Abstract
In this paper, the potential of sulfonated naphthalene formaldehyde (SNF) condensate as a depressant in the flotation separation of scheelite from calcite was verified and investigated. The results of microflotation experiments showed that SNF had a stronger depressant performance on calcite than a conventional depressant—water glass and had an excellent performance in fine-grained particles (−0.037 mm) treatment. Adsorption tests were conducted to quantitatively study the selective adsorption of SNF on the surface of scheelite and calcite. At 200 mg/L SNF, the adsorption density of SNF on the calcite surface reached 5.48 mg/g, which was more than four times than that of scheelite. In addition, compared with scheelite, the adsorption of SNF on the calcite surface had a more significant negative effect on the contact angle. Moreover, infrared (IR) measurements combined with X-ray photoelectron spectroscopy (XPS) analysis were performed to investigate the adsorption mechanisms of SNF on scheelite and calcite surfaces. The results showed that the adsorption of SNF on scheelite was more likely to be physical attraction, while the –SO3− group in SNF could chemically react with Ca species on the surface of calcite, resulting in a stronger adsorption than on scheelite.
1. Introduction
Scheelite, an ionic mineral with a theoretical WO3 content of 80.60%, is the primary source of industrial extraction of the element tungsten [1,2]. Due to the finely embedded particle sizes of minerals, flotation is the most commonly used technique for scheelite beneficiation [3,4,5,6]. Scheelite is easy to interact with various anionic collectors and has a good floatability. However, its gangue minerals such as apatite, fluorite, and calcite are all calcium-bearing minerals with similar surface physicochemical properties, which makes the high precision separation of scheelite from other calcium containing minerals difficult to achieve [7,8,9,10].
Depressants or inhibitors are the key to the selective separation of scheelite and its gangue minerals [11,12,13]. At present, water glass (WG; sodium silicate as the main ingredient) and its various modified products are the predominant depressants of interest [14]. Kupka and Rudolph demonstrated the synergistic effects of sodium carbonate and sodium silicate in scheelite flotation, as sodium carbonate refreshed the calcite surface which sodium silicate adsorbed preferentially [15]. Dong et al. verified that chemical bonds could be selectively formed between H2SiO42− and H3SiO4− and Ca2+ ions on the surface of calcite to depress calcite, and thereby, the flotation separation efficiency of scheelite was the highest in the NaOL/DDA system [16]. In addition, the combined use of metal ions and WG was studied to improve the selectivity of sodium silicate [17,18,19,20]. In the research of Wei et al., Al–Na2SiO3 was proved to be an effective depressant for the flotation separation of scheelite in the Pb–BHA system [21,22]. However, the flotation separation efficiency of scheelite and other calcium-bearing minerals is still unsatisfactory. Novel high-efficiency depressants are still expected for scheelite flotation.
Sulfonated naphthalene formaldehyde (SNF) condensate, a mature superplasticizer used in the concrete manufacturing industry, has shown its excellent ability of enhancing the surface wettability of silicate and carbonate minerals and improving the slurry rheology of concrete [23,24]. Figure 1 shows the chemical structure of SNF. It could be seen that the main hydrophilic group in SNF is the sulfonic group (–SO3Na), which makes it a potential depressant in flotation.
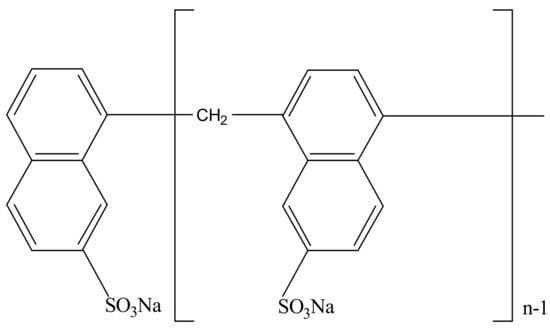
Figure 1.
Chemical structural diagram of sulfonated naphthalene formaldehyde (SNF) condensate.
However, there is no relevant research and application report on SNF in minerals flotation. To verify the potential of SNF as a depressant in the flotation separation of scheelite from calcite, microflotation tests were conducted to compare the effects of WG and SNF on the floatation recovery of calcite and scheelite. The adsorption tests were conducted to quantitatively study the selective adsorption of SNF on the surfaces of the two minerals. Contact angle tests as well as zeta potential measurements were used to further characterize the effects of SNF on mineral floatability and surface electrical properties. FTIR measurements combined with X-ray photoelectron spectroscopy (XPS) analysis were performed to investigate the adsorption mechanism of SNF on scheelite and calcite surfaces.
2. Materials and Method
2.1. Samples
The scheelite crystals in this paper were obtained from Yaoxiangang Tungsten Mine (Chenzhou, China), and the calcite samples were gained from Bamian Mountain Fluorite Mine (Jinhua, China). These crystals were crushed to −3.0 mm and hand-selected to remove impurity minerals. The chemical composition analysis combined with XRD was used to inspect the purity of scheelite (Table 1 and Figure 2a) and calcite (Table 1 and Figure 2b) samples. It is shown in Table 1 that the purities of the scheelite and calcite particles were more than 95%, as the WO3 content was 79.50% in scheecite samples and the CaCO3 content was 97.41% in calcite samples. No other characteristic peaks of the impurity minerals were observed because of their low contents, suggesting the satisfactory purity of the samples. Further, the particles were ground in a ceramic pot to obtain three main particle size fractions: −0.074 mm, −0.074 + 0.037 mm, and −0.037 mm.

Table 1.
Chemical composition analysis of the samples.
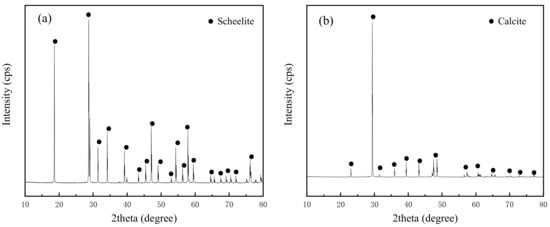
Figure 2.
XRD spectra of scheecite (a) and calcite (b) samples.
2.2. Chemicals
The details of the chemicals used in this study are shown in Table 2. Deionized water (DI water) with a resistivity of 18.2 MΩ·cm was used in each test. The SNF condensate was used as a depressant in this study to reduce the floatability of calcite in the sodium oleate flotation system. The infrared (IR) spectra of SNF are shown in Figure 3. The absorption band between 3000 cm−1 and 2800 cm−1 is the stretching vibration band of methyl and methylene. The peaks at 1631 cm−1 and 1401 cm−1 are the vibrational absorption peaks of the naphthalene ring. The peaks at 1038 cm−1 and 636 cm−1 belong to the bending vibration peak of the benzene ring. The two peaks at 1185 cm−1 and 1070 cm−1 are attributed to the asymmetric and symmetrical stretching vibration of –SO3−, respectively. Other peaks at 752 cm−1 and 677 cm−1 are the stretching vibration peaks of C–S and S–O, respectively [25,26,27].

Table 2.
Details of the chemicals.
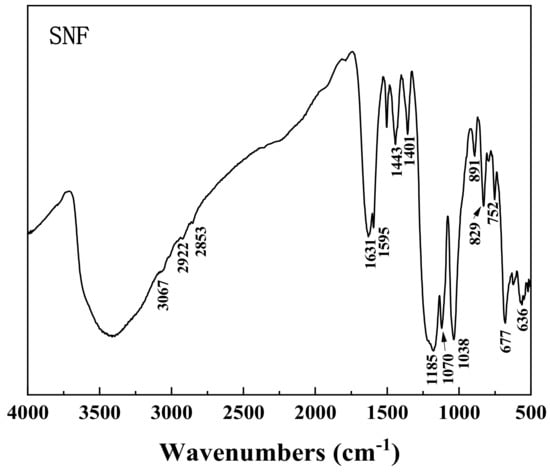
Figure 3.
FTIR spectra of the SNF condensate.
2.3. Method
2.3.1. Microflotation Test
The microflotation tests of the scheelite and calcite samples as well as the 1:1 (m:m) mixture of the two minerals were conducted in a 40 mL cell XFG flotation machine. In each test, 2 g particles were mixed with 35 mL DI water for 1 min at an agitation speed of 1800 rpm. Then, the pH of the slurry was modified at 9 ± 0.1 in 3 min by a NaOH and HCL solution with a mass concentration of 2%. Next, the depressant (WG or SNF) was added into a pulp and agitated for 3 min. Then, the mineralized froths were formed by a collector, and NaOL was introduced into a pump for 3 min. Forth products were collected for 3 min by scraping the froth layer every 5 s. Afterward, the floating and sinking samples were filtered, dried and weighed. Floatation recovery was calculated by Equation (1):
where and are the weights (g) of floating and sinking particles, respectively. In the case of the mixed minerals flotation, the samples were chemically analyzed by titration to obtain the WO3 grade and recovery.
2.3.2. Adsorption Test
The adsorption densities of SNF on scheelite and calcite samples were quantitatively studied by a TNL-L analyzer (Shimadzu, Japan). In each test, 2 g samples were mixed with 35 mL DI water in a magnetic stirring tank. Different amounts of SNF were introduced into a slurry separately and stirred for 10 min at pH 9. After stirring, the suspension was left to stand for 5 min, and then, the supernatant was transferred to a centrifuge field of 9000 rpm for 10 min to remove fine particles. The initial and residual amounts of the total organic carbon (TOC) in the solutions were detected by the TNL-L analyzer separately. Since there was no organic matter in the row pulp, the adsorption amount of SNF was directly related to the reduction of the TOC in the system. Therefore, the adsorption capacity of SNF on the particle surface was calculated by Equation (2):
where and are the volume (mL) and the volume concentration (mg/L) of the SNF solution, respectively, is the initial TOC (mg/L) of the SNF solution with a concentration of (mg/L), and is the residual TOC (mg/L) of the supernatant of the pulp after introducing mineral particles, is the mass (g) of the sample, and A is the specific surface area of the scheelite (0.618 m2/g) or calcite (0.707 m2/g) samples, which are obtained by BET analysis.
2.3.3. Contact Angle Test
Well-crystallized scheelite and calcite were cut and planished into 2 × 2 cm smooth flakes. Then, the flakes were immersed in a desired flotation system (pH = 9 ± 0.1) for 10 min. Finally, these flake samples were dried naturally in a nitrogen atmosphere, and the contact angle dates of them were determined by a MiniLab ILMS analyzer (GBX Corporation, Romans-sur-Isère, France). The contact angle was measured 3 times for each agent condition, and the average value with its standard deviation was recorded.
2.3.4. Zeta Potential Tests
The zeta potentials of scheelite and calcite particles in the presence of SNF with desired concentrations were measured by a ZSP microelectrophoresis analyzer (Malvern Corporation, Malvern, Worcestershire, UK). First, in each test, 2 mg mineral powder with a size of −5 μm was mixed with 50 mL of 0.01 mol/L KNO3 solution in a magnetic stirring tank. Then, the SNF solution with a desired concentration was introduced with 5 min stirring at pH 9. Another 5 min settling was conducted to remove coarse particles in the pulp system. Finally, the supernatant was transferred to the analyzer using a 5 mL medical syringe. Final data were the average of three measurements.
2.3.5. FTIR Measurements
The adsorption mechanisms of the depressant SNF on scheelite and calcite surfaces were investigated by FTIR measurements. Mineral samples were ground in an agate mortar to obtain the particles with a size below 5 μm. In each test, 0.2 g samples were conditioned with DI water in a floatation cell with the presence and absence of SNF at a desired pH value. Then, the samples were rinsed 5 times with DI water and dried in a vacuum-drying oven at 40 °C for 20 h. Finally, the particles were mixed with KBr powders and pressed into a cylinder to be detected by an FTIR spectrometer (Version IRAffinity–1, Tianjin Rui’an Technology Co., Ltd., Tianjin, China).
2.3.6. XPS Analysis
An X-ray photoelectron spectrometer (ESCALAB 250Xi, Thermo Fisher–VG Scientific, USA) was used to analyze the adsorption behavior of SNF on the mineral surfaces. First, the pulp was adjusted according to the process of the microflotation test. After adding the depressant and stirring the mixture for 3 min, the particles were transferred and washed 5 times with DI water. At last, the solid particles were transferred and dried in a vacuum-drying oven at 40 °C for 20 h to be tested.
3. Results and Discussion
3.1. Microflotation Tests
Microflotation experiments were performed to investigate the effects of the novel depressant—SNF—on the floatability of scheelite and calcite. At the same time, this study compared and analyzed the performance differences between SNF and conventional depressant—WG. The collector in the flotation process was sodium oleate with a concentration of 50 mg/L, and the pH of the suspension was modified to 9 ± 0.1. Figure 4 shows the functional relationship between the scheelite and calcite recoveries and the dosage of chemicals under different depressant systems (SNF vs. WG). The recovery difference between scheelite and calcite (ΔR = Rscheelite − Rcalcite) under these two systems is shown in Figure 5.
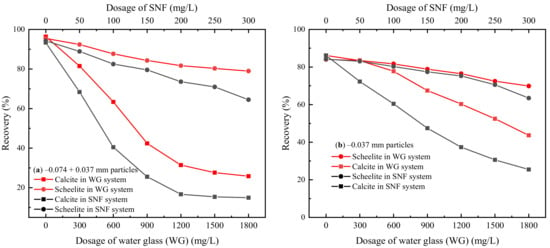
Figure 4.
Effects of the depressant dosage on the floatabilities of scheelite and calcite with size fractions of −0.074 + 0.037 mm (a) and −0.037 mm (b) (CNaOL = 50 mg/L, pH ≈ 9).
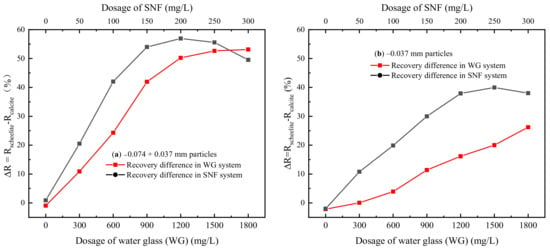
Figure 5.
Effects of the depressant dosage on the floatability deference between scheelite and calcite with size fractions of −0.074 + 0.037 mm (a) and −0.037 mm (b) (CNaOL = 50 mg/L, pH ≈ 9).
It is shown in Figure 4a that, for −0.074 + 0.037 mm particles, both recoveries of scheelite and calcite exceeded 95% without adding depressants, which is difficult to be separated directly by flotation. In a conventional WG system, the flotation recovery of −0.074 + 0.037 mm scheelite and calcite particles decreases with the increase of WG dosage. However, compared with scheelite, calcite was more sensitive to WG. When the amount of water glass increased from 0 mg/L to 1800 mg/L, the recovery of calcite dropped rapidly from 96.37% to 25.82%, while the scheelite recovery only went down from 95.42% to 16.48%. Further, replacing WG with SNF, declines in recoveries for both minerals became more obvious. In addition, from Figure 5a, a better separation performance of SNF was confirmed as the ΔR reached 56.95% at the SNF dosage of 200 mg/L, while employing WG as the depressant, the ΔR of 53.12% was obtained at a huge dosage of 1800 mg/L.
The effects of SNF and WG on the recovery of fine scheelite and calcite particles (−0.037 mm) are shown in Figure 4b and Figure 5b. Fine-grained minerals were more difficult to separate than those coarse-grained. WG had a limited ability to inhibit fine calcite particles. With the dosage of 1800 mg/L, calcite still had a recovery of 43.67%, and at the same time, the difference between the recovery of scheelite and calcite was only 26.5%. Notably, compared with WG, SNF had a stronger inhibitory effect on fine-grained calcite. The recovery of calcite decreased from 86.17% to 25.17% with 250 mg/L SNF, which provided a ΔR value of 39.36%.
Moreover, the flotation tests of the 1:1 (m:m) mixture of scheelite and calcite samples (particle size: −0.074 mm) were conducted to preliminarily verified the potential of using SNF as a depressant in scheelite separation from calcite. As is shown in Table 3, the concentrate grade of 55.60% with an 85.07% recovery could be achieved at 1800 mg/L WG. Notably, a significant increase (more than 10%) in the WO3 grade of the concentrate relative to that achieved by the water glass was obtained by SNF with little recovery loss (2.63% relative to that of WG), demonstrating the satisfactory performance of SNF in scheelite flotation.

Table 3.
Results of the mixed minerals flotation (CNaOL = 50 mg/L, pH ≈ 9).
3.2. Adsorption Test
The adsorption densities of the depressants on the mineral surface are directly related to their depressing effects. Therefore, adsorption tests were conducted to quantitatively study the adsorption densities of SNF on scheelite and calcite surfaces. The pH of the pulp was controlled around nine during the test. Figure 6 shows the functional relationship between SNF dosage and adsorption density on −0.074 mm scheelite and calcite surfaces. The results showed that although the SNF contents on the surfaces of both minerals increased with the increase of the depressant dosage, SNF exhibited selective adsorption on the calcite surface, which is in accordance with the flotation results in Figure 4. At 200 mg/L SNF, the adsorption density of SNF on the calcite surface reached 5.48 mg/m2, while its adsorption density on the scheelite surface was only 1.36 mg/m2.
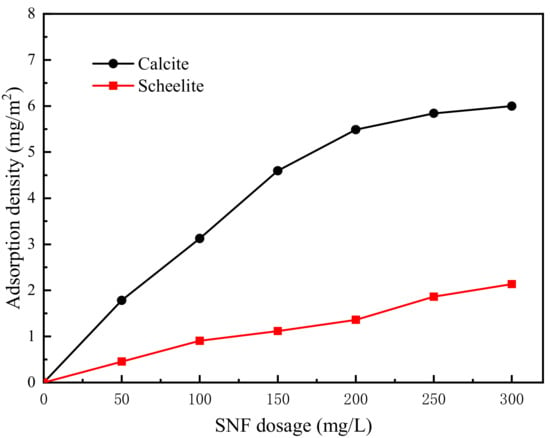
Figure 6.
Adsorption amounts of SNF on scheelite and calcite surfaces as a function of SNF dosage at pH 9.
3.3. Contact Angle Test
In this section of the paper, the effects of SNF on the wettabilities of scheelite and calcite surfaces were investigated by contact angle tests at pH 9. The results are shown in Figure 7. Both natural scheelite and calcite had small contact angles (<60°) at pH 9, indicating high wettability as well as poor natural floatability. After the addition of SNF as a depressant, the contact angles of both minerals were reduced. In addition, due to the selective adsorption of SNF on the calcite surface (studied in Section 3.2), the contact angle of calcite decreased more rapidly than that of scheelite. The difference of the contact angles between scheelite and calcite was the biggest at the SNF dosage range of 200–300 mg/L. It created a difference in the floatabilities of the two minerals before adding collectors, which is beneficial for subsequent selective separation.
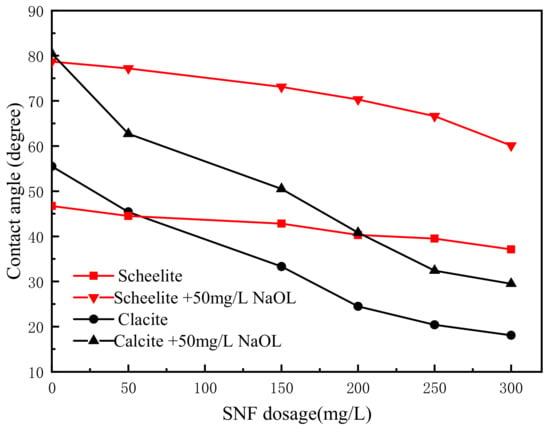
Figure 7.
Contact angles vs. SNF dosage in the presence and absence of 50 mg/L NaOL at pH 9.
Moreover, Figure 7 also shows the effects of collector (NaOL) on the mineral surface wettability with different dosages of SNF. In the absence of SNF, the addition of collectors increased the contact angles of both scheelite and calcite to about 80°, which made the flotation separation of the two minerals difficult to achieve. However, after the adsorption of SNF, the sensitivity of calcite to the collector was greatly reduced, while the effect on scheelite was weak. The contact angle difference between scheelite and calcite reached 40–50° in the depressant dosage range of 200–300 mg/L.
3.4. Zeta Potential Tests
Zeta potential tests were conducted to investigate the effects of SNF on mineral surface electrical properties in the absence and presence of the collector NaOL at pH 9. As is shown in Figure 8. In the absence of NaOL, the zeta potentials of pure scheelite and calcite exhibited dramatically different sensitivities to SNF. When the dosage of the depressant increased from 0 mg/L to 300 mg/L, the zeta potential of calcite dropped rapidly from −3.2 mV to −51.3 mV, while only a 10.1 mV decline was observed on that of scheelite. This was caused by the difference in the amounts of SNF adsorbed on the surface of calcite and scheelite, which is shown in Section 3.2.
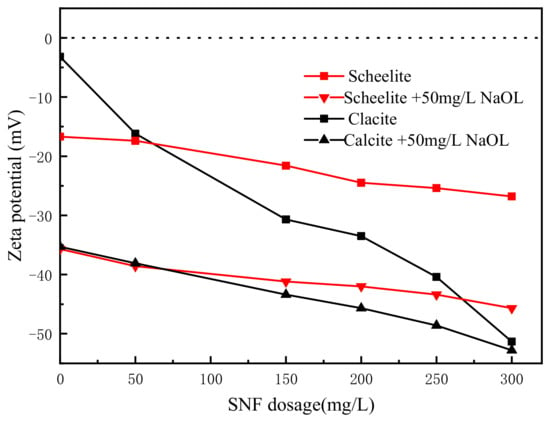
Figure 8.
Zeta potentials of scheelite and calcite as functions of the SNF dosage in the presence and absence of 50 mg/L NaOL at pH 9.
Moreover, the introduction of the collectors negatively charged the mineral surfaces as well, because of the functional groups of –COO− in NaOL. It is shown in Figure 8 that treating the samples with SNF prior to the addition of collectors could amplify the surface electrical differences between the two minerals. In the NaOL system, the 3–7 mV zeta potential difference between scheelite and calcite surfaces could be obtained at 200–300 mg/L SNF.
3.5. FTIR Analysis
Although the above studies demonstrated the selective adsorption of SNF on scheelite and calcite, the adsorption mechanism of SNF on mineral surfaces still needs further studies. FTIR was adopted to identify the modes of adsorption on mineral surfaces. The FTIR spectra of scheelite and calcite in the presence and absence of SNF at pH 9 are shown in Figure 9. As is shown in Figure 9a, the characteristic peaks in the IR spectrum of pure scheelite are mainly provided by WO42−. The antisymmetric stretching vibration peak of WO42− splits to two bands at the wavenumber of 799 cm−1 and 447 cm−1 [28]. In addition, the addition of SNF did not change the IR spectrum of scheelite, indicating that SNF cannot be chemically adsorbed on the mineral surface.
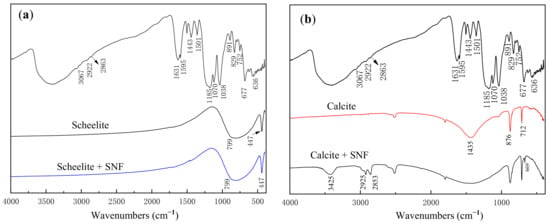
Figure 9.
FTIR spectra of scheelite (a) and calcite (b) in the presence and absence of SNF at pH 9.
The Ca–O bond in calcite belongs to ionic bond, which is easily broken and difficult to form characteristic peaks in IR spectra. Therefore, the characteristic peaks of pure calcite (shown in Figure 9b) are mainly formed by the C–O bonds in CO32− [29]. The peak at 1435 cm−1 is attributed to the antisymmetric stretching vibration of CO32−. The other two peaks at 876 cm−1 and 712 cm−1 are the out-plane and in-plane bending vibration peaks and of CO32−, respectively. After the calcite sample was treated by the depressant, several strong typical peaks belonging to SNF appeared in the IR spectra, indicating a strong adsorption of SNF on the calcite surface. The peak at 3425 cm−1 is the characteristic peak of O–H, and the other two peaks at 2925 cm−1 and 2853 cm−1 are attributed to the stretching vibration of H–C–H in SNF. Moreover, the new peak at 669 cm−1 is the vibrational absorption peak of S–O in SNF. An 8 cm−1 shift of this peak compared with the wavenumber of the S–O peak in SNF is an important indicator that SNF is chemisorbed on the calcite surface by –SO3−.
3.6. XPS Analysis
Based on the FTIR results, XPS analysis was adopted to investigate the interaction mechanism between the mineral surfaces and the depressant. Table 4 displays the relative atomic concentrations of the main elements on the surfaces of scheelite and calcite particles in the presence and absence of SNF. Although the adsorption test results in Section 3.2 showed that the adsorption amount of SNF on the mineral surface could reach 0.84 mg/g, the sulfur concentration on the surface of scheelite was 0 regardless of whether it was treated with SNF in the XPS tests, which is consistent with FTIR analysis. This is due to the fact that the mineral surface was cleaned by DI water during the IR and XPS sample preparation, indicating that the adsorption of SNF on the scheelite was more likely to be physical attraction rather than chemical bonds. Besides, sulfur was detected with a concentration of 0.54% on the surface of calcite treated with SNF, which further reflected the selective adsorption of SNF on the surfaces of these two minerals.

Table 4.
Relative contents of the main elements on the scheelite and calcite surfaces in the presence and absence of SNF.
Moreover, the high-resolution spectra of Ca elements were analyzed to confirm the main adsorption sites of SNF on calcite and provided an in-depth understanding of the adsorption of SNF on mineral surfaces. Figure 10 shows the Ca 2p XPS spectra of the scheelite and calcite surfaces with and without SNF treatment. The Ca 2p spectrum of scheelite (Figure 10a) could be fitted to two sets of peaks with binding energies (BEs) at 346.84 eV and 350.39 eV, which are the Ca2+ binding energies of the Ca 2p3/2 and 2p1/2 orbitals, respectively. After treatment with SNF, the BEs of the two orbitals were 346.90 eV and 350.45 eV (Figure 10b), respectively. The BEs were only shifted by 0.06 eV, which is within the error range. It suggested that SNF did not chemically interact with Ca2+ on the mineral surface.
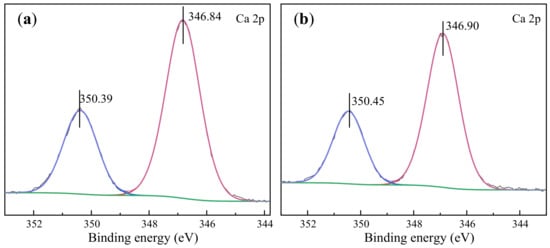
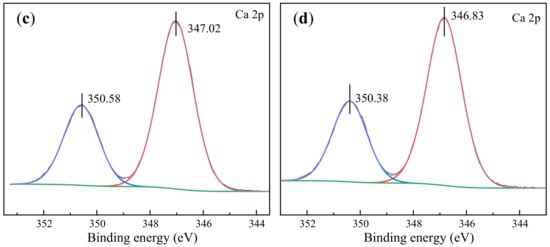
Figure 10.
XPS spectra of Ca 2p signals of scheelite and calcite in the presence and absence of SNF: (a) scheelite; (b) scheelite treated with SNF; (c) calcite; (d) calcite with SNF.
As is shown in Figure 10c, in the XPS spectrum, Ca2+ in pure calcite could be fitted to two related peaks. The binding energies of Ca2+ in the Ca 2p3/2 and 2p1/2 orbitals were 347.02 eV and 350.58 eV, respectively. For calcite treated by SNF, the binding energy shift of 0.2 eV was observed, compared to the pure calcite with higher binding energy of Ca 2p, indicating that the electron density of Ca2+ on the calcite surface was increased by SNF adsorption. It proved that the chemisorption of SNF occurred on the calcite surface, and the surface Ca species was an important adsorption site for SNF.
4. Conclusions
This paper demonstrated the potential of SNF as a depressant in the flotation separation of scheelite from calcite. The effects of conventional depressants, i.e., WG and SNF, on the floatabilities of calcite and scheelite were compared with sodium oleate as the collector. In addition, the adsorption mechanisms of SNF on the surface of the two minerals were investigated as well. The main conclusions are as follows:
(a) Compared with conventional WG inhibitors, SNF more strongly depressed calcite particles. A satisfactory result of a 66.20% WO3 grade of the concentrate with an 82.34% recovery was obtained by mixed minerals flotation tests;
(b) The adsorption test results showed that SNF was preferentially adsorbed on the surface of calcite. At 200 mg/L SNF, the adsorption density of SNF on the calcite surface reached 5.48 mg/m2, while its adsorption density on the scheelite surface was only 1.36 mg/m2;
(c) FTIR and XPS analyses were conducted to investigate the adsorption mechanisms of SNF on scheelite and calcite surfaces. The FTIR results showed that –SO3− groups were chemisorbed on the calcite surface, and the XPS analysis further revealed that the Ca component on the calcite surface was the main site for SNF adsorption, while SNF relied on physical attraction to be adsorbed on the surface of calcite.
Author Contributions
Y.W., conceptualization, methodology, and validation; G.P., data curation and writing of the original draft; H.C., writing—review and editing and validation; D.L., software; X.Z., methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Funds of China (grant numbers: 52174267 and 52174270), National Key Research and Development Program of China (No. 2021YFC2903202), Independent Exploration and Innovation of Central South University (No. 2020zzts202), Key Laboratory of Comprehensive Utilization of Vanadium and Titanium Resources (No. 2021P4FZG05A), Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources, and Open Foundation of State Key Laboratory of Mineral Processing.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foucaud, Y.; Filippov, L.; Filippov, I.; Badawi, M. The challenge of tungsten skarn processing by froth flotation: A review. Front. Chem. 2020, 230, 230. [Google Scholar] [CrossRef] [PubMed]
- Jewell, S.; Kimball, S.M. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2016; pp. 180–181. [Google Scholar]
- Chen, C.; Zhu, H.; Qin, W.; Chai, L.; Jia, W. Improving collecting performance of sodium oleate using a polyoxyethylene ether in scheelite flotation. J. Cent. South Univ. 2018, 25, 2971–2978. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Filippov, L.O.; Shokhin, V.N.; Yenbaeva, L.I.; Ignatkina, V.A. Improvement of engineering data for flotation of scheelite using combination of sodium oleate and Exol—B. Tsvetnye Met. 1993, 1, 60–64. [Google Scholar]
- Ilhan, S.; Kalpakli, A.O.; Kahruman, C.; Yusufoglu, I. The investigation of dissolution behavior of gangue materials during the dissolution of scheelite concentrate in oxalic acid solution. Hydrometallurgy 2013, 136, 15–26. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Cooper, T.G.; de Leeuw, N.H. A combined ab initio and atomistic simulation study of the surface and interfacial structures and energies of hydrated scheelite: Introducing a CaWO4 potential model. Surf. Sci. 2003, 531, 159–176. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W. Anisotropic surface properties of calcite: A consideration of surfacebroken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Li, Y.; Li, C. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. I. Selective flotation of scheelite. Inter. J. Min. Process. 1983, 10, 205–218. [Google Scholar]
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Liu, W. Selective flotation of scheelite from calcite using xanthan gum as depressant. Miner. Eng. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Selective depressive effect of sodium fluorosilicate on calcite during scheelite flotation. Miner. Eng. 2019, 131, 262–271. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Role of sodium carbonate in scheelite flotation—A multi–faceted reagent. Miner. Eng. 2018, 129, 120–128. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.L.; Sun, W.J.; Wang, J.J.; Gao, Z.Y.; Wang, L.; Zhang, C.Y. Selective Separation of Scheelite from Calcite by Self–Assembly of H2SiO3 Polymer Using Al3+ in Pb–BHA Flotation. Minerals 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Tohry, A.; Dehghani, A. Effect of sodium silicate on the reverse anionic flotation of a siliceous–phosphorus iron ore. Sep. Purif. Technol. 2016, 164, 28–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S. Beneficiation of fluorite by flotation in a new chemical scheme. Miner. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Luo, X.M.; Yin, W.Z.; Ma, Y.Q.; Sun, C.Y.; Yao, J.; Li, Q. New flotation technology research on carbonate—Containing hematite. Adv. Mater. Res. 2012, 454, 210–215. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodiumsilicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al–Na2SiO3 polymer as depressant and Pb–BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Redín, C.; Lange, F.T.; Brauch, H.J.; Eberle, S.H. Synthesis of Sulfonated Naphthalene—Formaldehyde Condensates and their Trace—Analytical Determination in Wastewater and River Water. Acta Hydrochim. Hydrobiol. 1999, 27, 136–142. [Google Scholar] [CrossRef]
- Ruckstuhl, S.; Suter, M.-J.F.; Giger, W. Sorption and mass fluxes of sulfonated naphthalene formaldehyde condensates in aquifers. J. Contam. Hydrol. 2003, 67, 1–12. [Google Scholar] [CrossRef]
- Pei, M.; Wang, D.; Hu, X.; Xu, D. Synthesis of sodium sulfanilate—Phenol—Formaldehyde condensate and its application as a superplasticizer in concrete. Cem. Concr. Res. 2000, 30, 1841–1845. [Google Scholar] [CrossRef]
- Packiaraj, M.; Kannan, K.; Kuma, S. High corrosion protective behavior of water–soluble conducting polyaniline–sulfonated naphthalene formaldehyde nanocomposites on 316L SS. J. Alloy. Compd. 2021, 864, 158345. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Zhang, Y.; Zhang, G.; Zhu, H. Composite application of naphthalene and melamine–based superplasticizers in alkali activated fly ash (AAFA). Constr. Build. Mater. 2021, 297, 123651. [Google Scholar] [CrossRef]
- Thongtem, T.; Phuruangrat, A.; Thongtem, S. Haracterization of MeWO4 (Me = Ba, Sr and Ca) nanocrystallines prepared by sonochemical method. Appl. Surf. Sci. 2008, 254, 7581–7585. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Kuz’kin, S.F.; Solynshkin, V.I. Infrared spectra of calcite, scheelite and apatite after treatment with flotation regulators. Izv. Vyssh. Uchebn. Zaved. Tsvetn. Metall. 1963, 6, 28–32. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).