Abstract
A laboratory scale study was conducted, aimed at finding an effective method for processing fine concentration tailings of copper-nickel ores. A sulfuric acid tailing granulation process followed by subsequent heap leaching of granules is proposed. Various methods of preparation and storage of the granular material are discussed. A solution of sulfuric acid was used as a binder. It was found that the addition of an oxidizing agent (Fe3+ and NO2−) when irrigating the granules had an effect on the recovery of metals. Changes in the recovery performance of non-ferrous metals into solution were studied under subsequent heap leaching of the material during a period of positive temperatures. The role of nitrogen compounds, in particular, nitrous acid, on the recovery of metals into solution after the preliminary storage of granules at below 0 °C temperatures is also discussed.
1. Introduction
The possibility of processing sulfide copper-nickel mineral feeds by heap leaching is becoming increasingly important for the mining and processing industry considering the environmental benefits of this approach. Heap leaching is mainly used in low-grade and secondary mineral feeds [1,2,3,4]. The increased attention to secondary raw materials is due to the deteriorating grade of copper-nickel ores currently processed by conventional methods. Particular attention was paid to the possibility of processing fine-grained feeds, namely, concentration tailings [4]. This is largely due to the fact that no additional grinding costs are incurred, and the operation has an additional economic incentive from the standpoint of processing its own waste.
However, existing studies of the mineral and phase compositions of the fresh and mature tailings of copper-nickel ore concentration circuits show that during the storage of fine-grained waste, irreversible changes occur that are associated with oxidation and natural leaching, as a result of which valuable components migrate to the surrounding natural environment [5,6]. Therefore, the loss of target metals in secondary mineral resources should be treated as lost profit, as well as a cause of a long-term negative impacts on the environment. This necessitates the development of a process approach supporting the most complete and prompt recovery of copper and nickel from ore concentration tailings.
The accumulated experience in the use of heap leaching justifies the need to coarsen the concentration tailings, in particular by granulation, to enhance the filtration properties of the stockpile [4,7]. In addition, the challenging climatic conditions in the Arctic complicate the year-round operation of the stockpile and containment of process solutions [8]. In this case, it is advisable to carry out preliminary preparation of the mineral feed (granulation) before a long period of below 0 °C ambient temperatures, to store stockpiles in winter and subsequently conduct leaching when the ambient temperature is positive.
Taking into account the long period of below 0 °C ambient temperatures in the Russian Arctic, one of the main prerequisites for the successful implementation of this method is to find ways to intensify the conversion of non-ferrous metals into a water-soluble form at below 0 °C temperatures. Chemical weathering processes involving atmospheric oxygen are known to noticeably accelerate in the presence of catalysts, whose role in sulfide tailings dumps can be taken by ferric iron ions and oxygen compounds of nitrogen. According to the existing literature, nitrous acid is the most kinetically reactive among nitrogen compounds. The fact of an increase in the oxidation rate under cryogenic conditions was found, which is caused by the fact that at below 0 °C temperatures, the reactivity of nitrous acid increases due to its higher stability even in a strongly acidic environment [9,10,11,12]. In addition, during freezing, the volume of the non-freezing liquid phase decreases sharply with decreasing temperature, which leads to the formation of highly concentrated areas where the liquid phase is surrounded by growing ice crystals, which creates a unique and reactive environment [13].
Considering the above, the features of cryochemical processes with the participation of oxygen nitrogen compounds can form the basis for the development of a heap leaching process for fine copper-nickel mineral feeds, adapted to harsh climatic conditions. In this study, the copper-nickel tailings accumulated at JSC Kola MMC were used. The company is the operator of a tailings storage facility at its concentrator in Zapolyarny (Murmansk region, Russia). The tailings storage facility has an area of approximately 1033 hectares and receives 7 million tons of tailings annually. Over the period of operation, it has accumulated 330 million tons (or 226 million m3) of tailings [14]. The tailings are characterized by a predominance of the −0.1 mm size fraction, with a significant share of the −0.044 mm fraction [15]. Mineralogical composition of tailings (%) is: serpentine—60, magnetite—13, olivine—5, augit—5, actinolite—5, kersutite—3, talc—3, sulfides—3, chlorite—2, and carbonates—1. The goal of this study was to develop a method for preparing fine-grained copper-nickel tailings for the heap leaching process, adapted to the climatic conditions prevalent in Russia’s Murmansk Region.
2. Materials and Methods
This study of sulfuric acid granulation of tailings followed by heap leaching followed the process sequence below: grinding of the mineral feed → granulation using sulfuric acid → irrigation of the obtained granules with an oxidizing agent → storage of granules for six months → heap leaching of granules.
In the course of the experiment, tailings with an initial copper grade of 0.07% and nickel grade of 0.20% were granulated using an FL015-1K-02 experimental granulator (LLC «Dzerzhinsktechnomash», Dzerzhinsk, Russia), and a 30% sulfuric acid solution was chosen as a binder at a S:L ratio of 6:1. The granulation product was pellets 3–4 mm in diameter and 1–1.5 mm thick. The strength of the obtained granules was determined in accordance with GOST 8269.0-97. The resulting granules were irrigated with a 0.5% sulfuric acid solution containing an oxidizing agent at 2 g/L at a granules-to-oxidizing agent ratio of 5:1.
The effect of two sulfide oxidation activators—ferric iron (Fe3+) and nitrite ion (NO2−)—was investigated. The nitrite ion was obtained by adding sodium nitrite into a sulfuric acid solution. The addition of sodium nitrite also implies the formation of a more reactive form—nitrous acid. The formation of nitrous acid proceeds according to Equation (1):
2NaNO2 + H2SO4 = 2HNO2 + Na2SO4
After the addition of the oxidizing agent, a storage stage followed, when excess moisture was removed and the granules acquired additional strength.
The study pursued the goal of developing an approach to tailings processing, adapted to the climatic conditions of the High Arctic. For this reason, an assessment was made of the effect of below 0 °C ambient temperatures on the storage of granules followed by leaching during a warmer period. One sample was kept for six months at a temperature of +19 °C, another one in a freezer at a temperature of −15 °C.
After long-term storage, the granules were heap leached. To simulate the heap leaching process, the granules were placed in glass percolators. The charge weight was 180 g, the stack height was 110 mm, and the column diameter was 45 mm. The granule bed was irrigated with 50 mL of distilled water five times a week for 20 days. This was followed by sulfuric acid leaching, where 50 mL of a 2% solution of sulfuric acid was fed to the surface of the heap with the same frequency. The ambient temperature during leaching was +19 °C. The total heap leaching time was 40 days.
The mineral composition of the copper-nickel tailings was identified using optical microscopy. The study was carried out in reflected polarized light on an Axioplan II polarizing microscope with a video recording unit (Carl Zeiss Ltd., Oberkochen, Germany).
To identify the phase composition of the tailings, we used powder X-ray diffraction (XRD) and a DRON-2.0 instrument, CuKα radiation (JSC «Burevestnik», Saint Petersburg, Russia). Surface morphology of the mineral particles was studied using a SEM Leo-420 scanning digital electron microscope (Carl Zeiss Ltd., Oberkochen, Germany).
The pH values of the pregnant liquors were monitored throughout the experiment using an I-160 MI ion meter (LLC «Izmeritel‘nayatekhnika», Moscow, Russia). The concentration of nickel and copper in the solutions was measured by atomic absorption spectrometry with electrothermal atomization using a Shimadzu-AA7000G instrument (to the PND F 14.1:2:4.140-98 standard). The measurement error did not exceed 5%. Metal recovery was estimated based on the concentrations in the pregnant liquors and the original grade of the tailings sample (Shimadzu Corp., Kyoto, Japan).
3. Results and Discussion
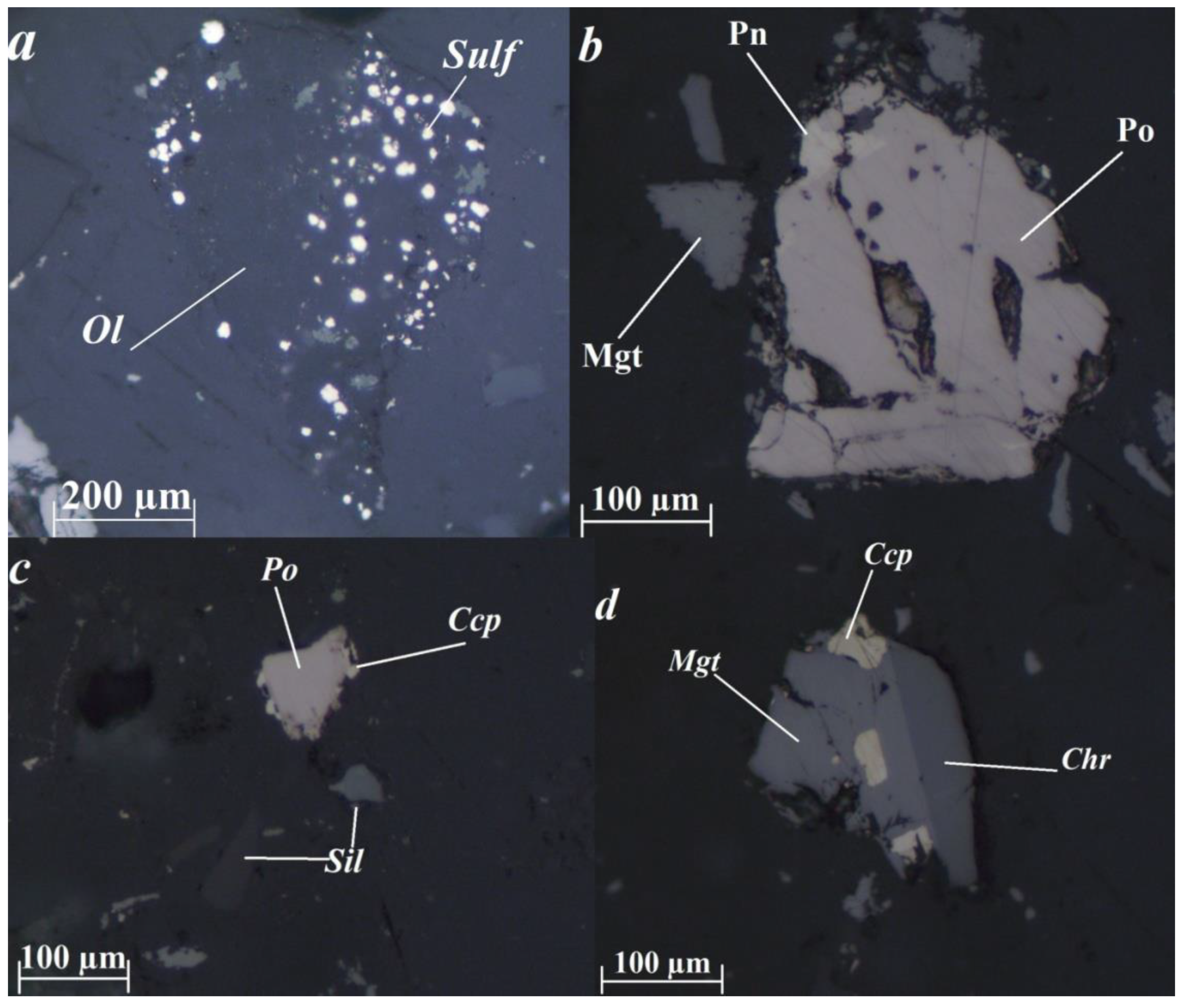
The mineral composition of the copper-nickel tailings is mostly rock-forming silicate minerals—olivine, serpentine, pyroxenes, chlorites, etc. The main ore minerals are sulfides, magnetite, chromite, and ilmenite (Figure 1). Sulfides are found as a finely-grained impregnation in olivine and with serpentine replacing the latter, as well as joint intergrowths with other ore and rock-forming minerals or individual grains. It was found that pyrrhotite (Fe1−xS) is the most common sulfide in the tailings, found mainly in the form of intergrowths with silicates, and less often with magnetite (Fe3O4), chalcopyrite (CuFeS2) or unlocked grains. Pyrrhotite contains flame-shaped ingrowth of pentlandite ((Fe, Ni)9S8). The pyrrhotite contains a minor nickel impurity (up to 1%). Pentlandite is also found as individual unlocked grains or intergrowths with magnetite or chalcopyrite. Secondaries to ore minerals include goethite which is secondary to magnetite, covellite which is secondary to chalcopyrite, and millerite and violarite which are secondary to pentlandite.
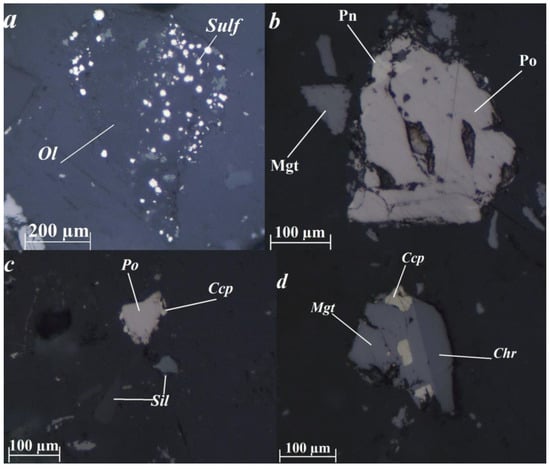
Figure 1.
Ore mineral morphology in the tailings. (a)—finely-grained sulfide impregnation in olivine; (b)—flame-shaped pentlandite ingrowth in pyrrhotite; (c)—an intergrowth of pyrrhotite and chalcopyrite; (d)—intergrowth of magnetite with inclusions of chalcopyrite and chromite. Image taken in reflected polarized light. Chr—chromite; Ccp—chalcopyrite; Mgt—magnetite; Pn—pentlandite; Po—pyrrhotite; Sil—silicate; Sulf—sulfide.
Figure 2a shows images of granules obtained from copper-nickel tailings. The granules are characterized by a high strength of 3.5 MPa. Their surface is characterized by minor porosity and fracturing (Figure 2b,c), which ensures good contact with leaching agents and does not allow the granules to break down at the stage of applying an oxidizing agent, subsequent storage and leaching.

Figure 2.
SEM images of the morphology (a) and the surface of copper-nickel tailings granules (b,c).
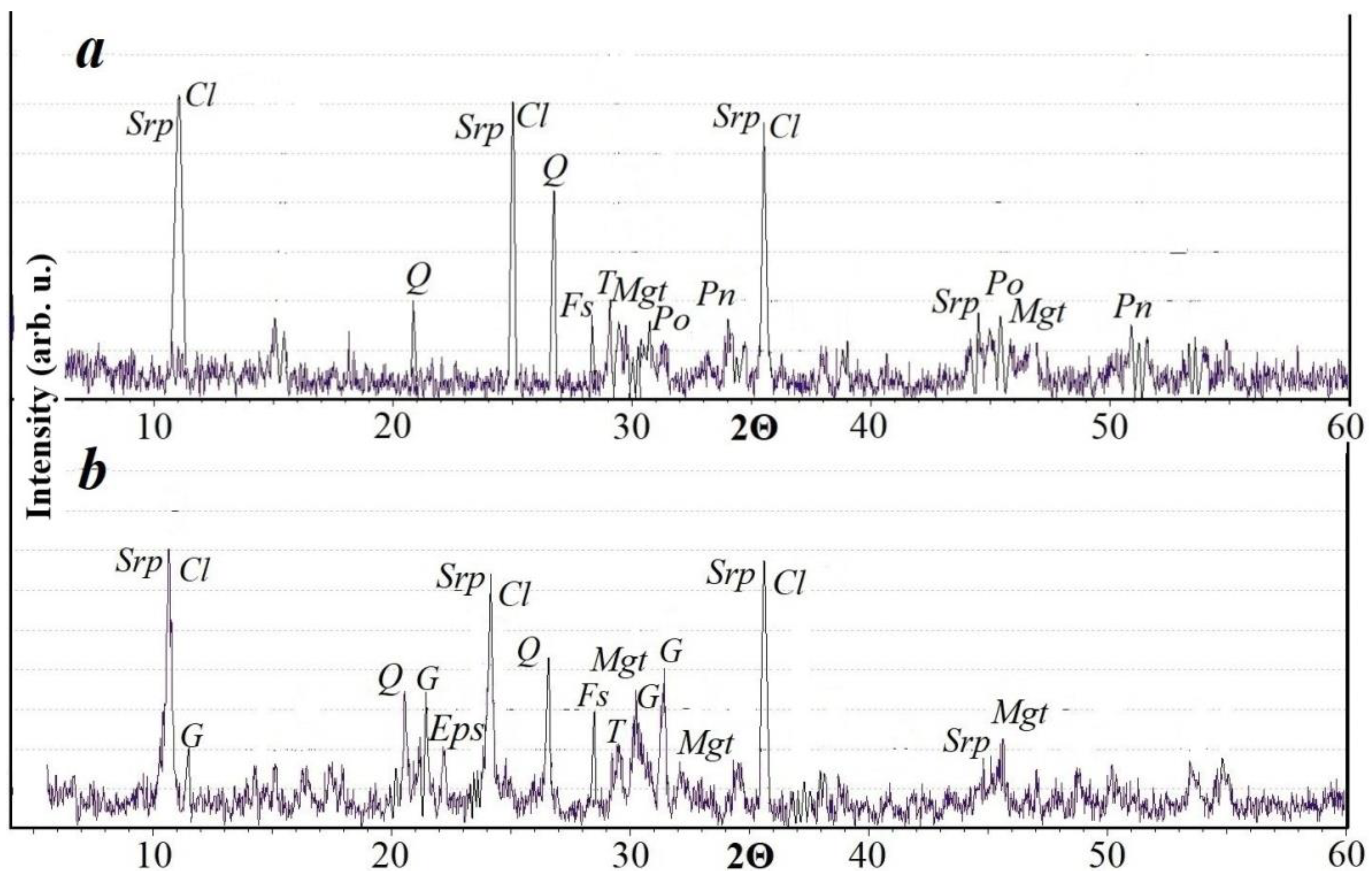
It was found by XRD that the silicates detected with an optical microscope belong to the following main phases—serpentine, chlorite, and quartz (Figure 3a). Reflexes of pyrrhotite and pentlandite were recorded in the sulfides in the sample. Reflexes of magnetite were found.
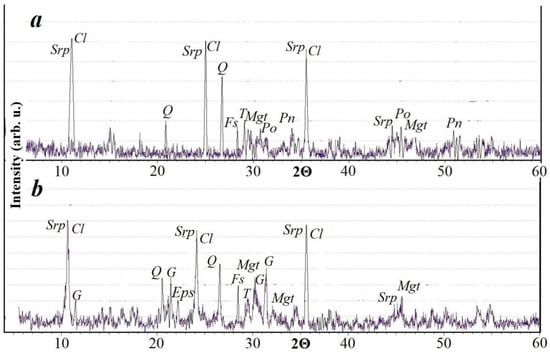
Figure 3.
XRD pattern of an original sample of the copper-nickel tailings (a) and same tailings after leaching for 40 days (b). Reflexes: Srp—serpentine, Cl—chlorite, Q—quartz, G—gypsum, Mgt—magnetite, Fs—feldspar, T—talc, Po—pyrrhotite, Pn—pentlandite, Eps—epsomite.
After treating the granules with an oxidizing agent, small amounts of epsomite (MgSO4·7H2O), a sulfate mineral, appeared, whose reflexes persist even after the leaching of the granules. This mineral is magnesium sulfate, but in this case it can contain a certain amount of nickel in the crystal lattice due to isomorphic substitution. Reflexes of other minerals after treatment with an oxidizing agent are retained in the diffraction pattern. During long-term storage, the granules retained their original shape, which will contribute to the successful filtration of the solution in the heap leaching process (Figure 4a). During the storage of granules, the formation of sulfate deposits on their surface was observed. Such crusts formed only during the storage of granules at a temperature of +19 °C (Figure 4b,c). Our X-ray phase analysis showed that the sulfate crust is composed of gypsum (Figure 3b). Gypsum is formed by the interaction of sulfuric acid and calcite, which is present in a small amount in the tailings.

Figure 4.
SEM images of the morphology (a) and the granule surface of copper-nickel tailings after storage for 180 days at +19 °C (b,c).
The XRD pattern of the sample after leaching does not show reflexes of sulfide minerals, which indicates a high intensity of their interaction with the leaching agent. The main minerals in the sample are silicates that are resistant to leaching agents.
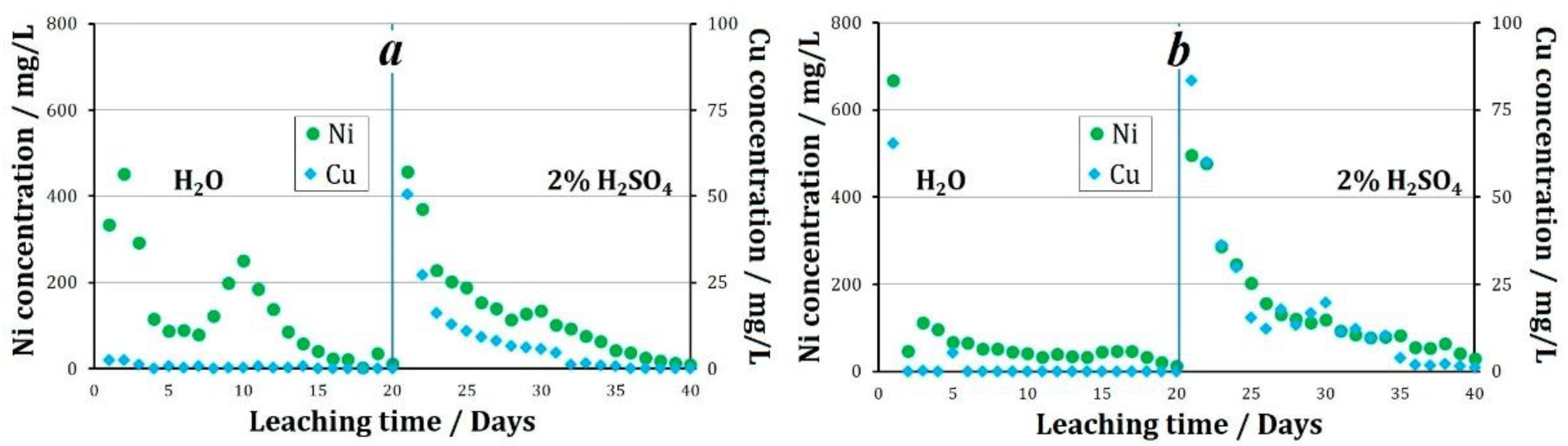
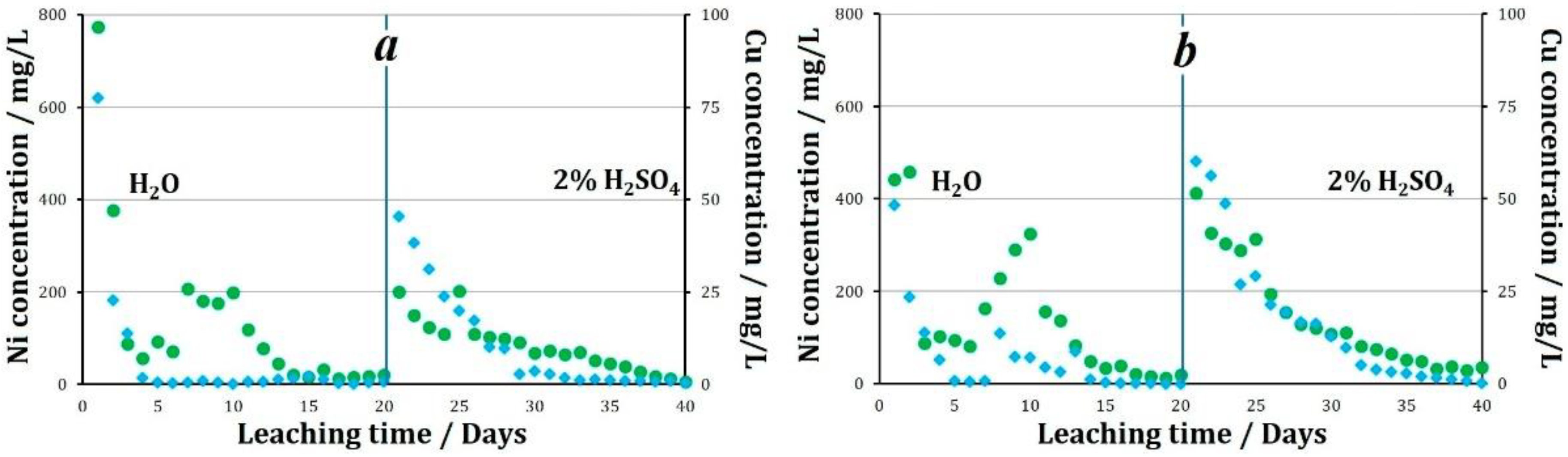
When leaching granules were treated with various oxidizing agents and stored at +19 °C, the following nickel and copper recovery performance was observed. When Fe3+ ions were used as an oxidizing agent, in the solutions collected at the column outlet during water leaching a change in the pH value from 3.8 to 5.5 was observed. The average concentration of nickel in the solution was 131.2 mg/L, and of copper was 0.6 mg/L. During water leaching of that sample, 28.2% of nickel and 0.3% of copper was recovered into the solution. When leaching with a sulfuric acid solution, the pH value of the pregnant liquors varied between 1.7 and 3.4, with an average of 2.2. The average concentration of nickel in the pregnant liquors was 129.5 mg/L, and of copper was 8.2 mg/L. By the end of the sulfuric acid leaching process, 27.9% of nickel and 4.5% of copper was recovered from the tailings. Thus, from the tailings that were pelletized using Fe3+ as an oxidizing agent, followed by storage at +19 °C, 56.1% of nickel and 4.8% of copper was recovered after 40 days of the experiment.
When using NO2− as an oxidizing agent followed by the storage of granules at +19 °C, the following results were obtained. During water leaching, the pH value of the solutions increases from 3.3 at the beginning of the experiment to 6.3 throughout the entire experiment. The average pH value during the entire experiment was 5.5. The average concentration of nickel in the solution was 79.3 mg/L, and of copper was 3.6 mg/L (Figure 5). During the experiment using NO2− 15.0% of nickel and 1.4% of copper was recovered into the solution. When leaching with a sulfuric acid solution, the pH value of the pregnant liquors averaged 2.3. The average concentration of metals in the solution was: Ni 150.0 mg/L, and Cu 18.0 mg/L. By the end of the sulfuric acid leaching process, 30.2% of nickel and 9.0% of copper was recovered from the tailings. Thus, from the tailings pelletized using NO2− as an oxidizing agent, followed by storage at +19 °C, 45.2% of nickel and 10.0% of copper was recovered after 40 days of the experiment.
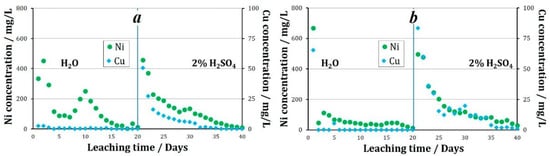
Figure 5.
The concentration of metals in pregnant liquors during leaching of granules treated with oxidizer: (a)—2% H2SO4 + Fe3+, (b)—2% H2SO4 + NO2−.
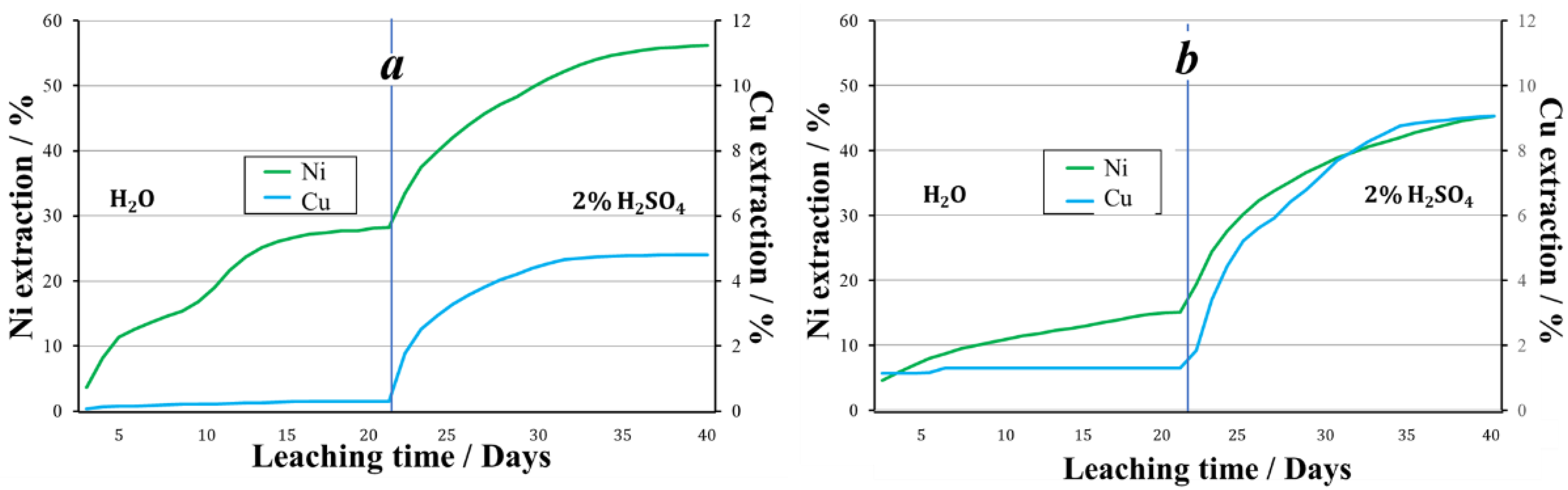
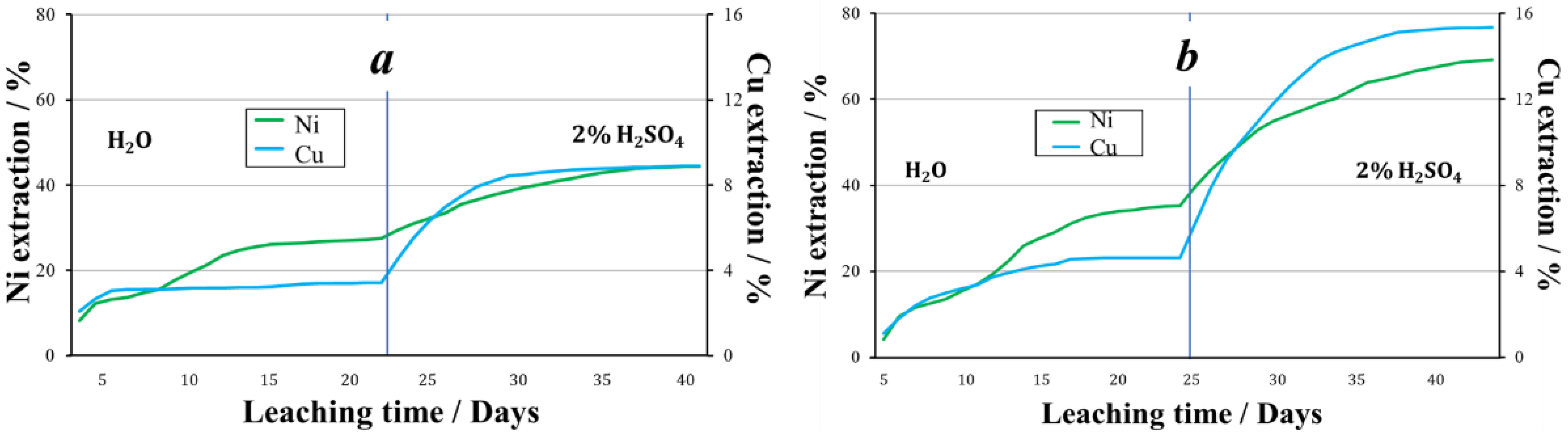
Thus, at a temperature of +19 °C, Fe3+ as an oxidizing agent promotes higher nickel recovery during aqueous leaching. When leaching with a 2% sulfuric acid solution, the recoveries of nickel were approximately equal. Over the entire 40 days of the experiment, copper had a higher recovery from the granules obtained using nitrite ions (Figure 6).
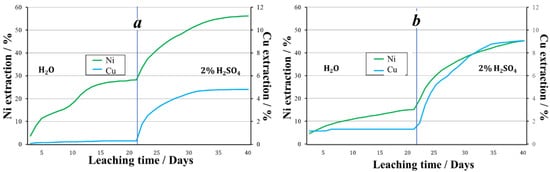
Figure 6.
The extraction of metals during the leaching of granules treated with oxidizer: (a)—2% H2SO4 + Fe3+, (b)—2% H2SO4 + NO2−.
The effect of using same oxidizing agents followed by the storage of granules at below 0 °C temperatures was studied. By the end of the aqueous leaching process of the granules prepared using Fe3+ as an oxidizing agent, 27.5% of nickel and 3.4% of copper was recovered. The recovery of metals into solution naturally decreased with an increase in the duration of the experiment. The average concentration of metals in the solution during the experiment was 129.5 mg/L Ni and 6.4 mg/L Cu. By the end of the aqueous leaching process, a major share of the granules retained their original shape. In the course of sulfuric acid leaching, the average pH of the pregnant liquors was 1.5. The average nickel concentration in the solution was 82.9 mg/L, and the maximum value was observed on the fifthday of leaching at 202.2 mg/L. The average copper concentration in the solution over 20 days of leaching was 10.7 mg/L (Figure 7). When leaching with a sulfuric acid solution, by the end of the experiment, 16.9% nickel and 5.5% copper was recovered. Thus, 44.4% of nickel and 8.9% of copper was recovered into the solution over the 40 days of leaching.
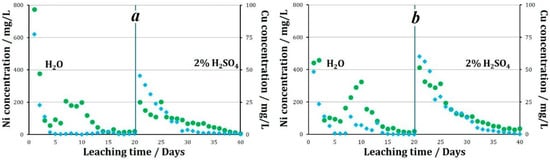
Figure 7.
The concentration of metals in pregnant liquors during leaching of granules prepared using various oxidizing agents followed by storage at below 0 °C ambient temperatures, (a)—2% H2SO4 + Fe3+, (b)—2% H2SO4 + NO2−.
The pH value of the pregnant liquors during the experiment using granules prepared using NO2− varied from 2.8 to 4.6, with an average value of 3.6. The concentration of metals in the solutions averaged: Ni 141.7 mg/L, Cu 7.0 mg/L.
In the aqueous leaching process, the recovery of nickel and copper was 44% and 27%, respectively. In the sulfuric acid leaching process of granules prepared using NO2−, the pH of the pregnant liquors averaged 1.7. The average concentration of nickel in the solution was 145.3 mg/L, of copper 16.9 mg/L. When leaching with a sulfuric acid solution, 30.8% nickel and 9.1% copper was recovered. Thus, 69.3% of nickel and 15.9% of copper was recovered from the tailings granulated using nitrite ion as an oxidizing agent (Figure 8).
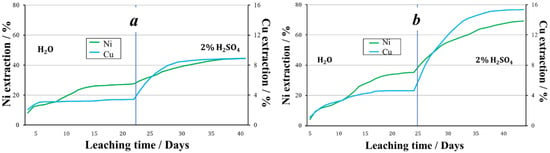
Figure 8.
The extraction of metals during leaching of granules prepared using various oxidizing agents followed by storage at below 0 °C ambient temperatures, (a)—2% H2SO4 + Fe3+, (b)—2% H2SO4 + NO2−.
Comparing the results obtained with the use of the studied oxidizing agents made it possible to establish that, under below 0 °C temperatures, nitrous acid promotes a higher recovery of metals into solution. This is due to the fact that nitrogen oxides and the products of their reaction with water have higher oxidation potentials than ferric ions. In addition, during oxidation, multiple nitrogen compounds with different catalytic activity and varying concentrations are supposedly present in the system (NO, N2O3, N2O4, HNO2, HNO3). The released nitrogen monoxide is able to oxidize to dioxide in the presence of oxygen, and, as a consequence, to form an acid again. In the presence of oxygen and water, nitrogen oxides are converted to nitrous acid as follows (reactions (2)–(5)):
2NO + O2→ 2NO2→ N2O4,
N2O4 + H2O → HNO3 + HNO2,
NO + NO2→ N2O3,
N2O2 + H2O → 2HNO2.
Therefore, the amount of the oxidizing agent and the duration of its interaction with sulfides when using the nitrite ion is higher in comparison with ferric iron. In addition, the high activity of nitrous acid is due to the properties of the HNO2 molecule that are expressed in its ability to be both an oxidizing agent and a reducing agent, depending on the composition of the system. Nitrous acid is characterized by high catalytic activity even at low concentrations and contributes to an increase in the intensity of oxidative sulfuric acid leaching of MeS by several times.
4. Conclusions
A potential method was proposed of processing the concentration tailings of copper-nickel ores providing further recovery of non-ferrous metals. The experiments carried out show the fundamental possibility of using sulfuric acid granulation as a pretreatment method, prior to subsequent heap leaching.
The possibility of accelerating chemical reactions during the storage of granular tailings at below 0 °C ambient temperatures and increasing the recovery of non-ferrous metals during subsequent heap leaching was experimentally validated. The highest recoveries of non-ferrous metals were observed when using nitrogen compounds as an oxidizing agent, followed by storing the granules at a temperature of −15 °C. Nickel and copper recoveries of 69.3% and 15.9%, respectively, were achieved. This can be explained, in addition to the described behavior of nitrogenous compounds under below 0 °C temperatures, by the processes of concentration of solutions during freezing, which noticeably accelerates the conversion of non-ferrous metals into a water-soluble form.
The results obtained demonstrate the prospects for the heap leaching of copper-nickel ore concentration tailings, where the granular mineral feed with the addition of an oxidizer is stored in the winter period, and the stockpile is leached during the relatively warm period of the year. This will significantly reduce the capital and operating costs involved in the cold Arctic climate and make the processing of low-grade mineral feeds cost-effective. The research results can be useful for enrichment processes outside the Russian Arctic in regions characterized by a cold climate. The described processes can be attributed to other sulfides, for example, PbS, ZnS, CoS, and FeS.
Author Contributions
Conceptualization, A.G., A.S. and D.M.; methodology, A.G., A.S. and A.K.; software, A.G., A.S. and A.K.; validation, A.G., A.S. and D.M.; formal analysis, A.G., A.S. and A.K.; investigation, A.G., A.S., A.K. and D.M.; resources, A.G., A.S., A.K. and D.M.; data curation, A.G. and A.S.; writing—original draft preparation, A.G., A.S. and A.K.; writing—review and editing, D.M.; visualization, A.G., A.S. and A.K.; supervision, A.S. and D.M.; project administration, A.S. and D.M.; funding acquisition, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian government grant nos. 1021051803680-5 and 0226-2019-0011.
Data Availability Statement
All the data used in this study can be found from the published manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khalezov, B.D. Heap Leaching of Copper and Copper-Zinc Ores (Domestic Experience): Monograph; Editorial and Publishing Department UB RAS: Ekaterinburg, Russia, 2013; 346p. [Google Scholar]
- Watling, H.R. The bioleaching of nickel-copper sulfides. Hydrometallurgy 2008, 91, 70–88. [Google Scholar] [CrossRef]
- Watling, H.R.; Collinson, D.M.; Watling, R.J.; Shiers, D.W. Simulated heap leaching and recovery of multiple elements from a mineralized black shale. Hydrometallurgy 2017, 167, 47–48. [Google Scholar] [CrossRef]
- Khabirov, E.T.; Shadrunova, I.V.; Garkavi, M.S. Formation of granules from sulfide waste for heap leaching. Min. Inf. Anal. Bull. 2007, 2, 309–314. [Google Scholar]
- Lindsay, M.B.J.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Masloboev, V.A.; Seleznev, S.G.; Makarov, D.V.; Svetlov, A.V. Assessment of eco-hazard ofcopper-nickel ore mining and processing waste. J. Min. Sci. 2014, 50, 559–572. [Google Scholar] [CrossRef]
- Ollakka, H.; Ruuska, J.; Taskila, S. The application of principal component analysis for bioheapleaching process–Case study: Talvivaara mine. Miner. Eng. 2016, 95, 48–58. [Google Scholar] [CrossRef]
- Masloboev, V.A.; Seleznev, S.G.; Svetlov, A.V.; Makarov, D.V. Hydrometallurgical processing of low-grade sulfide ore and mine waste in the Arctic regions: Perspectives and challenges. Minerals 2018, 8, 436. [Google Scholar] [CrossRef] [Green Version]
- Ptitsyn, A. Geochemical Foundations of Geotechnology of Metals in Permafrost Conditions; Nauka: Novosibirsk, Russia, 1992; pp. 90–93. [Google Scholar]
- Ptitsyn, A.B.; Sysoyeva, E.I. Cryogenic mechanism of formation of the Udokan oxidation zone. Geol. I Geofiz. 1995, 36, 90–97. [Google Scholar]
- Markovich, T.I.; Razvorotneva, L.I. Oxidative leaching of sulfide Udokan ore with the participation of oxygen compounds of nitrogen under cryogenic conditions. Vestn. ONZ RAS 2011, 3, NZ6071. [Google Scholar]
- Takenaka, N.; Ueda, A.; Maeda, Y. Acceleration of the rate of nitrite oxidation by freezing in aqueous solution. Nature 1992, 358, 736–738. [Google Scholar] [CrossRef]
- Takenaka, N.; Ueda, A.; Daimon, T.; Bandow, H.; Dohmaru, T.; Maeda, Y. Acceleration mechanism of chemical reaction by freezing: The reaction of nitrous acid with dissolve doxygen. J. Phys. Chem. 1996, 100, 13874–13884. [Google Scholar] [CrossRef]
- Annual Report of PJSC “MMC Norilsk Nickel” for 2018. “Sustainable Growth”. 332p. Available online: https://www.nornickel.ru/investors/reports-and-results/#2018 (accessed on 19 December 2021).
- Makarov, D.V.; Makarov, V.N.; Vasil’yeva, T.N.; Farvazova, E.R. Variation of the Ni, Cu, Fe, Mg content in the copper-nickel ores concentration tailings during storage. Inzhenernaya Ekol. 2004, 11, 18–28. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).