Reduction of Chlorinated Ethenes by Ag- and Cu-Amended Green Rust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Setup
3. Results and Discussion
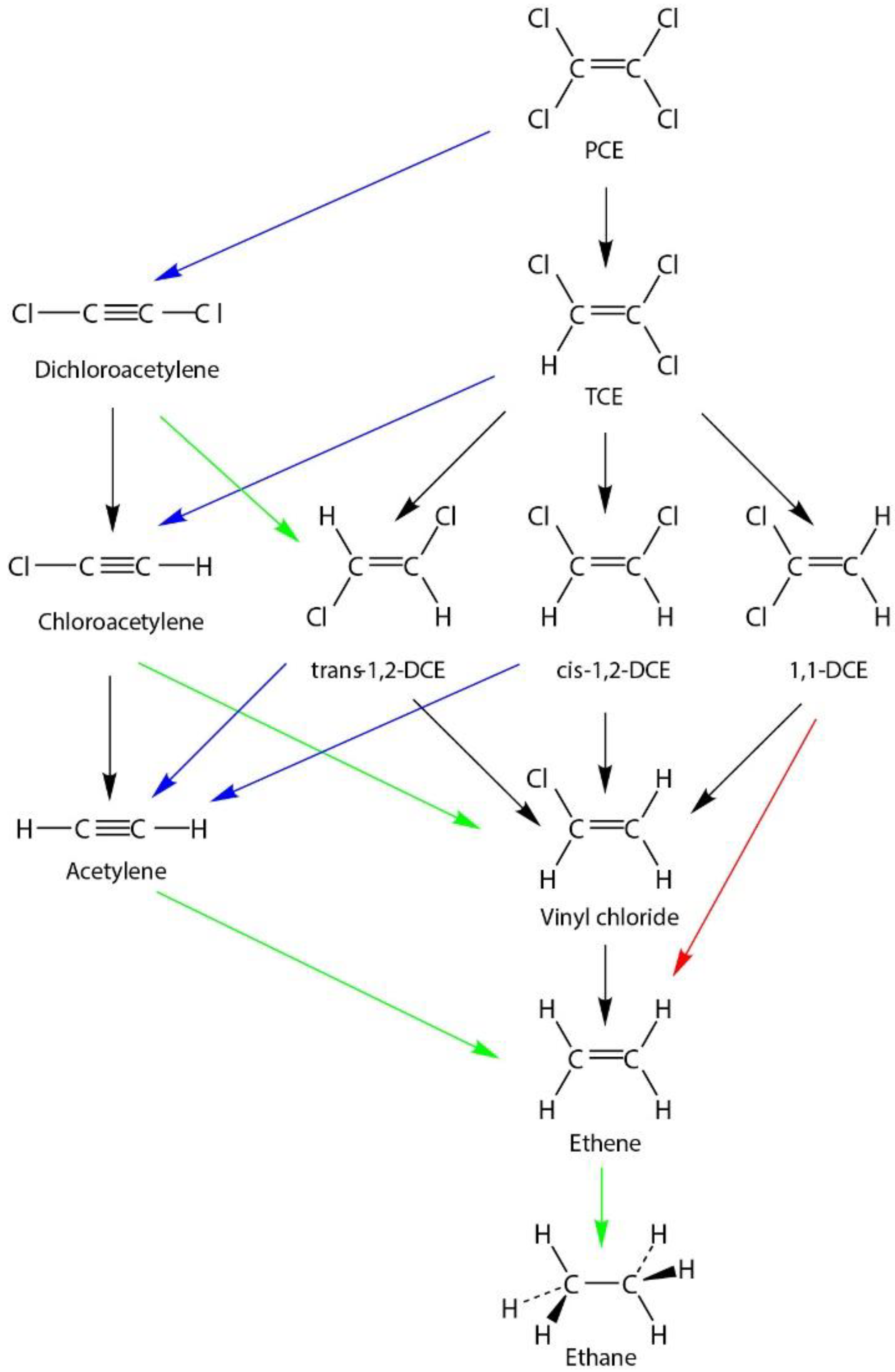
3.1. Reduction of Chlorinated Ethenes by GRSO4
3.2. Reduction of Chlorinated Ethenes by Ag-Amended GRSO4
3.3. Reduction of Chlorinated Ethenes by Cu-Amended GRSO4
3.4. Comparrison with Other Studies of Chlorinated Ethenes by Metal-Amended Green Rust
3.5. Potential Utility of Metal-Amended Green Rusts for Remediation of Chlorinated Ethenes
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usman, M.; Byrne, J.M.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S.-M. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef] [Green Version]
- Hansel, C.M.; Benner, S.G.; Neiss, J.; Dohnalkova, A.; Kukkadapu, R.K.; Fendorf, S. Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochim. Cosmochim. Acta 2003, 67, 2977–2992. [Google Scholar] [CrossRef] [Green Version]
- Borch, T.; Masue, Y.; Kukkadapu, R.K.; Fendorf, S. Phosphate imposed limitations on biological reduction and alteration of ferrihydrite. Environ. Sci. Technol. 2007, 41, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Zhou, J.; Schroder, C.; Obst, M.; Kappler, A.; Borch, T. Dissimilatory reduction and transformation of ferrihydrite-humic acid coprecipitates. Environ. Sci. Technol. 2013, 47, 13375–13384. [Google Scholar] [CrossRef] [PubMed]
- Ona-Nguema, G.; Abdelmoula, M.; Jorand, F.; Benali, O.; Géhin, A.; Block, J.-C.; Génin, J.-M.R. Iron (II,III) hydroxycarbonate green rust formation and stabilization from lepidocrocite bioreduction. Environ. Sci. Technol. 2002, 36, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Jorand, F.; Zegeye, A.; Landry, F.; Ruby, C. Reduction of ferric green rust by Shewanella putrefaciens. Lett. Appl. Microbiol. 2007, 45, 515–521. [Google Scholar] [CrossRef]
- Jung, J.; Bae, S.; Lee, W. Indirect contact of bio-transformation of lepidocrocite: Role of electron transfer mediator. Sustain. Environ. Res. 2012, 23, 193–198. [Google Scholar]
- Boyanov, M.I.; O’Loughlin, E.J.; Kemner, K.M. Iron phase transformations resulting from the respiration of Shewanella putrefaciens on a mixed mineral phase. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2009; Volume 190, p. 012193. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Boyanov, M.I.; Flynn, T.M.; Gorski, C.; Hofmann, S.M.; McCormick, M.L.; Scherer, M.M.; Kemner, K.M. Effects of bound phosphate on the bioreduction of lepidocrocite (g-FeOOH) and maghemite (g-Fe2O3) and formation of secondary minerals. Environ. Sci. Technol. 2013, 47, 9157–9166. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Flynn, T.M.; O’Loughlin, E.J.; Kemner, K.M.; George, S.; Fouke, K.E.; Li, S.; Huang, D.; et al. Controls on iron reduction and biomineralization over broad environmental conditions as suggested by the Firmicutes Orenia metallireducens strain Z6. Environ. Sci. Technol. 2020, 54, 10128–10140. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Boyanov, M.I.; Gorski, C.A.; Scherer, M.M.; Kemner, K.M. Effects of Fe(III) oxide mineralogy and phosphate on Fe(II) secondary mineral formation during microbial iron reduction. Minerals 2021, 11, 149. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lack, J.G.; Coates, J.D. Biogenic magnetite formation through anaerobic biooxidation of Fe(II). Appl. Environ. Microbiol. 2001, 67, 2844–2848. [Google Scholar] [CrossRef] [Green Version]
- Pantke, C.; Obst, M.; Benzerara, K.; Morin, G.; Ona-Nguema, G.; Dippon, U.; Kappler, A. Green rust formation during Fe(II) oxidation by the nitrate-reducing Acidovorax sp. strain BoFeN1. Environ. Sci. Technol. 2012, 46, 1439–1446. [Google Scholar] [CrossRef]
- Etique, M.; Jorand, F.P.; Zegeye, A.; Gregoire, B.; Despas, C.; Ruby, C. Abiotic process for Fe(II) oxidation and green rust mineralization driven by a heterotrophic nitrate reducing bacteria (Klebsiella mobilis). Environ. Sci. Technol. 2014, 48, 3742–3751. [Google Scholar] [CrossRef]
- Nordhoff, M.; Tominski, C.; Halama, M.; Byrne, J.M.; Obst, M.; Kleindienst, S.; Behrens, S.; Kappler, A. Insights into nitrate-reducing Fe(II) oxidation mechanisms through analysis of cell-mineral associations, cell encrustation, and mineralogy in the chemolithoautotrophic enrichment culture KS. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Bigham, J.M.; Tuovinen, O.H. Mineralogical, morphological, and microbiological characteristics of tubercles in cast iron water mains as related to their chemical activity. In Planetary Ecology; Caldwell, D.E., Brierley, J.A., Brierley, C.L., Eds.; Van Nostrand Reinhold Co.: New York, NY, USA, 1985; pp. 239–250. [Google Scholar]
- Génin, J.-M.R.; Refait, P.; Olowe, A.A.; Abdelmoula, M.; Fall, I.; Drissi, S.H. Identification of green rust compounds in the aqueous corrosion processes of steels; the case of microbially induced corrosion and use of 78 K CEMS. Hyperfine Interact. 1998, 112, 47–50. [Google Scholar] [CrossRef]
- Kumar, A.V.R.; Singh, R.; Nigam, R.K. Mössbauer spectroscopy of corrosion products of mild steel due to microbiologically influenced corrosion. J. Radioanal. Nucl. Chem. 1999, 242, 131–137. [Google Scholar] [CrossRef]
- Refait, P.; Abdelmoula, M.; Génin, J.-M.R. Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions. Corros. Sci. 1998, 40, 1547–1560. [Google Scholar] [CrossRef]
- Zegeye, A.; Bonneville, S.; Benning, L.G.; Sturm, A.; Fowle, D.A.; Jones, C.; Canfield, D.E.; Ruby, C.; MacLean, L.C.; Nomosatryo, S.; et al. Green rust formation controls nutrient availability in a ferruginous water column. Geology 2012, 40, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, B.C.; Balic-Zunic, T.; Dideriksen, K.; Stipp, S.L.S. Identification of green rust in groundwater. Environ. Sci. Technol. 2009, 43, 3436–3441. [Google Scholar] [CrossRef]
- Johnson, C.A.; Freyer, G.; Fabisch, M.; Caraballo, M.A.; Küsel, K.; Hochella, M.F. Observations and assessment of iron oxide and green rust nanoparticles in metal-polluted mine drainage within a steep redox gradient. Environ. Chem. 2014, 11, 377. [Google Scholar] [CrossRef]
- Feder, F.; Trolard, F.; Klingelhöfer, G.; Bourrié, G. In situ Mössbauer spectroscopy: Evidence for green rust (fougerite) in a gleysol and its mineralogical transformations with time and depth. Geochim. Cosmochim. Acta 2005, 69, 4463–4483. [Google Scholar] [CrossRef]
- Génin, J.-M.R.; Bourrié, G.; Trolard, F.; Abdelmoula, M.; Jaffrezic, A.; Refait, P.; Maitre, V.; Humbert, B.; Herbillon, A. Thermodynamic equilibria in aqueous suspensions of synthetic and natural Fe(II)-Fe(III) green rusts: Occurrences of the mineral in hydromorphic soils. Environ. Sci. Technol. 1998, 32, 1058–1068. [Google Scholar] [CrossRef]
- Refait, P.; Abdelmoula, M.; Trolard, F.; Génin, J.-M.R.; Ehrhardt, J.J.; Bourrié, G. Mössbauer and XAS study of a green rust mineral: The partial substitution of Fe2+ by Mg2+. Am. Mineral. 2001, 86, 731–739. [Google Scholar] [CrossRef]
- Trolard, F.; Génin, J.-M.R.; Abdelmoula, M.; Bourrié, G.; Humbert, B.; Herbillon, A. Identification of a green rust mineral in a reductomorphic soil by Mössbauer and Raman spectroscopies. Geochim. Cosmochim. Acta 1997, 61, 1107–1111. [Google Scholar] [CrossRef]
- Weatherington-Rice, J.; Bigham, J.M. Buried pre-Illinoian-age lacustrine deposits with “green rust” colors in Clermont County, Ohio. Ohio J. Sci. 2006, 106, 35–44. [Google Scholar]
- Latta, D.E.; Boyanov, M.I.; Kemner, K.M.; O’Loughlin, E.J.; Scherer, M.M. Abiotic reduction of uranium by Fe(II) in soil. Appl. Geochem. 2012, 27, 1512–1524. [Google Scholar] [CrossRef]
- Bearcock, J.M.; Perkins, W.T.; Dinelli, E.; Wade, S.C. Fe(II)/Fe(III) ‘green rust’ developed within ocherous coal mine drainage sediment in South Wales, UK. Mineral. Mag. 2006, 70, 731–741. [Google Scholar] [CrossRef]
- Bender Koch, C.; Mørup, S. Identification of green rust in an ochre sludge. Clay Miner. 1991, 26, 577–582. [Google Scholar] [CrossRef]
- Root, R.A.; Dixit, S.; Campbell, K.M.; Jew, A.D.; Hering, J.G.; O’Day, P.A. Arsenic sequestration by sorption processes in high-iron sediments. Geochim. Cosmochim. Acta 2007, 71, 5782–5803. [Google Scholar] [CrossRef]
- Gu, B.; Phelps, T.J.; Liang, L.; Dickey, M.J.; Roh, Y.; Kinsall, B.L.; Palumbo, A.V.; Jacobs, G.K. Biogeochemical dynamics in zero-valent iron columns: Implications for permeable reactive barriers. Environ. Sci. Technol. 1999, 33, 2170–2177. [Google Scholar] [CrossRef]
- Johnson, T.L.; Tratnyek, P.G. A column study of the geochemical factors affecting reductive dechlorination of chlorinated solvents by zero-valent iron. In Proceedings of the In-Situ Remediation: Scientific Basis for Current and Future Technologies. Thirty-Third Hanford Symposium on Health and the Environment, Pasco, WA, USA, 7–11 November 1994; Gee, G.W., Wing, N.R., Eds.; Battelle Press: Columbus, OH, USA, 1994; Volume 2, pp. 931–947. [Google Scholar]
- Roh, Y.; Lee, S.Y.; Elless, M.P. Characterization of corrosion products in the permeable reactive barriers. Environ. Geol. 2000, 40, 184–194. [Google Scholar] [CrossRef]
- Vogan, J.L.; Butler, B.J.; Odziemkowski, M.S.; Friday, G.; Gillham, R.W. Inorganic and biological evaluation of cores from permeable iron reactive barriers. In Designing and Applying Treatment Technolgies: Remediation of Chlorinated and Recalcitrant Compounds; Wickramanayake, G.B., Hinchee, R.E., Eds.; Battelle Press: Columbus, OH, USA, 1998; pp. 163–168. [Google Scholar]
- Furukawa, Y.; Kim, J.-W.; Watkins, J.; Wilkin, R.T. Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ. Sci. Technol. 2002, 36, 5469–5475. [Google Scholar] [CrossRef]
- Phillips, D.H.; Watson, D.B.; Roh, Y.; Gu, B. Mineralogical characteristics and transformation during long-term operation of a zerovalent iron reactive barrier. J. Environ. Qual. 2003, 32, 2033–2045. [Google Scholar] [CrossRef]
- Trolard, F.; Bourrié, G.; Abdelmoula, M.; Refait, P.; Feder, F. Fougerite, a new mineral of the pyroaurite-iowaite group: Description and crystal structure. Clays Clay Miner. 2007, 55, 323–334. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Génin, J.M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef] [Green Version]
- Génin, J.M.R.; Mills, S.J.; Christy, A.G.; Guérin, O.; Herbillon, A.J.; Kuzmann, E.; Ona-Nguema, G.; Ruby, C.; Upadhyay, C. Mössbauerite, Fe63+O4(OH)8[CO3]·3H2O, the fully oxidized ‘green rust’ mineral from Mont Saint-Michel Bay, France. Mineral. Mag. 2014, 78, 447–465. [Google Scholar] [CrossRef]
- Lee, W.; Batchelor, B. Reductive capacity of natural reductants. Environ. Sci. Technol. 2003, 37, 535–541. [Google Scholar] [CrossRef]
- Bond, D.L.; Fendorf, S. Kinetics and structural constraints of chromate reduction by green rusts. Environ. Sci. Technol. 2003, 37, 2750–2757. [Google Scholar] [CrossRef]
- Christiansen, B.C.; Geckeis, H.; Marquardt, C.M.; Bauer, A.; Römer, J.; Wiss, T.; Schild, D.; Stipp, S.L.S. Neptunyl (NpO2+) interaction with green rust, GRNa,SO4. Geochim. Cosmochim. Acta 2011, 75, 1216–1226. [Google Scholar] [CrossRef]
- Elsner, M.; Schwarzenbach, R.P.; Haderlein, S.B. Reactivity of Fe(II)-bearing minerals toward reductive transformation of organic contaminants. Environ. Sci. Technol. 2004, 38, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Erbs, M.; Hansen, H.C.B.; Olsen, C.E. Reductive dechlorination of carbon tetrachloride using iron(II) iron(III) hydroxide sulfate (green rust). Environ. Sci. Technol. 1999, 33, 307–311. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Bender Koch, C.; Nancke-Krogh, H.; Borggaard, O.K.; Sorensen, J. Abiotic nitrate reduction to ammonium: Key role of green rust. Environ. Sci. Technol. 1996, 30, 2053–2056. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Guldberg, S.; Erbs, M.; Bender Koch, C. Kinetics of nitrate reduction by green rusts—Effects of interlayer anion and Fe(II):Fe(III) ratio. Appl. Clay Sci. 2001, 18, 81–91. [Google Scholar] [CrossRef]
- Heasman, D.M.; Sherman, D.M.; Ragnarsdottir, K.V. The reduction of aqueous Au3+ by sulfide minerals and green rust phases. Am. Mineral. 2003, 88, 725–738. [Google Scholar] [CrossRef]
- Kone, T.; Hanna, K.; Abdelmoula, M.; Ruby, C.; Carteret, C. Reductive transformation and mineralization of an azo dye by hydroxysulphate green rust preceding oxidation using H2O2 at neutral pH. Chemosphere 2009, 75, 212–219. [Google Scholar] [CrossRef]
- Larese-Casanova, P.; Scherer, M.M. Abiotic transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by green rusts. Environ. Sci. Technol. 2008, 42, 3975–3981. [Google Scholar] [CrossRef]
- Latta, D.E.; Boyanov, M.I.; Kemner, K.M.; O’Loughlin, E.J.; Scherer, M.M. Reaction of uranium(VI) with green rusts: Effect of interlayer anion. Curr. Inorg. Chem. 2015, 5, 156–168. [Google Scholar] [CrossRef]
- Lee, W.; Batchelor, B. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 2. Green rust. Environ. Sci. Technol. 2002, 36, 5348–5354. [Google Scholar] [CrossRef]
- Legrand, L.; El Figuigui, A.; Mercier, F.; Chausse, A. Reduction of aqueous chromate by Fe(II)/Fe(III) carbonate green rust: Kinetic and mechanistic studies. Environ. Sci. Technol. 2004, 38, 4587–4595. [Google Scholar] [CrossRef]
- Loyaux-Lawniczak, S.; Refait, P.; Lecomte, P.; Ehrhardt, J.-J.; Génin, J.-M.R. The reduction of chromate ions by Fe(II) layered hydroxides. Hydrol. Earth Syst. Sci. 1999, 3, 593–599. [Google Scholar] [CrossRef]
- Myneni, S.C.B.; Tokunaga, T.K.; Brown, G.E., Jr. Abiotic selenium redox transformations in the presence of Fe(II,III) oxides. Science 1997, 278, 1106–1109. [Google Scholar] [CrossRef] [Green Version]
- O’Loughlin, E.J.; Burris, D.R. Reduction of halogenated ethanes by green rust. Environ. Toxicol. Chem. 2004, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.J.; Kelly, S.D.; Kemner, K.M.; Csencsits, R.; Cook, R.E. Reduction of AgI, AuIII, CuII, and HgII by FeII/FeIII hydroxysulfate green rust. Chemosphere 2003, 53, 437–446. [Google Scholar] [CrossRef]
- Pepper, S.E.; Bunker, D.J.; Bryan, N.D.; Livens, F.R.; Charnock, J.M.; Pattrick, R.A.D.; Collison, D. Treatment of radioactive wastes: An X-ray adsorption spectroscopy study of the treatment of technetium with green rust. J. Colloid Interface Sci. 2003, 268, 408–412. [Google Scholar] [CrossRef]
- Refait, P.; Simon, L.; Génin, J.-M.R. Reduction of SeO42− anions and anoxic formation of iron(II)-iron(III) hydroxy-selenate green rust. Environ. Sci. Technol. 2000, 34, 819–825. [Google Scholar] [CrossRef]
- Skovbjerg, L.L.; Stipp, S.L.S.; Utsunomiya, S.; Ewing, R.C. The mechanisms of reduction of hexavalent chromium by green rust sodium sulphate: Formation of Cr-goethite. Geochim. Cosmochim. Acta 2006, 70, 3582–3592. [Google Scholar] [CrossRef]
- Williams, A.G.B.; Scherer, M.M. Kinetics of Cr(VI) reduction by carbonate green rust. Environ. Sci. Technol. 2001, 35, 3488–3494. [Google Scholar] [CrossRef]
- Yan, S.; Boyanov, M.I.; Mishra, B.; Kemner, K.M.; O’Loughlin, E.J. U(VI) reduction by biogenic and abiotic hydroxycarbonate green rusts: Impacts on U(IV) speciation and stability over time. Environ. Sci. Technol. 2018, 52, 4601–4609. [Google Scholar] [CrossRef]
- Etique, M.; Zegeye, A.; Gregoire, B.; Carteret, C.; Ruby, C. Nitrate reduction by mixed iron(II-III) hydroxycarbonate green rust in the presence of phosphate anions: The key parameters influencing the ammonium selectivity. Water Res. 2014, 62, 29–39. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Boyanov, M.I.; Kemner, K.M. Reduction of vanadium(V) by iron(II)-bearing minerals. Minerals 2021, 11, 316. [Google Scholar] [CrossRef]
- Moran, M.J.; Zogorski, J.S.; Squillace, P.J. Chlorinated solvents in groundwater of the United States. Environ. Sci. Technol. 2007, 41, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.E. A History of the production and use of carbon tetrachloride, tetrachloroethylene, trichloroethylene and 1,1,1-trichloroethane in the United States: Part 1—Historical background; carbon tetrachloride and tetrachloroethylene. Environ. Forensics 2000, 1, 69–81. [Google Scholar] [CrossRef]
- Doherty, R.E. A History of the production and use of carbon tetrachloride, tetrachloroethylene, trichloroethylene and 1,1,1-trichloroethane in the United States: Part 2—Trichloroethylene and 1,1,1-trichloroethane. Environ. Forensics 2000, 1, 83–93. [Google Scholar] [CrossRef]
- Henschler, D. Toxicity of chlorinated organic compounds: Effects of the introduction of chlorine in organic molecules. Angew. Chem. Int. Ed. Engl. 1994, 33, 1920–1935. [Google Scholar] [CrossRef]
- Xiao, Z.; Jiang, W.; Chen, D.; Xu, Y. Bioremediation of typical chlorinated hydrocarbons by microbial reductive dechlorination and its key players: A review. Ecotoxicol. Environ. Saf. 2020, 202, 110925. [Google Scholar] [CrossRef]
- Ebrahimbabaie, P.; Pichtel, J. Biotechnology and nanotechnology for remediation of chlorinated volatile organic compounds: Current perspectives. Environ. Sci. Pollut. Res. Int. 2021, 28, 7710–7741. [Google Scholar] [CrossRef]
- Maithreepala, R.A.; Doong, R.-A. Enhanced dechlorination of chlorinated methanes and ethenes by chloride green rust in the presence of copper(II). Environ. Sci. Technol. 2005, 39, 4082–4090. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Kemner, K.M.; Burris, D.R. Effects of AgI, AuIII, and CuII on the reductive dechlorination of carbon tetrachloride by green rust. Environ. Sci. Technol. 2003, 37, 2905–2912. [Google Scholar] [CrossRef]
- Scherer, M.M.; O’Loughlin, E.; Parkin, G.F.; Valentine, R.; Al-Hosney, H.; Handler, R.; Just, C.; Larese-Casanova, P.; Pasakarnis, T.; Smith, S.L. Sustainability of Long-Term Abiotic Attenuation of Chlorinated Ethenes; SERDP Project ER-1369; Department of Defense Strategic Environmental Research and Development Program (SERDP): Alexandria, VA, USA, 2007; pp. 1–47. [Google Scholar]
- Choi, J.; Lee, W. Enhanced degradation of tetrachloroethylene by green rusts with platinum. Environ. Sci. Technol. 2008, 42, 3356–3362. [Google Scholar] [CrossRef]
- Liang, X.; Philp, R.P.; Butler, E.C. Kinetic and isotope analyses of tetrachloroethylene and trichloroethylene degradation by model Fe(II)-bearing minerals. Chemosphere 2009, 75, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Mangayayam, M.C.; Dideriksen, K.; Tobler, D.J. Can or cannot green rust reduce chlorinated ethenes? Energy Procedia 2018, 146, 173–178. [Google Scholar] [CrossRef]
- Ai, J.; Yin, W.; Hansen, H.C.B. Fast dechlorination of chlorinated ethylenes by green rust in the presence of bone char. Environ. Sci. Technol. Lett. 2019, 6, 191–196. [Google Scholar] [CrossRef]
- Choi, J.; Batchelor, B.; Chung, J. Reductive dechlorination of tetrachloroethylene by green rusts modified with copper. Water Air Soil Pollut. 2010, 212, 407–417. [Google Scholar] [CrossRef]
- Ayala-Luis, K.B.; Cooper, N.G.; Koch, C.B.; Hansen, H.C. Efficient dechlorination of carbon tetrachloride by hydrophobic green rust intercalated with dodecanoate anions. Environ. Sci. Technol. 2012, 46, 3390–3397. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Z.; Yin, Z.; Cooper, N.G.A.; Yin, W.; Bjerglund, E.T.; Strobel, B.W.; Hansen, H.C.B. Copper-mediated reductive dechlorination by green rust intercalated with dodecanoate. J. Hazard. Mater. 2018, 345, 18–26. [Google Scholar] [CrossRef]
- Huang, L.Z.; Hansen, H.C.; Daasbjerg, K. Graphene oxide-mediated rapid dechlorination of carbon tetrachloride by green rust. J. Hazard. Mater. 2017, 323, 690–697. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Kelly, S.D.; Csencsits, R.; Cook, R.E.; Kemner, K.M. Reduction of uranium(VI) by mixed iron(II)/iron(III) hydroxide (green rust): Formation of UO2 nanoparticles. Environ. Sci. Technol. 2003, 37, 721–727. [Google Scholar] [CrossRef]
- Campbell, T.J.; Burris, D.R. Analysis of chlorinated ethene reduction products in vapor/water phase systems by dual-column, single detector gas chromatography. Int. J. Environ. Anal. Chem. 1996, 63, 119–126. [Google Scholar] [CrossRef]
- Campbell, T.J.; Burris, D.R.; Roberts, A.L.; Wells, J.R. Trichloroethylene and tetrachloroethylene in a metallic iron-water vapor batch system. Environ. Toxicol. Chem. 1997, 16, 625–630. [Google Scholar] [CrossRef]
- Roberts, A.L.; Totten, L.A.; Arnold, W.A.; Burris, D.R.; Campbell, T.J. Reductive elimination of chlorinated ethylenes, by zero-valent metals. Environ. Sci. Technol. 1996, 30, 2654–2659. [Google Scholar] [CrossRef]
- Glod, G.; Brodmann, U.; Angst, W.; Holliger, C.; Schwarzenbach, R. Cobalamin-mediated reduction of cis- and trans-dichloroethene, 1,1-dichloroethene, and vinyl chloride in homogeneous aqueous solution: Reaction kinetics and mechanistic considerations. Environ. Sci. Technol. 1997, 31, 3154–3160. [Google Scholar] [CrossRef]
- Han, Y.S.; Hyun, S.P.; Jeong, H.Y.; Hayes, K.F. Kinetic study of cis-dichloroethylene (cis-DCE) and vinyl chloride (VC) dechlorination using green rusts formed under varying conditions. Water Res. 2012, 46, 6339–6350. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.A.; Roberts, A.L. Pathways of chlorinated ethylene and chlorinated acetylene reaction with Zn(0). Environ. Sci. Technol. 1998, 32, 3017–3025. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Ma, H.; Burris, D.R. Catalytic effects of Ni-humic complexes on the reductive dehalogenation of chlorinated C1 and C2 hydrocarbons. In Humic Substances: Nature’s Most Versatile Materials; Ghabbour, E.A., Davies, G., Eds.; Taylor and Francis, Inc.: New York, NY, USA, 2004; pp. 295–322. [Google Scholar]
- Bakac, A.; Espenson, J.H. Mechanistic investigation of carbon-carbon bond formation in the reduction of alkyl halides by organonickel complexes in aqueous solution. J. Am. Chem. Soc. 1986, 108, 719–723. [Google Scholar] [CrossRef]
- Arnold, W.A.; Roberts, A.L. Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environ. Sci. Technol. 2000, 34, 1794–1805. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Burris, D.R. Reductive dehalogenation of trichloroethene mediated by wetland DOC-transition metal complexes. In Wetlands and Remediation; Means, J.L., Hinchee, R.E., Eds.; Battelle Press: Columbus, OH, USA, 2000; pp. 1–8. [Google Scholar]
- Cheng, S.-F.; Wu, S.-C. The enhancement methods for the degradation of TCE by zero-valent metals. Chemosphere 2000, 41, 1263–1270. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.-X. Subcolloidal Fe/Ag particles for reductive dehalogenation of chlorinated benzenes. Ind. Eng. Chem. Res. 2000, 39, 2238–2244. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Wang, C.-B.; Lien, H.-L. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal. Today 1998, 40, 387–395. [Google Scholar] [CrossRef]
- Lien, H.-L.; Zhang, W.-X. Transformation of chlorinated methanes by nanoscale iron particles. J. Environ. Eng. 1999, 125, 1042–1047. [Google Scholar] [CrossRef]
- Muftikian, R.; Fernando, Q.; Korte, N. A method for the rapid dechlorination of low molecular weight chlorinated hydrocarbons in water. Water Res. 1995, 29, 2434–2439. [Google Scholar] [CrossRef]
- Wan, C.; Chen, Y.H.; Wei, R. Dechlorination of chloromethanes on iron an palladium-iron bimetallic surface in aqueous systems. Environ. Toxicol. Chem. 1999, 18, 1091–1096. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Elliott, D.W.; Zhang, W.-X. Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef]
- Liu, Z.; Arnold, R.G.; Betterton, E.A.; Festa, K.D. Electrolytic reduction of CCl4—Effects of cathode material and potential on kinetics, selectivity, and product stoichiometry. Environ. Eng. Sci. 1999, 16, 1–13. [Google Scholar] [CrossRef]

| Compound a | System | Final Sampling (h) | Compound Remaining | Products a | Carbon Recovery b |
|---|---|---|---|---|---|
| PCE | GR | 502 | 85.7% | ND | 85.7% |
| AgGR | 189 | 1.5% | TCE(87.4%), t12DCE(7.3%), AC(7.4%), and EE(0.5%) | 104.2% | |
| CuGR | 627 | 67.9% | TCE(6.3%), EE(3.3%), and EA(0.3%) | 77.7% | |
| TCE | GR | 501 | 97.4% | ND | 97.4% |
| AgGR | 501 | 87.6% | AC(1.8%) and EE(1.1%) | 89.9% | |
| CuGR | 625 | 79.9% | EE(11.1%) and EA(1.1%) | 92.1% | |
| 11DCE | GR | 499 | 95.7% | ND | 95.7% |
| AgGR | 501 | 93.8% | EE(0.3%) | 94.1% | |
| CuGR | 624 | 66.1% | EE(30.2%) and EA(2.8%) | 99.1% | |
| c12DCE | GR | 500 | 100.0% | ND | 100.0% |
| AgGR | 501 | 97.4% | EE(0.1%) | 97.5% | |
| CuGR | 625 | 83.1% | VC(1.0%), EE(14.9%), and EA(1.5%) | 100.5% | |
| t12DCE | GR | 504 | 95.2% | ND | 95.2% |
| AgGR | 499 | 94.2% | AC(0.8%), EE(0.3%), and EA(0.1%) | 95.3% | |
| CuGR | 623 | 48.9% | EE(42.6%) and EA(4.5%) | 96.9% | |
| VC | GR | 497 | 100.0% | ND | 100.0% |
| AgGR | 501 | 99.5% | ND | 99.5% | |
| CuGR | 622 | 34.2% | EE(58.2%) and EA(7.4%) | 99.8% |
| Compound a | Products a,b | This Study c AgGRSO4 | Choi and Lee [79] d PtGRF, Cl, SO4, CO3 | This Study e CuGRSO4 | Maithreepala and Doong [72] f CuGRCl |
|---|---|---|---|---|---|
| PCE | 2.30 ± 0.09 × 10−2 h−1 g | 9.93 ± 0.58 × 10−2 h−1 | 5.96 ± 0.69 × 10−4 h−1 | 3.06 ± 0.39 × 10−1 h−1 | |
| PCE | 1.5% | ~1–15% | 67.9% | 12.4% | |
| TCE | 87.4% | - | 6.3% | 16.6% | |
| t12DCE | 7.3% | - | - | - | |
| acetylene | 7.4% | ~71–90% | - | - | |
| ethene | 0.5% | - | 3.3% | 31.9% | |
| ethane | - | - | 0.3% | 0.1% | |
| Carbon recovery | 104.2% | 81-95% | 77.7% | 61.0% | |
| TCE | 2.44 ± 0.53 × 10−4 h−1 | - | 3.59 ± 0.23 × 10−4 h−1 | ~9.58 × 10−4 h−1 | |
| TCE | 87.6% | - | 79.9% | 51.0% | |
| acetylene | 1.8% | - | - | - | |
| ethene | 1.1% | - | 11.1% | 11.0% | |
| ethane | - | - | 1.1% | 1.0% | |
| Carbon recovery | 89.9% | - | 92.1% | 63.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Loughlin, E.J.; Burris, D.R. Reduction of Chlorinated Ethenes by Ag- and Cu-Amended Green Rust. Minerals 2022, 12, 138. https://doi.org/10.3390/min12020138
O’Loughlin EJ, Burris DR. Reduction of Chlorinated Ethenes by Ag- and Cu-Amended Green Rust. Minerals. 2022; 12(2):138. https://doi.org/10.3390/min12020138
Chicago/Turabian StyleO’Loughlin, Edward J., and David R. Burris. 2022. "Reduction of Chlorinated Ethenes by Ag- and Cu-Amended Green Rust" Minerals 12, no. 2: 138. https://doi.org/10.3390/min12020138
APA StyleO’Loughlin, E. J., & Burris, D. R. (2022). Reduction of Chlorinated Ethenes by Ag- and Cu-Amended Green Rust. Minerals, 12(2), 138. https://doi.org/10.3390/min12020138







