The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review
Abstract
1. Introduction
2. Global and Regional Carbon Dioxide Emissions
- Transportation (predominantly non-commercial motor vehicles);
- Electricity production (fossil fuel burning);
- Industry (manufacturing, processing, etc.);
- Residential (heating, cooking, etc.);
- Agriculture (farm equipment, agricultural machinery, etc.).
3. The Technologies for Carbon Dioxide Capture and Storage
- Absorption;
- Adsorption;
- Calcium looping;
- Cryogenic separation;
- Membrane separation;
- Biological separation with microalgae.
- Cryogenic distillation;
- Membrane purification;
- Electrochemical separation;
- Absorption with liquids;
- Adsorption using solid materials.
4. Materials for Carbon Dioxide Capture Technologies
- Metal organic frameworks (MOFs);
- Graphene organic frameworks (GOFs);
- Covalent organic frameworks (COFs);
- Metal oxides;
- Homogenous porous silica;
- Zeolites;
- Activated carbon;
- Clay minerals;
- Molecular basket sorbents (MBSs);
- Ionic liquids.
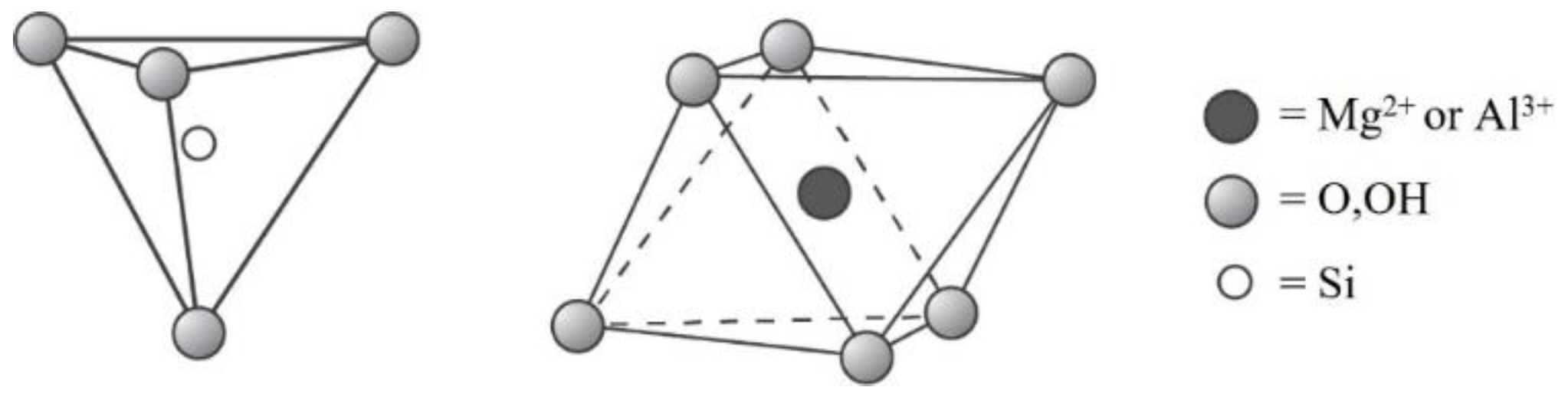
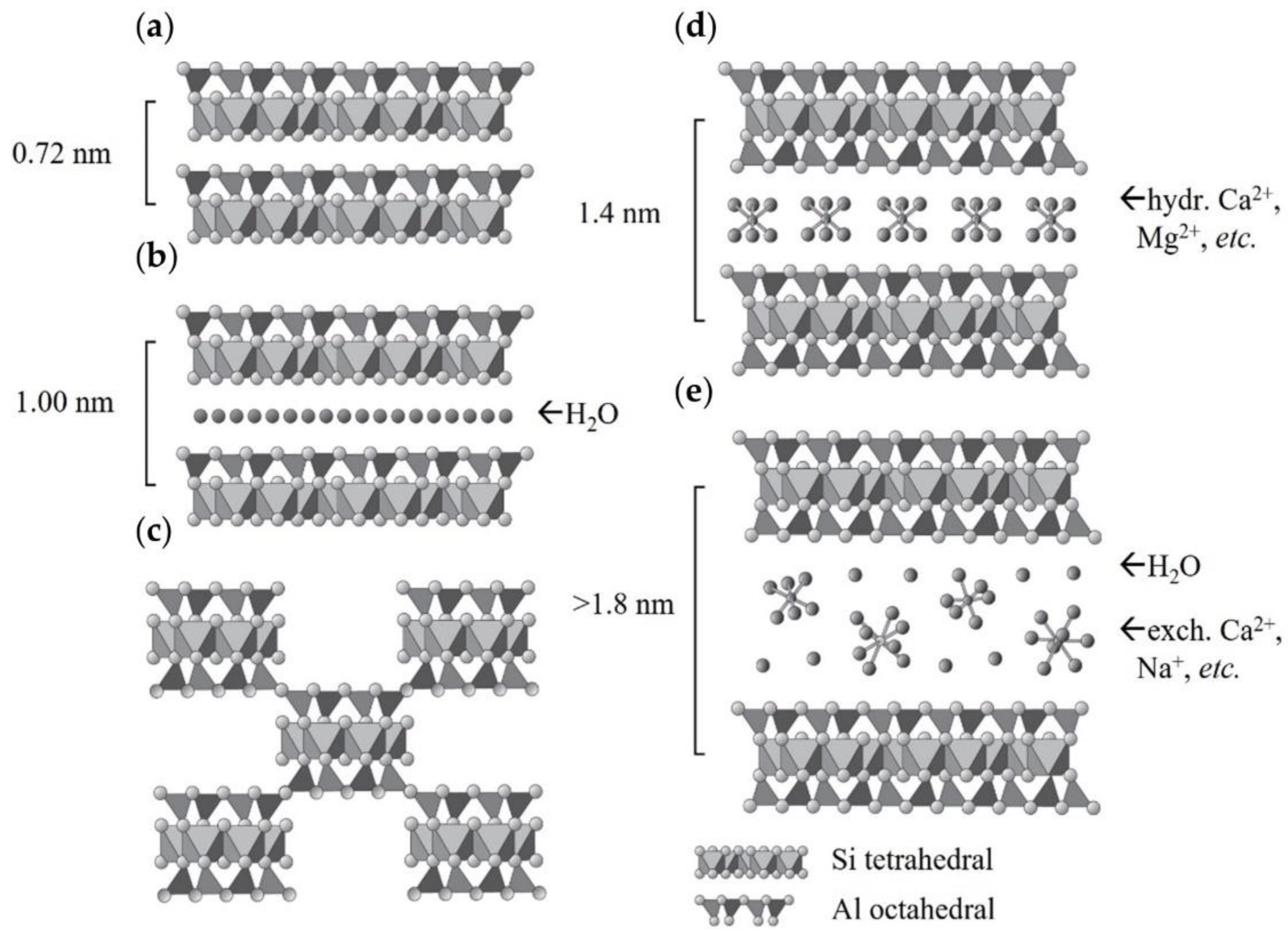
5. Structural and Property Features of Clay Minerals to Support Carbon Dioxide Capture
- Kaolin-serpentine (kaolinite, nacrite, dickite etc.);
- Pyrophyllite-talc;
- Mica;
- Vermiculite;
- Smectite (montmorillonite);
- Chlorite;
- Sepiolite-palygorskite;
- Interstratified clay minerals;
- Allophane-imogolite.
- 1:1 layer type, tetrahedral–octahedral sheet combination;
- 2:1 layer type, tetrahedral–octahedral–tetrahedral sheet combination;
- 2:1:1 layer type, tetrahedral–octahedral–tetrahedral sheet combination.
- Serpentine (e.g., chrysolite, lizardite);
- Kaolin (e.g., kaolinite, dickite, halloysite);
- Talc;
- Pyrophyllite;
- Smectite (e.g., saponite, hectorite, montmorillonite, beidellite);
- Vermiculite;
- True mica (e.g., illite, glauconite, phlogopite, biotite);
- Brittle mica (e.g., clintonite, margarite);
- Chlorite (e.g., clinochlore, chamosite, donbassite);
- Mixed layer group (e.g., chlorite-smectite, chlorite-vermiculite, illite-smectite).
6. Clay Mineral and Carbon Dioxide Interaction Depending on Mineral Type
7. Prospective for Clay Mineral Modification to Improve Carbon Dioxide Capture
8. Clay Mineral Activation
9. Clay Mineral Functionalization
- Impregnation of amines onto porous carriers;
- Formation of covalent bonds between amine containing functional groups and porous surface (grafting);
- In-situ polymerization.
10. Clay Mineral Assemblages in the Baltic States
11. Discussion
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atmospheric Carbon Dioxide Growth Rate. Available online: http://mlg.eng.cam.ac.uk/carl/words/carbon.html (accessed on 24 November 2021).
- Global Climate Report—Annual 2020. Available online: https://www.ncdc.noaa.gov/sotc/global/202013 (accessed on 24 November 2021).
- Wildfires and Climate Change. Available online: https://www.c2es.org/content/wildfires-and-climate-change/ (accessed on 24 November 2021).
- Allen, L.H. Effects of increasing carbon dioxide levels and climate change on plant growth. In Proceedings of a Colloquium “Managing Water Resources in the West under Conditions Climate Uncertainty”, Scottsdale, AZ, USA, 14–16 November 1990; The National Academies Press: Washington, DC, USA, 1991; pp. 101–147. [Google Scholar] [CrossRef]
- Taub, D.R. Effects of rising atmospheric concentrations of carbon dioxide on plants. Nat. Educ. Knowl. 2010, 3, 21. [Google Scholar]
- Lemordant, L.; Gentine, P.; Swann, A.S.; Cook, B.I.; Scheff, J. Critical impact of vegetation physiology on the continental hydrological cycle in response to increasing CO2. Proc. Natl. Acad. Sci. USA 2018, 115, 4093–4098. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Cecchi, L.; D’Amato, M.; Annesi-Maesano, I. Climate change and respiratory diseases. ERR 2014, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F.; Post, E. Climate change impacts on animal migration. Clim. Chang. Responses 2015, 2, 5. [Google Scholar] [CrossRef]
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Trends in Atmospheric Carbon Dioxide. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 18 October 2021).
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 1 November 2021).
- Outcomes of the Glasgow Climate Change Conference—Advance Unedited Versions (AUVs). Available online: https://unfccc.int/process-and-meetings/conferences/glasgow-climate-change-conference-october-november-2021/outcomes-of-the-glasgow-climate-change-conference (accessed on 17 November 2021).
- Alami, A.H.; Hawili, A.A.; Tawalbeh, M.; Hasan, R.; Al Mahmoud, L.; Chibib, S.; Aokal, K.; Mahmood, A.; Rattanapanya, P. Materials and logistics for carbon dioxide capture, storage and utilization. Sci. Total Environ. 2020, 717, 137221. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Krumins, J.; Klavins, M.; Delina, A.; Damkevics, R.; Seglins, V. Potential of the middle Cambrian aquifer for carbon dioxide storage in the Baltic States. Energies 2021, 14, 3681. [Google Scholar] [CrossRef]
- Pant, D.; Nadda, A.K.; Pant, K.K.; Agarwal, A.K. Advances in Carbon Capture and Utilization. In Advances in Carbon Capture and Utilization; Pant, D., Nadda, A.K., Pant, K.K., Agarwal, A.K., Eds.; Springer: Singapore, 2021; pp. 3–7. [Google Scholar]
- Sabri, M.A.; Al Jitan, S.; Bahamon, D.; Vega, L.F.; Palmisano, G. Current and future perspectives on catalytic-based integrated carbon capture and utilization. Sci. Total Environ. 2021, 790, 148081. [Google Scholar] [CrossRef]
- Hildenbrand, A.; Schlomer, S.; Krooss, B.M. Gas breakthrough experiments on fine-grained sedimentary rocks. Geofluids 2002, 2, 3–23. [Google Scholar] [CrossRef]
- Cole, D.R.; Chialvo, A.A.; Rother, G.; Vlcek, L.; Cummings, P.T. Supercritical fluid behaviour at nanoscale interfaces: Implications for CO2 sequestration in geologic formations. Phil. Mag. 2010, 90, 2339–2363. [Google Scholar] [CrossRef]
- Wollenweber, J.; Alles, S.; Busch, A.; Krooss, B.M.; Stanjek, H.; Littke, R. Experimental investigation of the CO2 sealing efficiency of caprocks. Int. J. Greenh. Gas Control 2010, 4, 231–241. [Google Scholar] [CrossRef]
- Nesse, W.D. Introduction to Mineralogy, 3rd ed.; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Hower, J.; Eslinger, E.V.; Hower, M.E.; Perry, E.A. Mechanism of burial metamorphism of argillaceous sediment: 1. Mineralogical and chemical evidence. GSA Bull. 1976, 87, 725–737. [Google Scholar] [CrossRef]
- de Jong, S.M.; Spiers, C.J.; Busch, A. Development of swelling strain in smectite clays through exposure to carbon dioxide. Int. J. Greenh. Gas Control 2014, 24, 149–161. [Google Scholar] [CrossRef]
- Dazas, B.; Lanson, B.; Breu, J.; Robert, J.-L.; Pelletier, M.; Ferrage, E. Smectite fluorination and its impact on interlayer water content and structure: A way to fine tune the hydrophilicity of clay surfaces? Microporous Mesoporous Mater. 2013, 181, 233–247. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benitez-Guerrero, M.; Lorente, M. Effectiveness of microwave assisted acid treatment on dioctahedral and trioctahedral smectites. The influence of octahedral composition. Appl. Clay Sci. 2016, 120, 70–80. [Google Scholar] [CrossRef]
- Kloproge, J.T. Synthesis of smectites and porous pillared clay catalysts: A review. J. Porous Mater. 1998, 5, 5–41. [Google Scholar] [CrossRef]
- Michels, L.; Fossum, J.O.; Rozynek, Z.; Hemmen, H.; Rustenberg, K.; Sobas, P.A.; Kalantzopoulos, G.N.; Knudsen, K.D.; Janek, M.; Plivelic, T.S.; et al. Intercalation and retention of carbon dioxide in a smectite clay promoted by interlayer cations. Sci. Rep. 2015, 5, 8775. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Platon, N.; Ghomari, K.; Shiao, T.C.; Hersant, G.; Bergron, J.-Y.; Roy, R. Truly reversible capture of CO2 by montmorillonite intercalated with soya oil-derived polyglycerols. Int. J. Greenh. Gas Control 2013, 17, 140–147. [Google Scholar] [CrossRef]
- Druckman, J.N.; McGrath, M.C. The evidence for motivated reasoning in climate change preference formation. Nat. Clim. Chang. 2019, 9, 111–119. [Google Scholar] [CrossRef]
- Nielsen, K.S.; van der Linden, S.; Stern, P.C. How behavioural interventions can reduce the climate impact of energy use. Joule 2020, 4, 1613–1616. [Google Scholar] [CrossRef]
- van der Linden, S. The social-psychological determinants of climate change risk perceptions, attitudes, and behaviours: A national study. Environ. Educ. Res. 2016, 22, 434–435. [Google Scholar] [CrossRef]
- Impact of Covid-19 Crisis on Industrial Production. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Impact_of_Covid-19_crisis_on_industrial_production (accessed on 24 November 2021).
- Impact of COVID-19 on CO2 Emissions. Available online: https://unfccc.int/sites/default/files/resource/1.GCP_.pdf (accessed on 18 October 2021).
- Largest Producers of Fossil Fuel CO2 Emissions Worldwide in 2019, by Share of Emissions. Available online: https://www.statista.com/statistics/271748/the-largest-emitters-of-co2-in-the-world/ (accessed on 27 October 2021).
- Keeling Curve. Available online: https://www.climatecentral.org/gallery/graphics/keeling_curve (accessed on 18 October 2021).
- Global CO2 Emissions Rebound by Nearly 5% in 2021, Approaching the 2018–2019 Peak. Available online: https://www.iea.org/reports/global-energy-review-2021/co2-emissions (accessed on 3 November 2021).
- Statistical Review of World Energy 2021. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 29 November 2021).
- Climate Change Mitigation Policies in Energy Sector of Baltic States. Available online: https://www.worldenergy.org/assets/downloads/PUB_Energy_and_Climate_Change_Annex_Baltic_states_2007_WEC.pdf (accessed on 15 November 2021).
- Greenhouse Gas Emissions by Source Sector. Available online: http://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 17 November 2021).
- Font-Palma, C.; Cann, D.; Udemu, C. Review of cryogenic carbon capture innovations and their potential applications. C 2021, 7, 58. [Google Scholar] [CrossRef]
- Lin, C.-C.; Liu, W.-T.; Tan, C.-S. Removal of carbon dioxide by absorption in a rotating packed bed. Ind. Eng. Chem. Res. 2003, 42, 2381–2386. [Google Scholar] [CrossRef]
- Dave, A.; Pathak, B.; Dave, M.; Rezvani, S.; Huang, Y.; Hewitt, N. Process design of CO2 desorption from physical solvent di-methyl-ether of poly-ethylene-glycol. Mater. Sci. Energy Technol. 2020, 3, 209–2017. [Google Scholar] [CrossRef]
- Yang, S.; Qian, Y.; Yang, S. Development of a full CO2 capture process based on the rectisol wash technology. Ind. Eng. Chem. Res. 2016, 55, 6186–6193. [Google Scholar] [CrossRef]
- Scherffius, J.R.; Reddy, S.; Klumpyan, J.P.; Armpriester, A. Large-scale CO2 capture demonstration plant using fluor’s econamine FG Plus technology at NRG’s WA parish electric generating station. Energy Procedia 2013, 37, 6553–6561. [Google Scholar] [CrossRef]
- Hochgesand, G. Rectisol and purisol. Ind. Eng. Chem. 1970, 62, 37–43. [Google Scholar] [CrossRef]
- Girimonte, R.; Testa, F.; Gallo, F.; Buscieti, R.; Leone, G.; Fromisani, B. Adsorption of CO2 on amine-modified silica particles in a confined-fluidized bed. Processes 2020, 8, 1531. [Google Scholar] [CrossRef]
- Auta, M.; Amat Darbis, N.D.; Mohd Din, A.T.; Harmeed, B.H. Fixed-bed column adsorption of carbon dioxide by sodium hydroxide modified activated alumina. Chem. Eng. J. 2013, 233, 80–87. [Google Scholar] [CrossRef]
- Casas, N.; Schell, J.; Pini, R.; Mazzotti, M. Fixed bed adsorption of CO2/H2 mixtures on activated carbon: Experiments and modelling. Adsorption 2012, 18, 143–161. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Bastin, L.; Barcia, P.S.; Hurtado, E.J.; Silva, J.A.C.; Rodrigues, A.E.; Chen, B. A microporous metal−organic framework for separation of CO2/N2 and CO2/CH4 by fixed-bed adsorption. J. Phys. Chem. C 2008, 112, 1575–1581. [Google Scholar] [CrossRef]
- Moreira, R.F.P.M.; Soares, J.L.; Casarin, G.L.; Rodrigues, A.E. Adsorption of CO2 on hydrotalcite-like compounds in a fixed bed. Sep. Sci. Technol. 2006, 41, 341–357. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, L.; Zheng, Y.; Zhu, T. Optimization of CO2 adsorption on solid-supported Amines and thermal regeneration mode comparison. ACS Omega 2020, 5, 9641–9648. [Google Scholar] [CrossRef]
- Yoro, K.O.; Singo, M.; Mulopo, J.L.; Daramola, M.O. Modelling and experimental study of the CO2 adsorption behaviour of polyaspartamide as an adsorbent during post-combustion CO2 capture. Energy Procedia 2017, 114, 1643–1664. [Google Scholar] [CrossRef]
- Siqueira, R.M.; Freitas, G.R.; Peixoto, H.R.; do Nascimento, J.F.; Musse, A.P.S.; Torres, A.E.B.; Azevedo, D.C.S.; Bastos-Neto, M. Carbon dioxide capture by pressure swing adsorption. Energy Procedia 2017, 114, 2182–2192. [Google Scholar] [CrossRef]
- Ntiamoah, A.; Ling, J.; Xiao, P.; Webley, P.A.; Zhai, Y. CO2 capture by temperature swing adsorption: Use of hot CO2-rich gas for regeneration. Ind. Eng. Chem. Res. 2016, 55, 703–713. [Google Scholar] [CrossRef]
- Elliott, J.E.; Copeland, R.J.; Leta, D.P.; McCall, P.P.; Bai, C.; DeRites, B.A. Carbon Dioxide Separation Using Adsorption with Steam Regeneration. US Patent 9504955, 29 November 2016. [Google Scholar]
- Dean, C.C.; Blamey, J.; Florin, N.H.; Al-Jeboori, M.J.; Fennell, P.S. The calcium looping cycle for CO2 capture from power generation, cement manufacture and hydrogen production. Chem. Eng. Res. Des. 2011, 89, 836–855. [Google Scholar] [CrossRef]
- Xu, J.; He, T.; Lin, W. Experimental and theoretical study of CO2 solubility in liquid CH4/H2 mixtures at cryogenic temperatures. J. Chem. Eng. Data 2021, 66, 2844–2855. [Google Scholar] [CrossRef]
- Chang, H.-M.; Chung, M.J.; Park, S.B. Cryogenic heat-exchanger design for freeze-out removal of carbon dioxide from landfill gas. J. Therm. Sci. Technol. 2009, 4, 362–371. [Google Scholar] [CrossRef]
- Babar, M.; Bustam, M.A.; Maulud, A.S.; Ali, A.; Mukhtar, A.; Ullah, S. Enhanced cryogenic packed bed with optimal CO2 removal from natural gas; a joint computational and experimental approach. Cryogenics 2020, 105, 103010. [Google Scholar] [CrossRef]
- Zhimin, H.; Zhigang, T.; Ataeivarjovi, E.; Dong, G.; Zhijun, Z.; Hongwei, L. Study on polydimethylsiloxane desorption membrane of CO2-dimethyl carbonate system. Energy Procedia 2017, 118, 210–215. [Google Scholar] [CrossRef]
- deMontigny, D.; Tontiwachwuthikul, P.; Chakma, A. Using polypropylene and polytetrafluoroethylene membranes in a membrane contactor for CO2 absorption. J. Membr. Sci. 2006, 277, 99–107. [Google Scholar] [CrossRef]
- Drioli, E.; Brunetti, A.; Barbieri, G. Ceramic membranes in carbon dioxide capture: Applications and potentialities. Adv. Sci. Technol. 2010, 72, 105–118. [Google Scholar] [CrossRef]
- Widayat, W.; Suzery, M.; Satriadi, H.; Wahyudi; Philia, J. Selection and cultivation of microalgae for CO2 biofixation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012132. [Google Scholar] [CrossRef]
- Xu, X.; Kentish, S.E.; Martin, G.J.O. Direct air capture of CO2 by microalgae with buoyant beads encapsulating carbonic anhydrase. ACS Sustain. Chem. Eng. 2021, 9, 9698–9706. [Google Scholar] [CrossRef]
- Pierre, A.C. Enzymatic carbon dioxide capture. Int. Sch. Res. Notices 2012, 2012, 753687. [Google Scholar] [CrossRef]
- Choi, S.; Dresse, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Ingrosso, S. Thermodynamic Framework for Cryogenic Carbon Capture. Comput. Aided Chem. Eng. 2020, 48, 475–480. [Google Scholar] [CrossRef]
- Shah, S.; Shah, M.; Shah, A.; Shah, M. Evolution in the membrane-based materials and comprehensive review on carbon capture and storage in industries. Emerg. Mater. 2020, 3, 33–44. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Gani, R.; Zhou, T. Comparative economic analysis of physical, chemical, and hybrid absorption processes for carbon capture. Ind. Eng. Chem. Res. 2020, 59, 2005–2012. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Dhir, A. Capture of carbon dioxide using solid carbonaceous and non-carbonaceous adsorbents: A review. Environ. Chem. Lett. 2021, 19, 851–873. [Google Scholar] [CrossRef]
- Vilarrasa-Garcia, E.; Cecilia, J.A.; Azevedo, D.C.S.; Cavalcante, C.L.; Rodriguez-Castellon, E. Evaluation of porous clay heterostructures modified with amine species as adsorbent for CO2 capture. Microporous Mesoporous Mater. 2017, 249, 25–33. [Google Scholar] [CrossRef]
- Park, S.; Wijaya, D.T.; Na, J.; Lee, C.W. Towards the large-scale electrochemical reduction of carbon dioxide. Catalysts 2021, 11, 253. [Google Scholar] [CrossRef]
- Pennline, H.W.; Granite, E.J.; Luebke, D.R.; Kitchin, J.R.; Landon, J.; Weiland, L.M. Separation of CO2 from flue gas using electrochemical cells. Fuel 2010, 89, 1307–1314. [Google Scholar] [CrossRef]
- Rinker, E.B.; Ashour, S.S.; Sandall, O.C. Absorption of carbon dioxide into aqueous blends of diethanolamine and methyldiethanolamine. Ind. Eng. Chem. Res. 2000, 39, 4346–4356. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Soares, J.L.; Moreira, R.F.P.M.; Jose, H.J.; Grande, C.A.; Rodrigues, A.E. Hydrotalcite materials for carbon dioxide adsorption at high temperatures: Characterization and diffusivity measurements. Sep. Sci. Technol. 2004, 39, 1989–2010. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; LeVan, M.D. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Mulfort, K.L.; Frost, H.; Ryan, P.; Punnathanam, S.; Broadbelt, L.J.; Hupp, J.T.; Snurr, R.Q. Separation of CO2 from CH4 using mixed-ligand metal-organic frameworks. Langmuir 2008, 24, 8592–8598. [Google Scholar] [CrossRef]
- Martavaltzi, C.S.; Lemonidou, A.A. Development of new CaO based sorbent materials for CO2 removal at high temperature. Microporous Mesoporous Mater. 2008, 110, 119–127. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, L.; Li, L. Adsorption of CO2 and N2 on synthesized NaY zeolite at high temperatures. Adsorption 2009, 15, 497. [Google Scholar] [CrossRef]
- Huang, C.-M.; Hsu, H.-W.; Liu, W.-H.; Cheng, J.-Y.; Chen, W.-C.; Wen, T.-W.; Chen, W. Development of post-combustion CO2 capture with CaO/CaCO3 looping in a bench scale plant. Energy Procedia 2011, 4, 1268–1275. [Google Scholar] [CrossRef]
- Bezzera, D.P.; da Silva, F.W.M.; de Moura, P.A.S.; Sousa, A.G.S.; Vieira, R.S.; Rodriguez-Castellon, E.; Azevedo, D.C.S. CO2 adsorption in amine-grafted zeolite 13X. Appl. Surf. Sci. 2014, 314, 314–321. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Edubilli, S.; Mishra, A.; Ghoshal, A.K. CO2 adsorption kinetics on mesoporous silica under wide range of pressure and temperature. Chem. Eng. J. 2014, 256, 1–8. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Olivera, S.; Venkatesh, K. Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: A review. J. Clean. Prod. 2015, 103, 171–196. [Google Scholar] [CrossRef]
- Chang, S.-C.; Chien, S.-Y.; Chen, C.-L.; Chen, C.-K. Analysing adsorption characteristics of CO2, N2 and H2O in MCM-41 silica by molecular simulation. Appl. Surf. Sci. 2015, 331, 225–233. [Google Scholar] [CrossRef]
- Montagnaro, F.; Silvestre-Albero, A.; Silvestre-Albero, J.; Rodriguez-Reinoso, F.; Erto, A.; Lancia, A.; Balsamo, M. Post-combustion CO2 adsorption on activated carbons with different textural properties. Microporous Mesoporous Mater. 2015, 209, 157–164. [Google Scholar] [CrossRef]
- Vilarrasa-Garcia, E.; Cecilia, J.A.; Santos, S.M.L.; Cavalcante, C.L.; Jimenez-Jimenez, J.; Azevedo, D.C.S.; Rodriguez-Castellon, E. CO2 adsorption on APTES functionalized mesocellular foams obtained from mesoporous silicas. Microporous Mesoporous Mater. 2014, 187, 125–134. [Google Scholar] [CrossRef]
- Vilarrasa-Garcia, E.; Moya, E.M.O.; Cecilia, J.A.; Cavalcante, C.L.; Jimenez-Jimenez, J.; Azevedo, D.C.S.; Rodriguez-Castellon, E. CO2 adsorption on amine modified mesoporous silicas: Effect of the progressive disorder of the honeycomb arrangement. Microporous Mesoporous Mater. 2015, 209, 172–183. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, L. Molecular insight into competitive adsorption of methane and carbon dioxide in montmorillonite: Effect of clay structure and water content. Fuel 2019, 239, 32–43. [Google Scholar] [CrossRef]
- Stanly, S.; Jelmy, E.J.; Nair, C.P.R.; John, H. Carbon dioxide adsorption studies on modified montmorillonite clay/reduced graphene oxide hybrids at low pressure. J. Environ. Chem. Eng. 2019, 7, 103344. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 capture using solid sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-Garcia, E.; Besghaier, S.; Chlendi, M.; Duro, F.I.F.; Castellon, R.R.; Bagane, M. CO2 adsorption of materials synthesized from clay minerals: A review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Luedtke, Z.; Aro, M.; Sun, Z.; Toan, S. Carbon Capture Materials and Technologies: A Review. Cur. Res. Mater. Chem. 2021, 3, 108. [Google Scholar] [CrossRef]
- Aniruddha, R.; Sreedhar, I.; Reddy, B.M. MOFs in carbon capture: Past, present and future. J. CO2 Util. 2020, 42, 101297. [Google Scholar] [CrossRef]
- Mutch, G.A.; Shulda, S.; McCue, A.J.; Menart, M.J.; Ciobanu, C.V.; Ngo, C.; Anderson, J.A.; Richards, R.M.; Vega-Maza, D. Carbon capture by metal oxides: Unleashing the potential of the (111) facet. J. Am. Chem. Soc. 2018, 140, 4736–4742. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Haque, E.; Islam, M.; Pourazadi, E.; Sarkar, S.; Harris, A.T.; Minett, A.I.; Yanmaz, E.; Alshehri, S.M.; Ide, Y.; Wu, K.C.-W.; et al. Boron functionalized graphene oxide-organic frameworks for highly efficient CO2 capture. Chem. Asian J. 2017, 12, 283–288. [Google Scholar] [CrossRef]
- Elhenawy, S.E.M.; Khraisheh, M.; AlMomani, F.; Walker, G. Metal-organic frameworks as a platform for CO2 capture and chemical processes: Adsorption, membrane separation, catalytic-conversion, and electrochemical reduction of CO2. Catalysts 2020, 10, 1293. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, J.; Wei, X.; Ding, J.; Wang, X.; Song, C. Development of a new clay supported polyethyleneimine composite for CO2 capture. Appl. Energy 2014, 113, 334–341. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells. Adv. Funct. Mat. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Composites of metal–organic frameworks: Preparation and application in adsorption. Mater. Today 2014, 17, 136–146. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photo electrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Wang, S.; Yao, W.; Lin, J.; Ding, Z.; Wang, X. Cobalt imidazolate metal–organic frameworks photosplit CO2 under mild reaction conditions. Angew. Chem. Int. Ed. 2013, 23, 1034–1038. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-driven heterogeneous reduction of carbon dioxide: Photocatalysts and photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Imidazolium ionic liquids, imidazolylidene heterocyclic carbenes, and zeolitic imidazolate frameworks for CO2 capture and photochemical reduction. Angew. Chem. Int. Ed. 2016, 55, 2308–2320. [Google Scholar] [CrossRef]
- Lanni, L.M.; Tilford, R.W.; Bharathy, M.; Lavigne, J.J. Enhanced hydrolytic stability of self-assembling alkylated two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2011, 133, 13975–13983. [Google Scholar] [CrossRef]
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Dai, Q.; Li, H.; Brédas, J.-L. Hydrolytic stability of boronate ester-linked covalent organic frameworks. Adv. Theory Sim. 2018, 1, 1700015. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, M.; Mao, H.; Pei, X.; Alshmimri, S.A.; Reimer, J.A.; Yaghi, O.M. Crystalline dioxin-linked covalent organic frameworks from irreversible reactions. J. Am. Chem. Soc. 2018, 140, 12715–12719. [Google Scholar] [CrossRef]
- Ramis, G.; Busca, G.; Lorenzelli, V. Low-temperature CO2 adsorption on metal oxides: Spectroscopic characterization of some weakly adsorbed species. Mater. Chem. Phys. 1991, 29, 425–435. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; Cavalcante, C.L.; Azevedo, D.C.S.; Franco, F.; Rodríguez-Castellón, E. Evaluation of two fibrous clay minerals (sepiolite and palygorskite) for CO2 Capture. J. Environ. Chem. Eng. 2018, 6, 4573–4587. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Arencibia, A.; Calleja, G.; San, R. Tuning the textural properties of HMS mesoporous silica. Functionalization towards CO2 adsorption. Microporous Mesoporous Mater. 2018, 260, 235–244. [Google Scholar] [CrossRef]
- Gomez-Pozuelo, G.; Sanz-Perez, E.S.; Arencibia, A.; Pizarro, P.; Sanz, R.; Serrano, D.P. CO2 adsorption on amine-functionalized clays. Microporous Mesoporous Mater. 2019, 282, 38–47. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Vilarrasa-Garcia, E.; Cecilia, J.A.; Moya, E.M.O.; Cavalcante, C.L.; Azevedo, D.C.S.; Rodriguez-Castellon, E. “Low cost” pore expanded SBA-15 functionalized with amine groups applied to CO2 adsorption. Materials 2015, 8, 2495–2513. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; García-Sancho, C.; Saboya, R.M.A.; Azevedo, D.C.S.; Cavalcante, C.L.; Rodríuez-Castellón, E. Functionalization of hollow silica microspheres by impregnation or grafted of amine groups for the CO2 capture. Int. J. Greenh. Gas Control 2016, 52, 344–356. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Zhang, Y.; Qiao, K.; Yan, Z.; Komarnemi, S. Amine-modified mesocellular silica foams for CO2 capture. Chem. Eng. J. 2011, 168, 918–924. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Webley, P.; Zhang, J.; Singh, R.; Marshall, M. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption 2008, 14, 415–422. [Google Scholar] [CrossRef]
- Merel, J.; Clausse, M.; Meurier, F. Experimental investigation on CO2 post-combustion capture by indirect thermal swing adsorption using 13X and 5A zeolites. Ind. Eng. Chem. Res. 2008, 47, 209–215. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arenillas, A.; Rubiera, F.; Pis, J.J. CO2 capture by adsorption with nitrogen enriched carbons. Fuel 2007, 86, 2204–2212. [Google Scholar] [CrossRef]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface modification of activated carbons for CO2 capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef]
- Peng, J.; Sun, H.; Wang, J.; Qiu, F.; Zhang, P.; Ning, W.; Zhang, D.; Li, W.; Wei, C.; Miao, S. Highly stable and recyclable sequestration of CO2 using supported melamine on layered-chain clay mineral. Appl. Mater. Interfaces 2021, 13, 10933–10941. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, L.P.; Kalantzopoulos, N.G.; Eckert, J.; Knudsen, K.D.; Fossum, J.O. A nano-silicate material with exceptional capacity for CO2 capture and storage at room temperature. Sci. Rep. 2018, 8, 11827. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Song, C.; Wei, X.; Ding, J.; Xiao, J. Sulfuric acid modified bentonite as the support of tetraethylenepentamine for CO2 capture. Energy Fuels 2013, 27, 1538–1546. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, Z.; Xu, G.; Zhang, K. Desorption kinetics and mechanisms of CO2 on amine-based mesoporous silica materials. Energies 2017, 10, 115. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P.T. Ionic liquids: Potential materials for carbon dioxide capture and utilization. Front. Mater. 2019, 6, 42. [Google Scholar] [CrossRef]
- Steinruck, H.-P.; Wasserscheid, P. Ionic liquids in catalysis. Catal. Lett. 2015, 145, 380–397. [Google Scholar] [CrossRef]
- DePaolo, D.J.; Cole, D.R. Geochemistry of geologic carbon sequestration: An overview. Rev. Mineral. Geochem. 2013, 77, 1–14. [Google Scholar] [CrossRef]
- Juzkow, J. Clay Materials Modified with Amino Acids for Purification Processes of Biogas and Natural Gas. Master’s Thesis, Technical University of Lisbon, Lisbon, Portugal, 2016. [Google Scholar]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of the AIPEA nomenclature and CMS nomenclature committees. Clays Clay Miner. 1995, 43, 255–256. [Google Scholar] [CrossRef]
- Schulze, D.G. Clay minerals. In Encyclopaedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Boston, MA, USA, 2005; pp. 246–254. [Google Scholar]
- Theng, B.K.G. The clay minerals. Dev. Clay Sci. 2012, 4, 3–45. [Google Scholar] [CrossRef]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Natural clay minerals as environmental cleaning agents. In Clay Materials for Environmental Remediation; Ismadji, S., Soetaredjo, F.E., Ayucitra, A., Eds.; Springer: Copenhagen, Denmark, 2015; pp. 5–37. [Google Scholar] [CrossRef]
- Christidis, G.E. Industrial clays. In European Mineralogical Union Notes in Mineralogy. Advances in the Characterization of Industrial Clays; Christidis, G.E., Ed.; Mineralogical Society of Great Britain and Ireland: London, UK, 2010; Volume 9, pp. 341–414. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Johnston, C.T.; Bergaya, F. Clay minerals and their surfaces. Dev. Clay Sci. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Clay Mineral. Available online: https://www.britannica.com/science/clay-mineral (accessed on 7 October 2021).
- Zhou, C.H.; Keeling, J. Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. App. Clay Sci. 2013, 74, 3–9. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.H. Structures and mineralogy of clay minerals. Dev. Clay Sci. 2006, 1, 19–86. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Malferrari, D.; Laurora, A.; Elmi, C.; Mottana, A. Structure and mineralogy of layer silicates: Recent perspectives and new trends. Layered mineral structures and their application in advanced technologies. EMU Notes Mineral. 2011, 11, 1–71. [Google Scholar]
- Martin, R.T.; Bailey, S.W.; Eberl, D.D.; Fanning, D.S.; Guggenheim, S.; Kodama, H.; Pevear, D.R.; Srodon, J.; Wicks, F.J. Report of the Clay Minerals Society Nomenclature Committee: Revised classification of clay materials. Clays Clay Miner. 1991, 39, 333–335. [Google Scholar] [CrossRef]
- Guggenheim, S.; Adams, J.M.; Bain, D.C.; Bergaya, F.; Brigatti, M.F.; Drits, V.A.; Formoso, M.L.L.; Galán, E.; Kogure, T.; Stanjek, H. Summary of recommendations of Nomenclature Committees relevant to clay mineralogy: Report of the association Internationale pour l’Etude des Argiles (AIPEA) Nomenclature Committee for 2006. Clays Clay Miner. 2006, 54, 761–772. [Google Scholar] [CrossRef]
- Schroeder, P. Clays in the Critical Zone; Cambridge University Press: Cambridge, UK, 2018; pp. 1–246. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. Dev. Clay. Sci. 2006, 1, 1–18. [Google Scholar] [CrossRef]
- Boulet, P.; Greenwell, H.C.; Stackhouse, S.; Coveney, P.V. Recent advances in understanding the structure and reactivity of clays using electronic structure calculations. J. Mol. Struc. Theochem 2006, 762, 33–48. [Google Scholar] [CrossRef]
- da Silva, G.J.; Fossum, J.O.; DiMasi, E.; Maloy, K.J.; Lutnaes, S.B. Synchrotron x-ray scattering studies of water intercalation in a layered synthetic silicate. Phys. Rev. E 2002, 66, 011303. [Google Scholar] [CrossRef] [PubMed]
- Bordallo, H.N.; Aldridge, L.P.; Churchman, G.J.; Gates, W.P.; Telling, M.T.F.; Kiefer, K.; Fouquet, P.; Seydel, T.; Kimber, S.A.J. Quasi-elastic neutron scattering studies on clay interlayer-space highlighting the effect of the cation in confined water dynamics. J. Phys. Chem. C. 2008, 112, 13982–13991. [Google Scholar] [CrossRef]
- Hansen, E.L.; Hemmen, H.; Fonseca, D.M.; Coutant, C.; Knudsen, K.D.; Plivelic, T.S.; Bonn, D.; Fossum, J.O. Swelling transition of a clay induced by heating. Sci. Rep. 2012, 2, 618. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.H. Applied Clay Mineralogy: Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–180. [Google Scholar]
- Yariv, S.; Cross, H. Organo-Clay Complexes and Interactions; Marcel Dekker: New York, NY, USA, 2002; pp. 1–688. [Google Scholar]
- Stinkule, A. Clays in the Subterranean of Latvia; RTU Publishing House: Riga, Latvia, 2014; pp. 1–121. [Google Scholar]
- Lan, Y.; Liu, Y.; Li, J.; Chen, D.; He, G.; Parkin, I.P. Natural clay-based materials for energy storage and conversion applications. Adv. Sci. 2021, 8, 2004036. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, D. CO2 capture by kaolinite and its adsorption mechanism. Appl. Clay Sci. 2015, 104, 221–228. [Google Scholar] [CrossRef]
- Birbara, P.J.; Nalette, T.A. Regenerable Supported Amine-Polyol Sorbent. US Patent 5,376,614, 27 December 1994. [Google Scholar]
- Yong, Z.; Rodrigues, A.E. Hydrotalcite-like compounds as adsorbents for carbon dioxide. Energy Convers. Manag. 2002, 43, 1865–1876. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Baltrus, J.; Stevens, R.W.; Toochinda, P.; Chuang, S.S.C. CO2 capture by amine-enriched fly ash carbon sorbents. Sep. Purif. Technol. 2004, 35, 31–36. [Google Scholar] [CrossRef]
- Santos-Costa, A.; Imae, T.; Takagi, K.; Kikuta, K. Intercalation of dendrimers in the interlayer of hydrotalcite clay sheets. In Surface and Colloid Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 128, pp. 113–119. [Google Scholar] [CrossRef]
- Sirwardane, R.V. Solid sorbents for removal of carbon dioxide from gas streams at low temperatures. US Patent 6908497, 21 June 2005. [Google Scholar]
- Chaffee, A.L.; Knowles, G.P.; Liang, Z.; Zhang, J.; Xiao, P.; Webley, P.A. CO2 capture by adsorption: Materials and process development. Int. J. Greenh. Gas Control 2007, 1, 11–18. [Google Scholar] [CrossRef]
- Thomas, J.; Bohor, B.F. Surface area of vermiculite with nitrogen and carbon dioxide as adsorbates. Clays Clay Miner. 1969, 17, 205–209. [Google Scholar] [CrossRef]
- Aylmore, L.A.G. Gas sorption in clay mineral systems. Clays Clay Miner. 1974, 22, 175–183. [Google Scholar] [CrossRef]
- Azzouz, A. Physicochimie des Tamis Moléculaires; Office des Publications Universitaires: Algiers, France, 1994. [Google Scholar]
- Rodlert, M.; Plummer, C.J.G.; Leterrier, Y.; Manson, J.-A.E.; Grunbauer, H.J.M. Rheological behaviour of hyperbranched polymer/montmorillonite clay nanocomposites. J. Rheol. 2004, 48, 1049–1065. [Google Scholar] [CrossRef]
- Lingaiah, S.; Shivakumar, K.N.; Sadler, R.; Sharpe, M. A method of visualization of dispersion of nanoplatelets in nanocomposites. Compos. Sci. Technol. 2005, 65, 2276–2280. [Google Scholar] [CrossRef]
- Azzouz, A.; Nistor, D.; Miron, D.; Ursu, A.-V.; Sajin, T.; Monette, F.; Niquette, P.; Hausler, R. Assessment of the acid–base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements. Thermochim. Acta 2006, 449, 27–34. [Google Scholar] [CrossRef]
- Liu, P. Hyperbranched aliphatic polyester grafted attapulgite via a melt polycondensation process. Appl. Clay Sci. 2007, 35, 11–16. [Google Scholar] [CrossRef]
- Azzouz, A.; Assaad, E.; Ursu, A.-V.; Sajin, T.; Nistor, D.; Roy, R. Carbon dioxide retention over montmorillonite-dendrimer materials. Appl. Clay Sci. 2010, 48, 133–137. [Google Scholar] [CrossRef]
- Irani, M.; Fan, M.; Ismail, H.; Tuwati, A.; Dutcher, B.; Russell, A.G. Modified nanosepiolite as an inexpensive support of tetraethylenepentamine for CO2 sorption. Nano Energy 2015, 15, 235–246. [Google Scholar] [CrossRef]
- Yuan, M.; Gao, G.; Hu, X.; Luo, X.; Huang, Y.; Jin, B.; Liang, Z. Pre-modified sepiolite functionalized with triethylenetetramine as an effective and inexpensive adsorbent for CO2 capture. Ind. Eng. Chem. Res. 2018, 57, 6189–6200. [Google Scholar] [CrossRef]
- Thomas, J.; Bohor, B.F. Surface area of montmorillonite from the dynamic sorption of nitrogen and carbon dioxide. Clays Clay Miner. 1968, 16, 83–91. [Google Scholar] [CrossRef]
- Fripiat, J.J.; Cruz, M.I.; Bohor, B.F.; Thomas, J. Interlamellar adsorption of carbon dioxide by smectites. Clays Clay Miner. 1974, 22, 23–30. [Google Scholar] [CrossRef]
- Chan, W.H.; Mazlee, M.N.; Ahmad, Z.A.; Ishak, M.A.M.; Shamsul, J.B. The development of low-cost adsorbents from clay and waste materials: A review. J. Mater. Cycles Waste Manag. 2017, 19, 293–301. [Google Scholar] [CrossRef]
- Jeon, P.R.; Choi, J.; Yun, T.S.; Lee, C.H. Sorption equilibrium and kinetics of CO2 on clay minerals from subcritical to supercritical conditions: CO2 sequestration at nanoscale interfaces. Chem. Eng. J. 2014, 255, 705–715. [Google Scholar] [CrossRef]
- Giesting, P.; Guggenheim, S.; Koster van Groos, A.F.; Busch, A. Interaction of carbon dioxide with Na-exchanged montmorillonite at pressures to 640 bars: Implications for CO2 sequestration. Int. J. Greenh. Gas Control 2012, 8, 73–81. [Google Scholar] [CrossRef]
- Schaef, H.T.; Ilton, E.S.; Qafoku, O.; Martin, P.F.; Felmy, A.R.; Rosso, K.M. In situ XRD study of Ca2+ saturated montmorillonite (STX-1) exposed to anhydrous and wet supercritical carbon dioxide. Int. J. Greenh. Gas Control 2012, 6, 220–229. [Google Scholar] [CrossRef]
- Rother, G.; Ilton, E.S.; Wallacher, D.; HauB, T.; Schaef, H.T.; Qafoku, O.; Rosso, K.M.; Felmy, A.R.; Krukowski, E.G.; Stack, A.G.; et al. CO2 sorption to sub-single hydration layer montmorillonite clay studied by excess sorption and neutron diffraction measurements. Env. Sci. Technol. 2013, 47, 205–211. [Google Scholar] [CrossRef]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Maruthi Sena, M.; Krishnan, M. Role of cations in adsorption of supercritical carbon dioxide at smectite mineral-water interfaces: Molecular dynamics and adaptive biasing fore simulation studies. J. Phys. Chem. C. 2018, 123, 1170–1184. [Google Scholar] [CrossRef]
- Narayanan Nair, A.K.; Cui, R.; Sun, S. Overview of the adsorption and transport properties of water, ions, carbon dioxide, and methane in swelling clays. ACS Earth Space Chem. 2021, 5, 2599–2611. [Google Scholar] [CrossRef]
- Mendel, N.; Sîretanu, D.; Sîretanu, I.; Brilman, D.W.; Mugele, F. Interlayer Cation-Controlled Adsorption of Carbon Dioxide in Anhydrous Montmorillonite Clay. J. Phys. Chem. C 2021, 125, 27159–27169. [Google Scholar] [CrossRef]
- Sato, K.; Hunger, M. Carbon dioxide adsorption in open nanospaces formed by overlap of saponite clay nanosheets. Comm. Chem. 2020, 3, 91. [Google Scholar] [CrossRef]
- Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; Ruı’z, B.; Rosa’rio, M. Carbon dioxide/methane separation by adsorption on sepiolite. J. Nat. Gas. Chem. 2007, 16, 235–243. [Google Scholar] [CrossRef]
- Pires, J.; Bestilleiro, M.; Pinto, M.; Gil, A. Selective adsorption of carbon dioxide, methane, and ethane by porous clays heterostructures. Sep. Purif. Technol. 2008, 61, 161–167. [Google Scholar] [CrossRef]
- Franco, F.; Cecilia, J.A.; Pozo, M.; Pardo, L.; Bellido, E.; Garcia-Sancho, C. Microwave assisted acid treatment of kerolitic clays from the Neogene Madrid Basin (Spain) and its use in CO2 capture processes. Microporous Mesoporous Mater. 2020, 292, 109749. [Google Scholar] [CrossRef]
- da Silva, G.J.; Fossum, J.O.; DiMasi, E.; Maloy, K.J. Hydration transitions in a nanolayered synthetic silicate: A synchrotron x-ray scattering study. Phys. Rev. B 2003, 67, 094114. [Google Scholar] [CrossRef]
- Jansson, M.; Eriksen, T.E. In situ anion diffusion experiments using radiotracers. J. Contam. Hydrol. 2004, 68, 183–192. [Google Scholar] [CrossRef]
- Tambach, T.J.; Hensen, E.J.M.; Smit, B. Molecular simulations of swelling clay minerals. J. Phys. Chem. B 2004, 108, 7586–7596. [Google Scholar] [CrossRef]
- Malikova, N.; Cadene, A.; Dubois, E.; Marry, C.; Durand-Vidal, S.; Turq, P.; Breu, J.; Longeville, S.; Zanotti, J.-M. Water diffusion in a synthetic hectorite clay studied by quasi-elastic neutron scattering. J. Phys. Chem. C 2007, 111, 17603–17611. [Google Scholar] [CrossRef]
- Porion, P.; Michot, L.J.; Faugere, A.M.; Delville, A. Structural and dynamical properties of the water molecules confined in dense clay sediments: A study combining 2H NMR spectroscopy and multiscale numerical modelling. J. Phys. Chem. C 2007, 111, 5441–5453. [Google Scholar] [CrossRef]
- Tenorio, R.P.; Alme, L.R.; Engelsberg, M.; Fossum, J.O.; Hallwass, F. Geometry and dynamics of intercalated water in Na-fluorhectorite clay hydrates. J. Phys. Chem. C 2008, 112, 575–580. [Google Scholar] [CrossRef]
- Tenorio, R.P.; Engelsberg, M.; Fossum, J.O.; da Silva, G.J. Intercalated water in synthetic fluorhectorite clay. Langmuir 2010, 26, 9703–9709. [Google Scholar] [CrossRef]
- Jimenez-Ruiz, M.; Ferrage, E.; Delville, A.; Michot, L.J. Anisotropy on the collective dynamics of water confined in swelling clay minerals. J. Phys. Chem. A 2012, 116, 2379–2387. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benitez-Guerrero, M.; Pozo, E.; Rubi, J.A.M. Microwave assisted acid treatment of sepiolite: The role of composition and “crystallinity”. Appl. Clay Sci. 2014, 102, 15–27. [Google Scholar] [CrossRef]
- Silva, V.C.; Araujo, M.E.B.; Rodrigues, A.M.; Cartazo, J.M.; Menezes, R.R.; Neves, G.A. adsorption behaviour of acid-treated Brazilian palygorskite for cationic and anionic dyes removal from water. Sustainability 2021, 13, 3954. [Google Scholar] [CrossRef]
- Wal, K.; Rutkowski, P.; Stawinski, W. Application of clay minerals and their derivatives in adsorption from gaseous phase. Appl. Clay Sci. 2021, 215, 106323. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lu, D.L. Amine modification on kaolinites to enhance CO2 adsorption. J. Colloid Interface Sci. 2014, 436, 47–51. [Google Scholar] [CrossRef]
- Horri, N.; Sanz-Pérez, E.S.; Arencibia, A.; Sanz, R.; Frini-Srasra, N. Amine grafting of acid-activated bentonite for carbon dioxide capture. Appl. Clay Sci. 2019, 180, 105195. [Google Scholar] [CrossRef]
- Ramadass, K.; Sathish, C.I.; Singh, G.; Ruban, S.J.; Ruban, A.M.; Bahadur, R.; Vinu, A. Morphologically tuneable nano architectonics of mixed kaolin-halloysite derived nitrogen-doped activated nanoporous carbons for supercapacitor and CO2 capture applications. Carbon 2022, 192, 133–144. [Google Scholar] [CrossRef]
- Bo Hunvik, K.W.; Loch, P.; Wallacher, D.; Kirch, A.; Cavalcanti, L.P.; Riess, M.; Fossum, J.O. CO2 adsorption enhanced by tuning the layer charge in a clay mineral. Langmuir 2021, 37, 14491–14499. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interface 2016, 8, 17312–17320. [Google Scholar] [CrossRef]
- Roth, E.A.; Agarwal, S.; Gupta, R.K. Nanoclay-based solid sorbents for CO2. Energy Fuels 2013, 27, 4129–4136. [Google Scholar] [CrossRef]
- Pinto, M.L.; Mafra, L.; Guil, J.M.; Pires, J.; Rocha, J. Adsorption and activation of CO2 by amine-modified nanoporous materials studied by solid-state NMR and 13CO2 adsorption. Chem. Mater. 2011, 23, 1387–1395. [Google Scholar] [CrossRef]
- Pinto, M.L.; Pires, J. Porous and hybrid clay-based materials for separation of hydrocarbons. Microporous Mesoporous Mater. 2012, 151, 403–410. [Google Scholar] [CrossRef]
- Garea, S.A.; Mihai, A.J.; Ghebaur, A.; Nistor, C.; Sarbu, A. Porous clay heterostructures: A new inorganic host for 5-fluorouracil encapsulation. Int. J. Pharm. 2015, 492, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, L.; Piwowarska, Z.; Kustrowski, P.; Wegrzyn, A.; Gil, B.; Kowalczyk, A.; Dudek, B.; Dziembaj, R.; Michalik, M. Comparison study of titania pillared interlayered clay and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process. Appl. Clay Sci. 2011, 53, 164–173. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Arango-Diaz, A.; Jimenez-Jimenez, J.; Storaro, L.; Moretti, E.; Rodriguez-Castellon, E. CuO-CeO2 supported on montmorillonite-derived porous clay heterostructures (PCH) for preferential CO oxidation in H2-rich stream. Catal. Today 2015, 253, 126–136. [Google Scholar] [CrossRef]
- Soriano, M.D.; Cecilia, J.A.; Natoli, A.; Jimenez-Jimenez, J.; Nieto, J.M.L.; Rodriguez-Castellon, E. Vanadium oxide supported on porous clay heterostructure for the partial oxidation of hydrogen sulphide to sulphur. Catal. Today 2015, 254, 36–42. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; Garcia-Sancho, C.; Luna, F.M.T.; Rodriguez-Castellon, E.; Cavalcante, C.L. WO3-based catalysts supported on porous clay heterostructures (PCH) with Si–Zr pillars for synthetic esters production. Appl. Clay Sci. 2016, 124–125, 69–78. [Google Scholar] [CrossRef]
- Azzouz, A.; Platon, N.; Nousir, S.; Ghomari, K.; Nistor, D.; Shiao, T.C.; Roy, R. OH-enriched organo montmorillonites for potential applications in carbon dioxide separation and concentration. Sep. Purif. Technol. 2013, 108, 181–188. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006, 61, 3893–3906. [Google Scholar] [CrossRef]
- Grande, C.A.; Rodrigues, A.E. Electric swing adsorption for CO2 removal from flue gas. Int. J. Greenh. Gas Control 2008, 2, 194–202. [Google Scholar] [CrossRef]
- Zhang, J.; Webley, P.A.; Xiao, P. Effect of process parameters on power requirements of vacuum swing adsorption technology for CO2 capture from flue gas. Energy Convers. Manag. 2008, 49, 346–356. [Google Scholar] [CrossRef]
- Gupta, S.K.; Lesslie, R.D.; King, A.D. Solubility of alcohols in compressed gases. A comparison of vapor-phase interactions of alcohols and homomorphic compounds with various gases I. Ethanol in compressed helium, hydrogen, argon, methane, ethylene, ethane, carbon dioxide, and nitrous oxide. J. Phys. Chem. 1973, 77, 2011–2015. [Google Scholar] [CrossRef]
- Massoudi, R.; King, A.D. Solubility of alcohols in compressed gases. A comparison of vapor-phase interactions of alcohols and homomorphic compounds with various gases II. 1-Butanol, diethyl ether, and n-pentane in compressed nitrogen, argon, methane, ethane, and carbon dioxide at 25.deg. J. Phys. Chem. 1973, 77, 2016–2018. [Google Scholar] [CrossRef]
- Saharay, M.; Balasubramanian, S. Electron donor–acceptor interactions in ethanol—CO2 mixtures: An ab initio molecular dynamics study of supercritical carbon dioxide. J. Phys. Chem. B 2006, 110, 3782–3790. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Ursu, A.-V.; Nistor, D.; Sajin, T.; Assaad, E.; Roy, R. TPD study of the reversible retention of carbon dioxide over montmorillonite intercalated with polyol dendrimers. Thermochim. Acta 2009, 496, 45–49. [Google Scholar] [CrossRef]
- Calderon, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Gassensmith, J.J.; Furukawa, H.; Smaldone, R.A.; Forgan, R.S.; Botros, Y.Y.; Yaghi, O.M.; Stoddart, J.F. Strong and reversible binding of carbon dioxide in a green metal–organic framework. J. Am. Chem. Soc. 2011, 133, 15312–15315. [Google Scholar] [CrossRef]
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Gouvea, V.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Sha, M.S.P. Graphene oxide as support for layered double hydroxides: Enhancing the CO2 adsorption capacity. Chem. Mater. 2012, 24, 4531–4539. [Google Scholar] [CrossRef]
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T.J. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1995, 374, 529–531. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Garcia-Sancho, C.; Franco, F. Montmorillonite based porous clay heterostructures: Influence of Zr in the structure and acidic properties. Microporous Mesoporous Mater. 2013, 176, 95–102. [Google Scholar] [CrossRef]
- Humphrey, J.; Boyd, D. Clay: Types, Properties and Uses; Nova Science Publishers, Inc.: New York, NY, USA, 2011. [Google Scholar]
- Nawani, P.; Gelfer, M.Y.; Hsiao, B.S.; Frenkel, A.; Gilman, J.W.; Khalid, S. Surface Modification of nanoclays by catalytically active transition metal Ions. Langmuir 2007, 23, 9808–9815. [Google Scholar] [CrossRef]
- Zope, I.S.; Dasari, A.; Camino, G. Elucidating the catalytic effect of metal ions in montmorillonite on thermal degradation of organic modifier. Mater. Chem. Phys. 2015, 157, 69–79. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.S.; Nagendrappa, G.; Jai Prakash, B.S. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef]
- Tang, T.; Chen, X.; Meng, X.; Chen, H.; Ding, Y. Synthesis of multiwalled carbon nanotubes by catalytic combustion of polypropylene. Angew. Chem. Int. Ed. 2005, 44, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Chen, X.; Chen, H.; Meng, X.; Jiang, Z.; Bi, W. Catalyzing carbonization of polypropylene itself by supported nickel catalyst during combustion of polypropylene/clay nanocomposite for improving fire retardancy. Chem. Mater. 2005, 17, 2799–2802. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Li, J. Regulating effect of exfoliated clay on intumescent char structure and flame retardancy of polypropylene composites. Ind. Eng. Chem. Res. 2016, 55, 5892–5901. [Google Scholar] [CrossRef]
- Vincente, M.A.; Gil, A.; Bergaya, F. Pillared clays and clay minerals. Dev. Clay. Sci. 2013, 5, 523–557. [Google Scholar] [CrossRef]
- Gil, A.; Vicente, M.A. Progress and perspectives on pillared clays applied in energetic and environmental remediation processes. Curr. Opin. Green Sustain. Chem. 2020, 21, 56–63. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Perez, E.S. CO2 adsorption on branched polyethyleneimine-impregnated mesoporous silica SBA-15. Appl. Surf. Sci. 2010, 256, 5323–5328. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Pinto, M.L.; Antunes, F.; Pires, J. L-histidine-based organoclays for the storage and release of therapeutic nitric oxide. J. Mater. Chem. B. 2015, 3, 3556–3563. [Google Scholar] [CrossRef]
- Elkhalifah, A.E.I.; Bustam, M.A.; Azmi, M.S.; Murugesan, T. Carbon dioxide retention on bentonite clay adsorbents modified by mono-, di- and triethanolamine compounds. Adv. Mater. Res. 2014, 917, 115–122. [Google Scholar] [CrossRef]
- Das, S.; Maity, A.; Pradhan, M.; Jana, S. Assessing atmospheric CO2 entrapped in clay nanotubes using residual gas analyser. Anal. Chem. 2016, 88, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Das, S.; Ghosh, C.; Maity, A.; Pradhan, M. Halloysite nanotubes capturing isotope selective atmospheric CO2. Sci. Rep. 2015, 5, 8711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Li, G.; Shi, C.; Wang, B.; Ling, Z. Exfoliated vermiculite nanosheets supporting tetraethylenepentamine for CO2 capture. Results Mater. 2020, 7, 100102. [Google Scholar] [CrossRef]
- Atilhan, M.; Atilhan, S.; Ullah, R.; Anaya, B.; Cagin, T.; Yavuz, C.T.; Aparicio, S. High-pressure methane, carbon dioxide and nitrogen adsorption on amine-impregnated porous montmorillonite nano clays. J. Chem. Eng. Data. 2016, 61, 2749–2760. [Google Scholar] [CrossRef]
- Ouyang, J.; Zheng, C.; Gu, W.; Zhang, Y.; Yang, H.; Suib, S.L. Textural properties determined CO2 capture of Tetraethylenepentamine loaded SiO2 nanowires from α-sepiolite. Chem. Eng. J. 2018, 337, 342–350. [Google Scholar] [CrossRef]
- Ouyang, J.; Gu, W.; Zheng, C.; Yang, H.; Zhang, X.; Jin, Y.; Chen, J.; Jiang, J. Polyethyleneimine (PEI) loaded MgO-SiO2 nanofibers from sepiolite minerals for reusable CO2 capture/release applications. Appl. Clay Sci. 2018, 152, 267–275. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H.; Shiko, E.; Fan, X.; Zhou, Y.; Zhang, G.; Luo, X.; Hu, X.E. Low-cost DETA impregnation of acid-activated sepiolite for CO2 capture. Chem. Eng. J. 2018, 353, 940–948. [Google Scholar] [CrossRef]
- Stevens, L.; Williams, K.; Han, W.Y.; Drage, T.; Snape, C.; Wood, J.; Wang, J. Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route. Chem. Eng. J. 2013, 215, 699–708. [Google Scholar] [CrossRef]
- Elkhalifah, A.E.I.; Bustam, M.A.; Shariff, A.M.; Murugesan, T. Selective adsorption of CO2 on a regenerable amine-bentonite hybrid adsorbent. Appl. Clay Sci. 2015, 107, 213–219. [Google Scholar] [CrossRef]
- Pires, J.; Juźków, J.; Pinto, M.L. Amino acid modified montmorillonite clays as sustainable materials for carbon dioxide adsorption and separation. Colloids Surf. A Physicochem. Eng. 2018, 544, 105–110. [Google Scholar] [CrossRef]
- Chen, C.; Park, D.W.; Ahn, W.S. Surface modification of a low-cost bentonite for post-combustion CO2 capture. Appl. Surf. Sci. 2013, 283, 699–704. [Google Scholar] [CrossRef]
- Horsch, S.; Serhatkulu, G.; Gulari, E.; Kannan, R.M. Supercritical CO2 dispersion of nano-clays and clay/polymer nanocomposites. Polymer 2006, 47, 7485–7496. [Google Scholar] [CrossRef]
- Gao, F. Clay/polymer composites: The story. Mater. Today 2004, 7, 50–55. [Google Scholar] [CrossRef]
- Ismail, N.M.; Ismail, A.F.; Mustafa, A.; Zulhairun, A.K.; Aziz, F.; Bolong, N.; Razali, A.R. Polymer clay nanocomposites for gas separation: A review. ECR 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Petersell, V.; Juriado, K.; Raukas, A.; Shtokalenko, M.; That-Kok, K. Quaternary deposits and weathered bedrock material as a source of dangerous radon emissions in Estonia. Geologos 2015, 21, 139–147. [Google Scholar] [CrossRef]
- Tuuling, I. The Leba Ridge-Riga-Pskov fault zone—A major East European Craton interior dislocation zone and its role in the early Palaeozoic development of the platform cover. Est. J. Earth Sci. 2019, 68, 161–189. [Google Scholar] [CrossRef]
- Raukas, A.; Teedumae, A. Geology of Mineral Resources of Estonia; Estonian Academy Publishers: Tallin, Estonia, 1997. [Google Scholar]
- Geology and Mineral Resources of Estonia. Available online: https://geoloogia.info/geology/text.html (accessed on 7 November 2021).
- Gorokhov, I.M.; Melnikov, N.N.; Kuznetsov, A.B.; Konstantinova, G.V.; Turchenko, T.L. Sm-Md systematics of fine-grained fractions of the Lower Cambrian blue clay in northern Estonia. Lithol. Miner. Resourc. 2007, 42, 482–495. [Google Scholar] [CrossRef]
- Lescinskis, O.; Svinka, R.; Svinka, V. Characterization of three different clay minerals from different geological periods. In Proceedings of the 2nd International Symposium “Clays and Ceramics”, Riga, Latvia, 29–31 January 2018; Dzene, L., Vircava, I., Eds.; University of Latvia: Riga, Latvia, 2018; p. 26. [Google Scholar]
- Stinkulis, G. Middle Devonian clays of the Liepa clay deposit, Latvia: Composition, sedimentary environment and deformation structures. In Proceedings of the 2nd International Symposium “Clays and Ceramics”, Riga, Latvia, 29–31 January 2018; Dzene, L., Vircava, I., Eds.; University of Latvia: Riga, Latvia, 2018; pp. 16–17. [Google Scholar]
- Burlakovs, J.; Ozola, R.; Kostjukovs, J.; Klavins, I.; Purmalis, O.; Klavins, M. Properties of the Jurassic clayey deposits of southwestern Latvia and northern Lithuania. Mater. Sci. Appl. Chem. 2015, 32, 5–12. [Google Scholar] [CrossRef][Green Version]
- Hamza, A.; Hussein, I.A.; Al-Marri, M.J.; Mahmoud, M.; Shawabkeh, R. Impact of clays on CO2 adsorption and enhanced gas recovery in sandstone reservoirs. Int. J. Greenh. Gas Control 2021, 106, 103286. [Google Scholar] [CrossRef]
- Hu, X.; Deng, H.; Lu, C.; Tian, Y.; Jin, Z. Characterization of CO2/CH4 competitive adsorption in various clay minerals in relation to shale gas recovery from molecular simulation. Energy Fuels 2019, 33, 8202–8214. [Google Scholar] [CrossRef]
- Zhou, C.H.; Shen, Z.-F.; Liu, L.-H.; Liu, S.-M. Preparation and functionality of clay-containing films. J. Mater. Chem. 2011, 21, 15132–15153. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Handbook of Clay Science. Developments in Clay Science 5, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Tahir, M.; Saidina, N. Photocatalytic CO2 reduction by CH4 over montmorillonite modified TiO2 nanocomposites in a continuous monolith photoreactor. Appl. Catal. B-Environ. 2013, 142–143, 512–523. [Google Scholar] [CrossRef]
- Christine, S.; Mana, A.; Hanafiah, M.M.; Khan, A.J. Environmental characteristics of clay and clay-based minerals. Geol. Ecol. Landsc. 2017, 1, 155–161. [Google Scholar] [CrossRef]
- Galan, E.; Aparicio, P.; Miras, A. Sepiolite and palygorskite as sealing materials for the geological storage of carbon dioxide. Dev. Clay. Sci. 2011, 3, 375–392. [Google Scholar] [CrossRef]
- Loganathan, N.; Yazaydin, A.O.; Bowers, G.M.; Ngouana-Wakou, B.F.; Kalinichev, A.G.; Kirkpatrick, R.J. Role of cations in the methane/carbon dioxide partitioning in nano- and mesopores of illite using constant reservoir composition molecular dynamics simulation. J. Phys. Chem. C 2020, 124, 2490–2500. [Google Scholar] [CrossRef]
- Zhang, J.; Clennell, M.B.; Liu, K.; Pervukhina, M.; Chen, G.; Dewhurst, D.N. Methane and carbon dioxide adsorption on illite. Energy Fuels 2016, 30, 10643–10652. [Google Scholar] [CrossRef]
- Jin, Z.; Firoozabadi, A. Methane and carbon dioxide adsorption in clay-like slit pores by Monte Carlo simulations. Fluid Phase Equilib. 2013, 360, 456–465. [Google Scholar] [CrossRef]
- 10 Carbon Capture Methods Compared: Costs, Scalability, Permanence, Cleanness. Available online: https://energypost.eu/10-carbon-capture-methods-compared-costs-scalability-permanence-cleanness/ (accessed on 16 November 2021).
- Rosenbloom, D.; Markard, J.; Geels, F.W.; Fuenfschilling, L. Opinion: Why carbon pricing is not sufficient to mitigate climate change—and how “sustainability transition policy” can help. Proc. Natl. Acad. Sci. USA 2020, 117, 8664–8668. [Google Scholar] [CrossRef]
- Stiglitz, J.E.; Stern, N.; Duan, M.; Edenhofer, O.; Giraud, G.; Heal, G.M.; Ia Rovere, E.L.; Morris, A.; Moyer, E.; Pangestu, M.; et al. Report of the High-Level Commission on Carbon Prices; International Bank for Reconstruction and Development and International Development Association/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Nordhaus, W.D. The Climate Casino: Risk, Uncertainty and Economics for a Warming World; Yale University Press: London, UK, 2013. [Google Scholar]
- Markard, J.; Raven, R.; Truffer, B. Sustainability transitions: An emerging field of research and its prospects. Res. Policy 2012, 41, 955–967. [Google Scholar] [CrossRef]
| Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth (ppm) | 1.92 | 2.65 | 1.99 | 2.22 | 2.90 | 3.03 | 1.92 | 2.86 | 2.48 | 2.31 | 2.46 1 |
| Share | Estonia | Latvia | Lithuania |
|---|---|---|---|
| Fossil fuel | 64.79 | 38.89 | 42.46 |
| Low-carbon sources (renewables) | 35.21 | 61.11 | 57.54 |
| Share | Estonia | Latvia | Lithuania |
|---|---|---|---|
| Oil | 18 | 22 | 38 |
| Coal | 39 | <1 | 2 |
| Gas | 4 | 13 | 22 |
| Hydropower | <1 | 5 | <1 |
| Wind | 2 | <1 | 4 |
| Solar | <1 | 0 | <1 |
| Other renewables | 3 | 2 | 1 |
| Approach | Costs (Euro/tCO2) | Scalability (GtCO2/y) |
|---|---|---|
| Reducing CO2 to its constituent components | −70 to 260 | 0.3–0.6 |
| Hydrocarbon fuel production | 590 | 1.0–4.2 |
| CO2 fixation with microalgae | 200 to 800 | 0.2–0.9 |
| Use in building materials | −25 to 60 | 0.1–1.4 |
| Enhanced oil recovery | −35 to 50 | 0.1–1.8 |
| Bioenergy with carbon capture | 50 to 140 | 0.5–5.0 |
| Forestry | −35 to 10 | 1.5 |
| Soil carbon sequestration | −80 to 20 | 0.9–1.9 |
| Biochar | −60 | 0.2–1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krūmiņš, J.; Kļaviņš, M.; Ozola-Davidāne, R.; Ansone-Bērtiņa, L. The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review. Minerals 2022, 12, 349. https://doi.org/10.3390/min12030349
Krūmiņš J, Kļaviņš M, Ozola-Davidāne R, Ansone-Bērtiņa L. The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review. Minerals. 2022; 12(3):349. https://doi.org/10.3390/min12030349
Chicago/Turabian StyleKrūmiņš, Jānis, Māris Kļaviņš, Rūta Ozola-Davidāne, and Linda Ansone-Bērtiņa. 2022. "The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review" Minerals 12, no. 3: 349. https://doi.org/10.3390/min12030349
APA StyleKrūmiņš, J., Kļaviņš, M., Ozola-Davidāne, R., & Ansone-Bērtiņa, L. (2022). The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review. Minerals, 12(3), 349. https://doi.org/10.3390/min12030349









