presence of calcium chloride. Metals 2018, 8, 69. [Google Scholar] [CrossRef]
- Sokhanvaran, S.; Paktunc, D. Method of Direct Reduction of Chromite with Cryolite Additive. U.S. Patent 20190119784A1, 25 April 2019. [Google Scholar]
- Canaguier, V. Synthesis and Reduction-Carburization of (Fe, Mg)(Cr, Al)2O4 Composite Spinel Solid Solution with CH4. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2018. [Google Scholar]
- Anacleto, N.; Ostrovski, O. Solid-state reduction of chromium oxide by methane-containing gas. Metall. Mater. Trans. B 2004, 35, 609–615. [Google Scholar] [CrossRef]
- Suzuki, A.; Yasuda, A.; Ozawa, K. Cr and Al diffusion in chromite spinel: Experimental determination and its implication for diffusion creep. Phys. Chem. Miner. 2008, 35, 433–445. [Google Scholar] [CrossRef]
- ICDA. Statistical Bulletin 2013. Available online: https://www.icdacr.com/download/ICDAActivityReport2013.pdf (accessed on 25 September 2021).
- Kleynhans, E.; Neizel, B.; Beukes, J.; Van Zyl, P. Utilisation of pre-oxidised ore in the pelletised chromite pre-reduction process. Miner. Eng. 2016, 92, 114–124. [Google Scholar] [CrossRef]
- Glastonbury, R.I.; Beukes, J.; Van Zyl, P.; Sadikit, L.; Jordaan, A.; Campbell, Q.; Stewart, H.; Dawson, N. Comparison of physical properties of oxidative sintered pellets produced with UG2 or metallurgical-grade South African chromite: A case study. J. S. Afr. Inst. Min. Metall. 2015, 115, 699–706. [Google Scholar] [CrossRef]
- Ringdalen, E.; Olsen, S.E. The effect of chromite ore mineralogy on reduction mechanisms and reducibility. INFACON 1998, 8, 147–152. [Google Scholar]
- Pandey, B.; Prajapati, Y.K.; Sheth, P.N. Recent progress in thermochemical techniques to produce hydrogen gas from biomass: A state of the art review. Int. J. Hydrogen Energy 2019, 44, 25384–25415. [Google Scholar] [CrossRef]
- Canavesio, C.; Nassini, D.; Nassini, H.E.; Bohé, A.E. Study on an original cobalt-chlorine thermochemical cycle for nuclear hydrogen production. Int. J. Hydrogen Energy 2019, 45, 26090–26103. [Google Scholar] [CrossRef]
- Falch, A.; Kriek, R.J. Laser induced H2 production employing Pt-TiO2 photocatalysts. J. Photochem. Photobiol. A Chem. 2013, 271, 117–123. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, C.; Shi, F. MoS2/Ti3C2 heterostructure for efficient visible-light photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 6291–6301. [Google Scholar] [CrossRef]
- Ziming, C.; Fuqiang, W.; Huaxu, L.; Shengpeng, H.; Bo, L.; Jianyu, T.; Hongyang, L. Photon-absorption-based explanation of ultrasonic-assisted solar photochemical splitting of water to improve hydrogen production. Int. J. Hydrogen Energy 2018, 43, 14439–14450. [Google Scholar] [CrossRef]
- Liu, W.-S.; Perng, T.-P. Ta2O5 hollow fiber composed of internal interconnected mesoporous nanotubes and its enhanced photochemical H2 evolution. Int. J. Hydrogen Energy 2019, 44, 17688–17696. [Google Scholar] [CrossRef]
- Patel, P.P.; Ghadge, S.D.; Hanumantha, P.J.; Datta, M.K.; Gattu, B.; Shanthi, P.M.; Kumta, P.N. Active and robust novel bilayer photoanode architectures for hydrogen generation via direct non-electric bias induced photo-electrochemical water splitting. Int. J. Hydrogen Energy 2018, 43, 13158–13176. [Google Scholar] [CrossRef]
- Manwar, N.R.; Borkar, R.G.; Khobragade, R.; Rayalu, S.S.; Jain, S.L.; Bansiwal, A.K.; Labhsetwar, N.K. Efficient solar photo-electrochemical hydrogen generation using nanocrystalline CeFeO3 synthesized by a modified microwave assisted method. Int. J. Hydrogen Energy 2017, 42, 10931–10942. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Hydrogen generation by means of hydrolysis using activated Al-In-Bi-Sn composites for electrochemical energy applications. Int. J. Electrochem. Sci. 2017, 12, 8663–8682. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Hydrogen generation of mechanochemically activated Al-Bi-In composites. Int. J. Hydrogen Energy 2017, 42, 16589–16602. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Hydrogen generation by the hydrolysis of mechanochemically activated aluminum-tin-indium composites in pure water. Int. J. Hydrogen Energy 2018, 43, 21398–21413. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. The effects of bismuth and tin on the mechanochemical processing of aluminum-based composites for hydrogen generation purposes. Int. J. Hydrogen Energy 2019, 44, 21896–21912. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Material Aspects Pertaining to Hydrogen Production from Aluminum: Opinion. Res. Dev. Mater. Sci. 2019, 44, 21896–21912. [Google Scholar] [CrossRef]
- Du Preez, S.P. Hydrogen Generation by the Reaction of Mechanochemically Activated Aluminium and Water; North-West University: Potchefstroom, South Africa, 2019. [Google Scholar]
- du Preez, S.P.; Bessarabov, D.G. On-demand hydrogen generation by the hydrolysis of ball-milled aluminum composites: A process overview. Int. J. Hydrogen Energy 2021, 46, 35790–35813. [Google Scholar] [CrossRef]
- Bessarabov, D.G.; Human, G.; Kruger, A.J.; Chiuta, S.; Modisha, P.M.; du Preez, S.P.; Oelofse, S.P.; Vincent, I.; Van Der Merwe, J.; Langmi, H.W.; et al. South African hydrogen infrastructure (HySA infrastructure) for fuel cells and energy storage: Overview of a projects portfolio. Int. J. Hydrogen Energy 2017, 42, 13568–13588. [Google Scholar] [CrossRef]
- Phillips, R.; Dunnill, C.W. Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Adv. 2016, 6, 100643–100651. [Google Scholar] [CrossRef]
- Falch, A.; Badets, V.A.; Labrugère, C.; Kriek, R.J. Co-sputtered PtxPdyAlz thin film electrocatalysts for the production of hydrogen via SO2 (aq) electro-oxidation. Electrocatalysis 2016, 7, 376–390. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of efficient membrane electrode assembly for low cost hydrogen production by anion exchange membrane electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Merwe, J.V.D.; Uren, K.; Schoor, G.V.; Bessarabov, D. A study of the loss characteristics of a single cell PEM electrolyser for pure hydrogen production. In Proceedings of the 2013 IEEE International Conference on Industrial Technology (ICIT), Cape Town, South Africa, 25–28 February 2013; pp. 668–672. [Google Scholar]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Bessarabov, D.G. Hydrogen production from ammonia decomposition over a commercial Ru/Al2O3 catalyst in a microchannel reactor: Experimental validation and CFD simulation. Int. J. Hydrogen Energy 2016, 41, 3774–3785. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Bessarabov, D.G. Performance evaluation of a high-throughput microchannel reactor for ammonia decomposition over a commercial Ru-based catalyst. Int. J. Hydrogen Energy 2015, 40, 2921–2926. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; van der Gryp, P.; Bessarabov, D.G. Reactor technology options for distributed hydrogen generation via ammonia decomposition: A review. Int. J. Hydrogen Energy 2013, 38, 14968–14991. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Engelbrecht, N.; du Preez, S.P.; Bessarabov, D. Thermophilic Biogas Upgrading via ex Situ Addition of H2 and CO2 Using Codigested Feedstocks of Cow Manure and the Organic Fraction of Solid Municipal Waste. ACS Omega 2020, 5, 17367–17376. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Daramola, M.O.; Mogwase, B.; Engelbrecht, N.; Yoro, K.O.; du Preez, S.P.; Mhlongo, S.; Ezeokoli, O.T.; Ghimire, A.; Ayeni, A.O.; et al. Revising the dark fermentative H2 research and development scenario—An overview of the recent advances and emerging technological approaches. Biomass Bioenergy 2020, 140, 105673. [Google Scholar] [CrossRef]
- Du Toit, M.H.; Avdeenkov, A.V.; Bessarabov, D. Reviewing H2 combustion: A case study for non-fuel-cell power systems and safety in passive autocatalytic recombiners. Energy Fuels 2018, 32, 6401–6422. [Google Scholar] [CrossRef]
- Gervilla, F.; Asta, M.P.; Fanlo, I.; Grolimund, D.; Ferreira-Sánchez, D.; Samson, V.A.; Hunziker, D.; Colas, V.; González-Jiménez, J.M.; Kerestedjian, T.N.; et al. Diffusion pathways of Fe2+ and Fe3+ during the formation of ferrian chromite: A µXANES study. Contrib. Mineral. Petrol 2019, 17, 65. [Google Scholar] [CrossRef]
- Du Preez, S.; Jones, D.; Warwick, M.; Falch, A.; Sekoai, P.; das Neves Quaresma, C.M.; Bessarabov, D.; Dunnill, C. Thermally stable Pt/Ti mesh catalyst for catalytic hydrogen combustion. Int. J. Hydrogen Energy 2020, 45, 16851–16864. [Google Scholar] [CrossRef]
- Algie, S.H.; Finn, C.W.P. Reaction Mechanisms in the Reduction of Winterveld Chrome Spinel with Graphite and Carbon; Council for Mineral Technology: Randburg, South Africa, 1984.
- Hazar-Yoruc, A. Reduction mechanism of chromite spinel with carbon. Min. Metall. Explor. 2007, 24, 115–120. [Google Scholar] [CrossRef]
- Ramos-Hernandez, J.J.; Porcayo-Calderon, J.; Salinas-Bravo, V.M.; Arrieta-Gonzalez, C.D.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Phase Stability Diagrams for High Temperature Corrosion Processes. Math. Probl. Eng. 2013, 2013, 542061. [Google Scholar] [CrossRef]
- Burcat, A.; Ruscic, B. Third Millenium Ideal Gas and Condensed Phase Thermochemical Database for Combustion (with Update from Active Thermochemical Tables); Argonne National Laboratory(ANL): Argonne, IL, USA, 2005.
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for chromium at 25–300 °C. Corros. Sci. 1997, 39, 43–57. [Google Scholar] [CrossRef]
- Khan, H.R.; Raub, C.J. Properties of chromium hydride. J. Less Common Met. 1976, 49, 399–406. [Google Scholar] [CrossRef]
- Venkatraman, M.; Neumann, J.P. The cr-h (chromium-hydrogen) system. J. Phase Equilibria 1991, 12, 672–677. [Google Scholar] [CrossRef]
- Dellien, I.; Hall, F.; Hepler, L.G. Chromium, molybdenum, and tungsten: Thermodynamic properties, chemical equilibriums, and standard potentials. Chem. Rev. 1976, 76, 283–310. [Google Scholar] [CrossRef]
- Soykan, O.; Eric, R.; King, R. Kinetics of the reduction of Bushveld complex chromite ore at 1416 C. Metall. Mater. Trans. B 1991, 22, 801–810. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Y.; Holappa, L.; Boom, R. Microstructure changes of chromite reduced with CO gas. INFACON XI New Delhi 2007, 1, 7–14. [Google Scholar]
- Słowiński, G.; Smoliński, A. Thermodynamic Feasibility of Hydrogen-Rich Gas Production Supported by Iron Based Chemical Looping Process. J. Chem. 2016, 2016, 1764670. [Google Scholar] [CrossRef]
- Chu, W.F.; Rahmel, A. The kinetics of the reduction of chromium oxide by hydrogen. Metall. Trans. B 1979, 10, 401–407. [Google Scholar] [CrossRef]
- Paktunc, A.; Cabri, L. A proton-and electron-microprobe study of gallium, nickel and zinc distribution in chromian spinel. Lithos 1995, 35, 261–282. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, P.; Jiang, M. Advances towards a clean hydrometallurgical process for chromite. Minerals 2016, 6, 7. [Google Scholar] [CrossRef]
- Xiao, Y.; Schuffeneger, C.; Reuter, M.; Holappa, L.; Seppala, T. Solid State Reduction of Chromite with CO; INFACON XI: Cape Town, South Africa, 2004; pp. 26–35. [Google Scholar]
- Roine, A.; Lamberg, P.; Katiranta, T.; Salminen, J. HSC Chemistry 7 Simulation; Outotec Research: Pori, Finland, 2009. [Google Scholar]
- Bondioli, F.; Ferrari, A.M.; Leonelli, C.; Manfredini, T.; Linati, L.; Mustarelli, P. Reaction Mechanism in Alumina/Chromia (Al2O3–Cr2O3) Solid Solutions Obtained by Coprecipitation. J. Am. Ceram. Soc. 2000, 83, 2036–2040. [Google Scholar] [CrossRef]
- Visser, M. An overview of the history and current operational facilities of Samancor Chrome. S. Afr. Pyrometall. 2006, 2006, 285–296. [Google Scholar]
- Naiker, O. The development and advantages of Xstrata’s Premus Process. In XI International Ferroalloys Congress; INFACON XI: New Delhi, India, 2006; pp. 113–119. [Google Scholar]
- Du Preez, S.P. Ferrochrome Waste Management: Addressing Current Gaps. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2018. [Google Scholar]
- Kozhukhova, A.E.; du Preez, S.P.; Malakhov, A.A.; Bessarabov, D.G. A Thermally Conductive Pt/AAO Catalyst for Hydrogen Passive Autocatalytic Recombination. Catalysts 2021, 11, 491. [Google Scholar] [CrossRef]
- Malakhov, A.; du Toit, M.H.; Du Preez, S.P.; Avdeenkov, A.; Bessarabov, D. Temperature profile mapping over a catalytic unit of a hydrogen passive autocatalytic recombiner: An experimental and computational fluid dynamics study. Energy Fuels 2020, 34, 11637–11649. [Google Scholar] [CrossRef]
- Du Preez, S.; Jones, D.; Bessarabov, D.; Falch, A.; das Neves Quaresma, C.M.; Dunnill, C. Development of a Pt/stainless steel mesh catalyst and its application in catalytic hydrogen combustion. Int. J. Hydrogen Energy 2019, 44, 27094–27106. [Google Scholar] [CrossRef]
- Kleynhans, E.L.J.; Beukes, J.P.; Van Zyl, P.G.; Bunt, J.R.; Nkosi, N.S.B.; Venter, M. The Effect of Carbonaceous Reductant Selection on Chromite Pre-reduction. Metall. Mater. Trans. B 2017, 48, 827–840. [Google Scholar] [CrossRef]


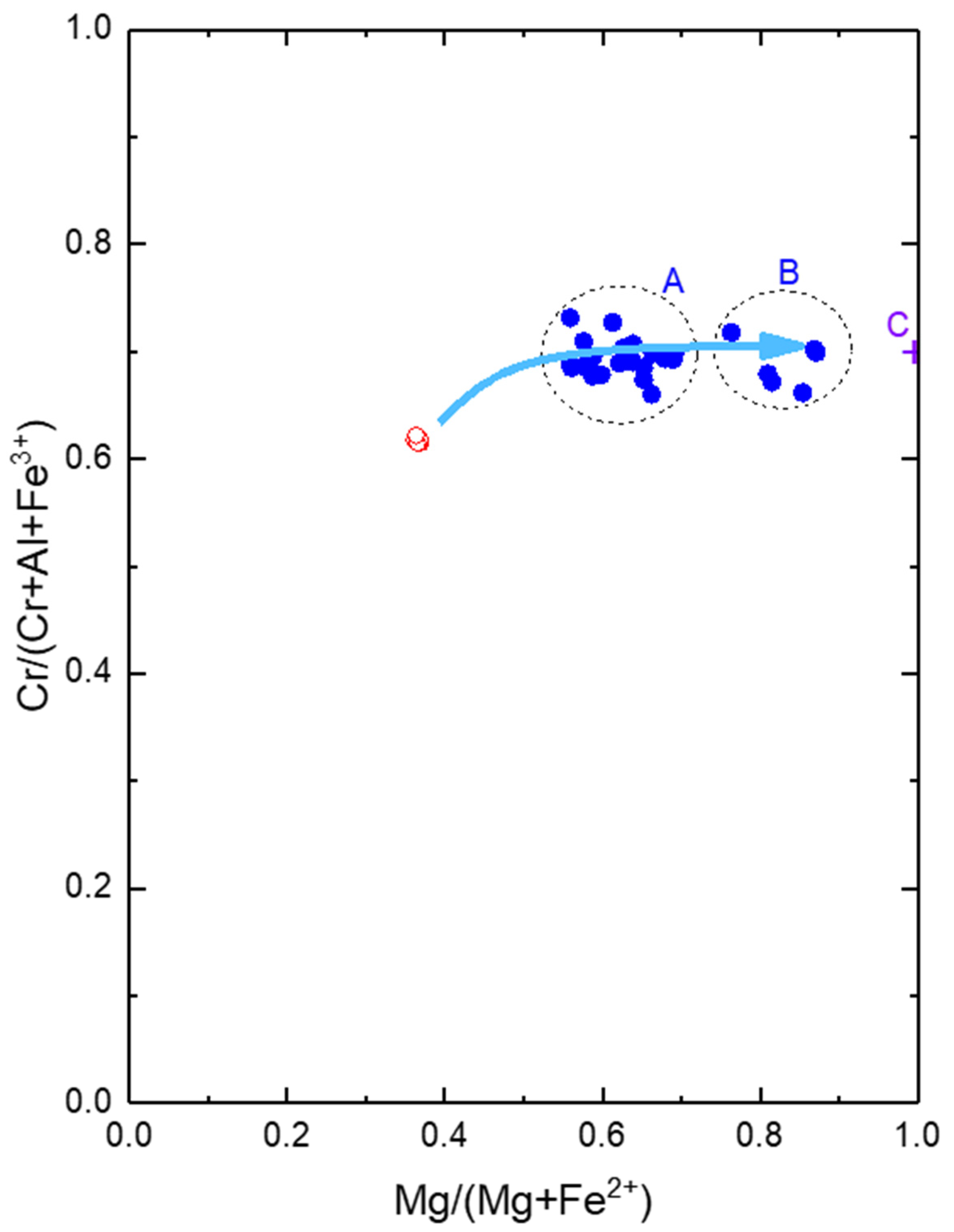
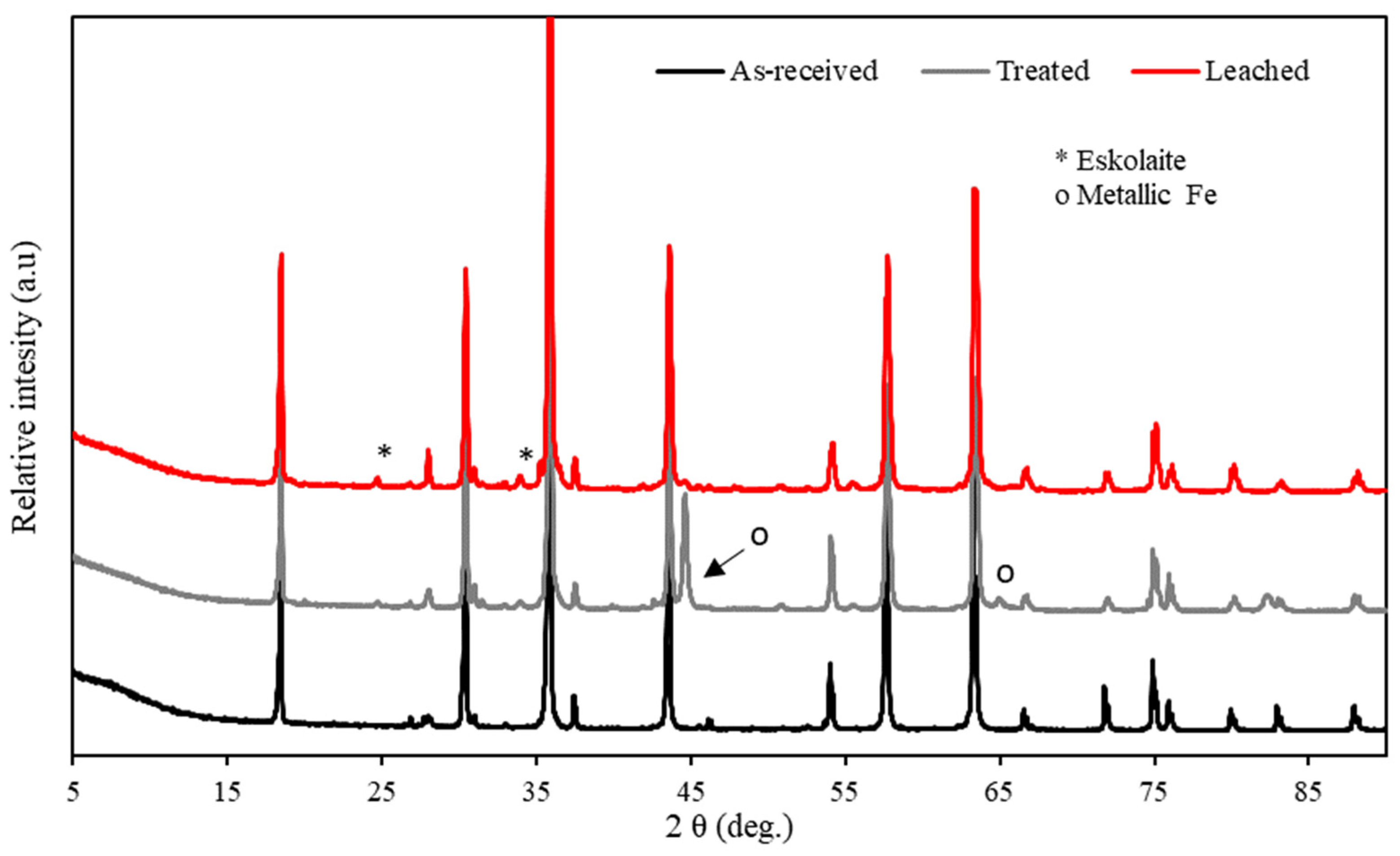
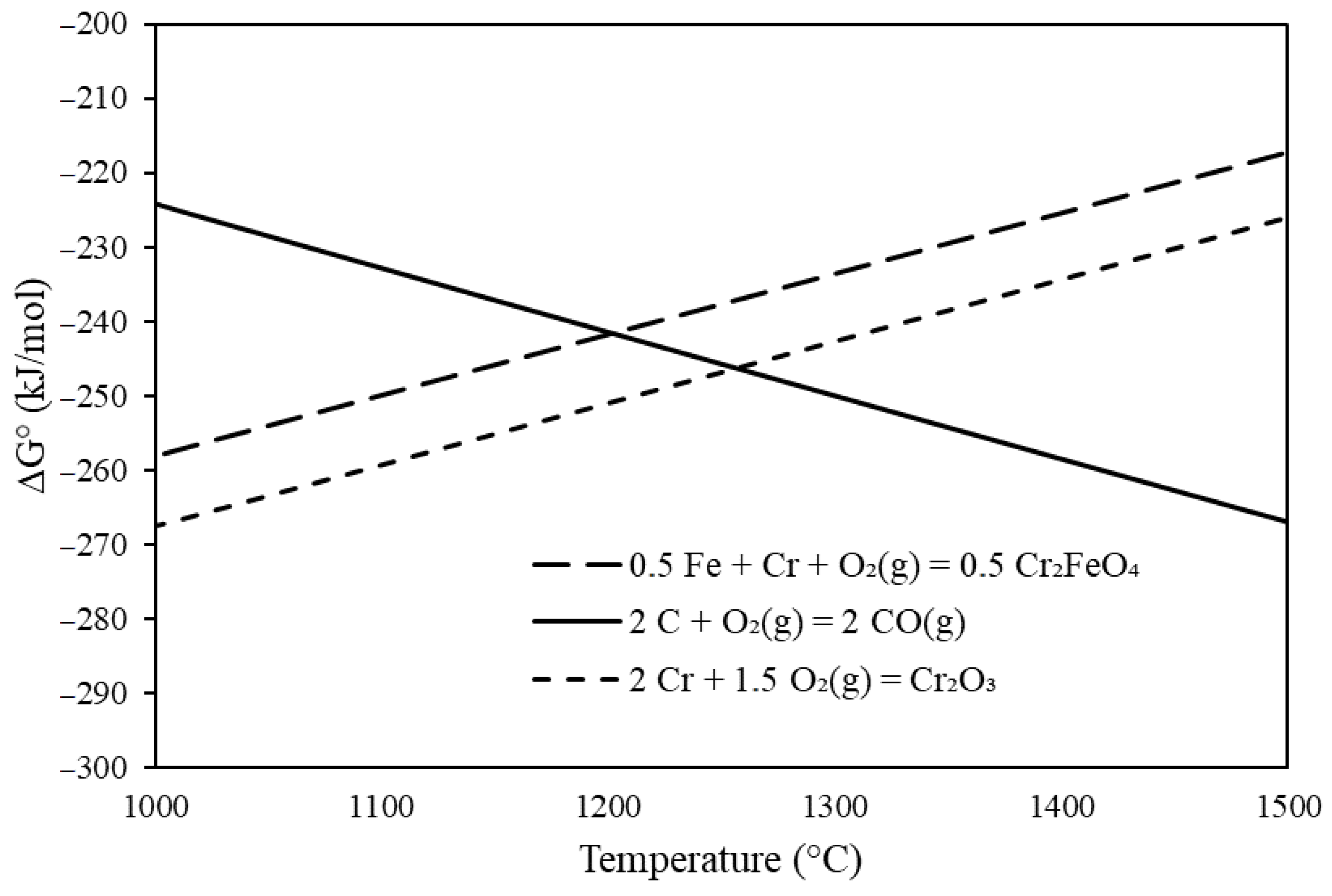
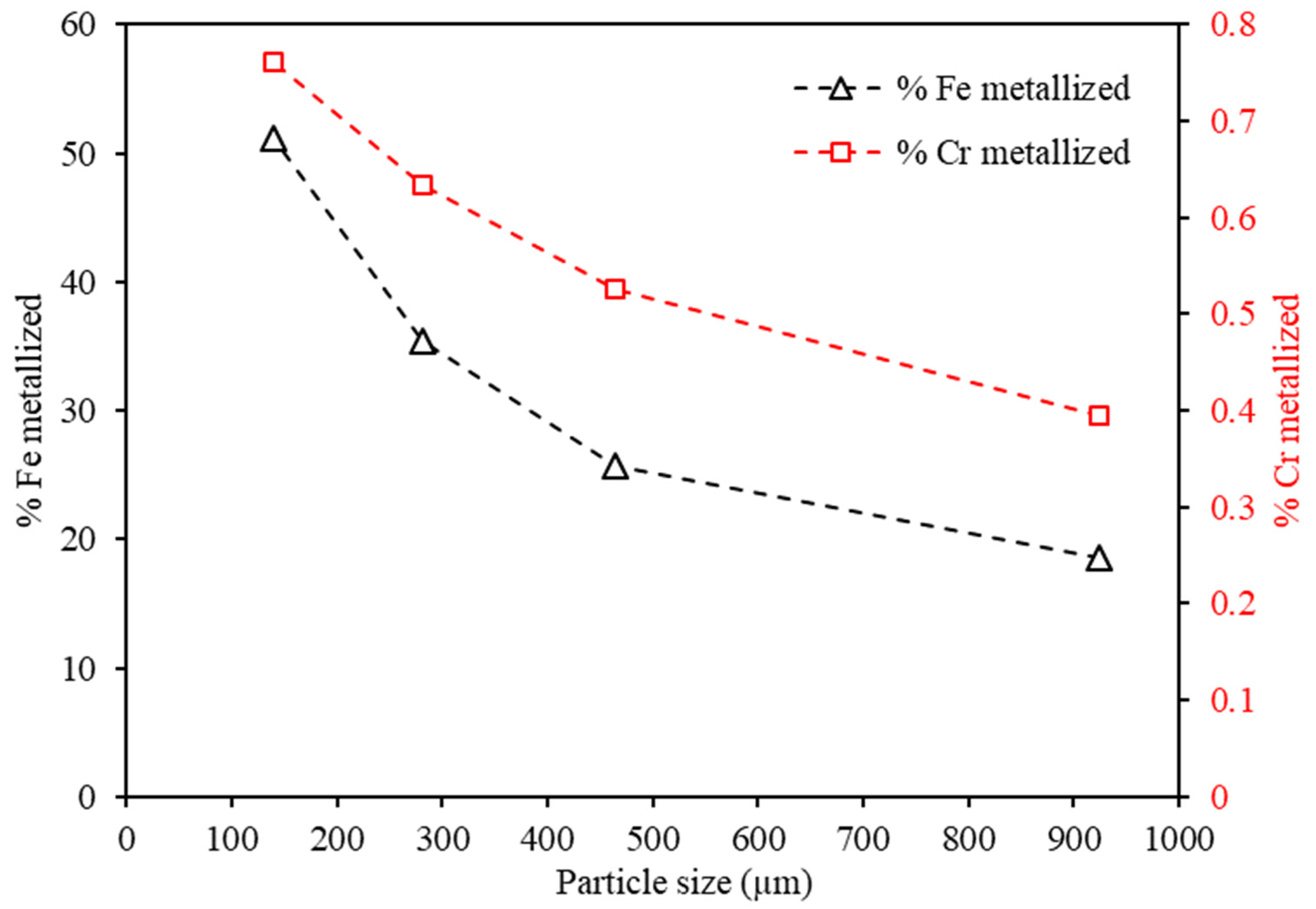
| ICP-OES | XRD | ||
|---|---|---|---|
| Compound | Metgrade | Compound | Metgrade |
| Cr2O3 | 44.19 | Chromite | 93.4 |
| FeO | 24.68 | Enstatite | 6.6 |
| SiO2 | 2.96 | ||
| Al2O3 | 14.71 | ||
| MgO | 10.31 | ||
| CaO | 0.16 | ||
| P | 0.001 | ||
| Cr/Fe | 1.58 | ||
| Size Equivalent | As-Received | Sieve Sizes (µm) | |||
|---|---|---|---|---|---|
| <106 | 106 to 250 | 250 to 500 | 500 to 1000 | ||
| d90 | 415.0 | 140.2 | 282.1 | 464.2 | 924.1 |
| d50 | 182.3 | 87.4 | 186.3 | 347.4 | 667.7 |
| d10 | 71.7 | 51.8 | 120.7 | 260.0 | 487.6 |
| Sample Surface | Detected Element (wt.%) | Cr/Fe Ratio | |||||
|---|---|---|---|---|---|---|---|
| O | Mg | Al | Si | Cr | Fe | ||
| As-received | 36.68 ± 1.41 | 6.29 ± 0.30 | 8.45 ± 0.23 | 0.29 ± 0.01 | 30.85 ± 1.49 | 17.44 ± 0.52 | 1.77 |
| Treated | 26.32 ± 3.69 | 2.12 ± 0.49 | 13.69 ± 0.48 | 0.42 ± 0.04 | 13.94 ± 1.35 | 43.53 ± 5.4 | 0.32 |
| Leached | 28.98 ± 0.68 | 2.43 ± 0.23 | 14.93 ± 0.63 | 0.66 ± 0.18 | 49.98 ± 0.74 | 3.01 ± 0.37 | 16.60 |
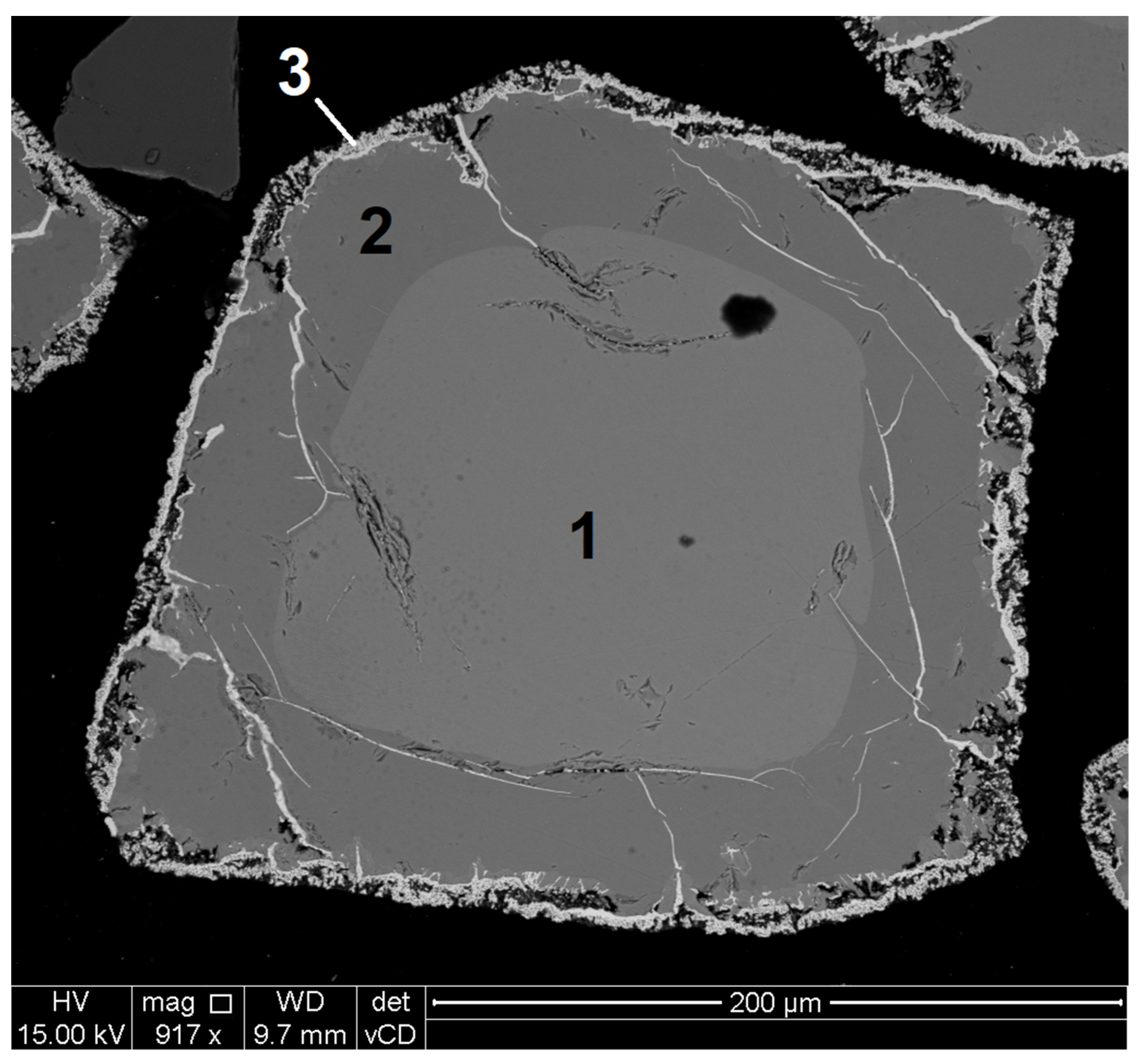
| Cross-Sectioned Particle | Detected Element (wt.%) | Cr/Fe Ratio | Mg/Fe Ratio | Al/Fe Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| O | Mg | Al | Si | Cr | Fe | ||||
| Area 1 | 31.64 ± 0.39 | 6.19 ± 0.07 | 8.24 ± 0.04 | 0.47 ± 0.06 | 33.41 ± 0.04 | 20.07 ± 0.31 | 1.66 | 0.31 | 0.41 |
| Area 2 | 32.42 ± 0.30 | 8.86 ± 0.08 | 8.79 ± 0.13 | 0.38 ± 0.02 | 40.29 ± 0.10 | 9.27 ± 0.33 | 4.35 | 0.96 | 0.95 |
| Area 3 | 3.44 ± 0.13 | 0.22 ± 0.30 | 1.53 ± 0.04 | 0 | 4.05 ± 0.51 | 90.38 ± 0.35 | 0.04 | 0.002 | 0.017 |
| Grain Area | Detected Phase (wt,%) | ||||||
|---|---|---|---|---|---|---|---|
| TiO2 | Al2O3 | Cr2O3 | FeO # | MnO | MgO | NiO | |
| Untreated * | 0.97 ± 0.04 | 11.97 ± 0.18 | 44.45 ± 0.34 | 32.14 ± 0.22 | 0.36 ± 0.02 | 4.45 ± 0.09 | 0.12 ± 0.02 |
| Area 1 | 0.59 | 16.69 | 56.14 | 12.28 | 0.34 | 13.71 | 0.01 |
| ±0.07 | ±0.25 | ±0.75 | ±0.28 | ±0.06 | ±0.17 | ±0.02 | |
| Area 2 | 3.31 | 21.47 | 71.82 | 0.62 | 0.04 | 0.67 | 0.01 |
| ±0.15 | ±0.28 | ±0.86 | ±0.08 | ±0.03 | ±0.04 | ±0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, J.; Paktunc, D.; Ramos-Hernandez, J.J.; Tangstad, M.; Ringdalen, E.; Beukes, J.P.; Bessarabov, D.G.; Du Preez, S.P. The Use of Hydrogen as a Potential Reductant in the Chromite Smelting Industry. Minerals 2022, 12, 534. https://doi.org/10.3390/min12050534
Davies J, Paktunc D, Ramos-Hernandez JJ, Tangstad M, Ringdalen E, Beukes JP, Bessarabov DG, Du Preez SP. The Use of Hydrogen as a Potential Reductant in the Chromite Smelting Industry. Minerals. 2022; 12(5):534. https://doi.org/10.3390/min12050534
Chicago/Turabian StyleDavies, Jamey, Dogan Paktunc, José Juan Ramos-Hernandez, Merete Tangstad, Eli Ringdalen, Johan P. Beukes, Dmitri G. Bessarabov, and Stephanus P. Du Preez. 2022. "The Use of Hydrogen as a Potential Reductant in the Chromite Smelting Industry" Minerals 12, no. 5: 534. https://doi.org/10.3390/min12050534
APA StyleDavies, J., Paktunc, D., Ramos-Hernandez, J. J., Tangstad, M., Ringdalen, E., Beukes, J. P., Bessarabov, D. G., & Du Preez, S. P. (2022). The Use of Hydrogen as a Potential Reductant in the Chromite Smelting Industry. Minerals, 12(5), 534. https://doi.org/10.3390/min12050534








