Investigation of the Flotation Separation of Scheelite from Fluorite with a Novel Chelating Agent: Pentasodium Diethylenetriaminepentaacetate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Flotation Tests
2.2. Adsorption Tests
2.3. Zeta Potential Measurement
2.4. FTIR Spectral Analysis
2.5. XPS Analysis
3. Results and Discussion
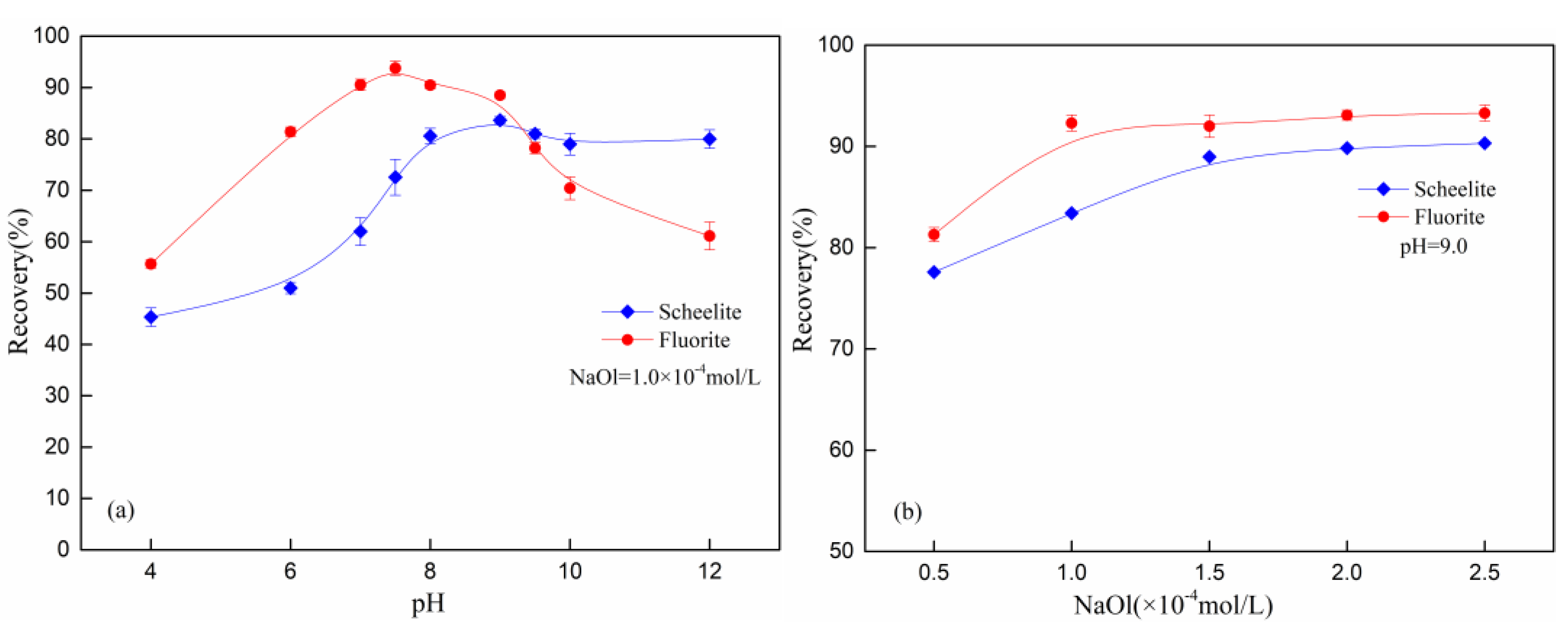
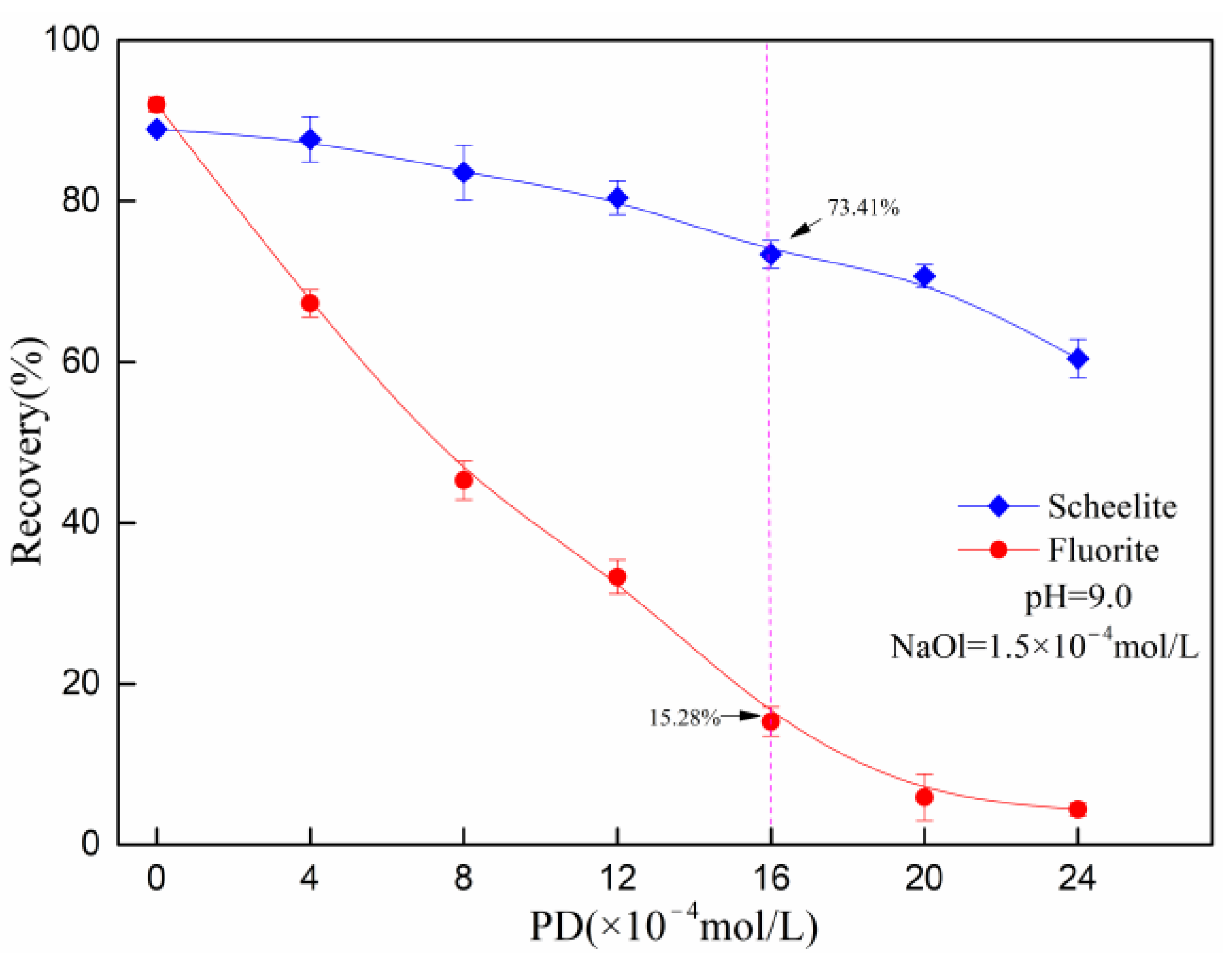
3.1. Micro-Flotation Results
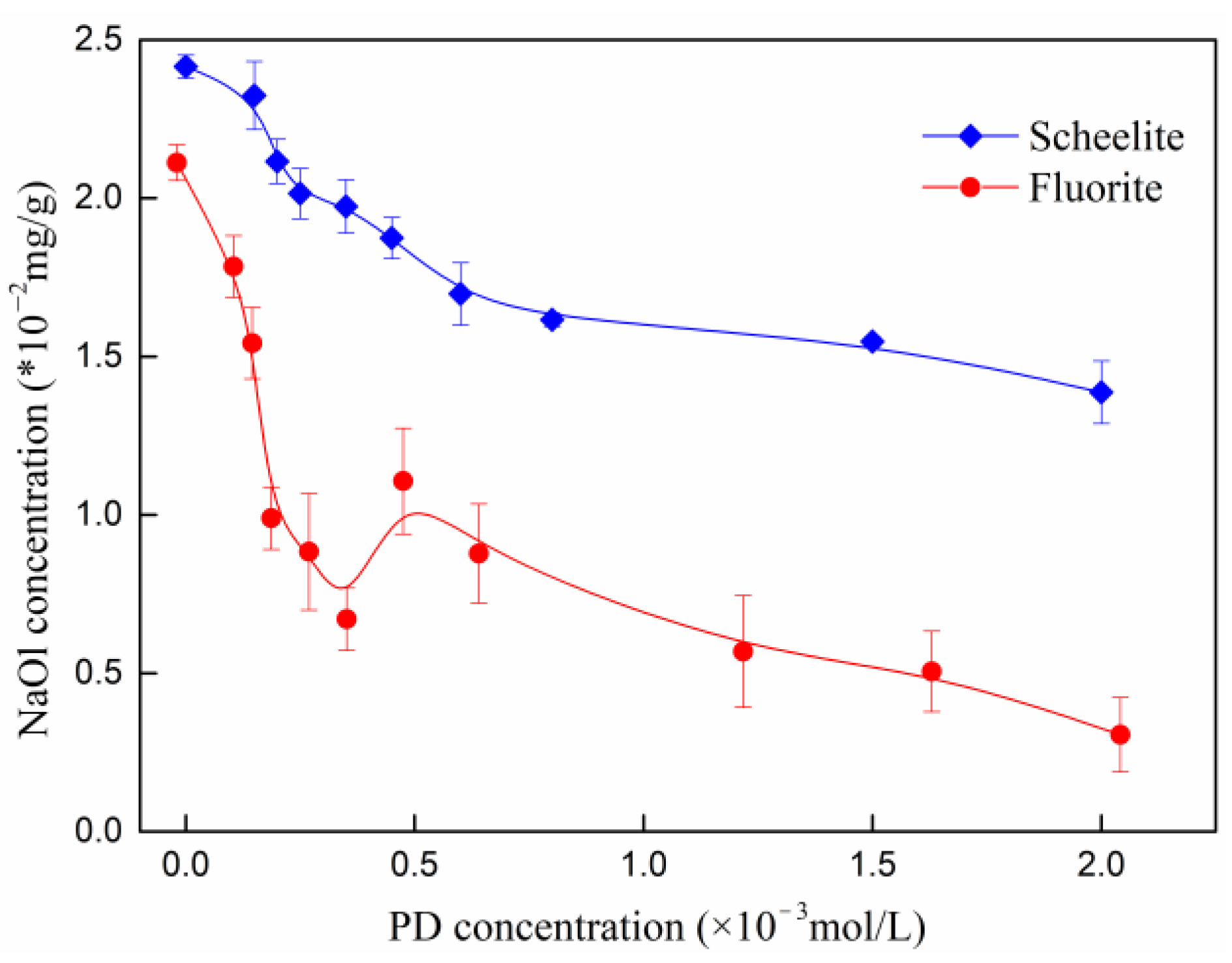
3.2. Adsorption Results
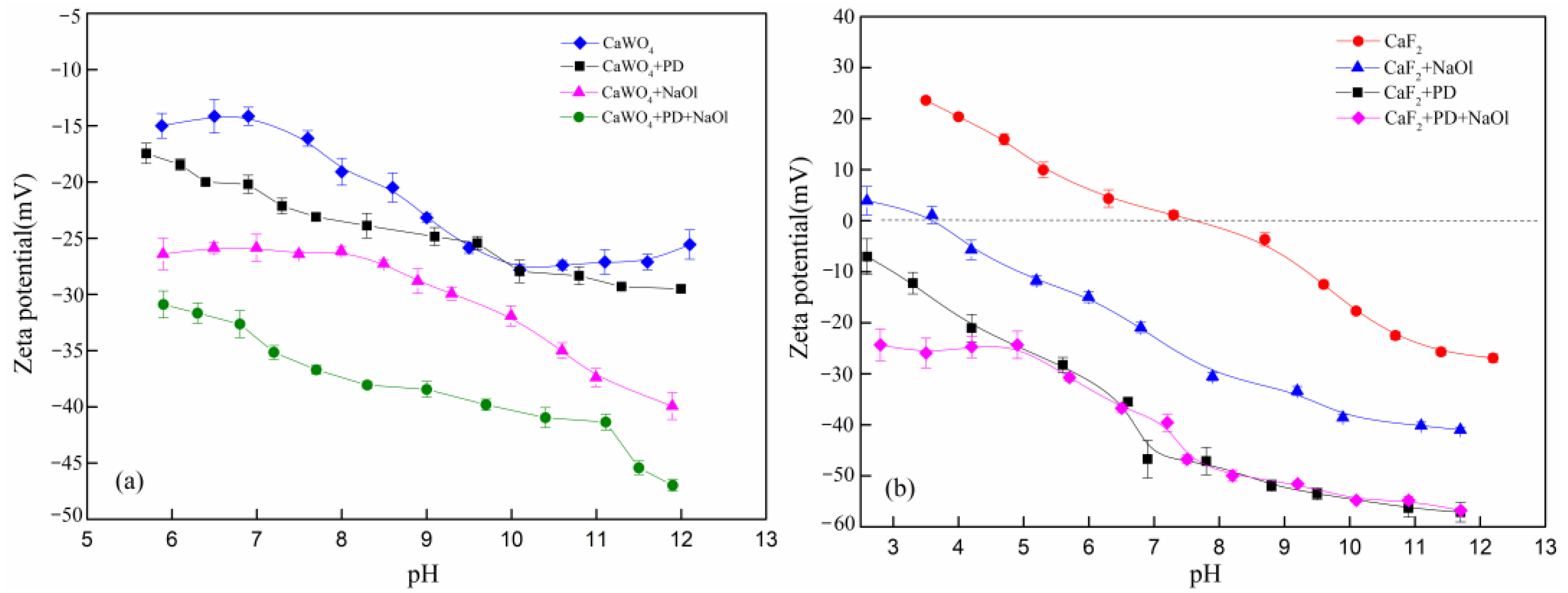
3.3. Zeta Potential Results
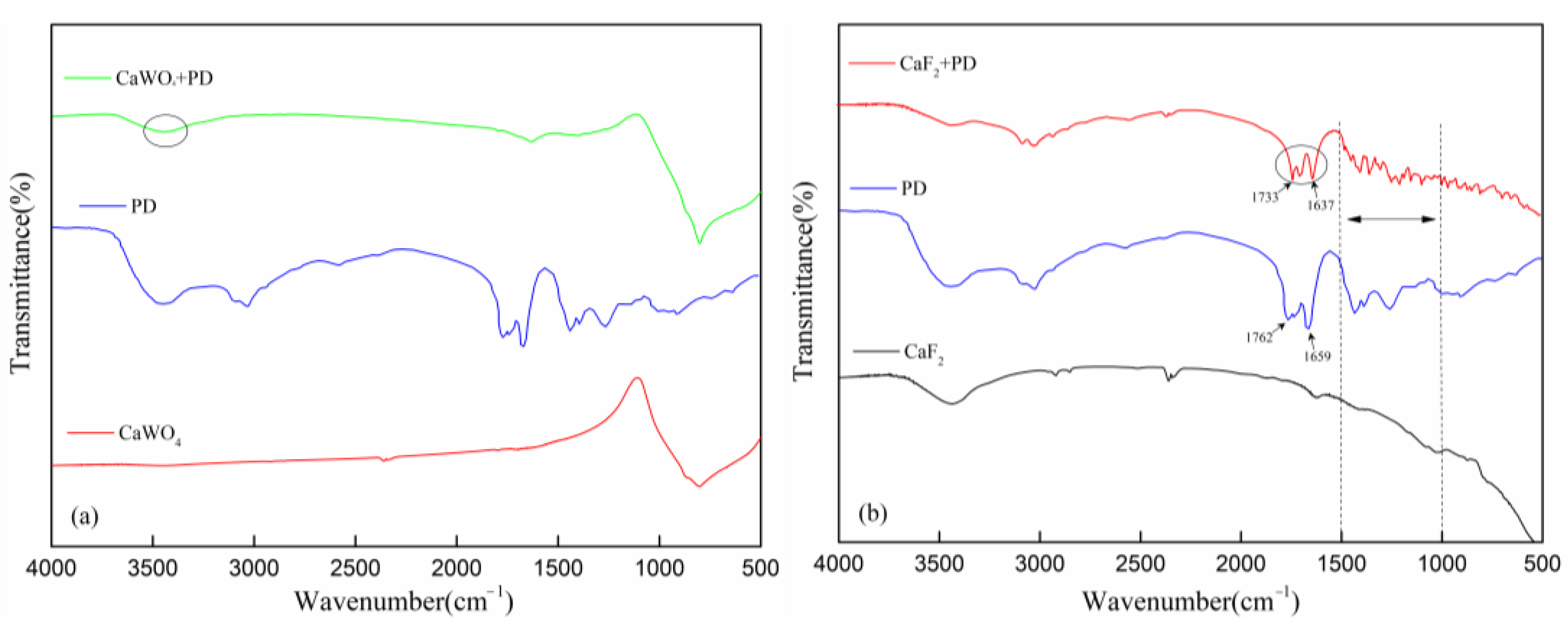
3.4. FTIR Results
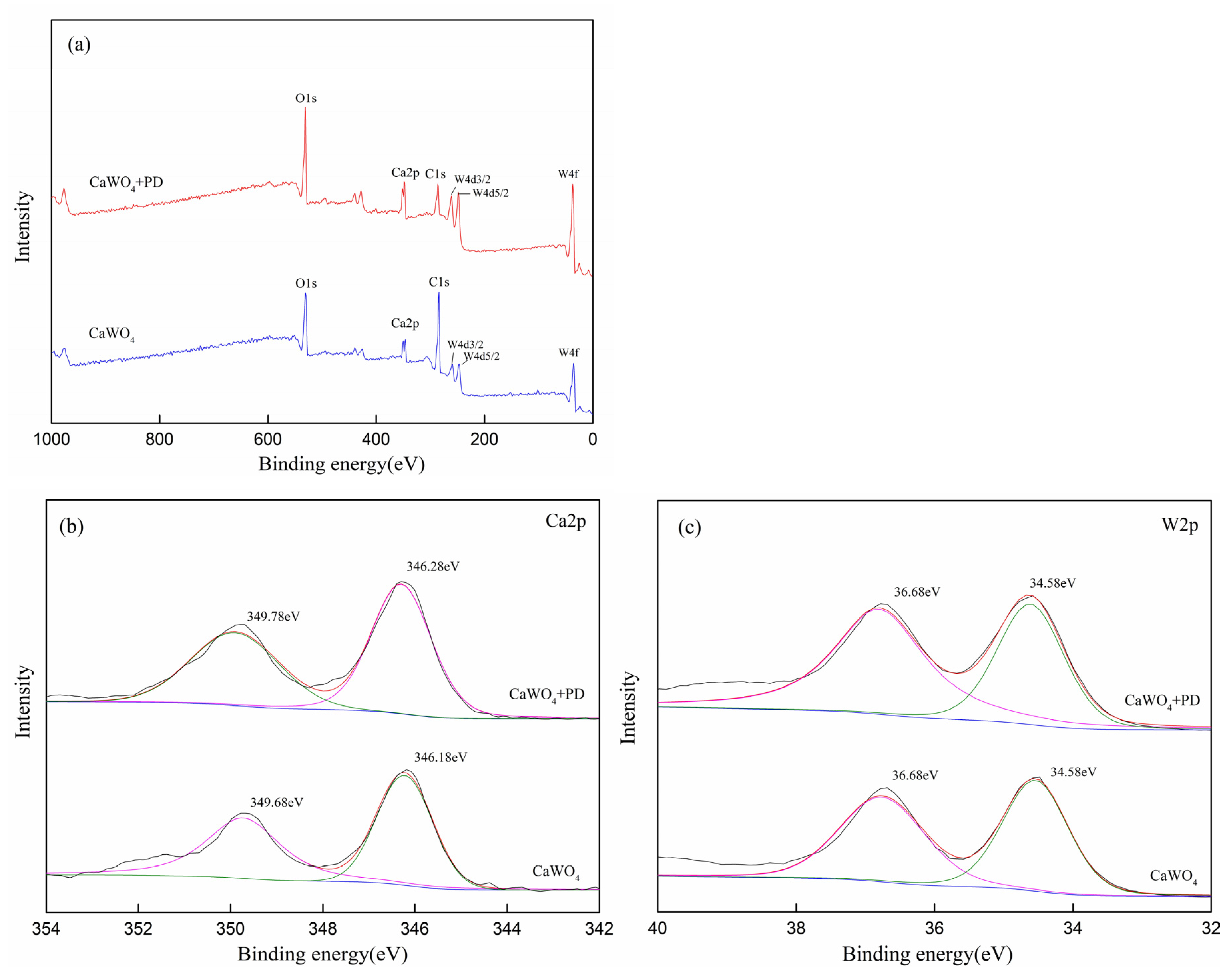
3.5. XPS Results
3.6. Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roundy, D.; Krenn, C.; Cohen, M.L.; Morris, J., Jr. The ideal strength of tungsten. Philos. Mag. A 2001, 81, 1725–1747. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J. From “strategic” tungsten to “green” neodymium: A century of critical met-als at a glance. Ore Geol. Rev. 2015, 64, 455–458. [Google Scholar] [CrossRef]
- Wu, C.-M.; Naseem, S.; Chou, M.-H.; Wang, J.-H.; Jian, Y.-Q. Recent Advances in Tungsten-Oxide-Based Materials and Their Applications. Front. Mater. 2019, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.-G.; Miyauchi, M. Shape Modulation of Tungstic Acid and Tungsten Oxide Hollow Structures. J. Phys. Chem. C 2009, 113, 6539–6546. [Google Scholar] [CrossRef]
- Mardare, C.C.; Hassel, A.W. Review on the Versatility of Tungsten Oxide Coatings. Phys. Status Solidi (a) 2019, 216, 1900047. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Feng, Q.; Shi, Q.; Ou, L. Studies on interaction mechanism of fine wolframite with octyl hydroxamic acid. Miner. Eng. 2015, 79, 133–138. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Zhong, H. Study on the Activation of Scheelite and Wolframite by Lead Nitrate. Minerals 2015, 5, 247–258. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Chen, W.; Chen, F.; Bu, X.; Zhang, G.; Zhang, C.; Song, Y. A significant improvement of fine scheelite flotation through rheological control of flotation pulp by using garnet. Miner. Eng. 2019, 138, 257–266. [Google Scholar] [CrossRef]
- Yin, W.-Z.; Wang, J.-Z. Effects of particle size and particle interactions on scheelite flotation. Trans. Nonferrous Met. Soc. China 2014, 24, 3682–3687. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Bo, F.; Xianping, L.; Jinqing, W.; Pengcheng, W. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel de-pressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Chen, W.; Chen, Y. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar] [CrossRef]
- Deng, R.; Yang, X.; Hu, Y.; Ku, J.; Zuo, W.; Ma, Y. Effect of Fe(II) as assistant depressant on flotation separation of scheel-ite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippova, I.; Filippov, L. Investigation of the depressants involved in the selective flotation of scheelite from apa-tite, fluorite, and calcium silicates: Focus on the sodium silicate/sodium carbonate system. Powder Technol. 2019, 352, 501–512. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Sun, W.; Zeng, X.; Fang, S.; Han, H.; Hong, K.; Hu, Y. Use of Al2(SO4)3 and acidified water glass as mixture depressants in flotation separation of fluorite from calcite and celestite. Miner. Eng. 2019, 137, 160–170. [Google Scholar] [CrossRef]
- Deng, J.; Liu, C.; Yang, S.; Li, H.; Liu, Y. Flotation separation of barite from calcite using acidified water glass as the de-pressant. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123605. [Google Scholar] [CrossRef]
- Silva, J.P.P.; Baltar, C.A.M.; Gonzaga, R.S.G.; Peres, A.E.C.; Leite, J.Y.P. Identification of sodium silicate species used as flotation depressants. Min. Metall. Explor. 2012, 29, 207–210. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Zhang, G.; Liu, D.; Liu, R. Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite. Minerals 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Michelmore, A.; Jenkins, P.; Ralston, J. The interaction of linear polyphosphates with zincite surfaces. Int. J. Miner. Process. 2003, 68, 1–16. [Google Scholar] [CrossRef]
- Rath, R.; Subramanian, S. Adsorption, electrokinetic and differential flotation studies on sphalerite and galena using dextrin. Int. J. Miner. Process. 1999, 57, 265–283. [Google Scholar] [CrossRef]
- de Castro, F.H.B.; de Hoces, M.C. Influence of quebracho and sodium silicate on flotation of celestite and calcite with sodium oleate. Int. J. Miner. Process. 1993, 37, 283–298. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Martinez-Martinez, C.; Torres-Armenta, R. Improving fluorite flotation from ores by disper-sion processing. Miner. Eng. 2006, 19, 912–917. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, S.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and impli-cations for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Sillanpää, M.; Meng, Y.; Yin, D.; Tang, W.Z. Green Synthesis of Magnetic EDTA- and/or DTPA-Cross-Linked Chitosan Adsorbents for Highly Efficient Removal of Metals. Ind. Eng. Chem. Res. 2015, 54, 1271–1281. [Google Scholar] [CrossRef]
- Means, J.L.; Kucak, T.; Crerar, D.A. Relative degradation rates of NTA, EDTA and DTPA and environmental implications. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 45–60. [Google Scholar] [CrossRef]
- Sillanpää, M.; Oikari, A. Assessing the impact of complexation by EDTA and DTPA on heavy metal toxicity using microtox bioassay. Chemosphere 1996, 32, 1485–1497. [Google Scholar] [CrossRef]
- Misra, S.N.; Gagnani, M.A.; Indira, D.M.; Shukla, R.S. Biological and Clinical Aspects of Lanthanide Coordination Compounds. Bioinorg. Chem. Appl. 2004, 2, 155–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactive components. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef] [PubMed]
- Repo, E.; Kurniawan, T.A.; Warchol, J.K.; Sillanpää, M.E. Removal of Co(II) and Ni(II) ions from contaminated water us-ing silica gel functionalized with EDTA and/or DTPA as chelating agents. J. Hazard. Mater. 2009, 171, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Katyal, J.; Sharma, B. DTPA-extractable and total Zn, Cu, Mn, and Fe in Indian soils and their association with some soil prop-erties. Geoderma 1991, 49, 165–179. [Google Scholar] [CrossRef]
- Atademir, M.; Kitchener, J.; Shergold, H. The surface chemistry and flotation of scheelite, II. Flotation “collectors”. Int. J. Miner. Process. 1981, 8, 9–16. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Role of sodium carbonate in scheelite flotation—A multi-faceted reagent. Miner. Eng. 2018, 129, 120–128. [Google Scholar] [CrossRef]
- Rao, K.; Forssberg, K. Mechanism of oleate interaction on salt-type minerals Part III. Adsorption, zeta potential and diffuse reflectance FT-IR studies of scheelite in the presence of sodium oleate. Colloids Surf. 1991, 54, 161–187. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Chen, K.; Zhu, Y.; Nguyen, A.V.; Tian, M.; Wang, L.; Yue, T.; et al. Novel catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the surface of scheelite particles. Miner. Eng. 2018, 119, 11–22. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhou, B.; Wang, C.; Zhao, X.; Deng, Y.; Wang, Z. In situ preparation of hydrophobic CaCO3 in the presence of sodium oleate. Appl. Surf. Sci. 2006, 253, 1983–1987. [Google Scholar] [CrossRef]
- Vučinić, D.R.; Radulović, D.S.; Deušić, S.Đ. Electrokinetic properties of hydroxyapatite under flotation conditions. J. Colloid Interface Sci. 2010, 343, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kellar, J.; Young, C.; Knutson, K.; Miller, J. Thermotropic phase transition of adsorbed oleate species at a fluorite surface byin situ FT-IR/IRS spectroscopy. J. Colloid Interface Sci. 1991, 144, 381–389. [Google Scholar] [CrossRef]
- Mielczarski, E.; de Donato, P.; Mielczarski, J.; Cases, J.; Barres, O.; Bouquet, E. Solution Chemistry in Adsorption Layer Formation of Oleate on Fluorite. J. Colloid Interface Sci. 2000, 226, 269–276. [Google Scholar] [CrossRef]
- Sivamohan, R.; De Donato, P.; Cases, J.M. Adsorption of oleate species at the fluorite-aqueous solution interface. Langmuir 1990, 6, 637–644. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Kołodyńska, D.; Gorbyk, P. Gd(III) adsorption on the DTPA-functionalized chitosan/magnetite nanocompo-sites. Sep. Sci. Technol. 2018, 53, 1006–1016. [Google Scholar] [CrossRef]
- Roosen, J.; Binnemans, K. Adsorption and chromatographic separation of rare earths with EDTA-and DTPA-functionalized chitosan biopolymers. J. Mater. Chem. A 2014, 2, 1530–1540. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hu, Y.; Sun, W.; Zhai, J.; Yin, Z.; Guan, Q. Effect of phytic acid on the surface properties of scheelite and fluorite for their selective flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 80–87. [Google Scholar] [CrossRef]
- Submeier, J.; Reilley, C.N. Nuclear Magnetic Resonance Studies of Protonation of Polyamine and Aminocarboxylate Com-pounds in Aqueous Solution. Anal. Chem. 1964, 36, 1698–1706. [Google Scholar] [CrossRef]
- Westin, K.-J.; Rasmuson, Å.C. Nucleation of calcium carbonate in presence of citric acid, DTPA, EDTA and pyromellitic acid. J. Colloid Interface Sci. 2005, 282, 370–379. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Dai, S.; Yang, T.; Duan, H.; Liu, W. Effect of Tween 80 on flotation separation of magnesite and dolo-mite using NaOL as the collector. J. Mol. Liquids 2020, 315, 113712. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Longhua, X. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Letkeman, P.; Martell, A.E. Nuclear magnetic resonance and potentiometric protonation study of polyaminopolyacetic acids containing from two to six nitrogen atoms. Inorg. Chem. 1979, 18, 1284–1289. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y.-H.; Liu, X. Anisotropic surface broken bond properties and wettability of calcite and fluorite crystals. Trans. Nonferrous Met. Soc. China 2012, 22, 1203–1208. [Google Scholar] [CrossRef]
- Zheng, R.; Ren, Z.; Gao, H.; Chen, Z.; Qian, Y.; Li, Y. Effects of crystal chemistry on sodium oleate adsorption on fluorite surface investigated by molecular dynamics simulation. Miner. Eng. 2018, 124, 77–85. [Google Scholar] [CrossRef]
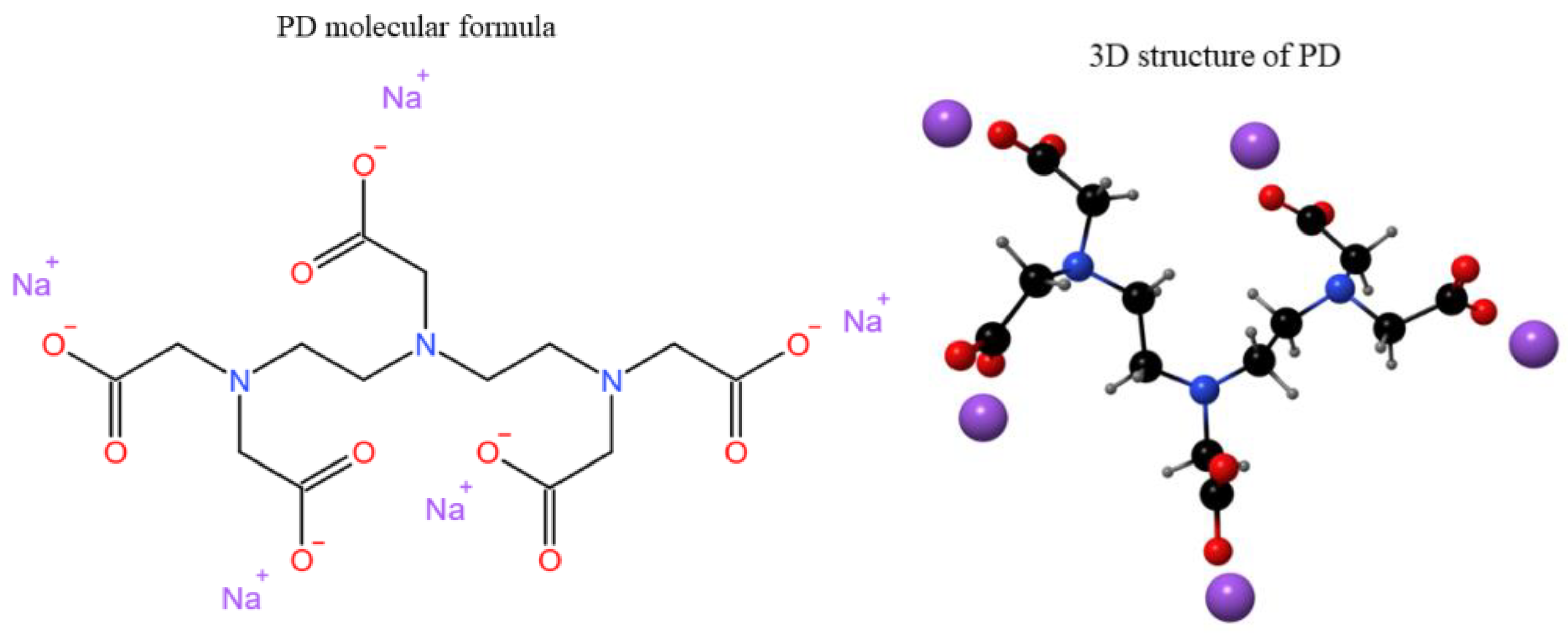
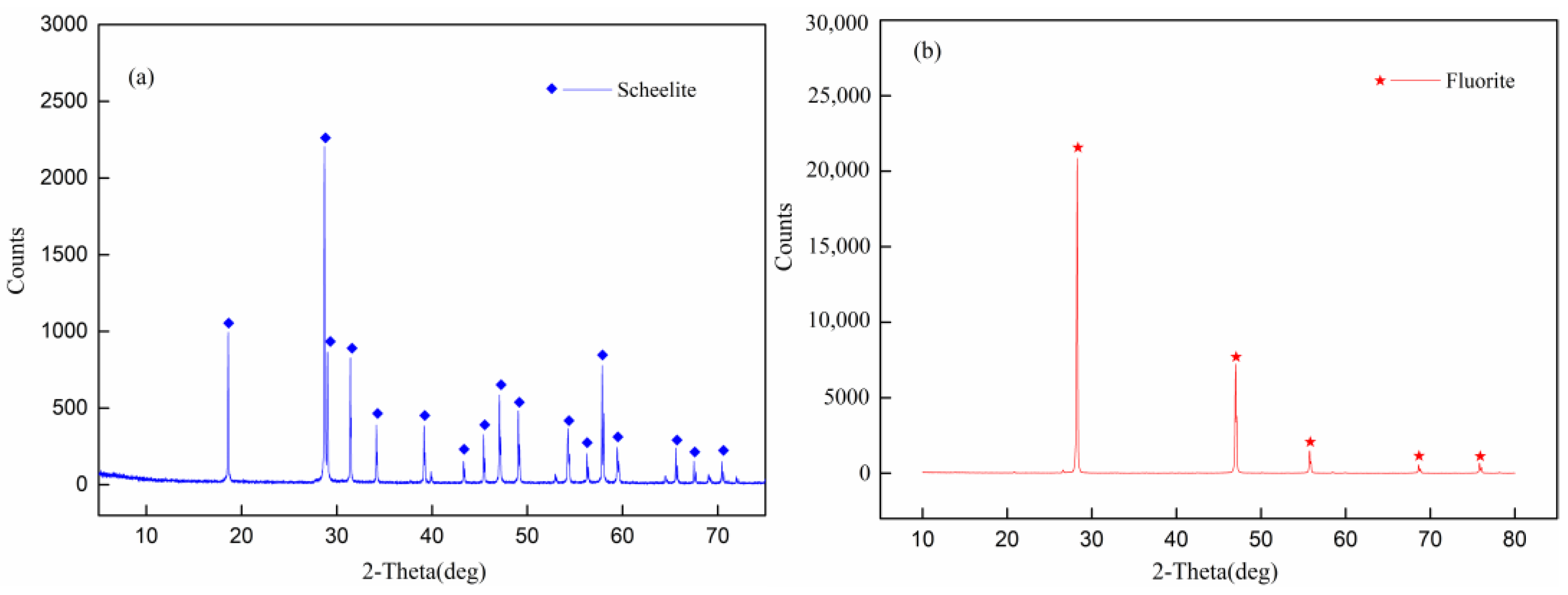

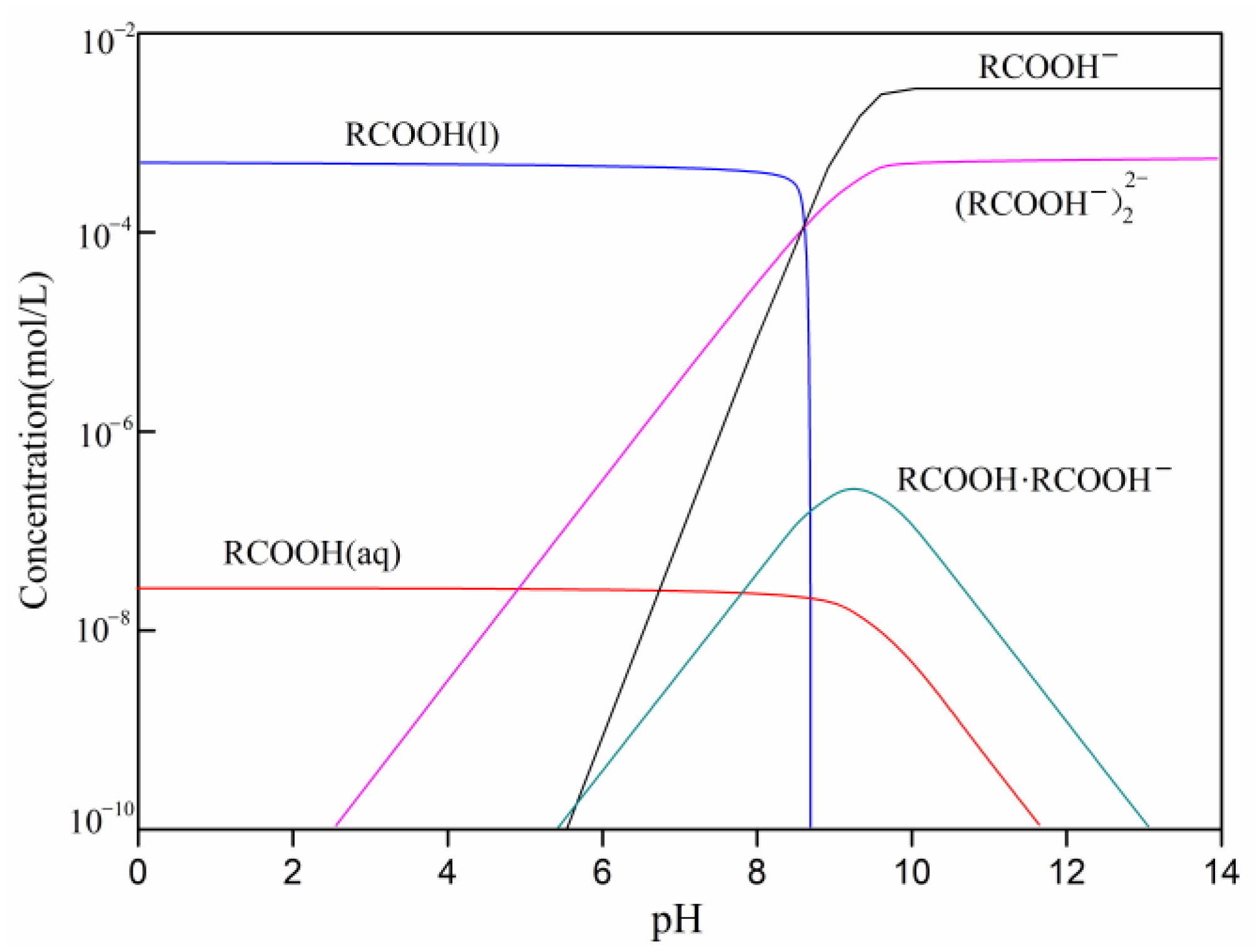
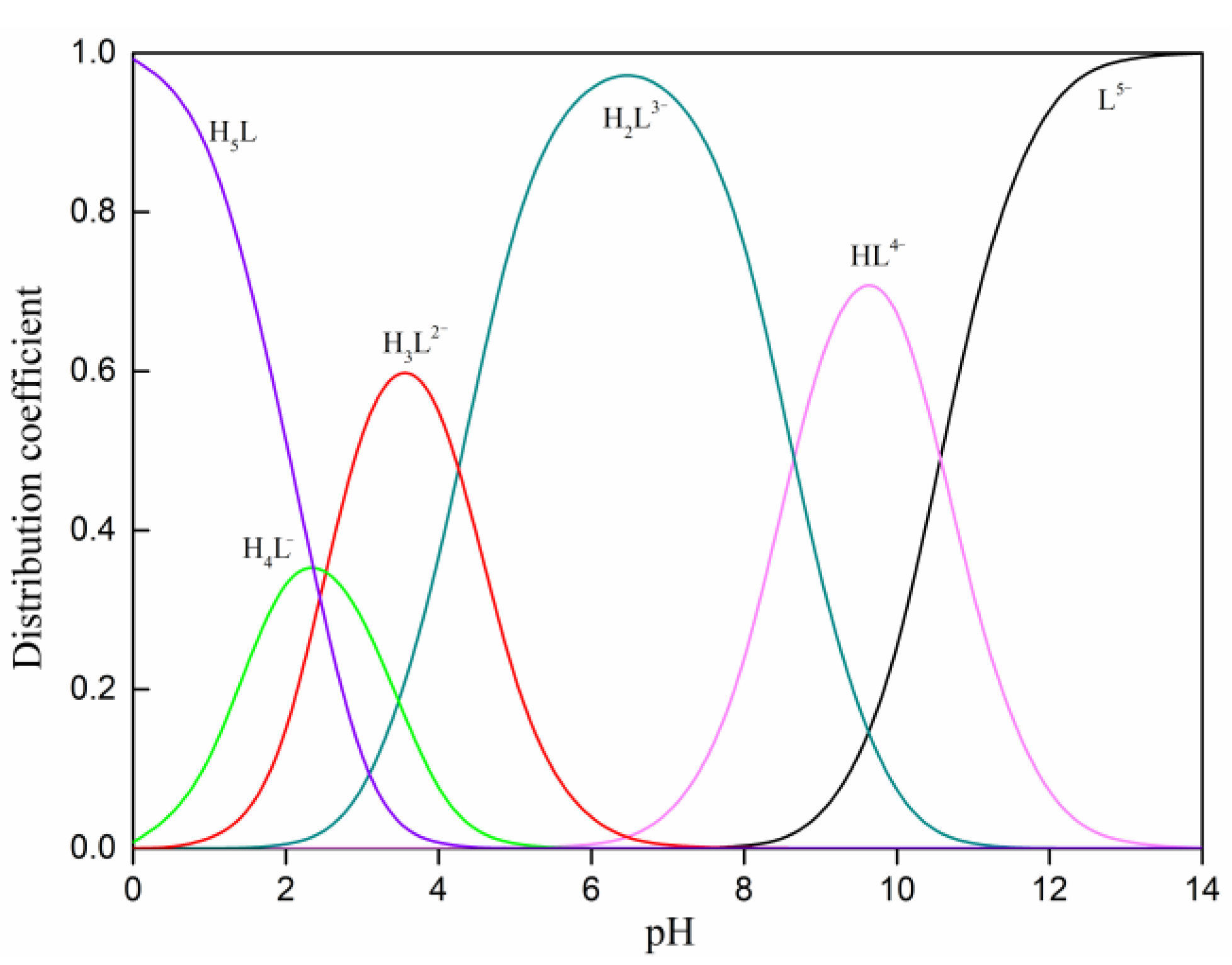
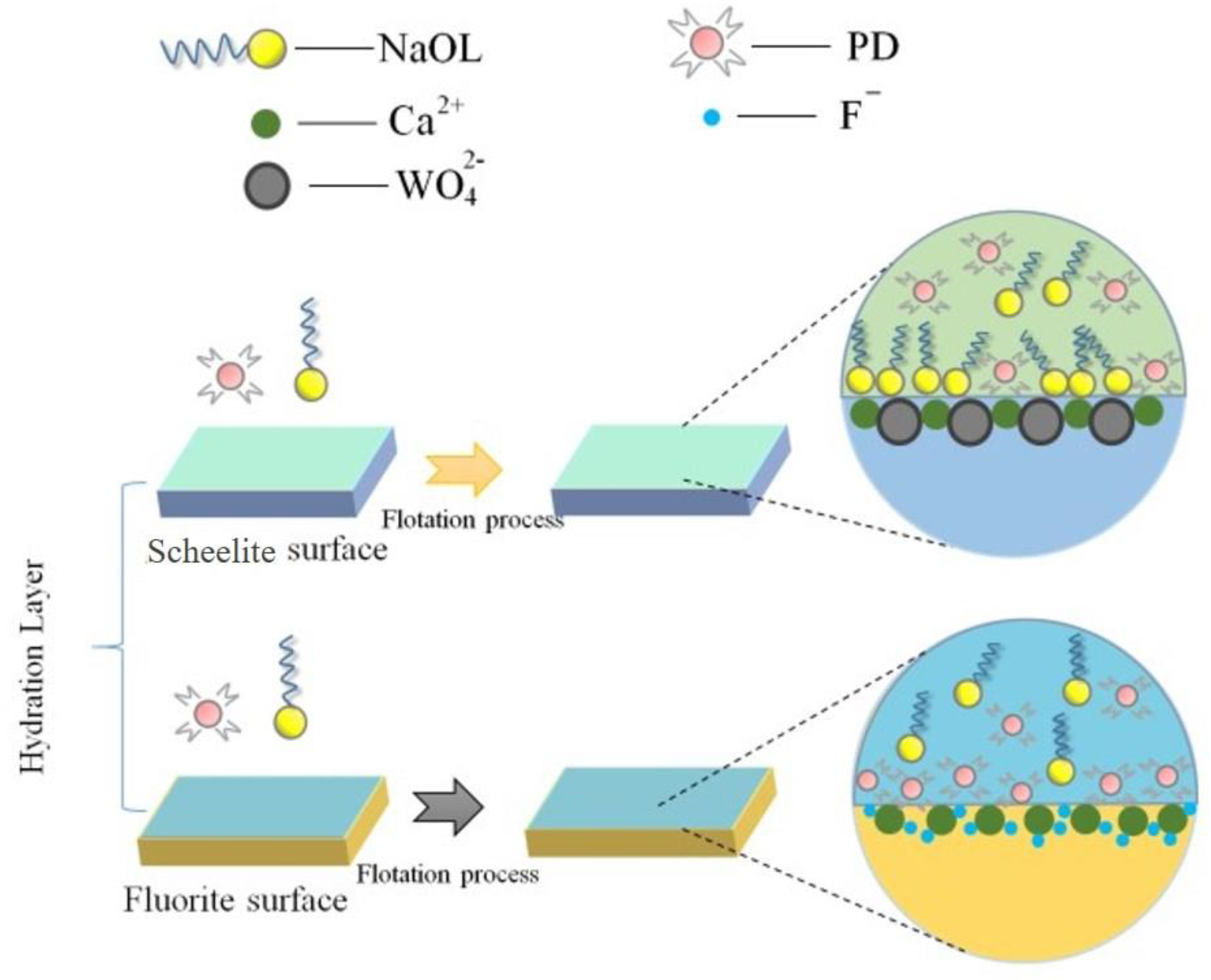
| Simple | Atomic Concentration (%) | ||||
|---|---|---|---|---|---|
| C | Ca | W | O | N | |
| CaWO4 (untreated) | 28.69 | 12.35 | 11.07 | 46.80 | 1.09 |
| CaWO4 + PD | 31.42 | 10.59 | 10.57 | 45.14 | 2.28 |
| Simple | Atomic Concentration (%) | |||
|---|---|---|---|---|
| C | Ca | F | N | |
| CaF2 (untreated) | 29.01 | 24.96 | 43.62 | 2.41 |
| CaF2 + PD | 45.50 | 17.79 | 30.64 | 6.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wu, H.; Sun, W.; Hu, Y.; Wang, C.; Zhu, S.; Chen, P. Investigation of the Flotation Separation of Scheelite from Fluorite with a Novel Chelating Agent: Pentasodium Diethylenetriaminepentaacetate. Minerals 2022, 12, 530. https://doi.org/10.3390/min12050530
Zhang C, Wu H, Sun W, Hu Y, Wang C, Zhu S, Chen P. Investigation of the Flotation Separation of Scheelite from Fluorite with a Novel Chelating Agent: Pentasodium Diethylenetriaminepentaacetate. Minerals. 2022; 12(5):530. https://doi.org/10.3390/min12050530
Chicago/Turabian StyleZhang, Chenhu, Haijun Wu, Wei Sun, Yuehua Hu, Chengyong Wang, Shan Zhu, and Peng Chen. 2022. "Investigation of the Flotation Separation of Scheelite from Fluorite with a Novel Chelating Agent: Pentasodium Diethylenetriaminepentaacetate" Minerals 12, no. 5: 530. https://doi.org/10.3390/min12050530
APA StyleZhang, C., Wu, H., Sun, W., Hu, Y., Wang, C., Zhu, S., & Chen, P. (2022). Investigation of the Flotation Separation of Scheelite from Fluorite with a Novel Chelating Agent: Pentasodium Diethylenetriaminepentaacetate. Minerals, 12(5), 530. https://doi.org/10.3390/min12050530








