A Hybrid Experimental and Theoretical Approach to Optimize Recovery of Rare Earth Elements from Acid Mine Drainage Precipitates by Oxalic Acid Precipitation
Abstract
:1. Introduction
2. Materials and Methods
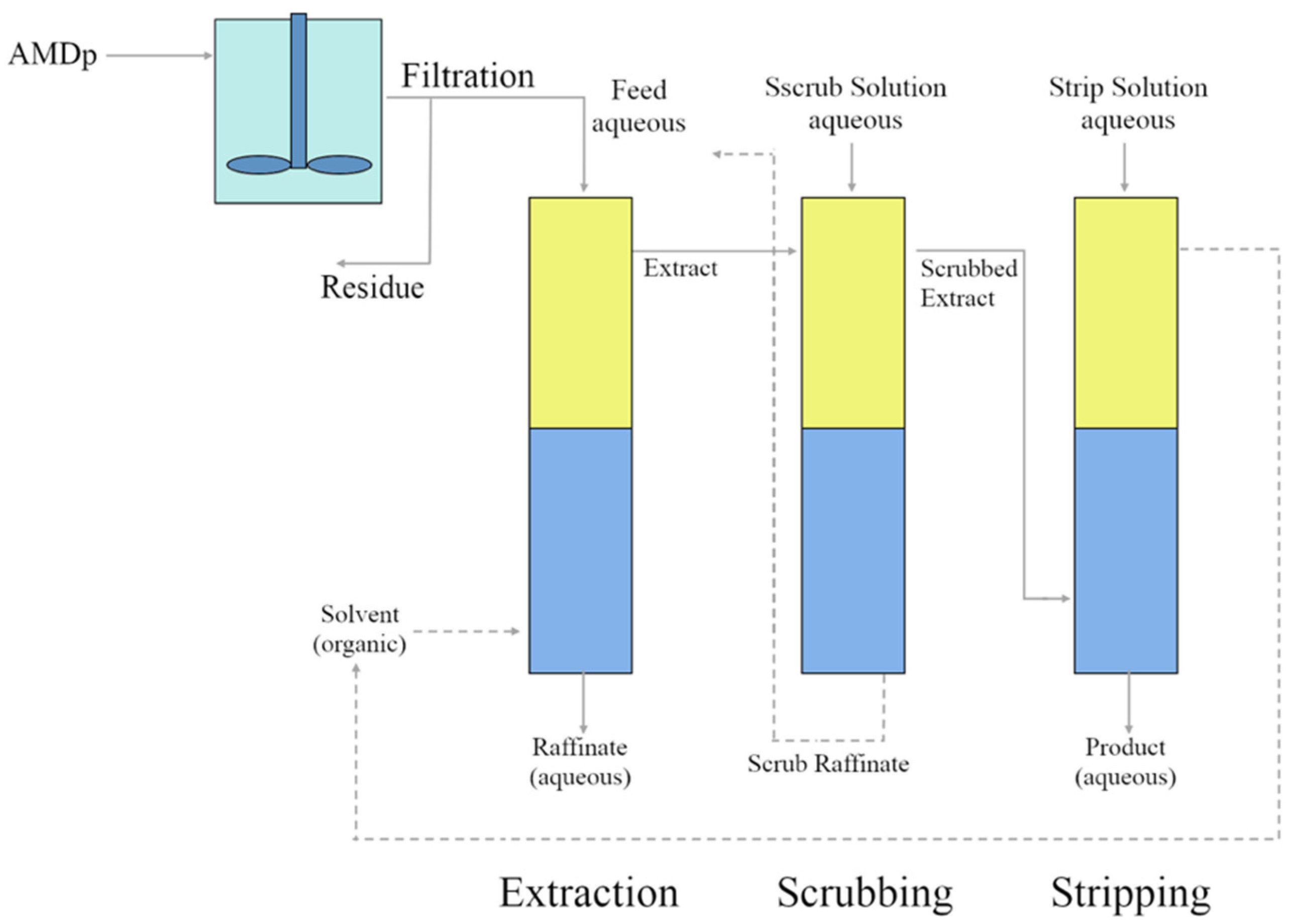
2.1. AMDp Sample Location, Acquisition
2.2. Sample Preparation
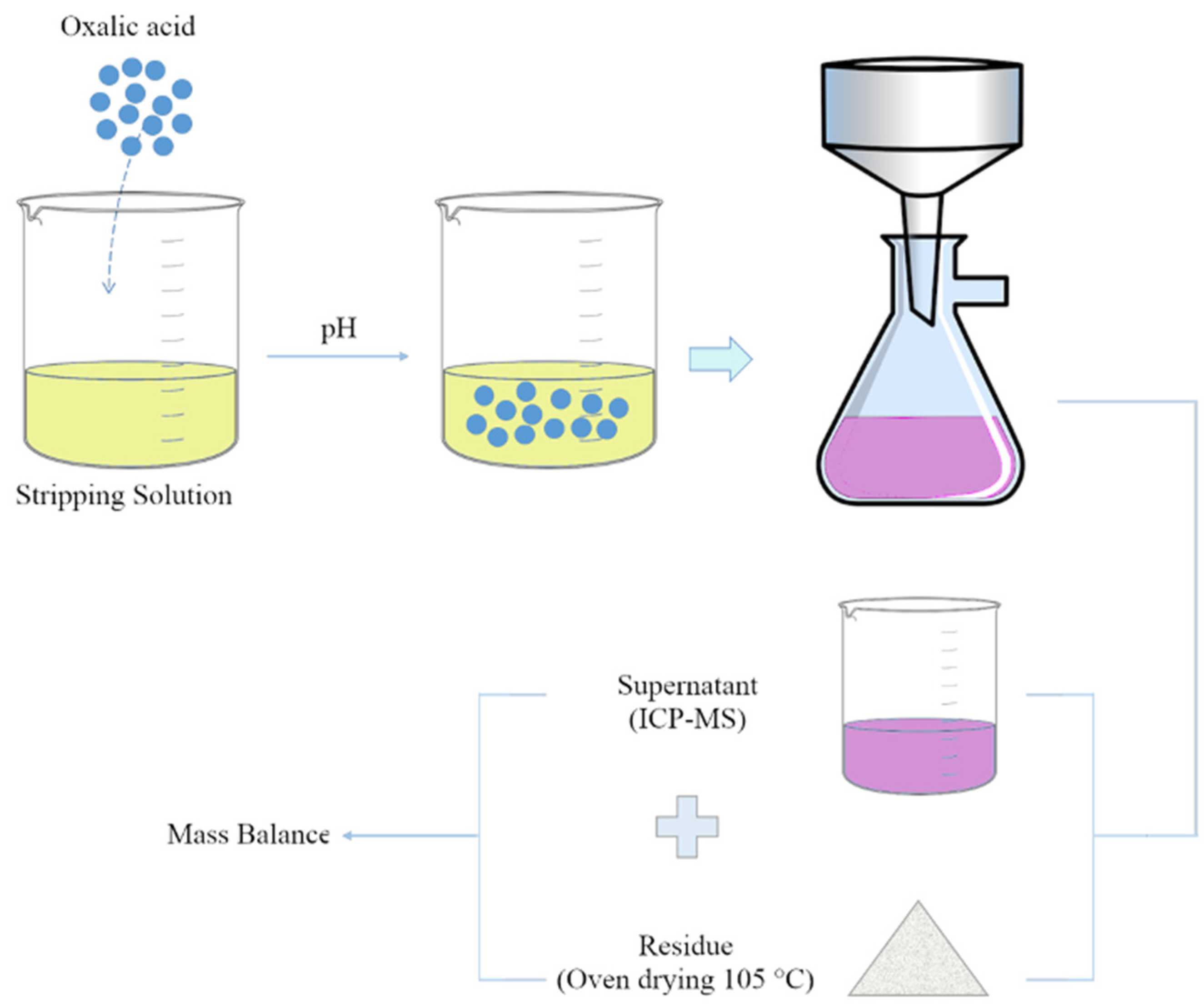
2.3. Oxalic Acid Precipitation Tests
2.4. Analytical Procedures
2.5. Solution Equilibrium Calculations Modeling
2.5.1. Oxalate Speciation
2.5.2. Oxalic Acid Consumption
3. Results and Discussion
3.1. Oxalic Acid Precipitation Tests
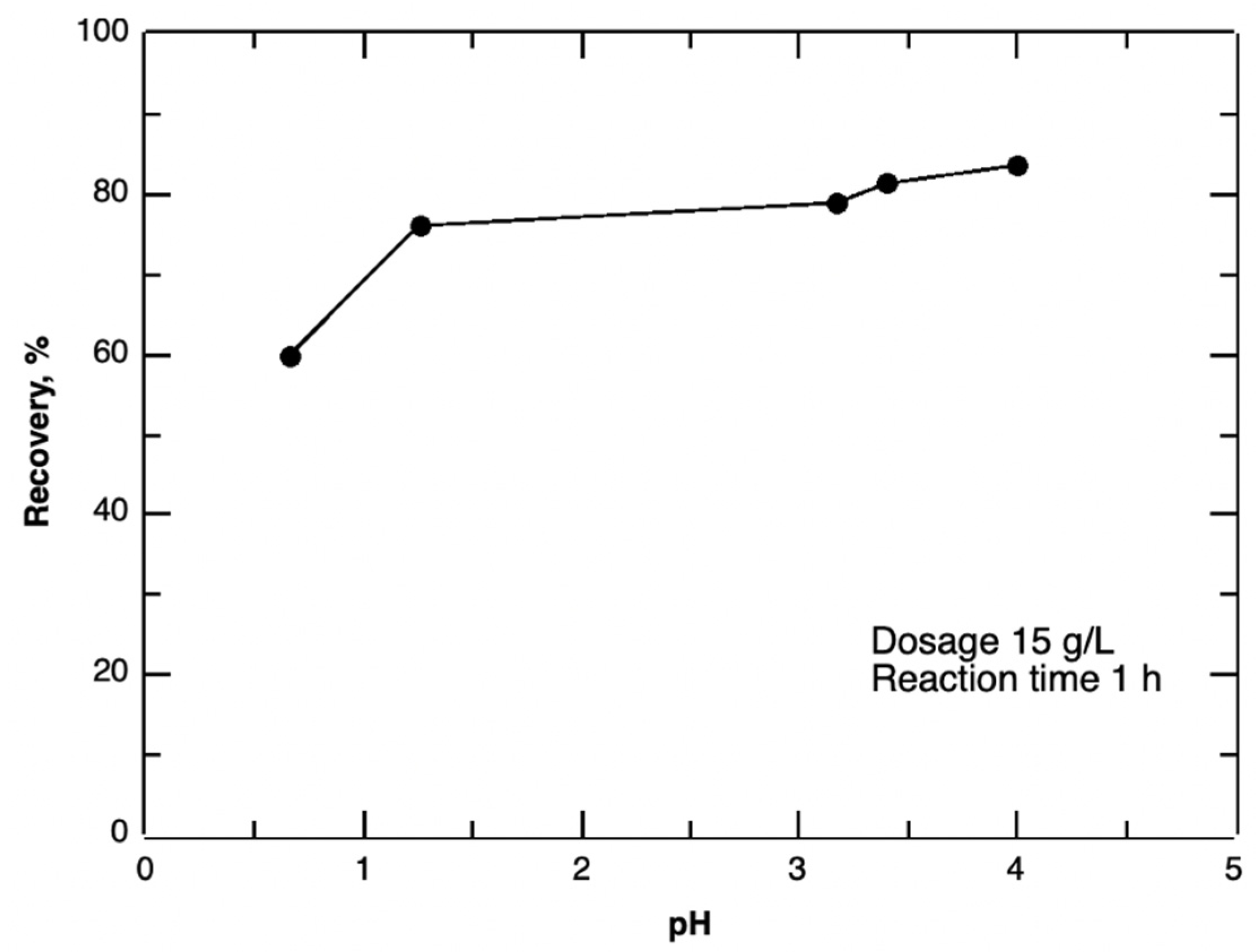
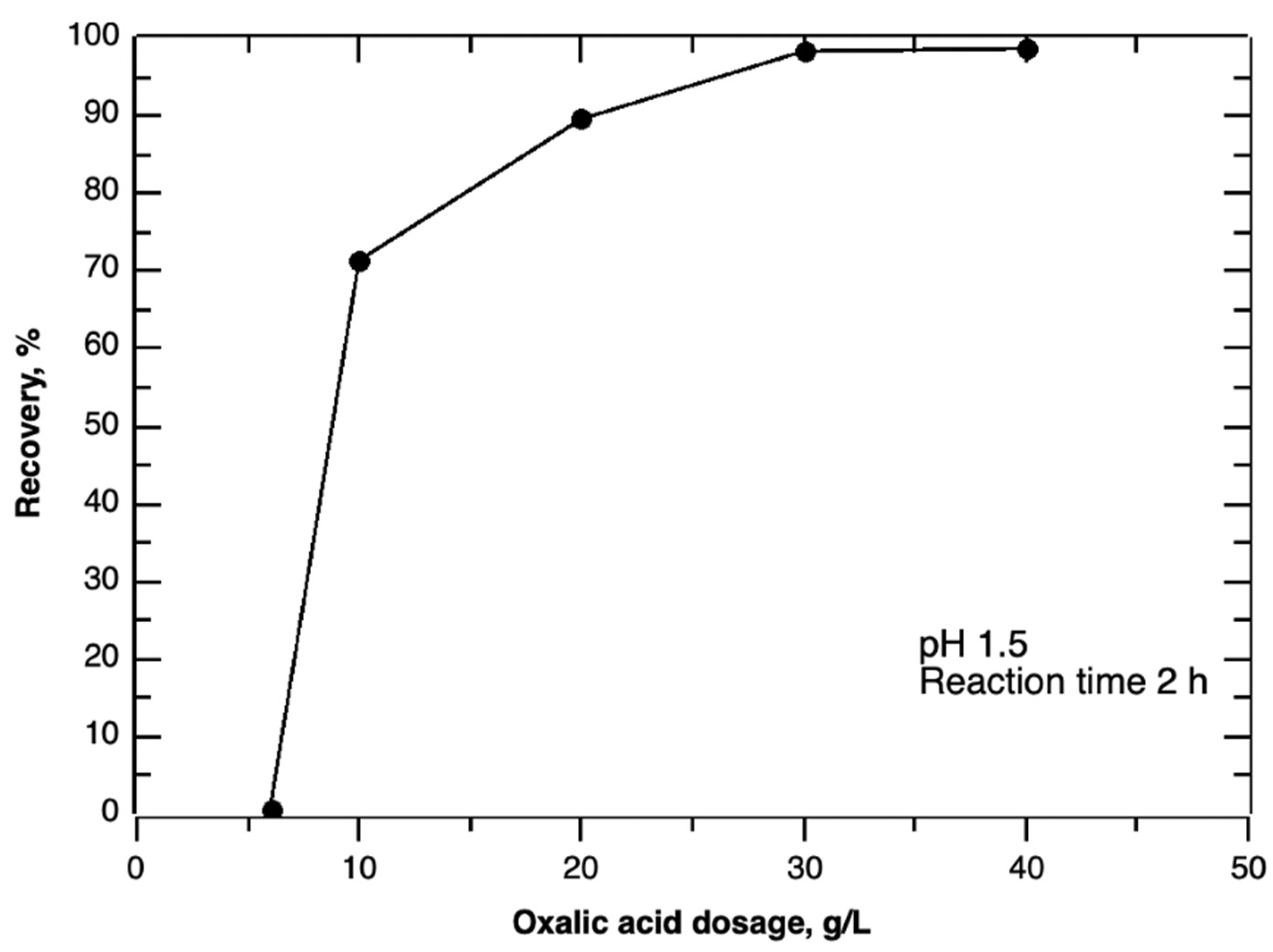
3.1.1. Effect of Reaction Time, pH and Oxalic Acid Dosage on Precipitation Efficiency
3.1.2. Effect of Reaction Time, pH and Oxalic Acid Dosage on REEs Recovery and Contaminants (Al, Ca, Fe, Mn, Si) Separation
3.2. Solution Equilibrium Calculations Modeling and Discussion
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chu, S. (Ed.) Critical Materials Strategy; DIANE Publishing: Collingdale, PA, USA, 2011. [Google Scholar]
- Humphries, M. Rare earth elements: The global supply chain; CRS Report for Congress R41347; Congressional Research Service: Washington, DC, USA, 2013.
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global produc-tion. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Bernhardt, D.; Reilly., J.F. Mineral Commodity Summaries 2020; US Geological Survey: Reston, VA, USA, 2020.
- Mancheri, N.A. World trade in rare earths, Chinese export restrictions, and implications. Resour. Policy 2015, 46, 262–271. [Google Scholar] [CrossRef]
- McLellan, B.; Yamasue, E.; Tezuka, T.; Corder, G.; Golev, A.; Giurco, D. Critical minerals and energy–impacts and limitations of moving to unconventional resources. Resources 2016, 5, 19. [Google Scholar] [CrossRef]
- Zhang, W.; Rezaee, M.; Bhagavatula, A.; Li, Y.; Groppo, J.; Honaker, R. A review of the occurrence and promising recovery methods of rare earth elements from coal and coal by-products. Int. J. Coal Prep. Util. 2015, 35, 295–330. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Vass, C.R.; Noble, A.; Ziemkiewicz, P.F. The occurrence and concentration of rare earth elements in acid mine drainage and treatment by-products: Part 1—Initial survey of the Northern Appalachian Coal Basin. Min. Eng. 2019, 71, 49–50. [Google Scholar] [CrossRef]
- Vass, C.R.; Noble, A.; Ziemkiewicz, P.F. The occurrence and concentration of rare earth elements in acid mine drainage and treatment by-products: Part 2: Regional survey of Northern and Central Appalachian Coal Basins. Min. Metall. Explor. 2019, 36, 917–929. [Google Scholar]
- Papirio, S.; Villa-Gomez, D.K.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Acid mine drainage treatment in fluidized-bed bioreactors by sulfate-reducing bacteria: A critical review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2545–2580. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid mine drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Groudev, S.N.; Georgiev, P.S.; Spasova, I.I.; Angelov, A.T.; Komnitsas, K. A pilot-scale passive system for the treatment of acid mine drainage. In Sustainable Solid Waste Management in The Southern Black Sea Region; Springer: Dordrecht, The Netherlands, 2000; pp. 189–194. [Google Scholar]
- Saito, T.; Sato, H.; Motegi, T. Recovery of rare earths from sludges containing rare-earth elements. J. Alloys Compd. 2006, 425, 145–147. [Google Scholar] [CrossRef]
- Kawasaki, A.; Kimura, R.; Arai, S. Rare earth elements and other trace elements in wastewater treatment sludges. Soil Sci. Plant Nutr. 1998, 44, 433–441. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kubo, K.; Katayama, Y.; Toshihiro, T.; Takaiku, Y. Extraction of rare earth elements as oxides from a Neodymium magnetic sludge. Metall. Mater. Trans. B 2012, 43, 468–476. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Fernández, F.J. Coal and sewage sludge ashes as sources of rare earth elements. Fuel 2017, 192, 128–139. [Google Scholar] [CrossRef]
- Stewart, B.W.; Capo, R.C.; Hedin, B.C.; Hedin, R.S. Rare earth element resources in coal mine drainage and treatment precipitates in the Appalachian basin, USA. Int. J. Coal Geol. 2017, 169, 28–39. [Google Scholar] [CrossRef]
- Rabatho, J.P.; Tongamp, W.; Takasaki, Y.; Haga, K.; Shibayama, A. recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. J. Mater. Cycles Waste Manag. 2013, 15, 171–178. [Google Scholar] [CrossRef]
- Ren, P. Recovery of rare earth elements (REEs) from coal mine drainage sludge leachate. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 2019. [Google Scholar]
- Smith, P.M. High-purity rare earth oxides produced via precipitation stripping. Metall. Mater. Trans. B 2007, 38, 763–768. [Google Scholar] [CrossRef]
- Kim, E.; Osseo-Asare, K. Aqueous stability of thorium and rare earth metals in monazite hydrometallurgy: Eh-pH diagrams for the systems Th-, Ce-, La-, Nd-(PO4)-(SO4)-H2O at 25 °C. Hydrometallurgy 2012, 113–114, 67–78. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R.Q. Rare earth elements recovery using staged precipitation from a leachate generated from coarse coal refuse. Int. J. Coal Geol. 2018, 195, 189–199. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Rezaee, M.; Pisupati, S.V. Precipitation of rare earth elements from acid mine drainage by CO2 mineralization process. Chem. Eng. J. 2020, 399, 125716. [Google Scholar] [CrossRef]
- Katalambula, H.; Gupta, R. Low-grade coals: A review of some prospective upgrading technologies. Energy Fuels 2009, 23, 3392–3405. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Characterization and recovery of rare earth elements and other critical metals (Co, Cr, Li, Mn, Sr, and V) from the calcination products of a coal refuse sample. Fuel 2020, 267, 117236. [Google Scholar] [CrossRef]
- Wang, Y.; Noble, A.; Vass, C.; Ziemkiewicz, P. Speciation of rare earth elements in acid mine drainage precipitates by sequential extraction. Miner. Eng. 2021, 168, 106827. [Google Scholar]
- Chi, R.; Xu, Z. A solution chemistry approach to the study of rare earth element precipitation by oxalic acid. Metall Mater Trans. B 1999, 30, 189–195. [Google Scholar] [CrossRef]
- US EPA. Method 3052: Microwave assisted acid digestion of siliceous and organically based matrices. In Test Methods for Evaluating Solid Waste; Office of Solid Waste and Emergency Response: Washington DC, USA, 1995. [Google Scholar]
- Christodoulou, E.; Panias, D.; Paspaliaris, I. Calculated solubility of trivalent iron and aluminum in oxalic acid solutions at 25 °C. Can. Metall. Q. 2001, 40, 421–432. [Google Scholar] [CrossRef]
- Bilinski, H.; Horvath, L.; Ingri, N.; Sjöberg, S. Equilibrium aluminium hydroxo-oxalate phases during initial clay formation; H+-Al3+-oxalic acid-Na+ System. Geochim. Cosmochim. Acta 1986, 50, 1911–1922. [Google Scholar] [CrossRef]


| REE | Stripping Solution (mg/L) |
|---|---|
| Y | 38.00 |
| La | 47.00 |
| Ce | 129.00 |
| Pr | 21.00 |
| Nd | 110.00 |
| Sm | 31.00 |
| Eu | 7.00 |
| Gd | 38.00 |
| Tb | 3.01 |
| Dy | 10.00 |
| Ho | 1.54 |
| Er | 2.84 |
| Tm | 0.24 |
| Yb | 0.84 |
| Lu | 0.10 |
| TREE | 440.00 |
| Parameter | Values | Constants |
|---|---|---|
| Reaction time | 10, 20, 40, 80, 120, 150 min | Dosage 15 g/L; pH 1.5 |
| pH | 0.5, 1, 1.5, 2, 3, 4 | Dosage 15 g/L; Reaction time 1 h. |
| Oxalic acid dosage | 6, 10, 20, 30, 40 g/L | pH 1.5; Reaction time 2 h. |
| Equation | Equilibrium Equation | Constants/Description |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | Total concentration of oxalic acid containing species | |
| 4 | to the total concentration | |
| 5 | to the total concentration | |
| 6 | to the total concentration | |
| 7 |
| Equation | Equilibrium Equation | Notes |
|---|---|---|
| 8 | Stoichiometric consumption by REEs —Total REEs concentration; —Remaining REEs concentration | |
| 9 | Excess consumption for complete precipitation by REEs | |
| 10 | Stoichiometric consumption; [M] represent the divalent and trivalent metal ions | |
| 11 | Excess consumption for the metal ion of [M] | |
| 12 | Total consumption for [M] | |
| 13 | Total consumption | |
| 14 | Oxalic acid efficiency | |
| 15 | Ksp = 2.32 × 10−9 | |
| 16 | β1 = 3.89 × 107 | |
| 17 | β2 = 5.01 × 1013 | |
| 18 | β3 = 3.55 × 1018 | |
| 19 | β1 = 5.37 × 107 | |
| 20 | β2 = 1.15 × 1013 | |
| 21 | β3 = 2.29 × 1016 | |
| 22 | β1 = 2.51 × 102 | |
| 23 | Ksp = 4.83 × 10−6 | |
| 24 | Ksp = 1.70 × 10−7 |
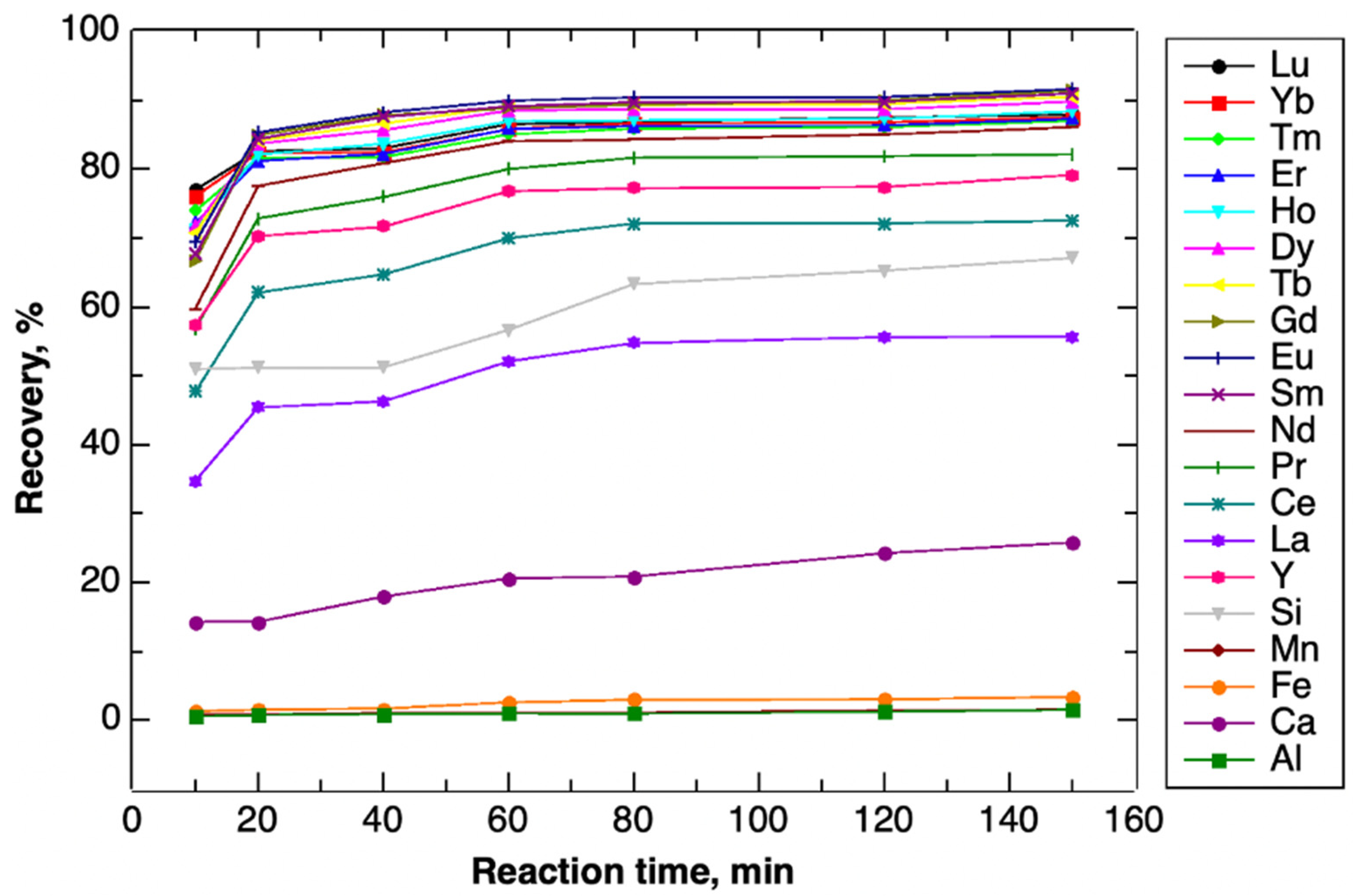
| Elemental Recovery, % | |||||||
|---|---|---|---|---|---|---|---|
| Reaction Time, min | 10 | 20 | 40 | 60 | 80 | 120 | 150 |
| Mg | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.91 | 1.09 |
| Al | 0.63 | 0.77 | 0.93 | 1.03 | 1.01 | 1.27 | 1.58 |
| Ca | 14.29 | 14.33 | 17.97 | 20.60 | 20.90 | 24.26 | 25.82 |
| Fe | 1.35 | 1.58 | 1.72 | 2.64 | 3.00 | 3.08 | 3.39 |
| Mn | 0.79 | 0.86 | 1.09 | 1.13 | 1.15 | 1.45 | 1.58 |
| Si | 50.96 | 51.19 | 51.22 | 56.66 | 63.32 | 65.27 | 67.09 |
| Y | 57.46 | 70.26 | 71.62 | 76.80 | 77.21 | 77.39 | 79.14 |
| La | 34.78 | 45.40 | 46.29 | 52.09 | 54.76 | 55.61 | 55.72 |
| Ce | 47.72 | 62.07 | 64.70 | 69.95 | 72.11 | 72.11 | 72.51 |
| Pr | 56.88 | 72.84 | 75.91 | 79.99 | 81.65 | 81.87 | 82.16 |
| Nd | 59.75 | 77.55 | 80.83 | 84.02 | 84.29 | 85.01 | 86.06 |
| Sm | 67.62 | 84.35 | 87.55 | 89.10 | 89.68 | 89.76 | 90.95 |
| Eu | 69.35 | 85.31 | 88.21 | 89.89 | 90.41 | 90.44 | 91.60 |
| Gd | 66.68 | 84.90 | 87.80 | 88.81 | 89.31 | 89.99 | 91.36 |
| Tb | 70.73 | 84.13 | 86.60 | 89.11 | 89.30 | 89.47 | 90.48 |
| Dy | 71.75 | 83.68 | 85.63 | 88.51 | 88.67 | 88.66 | 89.77 |
| Ho | 71.17 | 82.09 | 83.67 | 86.93 | 87.07 | 87.27 | 88.38 |
| Er | 72.22 | 81.17 | 82.21 | 85.80 | 86.24 | 86.33 | 87.26 |
| Tm | 73.94 | 81.59 | 81.74 | 85.03 | 85.87 | 86.08 | 87.00 |
| Yb | 76.07 | 82.33 | 82.42 | 85.78 | 86.66 | 86.76 | 87.50 |
| Lu | 76.99 | 82.61 | 83.00 | 86.51 | 86.83 | 87.48 | 87.89 |
| Elemental Recovery, % | |||||||
|---|---|---|---|---|---|---|---|
| pH | 0.6 | 1.28 | 2.05 | 3.17 | 4 | 5 | 6.1 |
| Mg | 0.00 | 0.00 | 0.00 | 0.00 | 16.67 | 22.16 | 53.70 |
| Al | 0.78 | 0.90 | 1.17 | 2.49 | 39.48 | 86.07 | 97.82 |
| Ca | 8.06 | 20.50 | 29.06 | 40.51 | 47.08 | 78.69 | 99.03 |
| Fe | 1.82 | 2.44 | 3.04 | 7.59 | 54.31 | 78.08 | 94.83 |
| Mn | 0.83 | 1.05 | 1.41 | 2.87 | 6.63 | 36.35 | 61.15 |
| Si | 29.68 | 31.61 | 52.37 | 73.70 | 85.92 | 96.40 | 96.14 |
| Y | 64.40 | 74.46 | 73.78 | 81.03 | 82.33 | 96.93 | 98.51 |
| La | 40.58 | 51.51 | 53.84 | 59.06 | 69.23 | 96.40 | 99.51 |
| Ce | 57.70 | 68.07 | 69.48 | 73.66 | 79.75 | 97.80 | 99.55 |
| Pr | 69.41 | 78.00 | 78.99 | 81.47 | 85.51 | 98.46 | 99.54 |
| Nd | 74.79 | 81.55 | 82.05 | 84.75 | 88.43 | 98.80 | 99.55 |
| Sm | 75.59 | 87.35 | 87.99 | 89.67 | 92.67 | 99.21 | 99.51 |
| Eu | 75.39 | 88.77 | 88.39 | 90.40 | 93.11 | 98.44 | 99.44 |
| Gd | 76.29 | 88.01 | 86.85 | 89.73 | 91.99 | 99.07 | 99.37 |
| Tb | 78.23 | 87.66 | 87.97 | 89.87 | 92.56 | 97.96 | 99.12 |
| Dy | 74.23 | 87.16 | 87.61 | 89.73 | 91.88 | 97.54 | 98.90 |
| Ho | 78.19 | 85.72 | 86.31 | 88.86 | 90.53 | 96.98 | 98.69 |
| Er | 76.66 | 84.91 | 85.80 | 88.44 | 89.63 | 96.52 | 98.54 |
| Tm | 76.65 | 85.19 | 85.63 | 88.84 | 89.60 | 96.39 | 98.47 |
| Yb | 77.48 | 85.93 | 86.71 | 89.42 | 90.04 | 96.47 | 98.42 |
| Lu | 78.34 | 86.43 | 87.24 | 89.67 | 89.91 | 96.53 | 98.51 |
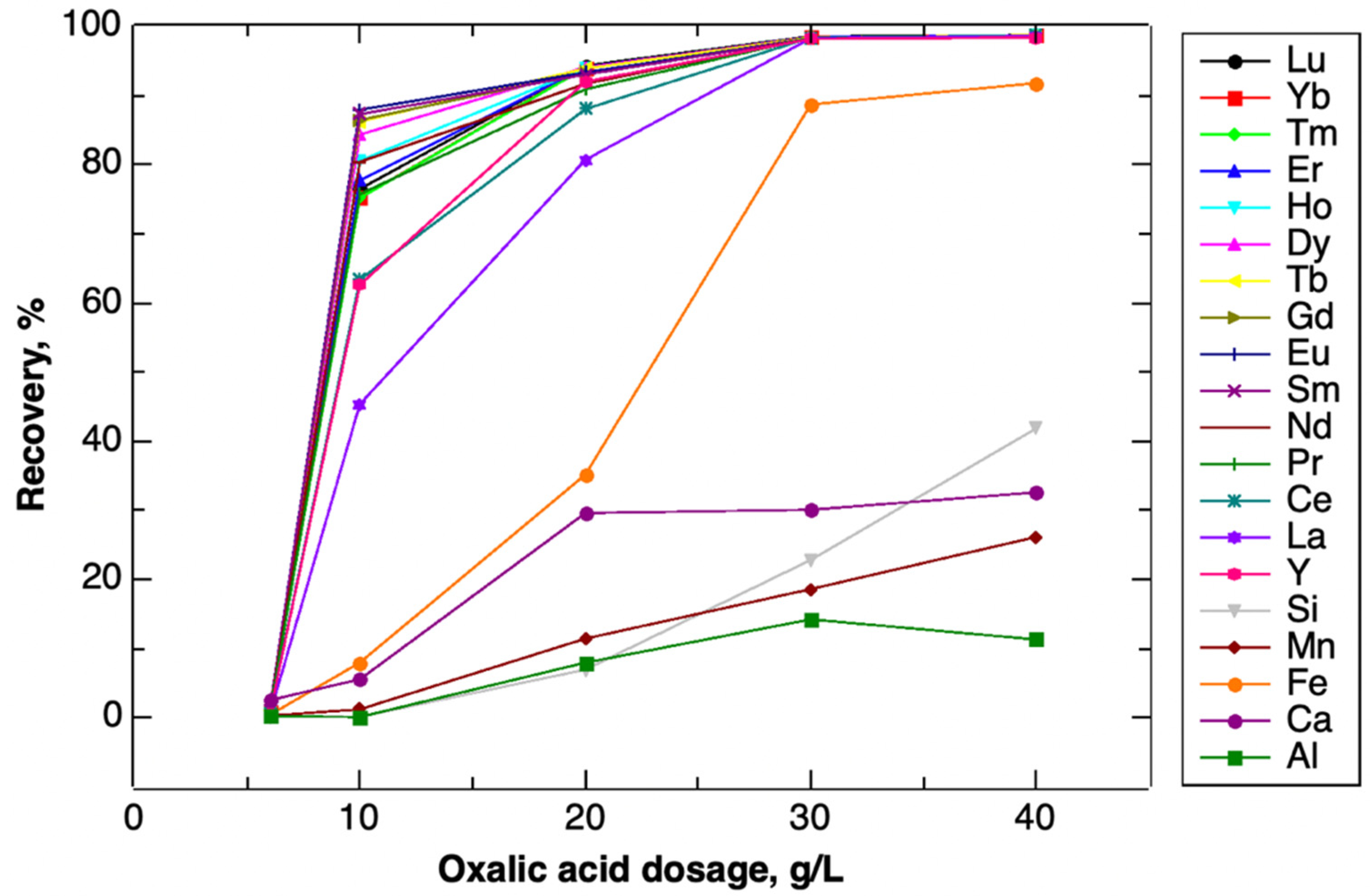
| Elemental Recovery, % | |||||
|---|---|---|---|---|---|
| Dosage, g/L | 6 | 10 | 20 | 30 | 40 |
| Mg | ND | ND | ND | ND | ND |
| Al | 0.22 | 0.13 | 7.99 | 14.24 | 11.33 |
| Ca | 0.42 | 8.00 | 35.16 | 88.69 | 91.78 |
| Fe | 0.32 | 1.24 | 11.46 | 18.62 | 26.16 |
| Mn | 0.20 | 0.16 | 6.98 | 22.84 | 41.85 |
| Si | 2.56 | 5.58 | 29.62 | 30.09 | 32.61 |
| Y | 0.53 | 62.62 | 91.98 | 98.16 | 98.36 |
| La | 0.47 | 45.37 | 80.69 | 98.25 | 98.61 |
| Ce | 0.63 | 63.37 | 88.02 | 98.36 | 98.69 |
| Pr | 0.82 | 75.73 | 90.88 | 98.35 | 98.69 |
| Nd | 0.90 | 80.39 | 91.67 | 98.30 | 98.67 |
| Sm | 1.15 | 87.28 | 93.00 | 98.25 | 98.66 |
| Eu | 1.19 | 87.91 | 93.32 | 98.25 | 98.67 |
| Gd | 1.04 | 86.44 | 93.27 | 98.28 | 98.69 |
| Tb | 1.12 | 86.21 | 93.87 | 98.35 | 98.73 |
| Dy | 1.07 | 84.33 | 94.05 | 98.38 | 98.62 |
| Ho | 0.96 | 80.56 | 93.94 | 98.36 | 98.70 |
| Er | 0.91 | 77.70 | 94.07 | 98.41 | 98.70 |
| Tm | 0.88 | 75.16 | 93.99 | 98.41 | 98.69 |
| Yb | 0.92 | 75.20 | 94.06 | 98.44 | 98.68 |
| Lu | 1.01 | 76.47 | 94.30 | 98.53 | 98.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ziemkiewicz, P.; Noble, A. A Hybrid Experimental and Theoretical Approach to Optimize Recovery of Rare Earth Elements from Acid Mine Drainage Precipitates by Oxalic Acid Precipitation. Minerals 2022, 12, 236. https://doi.org/10.3390/min12020236
Wang Y, Ziemkiewicz P, Noble A. A Hybrid Experimental and Theoretical Approach to Optimize Recovery of Rare Earth Elements from Acid Mine Drainage Precipitates by Oxalic Acid Precipitation. Minerals. 2022; 12(2):236. https://doi.org/10.3390/min12020236
Chicago/Turabian StyleWang, Yan, Paul Ziemkiewicz, and Aaron Noble. 2022. "A Hybrid Experimental and Theoretical Approach to Optimize Recovery of Rare Earth Elements from Acid Mine Drainage Precipitates by Oxalic Acid Precipitation" Minerals 12, no. 2: 236. https://doi.org/10.3390/min12020236
APA StyleWang, Y., Ziemkiewicz, P., & Noble, A. (2022). A Hybrid Experimental and Theoretical Approach to Optimize Recovery of Rare Earth Elements from Acid Mine Drainage Precipitates by Oxalic Acid Precipitation. Minerals, 12(2), 236. https://doi.org/10.3390/min12020236







