Revisiting Limestone Quality for Soil Liming Purpose
Abstract
1. Introduction
2. Materials and Methods
2.1. Strategy of Study
2.2. Limestone Powder Characterization
2.3. Mineral Composition
2.4. Particle Size Distribution Using Laser Diffraction
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analysis of Chemical Composition and Reactivity
3.2. Mineralogical Assemblage
3.3. Particle Size Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rheinheimer, D.S.; Tiecher, T.; Gonzatto, R.; Santanna, M.A.; Brunetto, G.; da Silva, L.S. Long-term effect of surface and incorporated liming in the conversion of natural grassland to no-till system for grain production in a highly acidic sandy-loam Ultisol from South Brazilian Campos. Soil Tillage Res. 2018, 180, 222–231. [Google Scholar] [CrossRef]
- Nolla, A.; Alves, E.O.; Silva, T.; Bordin, A.V. Correção da acidez e disponibilização de fósforo e potássio em Latossolo vermelho distrófico típico submetido à calagem incorporada e superficial. Braz. J. Anim. Environ. Res. 2020, 3, 2478–2487. [Google Scholar] [CrossRef]
- Kaminski, J.; Rheinheimer, D.S.; Gatiboni, L.C.; Brunetto, G.; Silva, L.S. Eficiência da calagem superficial e incorporada precedendo o sistema plantio direto em um Argissolo sob pastagem natural. Rev. Bras. Cienc. Solo 2005, 29, 573–580. [Google Scholar] [CrossRef][Green Version]
- Haby, V.A.; Leonard, A.T. Limestone quality and effectiveness for neutralizing soil acidity. Commun. Soil Sci. Plant Anal. 2002, 33, 2935–2948. [Google Scholar] [CrossRef]
- Bortoluzzi, E.C.; Poleto, C.; Baginski, A.J.; da Silva, V.R. Aggregation of subtropical soil under liming: A study using laser diffraction. Rev. Bras. Cienc. Solo 2010, 34, 725–734. [Google Scholar] [CrossRef][Green Version]
- Silva, S.R.; dos Santos, H.P.; Lollato, R.P.; Santi, A.; Fontaneli, R.S. Soybean Yield and Soil Physical Properties as Affected by Long-Term Tillage Systems and Liming in Southern Brazil. Int. J. Plant Prod. 2022, in press. [Google Scholar] [CrossRef]
- Olego, M.Á.; Quiroga, M.J.; López, R.; Garzón-Jimeno, E. The Importance of Liming with an Appropriate Liming Material: Long-Term Experience with a Typic Palexerult. Plants 2021, 10, 2605. [Google Scholar] [CrossRef] [PubMed]
- Embrapa. Características de Corretivos Agrícolas; Primavesi, A.C., Primavesi, O., Eds.; Embrapa Pecuária Sudeste: Brasília, Brazil, 2004; 28p. [Google Scholar]
- Rodrighero, M.B.; Barth, G.; Caires, E.F. Aplicação superficial de calcário com diferentes teores de magnésio e granulometrias em sistema plantio direto. Rev. Bras. Cienc. Solo 2015, 39, 1723–1736. [Google Scholar] [CrossRef]
- Van Raij, B.; Sacchetto, M.T.D.; Kupper, A. Correlações entre o pH e o grau de saturação em bases nos solos com horizonte B textural e horizonte B latossólico. Bragantia 1968, 27, 493–500. [Google Scholar]
- Bellinaso, R.J.; Tiecher, T.; Vargas, J.; Rheinheimer, D.S. Crop yields in no-tillage are severely limited by low availability of P and high acidity of the soil in depth. Soil Res. 2021, 60, 33–49. [Google Scholar] [CrossRef]
- Rheinheimer, D.S.; Tiecher, T.; Gonzatto, R.; Zafar, M.; Brunetto, G. Residual effect of surface-applied lime on soil acidity properties in a long-term experiment under no-till in a Southern Brazilian sandy Ultisol. Geoderma 2018, 313, 7–16. [Google Scholar] [CrossRef]
- Rheinheimer, D.S.; Santos, E.J.S.; Kaminski, J.; Bortoluzzi, E.C.; Gatiboni, L.C. Changes in acid soil properties by superficial and incorporated liming on natural pasture. Rev. Bras. Cienc. Solo 2000, 24, 797–805. [Google Scholar] [CrossRef]
- Embrapa. Embrapa Manual de Métodos de Análise de Solo, 3rd ed.; Empresa Brasileira de Pesquisa Agropecuária. Embrapa Solos; Embrapa: Brasília, Brazil, 2017; p. 577. [Google Scholar]
- Bortoluzzi, E.C.; Parize, G.L.; Korchagin, J.; Silva, V.R.; Rheinheimer, D.S.; Kaminski, J. Soybean root growth and crop yield in response to liming at the beginning of a no-tillage system. Rev. Bras. Cienc. Solo 2014, 38, 262–271. [Google Scholar] [CrossRef][Green Version]
- Bortoluzzi, E.C.; Garbozza, L.; Guareschi, C.; Rheinheimer, D.S. Efeito da calagem na relação entre solo e água. Rev. Bras. Cienc. Solo 2008, 32, 2621–2628. [Google Scholar] [CrossRef]
- Gomes, C.B. Os Carbonatitos Cretácicos da Plataforma Brasileira e Suas Principais Características; Instituto de Geociências da USP: São Paulo, Brazil, 2020; 253p. [Google Scholar] [CrossRef]
- Brasil Ministério da Agricultura, Pecuária e Abastecimento. Manual de Métodos Analíticos Oficiais Para Fertilizantes e Corretivos; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2017; p. 200. [Google Scholar]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification; Monograph No. 5; Mineralogical Society: London, UK, 1980; p. 495. [Google Scholar]
- Webster, R. Statistics to support soil research and their presentation. Eur. J. Soil Sci. 2001, 52, 331–340. [Google Scholar] [CrossRef]
- Eshel, G.; Levy, G.J.; Mingelgrin, U.; Singer, M.J. Critical Evaluation of the Use of Laser Diffraction for Particle-Size Distribution Analysis. Soil Sci. Soc. Am. J. 2004, 68, 736. [Google Scholar] [CrossRef]
- Tonello, M.S.; Hebner, T.S.; Sterner, R.W.; Brovold, S.; Tiecher, T.; Bortoluzzi, E.C.; Merten, G.H. Geochemistry and mineralogy of southwestern Lake Superior sediments with an emphasis on phosphorus lability. J. Soils Sedim. 2020, 20, 1060–1073. [Google Scholar] [CrossRef]
- Ramos, C.G.; Oliveira, M.L.S.; Pena, M.F.P.; Cantillo, A.M.; Ayarza, L.P.L.; Korchagin, J.; Bortoluzzi, E.C. Nanoparticles generated during volcanic rock exploitation: An overview. J. Environ. Chem. Eng. 2021, 9, 106441. [Google Scholar] [CrossRef]
- Morse, J.W.; Arvidson, R.S. The dissolution kinetics of major sedimentary carbonate minerals. Earth-Sci. Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D. The influence of experimental design on the rate of calcite dissolution. Geochim. Cosmochim. Acta 1983, 47, 2281–2285. [Google Scholar] [CrossRef]
- Grunwaldt, H.S.; Zimina, A.; Göttlicher, J.; Steininger, R.; Grunwaldt, J.D. Study of the relation between Mg content and dissolution kinetics of natural lime stone using μXRF, μXRD and μXAS. J. Phys. Conf. Ser. 2016, 712, 012144. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Processes at the magnesium-bearing carbonates/solution interface. II. Kinetics and mechanism of magnesite dissolution. Geochim. Cosmochim. Acta 1999, 63, 881–897. [Google Scholar] [CrossRef]
- Chou, L.; Garrels, R.M.; Wollast, R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem. Geol. 1989, 78, 269–282. [Google Scholar] [CrossRef]
- CQFS-RS/SC Comissão de Química e Fertilidade do Solo. Manual de Calagem e Adubação Para os Estados do Rio Grande do Sul e de Santa Catarina; Sociedade Brasileira de Ciência do Solo, Núcleo Regional Sul: Porto Alegre, Brazil, 2016. [Google Scholar]
- Viadé, A.; Fernández-Marcos, M.L.; Hernández-Nistal, J.; Alvarez, E. Effect of particle size of limestone on Ca, Mg and K contents in soil and in sward plants. Sci. Agric. 2011, 68, 200–208. [Google Scholar] [CrossRef]
- Scott, B.J.; Conyers, M.K.; Fisher, R.; Lill, W. Particle size determines the efficiency of calcitic limestone in amending acidic soil. Aust. J. Soil Res. 1992, 43, 1175–1185. [Google Scholar] [CrossRef]
- Korchagin, J.; Caner, L.; Bortoluzzi, E.C. Variability of amethyst mining waste: A mineralogical and geochemical approach to evaluate the potential use in agriculture. J. Clean. Prod. 2019, 210, 749–758. [Google Scholar] [CrossRef]
- Caires, E.F.; Barth, G.; Garbuio, F.J.; Churka, S. Soil acidity, liming and soybean performance under no-till. Sci. Agric. 2008, 65, 532–540. [Google Scholar] [CrossRef]
- Julien, J.-L.; Tessier, D. Rôles du pH, de la CEC effective et des cations échangeables sur la stabilité structurale et l’affinité pour l’eau du sol. Etude Gest. Sols 2021, 28, 159–179. [Google Scholar]
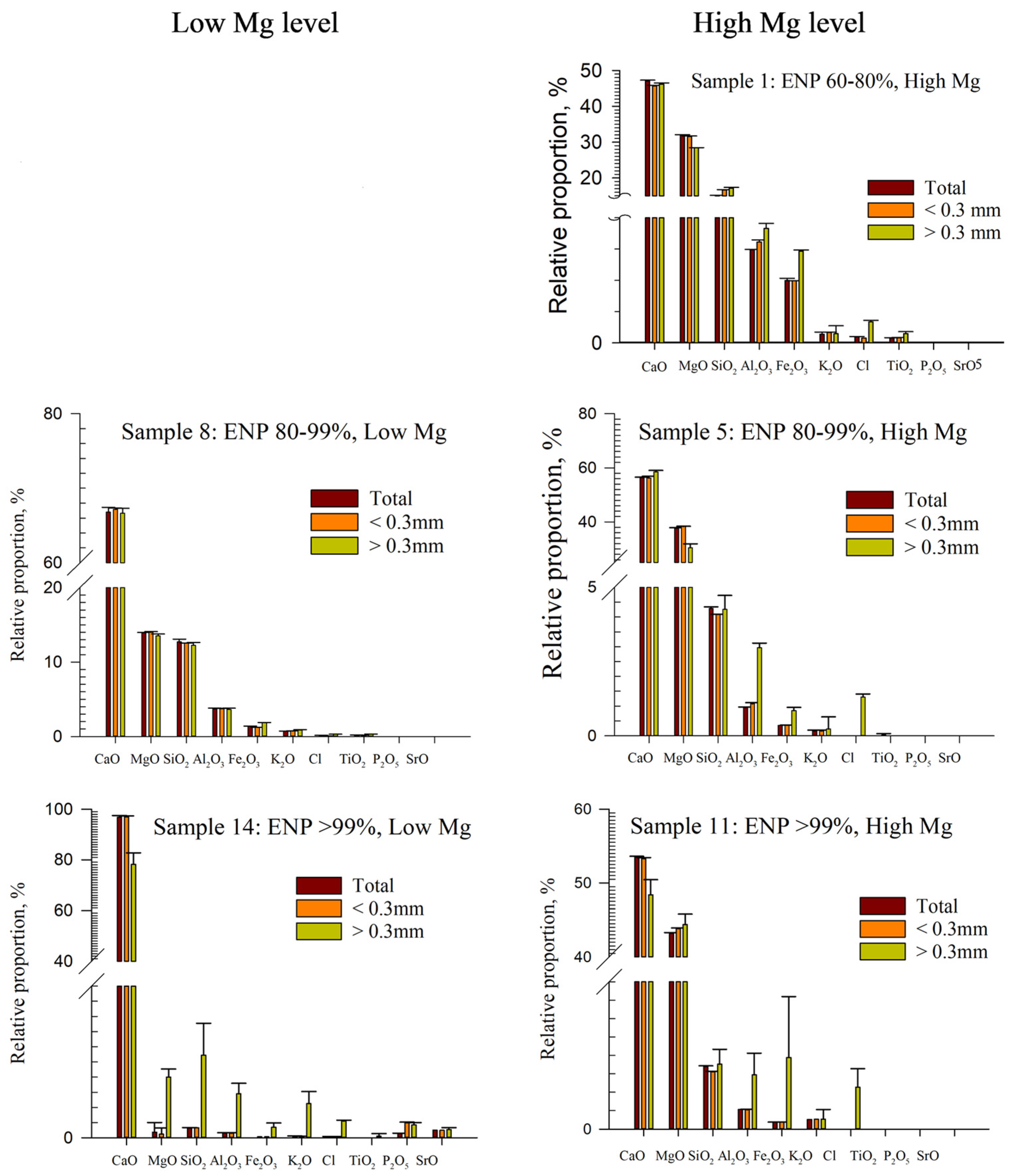
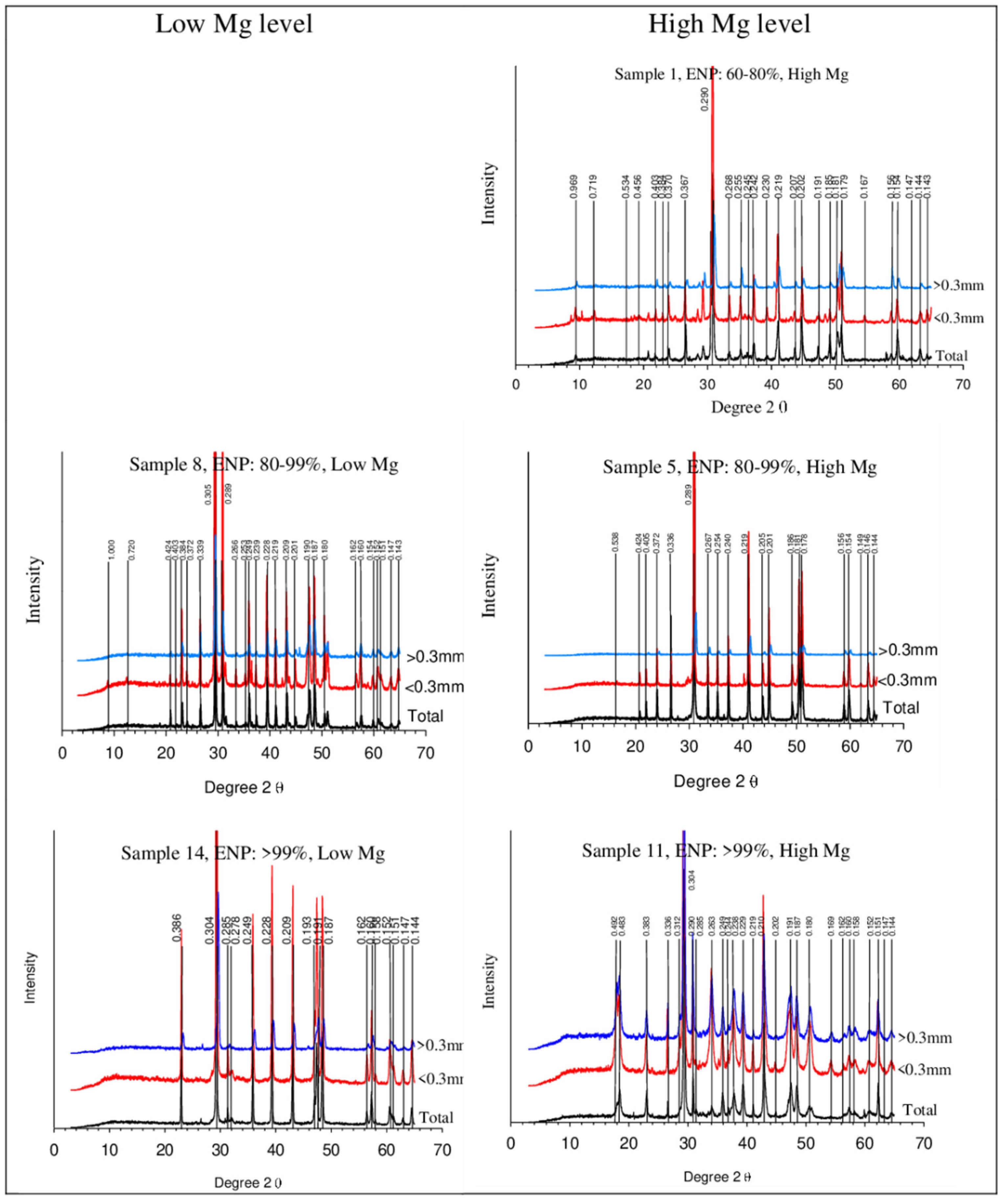
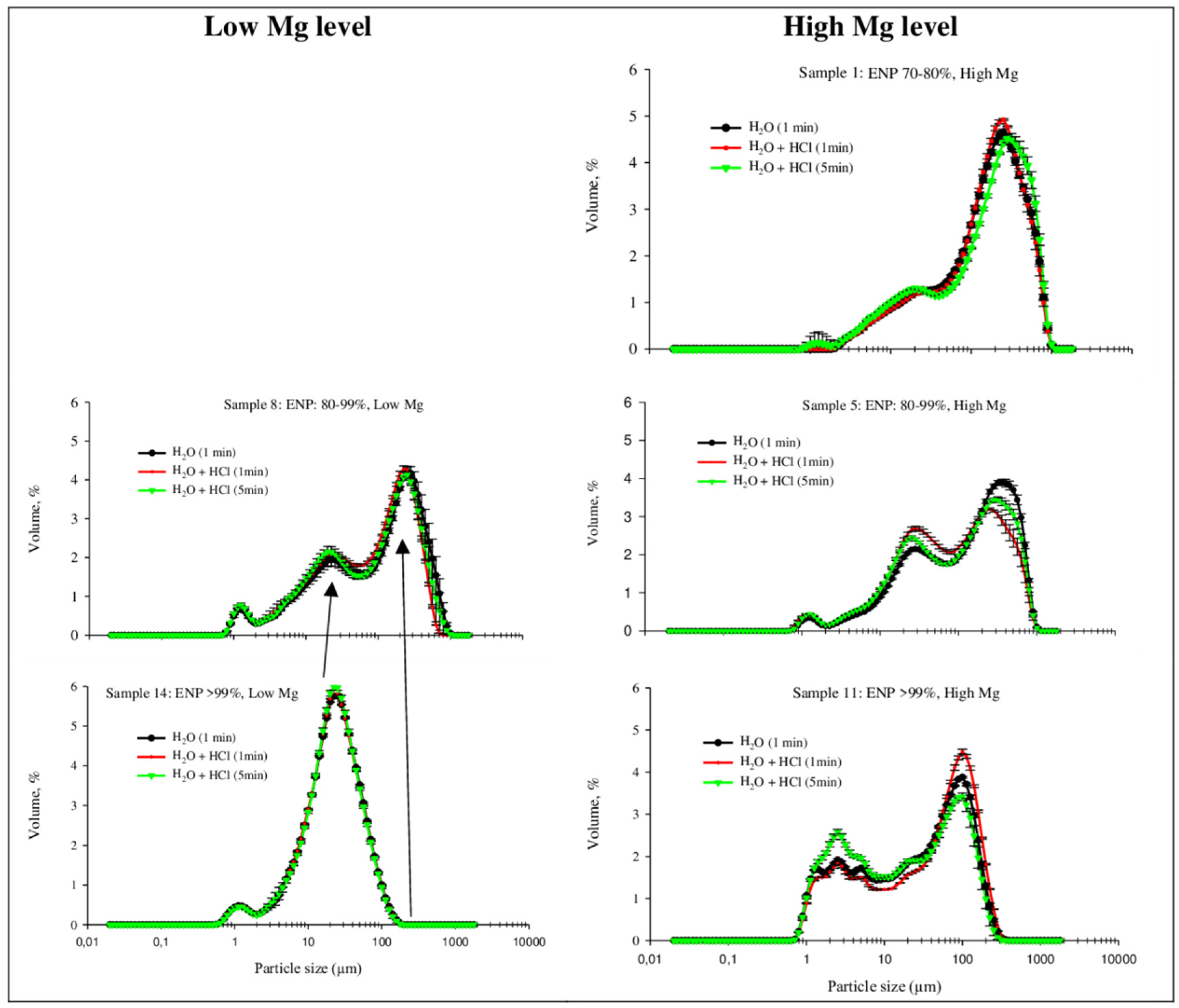

| Samples | ENP/MgO Contents | 2 mm Mesh | Retained 0.84 mm Mesh | 0.3 mm Mesh | NP (%) | ENP (%) | MgO (%) | CaO (%) | Ca/Mg Ratio | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70–80 | high Mg | 0 | 14.4 | 28.7 | 102.03 | 78.56 | 10.12 | 31.43 | 3.7 |
| 2 | 0 | 19.1 | 30.6 | 104.23 | 75.55 | 10.16 | 28.16 | 3.3 | ||
| 3 | 0 | 19.4 | 35.0 | 103.19 | 72.73 | 10.12 | 28.65 | 3.4 | ||
| 4 | 80–99 | high Mg | 0 | 6.5 | 13.0 | 108.88 | 97.56 | 10.09 | 29.28 | 3.4 |
| 5 | 0 | 12.0 | 22.5 | 111.44 | 90.71 | 10.06 | 30.08 | 3.5 | ||
| 6 | 0 | 7.0 | 15.3 | 110.51 | 97.56 | 10.45 | 29.99 | 3.4 | ||
| 7 | low Mg | 0 | 7.2 | 24.5 | 104.24 | 88.02 | 5.51 | 43.2 | 9.3 | |
| 8 | 0 | 9.2 | 25.6 | 100.29 | 82.64 | 6.55 | 37.92 | 6.9 | ||
| 9 | 0 | 8.6 | 20.4 | 104.35 | 88.66 | 7.01 | 45.16 | 7.6 | ||
| 10 | >99 | high Mg | 0 | 0 | 0 | 99.47 | 99.47 | 10.32 | 30.53 | 3.5 |
| 11 | 0 | 0 | 0 | 159.89 | 159.89 | 10.59 | 30.62 | 3.4 | ||
| 12 | 0 | 0 | 0 | 155.94 | 155.94 | 10.08 | 38.64 | 4.5 | ||
| 13 | low Mg | 0 | 0 | 0 | 105.37 | 105.37 | 0.61 | 43.1 | 83.7 | |
| 14 | 0 | 0 | 0 | 99.13 | 99.13 | 0.56 | 45.92 | 97.1 | ||
| 15 | 0 | 0 | 0 | 107.72 | 107.72 | 0.51 | 56.72 | 131.7 | ||
| min | n = 52 | 69.2 | 0.5 | 23.0 | ||||||
| mean | n = 52 | 88.2 | 5.6 | 31.6 | ||||||
| max | n = 52 | 159.9 | 10.6 | 56.7 | ||||||
| Samples | Mineral | Ideal Chemical Formula | XRD Peaks (d = nm) Intensity(I/Io) | Relative Proportion % |
|---|---|---|---|---|
| (1), 2, 3 | Ankerite | CaFe2 + 0.6Mg0.3Mn2 + 0.1(CO3)2 | 0.289(1), 0.181(0.06), 0.219(0.06) | 44.1 |
| Dolomite | CaMg(CO3)2 | 0.288(1), 0.178(0.6), 0.219(0.5) | 37.6 | |
| Sillimanite | (Al2O3)(SiO2) | 0.342(1), 0.337(0.65), 0.22(0.6) | 8.9 | |
| Otavite | Cd(CO3) | 0.295(1), 0.378(0.8), 0.246(0.35) | 3.8 | |
| Calcite Mica | CaCO3 | 0.303(1), 0.209(0.18), 0.228(0.18) 1.00 | 1.3 ~ | |
| 4, (5), 6 | Dolomite | CaMg(CO3)2 | 0.288(1), 0.178(0.6), 0.219(0.5) | 84.5 |
| Ankerite | CaFe2 + 0.6Mg0.3Mn2 + 0.1(CO3)2 | 0.289(1), 0.181(0.06), 0.219(0.06) | 7.4 | |
| Otavite | Cd(CO3) | 0.295(1), 0.378(0.8), 0.246(0.35) | 5.8 | |
| Quartzo | SiO2 | 0.6 | ||
| 7, (8), 9 | Calcite | CaCO3 | 0.303(1), 0.209(0.18), 0.228(0.18) | 54.0 |
| Dolomite | CaMg(CO3)2 | 0.288(1), 0.178(0.6), 0.219(0.5) | 36.1 | |
| Quartzo | SiO2 | 8.7 | ||
| Otavite | Cd(CO3) | 0.295(1), 0.378(0.8), 0.246(0.35) | 1.2 | |
| Mica | 1.00 | ~ | ||
| 10, (11), 12 | Calcite | CaCO3 | 0.303(1), 0.209(0.18), 0.228(0.18) | 66.3 |
| Portlandite | Ca(OH)2 | 0.262(1), 0.49(0.74), 0.192(0.42) | 14.3 | |
| Periclase | MgO | 0.210(1), 0.148(0.52), 0.122(0.12) | 13.8 | |
| 13, (14), 15 | Calcite | CaCO3 | 0.303(1), 0.209(0.18), 0.228(0.18) | 87.1 |
| Briartite | Cu2Zn0.75Fe2+0.25GeS4 | 0.306(1), 0.187(0.5), 0.189(0.5) | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortoluzzi, E.C.; Garibotti, A.; Tiecher, T.; dos Santos, D.R.; Moterle, D.F.; Fiorin, J.E. Revisiting Limestone Quality for Soil Liming Purpose. Minerals 2022, 12, 1522. https://doi.org/10.3390/min12121522
Bortoluzzi EC, Garibotti A, Tiecher T, dos Santos DR, Moterle DF, Fiorin JE. Revisiting Limestone Quality for Soil Liming Purpose. Minerals. 2022; 12(12):1522. https://doi.org/10.3390/min12121522
Chicago/Turabian StyleBortoluzzi, Edson Campanhola, Andressa Garibotti, Tales Tiecher, Danilo Rheinheimer dos Santos, Diovane Freire Moterle, and Jackson E. Fiorin. 2022. "Revisiting Limestone Quality for Soil Liming Purpose" Minerals 12, no. 12: 1522. https://doi.org/10.3390/min12121522
APA StyleBortoluzzi, E. C., Garibotti, A., Tiecher, T., dos Santos, D. R., Moterle, D. F., & Fiorin, J. E. (2022). Revisiting Limestone Quality for Soil Liming Purpose. Minerals, 12(12), 1522. https://doi.org/10.3390/min12121522







